Published online Mar 14, 2022. doi: 10.3748/wjg.v28.i10.1055
Peer-review started: September 14, 2021
First decision: November 16, 2021
Revised: November 29, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 14, 2022
Processing time: 177 Days and 23.7 Hours
It is unclear whether the Japan Narrow-Band Imaging Expert Team (JNET) classification and pit pattern classification are applicable for diagnosing neoplastic lesions in patients with ulcerative colitis (UC).
To clarify the diagnostic performance of these classifications for neoplastic lesions in patients with UC.
This study was conducted as a single-center, retrospective case-control study. Twenty-one lesions in 19 patients with UC-associated neoplasms (UCAN) and 23 lesions in 22 UC patients with sporadic neoplasms (SN), evaluated by magnifying image-enhanced endoscopy, were retrospectively and separately assessed by six endoscopists (three experts, three non-experts), using the JNET and pit pattern classifications. The results were compared with the pathological diagnoses to evaluate the diagnostic performance. Inter- and intra-observer agreements were calculated.
In this study, JNET type 2A and pit pattern type III/IV were used as indicators of low-grade dysplasia, JNET type 2B and pit pattern type VI low irregularity were used as indicators of high-grade dysplasia to shallow submucosal invasive carcinoma, JNET type 3 and pit pattern type VI high irregularity/VN were used as indicators of deep submucosal invasive carcinoma. In the UCAN group, JNET type 2A and pit pattern type III/IV had a low positive predictive value (PPV; 50.0% and 40.0%, respectively); however, they had a high negative predictive value (NPV; 94.7% and 100%, respectively). Conversely, in the SN group, JNET type 2A and pit pattern type III/IV had a high PPV (100% for both) but a low NPV (63.6% and 77.8%, respectively). In both groups, JNET type 3 and pit pattern type VI-high irregularity/VN showed high specificity. The inter-observer agreement of JNET classification and pit pattern classification for UCAN among experts were 0.401 and 0.364, in the same manner for SN, 0.666 and 0.597, respectively. The intra-observer agreements of JNET classification and pit pattern classification for UCAN among experts were 0.387, 0.454, for SN, 0.803 and 0.567, respectively.
The accuracy of endoscopic diagnosis using both classifications was lower for UCAN than for SN. Endoscopic diagnosis of UCAN tended to be underestimated compared with the pathological results.
Core Tip: This retrospective case-control study evaluated the diagnostic performance of the Japan Narrow-Band Imaging Expert Team (JNET) and pit pattern classifications for neoplastic lesions in patients with ulcerative colitis (UC). The JNET and pit pattern classifications did not show high accuracy in diagnosing the pathology and invasion depth of neoplastic lesions in patients with UC. Endoscopic diagnosis of UC-associated neoplasms tended to be underestimated when compared with pathological results. Endoscopic diagnosis of neoplastic lesions in patients with UC is still difficult, and treatment strategies need to be carefully determined.
- Citation: Kida Y, Yamamura T, Maeda K, Sawada T, Ishikawa E, Mizutani Y, Kakushima N, Furukawa K, Ishikawa T, Ohno E, Kawashima H, Nakamura M, Ishigami M, Fujishiro M. Diagnostic performance of endoscopic classifications for neoplastic lesions in patients with ulcerative colitis: A retrospective case-control study. World J Gastroenterol 2022; 28(10): 1055-1066
- URL: https://www.wjgnet.com/1007-9327/full/v28/i10/1055.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i10.1055
Patients with long-standing ulcerative colitis (UC) are at risk for colorectal tumors due to chronic inflammation. The cumulative risk of colorectal cancer at 10, 20, and 30 years after UC onset are reportedly 1.6%, 8.3%, and 18.4%, respectively[1]. Consequent to improvements in UC treatment, long-standing UC cases have gradually increased, and surveillance colonoscopy has become more important. UC patients are exposed to the risk of not only UC-associated neoplasms (UCAN) but also sporadic neoplasms (SN). As the treatment strategy for UCAN greatly differs from that for SN, distinguishing UCAN from SN is important[2]. In line with the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations (SCENIC) consensus statement, endoscopic resection now tends to be accepted as a treatment for endoscopically visible dysplasia[3]. With the support from the SCENIC consensus statement, endoscopic treatments for visible dysplasia have gradually increased and have attracted attention recently[4-6].
UCAN differentiation by endoscopic findings had been described previously. Prior studies revealed that features of surface structure and vascular pattern obtained by magnifying Narrow-Band Imaging (NBI) and chromoendoscopy are useful in diagnosing UCAN[7-11]. Additionally, multimodal endo
The Japan NBI Expert Team (JNET) and pit pattern classifications are useful for determining the pathology and invasion depth of colorectal tumors[13,14]. Both classifications have high reproducibility and good diagnostic accuracy in terms of pathology and invasion depth[15-19], as well as good intra- and inter-observer agreement rates for diagnosing colorectal tumors[18,20]. Dysplastic pit patterns are sometimes observed even in non-dysplastic lesions due to inflammation and regenerative changes in UC patients[21]. Surface and vascular patterns are modified by inflammation in UCAN[22]. These patterns are likely to be modified by inflammation not only in UCAN but also in SN located in the inflamed mucosa. Therefore, whether these endoscopic classifications apply to the diagnosis of neoplastic lesions in UC patients remains unclear. Only a few reports have described the usefulness of both classifications in diagnosing UCAN[22], and there have been no reports on their use for classifying SN in UC patients. Hence, the present retrospective case-control study aimed to evaluate the diagnostic performance of the JNET and pit pattern classifications for neoplastic lesions in UC patients.
A total of 89 UC patients who had neoplastic lesions that could be pathologically evaluated by biopsy, endoscopic resection, or surgery and who underwent colonoscopy at Nagoya University Hospital from August 2005 to April 2020 were consecutively registered. Neoplastic lesions located in the colonic mucosa outside the previously or currently inflamed mucosa were excluded. Additionally, lesions magnified using both NBI or Blue LASER imaging (BLI) and chromoendoscopy with indigo carmine or crystal violet were included. The present study ultimately enrolled 41 UC patients with 44 lesions that could be assessed using both the JNET and pit pattern classifications. According to pathological findings, these patients were divided into two groups—namely, the UCAN group, which comprised 19 patients with 21 lesions, and the SN group, which consisted of 22 UC patients with 23 lesions.
Endoscopists conducted routine white-light imaging observation. When neoplastic lesions were identified, magnifying NBI or BLI and magnifying chromoendoscopy using indigo carmine or crystal violet were performed. All lesions were endoscopically detectable, visually identified, and subsequently diagnosed using target biopsy. The morphological type of neoplasms was categorized in accordance with the SCENIC consensus statement[3]. The severity of inflammation in the mucosa surrounding neoplasms was assessed using the Ulcerative Colitis Endoscopic Index of Severity (UCEIS)[23]. The JNET and pit pattern classifications were employed to evaluate the pathology and invasion depth of neoplasms by endoscopy. With the JNET classification, lesions were categorized based on surface and vascular patterns into types 1, 2A, 2B, and 3. The pit pattern classification was used under indigo carmine or crystal violet observation; lesions were categorized based on form of crypt orifices into types I, II, III, IV, VI low irregularity, VI high irregularity, and VN. This study used JNET type 2A and pit pattern type III/IV as indicators of low-grade dysplasia (LGD), based on previous reports[22]. Furthermore, in the same manner, as LGD, JNET type 2B and pit pattern type VI low irregularity were utilized as indicators of high-grade dysplasia (HGD) to shallow submucosal invasive carcinoma (sSM), whereas JNET type 3 and pit pattern type VI high irregularity/VN were used as indicators of deep submucosal invasive carcinoma (dSM). Endoscopic images corresponding to the part that could be evaluated pathologically were extracted, and six endoscopists (three experts, three non-experts) each evaluated the endoscopic findings. Experts were defined as those with ≥ 5-year experience in magnifying image-enhanced endoscopy and who had managed more than 1000 cases[18]. Endoscopists independently evaluated the images obtained from 44 lesions; when individual diagnostic interpretations differed, they discussed the case until a consensus was reached. Diagnostic performance was assessed by consensus of the first diagnosis of three endoscopists. Inter- and intra-observer agreements were calculated for the diagnostic results of each endoscopist. The second diagnosis was performed by randomly switching the order of images at ≥ 1 mo after the first round of diagnosis to calculate for intra-observer agreement.
Two pathologists specializing in the gastrointestinal tract conducted pathological diagnosis of UCAN and SN according to the Riddell et al[24]’s pathological system. UCAN and SN were differentiated based on pathological results. If necessary, p53 and Ki-67 immunostaining were performed. UCAN was diagnosed for cases with diffuse and strong expression or complete absence of p53 immunostaining[25]. Differentiation of Ki-67-positive cells from the basal mucosal side toward the superficial mucosal side[25,26], called “bottom-up,” was also useful in diagnosing UCAN. Contrary to the UCAN, expression of p53 is low in SN. Moreover, Ki-67-positive cells are mainly distributed at the superficial zone of the mucosal layer, and tumor cells differentiate towards the basal side of the mucosa in the SN[26], also known as “top-down”. Dysplasia was classified into LGD and HGD according to the degree of cellular and nuclear dysplastic change. Submucosal invasive carcinoma was divided into dSM and sSM depending on whether the vertical invasion depth exceeded 1000 μm. When two pathologists had different diagnoses, they discussed the case until a conclusion was reached.
Clinical data, including age at UC onset, disease duration, sex, disease distribution (total colitis, left-sided colitis, proctitis), clinical type (relapse and remission, chronic persistent, first attack), primary sclerosing cholangitis (PSC), and family history of colorectal cancer, were retrospectively collected from medical records and investigated. Endoscopic findings, including location, color, lesion border, morphology, and UCEIS, were obtained from medical reports and evaluated.
Continuous variables were expressed as mean ± SD or as median with range and were compared using Student’s t-test or Mann–Whitney U test, depending on the normality of data distribution, as determined by the Shapiro–Wilk test. Categorial variables were compared using Fisher’s exact test or chi-square test. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for both the JNET and pit pattern classifications. Inter- and intra-observer agreements were calculated using κ coefficient and arbitrarily interpreted as follows: 0–0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.00, excellent. Statistical analysis was conducted using SPSS Statistics version 27 (IBM Corp., Armonk, NY, United States) and R3.6.3 (CRAN, freeware, https://personal.hs.hirosaki-u.ac.jp/pteiki/reserch/stat/R/), with P < 0.05 being indicative of statistical significance.
All patients were divided into two groups according to pathological findings. Inter-observer agreement in the diagnosis between UCAN and SN by the two pathologists was 0.531. The clinical characteristics of both groups are summarized in Table 1. The UCAN group had a significantly lower mean age at UC onset than the SN group (35.7 vs 48.8 years, P = 0.003). Pathological findings indicated 2 LGD lesions, 11 HGD lesions, 3 sSM lesions, and 5 dSM lesions in the UCAN group and 16 LGD lesions, 5 HGD lesions, and 2 dSM lesions in the SN group. No significant differences in the disease duration, sex, extent of disease, disease distribution, presence of PSC, and family history of colorectal cancer were identified between the two groups.
| UCAN group (n = 19) | SN group (n = 22) | P value | |
| Age at UC onset (yr) | 35.7 ± 10.9 | 48.8 ± 14.6 | 0.003a |
| Disease duration (yr) | 17.8 ± 9.4 | 12.9 ± 10.7 | 0.120a |
| Sex | 0.829b | ||
| Male | 11 | 12 | |
| Female | 8 | 10 | |
| Extent of disease | 0.231b | ||
| Total colitis | 16 | 14 | |
| Left-sided colitis | 3 | 6 | |
| Proctitis | 0 | 2 | |
| Clinical type | 0. 139b | ||
| Relapse and remission | 10 | 15 | |
| Chronic persistent | 9 | 5 | |
| First attack | 0 | 2 | |
| Pathological type | < 0.001b | ||
| LGD | 2 | 16 | |
| HGD | 11 | 5 | |
| Shallow submucosal invasive carcinoma | 3 | 0 | |
| Deep submucosal invasive carcinoma | 5 | 2 | |
| Primary sclerosing cholangitis | 1 | 0 | 0.463c |
| Family history of colorectal cancer | 1 | 2 | 0.639c |
The endoscopic findings for both groups are presented in Table 2. A total of 17 (81.0%) and 12 (52.2%) lesions were detected in the proctosigmoid colon in the UCAN and SN groups, respectively (P = 0.044). The UCAN group had a higher percentage of reddish lesions than the SN group (71.4% vs 39.1%, P = 0.032). All lesions in the SN group exhibited a clear border, whereas 16 lesions (76.2%) in the UCAN group showed an unclear border (P < 0.001). The UCAN group had a higher proportion of flat or depressed lesions than the SN group (28.6% vs 8.7%, P = 0.094). Inflammation in the mucosa surrounding neoplasms was more severe in the UCAN group than in the SN group [UCEIS (median): 2 vs 0, P < 0.001].
| UCAN group (n = 21) | SN group (n = 23) | P value | |
| Tumor location | 0.044a | ||
| Proctosigmoid colon | 17 | 12 | |
| Others | 4 | 11 | |
| Tumor color | 0.032a | ||
| Red | 15 | 9 | |
| Pale or the same as the surrounding mucosa | 6 | 14 | |
| Lesion border | < 0.001a | ||
| Clear | 5 | 23 | |
| Unclear | 16 | 0 | |
| Tumor morphology | 0.173a | ||
| Pedunculated | 0 | 0 | |
| Sessile | 8 | 7 | |
| Superficial elevated | 7 | 14 | |
| Flat | 2 | 0 | |
| Depressed | 4 | 2 | |
| UCEIS (median, range) | 2 (0–4) | 0 (0–5) | < 0.001b |
Diagnostic performance for each type in the JNET and pit pattern classifications is shown in Tables 3 and 4, respectively. Sensitivity, specificity, PPV, NPV, and accuracy were calculated for experts and non-experts separately.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
| UCAN JNET type 2A | |||||
| Experts | 50.0 (10.2–85.6) | 94.7 (90.5–98.5) | 50.0 (10.2–85.6) | 94.7 (90.5–98.5) | 90.5 (82.9–97.3) |
| Non-experts | 100 (36.9–100) | 78.9 (72.3–78.9) | 33.3 (12.3–33.3) | 100 (91.6–100) | 81.0 (68.9–81.0) |
| UCAN JNET type 2B | |||||
| Experts | 78.6 (64.6–89.5) | 57.1 (29.2–79.1) | 78.6 (64.6–89.5) | 57.1 (29.2–79.1) | 71.4 (52.8–86.1) |
| Non-experts | 78.6 (63.8–87.9) | 71.4 (41.9–90.0) | 84.6 (68.7–94.6) | 62.5 (36.6–78.8) | 76.2 (56.5–88.6) |
| UCAN JNET type 3 | |||||
| Experts | 60.0 (27.5–75.9) | 93.8 (83.6–98.7) | 75.0 (34.4–94.9) | 88.2 (78.7–92.9) | 85.7 (70.3–93.3) |
| Non-experts | 40.0 (14.9–40.0) | 100 (92.2–100) | 100 (37.3–100) | 84.2 (77.6–84.2) | 85.7 (73.8–85.7) |
| SN JNET type 2A | |||||
| Experts | 75.0 (62.6–75.0) | 100 (71.6–100) | 100 (83.4–100) | 63.6 (45.5–63.6) | 82.6 (65.3–82.6) |
| Non-experts | 87.5 (74.7–92.5) | 85.7 (56.4–97.1) | 93.3 (79.6–98.7) | 75.0 (49.3–85.0) | 87.0 (69.1–93.9) |
| SN JNET type 2B | |||||
| Experts | 100 (63.4–100) | 83.3 (73.2–83.3) | 62.5 (39.6–62.5) | 100 (87.8–100) | 87.0 (71.0–87.0) |
| Non-experts | 80.0 (42.6–96.1) | 83.3 (72.9–87.8) | 57.1 (30.4–68.7) | 93.8 (82.1–98.8) | 82.6 (66.4–89.6) |
| SN JNET type 3 | |||||
| Experts | 50.0 (11.2–50.0) | 100 (96.3–100) | 100 (22.4–100) | 95.5 (91.9–95.5) | 95.7 (88.9–95.7) |
| Non-experts | 50.0 (11.2–50.0) | 100 (96.3–100) | 100 (22.4–100) | 95.5 (91.9–95.5) | 95.7 (88.9–95.7) |
| Sensitivity, (%) | Specificity, (%) | PPV, (%) | NPV, (%) | Accuracy, (%) | |
| UCAN pit pattern type III/IV | |||||
| Experts | 100 (37.3–100) | 84.2 (77.6–84.2) | 40.0 (14.9–40.0) | 100 (92.2–100) | 85.7 (73.8–85.7) |
| Non-experts | 100 (36.4–100) | 57.9 (51.2–57.9) | 20.0 (7.3–20.0) | 100 (88.4–100) | 61.9 (49.8–61.9) |
| UCAN pit pattern type VI low irregularity | |||||
| Experts | 50.0 (35.2–59.8) | 71.4 (41.9–91.0) | 77.8 (54.8–93.0) | 41.7 (24.4–53.1) | 57.1 (37.5–70.2) |
| Non-experts | 42.9 (28.4–48.7) | 85.7 (56.7–97.3) | 85.7 (56.7–97.3) | 42.9 (28.4–48.7) | 57.1 (37.8–64.9) |
| UCAN pit pattern type VI high irregularity/VN | |||||
| Experts | 60.0 (26.1–85.8) | 81.3 (70.7–89.3) | 50.0 (21.8–71.5) | 86.7 (75.4–95.3) | 76.2 (60.1–88.5) |
| Non-experts | 60.0 (27.5–75.9) | 93.8 (83.6–98.7) | 75.0 (34.4–94.9) | 88.2 (78.7–92.9) | 85.7 (70.3–93.3) |
| SN pit pattern type III/IV | |||||
| Experts | 87.5 (75.6–87.5) | 100 (72.8–100) | 100 (86.4–100) | 77.8 (56.6–77.8) | 91.3 (74.8–91.3) |
| Non-experts | 81.3 (68.1–86.3) | 85.7 (55.6–97.3) | 92.9 (77.8–98.6) | 66.7 (43.3–75.6) | 82.6 (64.3–89.6) |
| SN pit pattern type VI low irregularity | |||||
| Experts | 100 (63.4–100) | 83.3 (73.2–83.3) | 62.5 (39.6–62.5) | 100 (87.8–100) | 87.0 (71.0–87.0) |
| Non-experts | 60.0 (26.0–85.9) | 83.3 (73.9–90.5) | 50.0 (21.7–71.6) | 88.2 (78.2–95.8) | 78.3 (63.5–89.5) |
| SN pit pattern type VI high irregularity/VN | |||||
| Experts | 50.0 (11.2–50.0) | 100 (96.3–100) | 100 (22.4–100) | 95.5 (91.9–95.5) | 95.7 (88.9–95.7) |
| Non-experts | 50.0 (10.2–85.6) | 95.2 (91.4–98.6) | 50.0 (10.2–85.6) | 95.2 (91.4–98.6) | 91.3 (84.4–97.5) |
In the UCAN group, JNET type 2A had a low PPV [experts vs non-experts: 50.0% (10.2–85.6) vs 33.3% (12.3–33.3)] and a high NPV [experts vs non-experts: 94.7% (90.5–98.5) vs 100% (91.6–100)] for both experts and non-experts. Conversely, in the SN group, JNET type 2A had a high PPV [experts vs non-experts: 100% (83.4–100) vs 93.3% (79.6–98.7)] and a low NPV [experts vs non-experts: 63.6% (45.5–63.6) vs 75.0% (49.3–85.0)]. In the UCAN group, the accuracy of diagnosis for JNET types 2A, 2B, and 3 by experts was 90.5%, 71.4%, and 85.7%, respectively, and that by non-experts was 81.0%, 76.2%, and 85.7%, respectively.
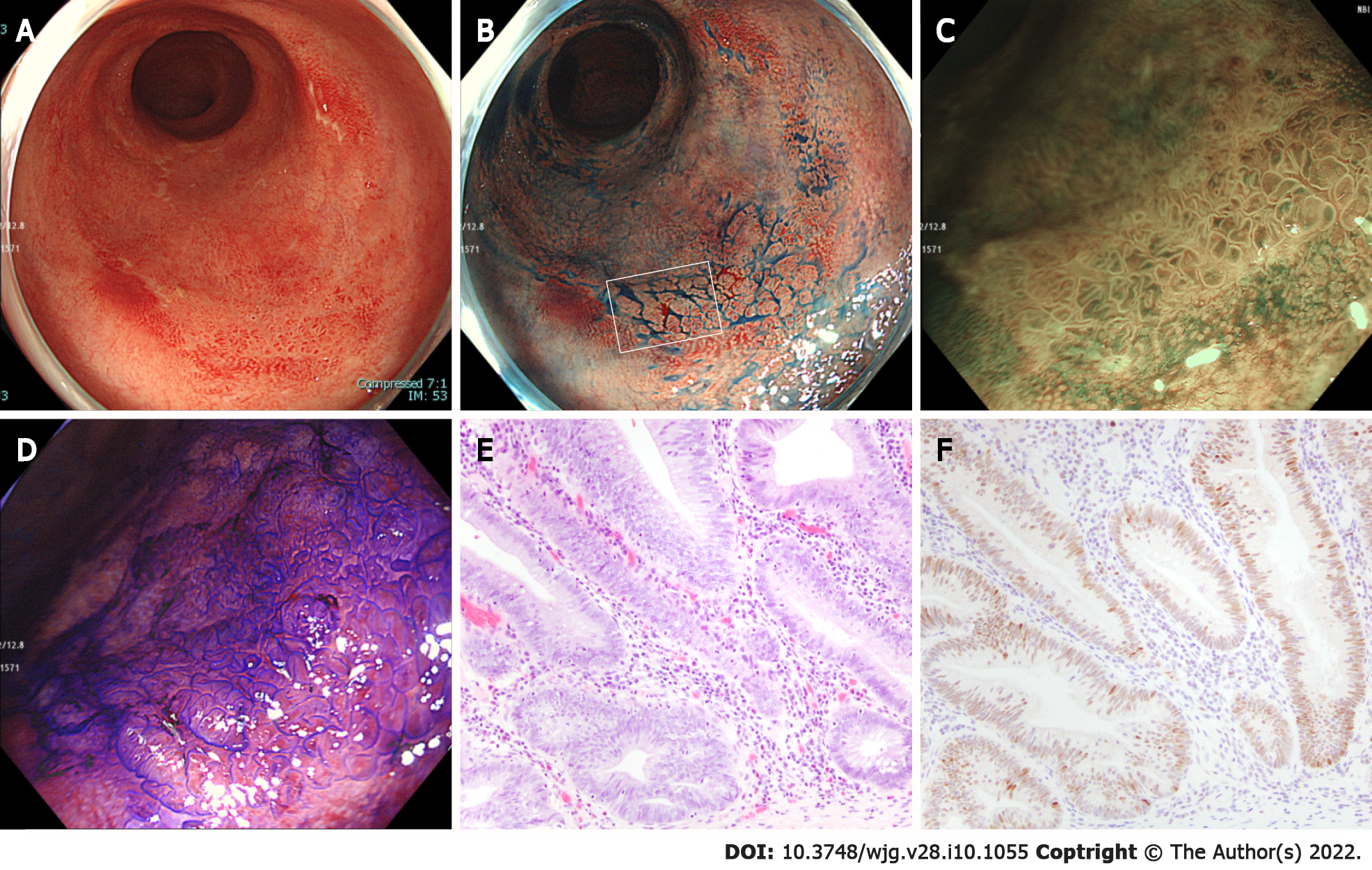
In the UCAN group, pit pattern type III/IV had a low PPV [experts vs non-experts: 40.0% (14.9–40.0) vs 20.0% (7.3–20.0)] and a high NPV [experts vs non-experts: 100% (92.2–100) vs 100% (88.4–100)] for both experts and non-experts. Conversely, in the SN group, pit pattern type III/IV had a high PPV [experts vs non-experts: 100% (86.4–100) vs 92.9% (77.8–98.6)] and a low NPV [77.8% (56.6–77.8) vs 66.7% (43.3–75.6)]. In the UCAN group, the accuracy of diagnosis for pit pattern type III/IV, type VI low irregularity, and type VI high irregularity/VN by experts was 85.7%, 57.1%, and 76.2%, respectively, and that by non-experts was 61.9%, 57.1%, and 85.7%, respectively. The accuracy of diagnosis for JNET type 3 and pit pattern type VI high irregularity/VN by both experts and non-experts was higher in the SN group than in the UCAN group. Figure 1 shows a representative case of UCAN misdiagnosed by all endoscopists.
Intra-observer agreement was separately calculated for experts and non-experts (Table 5). The intra-observer agreement among experts for the JNET classification of UCAN, pit pattern classification of UCAN, JNET classification of SN, and pit pattern classification of SN was 0.387, 0.454, 0.803, and 0.567, respectively. The corresponding values for non-experts were 0.640, 0.569, 0.828, and 0.628, respectively. The intra-observer agreement for SN was higher than that for UCAN. Among non-experts, the intra-observer agreement for both UCAN and SN was higher with the JNET classification than with the pit pattern classification.
| Experts | Non-experts | |
| UCAN | ||
| JNET classification | 0.387 (0.369–0.521) | 0.640 (0.566–0.708) |
| Pit pattern classification | 0.454 (0.391–0.509) | 0.569 (0.422–0.599) |
| SN | ||
| JNET classification | 0.803 (0.581–0.832) | 0.828 (0.686–0.849) |
| Pit pattern classification | 0.567 (0.477–0.595) | 0.628 (0.422–0.766) |
Inter-observer agreement was calculated similarly (Table 6). The inter-observer agreement among experts for the JNET classification of UCAN, pit pattern classification of UCAN, JNET classification of SN, and pit pattern classification of SN was 0.401, 0.364, 0.666, and 0.597, respectively. The corresponding values for non-experts were 0.237, 0.378, 0.503, and 0.437, respectively. Overall, the inter-observer agreement for SN was higher than that for UCAN among both experts and non-experts, irrespective of the classification system used.
| Experts | Non-experts | |
| UCAN | ||
| JNET classification | 0.401 | 0.237 |
| Pit pattern classification | 0.364 | 0.378 |
| SN | ||
| JNET classification | 0.666 | 0.503 |
| Pit-pattern classification | 0.597 | 0.437 |
In the present study, we evaluated the performance of the JNET and pit pattern classifications in patients with UC. The JNET classification evaluates the tumors’ surface and vascular patterns, whereas the pit pattern classification assesses the form of pits on the tumor surface. Colonic mucosal inflammation in UC patients modifies the tumors’ surface and vascular patterns and is considered to reduce the diagnostic accuracy of both classifications. Here, we revealed that the accuracy of diagnosing colorectal tumors using JNET and pit pattern classifications was lower in UC patients, particularly those with UCAN, than in non-UC patients[15-18]. The agreement rates were lower for both UCAN and SN patients than for non-UC patients. The diagnostic performance of both classifications in UC patients is substantially lower than their previously reported diagnostic performance in non-UC patients[15-18].
Previous reports revealed that, compared to SN, UCAN is more common in the proctosigmoid colon and features more redness, unclear border, flat and depressed lesions, and a higher degree of surrounding inflammation[2]. On magnifying chromoendoscopy, pit pattern types III, IV, and V, which are also caused by regenerative changes, are useful in diagnosing UCAN[9,21]. In the present study, the UCAN group had a significantly higher proportion of lesions with endoscopically unclear border and severe inflammation in the mucosa surrounding neoplasms than the SN group. As colorectal tumors can considerably impact the quality of life of UC patients, it is essential for endoscopists to understand these endoscopic features of UCAN.
In the UCAN group, JNET type 2A and pit pattern type III/IV had a low PPV but with a high NPV in LGD diagnosis, and JNET type 2B and pit pattern type VI low irregularity had a low NPV in the diagnosis of HGD to sSM. This was because several lesions in UCAN were diagnosed as JNET type 2A or pit pattern type III/IV, even though they were actually HGD to sSM. Additional detailed analysis revealed that most endoscopists diagnosed about one-quarter of HGD to sSM lesions as JNET type 2A and one-third of HGD to sSM lesions as pit pattern type III/IV. Furthermore, a small number of dSM lesions were diagnosed as JNET type 2A or pit pattern type III/IV (Table 7).
| Number | Pathological result | |||
| LGD (n = 2) | HGD-sSM (n = 14) | dSM (n = 5) | ||
| Endoscopist 1 | ||||
| JNET type 2A | 8 | 1 | 7 | 0 |
| Pit type III/IV | 7 | 2 | 3 | 2 |
| Endoscopist 2 | ||||
| JNET type 2A | 2 | 1 | 1 | 0 |
| Pit type III/IV | 5 | 2 | 3 | 0 |
| Endoscopist 3 | ||||
| JNET type 2A | 2 | 0 | 1 | 1 |
| Pit type III/IV | 7 | 2 | 5 | 0 |
| Endoscopist 4 | ||||
| JNET type 2A | 9 | 2 | 6 | 1 |
| Pit type III/IV | 8 | 2 | 5 | 1 |
| Endoscopist 5 | ||||
| JNET type 2A | 5 | 2 | 2 | 1 |
| Pit type III/IV | 8 | 2 | 6 | 0 |
| Endoscopist 6 | ||||
| JNET type 2A | 7 | 2 | 5 | 0 |
| Pit type III/IV | 9 | 2 | 6 | 1 |
In the SN group, JNET type 2A and pit pattern type III/IV had a high PPV but with a low NPV in LGD diagnosis, and JNET type 2B and pit pattern type VI low irregularity had a low PPV in the diagnosis of HGD to sSM. Because JNET type 2B and pit pattern type VI low irregularity include lesions from LGD to dSM, these types have low PPV even in non-UC patients[17,19,27]. Several LGD and dSM lesions in the SN group were diagnosed as JNET type 2B and pit pattern type VI low irregularity. JNET type 3 and pit pattern type VI high irregularity/VN in both UCAN and SN groups showed low sensitivity but with high specificity and accuracy. Previous studies showed that JNET type 3 and pit pattern type VI high irregularity/VN have high specificity for dSM diagnosis in both UC and non-UC patients[17-19,22]. Regardless of whether the surface and vascular patterns are modified by inflammation, JNET type 3 and pit pattern type VI high irregularity/VN have been confirmed to be useful in diagnosing dSM among UC patients. In UCAN, the tumors’ surface structure sometimes could not represent the dysplastic change due to the bottom-up growth pattern; hence, it is considered that several lesions are underestimated by endoscopic classifications. Additionally, SN located in the inflamed mucosa, especially SN in the severely inflamed mucosa, tends to be misdiagnosed due to the influence of inflammation.
Intra-observer agreement was higher among non-experts than among experts; however, the difference was not statistically significant. Inter-observer agreement did not also significantly differ but was higher in experts than in non-experts. Irrespective of the endoscopists’ experience, a consistent endoscopic diagnosis of neoplastic lesions in UC patients was difficult to achieve. In particular, the intra- and inter-observer agreements were lower for UCAN than for SN.
The present study has some limitations. First, our study was conducted on a small number of cases; however, given its retrospective nature, the same size could not be set a priori. We believe that the small number of typical LGDs in this study was responsible for the unsatisfactory diagnostic accuracy. Second, only neoplastic lesions evaluated using both the JNET and pit pattern classifications were included. While inflammation and regenerative changes might be evaluated as neoplastic patterns by both JNET and pit pattern classifications, our study could not include non-neoplastic lesions. Non-neoplastic lesions should be included in future studies. Third, differentiation between UCAN and SN was based on pathology; nevertheless, even in pathology, distinguishing UCAN from SN can be difficult.
The JNET and pit pattern classifications did not show high accuracy in diagnosing the pathology and invasion depth of neoplastic lesions in UC patients. Overall, the endoscopic diagnosis of UCAN tended to be underestimated as compared to the pathological results. Endoscopic diagnosis of neoplastic lesions in UC patients is still difficult, and treatment strategies need to be carefully determined.
Patients with long-standing ulcerative colitis (UC) have a risk of colorectal tumors due to chronic inflammation. Endoscopic treatments for patients with UC have gradually increased and have attracted attention recently.
Surface and vascular patterns of tumors located in the inflamed mucosa are likely to be modified by inflammation. For that reasons, it is unclear whether the Japan Narrow-Band Imaging Expert Team (JNET) classification and pit pattern classification are applicable to the diagnosis of neoplastic lesions in patients with UC.
The present study aimed to clarify the diagnostic performance of JNET and pit pattern classifications for neoplastic lesions in patients with UC.
We analyzed 41 UC patients with 44 lesions that could be assessed using both the JNET and pit pattern classifications. We devided them into the UC-associated neoplasms (UCAN) group (21 lesions) and sporadic neoplasms (SN) group (23 lesions) according to the pathological results. Six endoscopists each evaluated the endoscopic findings by using both endoscopic classifications.
In the UCAN group, the accuracy of diagnosis for JNET types 2A, 2B, and 3 by experts was 90.5%, 71.4%, and 85.7%, respectively. In the same manner, the accuracy of diagnosis for pit pattern type III/IV, type VI low irregularity, and type VI high irregularity/VN by experts was 85.7%, 57.1%, and 76.2%, respectively.
The JNET and pit pattern classifications did not show high accuracy in diagnosing the pathology and invasion depth of neoplastic lesions in patients with UC. Endoscopic diagnosis of UCAN tended to be underestimated, as compared to the pathological results.
Future prospective studies with a large number of UC patients are needed in clinical practice.
We would like to thank Go Kajikawa, Masaya Esaki, Issei Hasegawa, Kentaro Yamada, and Shuji Ikegami for evaluating the endoscopic data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Nakeep S, Zuo C S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2075] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 2. | Mutaguchi M, Naganuma M, Sugimoto S, Fukuda T, Nanki K, Mizuno S, Hosoe N, Shimoda M, Ogata H, Iwao Y, Kanai T. Difference in the clinical characteristic and prognosis of colitis-associated cancer and sporadic neoplasia in ulcerative colitis patients. Dig Liver Dis. 2019;51:1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489-501.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 270] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 4. | Matsumoto K, Oka S, Tanaka S, Tanaka H, Boda K, Yamashita K, Sumimoto K, Ninomiya Y, Arihiro K, Shimamoto F, Chayama K. Long-Term Outcomes after Endoscopic Submucosal Dissection for Ulcerative Colitis-Associated Dysplasia. Digestion. 2021;102:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Suzuki N, Toyonaga T, East JE. Endoscopic submucosal dissection of colitis-related dysplasia. Endoscopy. 2017;49:1237-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Yang DH, Kim J, Song EM, Chang K, Lee SH, Hwang SW, Park SH, Ye BD, Byeon JS, Myung SJ, Yang SK. Outcomes of ulcerative colitis-associated dysplasia patients referred for potential endoscopic submucosal dissection. J Gastroenterol Hepatol. 2019;34:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Nishiyama S, Oka S, Tanaka S, Sagami S, Hayashi R, Ueno Y, Arihiro K, Chayama K. Clinical usefulness of narrow band imaging magnifying colonoscopy for assessing ulcerative colitis-associated cancer/dysplasia. Endosc Int Open. 2016;4:E1183-E1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | East JE, Suzuki N, von Herbay A, Saunders BP. Narrow band imaging with magnification for dysplasia detection and pit pattern assessment in ulcerative colitis surveillance: a case with multiple dysplasia associated lesions or masses. Gut. 2006;55:1432-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 557] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 10. | Nishiyama S, Oka S, Tanaka S, Hayashi N, Hayashi R, Nagai K, Ueno Y, Shimamoto F, Arihiro K, Chayama K. Is it possible to discriminate between neoplastic and nonneoplastic lesions in ulcerative colitis by magnifying colonoscopy? Inflamm Bowel Dis. 2014;20:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Shinagawa T, Hata K, Morikawa T, Takiyama H, Emoto S, Murono K, Kaneko M, Sasaki K, Nishikawa T, Tanaka T, Kawai K, Fukayama M, Nozawa H. Pine-cone and villi patterns are endoscopic signs suggestive of ulcerative colitis-associated colorectal cancer and dysplasia. Gastrointest Endosc. 2019;89:565-575.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Iacucci M, McQuaid K, Gui XS, Iwao Y, Lethebe BC, Lowerison M, Matsumoto T, Shivaji UN, Smith SCL, Subramanian V, Uraoka T, Sanduleanu S, Ghosh S, Kiesslich R. A multimodal (FACILE) classification for optical diagnosis of inflammatory bowel disease associated neoplasia. Endoscopy. 2019;51:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 402] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 14. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 707] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 15. | Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007;39:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Li M, Ali SM, Umm-a-OmarahGilani S, Liu J, Li YQ, Zuo XL. Kudo's pit pattern classification for colorectal neoplasms: a meta-analysis. World J Gastroenterol. 2014;20:12649-12656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Kobayashi S, Yamada M, Takamaru H, Sakamoto T, Matsuda T, Sekine S, Igarashi Y, Saito Y. Diagnostic yield of the Japan NBI Expert Team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large-scale clinical practice database. United European Gastroenterol J. 2019;7:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Komeda Y, Kashida H, Sakurai T, Asakuma Y, Tribonias G, Nagai T, Kono M, Minaga K, Takenaka M, Arizumi T, Hagiwara S, Matsui S, Watanabe T, Nishida N, Chikugo T, Chiba Y, Kudo M. Magnifying Narrow Band Imaging (NBI) for the Diagnosis of Localized Colorectal Lesions Using the Japan NBI Expert Team (JNET) Classification. Oncology. 2017;93 Suppl 1:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Kanao H, Tanaka S, Oka S, Kaneko I, Yoshida S, Arihiro K, Yoshihara M, Chayama K. Clinical significance of type V(I) pit pattern subclassification in determining the depth of invasion of colorectal neoplasms. World J Gastroenterol. 2008;14:211-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Nakano A, Hirooka Y, Yamamura T, Watanabe O, Nakamura M, Funasaka K, Ohno E, Kawashima H, Miyahara R, Goto H. Comparison of the diagnostic ability of blue laser imaging magnification versus pit pattern analysis for colorectal polyps. Endosc Int Open. 2017;5:E224-E231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Hata K, Watanabe T, Motoi T, Nagawa H. Pitfalls of pit pattern diagnosis in ulcerative colitis-associated dysplasia. Gastroenterology. 2004;126:374-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Kawasaki K, Nakamura S, Esaki M, Kurahara K, Eizuka M, Nuki Y, Kochi S, Fujiwara M, Oshiro Y, Sugai T, Matsumoto T. Clinical usefulness of magnifying colonoscopy for the diagnosis of ulcerative colitis-associated neoplasia. Dig Endosc. 2019;31 Suppl 1:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lichtenstein GR, Marteau PR, Reinisch W, Sands BE, Yacyshyn BR, Schnell P, Bernhardt CA, Mary JY, Sandborn WJ. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013;145:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 24. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1348] [Cited by in RCA: 1213] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 25. | Wong NA, Mayer NJ, MacKell S, Gilmour HM, Harrison DJ. Immunohistochemical assessment of Ki67 and p53 expression assists the diagnosis and grading of ulcerative colitis-related dysplasia. Histopathology. 2000;37:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Mikami T, Yoshida T, Akino F, Motoori T, Yajima M, Okayasu I. Apoptosis regulation differs between ulcerative colitis-associated and sporadic colonic tumors. Association with survivin and bcl-2. Am J Clin Pathol. 2003;119:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Wada Y, Kashida H, Kudo SE, Misawa M, Ikehara N, Hamatani S. Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig Endosc. 2010;22:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |