Published online Jan 7, 2022. doi: 10.3748/wjg.v28.i1.154
Peer-review started: March 2, 2021
First decision: July 14, 2021
Revised: August 8, 2021
Accepted: December 25, 2021
Article in press: December 25, 2021
Published online: January 7, 2022
Processing time: 303 Days and 16.8 Hours
Wheat and other gluten-containing grains are widely consumed, providing approximately 50% of the caloric intake in both industrialised and developing countries. The widespread diffusion of gluten-containing diets has rapidly led to a sharp increase in celiac disease prevalence. This condition was thought to be very rare outside Europe and relatively ignored by health professionals and the global media. However, in recent years, the discovery of important diagnostic and pathogenic milestones has led to the emergence of celiac disease (CD) from obscurity to global prominence. These modifications have prompted experts worldwide to identify effective strategies for the diagnosis and follow-up of CD. Different scientific societies, mainly from Europe and America, have proposed guidelines based on CD's most recent evidence.
To identify the most recent scientific guidelines on CD, aiming to find and critically analyse the main differences.
We performed a database search on PubMed selecting papers published between January 2010 and January 2021 in the English language. PubMed was lastly accessed on 1 March 2021.
We distinguished guidelines from 7 different scientific societies whose reputation is worldwide recognized and representative of the clinical practice in different geographical regions. Differences were noted in the possibility of a no-biopsy diagnosis, HLA testing, follow-up protocols, and procedures.
We found a relatively high concordance between the guidelines for CD. Important modifications have occurred in the last years, especially about the possibility of a no-biopsy diagnosis in children. Other modifications are expected in the next future and will probably involve the extension of the non-invasive diagnosis to the adult population and the follow-up modalities.
Core Tip: Once considered a rare condition, celiac disease (CD) is becoming a significant health issue globally. An increasing number of studies have investigated this condition. International scientific societies have proposed guidelines for the management of CD to translate this evidence into clinical practice. In this review, we critically analyse both the converging and diverging points in the current clinical guidelines of CD, focusing on the diagnostic aspects and follow-up procedures.
- Citation: Raiteri A, Granito A, Giamperoli A, Catenaro T, Negrini G, Tovoli F. Current guidelines for the management of celiac disease: A systematic review with comparative analysis. World J Gastroenterol 2022; 28(1): 154-175
- URL: https://www.wjgnet.com/1007-9327/full/v28/i1/154.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i1.154
Celiac disease (CD) is an immune-mediated reaction to gluten characterised by an inflammatory injury to the small bowel in genetically predisposed subjects as a result of an inappropriate T cell-mediated immune response[1].The epidemiology of CD is well known, with an estimated worldwide prevalence of 0.6%-1% of the general population[2]. However, CD remains largely underdiagnosed in developing countries and has a higher impact on children[3,4]. Simultaneously, the misdiagnosis of CD is becoming an emergent problem worldwide[5].
An evidence-based approach is needed to optimise diagnostic accuracy to avoid life-threatening complications (including small bowel carcinoma and lymphoma)[6] resulting from unrecognised CD on the one hand, and unnecessary cost burden and impact on the quality of life due to incorrect prescription of a life-long gluten-free diet (GFD) on the other hand.
Simultaneously, follow-up of patients with CD who are on a GFD is of critical importance to assess the responsiveness to the GFD, detect complicated CD, find associated autoimmune diseases, and identify metabolic alterations induced by the GFD[7].
Thus, an increasing number of scientific societies have proposed guidelines for diagnosing and managing CD. In our systematic review, we identified the most recent and significant national and international guidelines and compared their recommendations. We also underlined the most apparent differences among these guidelines to identify ‘hot topics’ on CD and possible future developments.
The primary aim of this review was to identify the most recent national and international guidelines for CD by means of a systematic review and to compare their main recommendations.
We performed a database search on PubMed and selected papers published between January 2010 and January 2021 in the English language. PubMed was last accessed on 1 March 2021. The following keywords and terms were used: (1) Coeliac Diseaseor Celiac Disease; (2) Guideline; and (3) Management. The following string was used: (("coeliac disease"[All Fields] OR "celiac disease"[MeSH Terms] OR ("celiac"[All Fields] AND "disease"[All Fields]) OR "celiac disease"[All Fields] OR ("coeliac disease"[All Fields] OR "celiac disease"[MeSH Terms] OR ("celiac"[All Fields] AND "disease"[All Fields]) OR "celiac disease"[All Fields])) AND ("guideline"[Publication Type] OR "guidelines as topic"[MeSH Terms] OR "guideline"[All Fields] OR ("ma
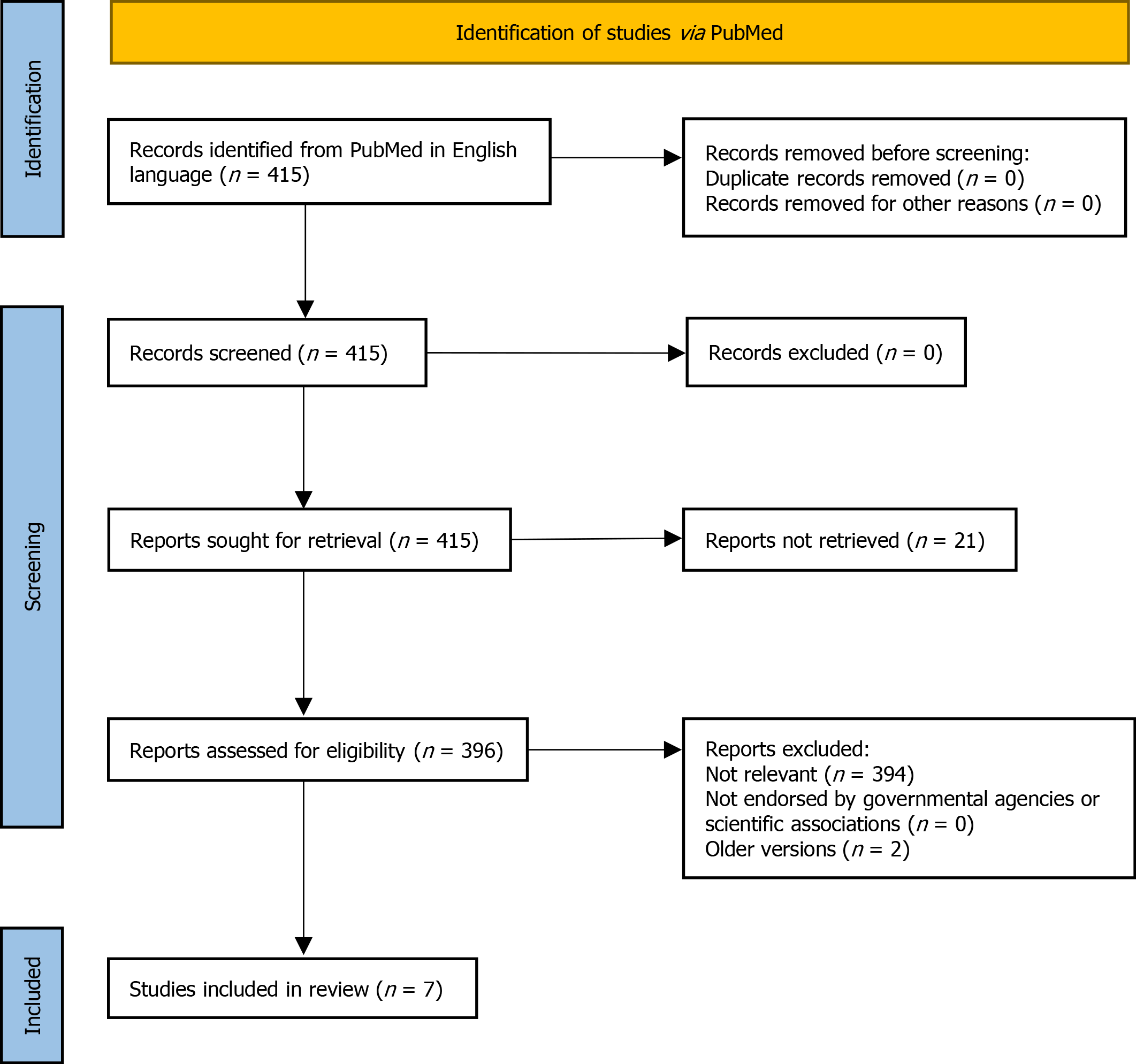
A total of 415 papers were identified with no duplicates, and, as a first step, no papers were excluded for other reasons (PRISMA flow diagram reported in Figure 1). However, twenty-one records were unavailable, leaving 396 papers for further evaluation. As a second step, we excluded papers that were not pertinent to any of the following criteria: (1) Clinical guidelines related to diagnosis and management of CD; and (2) Clinical guidelines published by governmental agencies and scientific associations. We included only the last version of the guidelines, excluding the previous updated versions.
According to the selection criteria, out of the 396 results of PubMed research assessed for eligibility, seven guidelines were finally included in this analysis. These guidelines strictly focus on the diagnosis and management of CD. These papers are presented in order of publication (newest to oldest): (1) European Society Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) 2020[8]; (2) European Society for the Study of Coeliac Disease (ECD) 2019[9]; (3) World Gastroenterology Organization (WGO) 2017[10]; (4) Central Research Institute of Gastroenterology, Russia, 2016[11]; (5) National Institute for Health and Care Excellence (NICE), 2015[12]; (6) British Society of Gastroenterology (BSG), 2014[13]; and (7) America College of Gastroenterology (ACG), 2013[14].
The recommendations provided by each selected guideline were systematically explored and classified into five categories: patients to be tested for CD, diagnostic tests (serology, duodenal biopsy, genetic test, no-biopsy diagnosis), potential/ silent/seronegative CD, refractory/complicated CD, and follow-up. These categories represent the most discussed topics of CD.
The results are reported in different paragraphs, containing both a brief intro
CD is a diagnostic challenge as it may develop at any age (even in older adults) and with a polymorphic clinical presentation[15]. The clinical spectrum of CD includes both symptomatic and silent forms revealed only by serological screening[16,17]. CD-related symptoms can be both intestinal and extraintestinal, reflecting the systemic nature of the disease. These manifestations are classified as ‘classical’ and ‘non-classical’ according to the historical presentation of first described cases. Table 1 reports the main manifestations of CD according to their categorization[1,17-26].
| Intestinal | Extraintestinal | |
| Classical | Diarroea | Iron deficiency anaemia |
| Failure to thrive | Muscle waisting | |
| Weight loss | Oedema | |
| Bloating | ||
| Non classical | Chronic abdominal pain | Short stature |
| Abdominal distension | Delayed puberty | |
| Constipation | Amenorrhea | |
| Vomiting | Irritability, unhappiness | |
| Chronic fatigue | ||
| Epilepsy | ||
| Peripheral neuropathy | ||
| Joint/muscle pain | ||
| Elevated aminotransferases | ||
| Aphtous stomatitis | ||
| Recurrent miscarriages | ||
| Reduced bone mineral density |
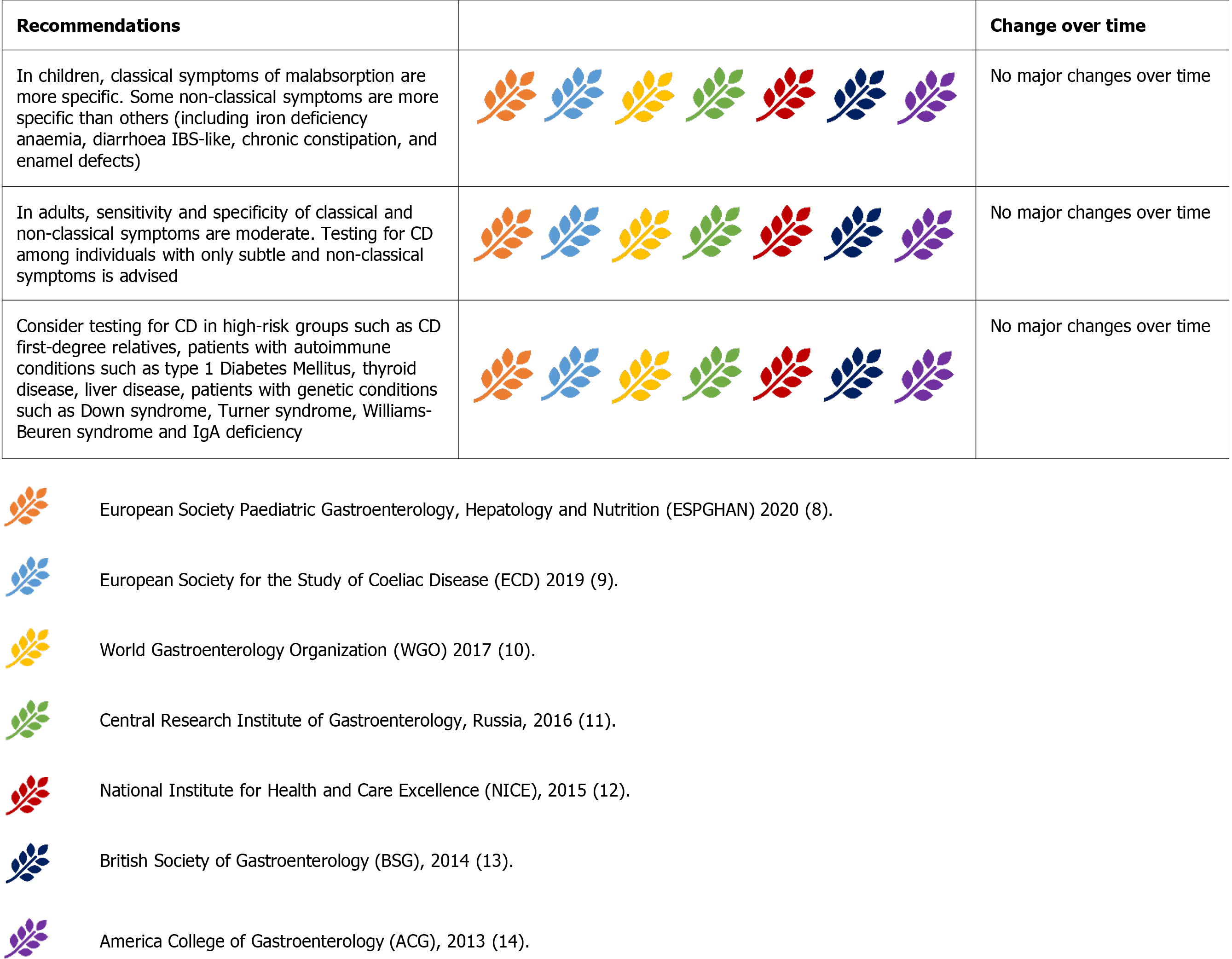
Some guidelines draw specific attention to some extraintestinal symptoms (Figure 2). In particular, the ESsCD 2019 guidelines focus on oral-dental and neuropsychiatric manifestations[9]. CD testing is advised in cases of dental enamel defects and recurrent oral aphthae. Special attention to neurological manifestations has also been drawn by the Russian Central Research Institute of Gastroenterology[11]. These guidelines also focus on reproductive disorders, such as delayed sexual development, amenorrhea, infertility, and miscarriage[11].
Despite these premises, all the guidelines agree on testing for CD in children, adolescents, and adults showing classical and non-classical symptoms of CD[7-13]. There is also a consensus on considering iron-deficiency anaemia and hypertransaminasemia as the most common laboratory abnormalities[8-14].
The high-risk group of patients did not change over time. These groups include first-degree relatives of patients with CD, patients with autoimmune conditions (such as type 1 diabetes mellitus and thyroid diseases) or genetic disorders such as IgA deficiency, Down syndrome, Turner syndrome, and Williams-Beuren syndrome[8-14].
There is no ‘gold standard’ for the diagnosis of CD. Clinical features, serology, or histology alone cannot provide a definitive diagnosis. Instead, the final diagnosis of CD relies on a combination of these elements. All the guidelines agree on a sequential approach to diagnosis, consisting of serology as a first-line test in high-risk patients, followed by duodenal biopsy in cases of positive serology or persistent suspicion of malabsorption (Figure 3). A positive serology paired with evidence of duodenal villous atrophy indicate a definite CD diagnosis, whereas cases with discordant findings should undergo HLA testing. All the guidelines also agree that patients with dis
We will report the guidelines’ detailed suggestions for obtaining key diagnostic elements from serology, histology, and genetic testing in the following paragraphs.
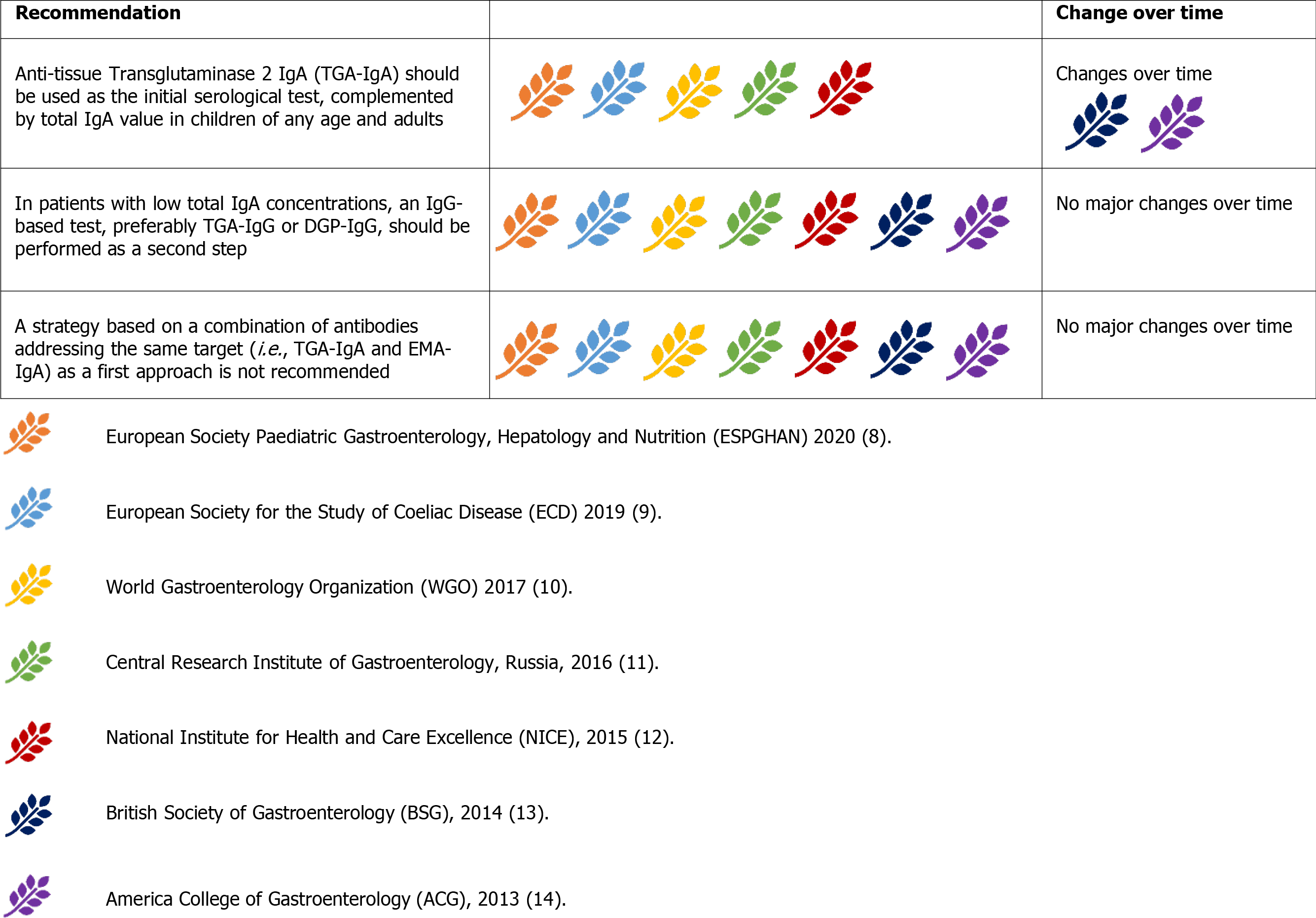
All diagnostic serological testing should be performed in patients on a gluten-containing diet[28]. Serum immunoglobulin A(IgA) anti-tissue transglutaminase antibody (anti-tTG-IgA) is widely accepted as the most sensitive test for CD diagnosis, although it suffers from low specificity, especially at low titres[29-33]. In contrast, IgA anti-endomysial antibodies (EMA-IgA) are nearly 100% specific for CD but are less sensitive, more expensive, and more operator-dependent than anti-tTG-IgA. There
Currently, the guidelines are concordant and suggest anti-tTG-IgA as the initial serological test, complemented by a determination of total IgA levels to rule out concurrent IgA deficiency (Figure 4)[8-14]. This initial approach was suggested for both children and adults.TheACG2013 guidelines suggest a combination of different IgA and IgG antibodies in children younger than two years of age (for instance, anti-tTG IgA and DGP-IgG)[14]. This approach is still accepted only by the WGO2017 guidelines[10]. The remaining guidelines advise against this strategy, as a combination of antibodies implies a higher sensitivity at the expense of a reduced specificity, often leading to the necessity of histological confirmation. This scenario represents an obstacle in the pursuit of a no-biopsy approach in children, for whom the anti-tTG-IgA + total IgA strategy fits better[8]. Alternatively, DGP-IgG (together with anti-tTG-IgG) maintained the unanimous recommendation as the test of choice in patients with IgA deficiency[8-14].
Further, EMA-IgA is considered a confirmatory test, particularly when TG2 has a low titre, i.e.,< 2x the upper normal limit (UNL)[9,10,12]. A positive result is also required for a no-biopsy CD diagnosis in children with anti-tTG IgA > 10x[8]. However, the use of paired anti-tTG and EMA-IgA as the first diagnostic test is not supported by any guideline.
Currently, all of the guidelines strongly discourage urine, stool, and saliva tests in clinical practice due to their low-performances[8-14] and the consequent risk of initiating a GFD without a firm diagnosis, impacting the final diagnosis[13].
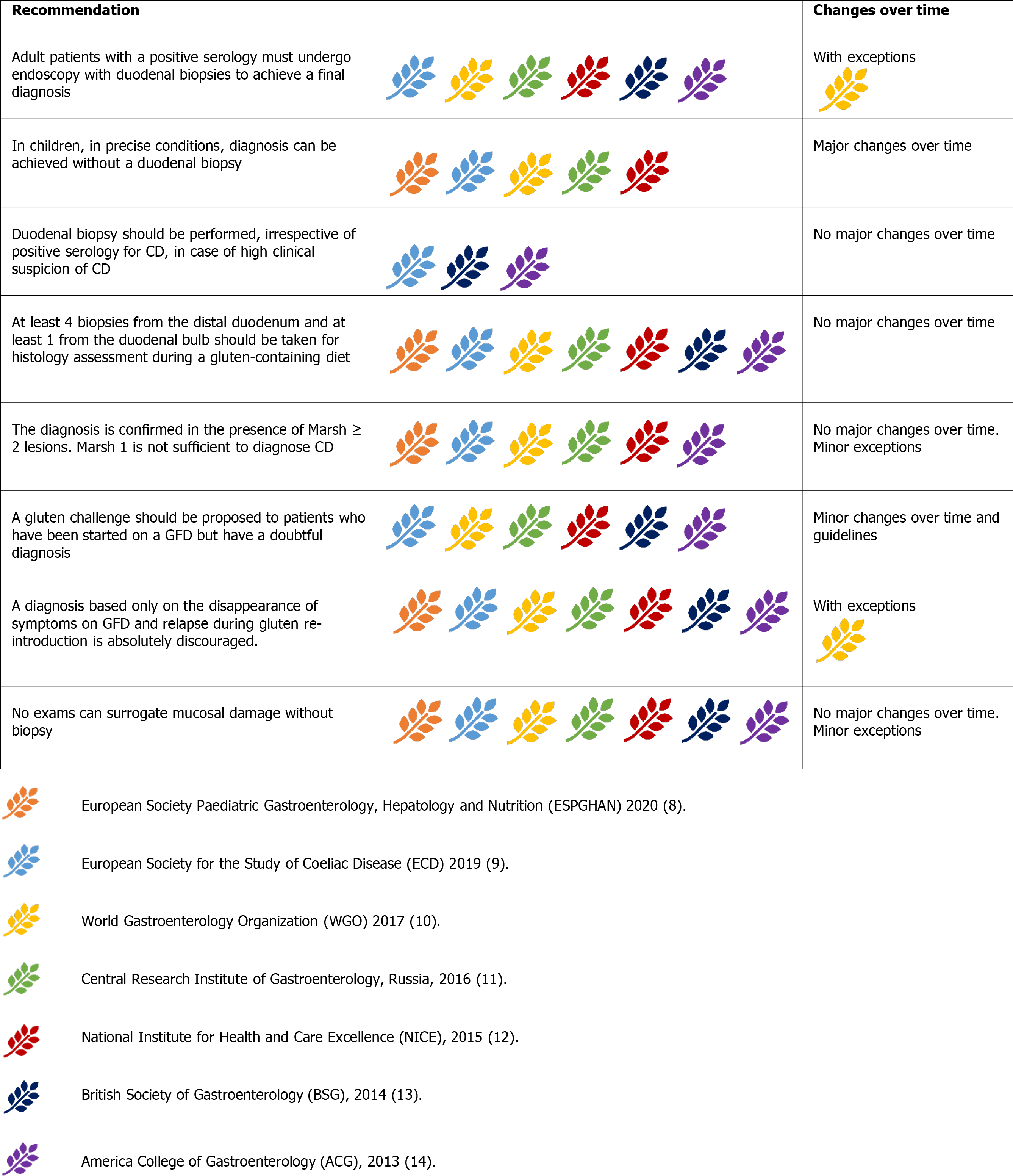
For a long time considered the ‘gold standard’ for diagnosing CD (ambiguously suggesting that other tests were of lesser importance), duodenal biopsies remain the mainstay of CD diagnosis, and all guidelines unanimously recognise this role. The presence of positive histology, however, was not considered CD-specific. Thus, clinical, and serological correlations are mandatory (Figure 5) [8-14].
Duodenal biopsies should be obtained from all patients with suspected CD. In high-risk symptomatic patients, duodenal biopsies should be performed irrespective of serology results for CD[9,13,14]. Some authors also suggested that duodenal biopsies should be considered in any individual undergoing endoscopy because of the relatively high prevalence of CD in the general population and its polymorphic presentation[13].
Histology samples should be collected from multiple sites, given the possible patchy distribution of CD lesions. Current evidence suggests collecting four biopsies from the second duodenal portion and two biopsies from the bulb[38]. Biopsy sample orientation using cellulose acetate Millipore filters is of paramount importance to avoid artefacts, potentially leading to a false diagnosis of villous atrophy[39].
The histological findings are currently categorised according to the classification proposed by Marsh and subsequently modified by Oberhuber[40]. Pathology findings are reported as Marsh-Oberhuber 0 (normal histology), 1, 2, or 3 (subdivided into 3a, 3b, and 3c).
An increase in intraepithelial lymphocytes (IELs) without villous atrophy defines Marsh 1 Lesion. In most cases, Marsh 1 Lesions (also called minimal lesions) are attributable to other causes, including lymphocytic colitis, bacterial and parasitic intestinal infections (especially Helicobacter pylori and Giardia lamblia), small intestinal bacterial overgrowth, Crohn’s disease, common variable immunodeficiency, and non-steroidal anti-inflammatory drugs[41]. While a Marsh 1 Lesion is not considered sufficient to diagnose CD, the BSG 2014 guidelines state that minimal lesions combined with positive serology could represent a probable CD. A trial with a GFD could be considered to support the diagnosis of CD[13]. When the increase in IELs is paired with hyperplasia of the duodenal crypts, the lesion is classified as Marsh 2. Conversely, increased IELs in combination with villous atrophy define the typical CD lesion (Marsh 3), subclassified as mild (3a), moderate (3b), or subtotal (3c)[40]. Some authors proposed a simplified histopathological grading, reducing the possible grades from five to three, thus reducing the possible inter-operator variability in the histological interpretation[42].This simplified classification is yet to be adopted by the international guidelines, which currently recommend the Marsh-Oberhuber classification[8-14].
At present, there is no alternative to duodenal biopsy for examining mucosal damage[8-14]. For instance, in children, video-capsule endoscopy (VCE) gives no indications[8], although in adults, VCE could support the diagnosis in cases of discordance between serology and biopsy[13] or if the patient is unwilling or unable to undergo traditional endoscopy[14]. VCE could also play a role in detecting CD complications (i.e., lymphoma, adenocarcinoma, ulcerative jejunitis)[9] and in helping to differentiate extended diseases (e.g., CD vs proximal Crohn’s disease)[11]. Anti-actin IgA antibodies have been shown to be predictive of severe villous atrophy in CD patients at the time of diagnosis[43]. Theoretically, they may also provide indirect information about villous recovery following the introduction of the GFD; however, data are still lacking in this setting. The available information about faecal and salivary microbiome, at present, is not sufficient to allow a reliable conclusion for the diagnosis of CD[44,45]. Intestinal fatty-acid binding protein (I-FABP) are higher in dietary non-adherence and unintentional gluten intake and could be used as a sensible blood marker of mucosal damage[46,47].This exam was first mentioned in the ESsCD guidelines[9].
A repeated small intestinal biopsy, including biopsies from the jejunum, could be considered in adults with discordance between histopathology and anti-tTG-IgA results[13]. In children, re-cutting biopsies and/or a second opinion from an expe
In adults, a gluten challenge should be proposed for patients with uncertain CD diagnosis, who have been started on a GFD[9-14]. In children, gluten challenge is discouraged before the age of 5 years and during puberty, and in general, it should be reserved for unusual cases[8].
Gluten challenge protocols are not homogeneous. A diet containing at least 10 g of gluten per day for 6-8 wk seems to be the most effective way to achieve disease relapse; however, the evidence is weak[28]. In shorter protocols, a diet containing at least 3 g of gluten per day for at least 2 wk seems to be sufficient for most patients[10,13,14]. Certainly, a shorter and lighter approach would fit better for highly symp
After reintroducing gluten, physical symptoms should not be used for diagnosis in the absence of other supportive evidence[8,9,11-14]. A diagnosis based only on the disappearance of symptoms on GFD and relapse during gluten re-introduction can be relevant in geographic areas where serology tests are not available, as the only way to confirm the diagnosis and treat the disease[10].
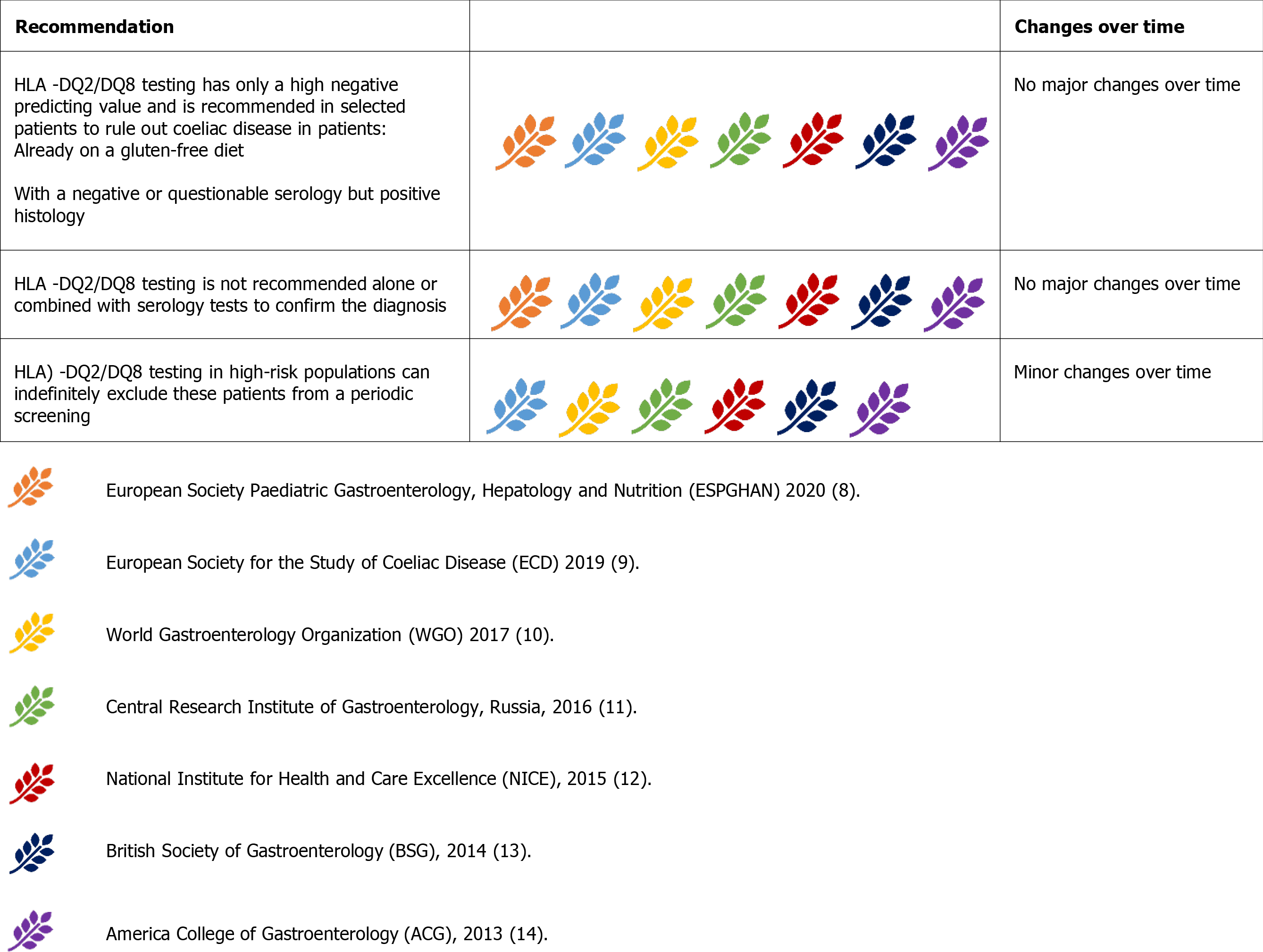
The strong genetic component of CD is testified by its high familial recurrence and high disease concordance among monozygotic twins (75%-80%)[48]. The presence of human leukocyte antigen (HLA) -DQ2/DQ8 is a pathogenic requisite for the development of the typical immune alterations found in CD. Simultaneously, HLA DQ2/DQ8 can be found in up to 30%-40% of the general population, so its specificity is remarkably poor[49]. In contrast, the absence of HLA DQ2/DQ8 virtually excludes CD diagnosis[48,49].Restricting this observation to the sole HLA DQ2 alleles, a recent systematic review of the literature confirmed that only 5.06% of patients with CD were completely lacking the HLA-DQB1*02 allelic variant[50].
Consequently, all the guidelines advise against using HLA testing as a first-line tool for the diagnosis of CD (Figure 6)[8-14]. They are also concordant in allocating this resource for: (1) Patients with uncertain diagnosis of CD, already on a GFD; (2) Patients with a flat intestinal mucosa but negative serology; and (3) In patients already on a GFD, serology and histology can be inconclusive. In this context, before embarking on a so-called ‘gluten-challenge’, it is advisable to verify the presence of HLA-DQ2/DQ8[8-14].
HLA tests would be useless for patients with positive serology before a gluten-challenge because virtually 100% of those patients would be positive. Therefore, HLA typing is no longer a criterion for the ‘no-biopsy’ approach of diagnosis in children with a TGA-IgA > 10x UNL[8]. In patients with positive histology (i.e., villous atrophy, though occasionally detected on esophagogastroduodenoscopy), and negative or questionable serology, HLA testing can exclude the diagnosis of CD[9]. In contrast, a positive result cannot confirm the diagnosis, which should be carefully evaluated on a patient-by-patient basis in expert centres.
The use of HLA typing in high-risk populations is controversial. HLA-DQ2/DQ8 can be found in more than 50% of first-degree relatives of patients with CD and in patients with other autoimmune or genetic disorders related to CD[14,49]. Most guidelines suggest excluding HLA-DQ2/DQ8 in CD first-degree relatives and high-risk patients, even if asymptomatic, to avoid periodic monitoring[9,10,13,14]. This strategy can be questioned in terms of resources and costs[10,11,14]. Some authors hav esuggested screening high-risk patients only if they complain of gastrointestinal or extraintestinal symptoms or have laboratory abnormalities[11]. In addition, a two-step genetic screening procedure starting with HLA-DQ β chains has been proposed[51].Thus, the choice of screening for symptomatic or asymptomatic first-degree relatives or high-risk patients, with or without a preliminary determination of HLA-type, remains debated, needing to take local resources and cost-benefit rates into account.
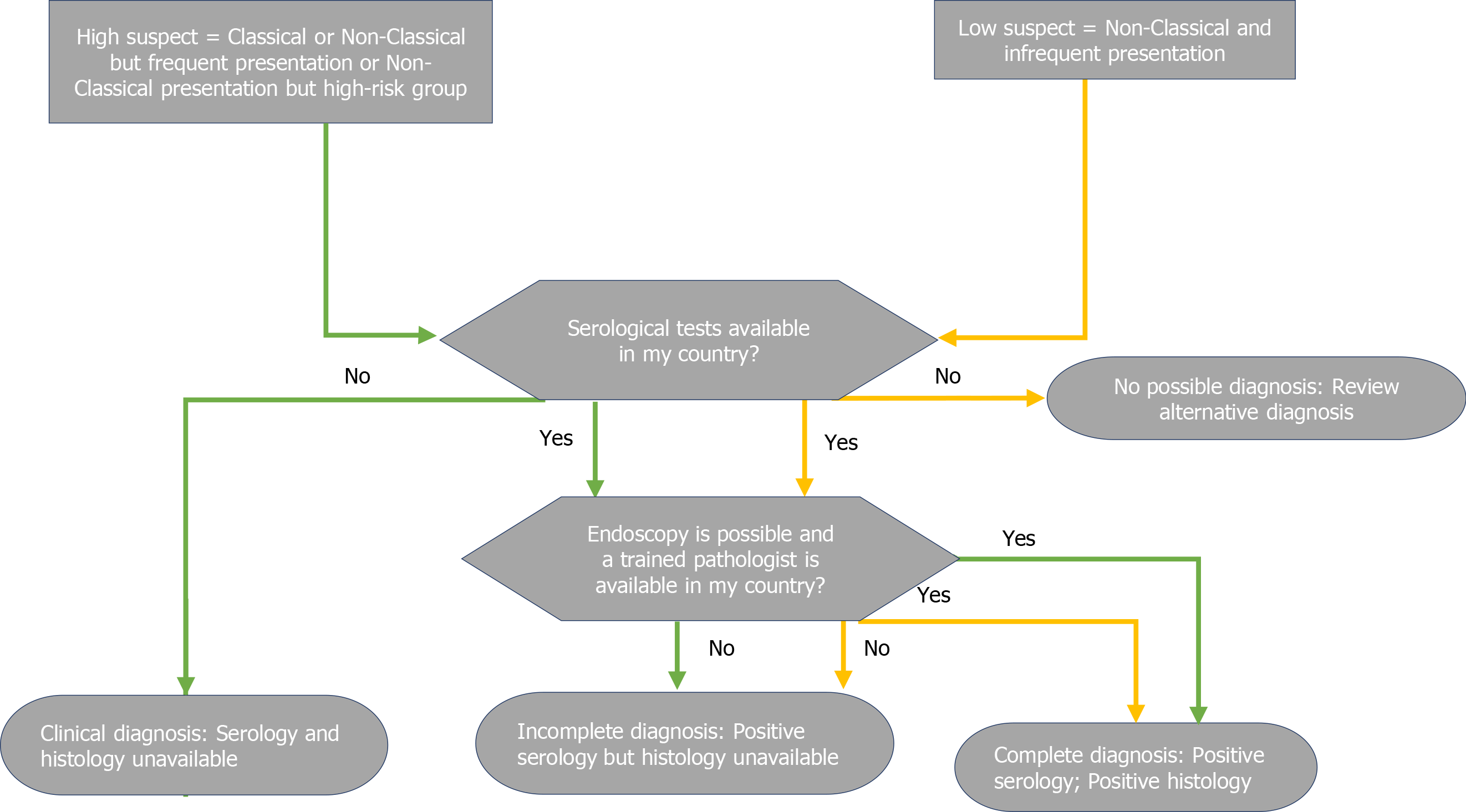
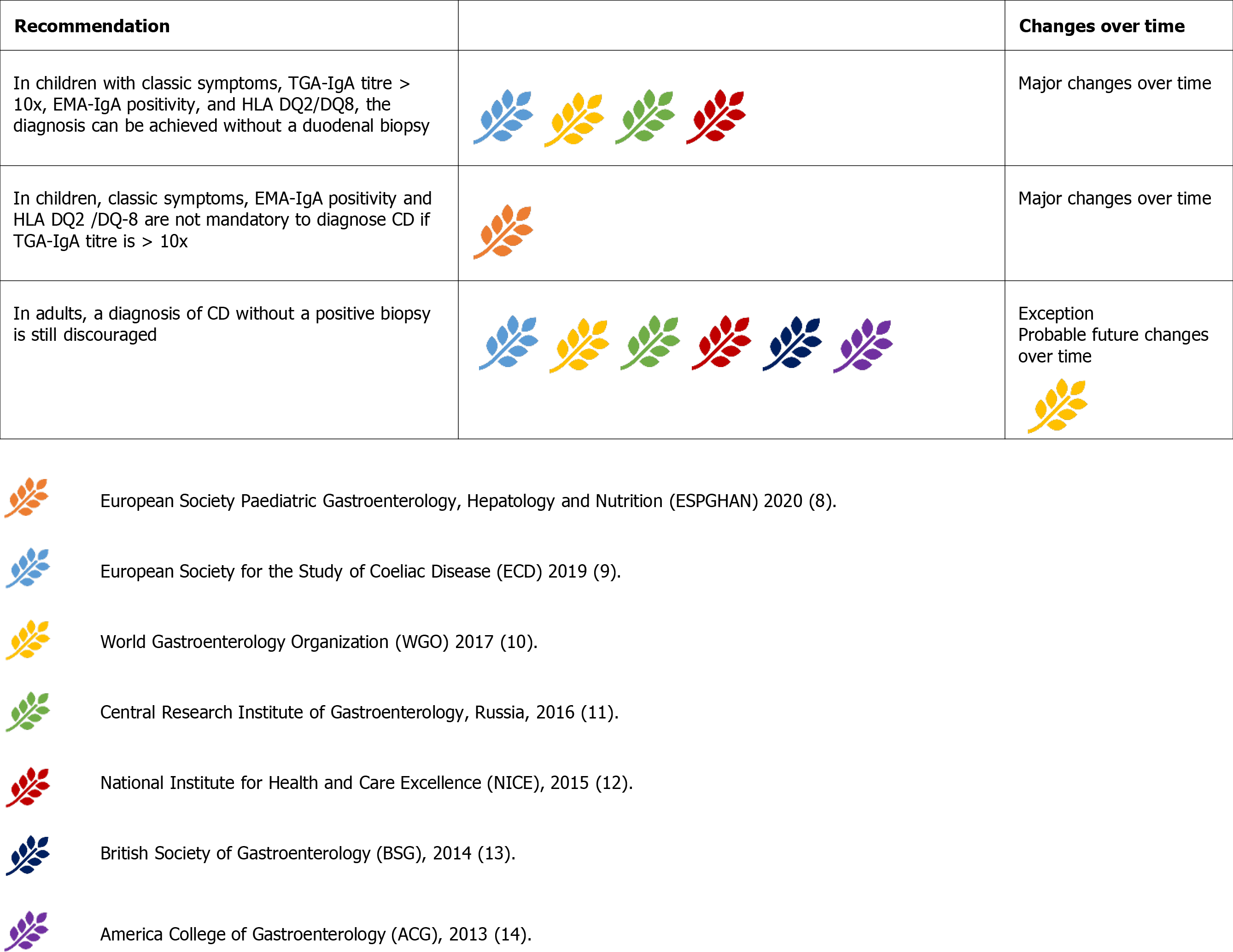
While most guidelines allow a no-biopsy diagnosis in children under strict conditions, endoscopy with duodenal biopsies is still mandatory to achieve a final diagnosis of CD in adults[9-14]. As the only exception, the WGO guidelines allow a diagnosis based on serology and clinical response to the GFD (Figure 7) in developing countries where endoscopy may not possible or trained pathologists may not be available[10].
The ESPGHAN2012 guidelines endorsed the possibility of a no-biopsy approach in children for the first time. This possibility was limited to certain conditions, which included the presence of classic symptoms, with tTG-IgA > 10x UNL, EMA-IgA positivity, and presence of permissive HLA[8].
This approach was subsequently adopted by a plurality of international guidelines[9-12]. although, the ACG2013 and BSG 2014 guidelines did not include this approach[13,14].
The 2020 update of the ESPGHAN guidelines removed classic symptoms, EMA-IgA positivity, and HLA DQ-2 or DQ-8 as crucial criteria for a diagnosis not based on biopsy[7]. However, EMA-IgA positivity is not discouraged[8,10]. The increasing confidence in diagnosing CD without biopsy in children has increased so rapidly that many recent studies consider tTGA > 10x as a new possible cut-off to further reduce the need for biopsies[52].
CD diagnosis without a positive duodenal biopsy has always been discouraged in adults[9-14]. This choice was not dictated by the reduced reliability of the serological tests in adults. In fact, large population studies concluded that tTG-IgA>10x could accurately predict villous atrophy[53]. Rather, other considerations currently prevent the extension of paediatric criteria into the adult population. First, CD at onset can be associated with complications. In the case of primary or secondary resistance, or slow response to the GFD, the absence of baseline histology may make the diagnosis of complications difficult[9]. Index histology may also predict the risk of future complications, such as lymphoma[54]. Moreover, endoscopy may help diagnose other treatable disorders associated with CD, such as eosinophilic esophagitis, autoimmune gastritis, and lymphocytic gastritis[9].
Both complicated CD and possible differential diagnoses of CD are virtually absent in children. However, they represent a serious concern in adults, thus justifying different diagnostic algorithms according to the age of presentation of the first symptoms.
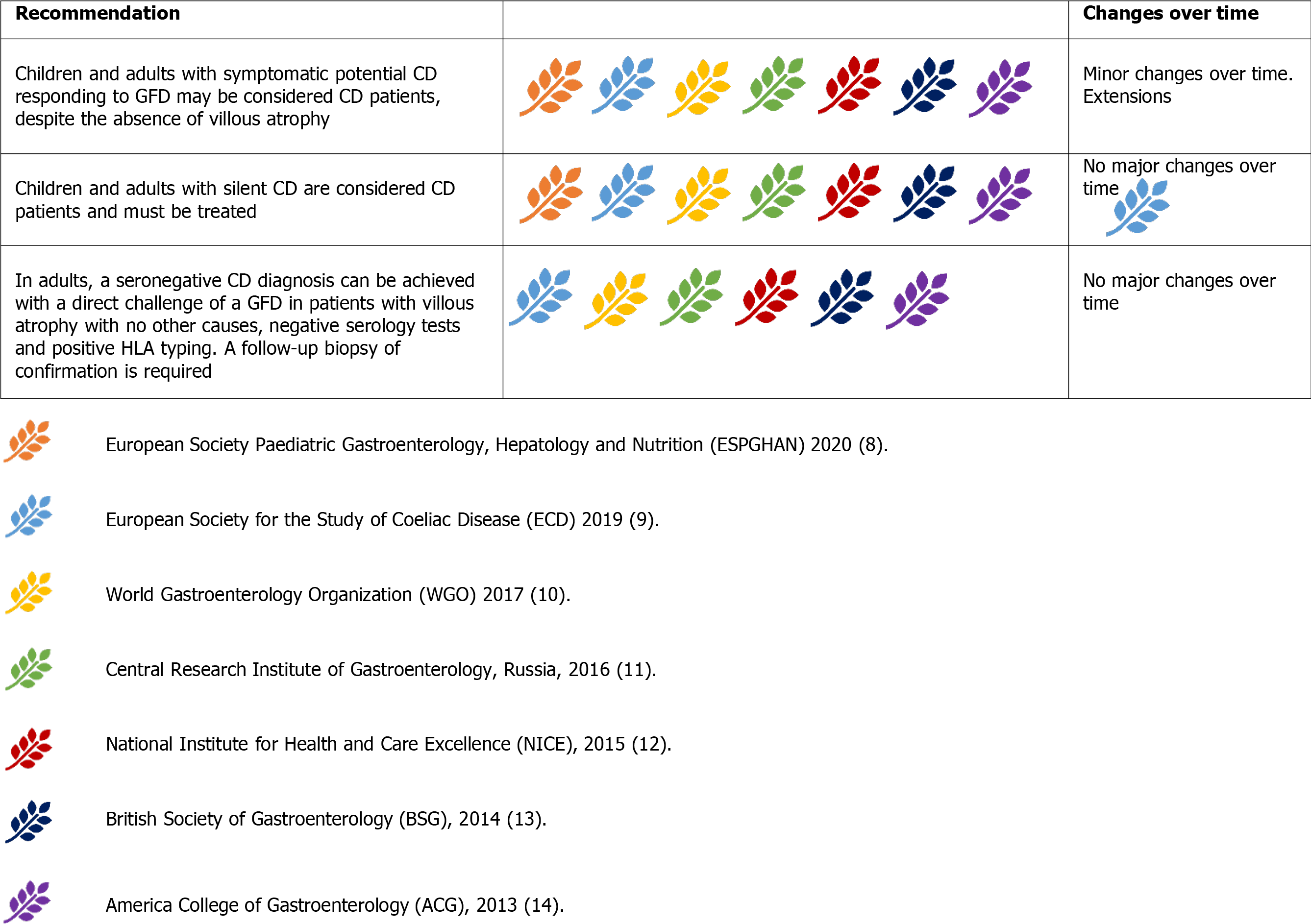
Potential CD is characterised by a positive serology for CD in the absence of mucosal damage at biopsy[1]. As stated above, Marsh 1 Lesions (i.e., an increased IELs count) are not suggestive of an active CD but may increase the risk of developing villous atrophy[41].
