Published online Mar 7, 2021. doi: 10.3748/wjg.v27.i9.815
Peer-review started: November 7, 2020
First decision: December 3, 2020
Revised: December 14, 2020
Accepted: February 1, 2021
Article in press: February 1, 2021
Published online: March 7, 2021
Processing time: 116 Days and 6.4 Hours
Our previous studies confirmed that abdominal paracentesis drainage (APD) attenuates intestinal mucosal injury in rats with severe acute pancreatitis (SAP), and improves administration of enteral nutrition in patients with acute pancreatitis (AP). However, the underlying mechanisms of the beneficial effects of APD remain poorly understood.
To evaluate the effect of APD on intestinal inflammation and accompanying apoptosis induced by SAP in rats, and its potential mechanisms.
SAP was induced in male adult Sprague-Dawley rats by 5% sodium taurocholate. Mild AP was induced by intraperitoneal injections of cerulein (20 μg/kg body weight, six consecutive injections). Following SAP induction, a drainage tube connected to a vacuum ball was placed into the lower right abdomen of the rats to build APD. Morphological changes, serum inflammatory mediators, serum and ascites high mobility group box protein 1 (HMGB1), intestinal barrier function indices, apoptosis and associated proteins, and toll-like receptor 4 (TLR4) signaling molecules in intestinal tissue were assessed.
APD significantly alleviated intestinal mucosal injury induced by SAP, as demonstrated by decreased pathological scores, serum levels of D-lactate, diamine oxidase and endotoxin. APD reduced intestinal inflammation and accompanying apoptosis of mucosal cells, and normalized the expression of apoptosis-associated proteins in intestinal tissues. APD significantly suppressed activation of the intestinal TLR4 signaling pathway mediated by HMGB1, thus exerting protective effects against SAP-associated intestinal injury.
APD improved intestinal barrier function, intestinal inflammatory response and accompanying mucosal cell apoptosis in SAP rats. The beneficial effects are potentially due to inhibition of HMGB1-mediated TLR4 signaling.
Core Tip: We provide the first evidence that abdominal paracentesis drainage (APD) plays a protective role in intestinal inflammation and accompanying apoptosis induced by severe acute pancreatitis (SAP). Our key findings were: (1) APD decreased serum levels of indices related to intestinal injury and improved intestinal barrier function; (2) APD alleviated intestinal inflammation and accompanying mucosal cell apoptosis; and (3) The beneficial effects of APD in ameliorating intestinal injury were due to suppressed activation of intestinal toll-like receptor 4/nuclear factor-κB signaling pathway mediated by high mobility group box protein 1. We elucidated the molecular mechanism underlying APD treatment of SAP and its complications.
- Citation: Huang SQ, Wen Y, Sun HY, Deng J, Zhang YL, Huang QL, Wang B, Luo ZL, Tang LJ. Abdominal paracentesis drainage attenuates intestinal inflammation in rats with severe acute pancreatitis by inhibiting the HMGB1-mediated TLR4 signaling pathway. World J Gastroenterol 2021; 27(9): 815-834
- URL: https://www.wjgnet.com/1007-9327/full/v27/i9/815.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i9.815
Severe acute pancreatitis (SAP) is a rapidly progressive abdominal disease, often accompanied by multiple organ damage, with a mortality of up to 25%[1]. Intestine is the most vulnerable extrapancreatic organ and is closely related to the severity of SAP[2]. It is reported that the gut, especially the small intestine is not just a target organ for SAP but a trigger for the initiation of systemic inflammatory responses[3]. Available evidence suggests that intestinal injury, including inflammation and apoptosis, caused by SAP promotes changes in intestinal permeability and the release of inflammatory factors, resulting in bacterial translocation, secondary infection of pancreatic tissue and the amplification of systemic inflammation, eventually contributing to the high mortality rate of SAP in the early stage[4]. Therefore, preventing intestinal injury is an important part of SAP treatment. Our previous studies have proved that elimination of pancreatitis-associated ascitic fluid (PAAF) through early abdominal paracentesis drainage (APD) attenuate intestinal mucosal injury and preserves intestinal barrier function, but the potential molecular mechanisms responsible for the protective effect are still unclear[5,6].
Toll-like receptor 4 (TLR4) is a member of the mammalian pattern recognition receptor family, and it is believed to be a key transmembrane protein that transmits extracellular antigen recognition information to cells and triggers an inflammatory response[7]. Recent studies have shown that TLR4 plays a vital role in inflammatory initiation and organ injury during SAP[8]. Through the myeloid differentiation primary response gene 88 (MyD88)-dependent pathway, TLR4 can mediate the activation of nuclear factor (NF)-κB, which further induces the expression and release of inflammatory cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-6, resulting in a vicious cycle of positive feedback of inflammation[9]. The TLR4/NF-κB proinflammatory pathway has been reported to be involved in intestinal inflammation and apoptosis induced by SAP, but TLR4 can mediate downstream signal transduction only by binding to its specific ligand[10].
High mobility group box protein 1 (HMGB1) as a member of the damage-associated molecular patterns family has been confirmed to be the most important endogenous ligand for TLR4, particularly in SAP[11]. Extracellular HMGB1 as a late mediator of lethality in systemic inflammation participates in the development of multiple organ injury caused by SAP[12]. Active neutralization with anti-HMGB1 antibodies can prevent intestinal mucosal barrier dysfunction in mice with SAP[13]. Accordingly, our recent study reported that APD can significantly reduce the level of HMGB1 by draining PAAF rich in high concentrations of HMGB1[14]. Therefore, we speculate that the reduced effect of HMGB1 by early APD treatment may have a protective role in SAP-induced intestinal injury.
Here, we explored the effect of APD on intestinal inflammation and accompanying apoptosis in rats with SAP. Furthermore, experiments were conducted to determine whether HMGB1 in PAAF had a regulatory role in the intestinal TLR4 signaling pathway to confirm the protective effect via inhibiting HMGB1-mediated TLR4 signaling.
Sodium taurocholate was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The antibody specific for apoptosis regulator Bax (cat. no. ab32503) and MyD88 (cat. no. ab2064) were purchased from Abcam (Cambridge, United Kingdom). The antibodies for caspase-3 (cat. no. 9662S), transcription factor p65 (cat. no. 8242) and phosphorylated (p-)p65 (serine 536; cat. no. 3033) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, United States). The antibodies for apoptosis regulator Bcl-2 (cat. no. GTX100064) and TLR4 (cat. no. GTX13867) were purchased from GeneTex Inc. (Irvine, CA, United States). The antibody for HMGB1 (cat. no. BM3965) was purchased from Boster Biological Technology, Inc. (Wuhan, China). All other chemicals and kits employed in this study were for experimental study and were commercially available.
Adult male Sprague-Dawley rats (weight, 190-210 g) were provided by Dashuo Animal Science and Technology Co., Ltd. (Chengdu, China). Three days prior to the experiments, all rats were housed in ventilated plastic cages, and rats were acclimated to a 12-h light/dark cycle under a standard room temperature of 23°C, and had free access to laboratory chow and water.
The animals were fasted overnight prior to the experiment but had free access to water. In the first part of the experiment, 45 rats were anesthetized with 5% isoflurane before surgery, and were randomly divided into three groups with 15 rats in each of the following groups: Sham operation, SAP, and SAP + APD. The SAP rat model was established under aseptic conditions through the retrograde infusion of 5% sodium taurocholate into the biliopancreatic duct using a pump (0.1 mL/100 g body weight, 12 mL/h) according to our previous study[15]. For the SAP + APD rat model, following SAP induction, we placed a drainage tube at the lower right abdomen which was connected to a negative-pressure suction ball. After the operation, rats received a subcutaneous injection of sterile saline every 6 h for fluid resuscitation (4 mL/100 g body weight). For the sham operation group, rats were treated in the same way without puncturing the biliopancreatic duct. All rats were killed under anesthesia at 24 h; blood samples and PAAF were obtained, and stored at -80 ℃ after centrifugation (1000 × g for 10 min). The pancreas and intestine were quickly harvested and washed with cold phosphate buffer saline (PBS) solution, and part of the pancreas and intestine were flash-frozen in liquid nitrogen or placed into 4% paraformaldehyde for subsequent experiments.
In the second part, experiments were conducted to evaluate the effect of HMGB1 in PAAF on the activity of the TLR4 signaling pathway in intestinal tissues. Fresh PAAF was aseptically collected from the SAP rats in the first part, and then centrifuged for 15 min at 4000 × g at 4 °C to obtain the sterile supernatant. Another 24 rats were injected intraperitoneally with cerulein (six consecutive injections, 20 μg/kg body weight, at 1-h intervals) to induce mild acute pancreatitis (MAP) as previously described[15], and were then randomly divided into the following four groups with 6 rats in each group: Controls (CN group), PAAF injection (PI group), PAAF + anti-HMGB1 neutralizing antibody (PIH group) and PAAF + control lgY (PIC group). CN group rats received an intraperitoneal injection of 8 mL saline solution; PI group rats received an intraperitoneal injection of 8 mL sterile supernatant of PAAF; PIH group rats received an intraperitoneal injection of 8 mL sterile supernatant of PAAF containing 200 μg anti-HMGB1 neutralizing antibody; PIC group rats received an intraperitoneal injection of 8 mL sterile supernatant of PAAF containing 200 µg control lgY. The dosage of anti-HMGB1 neutralizing antibody (rabbit anti-HMGB1 monoclonal antibody) was based on the results of our previous studies and has been proved to be effective in an in vivo experimental model[14]. Western blot analysis was adopted to confirm the neutralizing activity and specificity of this antibody. Before the intraperitoneal injection, PAAF and anti-HMGB1 neutralizing antibodies were incubated for 30 min at 36 ℃. The rats were anesthetized 8 h after the injections, blood and intestinal tissue samples were obtained and stored at -80 ℃ for subsequent detection.
The terminal pancreas and distal ileum tissue samples were treated with 4% paraformaldehyde, paraffin-embedded and then cut into sections 4 μm thick. Hematoxylin and eosin (HE) staining was used to examine the histology of pancreatic and intestinal samples under a Leica DM 3000 light microscope (Leica Microsystems GmbH, Wetzlar, Germany). Pathological scoring was performed by a consultant histopathologist blinded to the groups. Pathological damage of the pancreas, such as edema, hemorrhage, fat necrosis, acinar necrosis and inflammatory cell infiltrate were evaluated according to the literature[16]. With regard to the severity of intestinal injury, the grading system was utilized according to Chiu’s standard[17].
Biochemical indices included TNF-α, IL-6, IL-1β, HMGB1, amylase and lipase. Serum HMGB1, TNF-α, IL-6, D-lactate, diamine oxidase (DAO) and HMGB1 in PAAF were detected using an enzyme-linked immunosorbent assay (ELISA) kit (Nanjing Jiancheng Institute of Biological Engineering, China). Serum endotoxin was measured using a chromogenic limulus amebocyte lysate kit (Xiamen Bioendo Technology Co., Ltd., China). The serum levels of amylase and lipase were measured using an automatic biochemistry analyzer (TC6010L; Tecom Science Corporation, Jiangxi, China).
To determine the degree of inflammatory response in the intestine, TNF-α and IL-6 protein levels and myeloperoxidase (MPO) activity were measured in distal ileal homogenates. Distal ileal tissue (0.1 g) was obtained and homogenized in ice-cold 0.9% sodium chloride solution (weight/volume, 1:9). The homogenates were centrifuged at 800 g and the supernatants were collected. The concentrations of supernatants were measured using an enhanced BCA protein assay kit. MPO activity and TNF-α and IL-6 protein concentrations in the samples were measured with ELISA kits (both Nanjing Jiancheng Institute of Biological Engineering).
Immunohistochemistry was used to detect the expression of TLR4 in intestinal tissue of each group after 24 h of SAP induction. Briefly, the paraffin-embedded distal ileal tissue sections were dewaxed in xylene and rehydrated in gradient ethanol solutions, respectively, according to standard procedures. After peroxide-blocking enzyme reaction in 3% hydrogen peroxide for 10 min, the sections were boiled in sodium citrate solution (0.01 mol/L) for 5 min to repair the antibody. Subsequently, the antibody was blocked by 5% bovine serum (Boster Biological Technology Co., Ltd., Wuhan, China) for 30 min, and then incubated with rabbit anti-TLR4 at a 1:200 dilution overnight at 4 ℃. After rinsing with PBS, sections were incubated with biotin-labeled goat anti-rabbit IgG (Boster Biological Technology) for 30 min at 37 ℃, and horseradish peroxidase (HRP)-labeled streptavidin (Boster Biological Technology) for 30 min at 37 ℃. Finally, the specimens were stained with diaminobenzidine tetrahydrochloride (Boster Biological Technology), nuclei were counterstained with hematoxylin, and images were captured under a Leica DM 3000 microscope which were analyzed by two pathologists in a blind manner.
We used a terminal deoxynucleotidyl-transferase-mediated dUTP nick end labeling (TUNEL) assay kit (Fluorescein in situ Cell Death Detection Kit; Boster Biological Technology) to detect intestinal mucosa cell apoptosis in frozen intestinal sections (10 μm). All cells exhibited blue nuclear 4′,6-diamidino-2-phenylindole staining, but the TUNEL-positive cells displayed green/yellow nuclear staining. The stained specimens were captured and analyzed by confocal laser scanning microscopy (Eclipse Ti2; Nikon Corporation, Tokyo, Japan).
TRIzol reagent (Beyotime Institute of Biotechnology, China) was used to extract total RNA of tissues, and the Thermo NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, United States) was adopted to determine the concentration and purity of the extracted RNA. The primers were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) and their sequences were as follows: TLR4 forward 5’-TTTATTCAGAGCCGTTGG-3’ and reverse 5’-AGGCGATACAATT CCACC-3’; GAPDH forward 5’-GCAGAATTCCTGGCCAAGGTCATCCATGACA-3’ and reverse 5’-GCAGGTACCGGGGCCATCCACAGTCTTCTG-3’. The One Step SYBR® PrimeScript™ RT-PCR kit II (Takara Biotechnology Co., Ltd., Dalian, China) was used to perform real-time quantitative polymerase chain reaction (qPCR) on a C1000™ Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, United States) according to the manufacturer’s instructions. Each reaction was performed 3 times for each sample. The expression of TLR4 was analyzed using CFX Manager™ software version 1.6 (Bio-Rad Laboratories) and expressed as the ratio of TLR4 over the levels of GAPDH in the samples.
Proteins from the distal ileal tissues were extracted using ice-cold lysis buffer, and subsequently we used an enhanced BCA protein detection kit (both Nanjing Jiancheng Bioengineering Institute) to determine the concentrations. Equal amounts of protein samples (30 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel in a MiniPROTEAN II minigel apparatus (Bio-Rad Laboratories) and were transferred to a 0.22- or 0.40-μm polyvinylidene difluoride membrane using wet transfer at 300-mA or 200-mA. Protein was blocked with 0.05% Tween-20 Tris-buffered saline containing 5% nonfat milk or bovine serum albumin for 60 min at room temperature, and then incubated with the primary antibodies such as anti-caspase-3 (1:1000 dilution), anti-Bax (1:2000 dilution), anti-Bcl-2 (1:1000 dilution), anti-TLR4 (1:500 dilution), anti-p65 (1:1000 dilution), anti-p-p65 (1:1000 dilution) and anti-GAPDH (1:5000 dilution; loading control) antibodies overnight at 4 ℃. The HRP-conjugated secondary antibody was then used, the bands were developed with an electrochemiluminescence solution (Millipore, Billerica, MA, United States), and exposed to the BioSpectrum4 apparatus (UVP, Upland, CA, United States).
SPSS version 19 statistical software (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. Measurement data are presented as mean ± SD, one-way analysis of variance (Tukey method) was used for comparison between multiple groups, and P < 0.05 was considered statistically significant.
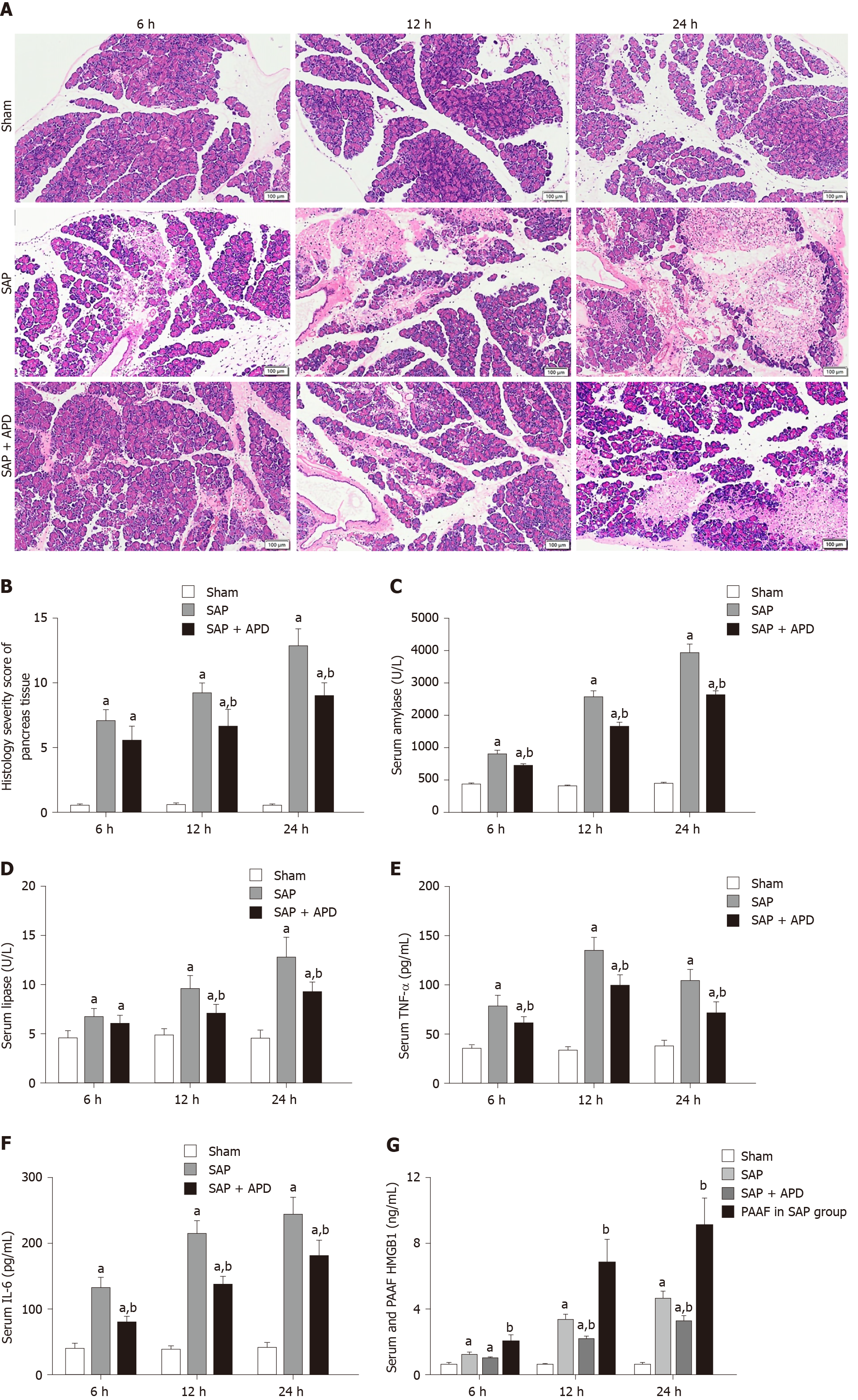
To evaluate the effects of APD treatment in a rat model of SAP induced by the retrograde injection of 5% sodium taurocholate, the changes in pancreatic histopathology, amylase and lipase activity, and levels of inflammatory mediators were measured. For pancreatic histopathology, HE-stained sections of rat pancreatic tissue were analyzed (Figure 1A and B). The samples obtained from the sham group exhibited intact pancreatic characteristics with normal structures. However, the SAP group samples showed large areas of tissue necrosis and notable inflammatory infiltration and hemorrhage, supported by the raised histological severity scores. Compared with the histological features of the SAP group, samples from the SAP + APD group exhibited reduced pancreatic injury and had a lower severity score.
In addition, compared with the sham group, serum amylase and lipase activity and levels of IL-6, TNF-α and HMGB1 in the SAP group were significantly increased which were consistent with pathological changes. In particular, HMGB1 level in PAAF was higher than that in serum in the SAP group (Figure 1C-G) (P < 0.05). APD treatment following SAP induction resulted in a marked decrease in the levels of these indices (P < 0.05). These results indicate that APD treatment reduces tissue damage and alleviates the inflammatory process induced by SAP.
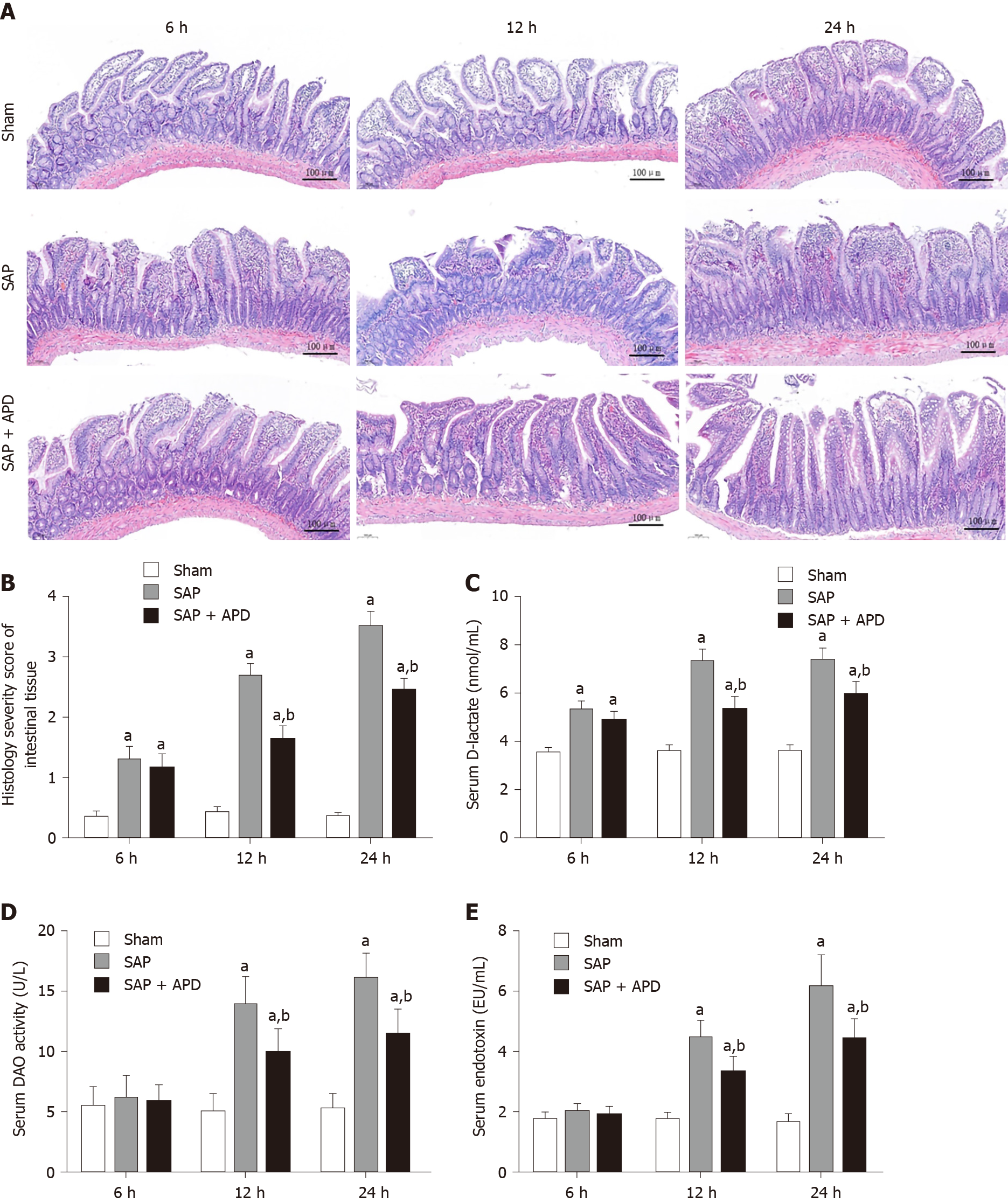
To evaluate the therapeutic effects of APD on intestinal injury in rats with SAP, distal ileal histopathology and intestinal mucosal-injury-associated indices, such as serum D-lactate, DAO activity and endotoxin were investigated. Tissue from the sham group displayed regular intestinal architecture and villous shape, whereas the samples from the SAP rats showed notable changes in morphology and structure, including denuded villi, mucosal and submucosal swelling, congestion, inflammatory infiltration and increased granules in Paneth cells (Figure 2A and B). In contrast, the intestinal lesions in the SAP + APD group were mild with relatively normal structure, and their severity score was significantly lower compared with the SAP group (P < 0.05). Serum DAO activity, D-lactate and endotoxin levels proved to be important indicators of intestinal mucosal integrity and barrier function. Compared with the sham group, the serum levels of the above indicators in the SAP group were obviously elevated following SAP induction at different time points (P < 0.05) (Figure 2C-E), suggesting that the intestinal integrity was partially destroyed and the permeability was markedly increased. However, APD treatment significantly decreased serum DAO activity, D-lactate and endotoxin levels (P < 0.05). These results indicate that APD treatment improves histopathology and preserves the integrity of intestinal mucosa during SAP.
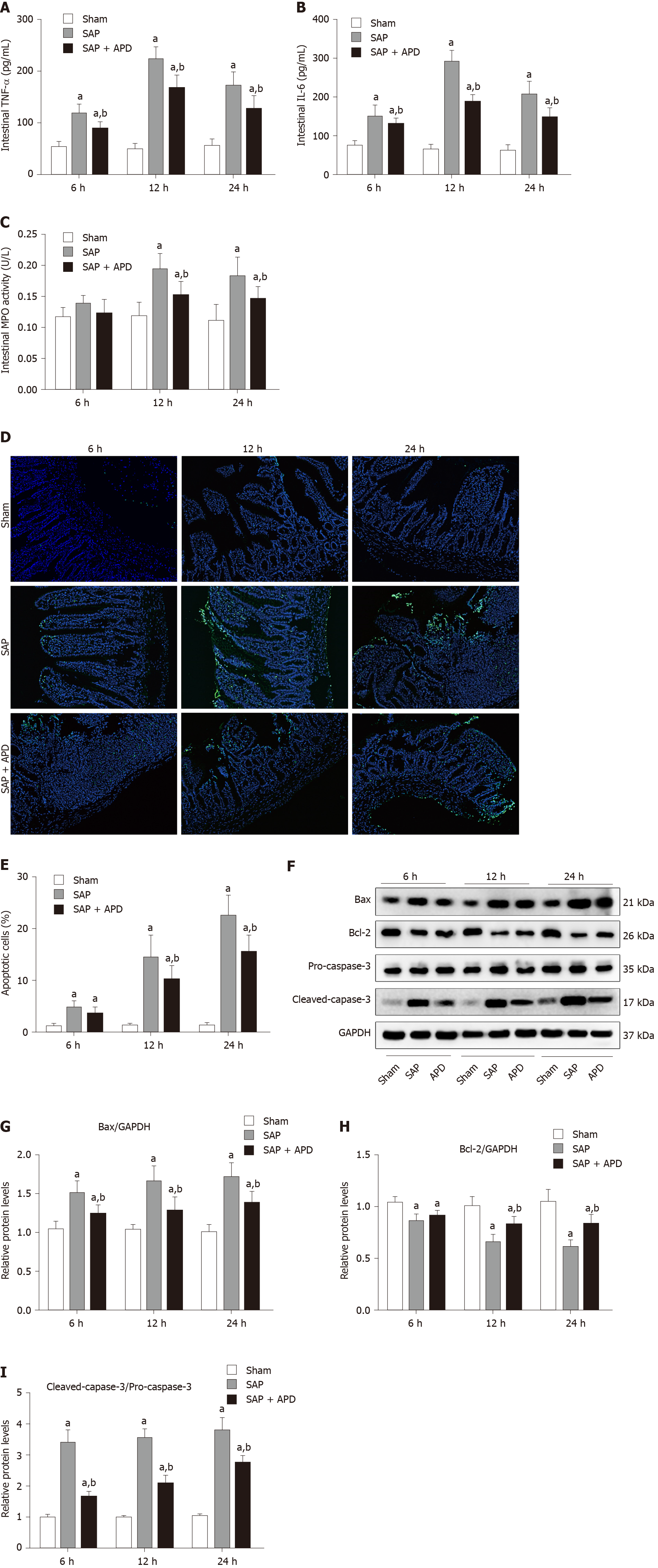
Given that inflammation is the main initiator of intestinal injury caused by SAP, we sought to explore whether APD treatment influenced intestinal inflammation and apoptosis during SAP. Expression of TNF-α, IL-6 and MPO activity was examined to evaluate the level of inflammation in the distal ileum. After SAP induction, protein expression of TNF-α and IL-6 and MPO activity were significantly elevated compared with the sham group (P < 0.05) (Figure 3A-C). However, APD treatment significantly inhibited this elevation (P < 0.05). Intestinal epithelial apoptosis has a direct link with the inflammatory response, which can reflect the severity of intestinal inflammation. Thus, a TUNEL assay was used to measure apoptosis in the myocardium, and western blot analysis was conducted to determine the expression of apoptosis-associated proteins. The apoptotic indices were significantly increased in the SAP group compared with the sham group at different time points (P < 0.05), and following the intervention of APD, the incidence of apoptosis was significantly decreased compared with the SAP group (P < 0.01) (Figure 3D and E). Western blotting revealed that APD treatment led to downregulation of the proapoptotic markers cleaved caspase-3 and Bax, and upregulation of the antiapoptotic protein Bcl-2 (P < 0.05) (Figure 3F-I). These data suggested that APD treatment lessened SAP-induced intestinal inflammation and accompanying apoptosis of intestinal epithelial cells.
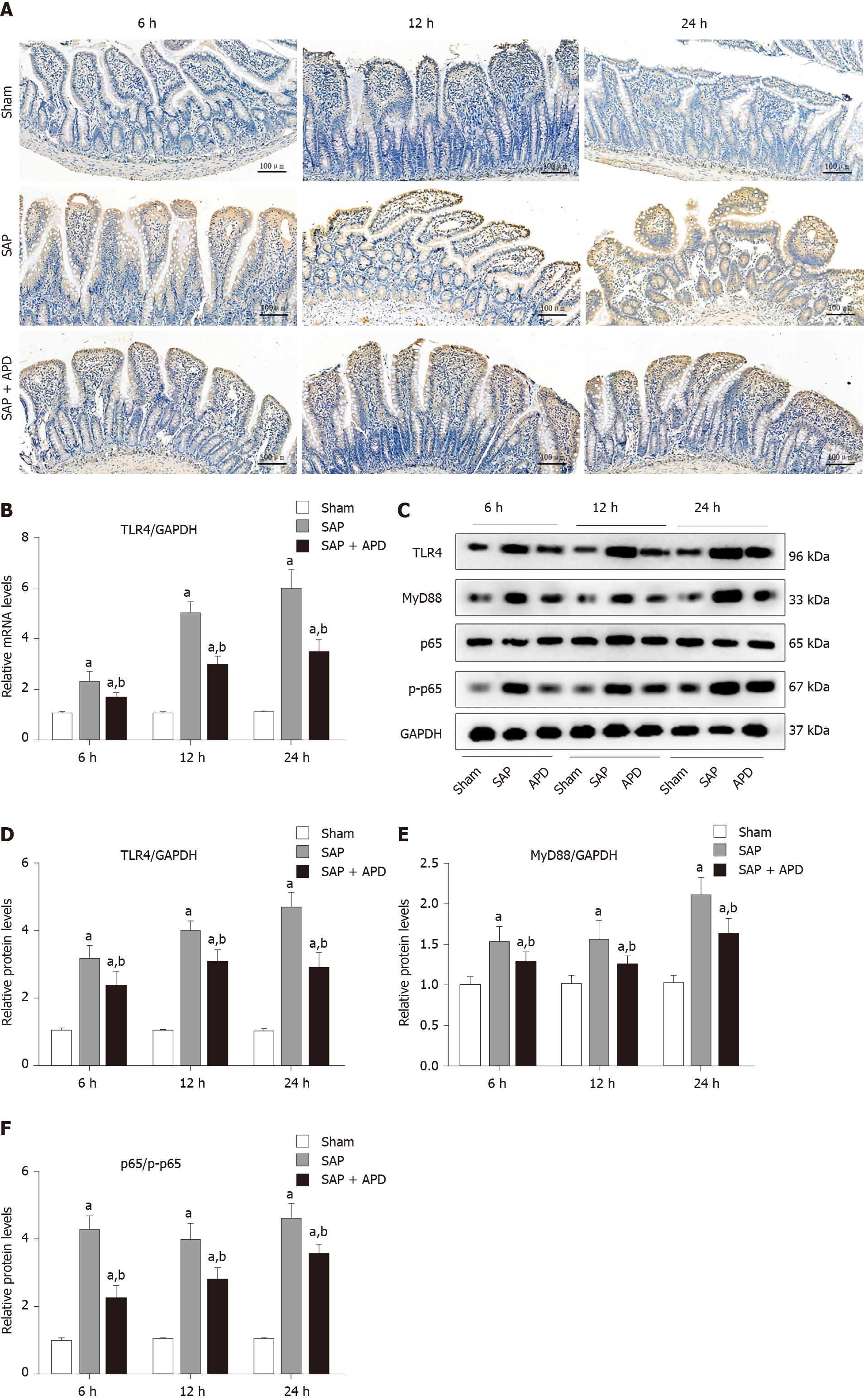
Activation of TLR4 and its downstream signaling pathway has been reported to exert an essential role in the regulation of intestinal inflammation and accompanying cell apoptosis induced by SAP. Therefore, we sought to investigate whether the signaling pathway was involved in the molecular mechanism of APD-mediated protection. Western blotting showed a significant decline in the expression of intestinal TLR4 in the APD group compared with the SAP group from 6 h to 24 h (P < 0.05) (Figure 4). This effect of APD treatment on TLR4 expression was further confirmed by immunohistochemical detection that showed the same trend. Compared with the SAP group, expression of MyD88 and phosphorylation of NF-κB p65, known as the downstream signaling molecules, were markedly reduced in the APD group (P < 0.05). The above results showed that APD treatment suppressed the TLR4/MyD88/NF-κB signaling pathway in SAP rat intestine, indicating that the inhibition of TLR4-mediated signaling may be involved in the protection of APD in SAP-induced intestinal injury.
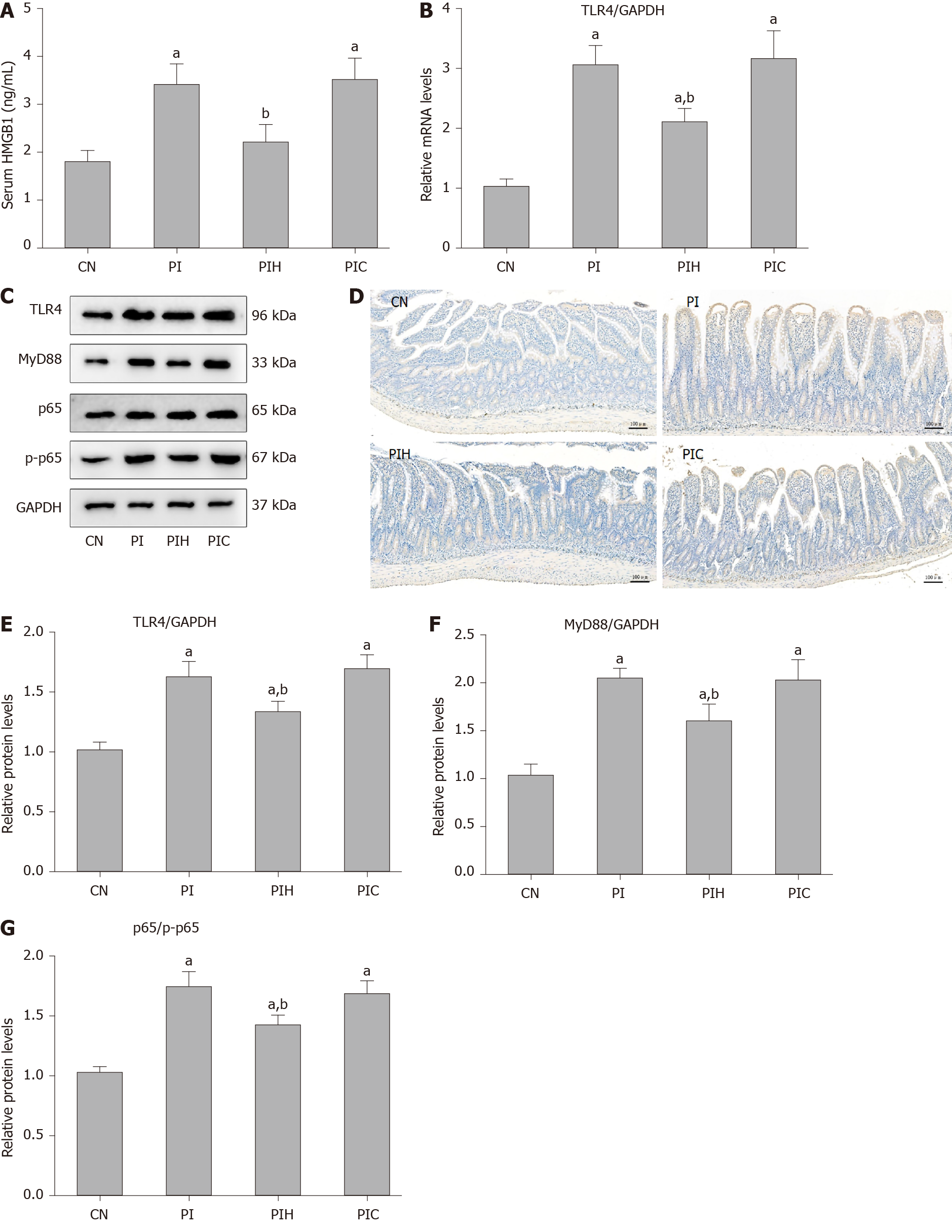
HMGB1 is the most important natural ligand of TLR4 and acts as a key inflammatory mediator during SAP; therefore, we determined whether the PAAF which contains a high concentration of HMGB1 participated in regulation of the TLR4 signaling pathway. Therefore, rats were intraperitoneally injected with the supernatant of PAAF (PI group) or supernatant containing the anti-HMGB1 neutralizing antibody (PIH group) and TLR4 signaling was detected in intestinal samples from rats with MAP. We then assessed the HMGB1 level in rat circulation in each group. As shown in Figure 5A, compared with the control group, the serum level of HMGB1 in the PI group was obviously increased (P < 0.05), indicating that HMGB1 following intraperitoneal injection of PAAF could permeate into the circulation under a microinflammatory environment. With blockade of HMGB1 in the PAAF, the level of HMBG1 in serum was significantly decreased compared with that in the PI group (P < 0.05). Next, we evaluated the effect of HMGB1 in PAAF on the TLR4 signaling pathway (Figure 5B-G). Eight hours after injection, compared with the MAP control group, expression of TLR4, MyD88 and NF-κB p65 phosphorylation level were significantly increased in the PI group, suggesting that PAAF activated the TLR4 signaling pathway in intestinal tissues during MAP. When treated with the anti-HMGB1 neutralizing antibody, the TLR4 signaling pathway was significantly inhibited compared with that in the PI group. These data demonstrated that APD treatment attenuated intestinal injury via inhibition of HMGB1-mediated TLR4 signaling by draining PAAF.
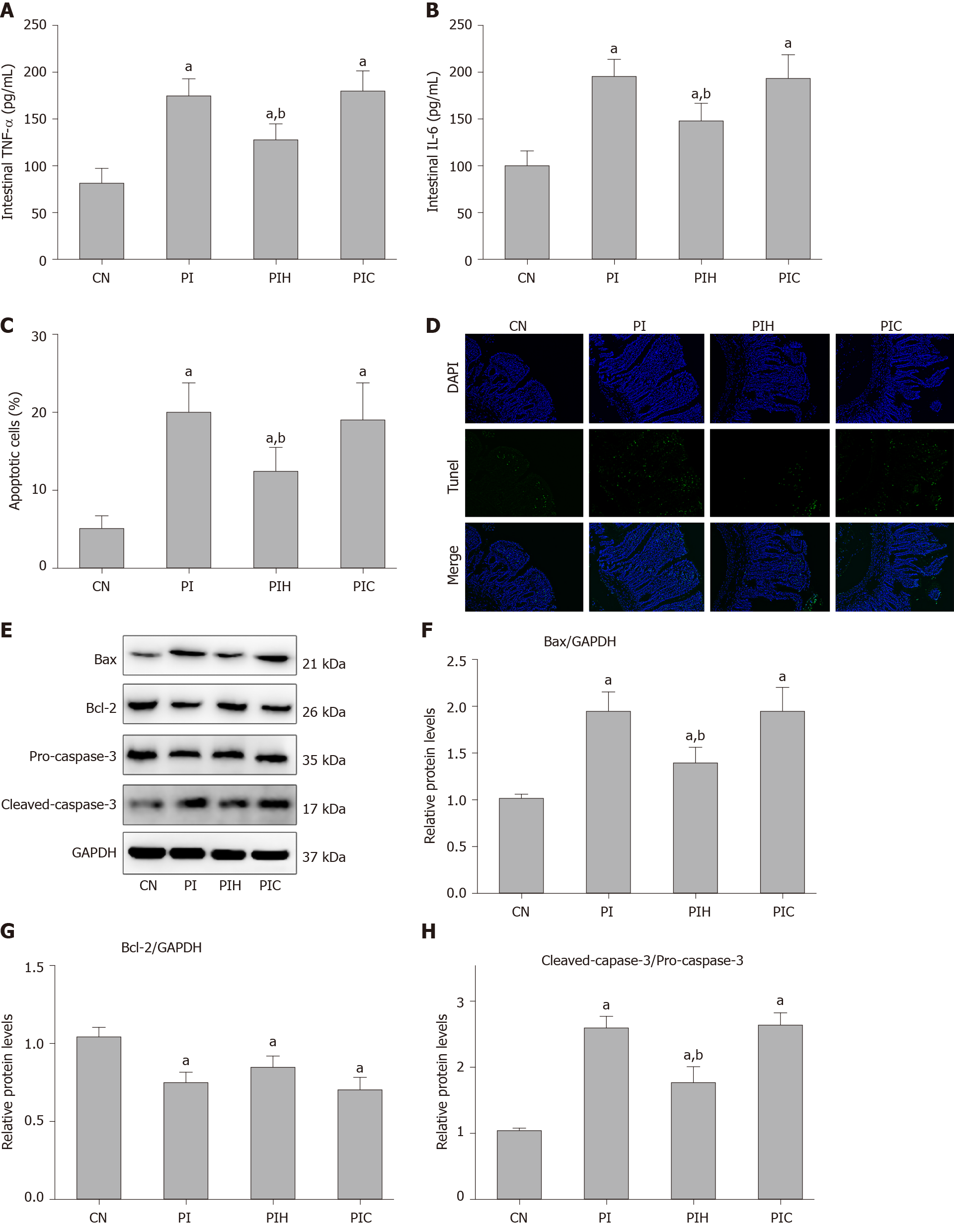
The present data showed that APD treatment reduced intestinal inflammation and accompanying cell apoptosis, and inhibited HMGB1-mediated TLR4 signaling in intestinal tissues during SAP. However, we are still unclear whether there is any potential relationship between these results. To clarify this, the effects of PAAF on intestinal inflammation and cell apoptosis in rats with MAP were investigated. The expression of TNF-α and IL-6 was significantly increased in the PI group compared with the CN group, along with upregulated apoptosis detected by TUNEL staining, which was confirmed by the changes in caspase-3, Bax and Bcl-2 expression measured by western blotting (Figure 6). However, the increased expression of proinflammatory cytokines and the incidence of apoptosis in intestinal samples were significantly downregulated in the PIH group compared with the PI group. These results indicated that administration of PAAF aggravated the intestinal inflammatory response and promoted apoptosis in MAP. This suggested that APD treatment significantly attenuated inflammation and apoptosis of intestinal tissues by suppressing HMGB1-mediated TLR4 signaling by removing PAAF.
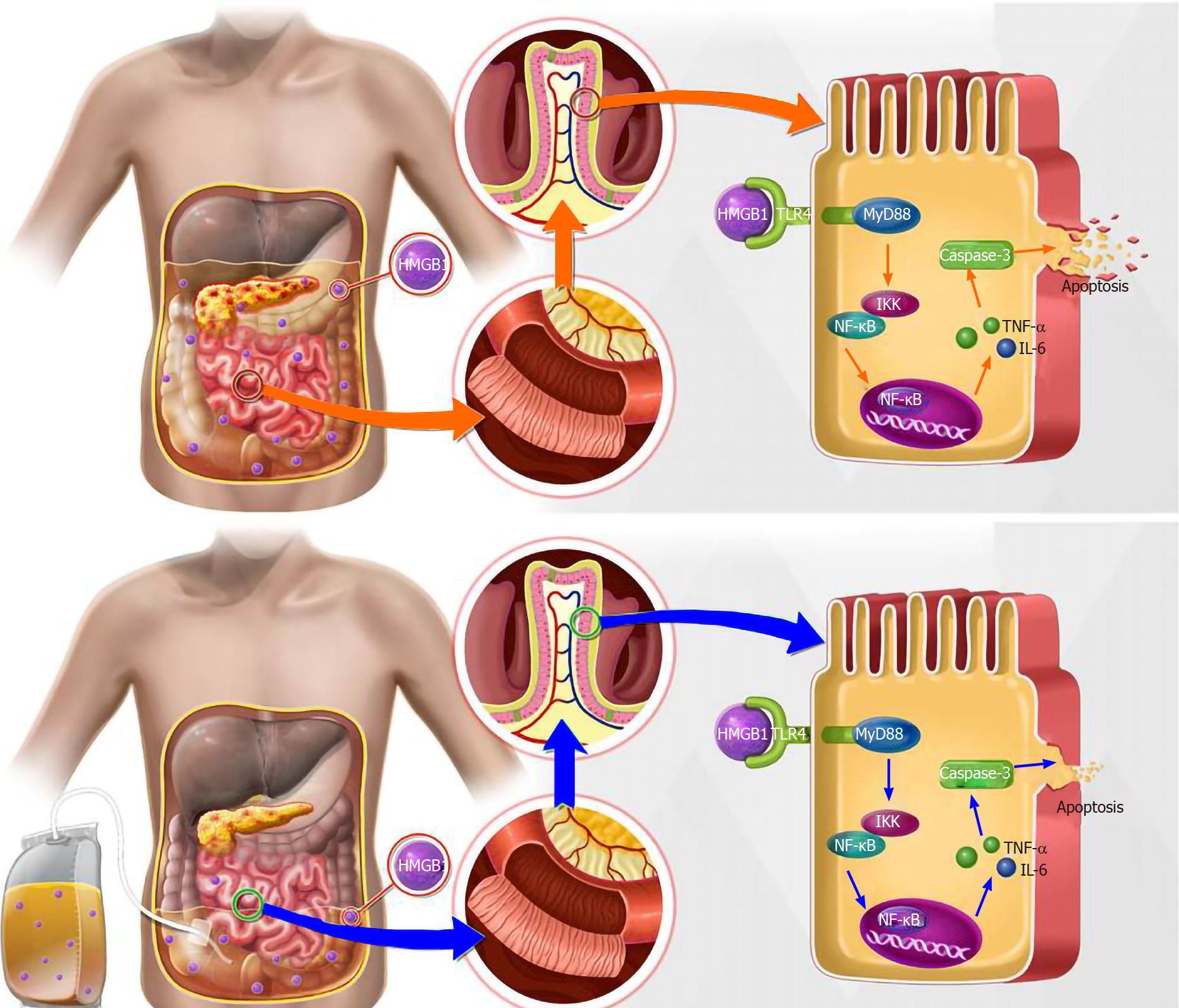
According to the above research results, we proposed a schematic diagram of the underlying mechanism of the influence of APD treatment on SAP-induced intestinal injury (Figure 7). Once SAP occurs, a large amount of HMGB1 enters the blood circulation through peritoneal reabsorption, which is involved in the development of remote organ injury. Following the implementation of APD treatment, HMGB1 concentrations in the circulation decrease markedly, inducing a downregulation of TLR4 expression in intestinal tissues, which can effectively alleviate intestinal inflammation and accompanying cell apoptosis via inhibiting activation of the NF-κB signaling, and thereby protecting against SAP-induced intestinal injury.
In the present study, the primary goal was to investigate the effect of APD treatment on intestinal inflammation and accompanying apoptosis in rats with SAP, and explore the potential mechanism of this condition. The important findings were that (1) APD treatment lessened the intestinal inflammatory response and accompanying mucosa cell apoptosis by removing PAAF; and (2) the protective effects of APD treatment in attenuating SAP-induced intestinal injury were due to inhibition of HMGB1-mediated TLR4 signaling. The above findings present new insights into the therapeutic mechanism underlying APD.
SAP is a dangerous acute abdominal disease that is usually complicated by multiple organ injury[18]. The small intestine is most frequently affected by SAP[19,20]. In the present study, we found that rats with SAP exhibited serious pancreatic injury and changes in the structure of the small intestine mucosa, which is consistent with previous research[21,22]. Simultaneously, following SAP induction, serum DAO activity, D-lactate and endotoxin levels in rats were significantly increased, and these are useful markers of intestinal mucosal integrity and barrier function. In particular, DAO is specific in the cytoplasm of mammalian intestinal mucosal epithelial cells and its basal level in peripheral blood is positively correlated with integrity of the intestinal mucosa[23]. Disruption of the integrity of the intestinal mucosa during SAP is accepted as the main reason for bacterial translocation and endotoxin into the circulation, which further stimulates activated monocytes and macrophages to release excessive cytokines and inflammatory mediators, promote the occurrence of systemic inflammatory reaction syndrome, and constitute a serious “second blow” to the pancreas and other organs[4]. Therefore, it is important to control the occurrence and development of intestinal mucosal injury in SAP to improve prognosis. We demonstrated that APD attenuated intestinal mucosal injury in SAP rats via elimination of PAAF, which has been shown to play an important role in the severity of SAP[24]. Clinically, APD improves the administration of enteral nutrition in acute pancreatitis (AP) patients[25]. Thus, we devoted our attention to further exploration of the potential therapeutic mechanisms of APD in SAP-evoked intestinal injury.
TLR4 is the best-characterized member of the pattern-recognition receptor family, and has been shown to play important roles in innate immunity and in inducing the inflammatory response by acting as a transmembrane signal transduction receptor during SAP[26]. It is reported that the downstream effects of TLR4 are mainly dependent on the interaction with the adaptor molecule MyD88, through a series of signal transductions leading to activation of NF-κB, ultimately resulting in massive synthesis and release of inflammatory cytokines, such as TNF-α and IL-6, which is widely accepted as the primary mode of action[27,28]. Under physiological conditions, to avoid an excessive inflammatory response caused by some stimuli, TLR4 expression in the intestinal epithelium is low. However, the expression of intestinal TLR4 can be upregulated significantly in the course of SAP[21,29]. In this study, we verified that TLR4 expression is markedly increased in distal ileal tissue in rats with SAP. Correspondingly, the activation of MyD88/NF-κB was demonstrated to aggravate the inflammatory response in the intestinal mucosa, along with the upregulated expression of TNF-α and IL-6. In contrast, previous studies have reported that inhibition of the TLR4 signaling pathway can significantly reduce the expression of inflammatory cytokines and ameliorate intestinal injury induced by SAP, indicating that activation of TLR4 plays a critical role in intestinal barrier disruption and inflammation[8,21]. When measurements were implemented at different timepoints after SAP induction, we found that APD treatment markedly decreased intestinal TLR4 and MyD88 expression detected by western blotting, thereby inhibiting involvement of downstream NF-κB in the nucleus, which was supported by the decreased expression of TNF-α and IL-6. Intestinal mucosal cell apoptosis secondary to the inflammatory response that accounts for destroyed integrity and reduced defense has been confirmed in the course of SAP[30,31]. Activation of the NF-κB signaling pathway can lead to an imbalance in the expression of apoptosis-related proteins[32]. In particular, the proapoptotic protein caspase-3 is a key protein that induces cell death, and its activated lysates further activate the caspase signaling pathway, ultimately leading to apoptosis[33]. In the present study, we observed through TUNEL staining that the number of apoptotic cells in intestinal mucosa was significantly increased along with aggravation of intestinal inflammation in rats with SAP, which was further demonstrated by the increased expression of proapoptotic cleaved caspase-3 and Bax, and decreased expression of antiapoptotic protein Bcl-2. After treatment with APD, intestinal mucosal cell apoptosis and the imbalance between proapoptotic and antiapoptotic proteins were markedly improved. These data demonstrated that downregulation of TLR4 expression via APD treatment alleviated inflammatory injury and intestinal mucosal cell apoptosis caused by SAP, which indicates a therapeutic mechanism of APD in preserving intestinal barrier function. However, it is still unclear how APD treatment suppresses intestinal TLR4 signaling by removing PAAF, and it may be related to the reduction in the levels of some lethal factors by APD that can act on intestinal TLR4.
Activation of TLR4 requires binding to its specific ligand. Extracellular HMGB1 has been confirmed as the most important endogenous ligand for TLR4 during SAP and to act as a key inflammatory mediator[11,34]. Accumulated evidence indicates that HMGB1 promotes the development of AP from local inflammation to a lethal systemic inflammatory response, and participates in multiple organ injury[11,35]. Clinical studies have shown that elevated serum level of HMGB1 is positively related to the outcome of patients with SAP as well as organ dysfunction[36]. It is reported that blockade of HMGB1 attenuates intestinal mucosal barrier dysfunction in experimental AP, suggesting a contribution by HMGB1 to intestinal mucosal biological dysfunction[13]. In the present study, the serum levels of HMGB1 in SAP rats were significantly increased with time after SAP induction, which was in line with earlier studies[37,38]. In particular, HMGB1 concentration in PAAF was higher than that in serum at the same time point, which may be because HMGB1 was mainly derived from activated intraperitoneal macrophages and damaged pancreatic tissue during the course of SAP[37]. PAAF is a common local complication of SAP, and our previous studies have shown that timely drainage of PAAF through APD is an important part of SAP therapy and plays a significant role in organ protection[7]. Therefore, we speculate that the high concentration of HMGB1 in PAAF may have a detrimental effect on the intestine in SAP, and the protective mechanism of APD treatment may involve HMGB1 mediation of the TLR4 signaling pathway.
To confirm that the effect of HMGB1 in PAAF drained by APD treatment can alleviate hyperactivity of the intestinal TLR4 signaling pathway, rats with MAP received an intraperitoneal injection of PAAF or PAAF with anti-HMGB1 neutralizing antibody. Eight hours after PAAF injection, compared with the MAP control group, we found that the serum level of HMGB1 in the PI group was significantly increased, suggesting that HMGB1 in PAAF enters the bloodstream under a microinflammatory state in the abdominal cavity induced by cerulein pancreatitis. In line with that, mRNA and protein expression of intestinal TLR4 was significantly increased, along with expression of MyD88 protein and NF-κB p65 phosphorylation level, indicating that administration of PAAF activates the TLR4 signaling pathway in intestinal tissues during MAP. When treated with the anti-HMGB1 neutralizing antibody, we observed that the level of active HMGB1 in serum was significantly reduced, and the TLR4 signaling pathway was also significantly inhibited in the PIH group compared with the PI group, in conjunction with the downregulated expression of TNF-α and IL-6, and reduced intestinal mucosal cell apoptosis. Overall, our results indicate that APD treatment can suppress overactivation of the intestinal TLR4 signaling pathway by draining HMGB1 from PAAF, thereby reducing the release of proinflammatory cytokines and the incidence of apoptosis, and ultimately reducing the severity of SAP-induced intestinal injury.
Our study had several limitations. Firstly, we focused on the effect of APD on intestinal inflammation and apoptosis, but whether APD also has an effect on intestinal motility remains to be studied. Secondly, we did not detect changes in the expression of HMGB1 in intestinal tissue, and future experiments should be carried out to verify the relationship between intestinal HMGB1 and PAAF.
Our data revealed that APD treatment ameliorated intestinal injury and improved intestinal barrier function in an SAP rat model, and ultimately improved outcome. The protective effect was potentially achieved through reducing intestinal inflammatory response and accompanying apoptosis of mucosal cells by downregulation of HMGB1-mediated TLR4 signaling. This study provides a novel molecular mechanism associated with the effect of APD on SAP-induced intestinal injury. SAP is characterized by extensive inflammation of the pancreas with tissue necrosis.
Severe acute pancreatitis (SAP) is an abdominal disease characterized by extensive inflammation and tissue necrosis of the pancreas and usually accompanied by multiple organ damage, resulting in high mortality. Intestinal injury is the most common extrapancreatic complication and is closely related to the severity of SAP. Our previous studies proved that elimination of pancreatitis associated ascitic fluids (PAAF) through early abdominal paracentesis drainage (APD) attenuates intestinal mucosal injury and protects intestinal barrier effectively, but the potential molecular mechanisms responsible for the beneficial effect are yet to be clarified.
The inflammatory response plays an essential role in the pathological process of SAP-induced intestinal injury. High concentration of high mobility group box protein 1 (HMGB1) participates in remote organ damage and has been confirmed during SAP. Based on our previous research, we further investigate whether HMGB1 in ascites is related to intestinal inflammation and apoptosis under SAP conditions, which may reveal the underlying mechanism of APD in SAP.
The purpose of the present experiment was to explore the effect of APD treatment on intestinal inflammation and apoptosis induced by SAP in rats, and its potential mechanisms.
SAP was induced in male adult Sprague-Dawley rats by 5% sodium taurocholate. Mild acute pancreatitis was induced by intraperitoneal injections of cerulein (20 μg/kg body weight, six consecutive injections). Following SAP induction, a drainage tube connected to a vacuum ball was placed into the lower right abdomen of the rats for APD. Morphological staining, serum amylase, lipase and inflammatory mediators, serum and ascites HMGB1, serum indices which reflect intestinal barrier function, apoptosis and associated proteins in intestinal tissue were assessed. The expression levels of key proteins in the toll-like receptor 4 (TLR4) signaling pathway were also examined.
The results demonstrated that APD notably alleviated the changes in pancrease and intestinal mucosa, attenuated the alterations in serum amylase, lipase and inflammatory mediators, improved intestinal barrier function, lessened intestinal inflammation and accompanying apoptosis of mucosal cells, and reversed the expression of apoptosis-associated proteins. APD significantly inhibited activation of the intestinal TLR4 signaling pathway mediated by HMGB1 in intestinal tissue, and thus plays a protective role in SAP-associated intestinal injury.
APD treatment effectively improved intestinal barrier function, ameliorated the intestinal inflammatory response and mucosa cell apoptosis in rats with SAP. The protective effects are potentially due to the inhibition of the HMGB1-mediated TLR4 signaling pathway.
The present study provided new evidence of the efficacy and safety of APD treatment on SAP, and provided a novel molecular mechanism associated with the effect of APD on SAP-induced intestinal injury. However, in this research we did not detect the expression change of HMGB1 in intestinal tissue. In our next experiments, we will attempt to verify the relationship between intestinal HMGB1 and PAAF to further reveal the precise mechanisms of APD treatment in SAP.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Novita BD S-Editor: Fan JR L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375:1972-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 556] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 2. | Guo ZZ, Wang P, Yi ZH, Huang ZY, Tang CW. The crosstalk between gut inflammation and gastrointestinal disorders during acute pancreatitis. Curr Pharm Des. 2014;20:1051-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | de Jong PR, González-Navajas JM, Jansen NJ. The digestive tract as the origin of systemic inflammation. Crit Care. 2016;20:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 4. | Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, Delle Fave G. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 2012;46 Suppl:S46-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Deng C, Wang T, Cui J, Zhang S, Jiang Z, Yan H, Liang H, Tang L, Dai R. Effect of Early Abdominal Paracentesis Drainage on the Injury of Intestinal Mucosa and Intestinal Microcirculation in Severe Acute Pancreatitis Rats. Pancreas. 2019;48:e6-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Liu WH, Ren LN, Chen T, Liu LY, Jiang JH, Wang T, Xu C, Yan HT, Zheng XB, Song FQ, Tang LJ. Abdominal paracentesis drainage ahead of percutaneous catheter drainage benefits patients attacked by acute pancreatitis with fluid collections: a retrospective clinical cohort study. Crit Care Med. 2015;43:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Garcia MM, Goicoechea C, Molina-Álvarez M, Pascual D. Toll-like receptor 4: A promising crossroads in the diagnosis and treatment of several pathologies. Eur J Pharmacol. 2020;874:172975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Role of toll-like receptor 4 in the pathophysiology of severe acute pancreatitis in mice. Surg Today. 2007;37:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Kiziltas S. Toll-like receptors in pathophysiology of liver diseases. World J Hepatol. 2016;8:1354-1369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (2)] |
| 10. | Wang W, Xia T, Yu X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm Res. 2015;64:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Shen X, Li WQ. High-mobility group box 1 protein and its role in severe acute pancreatitis. World J Gastroenterol. 2015;21:1424-1435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Yuan H, Jin X, Sun J, Li F, Feng Q, Zhang C, Cao Y, Wang Y. Protective effect of HMGB1 a box on organ injury of acute pancreatitis in mice. Pancreas. 2009;38:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Chen X, Zhao HX, Bai C, Zhou XY. Blockade of high-mobility group box 1 attenuates intestinal mucosal barrier dysfunction in experimental acute pancreatitis. Sci Rep. 2017;7:6799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Wen Y, Sun HY, Tan Z, Liu RH, Huang SQ, Chen GY, Qi H, Tang LJ. Abdominal paracentesis drainage ameliorates myocardial injury in severe experimental pancreatitis rats through suppressing oxidative stress. World J Gastroenterol. 2020;26:35-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Marciniak A, Walczyna B, Rajtar G, Marciniak S, Wojtak A, Lasiecka K. Tempol, a Membrane-Permeable Radical Scavenger, Exhibits Anti-Inflammatory and Cardioprotective Effects in the Cerulein-Induced Pancreatitis Rat Model. Oxid Med Cell Longev. 2016;2016:4139851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 674] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 17. | Chiu CJ, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. II. The protective effect of intraluminal glucose as energy substrate. Arch Surg. 1970;101:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Rau BM, Bothe A, Kron M, Beger HG. Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis. Clin Gastroenterol Hepatol. 2006;4:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Tan Y, Zhang W, Wu HY, Xia J, Zhang HB, Liu MW, Qian CY. Effects of emodin on intestinal mucosal barrier by the upregulation of miR-218a-5p expression in rats with acute necrotizing pancreatitis. Int J Immunopathol Pharmacol. 2020;34:2058738420941765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Zhang XP, Jiang J, Cheng QH, Ye Q, Li WJ, Zhu H, Shen JY. Protective effects of Ligustrazine, Kakonein and Panax Notoginsenoside on the small intestine and immune organs of rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2011;10:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Junyuan Z, Hui X, Chunlan H, Junjie F, Qixiang M, Yingying L, Lihong L, Xingpeng W, Yue Z. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatology. 2018;18:742-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Chen J, Huang C, Wang J, Zhou H, Lu Y, Lou L, Zheng J, Tian L, Wang X, Cao Z, Zeng Y. Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing pancreatitis in rats. PLoS One. 2017;12:e0176583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Wolvekamp MC, de Bruin RW. Diamine oxidase: an overview of historical, biochemical and functional aspects. Dig Dis. 1994;12:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 133] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Samanta J, Rana A, Dhaka N, Agarwala R, Gupta P, Sinha SK, Gupta V, Yadav TD, Kochhar R. Ascites in acute pancreatitis: not a silent bystander. Pancreatology. 2019;19:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 25. | Hongyin L, Zhu H, Tao W, Ning L, Weihui L, Jianfeng C, Hongtao Y, Lijun T. Abdominal paracentesis drainage improves tolerance of enteral nutrition in acute pancreatitis: a randomized controlled trial. Scand J Gastroenterol. 2017;52:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Hall JC, Crawford HC. The conspiracy of autophagy, stress and inflammation in acute pancreatitis. Curr Opin Gastroenterol. 2014;30:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Noreen M, Shah MA, Mall SM, Choudhary S, Hussain T, Ahmed I, Jalil SF, Raza MI. TLR4 polymorphisms and disease susceptibility. Inflamm Res. 2012;61:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 28. | Wullaert A. Role of NF-kappaB activation in intestinal immune homeostasis. Int J Med Microbiol. 2010;300:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Yao P, Cui M, Li Y, Deng Y, Wu H. Effects of rhubarb on intestinal flora and toll-like receptors of intestinal mucosa in rats with severe acute pancreatitis. Pancreas. 2015;44:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Nakajima T, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Sawa H, Kuroda Y. Protective effects of vascular endothelial growth factor on intestinal epithelial apoptosis and bacterial translocation in experimental severe acute pancreatitis. Pancreas. 2007;34:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Zhang XP, Jiang J, Yu YP, Cheng QH, Chen B. Effect of Danshen on apoptosis and NF-κB protein expression of the intestinal mucosa of rats with severe acute pancreatitis or obstructive jaundice. Hepatobiliary Pancreat Dis Int. 2010;9:537-546. [PubMed] |
| 32. | Xie MY, Hou LJ, Sun JJ, Zeng B, Xi QY, Luo JY, Chen T, Zhang YL. Porcine Milk Exosome MiRNAs Attenuate LPS-Induced Apoptosis through Inhibiting TLR4/NF-κB and p53 Pathways in Intestinal Epithelial Cells. J Agric Food Chem. 2019;67:9477-9491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 33. | Van Opdenbosch N, Lamkanfi M. Caspases in Cell Death, Inflammation, and Disease. Immunity. 2019;50:1352-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 848] [Article Influence: 141.3] [Reference Citation Analysis (0)] |
| 34. | Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34:1503-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 302] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 35. | Yang R, Tenhunen J, Tonnessen TI. HMGB1 and Histones Play a Significant Role in Inducing Systemic Inflammation and Multiple Organ Dysfunctions in Severe Acute Pancreatitis. Int J Inflam. 2017;2017:1817564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Yasuda T, Ueda T, Takeyama Y, Shinzeki M, Sawa H, Nakajima T, Ajiki T, Fujino Y, Suzuki Y, Kuroda Y. Significant increase of serum high-mobility group box chromosomal protein 1 levels in patients with severe acute pancreatitis. Pancreas. 2006;33:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Yasuda T, Ueda T, Shinzeki M, Sawa H, Nakajima T, Takeyama Y, Kuroda Y. Increase of high-mobility group box chromosomal protein 1 in blood and injured organs in experimental severe acute pancreatitis. Pancreas. 2007;34:487-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Zhou H, Zhu ZH, Liu Y, Liu YY. Effects of midazolam combined with sufentanil on injury and expression of HMGB1 and NF-κB in rats with pancreatitis. Eur Rev Med Pharmacol Sci. 2020;24:2102-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |