Published online Jan 7, 2022. doi: 10.3748/wjg.v28.i1.76
Peer-review started: May 30, 2021
First decision: June 11, 2021
Revised: June 26, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 7, 2022
Processing time: 214 Days and 18.6 Hours
Viral hepatitis results in 1.4 million deaths annually. The World Health Orga
Core Tip: Viral hepatitis results in 1.4 million deaths annually. The World Health Organization set an ambitious target to eliminate viral hepatitis by 2030, but significant challenges remain. These include inequalities in access to healthcare, reaching at risk populations and providing access to screening and effective treatment. In this review article, we discuss the advances in the field of viral hepatitis over the past decade. We also discuss the remaining challenges relating to viral hepatitis A to E, and suggest strategies and pathways for their resolution.
- Citation: Dunn R, Wetten A, McPherson S, Donnelly MC. Viral hepatitis in 2021: The challenges remaining and how we should tackle them. World J Gastroenterol 2022; 28(1): 76-95
- URL: https://www.wjgnet.com/1007-9327/full/v28/i1/76.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i1.76
Our understanding of the epidemiology of viral hepatitis and associated treatment strategies has advanced significantly over the past decade. Arguably, the most significant advances have occurred in the treatment of chronic hepatitis C, which is now curable with a short course of all oral antiviral therapy. Despite this, viral hepatitis still kills more than 1.4 million people a year[1]. As such, viral hepatitis has become a global health priority and a number of large-scale public health policies have been implemented. The World Health Organization (WHO) has set out an ambitious global elimination strategy for viral hepatitis, aiming to eliminate viral hepatitis as a public health threat by 2030[2]. Key interventions for viral elimination have been identified and include hepatitis B vaccination, facilitation of safe injection practices and safe blood transfusions, promotion of safe sex, hepatitis B treatment and hepatitis C cure. However, modelling studies suggest that up to 80% of high-income countries will not meet the WHO target[3].
This review article will focus on hepatitis A-E, highlighting problems that have been resolved in the field over the past decade, those that remain to be resolved and suggest directions for future problem solving and research. We will also discuss the impact of the coronavirus 2019 (COVID-19) pandemic on viral elimination.
A PubMed search was performed using the following terms: “hepatitis A”; “hepatitis B”; “hepatitis C”; “hepatitis D”; “delta agent”; “hepatitis E”; “cirrhosis”; “direct acting antivirals”; “chronic kidney disease”; “chronic liver disease”; “functional cure”; “hepatocellular carcinoma”; “liver transplant”; “reinfection”; “ribavirin”; “viral elimination”; “viral resistance”; “virologic cure”. Only English-language articles were included in this review. Reference lists of selected articles were reviewed for relevant studies. Published abstracts were included.
Worldwide, the incidence of hepatitis A virus (HAV) is decreasing[4,5], but with increasing globalization there are significant shifts in the epidemiology of HAV infection[6]. Due to a large number of cases being asymptomatic and an estimated under-reporting of up to 80% of cases, it is acknowledged that the true incidence is difficult to quantify[7]. The incidence rate of HAV infection is strongly correlated with socioeconomic indicators; the incidence decreases with increasing access to clean water and sanitation. HAV infection is commonly reported in countries where conflict leads to the displacement of people, resulting in poor sanitation and overcrowding[8].
Recent studies have expanded our understanding of the molecular virology and pathobiology of HAV. It is likely that multiple immune mechanisms contribute to the development of acute liver injury due to HAV infection, including decreased fre
The WHO estimated that HAV infection caused approximately 7134 deaths in 2016[11]. In the United States, case-fatality estimates range from 0.3% to 0.6% for all age groups, rising to 1.8% amongst patients aged > 50 years[12]. A safe and effective inactivated vaccine has been in use for almost 30 years[13]. It was initially developed for individual prophylaxis, but now is used to control endemics[13]. A live attenuated vaccine has been developed and licensed in China and it is used in the Chinese national vaccination program. Use of this vaccine in children has reportedly reduced the incidence of HAV infection by 80%[14]. There are now 34 countries that use or are planning to introduce HAV vaccination into routine immunization of children in specific risk groups[11]. Within the United Kingdom, persons who are considered high-risk for HAV infection and should be offered vaccination include those in close contact with someone with HAV infection, travelers who plan to travel to parts of the world where HAV is highly endemic, persons with chronic liver disease, men who have sex with other men (MSM), people who inject drugs (PWIDs) and those who are likely to be exposed to HAV from their employment, for example workers who are exposed to raw sewage such as within the construction industry.
Another advance in the past decade has been in the area of post-exposure prophylaxis (PEP) against HAV. PEP is recommended for persons who are immunocompromised and those who have chronic liver disease[15]. Immunoglobulin was previously the only recommended PEP however due to a number of factors including declining anti-HAV IgG titres in donor pools, new strategies were sought. Recent data support post-exposure immunization with an inactivated HAV vaccine as being effective in preventing infection when given within 14 d of exposure[13].
Prevention of infection in high-risk populations (including targeted vaccination): With increasing numbers of forcibly displaced persons in certain parts of the world[16], endemic HAV infection will continue to be an ongoing but preventable issue that requires a global response to provide public health infrastructure, sanitation and free HAV vaccination programmes. This approach requires significant input from public health agencies and politicians alike.
Person to person transmission is described, with infection reported amongst PWIDs and homeless populations. These populations can be difficult to engage, and vac
Treatment of severe liver injury due to HAV infection: Although rare, patients with acute HAV infection can progress to acute liver failure (ALF)[7]. Whilst these patients can recover with supportive management, a small number of patients may require transplantation. Patients progressing to ALF are typically older and may not be suitable candidates for liver transplantation, and therefore other specific treatment strategies are required. Furthermore, liver transplantation is not accessible to those most at risk in displaced communities. Ribavirin has successfully been used in treatment of acute hepatitis E infection; it has been shown to have an inhibitory effect on HAV in vitro but has not been assessed in vivo for therapeutic activity[22].
Chronic hepatitis B infection is a global problem, but the burden of disease is mostly in low to middle income countries, with 248 million of the estimated 292 million people affected residing in Asia, Africa, the Pacific and Latin America. Chronic hepatitis B virus (HBV) accounts for approximately 47% of all viral hepatitis related deaths, the vast majority of which are secondary to complications of chronic liver disease[23,24].
In 2017 the nomenclature to describe the different phases of chronic HBV changed within the updated European Association for the Study of the Liver (EASL) hepatitis B guidelines[25]. This was to better reflect and highlight the two main pathological processes of chronic infection and chronic hepatitis, in particular taking into account the presence of hepatitis B e antigen (HBeAg), HBV DNA levels, alanine aminotransferase (ALT) values and the presence or absence of liver inflammation. The new definition of phases highlights the increased risk of advancing liver disease in both chronic hepatitis phases - even in HBeAg negative patients - where there is elevated HBV DNA levels and/or elevated ALT, removing the somewhat misleading term “inactive carrier”. These changes in nomenclature have now been widely adopted[24].
Multiple societies now provide guidance on when to initiate treatment. Viral resistance to treatment is a problem which has now been largely overcome. The nucleos(t)ide analogues (NAs) entecavir (ETV), tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide are recommended as first-line treatment in both American and European HBV guidelines[25,26]. These agents show high rates of viral supp
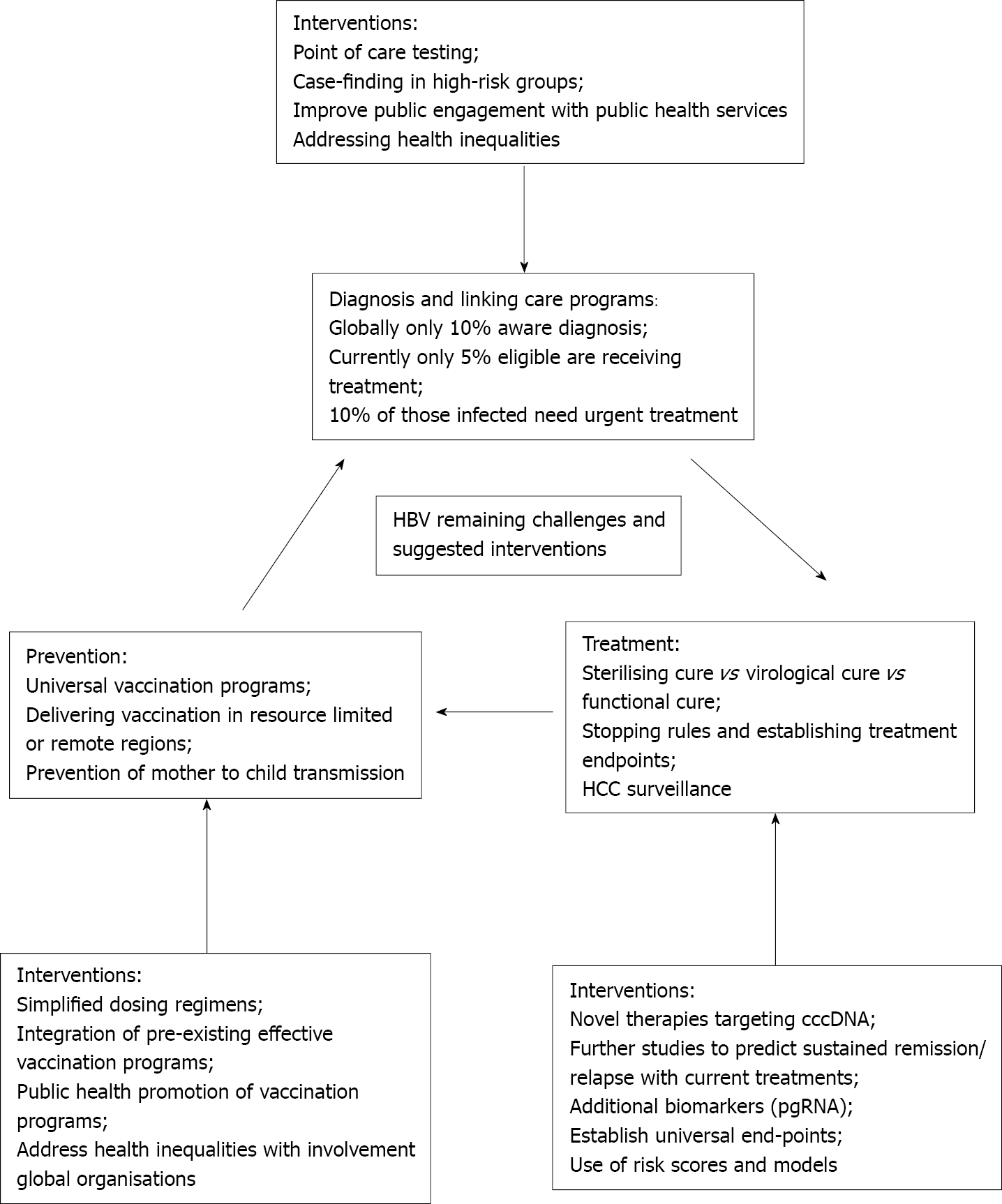
There remain a number of challenges in the diagnosis and management of patients with chronic hepatitis B infection - Figure 1.
Diagnosing and linking infected patients to care programmes: A significant pro
New point of care (POC) tests are also becoming available, making diagnosing infection easier and quicker. For example, the Determine HBsAg 2 test provides a HBsAg result in 15 min with high sensitivity and specificity[33]. POC tests allow testing and diagnosis to move out of established health care settings and may be of particular utility in resource poor settings and high-risk communities.
Increasing testing and subsequent diagnosis rates relies on public engagement to break down stereotypes and address stigma, improved interactions with health care services and addressing health inequalities arising from poverty and language barriers[34,35]. Collaboration and integration with other successful public health programs such as human immunodeficiency virus (HIV) services is also likely to be effective.
Defining cure: A ‘cure’ for HBV might be considered as one where the virus is completely eliminated [undetectable HBsAg, HBeAg, HBV DNA and hepatic co
The term ‘sterilising cure’ (complete eradication of the virus) has been replaced with ’functional cure’. Functional cure is currently defined as sustained HBsAg loss, undetectable HBV DNA, with or without seroconversion to hepatitis B surface antibody, following a finite course of treatment[25] and it occurs in 1% of chronically infected patients annually[37]. However, HBV genomes can persist in the liver even if HBsAg is undetectable questioning the true value of achieving a functional cure. A ‘partial functional cure’ is considered an intermediate goal of therapy and signifies detectable HBsAg but persistent undetectable HBV DNA 6 mo post-treatment. Virologic cure is essentially ‘halting’ all forms of HBV replication, however difficulties with obtaining virologic cure remain due to the persistence of cccDNA in hepatocytes. To obtain virologic cure, treatments inhibiting both cccDNA and viral replication are required[38].
An agreed definition of cure remains elusive, however with clearly defining treatment endpoints and new therapies targeting different aspects of the HBV life cycle, virologic cure may be achievable in the future.
Striving for prevention rather than cure: To prevent HBV infection, there needs to be a focus on improving vaccination strategies. Barriers to HBV vaccination, particularly in resource limited or remote regions, can be attributed to inadequate resources to acquire vaccinations, current dosing regimens, insufficient trained health staff for administration of the vaccine and lack of facilities to keep vaccinations between 2-8 oC. One study of a two-dose regime of HBsAg-1018 (containing HBsAg plus a toll-like receptor 9 agonist adjuvant) demonstrated a higher seroprotection rate at one year compared with the standard three dose regimen[39]. Simplified regimens with fewer doses over a shorter time period (HBsAg-1018 given at 0 and 4 wk) are likely to be associated with increased uptake[39]. Many countries have now instituted effective COVID-19 vaccination programmes, and similar systems could be used to roll out simplified HBV vaccination regimens.
Preventing mother to child (vertical) transmission of hepatitis B is vital if global elimination is to be achieved[40,41]. High maternal viral load is the greatest risk factor for mother to child transmission; HBeAg positivity also increases risk[41]. In resource poor settings the WHO-recommended vaccine strategy may be difficult to deliver, and diagnostic assays for HBV testing may not be readily available. A potential strategy in these settings is POC testing to establish HBeAg status, followed by empirical treatment with tenofovir in the 3rd trimester in those who are HBeAg positive to reduce viral load and the risk of perinatal transmission[42], however such diagnostic assays are not readily available and remain costly.
Defining ‘stopping rules’ for HBeAg negative patients treated with NAs: Where seroconversion of HBeAg occurs, 67%-85% of patients have a sustained inactive state (HBeAg negative chronic infection); this is particularly the case where seroconversion occurs below the age of 30 years and where a low or undetectable HBV DNA level has been maintained[43]. However, given significant relapse rates it remains controversial as to whether NA treatment can be stopped after HBeAg loss. A HBeAg negative state is associated with higher rates of regression of fibrosis but some patients will develop HBeAg negative hepatitis, the risk of which increases with time (22% at 10 years) and increases the risk of progression to advanced liver disease[44].
Given the low rate of clearance of HBsAg, HBeAg seroconversion is considered as a potential endpoint of treatment, where undetectable HBV DNA is achieved on three separate occasions in a 6[25] or 12-mo[26] period. If treatment is stopped at this endpoint, 50% will undergo HBeAg reversion requiring treatment with NAs to restart; close biochemical monitoring is therefore required. There is evidence to suggest that longer treatment with NAs results in a higher chance of persistent remission, with viral remission for 24-mo on NAs offering the most likely chance of sustained re
Therefore, currently there is no universal stopping rule. In real-world practice, many different factors are taken in to consideration when making the decision to stop treatment with NAs, including the stage of fibrosis and family history of hepatocellular carcinoma (HCC). Further studies are needed to more clearly define the pre
Establishing treatment endpoints - aiming for viral suppression vs cure: Currently, long-term suppression of HBV DNA levels is the main endpoint of treatment (+/- HBeAg loss in HBeAg positive patients). It remains a subject of debate as to whether the endpoint of treatment should be viral suppression, functional cure, partial fun
Biomarkers continue to be developed and may prove useful in defining future treatment endpoints. These biomarkers are likely to be used in conjunction with currently utilised clinical markers. The development of hepatitis B core-related antigen (HBcrAg) as a potential serological marker for cccDNA levels may identify patients who could discontinue NA therapy, those at risk of HCC development or of recur
Establishing a universally accepted endpoint of treatment along with biomarkers to help predict or confirm the achievement of this endpoint would be an important advance in the treatment of chronic HBV infection.
Risk of HCC and surveillance in patients on long term NAs: Chronic HBV infection is a leading cause of HCC; it is responsible for around 25% of liver cancer cases in developed countries and up to 60% of cases in developing countries[48]. NA therapy has been reported to decrease incidence of HCC[49,50]. While HBsAg loss after the development of advanced fibrosis minimizes the risk of the development of HCC, it does not negate it completely[49]. A number of factors are taken into consideration when deciding which patient to survey for HCC including disease phase, age, ethnicity and family history of HCC[49]; international guidelines do not agree on the populations for surveillance however, promoting inequalities in care.
In those on NA therapy, risk scores such as the REACH-B score[51] or PAGE-B score[52] are used to identify patients who would benefit from HCC surveillance. The REACH-B scoring system was developed in a cohort of Asian patients with chronic HBV infection who were treatment naïve; no patients with cirrhosis were included in the development of this score[51]. This score does not offer good predictability in Caucasian patients with chronic HBV infection[53]. The modified REACH-B score substituted HBV DNA levels for the liver stiffness value which increased its accuracy[54]. The PAGE-B score was developed for use in Caucasian populations receiving tenofovir or ETV. A modified PAGE-B score (addition of serum albumin) has recently been tested in Asian patients on NA therapy, with an area under the receiver ope
Quantitative HBsAg and HBcrAg have been proposed as new biomarkers for HCC risk which might influence patient selection for HCC surveillance[55]. Risk models incorporating these biomarkers would be an advance in the field of HBV. New models could also incorporate other novel markers such as specific HBV mutations, presence of the metabolic syndrome and HBV genotype.
Identifying new treatments with finite duration and high cure rates: Most patients with chronic HBV currently require lifelong therapy, achieving viral suppression rather than cure[25,26]. To achieve cure, combinations of therapy targeting different aspects of the HBV lifecycle are likely to be required including inhibition of cccDNA and viral replication[38].
A number of new treatments are being investigated for HBV and these are aiming to achieve clearance of HBsAg rather than just suppressing HBV DNA[36]. A detailed description of these treatments is beyond the scope of this review, but these include the development of new NAs (besifovir and metacavir), cccDNA silencers (e.g., lymphotoxin beta receptor agonist) and HBV entry inhibitors (Myrcludex B)[28,38,56]. There may also be a role for immunomodulatory therapies such as toll-like receptor agonists (acting via activating the innate immune response), check point inhibitors (helping to restore T-cell dysfunction) or therapeutic vaccines such as TherVacB[56,57]. Gene editing strategies and RNA interference may be other potential treatment strategies[56]. Where eligible, patients should be considered for entry into clinical trials of novel therapies.
The current burden of hepatitis D virus (HDV) infection is unknown; estimates from a recent meta-analysis vary considerably, ranging from 12 million to 72 million in
Identification of infected patients: A positive HDV antibody should be accompanied by detectable serum HDV RNA to detect active infection. However, some guidelines do not explicitly make recommendations for HDV testing and therefore many patients who are HBsAg positive are not tested for HDV. One study looking at clinic-led anti-HDV testing identified that only 40% of HBV patients were tested[61]. The same study looked at a different centre offering reflex laboratory testing and found that 99.4% of first HBsAg positive samples were tested for anti-HDV. This is a potentially reliable approach to increasing detection of patients with HDV infection, as all patients who are newly diagnosed with HBsAg positivity should be tested for serological evidence of HDV infection.
There is an epidemiological association between anti-HDV seroprevalence and PWIDs, commercial sex workers, MSM and recipients of haemodialysis[58,62]. Suggested patient groups who should be prioritised for screening for HDV include: Patients who are HBsAg positive, patients with HIV, PWIDs, MSM and migrants from highly endemic regions.
Treatment for HDV infection: Pegylated-interferon (PEG-IFN) is the only treatment proven to have antiviral efficacy against chronic HDV infection, however viral supp
Establishing treatment endpoints: Unfortunately, endpoints for HDV treatment and indicators of response to treatment have not been well established[38]. Cure may not be feasible. ALT normalization, changes in HDV RNA and qHBsAg are markers of response to treatment. Barriers to establishing treatment endpoints include lack of widespread availability of HDV diagnostics and lack of standardization of HDV RNA assays. Composite endpoints are likely to be more useful than singular end-points.
Perhaps the greatest advances in our understanding of virology and development of treatment strategies over the past decade have occurred in relation to hepatitis C virus (HCV) infection. Despite these advances a number of challenges remain, including targeting difficult to reach populations and expanding HCV testing and treatment programmes in resource poor countries. Addressing these areas will be critical if global elimination of HCV is to be achieved by 2030.
Treatment and cure: HCV treatment has evolved rapidly in the last 10 years, with the emergence of direct acting antiviral (DAA) regimens. These drugs are very well tolerated and highly effective in achieving sustained virologic response (SVR), even in patients who were previously considered ‘hard to treat’ or in whom interferon-based treatment was contraindicated. As a result, antiviral treatment with DAAs is recom
In 2011 the first protease-inhibitors (telaprevir and boceprevir) were approved for use in HCV infected individuals in combination with pegylated-interferon and ribavirin, but whilst SVR rates improved so did the frequency of side effects[65]. This was quickly followed by the approval of the first interferon-free regimens for the treatment of genotype 1 HCV infection in 2014, followed by the first pangenotypic regimen, sofosbuvir-velpatasvir, in 2016[66]. Pangenotypic regimens are advantageous because they remove the need for genotype testing prior to the commencement of treatment which simplifies treatment regimens, thus reducing the frequency of patients dropping out before they start antiviral treatment.
Presently, the availability of safe and highly effective DAA regimens supports a strategy of treating all individuals with chronic HCV infection over the age of 12, irrespective of the stage of disease[67]. Current regimens offer a number of advantages over previous interferon-containing regimens including much greater efficacy, few side-effects, oral once daily dosing and shorter duration of treatment. For current DAA regimes, SVR rates (undetectable HCV RNA at 12 or 24 wk after treatment) well exceed 90% for most patient cohorts, compared with approximately 50% of patients treated with PEG-interferon and ribavirin. Patients with chronic kidney disease (including dialysis-dependent patients) and cirrhosis were previously considered difficult to treat but now have similar SVRs when treated with DAAs to those without chronic kidney disease and cirrhosis[68,69].
Significant improvements in SVR rates with DAAs has translated into a reduction in morbidity and mortality rates in patients with HCV. A systemic review and meta-analysis concluded that there was an 87% reduction in the incidence of HCC and a 75% reduction in all-cause mortality in those who achieved SVR when compared with those who did not[70]. By 2019 in the United Kingdom, the incidence of HCV-related end stage liver disease and HCC had fallen by 24% following the introduction of DAAs and the associated increase in the number of patients completing treatment. In Scotland, new presentations of HCV-related decompensated cirrhosis decreased by 51% in the DAA area with an estimated avoidance of 330 cases of decompensated cirrhosis[73].
Prevention: The ideal preventative treatment for HCV would be a vaccine. However, development of an HCV vaccine has been challenging due to the genetic diversity of the virus, the virus’ ability to avoid the host immune response and a lack of in vitro and in vivo models of infection[71]. Some progress has been made, and a recent trial of a vaccine regimen to prevent chronic HCV infection was safe and induced HCV-specific T-cell responses but it did not prevent chronic HCV infection in a cohort of patients with a recent history of intravenous drug use[72]. It is therefore unlikely that an available efficacious vaccine will be available in the short-term. Work to develop a vaccine is ongoing.
In the absence of a vaccine, improving harm reduction approaches for PWIDs is vital. Existing strategies include promotion of sterile injection equipment use through needle exchange programmes and opioid substitution therapy. These services are often poorly provided and under-utilized, but they have been shown to be highly cost-effective[73]. It is been estimated that eliminating non-sterile injection techniques could prevent 43% of incident HCV infections between 2018 and 2030[74].
Difficult to reach populations: Despite advances in the medical treatment of hepatitis C, global elimination is unlikely to be achieved unless all infected patients are identified and then complete their treatment regimen. A significant proportion of people with HCV infection are unaware of their diagnosis, and our ability to find these patients is becoming increasingly challenging. Previous work has shown that HCV testing is concentrated in areas with lower risk of infection[75], commonly settings where patients are either in recovery from previous drug use or ongoing drug use is more ‘controlled’. Testing needs to be expanded among ‘difficult to reach’ popula
One important area to target to increase testing and treatment is in the prison population. Prison populations have a high prevalence of HCV infection with many studies reporting an incidence > 10 times that of the general population[78]. Drug use prior to or during imprisonment is common, yet harm reduction methods such as access to clean injecting equipment is non-existent or inadequate in the majority of prisons. Opt-out screening for blood borne viruses (BBVs) is recommended in the EASL HCV guidelines for all prison inmates[79], but even where this is practiced rates of testing are suboptimal[78]. Opt-in testing is more commonly practiced but is a far less effective approach. BBV testing can be challenging, particularly in reception prisons (prisoners awaiting sentencing) because these typically have a very large throughput of inmates and periods of incarceration can be short. However, these challenges can be effectively overcome with investment and an organized approach to testing. Effective approaches to increasing testing for HCV and scaling up of treatment with DAAs can also be used as ‘treatment as prevention’. This approach was practiced in an Australian prison population and led to a significant reduction in incidence of new HCV infections[80].
Another approach that could be considered to identify undiagnosed patients with HCV is a ‘track and trace’ approach by mapping the social networks of individuals with a history of injecting drug use and offering HCV testing to those in a group who may not have been tested. Whilst this may sound like a practical solution, one study showed that this was ineffective in real world clinical practice with only one par
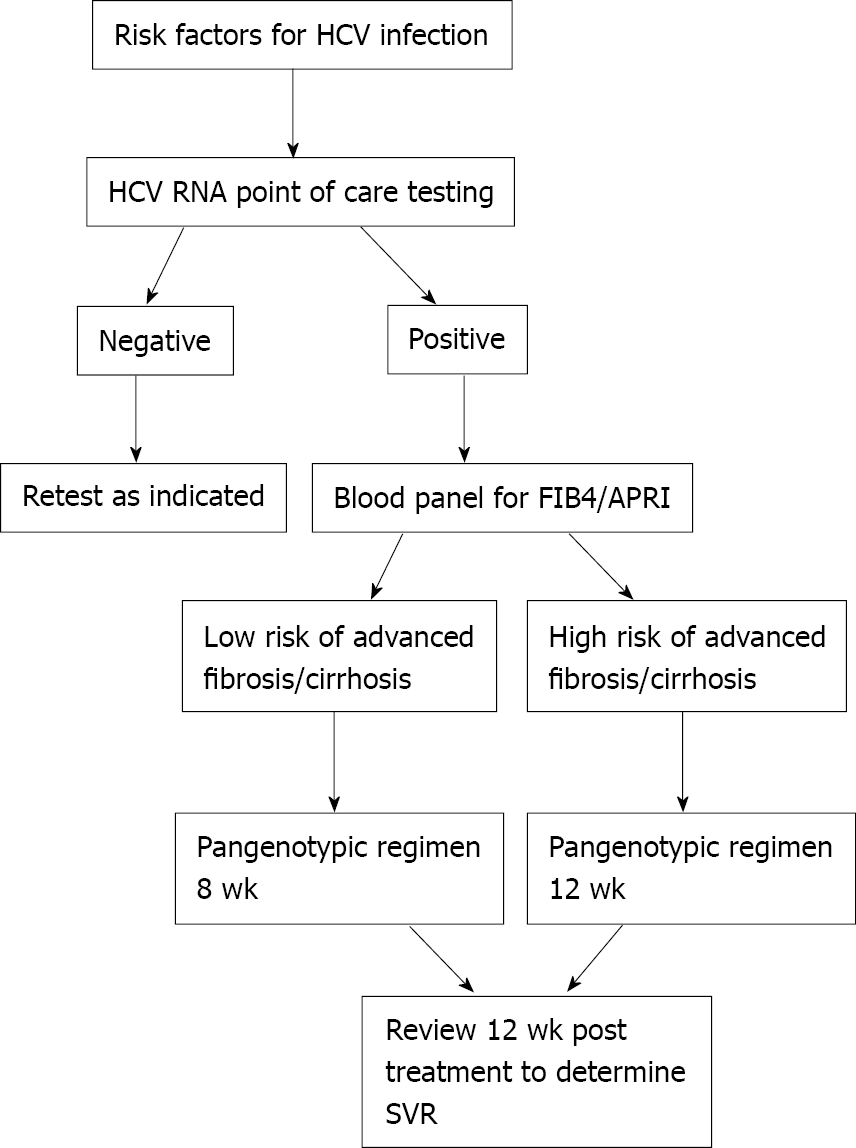
Attrition: Increasing detection rates will only help in the strive for global elimination if these are translated into increased treatment rates. An analysis of two large national laboratory databases from 2013 to 2016 found that 89.4% of patients diagnosed with chronic HCV infection did not receive a prescription for antiviral therapy[82]. In Spain, 49.8% of those with a positive anti-HCV result were not then linked into specialist care[75]. One reason for this is that care pathways have been unnecessarily complex including multiple investigations prior to treatment, which leads to patients frequently being lost to follow up and never completing treatment. Attrition appears to occur early in the treatment cascade; in one study 57.3% of patients dropped off prior to having liver enzymes checked[83]. With the advent of pangenotypic regimens and simple non-invasive fibrosis scores [e.g., fibrosis-4 (FIB-4) and aminotransferase-platelet ratio index] pathways can effectively be simplified, which is likely to reduce attrition. An ideal pathway is shown in Figure 2.
Moving care delivery out of hospital settings may improve attrition rates. One study from the North-East of England found that distance from a HCV treatment service was a major predictor of patients not commencing antivirals[84]. DAAs can be effectively delivered in non-hospital settings, increasing access to treatment. A cluster-ran
Reinfections: Re-infection with HCV after SVR, detected by the presence of HCV RNA rather than HCV antibodies, is largely related to an individuals’ ongoing high risk behavior, inadequate harm reduction knowledge and/or lack of availability of clean injecting equipment. The true rate of reinfection is not known and is likely to vary significantly depending on the population studied. Individuals who continue to actively inject drugs after treatment have the highest rates. Very high rates of rein
Long term impact of hepatitis C infection: HCV infection is associated with multiple extrahepatic complications including increased risk of autoimmune disorders, cryoglobulinaemia and lymphoma. In addition, there is increased risk of type II diabetes, cardiovascular disease, chronic fatigue and psychological morbidity. Many of these comorbidities persist following SVR and one study found that nearly all patients have at least one co-morbidity that remains long-term[88].
Despite individuals with HCV having a significantly increased risk of cardio
HCC surveillance in non-cirrhotic patients: The risk of development of HCC in individuals with HCV-related cirrhosis falls following SVR, but remains approximately 2% per year and as a result, surveillance is recommended for these individuals[91]. HCC may also occur in patients with advanced fibrosis, but at a lower rate than in those with cirrhosis and it remains uncertain whether HCC surveillance is clinically effective and cost effective in this group. This is further complicated by the fact that many patients are staged using transient elastography and relevant cut-offs to identify those who are likely to benefit from HCC surveillance have not been defined. There is a clear cut need to develop better models to predict the development of HCC in individuals following SVR. International societies have different recommendations regarding HCC surveillance in those achieving SVR which reflects the overall uncertainty - Table 1.
There have been some recent studies that have attempted to more clearly define patients who would benefit from HCC surveillance post SVR[90,91]. One study developed a model to predict patients with advanced fibrosis who have a low risk of HCC and may therefore not benefit from surveillance. The model used a combination of baseline and dynamic changes in liver stiffness measurement, FIB-4 score and serum albumin after SVR and identified that nearly 20% of their cohort of patients with compensated advanced fibrosis had a very low risk of developing HCC[90]. Dynamic assessment of the FIB-4 score in isolation may also predict the risk of development of HCC after SVR[98]. In one study, no patients with a FIB-4 < 1.45 after SVR developed HCC[92]. A number of studies are underway with the aim of de
Increased use of hepatitis C positive donor organs: The advent of safe and highly effective DAAs for HCV infection has increased the potential to use HCV-positive organs even when the donor is viraemic, expanding the donor pool[93]. HCV positive (HCV RNA +) donor organs universally transmit HCV to the recipient[94] so prior to the widespread availability of DAAs use of these organs was restricted to those who already had HCV viraemia. However, given the efficacy of DAAs it is now possible to transplant HCV RNA + organs in to HCV negative recipients and then treat the HCV infection in the recipient.
In 2019, Kwong et al[95] assessed the outcomes from HCV treatment with DAAs in 10 non-viraemic patients who received HCV RNA + livers. Short-term outcomes were excellent with 100% achieving SVR at 12 wk post treatment. The practice of using HCV RNA + organs with subsequent DAA treatment is now routine in some countries around the world.
Hepatitis E virus (HEV) is the most common cause of acute hepatitis worldwide and carries a significant global burden of disease. HEV genotypes 1 and 2 account for approximately 20.1 million HEV infections, 3.4 million symptomatic cases, 70000 deaths, and 3000 stillbirths annually[96]. Our understanding of the impact of hepatitis E infection has advanced significantly over the past decade, with the recognition of chronic infection, risk of progression to cirrhosis, risk factors for transmission and treatment strategies. Despite these advances, there are problems that remain to be resolved.
There are now eight recognized genotypes of HEV. Genotypes 1-4 and 7 cause human infection. Genotypes 1 and 2 are obligate human pathogens transmitted by the faeco-oral route and cause both sporadic infection and large outbreaks. In the developed world, sporadic infections are mainly caused by genotype 3 infection.
Ingestion of raw or under-cooked meat (particularly pork products), shellfish and contaminated fruits is a significant risk factor for locally-acquired infection in the Western world. Genotype 3 and 4 HEV infection can be transmitted via transfusion of infected blood products and solid organ transplantation, and may have a significant clinical impact upon immunosuppressed individuals. A French study looked at 23 cases of reported transfusion related HEV infections in France between 2006-2016. It reported that 14 of these cases, all of whom were immunosuppressed, went on to develop chronic HEV infection[97]. The United Kingdom introduced a universal screening policy for blood products in 2017 and also screens deceased and live organ donors for HEV RNA[98]. Other countries have a more selective strategy and only screen blood products intended for high-risk patients[99]. Universal screening has been shown to be more cost effective than selective screening if the incidence of HEV infection is above 1 in 10000 blood donations[100]. Sexual transmission in MSM has also been reported more recently[101].
Prior to 2008, HEV was recognized to cause an acute, self-limiting illness. Genotype 3 HEV was first reported to cause chronic infection in 2008 and chronic infection has now been reported in immunocompromised individuals including solid organ transplant (SOT) recipients, patients receiving chemotherapy for haematological malignancies, HIV-1 infected patients and patients receiving immunomodulating drugs. In immunocompromised patients, the detection of HEV RNA in plasma or stool after 3 mo is defined as chronic infection[102]. Progression to cirrhosis in those with chronic hepatitis E infection occurs in 10%-15% and can occur rapidly, within 2-3 years[103]. In a study of 85 patients with chronic HEV infection in 17 transplant centres across Europe and North America, almost 66% of transplant recipients who contracted HEV developed chronic infection and 10% progressed to cirrhosis[103,104]. Chronic infection and the risk of cirrhosis is not seen with genotype 1 or 2 infection.
Most published data regarding treatment of chronic HEV infection are from case series and reports in SOT recipients[105]. Reducing immunosuppression dose by around 30% has been shown to be effective in clearing HEV in around one third of patients[106]. Both PEGylated interferon and ribavirin are effective in treating chronic HEV infection. Interferon increases the risk of organ rejection in transplant recipients and therefore ribavirin monotherapy is the preferred option[107]. A systematic review has shown that 64% of patients were HEV RNA negative at 6 mo after the end of treatment with ribavirin monotherapy[108]. The optimal dose and duration of treatment is still to be determined but 3 mo courses have been used most commonly[107]. A multi-centre case series of 59 transplant recipients infected with HEV showed that ribavirin monotherapy, at a median dose of 600 mg/d for 3 mo achieved SVR in 78% of cases[107].
Non-response to ribavirin: The main problem to be solved in relation to chronic HEV infection is how to manage non-response to ribavirin. Sofosbuvir has been proposed as an alternative agent to treat chronic HEV infection. It has shown promise in inhibiting HEV replication in vitro[109] but it had a negligible effect on improving viraemia in a case report[110]. A later study of sofosbuvir monotherapy in nine patients demon
We also need to understand the relevance of HEV mutations and their effect on ribavirin resistance. Mutations have been identified in ribavirin non-responders but their impact on the treatment of these and other individuals has yet to be established. For example, the G1634R mutation does not lead to absolute ribavirin resistance and does not appear to compromise the response to a second course of treatment with ribavirin[112]. New treatments are ultimately required for those who fail treatment with ribavirin.
The COVID-19 pandemic has compromised efforts to progress towards the WHO goal of elimination of viral hepatitis. This impact of the pandemic is likely to be felt for years to come and during the initial peaks has resulted in delays in diagnosis and treatment, and reduced access to harm reduction services. In April 2020 in the United Kingdom, new diagnoses of HCV were down 85% and new treatment initiations had also fallen by 63% compared with the year prior[113]. Although there has been some recovery, pre-COVID 19 levels of testing and treatment have not yet been reached. Funding and resources have also been re-allocated to fighting the COVID-19 pandemic. In addition to the impact on global elimination, the COVID-19 pandemic has significantly impacted upon the provision of HCC surveillance programmes for patients with viral hepatitis.
However, during the pandemic many new ways of working (such as telemedicine) and care cascades have been adopted, which may in fact positively impact upon the delivery of viral hepatitis services in the years to come. For example, in some centres patients have been commenced on HCV treatment remotely using telemedicine (personal communication). The vaccination programmes and ‘track and trace’ systems set up during the COVID-19 pandemic could be extrapolated to viral hepatitis to improve service delivery.
The global hepatology community is well placed to set public health and research priorities in viral hepatitis for the forthcoming decade, striving towards global elimination and reduced health care burden. Potential priorities for each individual virus are proposed in Table 2.
| Virus | Public health priorities | Research priorities |
| Hepatitis A | Increased vaccination of high-risk individuals; Improved sanitation and vaccination in camps for displaced persons | Medical treatments for those with acute liver failure |
| Hepatitis B | Increase uptake of vaccination; Identifying undiagnosed individuals; Linkage to care | Establishing treatment end-points; Identifying curative treatment |
| Hepatitis C | Microelimination; Reducing re-infection rates; Identifying undiagnosed individuals; Harm reduction | Vaccination; Confirming most effective HCC surveillance strategies |
| Hepatitis D | Identification of infected individuals; Clarifying current disease burden of HDV | Novel therapies |
| Hepatitis E | Increased screening of blood products/change in donor policies; Educating immunosuppressed patients of risk of food-borne transmission; Further understanding of sources of infection | RCT to confirm optimal dose and duration of ribavirin therapy; Novel treatments; Vaccination; Greater understanding of genetic mutations |
Significant advances have occurred in the field of viral hepatitis over the past decade, particularly in relation to the treatment and cure of hepatitis C. Over the next decade – as we strive towards global elimination of viral hepatitis – the gastroenterology and hepatology community must focus on identifying the undiagnosed and engaging these individuals in to treatment programmes whilst continuing to develop novel treatments with the ultimate aim of cure.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jackson K S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, Forouzanfour MH, Groeger J, Hanafiah KM, Jacobsen KH, James SL, MacLachlan J, Malekzadeh R, Martin NK, Mokdad AA, Mokdad AH, Murray CJL, Plass D, Rana S, Rein DB, Richardus JH, Sanabria J, Saylan M, Shahraz S, So S, Vlassov VV, Weiderpass E, Wiersma ST, Younis M, Yu C, El Sayed Zaki M, Cooke GS. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 989] [Article Influence: 109.9] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Global health sector stratergy on viral hepatitis 2016-2021: Towards ending viral hepatitis: World Health Organisation; 2016. [cited 10 May 2021]. Available from: https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf?sequence=1. |
| 3. | Razavi H, Sanchez Gonzalez Y, Yuen C, Cornberg M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020;40:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 4. | Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28:6653-6657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (3)] |
| 5. | Jacobsen KH, Koopman JS. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol. 2005;34:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Jacobsen KH. Globalization and the Changing Epidemiology of Hepatitis A Virus. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 7. | World Health Organization. World Health Organisation Immunological basis for Immunization Series. Module 18: Hepatitis A Update 2019. [cited 10 May 2021]. Available from: https://apps.who.int/iris/handle/10665/326501. |
| 8. | Kaddoura M, Allaham R, Abubakar A, Ezzeddine A, Barakat A, Mala P, Zaraket H. Hepatitis A Virus Genotype IB Outbreak among Internally Displaced Persons, Syria. Emerg Infect Dis. 2020;26:369-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Choi YS, Lee J, Lee HW, Chang DY, Sung PS, Jung MK, Park JY, Kim JK, Lee JI, Park H, Cheong JY, Suh KS, Kim HJ, Lee JS, Kim KA, Shin EC. Liver injury in acute hepatitis A is associated with decreased frequency of regulatory T cells caused by Fas-mediated apoptosis. Gut. 2015;64:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Fujiwara K, Kojima H, Yonemitsu Y, Yasui S, Imazeki F, Miki M, Suzuki K, Sakaida I, Okita K, Tanaka E, Omata M, Yokosuka O. Phylogenetic analysis of hepatitis A virus in sera from patients with hepatitis A of various severities. Liver Int. 2009;29:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | World Health Organization. Hepatitis A. 2020. [cited 27 April 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-a. |
| 12. | Advisory Committee on Immunization Practices (ACIP); Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55:1-23. [PubMed] |
| 13. | Herzog C, Van Herck K, Van Damme P. Hepatitis A vaccination and its immunological and epidemiological long-term effects - a review of the evidence. Hum Vaccin Immunother. 2021;17:1496-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Sun X, Wang F, Zheng H, Miao N, Yuan Q, Cui F, Yin Z, Zhang G, Levine H. The impact of expanded program on immunization with live attenuated and inactivated Hepatitis A vaccines in China, 2004-2016. Vaccine. 2018;36:1279-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | National Institute for Health and Care Excellence. Scenario: Prevention of infection with hepatitis A. [cited 20 May 2021]. London: NICE; 2021. Available from: https://cks.nice.org.uk/topics/hepatitis-a/management/prevention-of-infection-with-hepatitis-a/. |
| 16. | United Nations High Commissioner for Refugees. Global Trends: Forced Displacement in 2019: The United Nations Refugee Agency; 2019. [cited 20 May 2021]. Available from: https://www.unhcr.org/globaltrends2019/. |
| 17. | Zimmermann R, Faber M, Dudareva S, Ingiliz P, Jessen H, Koch J, Marcus U, Michaelis K, Rieck T, Ruscher C, Schilling B, Schumacher J, Sissolak D, Thoulass J, Wenzel JJ, Werber D, Sagebiel D. Hepatitis A outbreak among MSM in Berlin due to low vaccination coverage: Epidemiology, management, and successful interventions. Int J Infect Dis. 2021;103:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Hu X, Collier MG, Xu F. Hepatitis A Outbreaks in Developed Countries: Detection, Control, and Prevention. Foodborne Pathog Dis. 2020;17:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Yin S, Barker L, Ly KN, Kilmer G, Foster MA, Drobeniuc J, Jiles RB. Susceptibility to Hepatitis A Virus Infection in the United States, 2007-2016. Clin Infect Dis. 2020;71:e571-e579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Jacobs RJ, Meyerhoff AS, Saab S. Immunization needs of chronic liver disease patients seen in primary care versus specialist settings. Dig Dis Sci. 2005;50:1525-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Shim M, Khaykis I, Park J, Bini EJ. Susceptibility to hepatitis A in patients with chronic liver disease due to hepatitis C virus infection: missed opportunities for vaccination. Hepatology. 2005;42:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Chaudhary RK, Andonov AP. Effect of ribavirin on hepatitis A virus replication in vitro. Can J Infect Dis. 1992;3:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Howell J, Pedrana A, Schroeder SE, Scott N, Aufegger L, Atun R, Baptista-Leite R, Hirnschall G, 't Hoen E, Hutchinson SJ, Lazarus JV, Olufunmilayo L, Peck R, Sharma M, Sohn AH, Thompson A, Thursz M, Wilson D, Hellard M. A global investment framework for the elimination of hepatitis B. J Hepatol. 2021;74:535-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | World Health Organization. Hepatitis B. 2020. [cited 20 May 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. |
| 25. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 26. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2845] [Article Influence: 406.4] [Reference Citation Analysis (0)] |
| 27. | Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137:1593-608.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 540] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 28. | Do A, Reau NS. Chronic Viral Hepatitis: Current Management and Future Directions. Hepatol Commun. 2020;4:329-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, Hüppe D, Stein K, Trojan J, Sarrazin C, Böcher WO, Spengler U, Wasmuth HE, Reinders JG, Möller B, Rhode P, Feucht HH, Wiedenmann B, Berg T. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 274] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 30. | Tan M, Bhadoria AS, Cui F, Tan A, Van Holten J, Easterbrook P, Ford N, Han Q, Lu Y, Bulterys M, Hutin Y. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:106-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 31. | McPherson S, Valappil M, Moses SE, Eltringham G, Miller C, Baxter K, Chan A, Shafiq K, Saeed A, Qureshi R, Hudson M, Bassendine MF. Targeted case finding for hepatitis B using dry blood spot testing in the British-Chinese and South Asian populations of the North-East of England. J Viral Hepat. 2013;20:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Martin NK, Vickerman P, Khakoo S, Ghosh A, Ramsay M, Hickman M, Williams J, Miners A. Chronic hepatitis B virus case-finding in UK populations born abroad in intermediate or high endemicity countries: an economic evaluation. BMJ Open. 2019;9:e030183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Avellon A, Ala A, Diaz A, Domingo D, Gonzalez R, Hidalgo L, Kooner P, Loganathan S, Martin D, McPherson S, Munoz-Chimeno M, Ryder S, Slapak G, Ryan P, Valbuena M, Kennedy PT. Clinical performance of Determine HBsAg 2 rapid test for Hepatitis B detection. J Med Virol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 34. | Nguyen MH, Wong G, Gane E, Kao JH, Dusheiko G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin Microbiol Rev. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 35. | McNaughton AL, Lourenço J, Bester PA, Mokaya J, Lumley SF, Obolski U, Forde D, Maponga TG, Katumba KR, Goedhals D, Gupta S, Seeley J, Newton R, Ocama P, Matthews PC. Hepatitis B virus seroepidemiology data for Africa: Modelling intervention strategies based on a systematic review and meta-analysis. PLoS Med. 2020;17:e1003068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, Hu J, Kramvis A, Lampertico P, Janssen HLA, Levrero M, Li W, Liang TJ, Lim SG, Lu F, Penicaud MC, Tavis JE, Thimme R; Members of the ICE-HBV Working Groups; ICE-HBV Stakeholders Group Chairs; ICE-HBV Senior Advisors, Zoulim F. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol. 2019;4:545-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 380] [Article Influence: 63.3] [Reference Citation Analysis (1)] |
| 37. | Zhou K, Contag C, Whitaker E, Terrault N. Spontaneous loss of surface antigen among adults living with chronic hepatitis B virus infection: a systematic review and pooled meta-analyses. Lancet Gastroenterol Hepatol. 2019;4:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 38. | Cornberg M, Lok AS, Terrault NA, Zoulim F; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference‡. J Hepatol. 2020;72:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 39. | Hyer R, McGuire DK, Xing B, Jackson S, Janssen R. Safety of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant in adults. Vaccine. 2018;36:2604-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Wen WH, Lai MW, Chang MH. A review of strategies to prevent mother-to-infant transmission of hepatitis B virus infection. Expert Rev Gastroenterol Hepatol. 2016;10:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Funk AL, Lu Y, Yoshida K, Zhao T, Boucheron P, van Holten J, Chou R, Bulterys M, Shimakawa Y. Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21:70-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 42. | World Health Organization. Prevention of Mother-To-Child Transmission of Hepatitis B virus: Guidelines on antiviral prophylaxis. [cited 20 May 2021]. Available from: https://apps.who.int/iris/bitstream/handle/10665/333391/9789240002708-eng.pdf?sequence=1&isAllowed=y. |
| 43. | Liaw YF. HBeAg seroconversion as an important end point in the treatment of chronic hepatitis B. Hepatol Int. 2009;3:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 45. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1216] [Article Influence: 173.7] [Reference Citation Analysis (2)] |
| 46. | Mak LY, Wong DK, Cheung KS, Seto WK, Lai CL, Yuen MF. Review article: hepatitis B core-related antigen (HBcrAg): an emerging marker for chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2018;47:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 47. | van Bömmel F, Bartens A, Mysickova A, Hofmann J, Krüger DH, Berg T, Edelmann A. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology. 2015;61:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 48. | MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5:a021410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 49. | Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 349] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 50. | Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 51. | Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK; REACH-B Working Group. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 521] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 52. | Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 393] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 53. | Voulgaris T, Papatheodoridi M, Lampertico P, Papatheodoridis GV. Clinical utility of hepatocellular carcinoma risk scores in chronic hepatitis B. Liver Int. 2020;40:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 54. | Lee HW, Ahn SH. Prediction models of hepatocellular carcinoma development in chronic hepatitis B patients. World J Gastroenterol. 2016;22:8314-8321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 55. | Suzuki Y, Maekawa S, Komatsu N, Sato M, Tatsumi A, Miura M, Matsuda S, Muraoka M, Nakakuki N, Shindo H, Amemiya F, Takano S, Fukasawa M, Nakayama Y, Yamaguchi T, Inoue T, Sato T, Sakamoto M, Yamashita A, Moriishi K, Enomoto N. Hepatitis B virus (HBV)-infected patients with low hepatitis B surface antigen and high hepatitis B core-related antigen titers have a high risk of HBV-related hepatocellular carcinoma. Hepatol Res. 2019;49:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 56. | Ligat G, Goto K, Verrier E, Baumert TF. Targeting Viral cccDNA for Cure of Chronic Hepatitis B. Cur Hepatol Rep. 2020;19:235-44. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 57. | European Union's Horizon 2020 research and innovation programme. TherVacB - A Therapeutic vaccine to cure Hepatitis B. [cited 20 May 2021]. Available from: https://www.thervacb.eu/. |
| 58. | Stockdale AJ, Kreuels B, Henrion MYR, Giorgi E, Kyomuhangi I, de Martel C, Hutin Y, Geretti AM. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020;73:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 428] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 59. | Chen HY, Shen DT, Ji DZ, Han PC, Zhang WM, Ma JF, Chen WS, Goyal H, Pan S, Xu HG. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019;68:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 60. | Miao Z, Zhang S, Ou X, Li S, Ma Z, Wang W, Peppelenbosch MP, Liu J, Pan Q. Estimating the Global Prevalence, Disease Progression, and Clinical Outcome of Hepatitis Delta Virus Infection. J Infect Dis. 2020;221:1677-1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 227] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 61. | El Bouzidi K, Elamin W, Kranzer K, Irish DN, Ferns B, Kennedy P, Rosenberg W, Dusheiko G, Sabin CA, Smith BC, Nastouli E. Hepatitis delta virus testing, epidemiology and management: a multicentre cross-sectional study of patients in London. J Clin Virol. 2015;66:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Ferrante ND, Lo Re V 3rd. Epidemiology, Natural History, and Treatment of Hepatitis Delta Virus Infection in HIV/Hepatitis B Virus Coinfection. Curr HIV/AIDS Rep. 2020;17:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Urban S, Neumann-Haefelin C, Lampertico P. Hepatitis D virus in 2021: virology, immunology and new treatment approaches for a difficult-to-treat disease. Gut. 2021;70:1782-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 64. | World Health Organization. Hepatitis C. 2020. [cited 20 May 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. |
| 65. | Park C, Jiang S, Lawson KA. Efficacy and safety of telaprevir and boceprevir in patients with hepatitis C genotype 1: a meta-analysis. J Clin Pharm Ther. 2014;39:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Martinello M, Bajis S, Dore GJ. Progress Toward Hepatitis C Virus Elimination: Therapy and Implementation. Gastroenterol Clin North Am. 2020;49:253-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | World Health Organization. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection. [cited 20 May 2021]. Available from: http://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345-eng.pdf?ua=1. |
| 68. | Iliescu EL, Mercan-Stanciu A, Toma L. Safety and efficacy of direct-acting antivirals for chronic hepatitis C in patients with chronic kidney disease. BMC Nephrol. 2020;21:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Velosa J. Why is viral eradication so important in patients with HCV-related cirrhosis? Antivir Ther. 2017;22:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Bang CS, Song IH. Impact of antiviral therapy on hepatocellular carcinoma and mortality in patients with chronic hepatitis C: systematic review and meta-analysis. BMC Gastroenterol. 2017;17:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Stoll-Keller F, Barth H, Fafi-Kremer S, Zeisel MB, Baumert TF. Development of hepatitis C virus vaccines: challenges and progress. Expert Rev Vaccines. 2009;8:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 72. | Page K, Melia MT, Veenhuis RT, Winter M, Rousseau KE, Massaccesi G, Osburn WO, Forman M, Thomas E, Thornton K, Wagner K, Vassilev V, Lin L, Lum PJ, Giudice LC, Stein E, Asher A, Chang S, Gorman R, Ghany MG, Liang TJ, Wierzbicki MR, Scarselli E, Nicosia A, Folgori A, Capone S, Cox AL. Randomized Trial of a Vaccine Regimen to Prevent Chronic HCV Infection. N Engl J Med. 2021;384:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 73. | US Department of Health and Human Services (HHS). Facing addiction in America: the Surgeon General's spotlight on opioids. [cited 19 May 2021]. Available from: https://addiction.surgeongeneral.gov/sites/default/files/OC_SpotlightOnOpioids.pdf. |
| 74. | Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, Jordan A, Degenhardt L, Hope V, Hutchinson S, Maher L, Palmateer N, Taylor A, Bruneau J, Hickman M. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction. 2018;113:545-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 255] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 75. | Reyes-Urueña J, Celly A, Moreno S, Majó X, Colom J, Casabona J. Hepatitis C virus: Testing rate and attrition at linkage to specialized care, Catalonia, Spain 2011-2016. J Viral Hepat. 2021;28:288-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Nyamathi AM, Dixon EL, Robbins W, Smith C, Wiley D, Leake B, Longshore D, Gelberg L. Risk factors for hepatitis C virus infection among homeless adults. J Gen Intern Med. 2002;17:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | The Hepatitis C Trust. Community Peer Programme. [cited 20 may 2021]. Available from: http://www.hepctrust.org.uk/services/community-peer-programme. |
| 78. | Gallacher J, McPherson S. Progress towards micro-elimination of hepatitis C in the custodial setting. J Viral Hepat. 2021;28:300-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1281] [Cited by in RCA: 1212] [Article Influence: 173.1] [Reference Citation Analysis (0)] |
| 80. | Hajarizadeh B, Grebely J, Byrne M, Marks P, Amin J, McManus H, Butler T, Cunningham EB, Vickerman P, Martin NK, McHutchison JG, Brainard DM, Treloar C, Chambers GM, Grant L, Mcgrath C, Lloyd AR, Dore GJ; SToP-C study group. Evaluation of hepatitis C treatment-as-prevention within Australian prisons (SToP-C): a prospective cohort study. Lancet Gastroenterol Hepatol. 2021;6:533-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 81. | Donaldson SR, Radley A, Dillon JF. Identifying the Hidden Population: Former Intravenous Drug Users Who Are No Longer in Contact with Services. "Ask a Friend". Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Papaluca T, McDonald L, Craigie A, Gibson A, Desmond P, Wong D, Winter R, Scott N, Howell J, Doyle J, Pedrana A, Lloyd A, Stoove M, Hellard M, Iser D, Thompson A. Outcomes of treatment for hepatitis C in prisoners using a nurse-led, statewide model of care. J Hepatol. 2019;70:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 83. | Rege SM, Gonzalez, Sanchez Y PhD, Marx, PharmD2 S; Reau, Nancy MD, FACG3. Patient Flow Across Physician Specialties Over the Course of the Hepatitis C Care Cascade: A Real World Analysis From the United States. Am J Gastroenterol. 2019;114:s561. |
| 84. | Simpson H, Manley P, Lawler J, Morey S, Buchanan E, Hewett M, Knowles J, Miller C, McCarron B, Valappil M, McPherson S. Distance to treatment as a factor for loss to follow up of hepatitis C patients in North East England. J Public Health (Oxf). 2019;41:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Radley A, de Bruin M, Inglis SK, Donnan PT, Hapca A, Barclay ST, Fraser A, Dillon JF. Clinical effectiveness of pharmacist-led versus conventionally delivered antiviral treatment for hepatitis C virus in patients receiving opioid substitution therapy: a pragmatic, cluster-randomised trial. Lancet Gastroenterol Hepatol. 2020;5:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 86. | Hashim A, O'Sullivan M, Williams H, Verma S. Developing a community HCV service: project ITTREAT (integrated community-based test - stage - TREAT) service for people who inject drugs. Prim Health Care Res Dev. 2018;19:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62:683-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 88. | Louie KS, St Laurent S, Forssen UM, Mundy LM, Pimenta JM. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infect Dis. 2012;12:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 89. | McPherson S, Gosrani S, Hogg S, Patel P, Wetten A, Welton R, Hallsworth K, Campbell M. Increased cardiovascular risk and reduced quality of life are highly prevalent among individuals with hepatitis C. BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Alonso López S, Manzano ML, Gea F, Gutiérrez ML, Ahumada AM, Devesa MJ, Olveira A, Polo BA, Márquez L, Fernández I, Cobo JCR, Rayón L, Riado D, Izquierdo S, Usón C, Real Y, Rincón D, Fernández-Rodríguez CM, Bañares R. A Model Based on Noninvasive Markers Predicts Very Low Hepatocellular Carcinoma Risk After Viral Response in Hepatitis C Virus-Advanced Fibrosis. Hepatology. 2020;72:1924-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 91. | Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, Sterling RK, Feld JJ, Kaplan DE, Taddei TH, Berry K. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology. 2019;157:1264-1278.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 92. | Toyoda H, Tada T, Yasuda S, Mizuno K, Ito T, Kumada T. Dynamic Evaluation of Liver Fibrosis to Assess the Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis C Who Achieved Sustained Virologic Response. Clin Infect Dis. 2020;70:1208-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Kahn JA. The use of organs from hepatitis C virus-viremic donors into uninfected recipients. Curr Opin Organ Transplant. 2020;25:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Ting PS, Hamilton JP, Gurakar A, Urrunaga NH, Ma M, Glorioso J, King E, Toman LP, Wesson R, Garonzik-Wang J, Ottmann S, Philosophe B, Sulkowski M, Cameron AM, Durand CM, Chen PH. Hepatitis C-positive donor liver transplantation for hepatitis C seronegative recipients. Transpl Infect Dis. 2019;21:e13194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 95. | Kwong AJ, Wall A, Melcher M, Wang U, Ahmed A, Subramanian A, Kwo PY. Liver transplantation for hepatitis C virus (HCV) non-viremic recipients with HCV viremic donors. Am J Transplant. 2019;19:1380-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 96. | Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E genotypes 1 and 2 in 2005. Hepatology. 2012;55:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (1)] |
| 97. | Gallian P, Pouchol E, Djoudi R, Lhomme S, Mouna L, Gross S, Bierling P, Assal A, Kamar N, Mallet V, Roque-Afonso AM, Izopet J, Tiberghien P. Transfusion-Transmitted Hepatitis E Virus Infection in France. Transfus Med Rev. 2019;33:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 98. | Harvala H, Hewitt PE, Reynolds C, Pearson C, Haywood B, Tettmar KI, Ushiro-Lumb I, Brailsford SR, Tedder R, Ijaz S. Hepatitis E virus in blood donors in England, 2016 to 2017: from selective to universal screening. Euro Surveill. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 99. | Dreier J, Knabbe C, Vollmer T. Transfusion-Transmitted Hepatitis E: NAT Screening of Blood Donations and Infectious Dose. Front Med (Lausanne). 2018;5:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 100. | Domanović D, Tedder R, Blümel J, Zaaijer H, Gallian P, Niederhauser C, Sauleda Oliveras S, O'Riordan J, Boland F, Harritshøj L, Nascimento MSJ, Ciccaglione AR, Politis C, Adlhoch C, Flan B, Oualikene-Gonin W, Rautmann G, Strengers P, Hewitt P. Hepatitis E and blood donation safety in selected European countries: a shift to screening? Euro Surveill. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 101. | Lawrence D GY, Clarke A, Fisher M, Richardson D. P34 Two cases of acute hepatitis e causing a transient transaminitis in hiv infected msm. Sexually Transmitted Infections. 2015;A26-A7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 102. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018;68:1256-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 103. | Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 489] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 104. | Kamar N, Selves J, Mansuy JM, Ouezzani L, Péron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 999] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 105. | Kamar N, Abravanel F, Behrendt P, Hofmann J, Pageaux GP, Barbet C, Moal V, Couzi L, Horvatits T, De Man RA, Cassuto E, Elsharkawy AM, Riezebos-Brilman A, Scemla A, Hillaire S, Donnelly MC, Radenne S, Sayegh J, Garrouste C, Dumortier J, Glowaki F, Matignon M, Coilly A, Figueres L, Mousson C, Minello A, Dharancy S, Rerolle JP, Lebray P, Etienne I, Perrin P, Choi M, Marion O, Izopet J; Hepatitis E Virus Ribavirin Study Group. Ribavirin for Hepatitis E Virus Infection After Organ Transplantation: A Large European Retrospective Multicenter Study. Clin Infect Dis. 2020;71:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 106. | Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D'Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 374] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 107. | Narayanan S, Abutaleb A, Sherman KE, Kottilil S. Clinical features and determinants of chronicity in hepatitis E virus infection. J Viral Hepat. 2019;26:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat. 2015;22:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Dao Thi VL, Debing Y, Wu X, Rice CM, Neyts J, Moradpour D, Gouttenoire J. Sofosbuvir Inhibits Hepatitis E Virus Replication In Vitro and Results in an Additive Effect When Combined With Ribavirin. Gastroenterology. 2016;150:82-85.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 110. | Donnelly MC, Imlach SN, Abravanel F, Ramalingam S, Johannessen I, Petrik J, Fraser AR, Campbell JD, Bramley P, Dalton HR, Hayes PC, Kamar N, Simpson KJ. Sofosbuvir and Daclatasvir Anti-Viral Therapy Fails to Clear HEV Viremia and Restore Reactive T Cells in a HEV/HCV Co-Infected Liver Transplant Recipient. Gastroenterology. 2017;152:300-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 111. | Cornberg M, Pischke S, Müller T, Behrendt P, Piecha F, Benckert J, Todt D, Steinmann E, Papkalla A, von Karpowitz M, Koch A, Lohse A, Hardtke S, Manns MP, Wedemeyer H. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E - The HepNet SofE pilot study. J Hepatol. 2020;73:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 112. | Lhomme S, Kamar N, Nicot F, Ducos J, Bismuth M, Garrigue V, Petitjean-Lecherbonnier J, Ollivier I, Alessandri-Gradt E, Goria O, Barth H, Perrin P, Saune K, Dubois M, Carcenac R, Lefebvre C, Jeanne N, Abravanel F, Izopet J. Mutation in the Hepatitis E Virus Polymerase and Outcome of Ribavirin Therapy. Antimicrob Agents Chemother. 2015;60:1608-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 113. | Public Health England. Hepatitis C in Endland 2020 Working to eliminate hepatitis C as a major public health threat. [cited 20 May 2021]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/898221/HCV_in_England_2020_report.pdf. |
| 114. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair:; EASL Governing Board representative:; Panel members:. EASL recommendations on treatment of hepatitis C: Final update of the series☆. J Hepatol. 2020;73:1170-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 779] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 115. | AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 479] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 116. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1646] [Article Influence: 205.8] [Reference Citation Analysis (0)] |