It is widely accepted that symptomatic potential CD may benefit from a GFD, and a direct challenge would be run[8-14]. In adult patients with both positive TGA-IgA and EMA-IgA CD is likely, and a GFD may be initiated irrespective of symptoms[9]. A serological response after a period of approximately 12 mo confirms the diagnosis of CD[9]. In EMA-IgA negativity, HLA-typing may exclude the diagnosis before em
Silent CD is characterised by the presence of both positive serology and histology for CD in the absence of classical or non-classical symptoms[1]. It is widely re
Seronegative CD is characterised by the presence of active enteropathy and negative serology for CD, with no other causes, and with clinical and histological responses to a GFD[1,37]. In these cases, other causes of enteropathy should be excluded before embarking on the direct challenge of a GFD[37,55]. HLA-typing can also rule out the diagnosis of CD in seronegative enteropathies[9,14,37]. Finally, the direct challenge of a GFD is advised only in patients with seronegative enteropathy, positive HLA typing with no other causes. A documented histological response after 1-3 years of GFD is needed to confirm the diagnosis[9,14,37]. No major changes occurred over time in the management of seronegative CD[9,14].
CD can be complicated by a persistent active form of the disease, independent of gluten intake, known as refractory CD (RCD)[1]. Other rare complications of CD can be neoplastic. Primarily, enteropathy-associated T-cell lymphoma (EATL) is a rare T-cell lymphoma associated with untreated CD. EATL has an abysmal prognosis and can occur primarily at diagnosis or as an evolution of RCD type 2[56]. Duodenal adenocarcinoma is possible, albeit less frequent in the CD population[57].
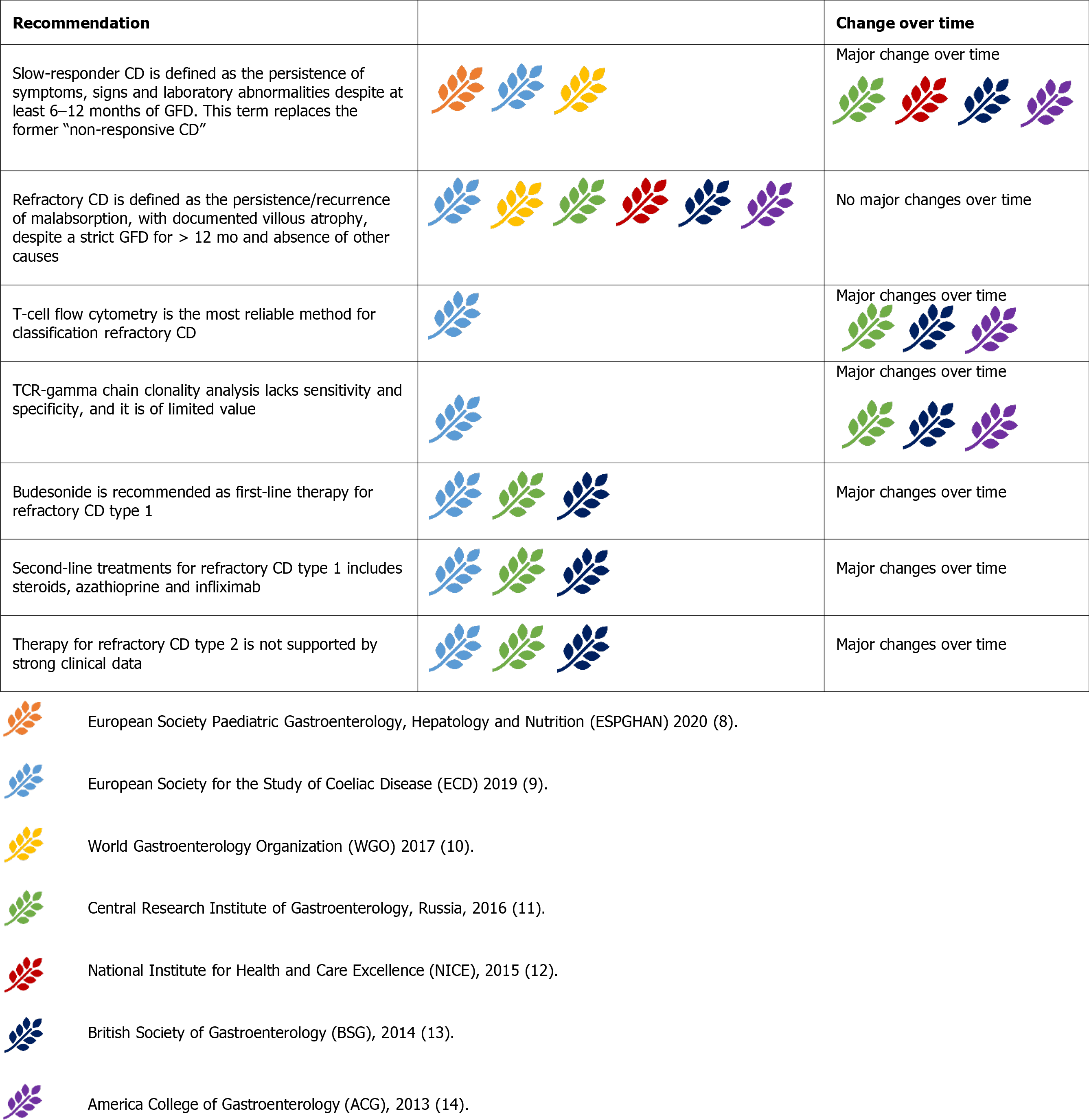
Refractory CD (RCD) is characterised by the persistence or recurrence of symptoms and signs of malabsorption, with documented villous atrophy, despite a strict GFD for more than 12 mo and in the absence of other causes[9-14]. No major changes occurred in this definition over time (Figure 9).
RCD can be primary (refractory at the time of the first diagnosis), or secondary (occurring after a period of response to the GFD)[1]. The first step in evaluating suspected RCD is to re-evaluate the initial diagnosis of CD by reviewing biopsies and serology tests obtained at the time of diagnosis[58]. The most common cause of GFD failure is inadvertent gluten ingestion[59].Therefore, evaluation by an expert dietitian should always be included[9,10,13,14]. Other associated or concomitant pathological conditions should be excluded before RCD diagnosis. These include lactose and fructose intolerance, small intestinal bacterial overgrowth, microscopic colitis, pancreatic insufficiency, and inflammatory bowel diseases[59,60]. All guidelines recommend this strategy[9,10,13,14].
RCD is further classified into type 1 (RCD-1) and type 2 (RCD-2)[1]. T-cell flow cytometry is the most reliable method for classifying RCDs. Aberrant T cells lose the normal surface markers CD3 and CD8 with preserved expression of intracytoplasmic CD3. In RCD-1, the percentage of aberrant T cells is below 20%, whereas in RCD-2, they represent more than 20% of the total IELs[58]. RCD-2 can be considered a pre-lymphoma or low-grade lymphoma[54]. T-cell receptor (TCR) g chain clonality analysis lacks sensitivity and specificity, and is of limited value in separating RCD-1 from RCD-2[54]. TCR analysis has been formerly indicated as a criterion for differentiating RCD-1 from RCD-2[11,13,14]. The latest ESsCD guidelines exclude TCR analysis in the RCD classification[9].
RCD-1 has an extremely high 5-year survival rate (> 90%)[54,59,60]. In RCD-1, the first-line therapy should be ‘open-capsule’ budesonide (OCB), 3 mg, 3 times a day[61]. Budesonide (open capsule or not) has been progressively accepted as the first-line therapy for RCD-1[9,11,13,14]. In the ACG 2013 guidelines, systemic steroids are considered the first-line therapy for RCD-1[14]. Second-line treatment for RCD-1 includes immunosuppressive drugs such as steroids (prednisone 0.5-1 mg/kg/day) and azathioprine (2-2.5 mg/kg/day)[60]. Most guidelines agree with this strategy[9,11,12]. Systemic steroids can also be considered as first-line treatment while waiting for a specialist’s advice[12]. Infliximab may be the preferred biological therapy for second-line treatment of RCD-1[62]. Evidence is still weak, and only one guideline includes infliximab as an RCD-1 treatment[9].Withdrawing of immunosuppressive therapy after 2-3 years of complete response may be considered[9,54].
RCD-2 is rarer than RCD-1, has a much higher mortality rate, and treatment is less well defined. Systemic steroids or open-capsule budesonide should be the first choice for milder presentations. In severe cases, cytoreductive therapies such as cladribine and fludarabine or autologous hematopoietic stem cell transplantation should be chosen[59,60]. Guidelines are mostly aligned with this strategy[9,13,14]. Some gui
Transformation to enteropathy-associated T-cell lymphoma (EATL) is likely in RCD-2[59]. VCE, positron-emission tomography (PET), and magnetic resonance (MR) enterography can be useful in cases of suspected progression to EATL to assess the extent of the disease[63]. All guidelines advise the use of these tools in RCD-2 staging[9-14]. Severe RCD-2 and EATL may require surgery, chemotherapy, or bone marrow transplantation[64]. The former therapeutic strategies are mostly based on case reports, and only one guideline extensively discusses them[9].
Since CD is the only autoimmune disease with a known environmental trigger (i.e., gluten), a periodical assessment of compliance to a GFD is essential[65]. Poor GFD compliance is not infrequent, and mucosal damage can persist despite negative serology and the absence of symptoms[66]. Follow-up is also essential for evaluating possible complications[54]. Osteoporosis and metabolic complications of GFD should also be evaluated during follow-up[67-69]. Suggested follow-up schedules are based on the frequency of complications, risk of GFD non-compliance, and reported quality of life[70].
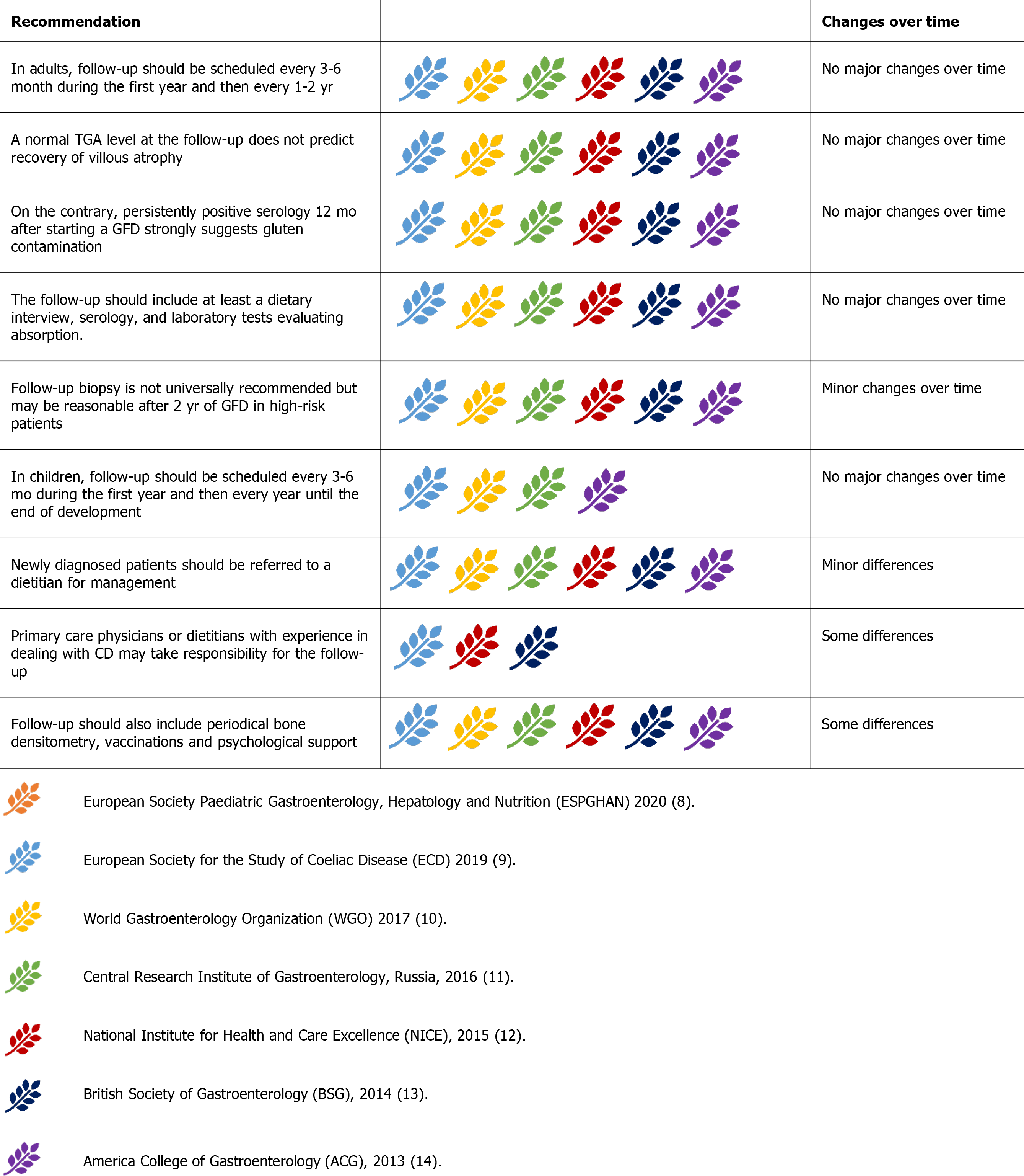
Therefore, there is universal agreement on the necessity of long-term monitoring of patients with CD to assess the compliance and responsiveness to the GFD and allow early detection of complicated CD (Figure 10)[8-14]. Follow-up evaluations should be scheduled every 3-6 mo during the first year and then every 1-2 years[9-14]. In children, follow-up should continue until they reach their final height[9-11,14], focusing on normal growth and development[9,10,14].
There is disagreement about who should oversee follow-up. While most guidelines show no preference between primary care physicians, specialists, or dietitians[9-11,13,14], the NICE 2015 guidelines suggest that dietitians with expertise in CD may be best suited to carry out an annual follow-up[12]. However, on a general principle, all guidelines agree that newly diagnosed patients should be referred to a dietitian[9-14]. Some guidelines suggest that nutritionist counselling should coincide with medical visits during follow-up[10,13]. The inclusion of a dietitian assessment at diagnosis and during follow-up was supported by clinical data[71]. Indeed, nutritional counselling could also help manage metabolic alterations, which frequently appear during the first years of the GFD[67].
All guidelines also provide information about the essential information that should be collected during follow-up evaluations. These evaluations should include a dietary interview, serology (TTG-IgA if normal IgA), and laboratory tests[9-14]. Laboratory tests should evaluate the presence of micronutrients malabsorption, including complete blood count, iron status, folate, vitamin B12, calcium, phosphate, vitamin D, and should monitor associated autoimmune conditions (thyroid-stimulating hormone and serum glucose) and liver disorders (aspartate aminotransferase/alanine amino
The inability of serology alone to predict mucosal healing automatically leads to consider the opportunity of repeating duodenal biopsies after the start of the GFD. While the general agreement is that follow-up biopsies are not mandatory in asymptomatic patients on a GFD and without an increased risk of complications[9-14], the guidelines diverge regarding other points. Many guidelines consider it reasonable to repeat biopsy after 2 years of GFD to assess mucosal healing[9,11,14]. Other guidelines suggest repeating biopsies only for persistent symptoms or se
Some guidelines also provide suggestions for further examinations to be performed during follow-up. According to the ECD and Russian guidelines, bone densitometry should be offered to every patient at the time of diagnosis and should be repeated after 3 years if abnormal, or 5 years if normal[9,11]. Other guidelines suggest performing bone densitometry only in patients with a high risk of osteoporosis or those older than 55 years[12,13].
While there is a general agreement in recommending a pneumococcal vaccine[8-10,12], the WGO2017 guidelines also recommend vaccinations against Haemophilus influenzae typeB, and Meningococcus, while other guidelines state that these vaccines have a less clear indication to be given to every patient with CD[9,11-13].
Mood disorders are another common problem in patients with dietary restrictions. Anxiety, depression, and fatigue may be associated with CD before and after diagnosis and can affect the quality of life[73]. In this context, most guidelines agree on advising patients to join CD support groups and associations[9,10,12,13]. Some of them also suggest that psychological support provided by a specialist may be offered[12,13].
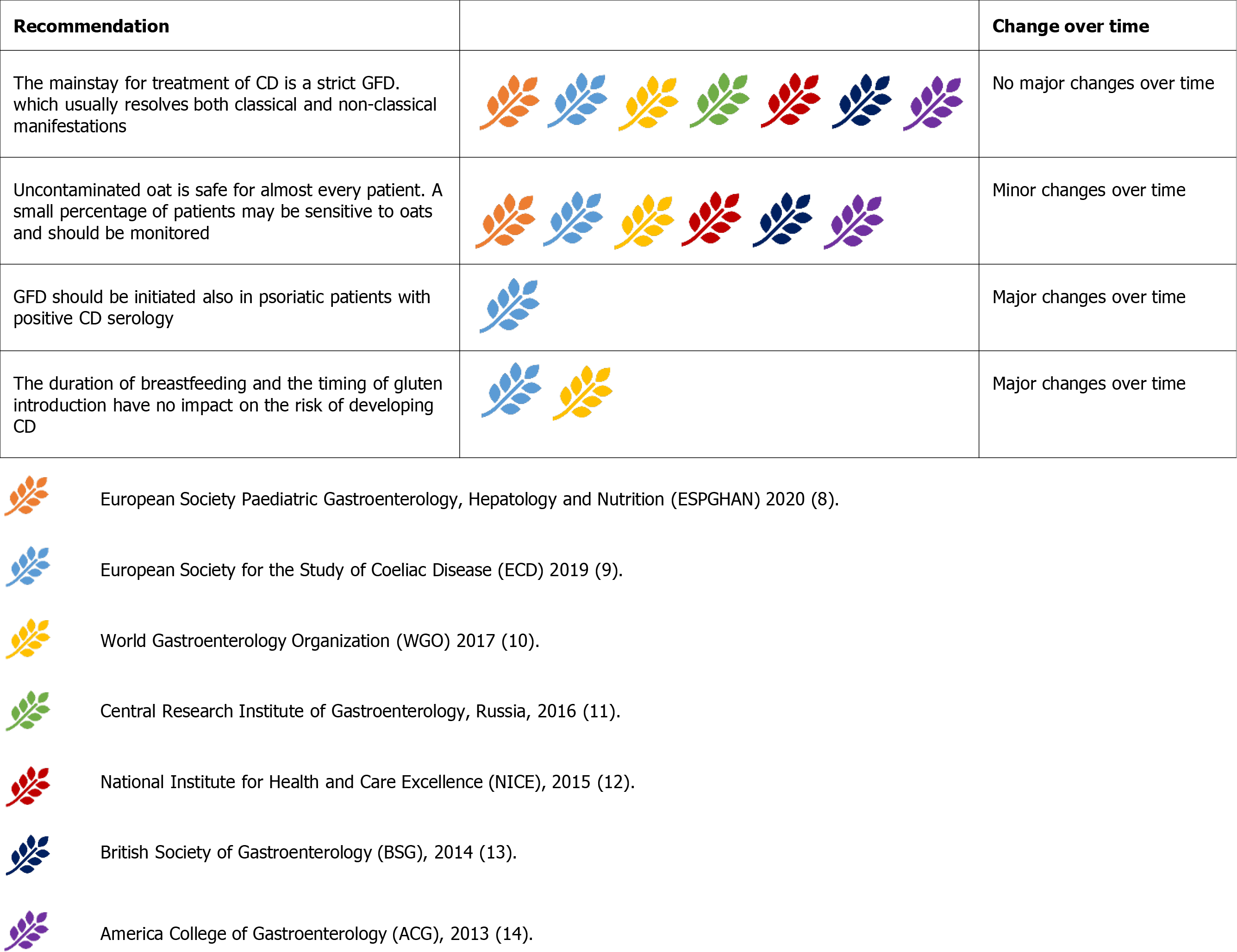
Gluten is a protein with high proline and glutamine content, primarily found in wheat. Rye and barley belong to the same tribe as wheat and are known to contain gluten. In contrast, oats are derived from a different tribe and do not contain pure gluten[1].
Uncontaminated oats are safe for almost all patients with CD, but a small percentage of patients may be sensitive to some oat cultivars[74] and should be monitored[9,10,12-14]. Some guidelines advise the initiation of a Gluten-free diet (GFD), excluding oats, and recently introduced them[10,13,14]. The Russian guidelines (2016)are against oat consumption in patients with CD because of the high risk of contamination[11]. Even if not stated, oat consumption would be safe in many countries, though it may be discouraged in developing countries where contamination could be widespread (Figure 11).
WHO guidelines on ‘Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten’ state that foods labelled as ‘gluten free’ should contain ≤ 20 parts per million (ppm) of gluten[75].
Patients should be instructed to avoid contaminating their gluten-free food by using separate cooking utensils and cooking surfaces[9,10]. At present, shared items can be safely used if thoroughly cleaned with soap and water between use[9,76].
The duration of breastfeeding and the timing of gluten introduction to the infant seem to have no impact on the risk of developing CD, even in those at high risk[77]. Therefore, there are no strict indications for gluten introduction in infant diets[9]. Formerly, it was advised to avoid either early or late gluten introduction in children at risk of CD[13].
Dermatitis herpetiformis (DH) is a bullous cutaneous disease triggered by gluten consumption like CD[1]. DH and CD often coexist and share the same treatment, GFD[9,10,13,14]. Interestingly, the ESsCD guidelines suggest that psoriasis could also benefit from GFD in the case of documented CD serology, even in the absence of mucosal damage[9].
Our comparative analysis of the currently adopted CD guidelines underlined differences in diagnostic aspects and the management of the follow-up. These differences mirror some relevant clinical points in both developing and developed countries.
First, the differences in the diagnostic process of CD are important. The possibility of a no-biopsy diagnosis has relevant repercussions in developing countries. Most guidelines are still cautious in this regard, with the WGO2017 guidelines being the only ones contemplating this possibility in geographical areas with a paucity of resources. As correctly underlined by these guidelines, some absolute recommendations may not be valid for developing countries where the availability of serology or endoscopy may be lacking[10]. CD seems to have a non-negligible prevalence in Asia and sub-Saharan Africa[77,78]. Especially in Russia and Central Asia, the prevalence of CD is very likely to be underestimated due to poor disease awareness among physicians and/or patients, limited access to diagnostic resources, inappropriate use or interpretation of the serological tests, absence of standardised diagnostic and endoscopic protocols, and insufficient expertise in histopathological interpretation[3]. Specific guidelines are lacking in these geographical areas[79]. In addition, the incidence of undiagnosed CD in children can be extremely high[80]. Knowing the high mortality and disability related to untreated CD in childhood, it would be advisable to develop specific protocols for specific geographical areas.
The no-biopsy approach has been discouraged for a long time, especially in adults[13,14]. In contrast, most recent guidelines have incorporated the ESPGHAN 2012 recommendations for a no-biopsy approach in children[9,10]. The possibility of an outright extension of these criteria into the adult population still meets key obstacles. However, in an era during which the COVID-19 pandemic has caused a staggering drop in new CD diagnoses even in industrialised countries[81], ESPGHAN released the advice to lower the TGA-IgA threshold for diagnosing CD without biopsy[52]. Moreover, retrospective data on a possible no-biopsy approach in adults are increasing[53]. Prospective data will probably lead to the integration of such an approach to future guidelines over the next decade.
Second, the differences in follow-up recommendations reflect a relatively low interest in this topic in the past. Arguably, the search for more reliable diagnostic tools was the right priority in an era characterised by a severe under-diagnosis of CD .Nowadays, significant diagnostic delays can still occur in a minority of Central European children[82], with socioeconomically deprived children being more likely to be underdiagnosed despite improved and easily available serological testing[4].
Nonetheless, the current physicians’ awareness of CD has reached fairly high levels, and the case-detection strategy has significantly contributed to the increased number of diagnoses. Consequently, the correct management of follow-up is crucial. This topic is of special interest in developed countries, in which metabolic problems possibly caused by an unbalanced GFD are particularly prevalent. Uncontrolled weight gain, metabolic syndrome, and non-alcoholic fatty liver disease are epidemic in these countries and can also be facilitated by the GFD[67,69,83-85]. In addition, quick detection of associated autoimmune conditions can prove highly beneficial, especially in autoimmune liver diseases[86]. Finally, early detection of complicated CD requires particular attention, as both neoplastic and non-neoplastic complications may arise years after the diagnosis[6].
We found a relatively high concordance between CD guidelines. Important modifications have occurred in recent years, especially regarding the possibility of a no-biopsy diagnosis in children. Other modifications are expected in the future and will probably involve the extension of the non-invasive diagnosis to the adult population and the follow-up modalities.
Celiac disease (CD) has risen from obscurity to global prominence in a few decades. These modifications have prompted experts from all over the world to identify effective strategies for the diagnosis and follow-up of CD. Different scientific societies, mainly from Europe and America regions, have proposed different guidelines.
CD guidelines are consistent when they deal key points in the diagnosis and follow-up of this condition. However, they differ in a number of other points.
To identify all of the existing guidelines across the globe and perform a comparative analysis to verify similarities and differences and, thus, discuss the most debated topics and the possible innovations in the next future.
We searched PubMed for a complex string containing the terms “celiac disease”, “management”, and “guidelines”. The results were subsequently explored to identify the most recent versions of existing guidelines of governmental agencies and scientific societies. The recommendations provided by each selected guideline were systematically explored and classified under five categories: Patients to be tested for CD, diagnostic tests (serology, duodenal biopsy, genetic test, no-biopsy diagnosis), potential/silent/seronegative CD, refractory/complicated CD, follow-up.
We identified 7 different guidelines [European Society Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) 2020; European Society for the Study of Coeliac Disease (ECD) 2019; World Gastroenterology Organization (WGO) 2017; Central Research Institute of Gastroenterology, Russia, 2016; National Institute for Health and Care Excellence (NICE), 2015; British Society of Gastroenterology (BSG), 2014; and America College of Gastroenterology (ACG), 2013]. These guidelines were mostly concordant but differed under certain recommendation for no-biopsy diag
We found a relatively high concordance between the guidelines for CD. Important modifications have occurred in the last years, especially about the possibility of a no-biopsy diagnosis in children.
Modifications of the current guidelines are expected in the near future. These modification will probably regard the possibility of a no-biopsy diagnosis (especially in developing countries) and the modalities of follow-up.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poddighe D, Sabelnikova EA, Sahin Y, Vasudevan A S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PHR, Hadjivassiliou M, Kaukinen K, Kelly CP, Leonard JN, Lundin KEA, Murray JA, Sanders DS, Walker MM, Zingone F, Ciacci C. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43-52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1254] [Cited by in RCA: 1162] [Article Influence: 96.8] [Reference Citation Analysis (1)] |
| 2. | Fasano A, Catassi C. Clinical practice. Celiac disease. N Engl J Med. 2012;367:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 457] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 3. | Poddighe D, Abdukhakimova D. Celiac Disease in Asia beyond the Middle East and Indian subcontinent: Epidemiological burden and diagnostic barriers. World J Gastroenterol. 2021;27:2251-2256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 4. | Whitburn J, Rao SR, Paul SP, Sandhu BK. Diagnosis of celiac disease is being missed in over 80% of children particularly in those from socioeconomically deprived backgrounds. Eur J Pediatr. 2021;180:1941-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Ianiro G, Bibbò S, Bruno G, Ricci R, Arena V, Gasbarrini A, Cammarota G. Prior Misdiagnosis of Celiac Disease Is Common Among Patients Referred to a Tertiary Care Center: A Prospective Cohort Study. Clin Transl Gastroenterol. 2016;7:e139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Biagi F, Schiepatti A, Maiorano G, Fraternale G, Agazzi S, Zingone F, Ciacci C, Volta U, Caio G, Tortora R, Klersy C, Corazza GR. Risk of complications in coeliac patients depends on age at diagnosis and type of clinical presentation. Dig Liver Dis. 2018;50:549-552. [PubMed] |
| 7. | D'Avino P, Serena G, Kenyon V, Fasano A. An updated overview on celiac disease: from immuno-pathogenesis and immuno-genetics to therapeutic implications. Expert Rev Clin Immunol. 2021;17:269-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 8. | Husby S, Koletzko S, Korponay-Szabó I, Kurppa K, Mearin ML, Ribes-Koninckx C, Shamir R, Troncone R, Auricchio R, Castillejo G, Christensen R, Dolinsek J, Gillett P, Hróbjartsson A, Koltai T, Maki M, Nielsen SM, Popp A, Størdal K, Werkstetter K, Wessels M. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J Pediatr Gastroenterol Nutr. 2020;70:141-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 693] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 9. | Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, Mulder CJ, Lundin KEA. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 598] [Article Influence: 99.7] [Reference Citation Analysis (1)] |
| 10. | Bai JC, Ciacci C. World Gastroenterology Organisation Global Guidelines: Celiac Disease February 2017. J Clin Gastroenterol. 2017;51:755-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Parfenov AI, Bykova SV, Sabel'nikova EA, Maev IV, Baranov AA, Bakulin IG, Krums LM, Bel'mer SV, Borovik TE, Zakharova IN, Dmitrieva YA, Roslavtseva EA, Kornienko EA, Khavkin AI, Potapov AS, Revnova MO, Mukhina YG, Shcherbakov PL, Fedorov ED, Belousova EA, Khalif IL, Khomeriki SG, Rotin DL, Vorob'eva NG, Pivnik AV, Gudkova RB, Chernin VV, Vokhmyanina NV, Pukhlikova TV, Degtyarev DA, Damulin IV, Mkrtumyan AM, Dzhulai GS, Tetruashvili NK, Baranovsky AY, Nazarenko LI, Kharitonov AG, Loranskaya ID, Saifutdinov RG, Livzan MA, Abramov DA, Osipenko MF, Oreshko LV, Tkachenko EI, Sitkin SI, Efremov LI. All-Russian Consensus on Diagnosis and Treatment of Celiac Disease in Children and Adults. Ter Arkh. 2017;89:94-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Downey L, Houten R, Murch S, Longson D; Guideline Development Group. Recognition, assessment, and management of coeliac disease: summary of updated NICE guidance. BMJ. 2015;351:h4513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, Green PH, Hadjivassiliou M, Holdoway A, van Heel DA, Kaukinen K, Leffler DA, Leonard JN, Lundin KE, McGough N, Davidson M, Murray JA, Swift GL, Walker MM, Zingone F, Sanders DS; BSG Coeliac Disease Guidelines Development Group; British Society of Gastroenterology. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63:1210-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 689] [Cited by in RCA: 783] [Article Influence: 71.2] [Reference Citation Analysis (3)] |
| 14. | Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA; American College of Gastroenterology. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656-676; quiz 677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 1157] [Article Influence: 96.4] [Reference Citation Analysis (0)] |
| 15. | Vilppula A, Kaukinen K, Luostarinen L, Krekelä I, Patrikainen H, Valve R, Mäki M, Collin P. Increasing prevalence and high incidence of celiac disease in elderly people: a population-based study. BMC Gastroenterol. 2009;9:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Vivas S, Ruiz de Morales JM, Fernandez M, Hernando M, Herrero B, Casqueiro J, Gutierrez S. Age-related clinical, serological, and histopathological features of celiac disease. Am J Gastroenterol. 2008;103:2360-2365; quiz 2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 17. | Fasano A. Celiac disease--how to handle a clinical chameleon. N Engl J Med. 2003;348:2568-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Baydoun A, Maakaron JE, Halawi H, Abou Rahal J, Taher AT. Hematological manifestations of celiac disease. Scand J Gastroenterol. 2012;47:1401-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Kamycheva E, Goto T, Camargo CA Jr. Celiac disease is associated with reduced bone mineral density and increased FRAX scores in the US National Health and Nutrition Examination Survey. Osteoporos Int. 2017;28:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Volta U, De Franceschi L, Lari F, Molinaro N, Zoli M, Bianchi FB. Coeliac disease hidden by cryptogenic hypertransaminasaemia. Lancet. 1998;352:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Luostarinen L, Pirttilä T, Collin P. Coeliac disease presenting with neurological disorders. Eur Neurol. 1999;42:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Schiepatti A, Sprio E, Sanders DS, Lovati E, Biagi F. Coeliac disease and obstetric and gynaecological disorders: where are we now? Eur J Gastroenterol Hepatol. 2019;31:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Agardh D, Lee HS, Kurppa K, Simell V, Aronsson CA, Jörneus O, Hummel M, Liu E, Koletzko S; TEDDY Study Group. Clinical features of celiac disease: a prospective birth cohort. Pediatrics. 2015;135:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Imanzadeh F, Sayyari AA, Yaghoobi M, Akbari MR, Shafagh H, Farsar AR. Celiac disease in children with diarrhea is more frequent than previously suspected. J Pediatr Gastroenterol Nutr. 2005;40:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Reilly NR, Fasano A, Green PHR. Presentation of Celiac Disease. Gastrointest Endosc Clin N Am. 2012;22:613-621. [RCA] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Leffler DA, Green PHR, Fasano A. Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol. 2015;12:561-571. [RCA] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 27. | Catassi C, Fasano A. Celiac disease diagnosis: simple rules are better than complicated algorithms. Am J Med. 2010;123:691-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Hischenhuber C, Crevel R, Jarry B, Maki M, Moneret-Vautrin DA, Romano A, Troncone R, Ward R. Review article: safe amounts of gluten for patients with wheat allergy or coeliac disease. Aliment Pharmacol Ther. 2006;23:559-575. [RCA] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Reeves GEM, Squance ML, Duggan AE, Murugasu RR, Wilson RJ, Wong RC, Gibson RA, Steele RH, Pollock WK. Diagnostic accuracy of coeliac serological tests: a prospective study. Eur J Gastroenterol Hepatol. 2006;18:493-501. [RCA] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Volta U, Fabbri A, Parisi C, Piscaglia M, Caio G, Tovoli F, Fiorini E. Old and new serological tests for celiac disease screening. Expert Rev Gastroenterol Hepatol. 2010;4:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Stern M; Working Group on Serologic Screening for Celiac Disease. Comparative evaluation of serologic tests for celiac disease: a European initiative toward standardization. J Pediatr Gastroenterol Nutr. 2000;31:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Sood A, Khurana MS, Mahajan R, Midha V, Puri S, Kaur A, Gupta N, Sharma S. Prevalence and clinical significance of IgA anti-tissue transglutaminase antibodies in patients with chronic liver disease. J Gastroenterol Hepatol. 2017;32:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Granito A, Muratori L, Muratori P, Petrolini N, Bianchi FB, Volta U. Antitransglutaminase antibodies and giardiasis. Am J Gastroenterol. 2004;99:2505-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Carroccio A, Vitale G, Di Prima L, Chifari N, Napoli S, La Russa C, Gulotta G, Averna MR, Montalto G, Mansueto S, Notarbartolo A. Comparison of anti-transglutaminase ELISAs and an anti-endomysial antibody assay in the diagnosis of celiac disease: a prospective study. Clin Chem. 2002;48:1546-1550. [PubMed] |
| 35. | Korponay-Szabó IR, Dahlbom I, Laurila K, Koskinen S, Woolley N, Partanen J, Kovács JB, Mäki M, Hansson T. Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut. 2003;52:1567-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Mozo L, Gómez J, Escanlar E, Bousoño C, Gutiérrez C. Diagnostic value of anti-deamidated gliadin peptide IgG antibodies for celiac disease in children and IgA-deficient patients. J Pediatr Gastroenterol Nutr. 2012;55:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Leonard MM, Lebwohl B, Rubio-Tapia A, Biagi F. AGA Clinical Practice Update on the Evaluation and Management of Seronegative Enteropathies: Expert Review. Gastroenterology. 2021;160:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Pais WP, Duerksen DR, Pettigrew NM, Bernstein CN. How many duodenal biopsy specimens are required to make a diagnosis of celiac disease? Gastrointest Endosc. 2008;67:1082-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Corazza GR, Villanacci V. Coeliac disease. J Clin Pathol. 2005;58:573-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 40. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1205] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 41. | Hammer ST, Greenson JK. The clinical significance of duodenal lymphocytosis with normal villus architecture. Arch Pathol Lab Med. 2013;137:1216-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Corazza GR, Villanacci V, Zambelli C, Milione M, Luinetti O, Vindigni C, Chioda C, Albarello L, Bartolini D, Donato F. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin Gastroenterol Hepatol. 2007;5:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 43. | Granito A, Muratori P, Cassani F, Pappas G, Muratori L, Agostinelli D, Veronesi L, Bortolotti R, Petrolini N, Bianchi FB, Volta U. Anti-actin IgA antibodies in severe coeliac disease. Clin Exp Immunol. 2004;137:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Poddighe D, Kushugulova A. Salivary Microbiome in Pediatric and Adult Celiac Disease. Front Cell Infect Microbiol. 2021;11:625162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 45. | Abdukhakimova D, Dossybayeva K, Poddighe D. Fecal and Duodenal Microbiota in Pediatric Celiac Disease. Front Pediatr. 2021;9:652208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 46. | Oldenburger IB, Wolters VM, Kardol-Hoefnagel T, Houwen RHJ, Otten HG. Serum intestinal fatty acid-binding protein in the noninvasive diagnosis of celiac disease. APMIS. 2018;126:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Vreugdenhil AC, Wolters VM, Adriaanse MP, Van den Neucker AM, van Bijnen AA, Houwen R, Buurman WA. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand J Gastroenterol. 2011;46:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Lundin KE, Wijmenga C. Coeliac disease and autoimmune disease-genetic overlap and screening. Nat Rev Gastroenterol Hepatol. 2015;12:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 49. | Megiorni F, Pizzuti A. HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: practical implications of the HLA molecular typing. J Biomed Sci. 2012;19:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 50. | Poddighe D, Rebuffi C, De Silvestri A, Capittini C. Carrier frequency of HLA-DQB1*02 allele in patients affected with celiac disease: A systematic review assessing the potential rationale of a targeted allelic genotyping as a first-line screening. World J Gastroenterol. 2020;26:1365-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (4)] |
| 51. | De Silvestri A, Capittini C, Poddighe D, Valsecchi C, Marseglia G, Tagliacarne SC, Scotti V, Rebuffi C, Pasi A, Martinetti M, Tinelli C. HLA-DQ genetics in children with celiac disease: a meta-analysis suggesting a two-step genetic screening procedure starting with HLA-DQ β chains. Pediatr Res. 2018;83:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Trovato CM, Montuori M, Cucchiara S, Oliva S. ESPGHAN 'biopsy-sparing' guidelines for celiac disease in children with low antitransglutaminase during COVID-19. Eur J Gastroenterol Hepatol. 2020;32:1523-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Penny HA, Raju SA, Lau MS, Marks LJ, Baggus EM, Bai JC, Bassotti G, Bontkes HJ, Carroccio A, Danciu M, Derakhshan MH, Ensari A, Ganji A, Green PHR, Johnson MW, Ishaq S, Lebwohl B, Levene A, Maxim R, Mohaghegh Shalmani H, Rostami-Nejad M, Rowlands D, Spiridon IA, Srivastava A, Volta U, Villanacci V, Wild G, Cross SS, Rostami K, Sanders DS. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut. 2021;70:876-883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 54. | Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, Bouhnik Y, Colombel JF, Delchier JC, Allez M, Cosnes J, Lavergne-Slove A, Meresse B, Trinquart L, Macintyre E, Radford-Weiss I, Hermine O, Brousse N, Cerf-Bensussan N, Cellier C. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 253] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 55. | Schiepatti A, Biagi F, Fraternale G, Vattiato C, Balduzzi D, Agazzi S, Alpini C, Klersy C, Corazza GR. Short article: Mortality and differential diagnoses of villous atrophy without coeliac antibodies. Eur J Gastroenterol Hepatol. 2017;29:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Delabie J, Holte H, Vose JM, Ullrich F, Jaffe ES, Savage KJ, Connors JM, Rimsza L, Harris NL, Müller-Hermelink K, Rüdiger T, Coiffier B, Gascoyne RD, Berger F, Tobinai K, Au WY, Liang R, Montserrat E, Hochberg EP, Pileri S, Federico M, Nathwani B, Armitage JO, Weisenburger DD. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 57. | Biagi F, Gobbi P, Marchese A, Borsotti E, Zingone F, Ciacci C, Volta U, Caio G, Carroccio A, Ambrosiano G, Mansueto P, Corazza GR. Low incidence but poor prognosis of complicated coeliac disease: a retrospective multicentre study. Dig Liver Dis. 2014;46:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | van Wanrooij RL, Schreurs MW, Bouma G, von Blomberg BM, Tack GJ, Verbeek WH, Mulder CJ. Accurate classification of RCD requires flow cytometry. Gut. 2010;59:1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 60. | Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99-107; quiz 352-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 61. | Mukewar SS, Sharma A, Rubio-Tapia A, Wu TT, Jabri B, Murray JA. Open-Capsule Budesonide for Refractory Celiac Disease. Am J Gastroenterol. 2017;112:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 62. | Chaudhary R, Ghosh S. Infliximab in refractory coeliac disease. Eur J Gastroenterol Hepatol. 2005;17:603-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Daum S, Wahnschaffe U, Glasenapp R, Borchert M, Ullrich R, Zeitz M, Faiss S. Capsule endoscopy in refractory celiac disease. Endoscopy. 2007;39:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Al-toma A, Visser OJ, van Roessel HM, von Blomberg BM, Verbeek WH, Scholten PE, Ossenkoppele GJ, Huijgens PC, Mulder CJ. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood. 2007;109:2243-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 65. | Pietzak MM. Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology. 2005;128:S135-S141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Ciacci C, Cirillo M, Cavallaro R, Mazzacca G. Long-term follow-up of celiac adults on gluten-free diet: prevalence and correlates of intestinal damage. Digestion. 2002;66:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 67. | Tovoli F, Negrini G, Farì R, Guidetti E, Faggiano C, Napoli L, Bolondi L, Granito A. Increased risk of nonalcoholic fatty liver disease in patients with coeliac disease on a gluten-free diet: beyond traditional metabolic factors. Aliment Pharmacol Ther. 2018;48:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 68. | Kemppainen T, Kröger H, Janatuinen E, Arnala I, Kosma VM, Pikkarainen P, Julkunen R, Jurvelin J, Alhava E, Uusitupa M. Osteoporosis in adult patients with celiac disease. Bone. 1999;24:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 69. | Valletta E, Fornaro M, Cipolli M, Conte S, Bissolo F, Danchielli C. Celiac disease and obesity: need for nutritional follow-up after diagnosis. Eur J Clin Nutr. 2010;64:1371-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 70. | Hughey JJ, Ray BK, Lee AR, Voorhees KN, Kelly CP, Schuppan D. Self-reported dietary adherence, disease-specific symptoms, and quality of life are associated with healthcare provider follow-up in celiac disease. BMC Gastroenterol. 2017;17:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Johansson K, Malmberg Hård Af Segerstad E, Mårtensson H, Agardh D. Dietitian visits were a safe and cost-effective form of follow-up care for children with celiac disease. Acta Paediatr. 2019;108:676-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | van Wanrooij RL, Bouma G, Bontkes HJ, Neefjes-Borst A, van Grieken NC, von Blomberg BM, Mulder CJ. Outcome of Referrals for Non-Responsive Celiac Disease in a Tertiary Center: Low Incidence of Refractory Celiac Disease in the Netherlands. Clin Transl Gastroenterol. 2017;8:e218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Lee A, Newman JM. Celiac diet: its impact on quality of life. J Am Diet Assoc. 2003;103:1533-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 74. | Comino I, Moreno Mde L, Sousa C. Role of oats in celiac disease. World J Gastroenterol. 2015;21:11825-11831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Standard for Foods for Special Dietary Use for Persons Intolerant to Gluten, CXS 118-1979, Adopted in 1979. [cited 22 February 2021]. Available from: http://www.fao.org/fao-who-codexalimentarius. |
| 76. | Studerus D, Hampe EI, Fahrer D, Wilhelmi M, Vavricka SR. Cross-Contamination with Gluten by Using Kitchen Utensils: Fact or Fiction? J Food Prot. 2018;81:1679-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Aronsson CA, Lee H-S, Liu E, Uusitalo U, Hummel S, Yang J, Hummel M, Rewers M, She J-X, Simell O, Toppari J, Ziegler A-G, Krischer J, Virtanen SM, Norris JM, Agardh D, for the TEDDY STUDY GROUP. Age at Gluten Introduction and Risk of Celiac Disease. Pediatrics. 2015;135:239-245. [RCA] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 78. | Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, Kelly CP, Ahuja V, Makharia GK. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:823-836.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 948] [Article Influence: 135.4] [Reference Citation Analysis (1)] |
| 79. | Dhawan A, Agarwal A, Mulder CJ, Makharia GK. Celiac disease in the East and the West: Bridging the gaps between the guidelines and their implementation in daily practice is mandatory. Indian J Gastroenterol. 2019;38:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Biagi F, Raiteri A, Schiepatti A, Klersy C, Corazza GR. The Relationship Between Child Mortality Rates and Prevalence of Celiac Disease. J Pediatr Gastroenterol Nutr. 2018;66:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Valitutti F, Troncone R, Pisano P, Ciacci C; Campania Coeliac Disease Network. Where have all the other coeliacs gone in 2020? Dig Liver Dis. 2021;53:504-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Riznik P, De Leo L, Dolinsek J, Gyimesi J, Klemenak M, Koletzko B, Koletzko S, Korponay-Szabó IR, Krencnik T, Not T, Palcevski G, Sblattero D, Werkstetter KJ. Clinical Presentation in Children With Coeliac Disease in Central. Europe J Pediatr Gastroenterol Nutr. 2021;72:546-551. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 83. | Reilly NR, Lebwohl B, Hultcrantz R, Green PH, Ludvigsson JF. Increased risk of non-alcoholic fatty liver disease after diagnosis of celiac disease. J Hepatol. 2015;62:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 84. | Reilly NR, Aguilar K, Hassid BG, Cheng J, Defelice AR, Kazlow P, Bhagat G, Green PH. Celiac disease in normal-weight and overweight children: clinical features and growth outcomes following a gluten-free diet. J Pediatr Gastroenterol Nutr. 2011;53:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 85. | Tortora R, Capone P, De Stefano G, Imperatore N, Gerbino N, Donetto S, Monaco V, Caporaso N, Rispo A. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther. 2015;41:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 86. | Rubio-Tapia A, Murray JA. The Liver and Celiac Disease. Clin Liver Dis. 2019;23:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |