Published online Jan 7, 2022. doi: 10.3748/wjg.v28.i1.123
Peer-review started: July 14, 2021
First decision: October 3, 2021
Revised: October 22, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 7, 2022
Processing time: 170 Days and 11.5 Hours
Hepatic stellate cell (HSC) hyperactivation is a central link in liver fibrosis development. HSCs perform aerobic glycolysis to provide energy for their activation. Focal adhesion kinase (FAK) promotes aerobic glycolysis in cancer cells or fibroblasts, while FAK-related non-kinase (FRNK) inhibits FAK phos
To elucidate the effect of FRNK on liver fibrosis at the level of aerobic glycolytic metabolism in HSCs.
Mouse liver fibrosis models were established by administering CCl4, and the effect of FRNK on the degree of liver fibrosis in the model was evaluated. Transforming growth factor-β1 was used to activate LX-2 cells. Tyrosine phosphorylation at position 397 (pY397-FAK) was detected to identify activated FAK, and the expression of the glycolysis-related proteins monocarboxylate transporter 1 (MCT-1) and enolase1 (ENO1) was assessed. Bioinformatics analysis was per
The pY397-FAK level was increased in human fibrotic liver tissue. FRNK knock
FRNK inhibits aerobic glycolysis in HSCs by inhibiting the FAK/Ras/c-myc/ ENO1 pathway, thereby improving liver fibrosis. FRNK might be a potential target for liver fibrosis treatment.
Core Tip: We show that focal adhesion kinase-related non-kinase (FRNK) limits hepatic stellate cell (HSC) activation, proliferation, and migration and promotes HSC apoptosis by inhibiting aerobic glycolysis, thereby ameliorating liver fibrosis. FRNK may represent a potential therapeutic candidate for liver fibrosis treatment.
- Citation: Huang T, Li YQX, Zhou MY, Hu RH, Zou GL, Li JC, Feng S, Liu YM, Xin CQ, Zhao XK. Focal adhesion kinase-related non-kinase ameliorates liver fibrosis by inhibiting aerobic glycolysis via the FAK/Ras/c-myc/ENO1 pathway. World J Gastroenterol 2022; 28(1): 123-139
- URL: https://www.wjgnet.com/1007-9327/full/v28/i1/123.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i1.123
Long-term damage to liver function by hepatitis viruses, alcohol, and diet may cause chronic hepatic injuries leading to liver fibrosis and cirrhosis[1-4], which is characterized by the activation of hepatic stellate cells (HSCs) and their transformation into myofibroblasts[5]. This process continuously damages the liver and disrupts the balance of liver self-repair, causing increased cell proliferation and migration and a reduced apoptosis rate[6-8]. At present, the pathological changes associated with chronic liver injuries in individuals without cirrhosis can be reversed after removing the etiological agent, such as in a small proportion of patients with hepatitis B and alcoholic fatty liver disease and most patients with hepatitis C[1,9], but the remaining patients with hepatic fibrosis develop irreversible cirrhosis due to an inability to completely and effectively reverse the pathogenesis and a lack of effective antifibrotic drugs[10]. Therefore, studies of the treatment of liver fibrosis are particularly critical, among which the regulation of the relevant biological functions of HSCs is the most important antifibrotic approach[11,12].
Focal adhesion kinases (FAKs) are a class of nonreceptor cytosolic protein tyrosine kinases that belong to the protein tyrosine kinase superfamily[13,14]. FAK plays an important role in cellular signal transduction and enhances biological behaviors such as proliferation, migration, wound healing and angiogenesis in cells and tissues after integrating signals from integrins, growth factors and mechanical stimuli[15,16]. FAK binds to extracellular matrix (ECM) proteins through an accumulation of integrin receptors to form FAK dimers, which further induce tyrosine phosphorylation at position 397 (pY397-FAK); pY397-FAK regulates these biological functions in cells[17,18] and therefore plays an important role in a variety of malignant tumor cells[18,19]. FAK-related non-kinase (FRNK), which has a nucleotide sequence corresponding to the C-terminus of FAK but lacks the N-terminal functional site of FAK, is an independently expressed protein[20] with the main function of inhibiting FAK phosphorylation, thereby inversely regulating the function of FAK after cell activation[21,22]. FRNK negatively regulates FAK signaling axis function, thereby improving pulmonary fibrosis in an experimental mouse model[23].
FAK is also overexpressed in pancreatic ductal adenocarcinoma cells[24,25], promoting the conversion of pyruvate into lactate by increasing enolase1 (ENO1), pyruvate kinase 2, lactate dehydrogenase, and monocarboxylate transporter (MCT)-1 expression and lactate transport, enhancing aerobic glycolysis in cancer cells, and inhibiting mitochondrial oxidative phosphorylation in cancer cells[16]. This switch to aerobic glycolysis is an important mechanism by which tumor cells acquire energy[17,26], as shown by the fact that oxidative phosphorylation simultaneously provides energy to cells performing aerobic glycolysis even in the presence of sufficient oxygen and normal mitochondrial function[27,28]. HSCs also exhibit increased aerobic glycolysis, resulting in lactate accumulation and gluconeogenesis inhibition when they differentiate into myofibroblasts[8,28]. This phenomenon also occurs in individuals with congenital pulmonary fibrosis[29]. Therefore, the inhibition of FAK-related pathways by FRNK may reduce energy acquisition through aerobic glycolysis during HSC activation and could be used as a targeted therapy to ameliorate liver fibrosis. Nevertheless, few studies have focused on the physiological or pathological role of FRNK in obtaining energy during hepatic fibrosis, and its mechanism remains unclear.
In the present study, we first showed that FRNK was downregulated in human liver fibrotic tissues. Then, we verified that FRNK knockout in vivo and in vitro promoted aerobic glycolysis and hepatic fibrosis. Exogenous FRNK inhibited aerobic glycolysis by inhibiting the FAK/Ras/c-myc/ENO1 pathway, limiting HSC activation, migra
Paraffin blocks of liver tissues from 15 patients with liver fibrosis were collected from the Department of Infectious Diseases, Affiliated Hospital of Guizhou Medical University (Guiyang, China) between March 2019 and September 2019; none of the patients had any other organ-specific or systemic diseases, and liver fibrosis was diagnosed by pathological biopsy. Fifteen healthy liver samples were obtained from distal hepatocarcinoma liver tissue without any abnormalities in specimens surgically resected from patients at the Department of Hepatobiliary Surgery, Affiliated Hospital of Guizhou Medical University. None of the aforementioned subjects had contraindications to liver biopsy, and the study was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University (Approval 2018 Ethics Review No. 032) and conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Written informed consent was obtained from the patients.
FRNK knockout (FRNK-/-) mice were a gift from the Respiratory and Critical Care Medicine Center, School of Medicine, University of Alabama at Birmingham, Birmingham, AL, United States. All mouse interventions were approved by the Animal Care Committee (IACUC) of Guizhou Medical University (No. 1801109), and the methods and experimental procedures were performed in accordance with the relevant guidelines and regulations. Wild-type (WT) mice of the same genotype were used as controls, and all experimental mice were on the C57BL/6 background.
Mice were maintained under pathogen-free conditions at a controlled temperature (22 ± 2 °C) with a consistent photoperiod (12:12 h light-dark cycle); five mice were housed in each cage, with cages containing soft bedding. The mice were habituated to these conditions for 2 d before inclusion in an experiment. Healthy male mice (aged 8-11 wk, weighing 20 ± 3 g) were selected and intraperitoneally injected with 1.5 μL/g of a 10% Carbon tetrachloride (CCl4) in corn oil solution three times a week to establish a liver fibrosis model. Mice in the control group were injected with a 1.5 μL/g solution of corn oil three times a week. Livers were harvested at each time point, namely, 0, 2, 4 and 6 wk, for experiments. Six mice per group were used.
CCl4, corn oil, and OptiPrep were purchased from Sigma-Aldrich (St. Louis, MO, United States). Transforming growth factor-β1 (TGF-β1) was purchased from R&D Systems (Minneapolis, MN, United States). Primary antibodies specific for the follo
H&E staining kits, Masson’s trichrome staining solution and Sirius Red staining solution were purchased from Solarbio Biotechnology Co., Ltd. (Beijing, China) and used according to the manufacturer’s guidelines. A hydroxyproline assay was performed using a Nanjing Jiancheng Biotechnology (Nanjing, China) hydroxyproline kit. All kits were used according to the instructions for use. Liver samples were fixed with neutral buffered formalin and embedded in paraffin for IHC. Briefly, sections were incubated with the indicated antibodies. Horseradish peroxidase-conjugated antibodies were used as the secondary antibodies. Finally, a diaminobenzidine colorimetric reagent solution was applied, followed by hematoxylin counterstaining. The slides were then scanned, and representative images were acquired.
LX-2 cells were purchased from Zhongqiao Xinzhou (Shanghai, China). Human HSCs (ZQ0026) and LX-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS; Biological Industries, Kibbutz Beit-Haemek, Israel). Primary hepatic stellate cells (pHSCs) were extracted from C57BL/6 WT or FRNK-/- mice aged 8-11 wk, as previously described[30,31]. Briefly, the abdo
An adenovirus-mediated gene delivery system was used to effectively deliver the FRNK cDNA into HSCs. An adenoviral vector carrying the FRNK protein and green fluorescent protein (Ad-FRNK) as well as a green fluorescent protein-carrying adenovirus (Ad-GFP) were purchased from Jikai Gene (Shanghai, China). All trans
Transwell migration experiments used 8.0-μm pore size membranes (Corning, United States) according to the manufacturer's protocol. A total of 105 cells were seeded in the upper chamber of each well in 100 μL of serum-free medium, while 600 μL of complete medium was added to the lower chamber as a chemoattractant. After a 6-h incubation at 37 °C, the cells remaining on the upper surface of the membrane were removed with a cotton swab, and the cells on the lower surface of the membrane were considered migrated cells. After fixation with 4% paraformaldehyde and staining with a 0.1% crystal violet solution, images were acquired under an inverted microscope. Cell Counting Kit-8 (CCK-8) was purchased from Dojindo (Shanghai, China), and 104 cells (100 μL/well) were seeded in 96-well plates. After placing the culture plate in an incubator for preincubation (37 °C, 5% CO2), 10 μL of CCK-8 solution was added to each well, and then the culture plate was evaluated with a microplate reader to detect the absorbance at 450 nm. A total of 105 cells in each group were stained with an Annexin V-PE/7-AAD apoptosis kit (Hangzhou Lianke, Hangzhou, Zhejiang Province, China) according to the instructions for use, sorted with a flow cytometer (Beckman, United States) and analyzed using Flow Jo software (Tree Star); dead cells were excluded based on forward scatter and side scatter data.
Western blot analysis was performed as previously described[30]. Briefly, 1% NP-40-treated whole-liver tissue lysates or whole-cell lysates were used for Western blot analysis. Protein levels were quantified using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA) after total protein extraction. Twenty milligrams of each protein sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). GAPDH was used as a loading control for all blots. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes, which were incubated with primary antibodies overnight at 4 °C. The next day, after an incubation with an appropriate secondary antibody, signals were generated with an electrochemiluminescence detection kit.
The lactate level in culture medium was detected with the Lactate Colorimetric Assay Kit (BioVision, Milpitas, CA, United States) according to the manufacturer's ins
JASPAR (http://jaspar.genereg.net) and PROMO (http://alggen.lsi.upc.es) database analyses predicted two putative c-myc binding sites in the ENO1 promoter region. A total of 107 cells fixed with formaldehyde were collected in 500 μL of lysis buffer from the Magna ChIP HiSens Kit (Millipore, Bedford, Massachusetts, United States) according to the manufacturer's manual. Cells were then sonicated for 25 cycles with a 6-s power-on interval of 30 s and an intensity of 200 W. Afterward, the supernatant was diluted and thoroughly mixed with Protein A/G beads. Then, 5 μg of IgG or an anti-c-myc antibody was added and incubated with the mixture overnight at 4 °C. After washing the beads the next day, the mixture was incubated with elution buffer at 62 °C for 2 h and then 95 °C for 10 min. The eluted DNA was then purified and subjected to a PCR assay to assess the binding sequence. Specific primer sequences were used to perform PCR.
The effect of c-myc on the ENO1 promoter was determined by cotransfecting pcDNA-c-myc or pcDNA-vector (NC) into LX-2 cells with pGL3-based constructs containing an empty sequence (NC) or the WT or MT1/MT2 ENO1 promoter sequences, and Renilla luciferase reporter plasmids. Twenty-four hours after transfection, firefly and Renilla luciferase activities were measured with a luciferase reporter assay kit (Genomeditech, Shanghai, China). Fluorescence detection was performed according to the instructions of the instrument, the parameters were set, the measurement time was 10 s, and the measurement interval was 2 s. Each sample was added into a measuring tube in a total volume of 20 μL (the sample volume was consistent in each mea
Data were statistically analyzed using GraphPad Prism 5.0 software, and a two-tailed Student's t test was used for comparisons between different groups. P < 0.05 was considered statistically significant. Data are presented as the mean ± SD.
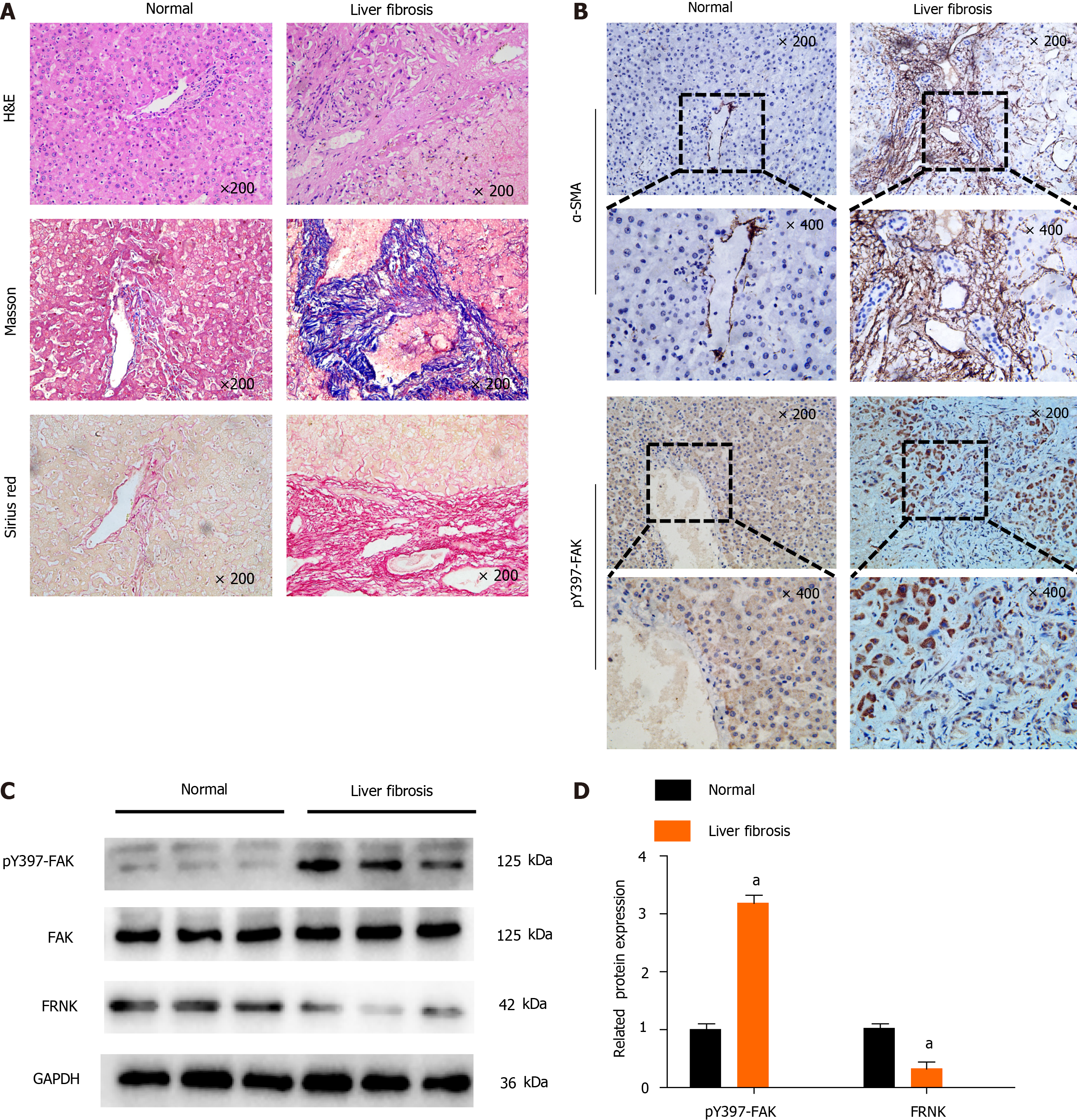
We first investigated the level of pY397-FAK in fibrotic liver tissue to explore the role of pY397-FAK in liver fibrosis. Compared with normal liver tissue, liver tissue samples from patients in the liver fibrosis group showed typical pathological features, including significant steatosis, inflammatory necrosis, significant collagen deposition, hepatic fibrosis and hepatocyte loosening. Masson’s trichrome staining showed less collagen deposition and a normal cell morphology in the normal group, while a large number of blue-stained collagen fibers was observed in the liver fibrosis group, and the tissue had accumulated a wide band of collagen fibers that extended into and was distributed in the hepatic lobules. Sirius Red staining showed less collagen deposition in normal subjects and a normal cell morphology but substantial red staining indicating collagen deposition in portal areas in fibrotic liver tissues. Notably, IHC showed higher α-smooth muscle actin (α-SMA) and pY397-FAK expression in fibrotic liver tissue than in normal liver tissues (Figure 1A and B). Western blot analysis showed higher levels of the pY397-FAK protein in fibrotic liver tissues than in normal liver tissues. Conversely, in fibrotic liver tissue, FRNK was expressed at lower levels than that in normal tissues (P < 0.05, Figure 1C and D). These results suggest that pY397-FAK protein expression is increased and FRNK protein expression is decreased in fibrotic liver tissue.
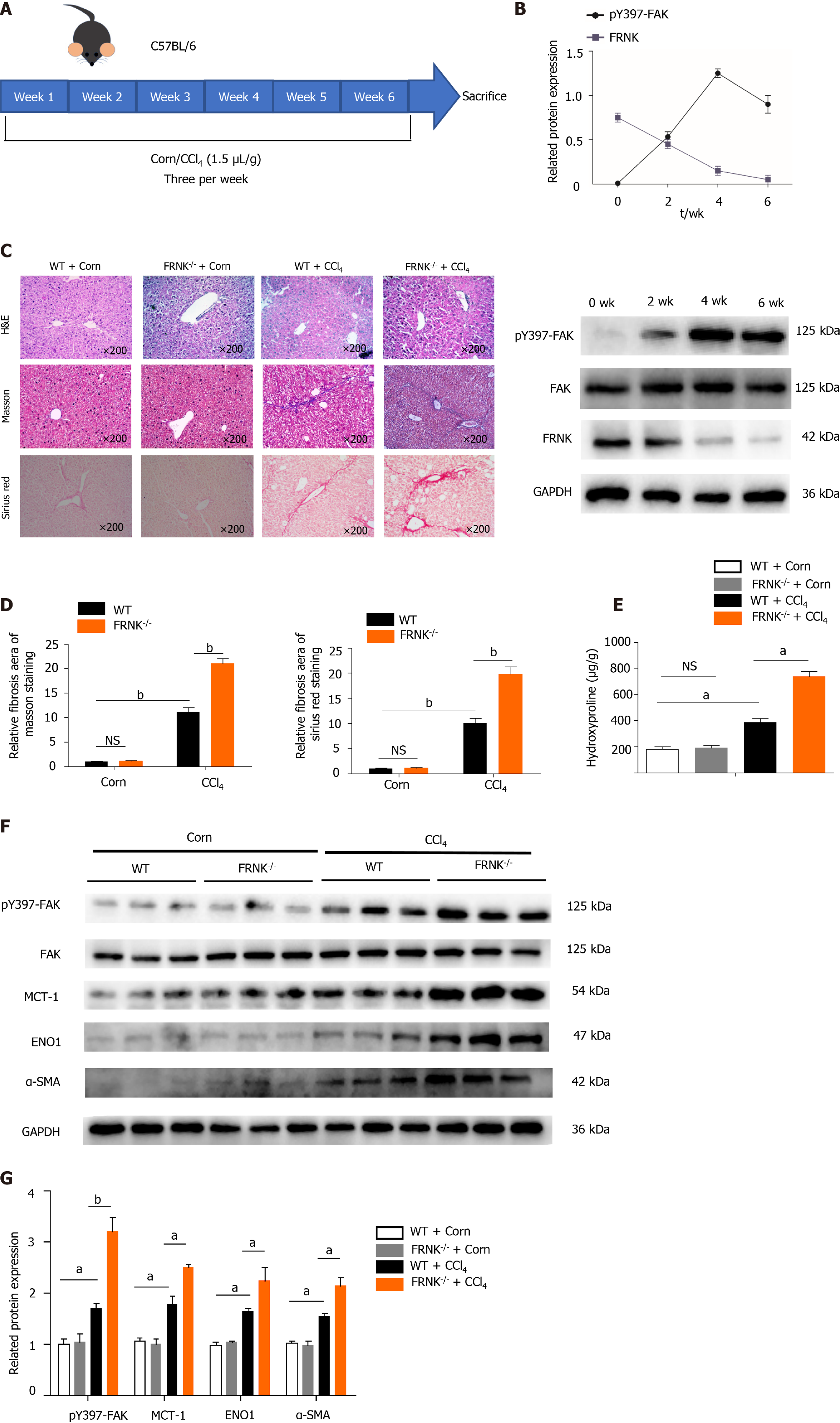
We established a fibrosis model by injecting CCl4 into WT mice and FRNK-/- mice. After two fortnights, the expression of the pY397-FAK protein peaked, while the FRNK protein was expressed at a low level (P < 0.05, Figure 2A and B). Therefore, mouse models with four weeks of injection were used in subsequent experiments. By performing H&E, Masson’s trichrome and Sirius Red staining, we found a greater liver fibrosis area and more extensive liver fibrosis in FRNK-/- mice than in WT mice after the CCl4 intervention (P < 0.05, Figure 2C and D), while the hydroxyproline content in FRNK-/- mice with fibrosis was greater than that in WT mice with fibrosis (P < 0.05, Figure 2E). Western blot analysis revealed higher levels of the pY397-FAK, MCT-1, ENO1 and α-SMA proteins in the liver tissues from FRNK-/- mice treated with CCl4 than in WT mice (P < 0.05, Figure 2F and G). Based on these results, FRNK-/- mice develop more severe liver fibrosis after the CCl4 intervention, along with increased expression of the aerobic glycolysis-related proteins MCT-1 and ENO1. It suggests that there may be more active aerobic glycolysis in the liver.
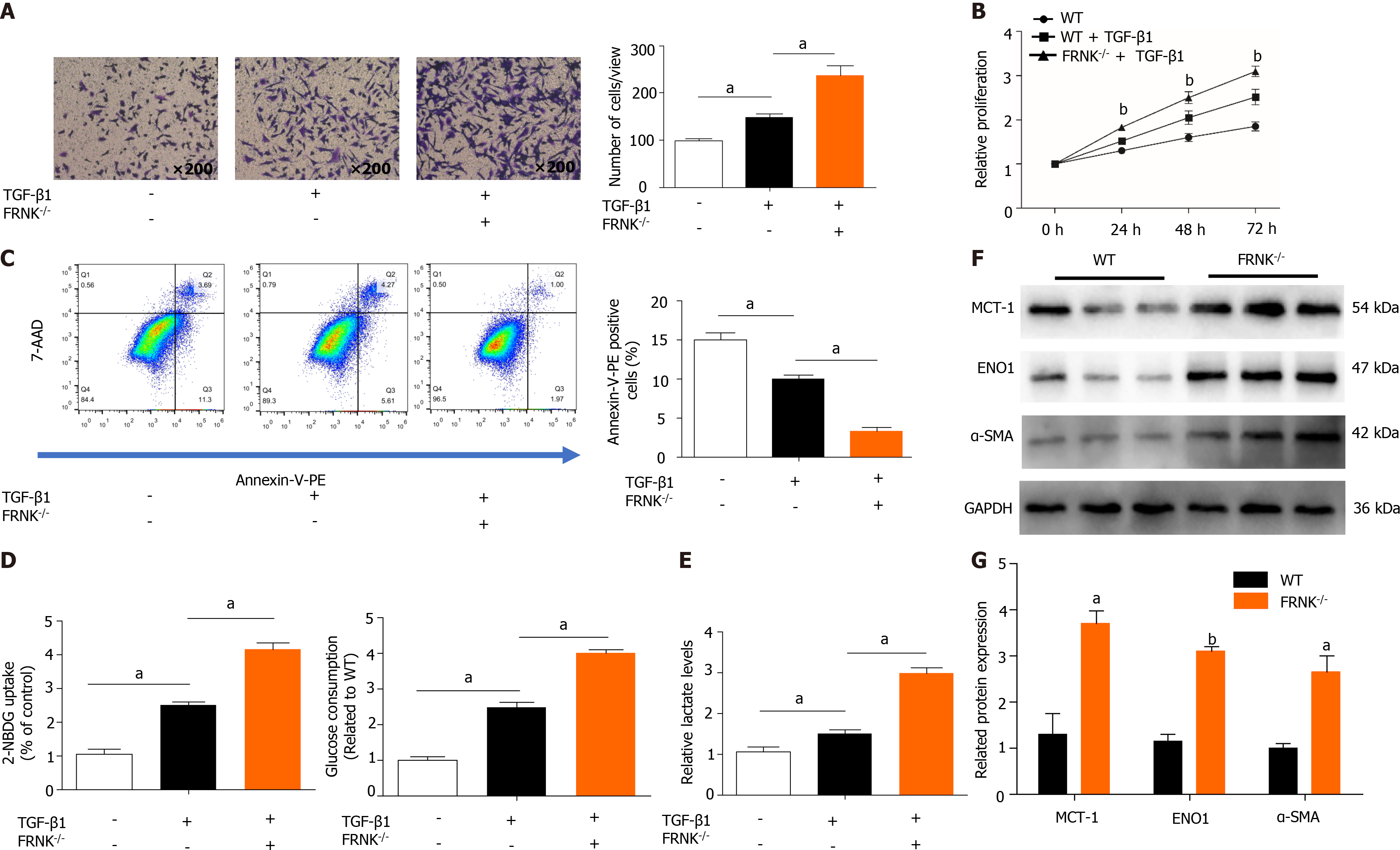
We extracted pHSCs from WT mice and FRNK-/- mice for in vitro experiments (Supplementary Figure 1). After 36 h of TGF-β1 treatment, the migration of pHSCs from the FRNK-/- groups in a Transwell chamber was increased (P < 0.05, Figure 3A). As indicated by the level of cell proliferation, pHSCs from the FRNK-/- group exhibited increased cellular activity (P < 0.05, Figure 3B). After adding TGF-β1 to pHSCs from FRNK-/- mice for 36 h, the apoptosis rate was lower than that of pHSCs from the control mice (P < 0.05, Figure 3C). Moreover, pHSCs from FRNK-/- mice showed increased glucose uptake and consumption compared with control pHSCs. Addi
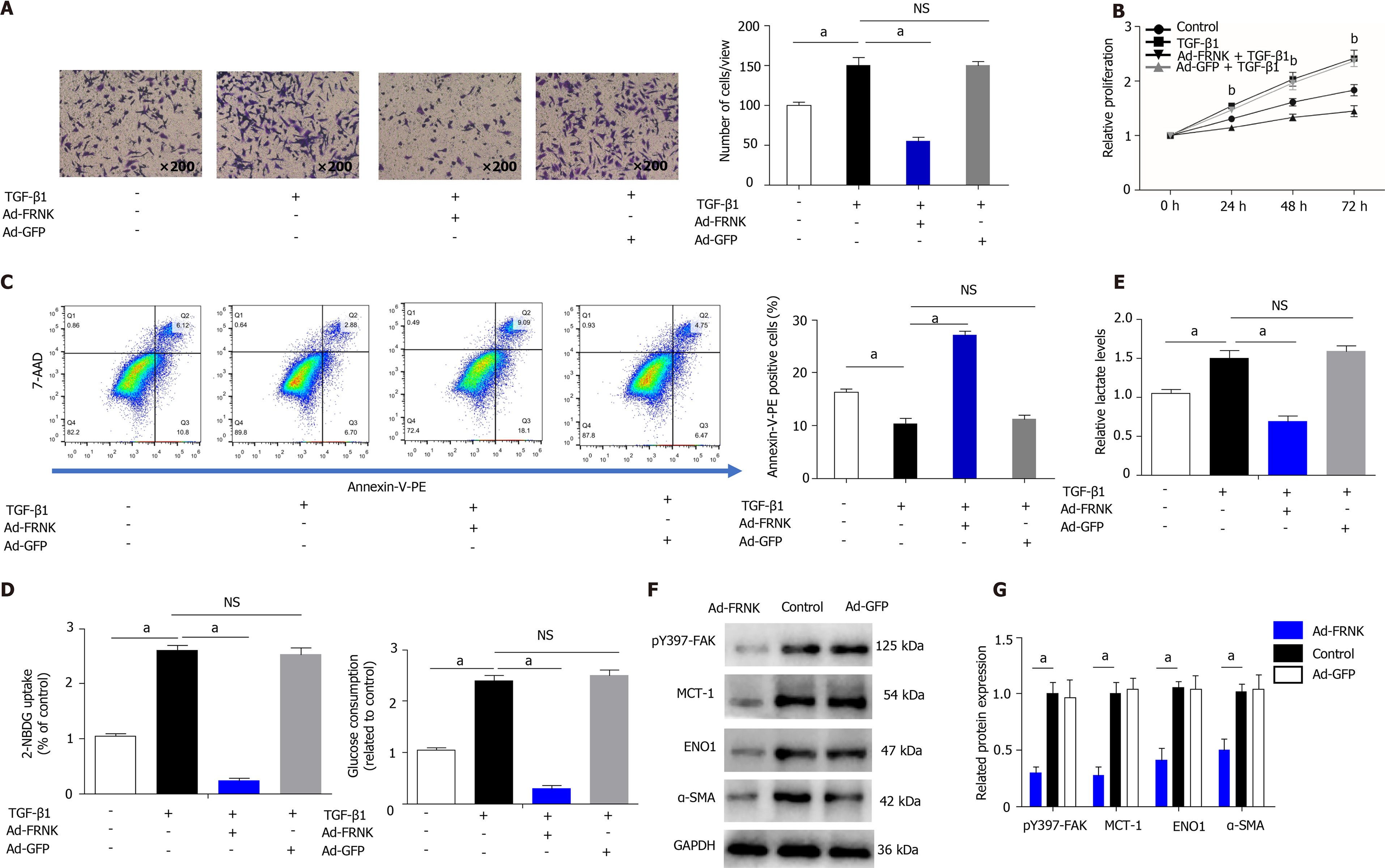
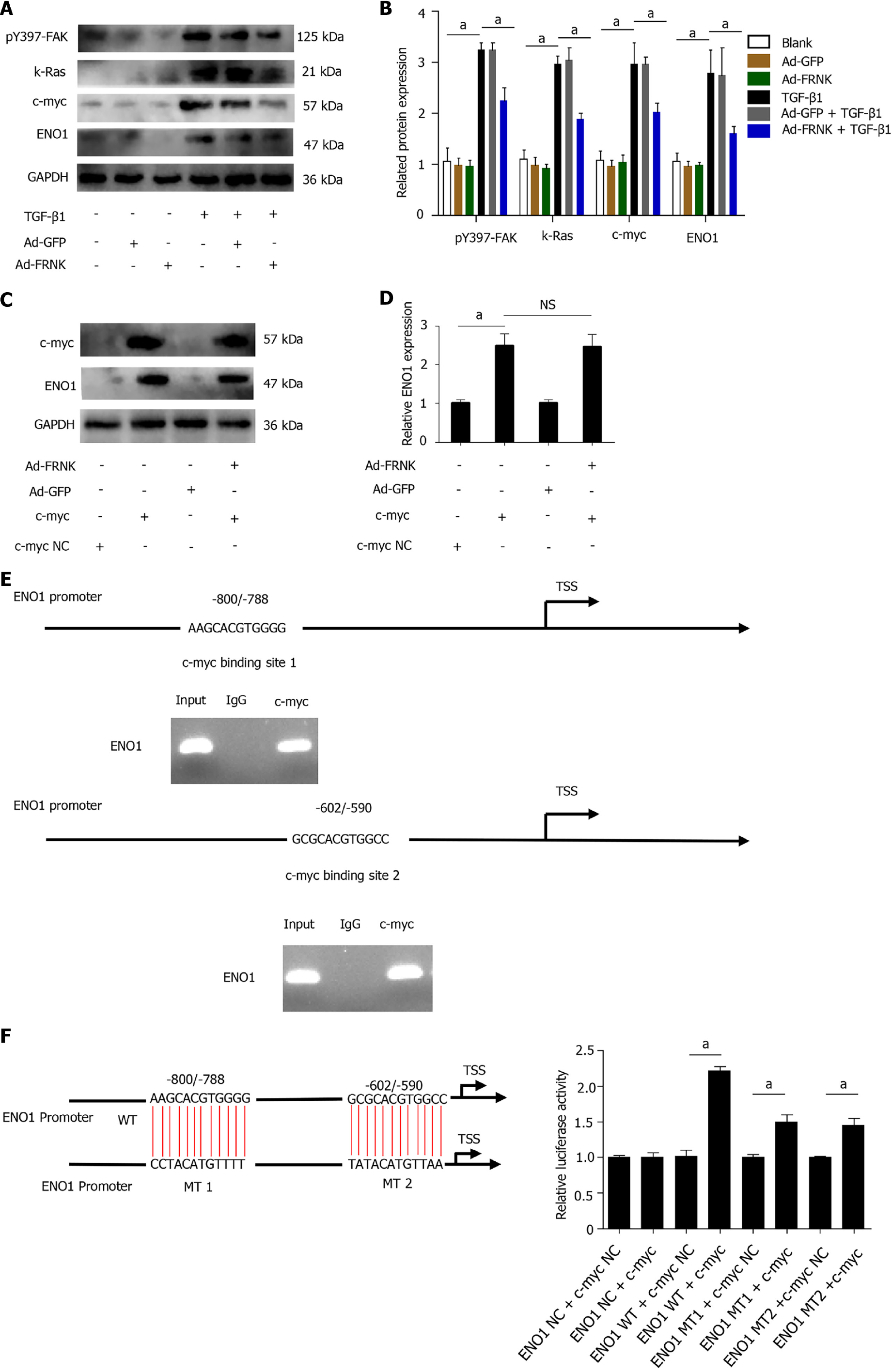
We transfected LX-2 cells with an adenovirus containing FRNK, induced the ex
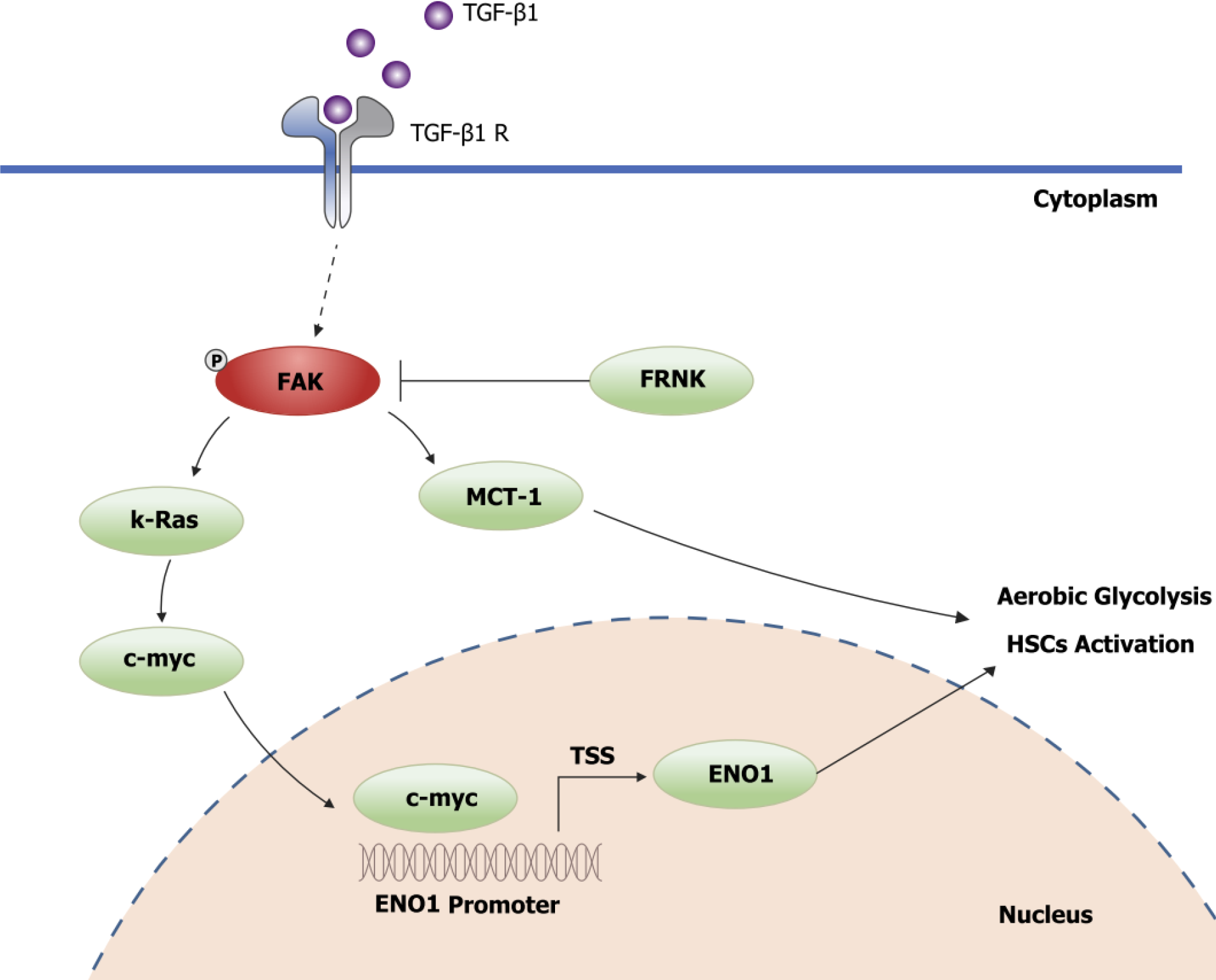
To explore the precise molecular mechanism by which FRNK regulates the ENO1 protein, TGF-β1 was used to stimulate LX-2 cells to activate FAK. The level of pY397-FAK was increased after stimulation with TGF-β1. The expression of the K-Ras, c-myc and ENO1 proteins downstream of FAK was also examined (P < 0.05, Figure 5A and B). While examining whether FRNK directly inhibits ENO1 protein expression, increased ENO1 protein expression was observed after introducing exogenous c-myc into LX-2 cells, but ENO1 protein expression was not reduced after the continued introduction of exogenous FRNK (P < 0.05, Figure 5C and D), suggesting that exogenous FRNK does not directly inhibit ENO1 protein expression to exert its biological function. As a method to investigate whether c-myc directly regulates ENO1, a bioinformatics analysis of the ENO1 promoter was performed to predict the putative binding site for c-myc in the ENO1 promoter, followed by ChIP and dual-luciferase reporter assays to verify that c-myc transcriptionally activates the ENO1 promoter (P < 0.05, Figure 5E and F). The results suggested that TGF-β1 activates FAK by inducing FAK phosphorylation at position 397. Then, pY397-FAK increased the expression of downstream K-Ras and c-myc proteins, followed by transcriptional activation of ENO1 expression by c-myc, promoting aerobic glycolysis and activation in HSCs. pY397-FAK and its biological functions were inhibited by the introduction of exogenous FRNK, limiting aerobic glycolysis and activation in HSCs and ameliorating liver fibrosis (Figure 6).
The imbalance between liver injury and self-repair is the key to the development of liver fibrosis, and restoring the balance from an imbalanced state is a potential treatment for liver diseases. In the present study, we verified that FRNK alters the activation, proliferation, migration and apoptosis of HSCs by regulating aerobic glycolysis during liver fibrosis. FRNK inhibits aerobic glycolysis in HSCs by sup
Early studies by our group verified that FAK plays important roles in the activation of HSCs and the development of liver fibrosis and that inhibition of FAK gene expression inhibits liver fibrogenesis[30]. Ding et al[23] verified that FRNK negatively regulates pulmonary fibrosis induced by FAK phosphorylation during pulmonary fibrosis. If FRNK inhibits the biological function of FAK in pulmonary fibrosis and uses a similar mechanism to repress liver fibrosis, it may represent a potential therapeutic target in liver fibrosis. Previous studies on FRNK have focused on the inhibition of the migratory function of vascular smooth muscle[32,33], combined with the presence of extracellular lactate accumulation during HSC activation[28,34] and FAK activation of aerobic glycolytic function in tumor cells[35-37]. FRNK may improve liver fibrosis by inhibiting aerobic glycolysis and inhibiting FAK activation in HSCs, but the mechanism by which FRNK exerts this effect remains unclear.
In the current study, we first observed increased expression of the pY397-FAK protein and decreased expression of the FRNK protein in tissues from patients with liver fibrosis (Figure 1). Subsequent experiments using CCl4 to replicate liver fibrosis in a mouse model yielded the same results (Figure 2B). Therefore, we speculated that a correlation between the occurrence of liver fibrosis and the downregulation of FRNK expression may exist and subsequently performed experiments in WT mice and FRNK-/- mice. After the CCl4 intervention, the degree of liver fibrosis in WT mice was lower than that in FRNK-/- mice (Figure 2C and D). The expression of the aerobic glycolysis-related proteins MCT-1 and ENO1 in the liver tissue of FRNK-/- mice was increased (Figure 2F and G), suggesting that FRNK gene deletion may promote in
In conclusion, this study is the first to reveal the effect of FRNK on liver fibrosis at the metabolic level. The experimental results suggest that the FAK/FRNK genes are potentially useful therapeutic targets in liver fibrosis and provide some rationale for the development of related drugs in the future.
Hepatic stellate cell (HSC) hyperactivation is a central link in liver fibrosis deve
Focal adhesion kinase (FAK) promotes aerobic glycolysis in cancer cells or fibroblasts, while FAK-related non-kinase (FRNK) inhibits FAK phosphorylation and biological functions.
To elucidate the effect of FRNK on liver fibrosis at the level of aerobic glycolytic metabolism in HSCs.
Mouse liver fibrosis models were established by administering CCl4, and the effect of FRNK on the degree of liver fibrosis in the model was evaluated. Transforming growth factor-β1 was used to activate LX-2 cells. Tyrosine phosphorylation at position 397 (pY397-FAK) was detected to identify activated FAK, and the expression of the glycolysis-related proteins monocarboxylate transporter 1 (MCT-1) and enolase1 (ENO1) was assessed. Bioinformatics analysis was performed to predict putative binding sites for c-myc in the ENO1 promoter region, which were validated with chromatin immunoprecipitation (ChIP) and dual-luciferase reporter assays.
The pY397-FAK level was increased in human fibrotic liver tissue. FRNK knockout promoted liver fibrosis in mouse models. It also increased the activation, migration, proliferation and aerobic glycolysis of primary hepatic stellate cells (pHSCs) but inhibited pHSC apoptosis. Nevertheless, opposite trends for these phenomena were observed after exogenous FRNK treatment in LX-2 cells. Mechanistically, the FAK/ Ras/c-myc/ENO1 pathway promoted aerobic glycolysis, which was inhibited by exogenous FRNK.
FRNK inhibits aerobic glycolysis in HSCs by inhibiting the FAK/Ras/c-myc/ENO1 pathway, thereby improving liver fibrosis. FRNK might be a potential target for liver fibrosis treatment.
The molecular mechanism by which FRNK regulates ENO1 and MCT-1 expression should be confirmed by conducting more complicated investigations in the future, and our group will be dedicated to studying this pathway.
The authors thank Professor Qiang Ding's laboratory at the University of Alabama at Birmingham, School of Medicine, Birmingham, AL, United States, for the gift of FRNK-/- mice and providing experimental technical guidance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morozov S, Truong NH S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Testino G, Leone S, Fagoonee S, Pellicano R. Alcoholic liver fibrosis: detection and treatment. Minerva Med. 2018;109:457-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Stål P. Liver fibrosis in non-alcoholic fatty liver disease - diagnostic challenge with prognostic significance. World J Gastroenterol. 2015;21:11077-11087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 100] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (3)] |
| 3. | Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol. 2014;20:11033-11053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 5. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 783] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 6. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4119] [Article Influence: 206.0] [Reference Citation Analysis (3)] |
| 7. | Campana L, Iredale JP. Regression of Liver Fibrosis. Semin Liver Dis. 2017;37:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 286] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 8. | Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1221] [Cited by in RCA: 1995] [Article Influence: 249.4] [Reference Citation Analysis (0)] |
| 9. | Rocco A, Compare D, Angrisani D, Sanduzzi Zamparelli M, Nardone G. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652-14659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (5)] |
| 10. | Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68-69:435-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 11. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 12. | Dhar A, Sadiq F, Anstee QM, Levene AP, Goldin RD, Thursz MR. Thrombin and factor Xa link the coagulation system with liver fibrosis. BMC Gastroenterol. 2018;18:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Lai IR, Chu PY, Lin HS, Liou JY, Jan YJ, Lee JC, Shen TL. Phosphorylation of focal adhesion kinase at Tyr397 in gastric carcinomas and its clinical significance. Am J Pathol. 2010;177:1629-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Tapial Martínez P, López Navajas P, Lietha D. FAK Structure and Regulation by Membrane Interactions and Force in Focal Adhesions. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 15. | Sakurai M, Ohtake J, Ishikawa T, Tanemura K, Hoshino Y, Arima T, Sato E. Distribution and Y397 phosphorylation of focal adhesion kinase on follicular development in the mouse ovary. Cell Tissue Res. 2012;347:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Mikuriya K, Kuramitsu Y, Ryozawa S, Fujimoto M, Mori S, Oka M, Hamano K, Okita K, Sakaida I, Nakamura K. Expression of glycolytic enzymes is increased in pancreatic cancerous tissues as evidenced by proteomic profiling by two-dimensional electrophoresis and liquid chromatography-mass spectrometry/mass spectrometry. Int J Oncol. 2007;30:849-855. [PubMed] |
| 17. | Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, Li Y, You W, Dong Q, Hong T, Yan Z, Jin S, Wang T, Zhao W, Mai H, Huang J, Han X, Ji Q, Song Q, Yang C, Zhao S, Xu X, Ye Q. Transcriptional Regulation of the Warburg Effect in Cancer by SIX1. Cancer Cell. 2018;33:368-385.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 18. | Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1910] [Cited by in RCA: 3260] [Article Influence: 362.2] [Reference Citation Analysis (1)] |
| 19. | Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 1043] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 20. | Sayers RL, Sundberg-Smith LJ, Rojas M, Hayasaka H, Parsons JT, Mack CP, Taylor JM. FRNK expression promotes smooth muscle cell maturation during vascular development and after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:2115-2122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res. 2002;90:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Hayasaka H, Martin KH, Hershey ED, Parsons JT. Disruption of FRNK expression by gene targeting of the intronic promoter within the focal adhesion kinase gene. J Cell Biochem. 2007;102:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Ding Q, Cai GQ, Hu M, Yang Y, Zheng A, Tang Q, Gladson CL, Hayasaka H, Wu H, You Z, Southern BD, Grove LM, Rahaman SO, Fang H, Olman MA. FAK-related nonkinase is a multifunctional negative regulator of pulmonary fibrosis. Am J Pathol. 2013;182:1572-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Jiang H, Liu X, Knolhoff BL, Hegde S, Lee KB, Jiang H, Fields RC, Pachter JA, Lim KH, DeNardo DG. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut. 2020;69:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 25. | Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, DeNardo DG. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 774] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 26. | Yang J, Ren B, Yang G, Wang H, Chen G, You L, Zhang T, Zhao Y. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 2020;77:305-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 27. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11812] [Article Influence: 738.3] [Reference Citation Analysis (0)] |
| 28. | Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, Xie G, Moylan CA, Garibaldi F, Premont R, Suliman HB, Piantadosi CA, Diehl AM. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319-1329.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 29. | Chen Z, Liu M, Li L, Chen L. Involvement of the Warburg effect in non-tumor diseases processes. J Cell Physiol. 2018;233:2839-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 30. | Zhao XK, Yu L, Cheng ML, Che P, Lu YY, Zhang Q, Mu M, Li H, Zhu LL, Zhu JJ, Hu M, Li P, Liang YD, Luo XH, Cheng YJ, Xu ZX, Ding Q. Focal Adhesion Kinase Regulates Hepatic Stellate Cell Activation and Liver Fibrosis. Sci Rep. 2017;7:4032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Zou GL, Zuo S, Lu S, Hu RH, Lu YY, Yang J, Deng KS, Wu YT, Mu M, Zhu JJ, Zeng JZ, Zhang BF, Wu X, Zhao XK, Li HY. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-β/Smad signaling pathway. World J Gastroenterol. 2019;25:4222-4234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Koshman YE, Engman SJ, Kim T, Iyengar R, Henderson KK, Samarel AM. Role of FRNK tyrosine phosphorylation in vascular smooth muscle spreading and migration. Cardiovasc Res. 2010;85:571-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Koshman YE, Kim T, Chu M, Engman SJ, Iyengar R, Robia SL, Samarel AM. FRNK inhibition of focal adhesion kinase-dependent signaling and migration in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2010;30:2226-2233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Wang YH, Israelsen WJ, Lee D, Yu VWC, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158:1309-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 35. | Demircioglu F, Wang J, Candido J, Costa ASH, Casado P, de Luxan Delgado B, Reynolds LE, Gomez-Escudero J, Newport E, Rajeeve V, Baker AM, Roy-Luzarraga M, Graham TA, Foster J, Wang Y, Campbell JJ, Singh R, Zhang P, Schall TJ, Balkwill FR, Sosabowski J, Cutillas PR, Frezza C, Sancho P, Hodivala-Dilke K. Cancer associated fibroblast FAK regulates malignant cell metabolism. Nat Commun. 2020;11:1290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 36. | Cao D, Qi Z, Pang Y, Li H, Xie H, Wu J, Huang Y, Zhu Y, Shen Y, Dai B, Hu X, Ye D, Wang Z. Retinoic Acid-Related Orphan Receptor C Regulates Proliferation, Glycolysis, and Chemoresistance via the PD-L1/ITGB6/STAT3 Signaling Axis in Bladder Cancer. Cancer Res. 2019;79:2604-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 37. | Siu MKY, Jiang YX, Wang JJ, Leung THY, Han CY, Tsang BK, Cheung ANY, Ngan HYS, Chan KKL. Hexokinase 2 Regulates Ovarian Cancer Cell Migration, Invasion and Stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 Signaling Cascades. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 38. | Luo W, Chen J, Li L, Ren X, Cheng T, Lu S, Lawal RA, Nie Q, Zhang X, Hanotte O. c-Myc inhibits myoblast differentiation and promotes myoblast proliferation and muscle fibre hypertrophy by regulating the expression of its target genes, miRNAs and lincRNAs. Cell Death Differ. 2019;26:426-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Xiao ZD, Han L, Lee H, Zhuang L, Zhang Y, Baddour J, Nagrath D, Wood CG, Gu J, Wu X, Liang H, Gan B. Energy stress-induced lncRNA FILNC1 represses c-Myc-mediated energy metabolism and inhibits renal tumor development. Nat Commun. 2017;8:783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |