Published online Feb 28, 2021. doi: 10.3748/wjg.v27.i8.737
Peer-review started: November 28, 2020
First decision: January 17, 2021
Revised: January 20, 2021
Accepted: February 1, 2021
Article in press: February 1, 2021
Published online: February 28, 2021
Processing time: 90 Days and 1.2 Hours
Lymph node metastasis (LNM) affects the application and outcomes of endoscopic resection in T1 esophageal squamous cell carcinoma (ESCC). However, reports of the risk factors for LNM have been controversial.
To evaluate risk factors for LNM in T1 ESCC.
We searched Embase, PubMed and Cochrane Library to select studies related to LNM in patients with T1 ESCC. Included studies were divided into LNM and non-LNM groups. We performed a meta-analysis to examine the relationship between LNM and clinicopathologic features. Odds ratio (OR), mean differences and 95% confidence interval (CI) were assessed using a fixed-effects or random-effects model.
Seventeen studies involving a total of 3775 patients with T1 ESCC met the inclusion criteria. After excluding studies with heterogeneity based on influence analysis, tumor size (OR = 1.93, 95%CI = 1.49-2.50, P < 0.001), tumor location (OR = 1.46, 95%CI = 1.17-1.82, P < 0.001), macroscopic type (OR = 3.17, 95%CI = 2.33-4.31, P < 0.001), T1 substage (OR = 6.28, 95%CI = 4.93-8.00, P < 0.001), differentiation (OR = 2.11, 95%CI = 1.64-2.72, P < 0.001) and lymphovascular invasion (OR = 5.86, 95%CI = 4.60-7.48, P < 0.001) were found to be significantly associated with LNM. Conversely, sex, age and infiltrative growth pattern were not identified as risk factors for LNM.
A tumor size > 2 cm, lower location, nonflat macroscopic type, T1b stage, poor differentiation and lymphovascular invasion were associated with LNM in patients with T1 ESCC.
Core Tip: No consensus is available in the literature about risk factors for lymph node metastasis (LNM) in T1 esophageal squamous cell carcinoma. This meta-analysis is the first to comprehensively evaluate LNM only in esophageal squamous cell carcinoma patients with T1 stage. We investigated the relationship between LNM and the factors of demographic and clinicopathological characteristics. The results showed that risk factors associated with LNM were tumor size, tumor location, T1 substage, tumor differentiation, lymphovascular invasion and macroscopic type.
- Citation: Jiang KY, Huang H, Chen WY, Yan HJ, Wei ZT, Wang XW, Li HX, Zheng XY, Tian D. Risk factors for lymph node metastasis in T1 esophageal squamous cell carcinoma: A systematic review and meta-analysis. World J Gastroenterol 2021; 27(8): 737-750
- URL: https://www.wjgnet.com/1007-9327/full/v27/i8/737.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i8.737
The morbidity and mortality of esophageal cancer (EC) rank 7th and 6th among cancers globally[1]. In the last few decades, there has been great progress in the early diagnosis, surgical techniques and comprehensive treatment of EC. However, long-term efficacy remains poor, and the 5-yr survival rate is only 10%-30%[2]. In general, the opportunity for a cure in cancer is determined by early screening and intervention.
Esophageal squamous cell carcinoma (ESCC) is the most common histological type of EC in Asia[3]. T1 EC are stratified into T1a (mucosal layer) and T1b (submucosal layer) based on the 8th edition EC tumor, node, metastasis staging. For patients with T1a or T1b tumors, the 5-yr survival rate can be higher than 85%[4]. Although radical esophagectomy with lymphadenectomy is still the gold standard, endoscopic imaging with resection offers a new model for the treatment of early ESCC. In addition, evaluation of the clinical stage, disease extent, tumor grade and risk of lymph node metastasis (LNM) is critical when determining eligibility for endoscopic treatment of T1 ESCC[5]. LNM rates in T1a and T1b are reportedly 7.0%-16.0% and 16.0%-41.1%, respectively[6-8], and thus endoscopic resection (ER) for the treatment of T1 ESCC should be considered carefully[9]. Furthermore, LNM may be regarded as the most important prognostic factor in ESCC[10], and accurate assessment of the risk of LNM in T1 ESCC is particularly important.
Risk factors for LNM in T1 ESCC have been reported without consensus. We previously demonstrated that clinicopathological and hematological parameters can predict the risk of LNM in T1 ESCC, though some features were not included and the sample size in that study was limited. Herein, we present a meta-analysis to evaluate risk factors of LNM alone in stage T1 ESCC.
This meta-analysis was performed in accordance with the guidelines proposed by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (http://www.prisma-statement.org/)[11].
To select relevant studies for the meta-analysis, we searched the following electronic databases: PubMed, EMBASE, and the Cochrane Library. The last search was performed on July 1, 2020. The search terms were “T1,” “early,” “mucosal,” “submucosal,” “superficial,” “intramucosal,” “esophageal,” “esophagus,” “squamous,” “cancer” and “carcinoma.” Two authors (Tian D and Jiang KY) independently performed the selection and evaluation of references. When necessary, a third author (Huang H) independently resolved any discrepancies between the two review authors. The full text of studies meeting the inclusion criteria were reviewed to identify whether they contained useful information.
The criteria for inclusion in this meta-analysis were as follows: (1) A case-control study of risk factors for LNM in stage T1 ESCC; (2) All patients underwent 2- or 3-field lymphadenectomy; (3) Published in English; (4) Contained original data, odds ratio (OR), mean difference and 95% confidence intervals (CI); (5) The definition and classification standard of tumors were basically the same; and (6) When overlapping data resources were presented in several articles, only the study with the highest quality and largest sample size was selected. Studies were excluded from the meta-analysis for the following reasons: (1) References were abstracts, comments, reviews and editorials; (2) Patients with esophagogastric junction adenocarcinoma or esophageal adenocarcinoma; or (3) Low reliability and poor quality Newcastle-Ottawa Scale score ≤ 5).
According to the above criteria, qualified literature was selected by reading the title, abstract and full text. The following data were extracted: first author, publication year, location, research period, study design, case number, operation protocol, lymph node status (LNM/non-LNM), age, sex, tumor size, tumor location, macroscopic type, T1 substage, tumor differentiation, lymphovascular invasion (LVI) and infiltrative growth pattern (INF). A structured table was employed to help extract relevant data from the included studies. We used the Newcastle-Ottawa Scale for evaluating the quality of references. The scale includes three aspects (selection, comparability and outcome) ranging from 0 to 9 stars, and studies with a score of 6 were considered to have adequate methodologic quality for inclusion[12].
Patients in the literature included underwent open esophagectomy or thoracoscopy-assisted esophagectomy or ER and 2- or 3-field lymph node dissection. Postoperative pathological results confirmed that the tumor invaded the mucosa or submucosa. T1 ESCC was stratified as T1a, which included T1a-carcinoma in situ, T1a-lamina propria mucosa, T1a-MM (muscularis mucosa), and T1b, which include SM1 (the upper third of the submucosal layer), SM2 (the middle third of the submucosal layer) and SM3 (the lower third of the submucosal layer). Tumor locations were classified as upper, middle and lower. Tumor differentiation was stratified into well differentiated, moderately differentiated and poorly differentiated. Macroscopic type was divided into flat and nonflat types based on visual observation. LVI (absent/present) and INF (a/b/c) were determined by postoperative immunohistochemistry or hematoxylin-eosin staining. The INF division was as follows: INF-a (expansive growth of tumor nests with a well-demarcated border from the surrounding tissue), INF-b (growth pattern intermediate between that of INF-a and INF-c) and INF-c (infiltrative growth of tumor nests with an ill-defined border from the surrounding tissue).
Statistical software Review Manager 5.3 and Stata 15.0 were used for data analyses. Dichotomous data were analyzed using the OR with 95%CI. For continuous data, the mean difference and 95%CI were calculated. The Mantel-Haenszel method and inverse variance method were applied for dichotomous and continuous data, respectively. Heterogeneity among the included studies was preliminarily examined by a Galbraith plot. If heterogeneity was detected, influence analysis was performed to explore individual studies with heterogeneity. After excluding studies with heterogeneity, data were graphically plotted using forest plots to evaluate the treatment effects and heterogeneity of the trials. Heterogeneity was quantified with the χ2(Cochrane Q) test and the I2statistic, whereby low, moderate and high degrees of heterogeneity corresponded to I2values of 25%, 50% and 75%, respectively. When P > 0.1 and I2< 50%, a fixed-effects model was utilized; otherwise, a random-effects model was applied. After the heterogeneity test of outcome indicators, the P value, OR value and 95%CI of the combined statistics were then calculated. Funnel plots were used to evaluate the presence of publication bias.
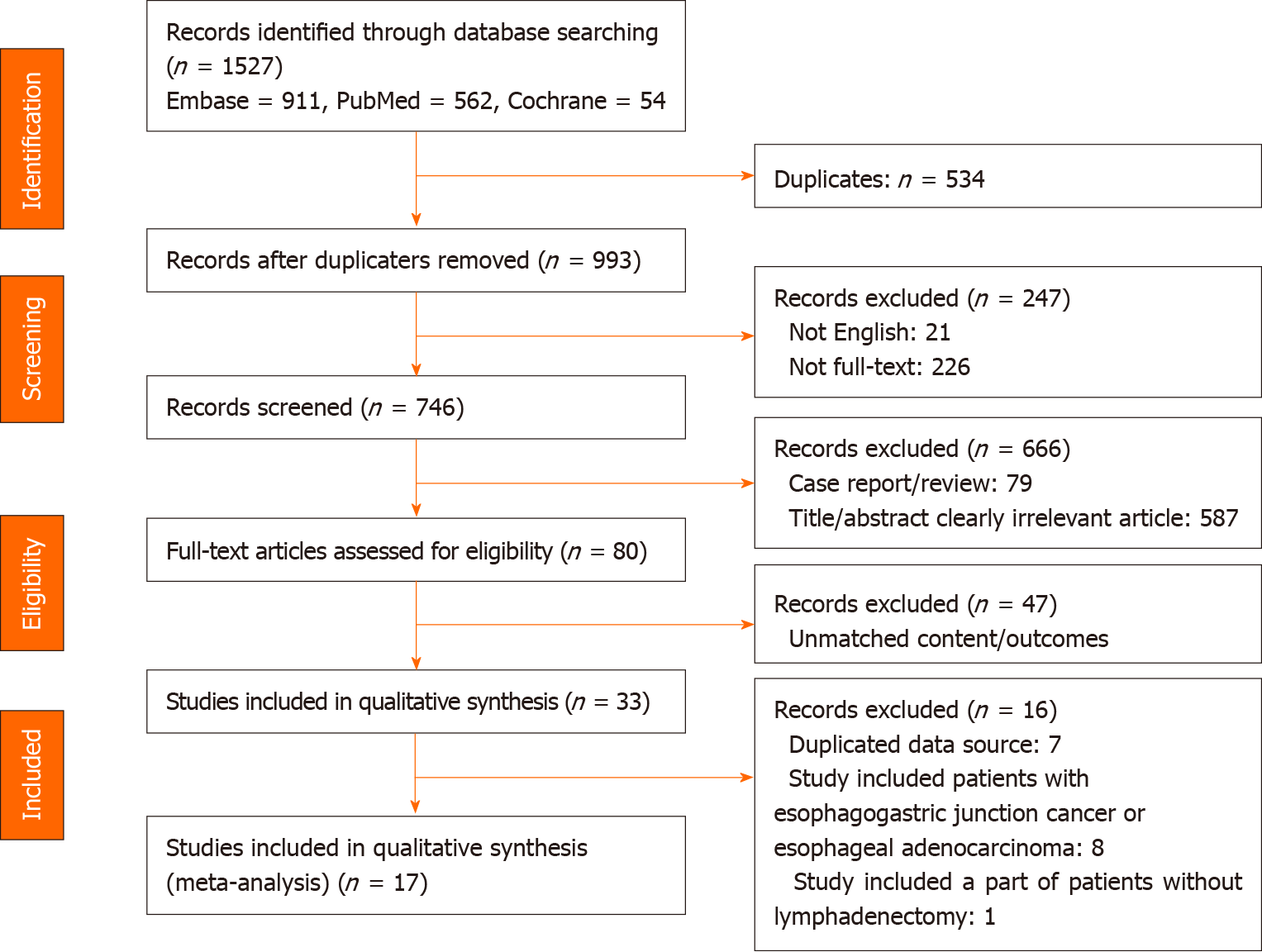
A flow chart of the selection process for the studies included in this meta-analysis is presented in Figure 1. The search strategy identified 1527 records from all sources (Embase, n = 911; PubMed, n = 562; and Cochrane, n = 54). Of these, 537 duplicate records, 21 non-English articles and 226 non-full texts were excluded. In addition, 666 studies were excluded after scanning the article types, titles and abstracts, including 79 case reports/reviews and 587 title/abstract clearly irrelevant articles; thus, 80 studies remained for full-text assessment. Thereafter, 33 studies were included for qualitative synthesis. Seven studies had duplicate data sources, eight studies included patients with esophagogastric junction adenocarcinoma or esophageal adenocarcinoma, and one study included some patients without lymphadenectomy. Ultimately, 17 studies meeting all inclusion criteria were selected for the meta-analysis.
Table 1 shows the baseline characteristics and quality assessment scores of the 17 included retrospective studies. Three[13-15] were multicenter studies, and fourteen[16-29] were single-center studies. All studies were conducted in Asian countries, including seven in China[14,15,20,21,23,26,29], seven in Japan[16,18,19,22,24,27,28] and three in South Korea[13,17,25]. All patients underwent open esophagectomy/thoracoscopy-assisted esophagectomy/ER and 2- or 3-field lymphadenectomy. Moreover, postoperative pathological results confirmed T1 ESCC in all cases. A total of 3775 patients were enrolled, of whom 914 had LNM and 2861 were non-LNM, with a metastasis rate of 24.2%. In terms of the assessment of the Newcastle-Ottawa Scale, 11 studies[8,13,16-22,25,27] scored 6, and 6 studies[14,15,23,24,26,28] scored 7.
| Reference | Location | Period | Design | Case | Operation protocol | Lymphadenectomy strategy | LNM/non-LNM | Quality assessment |
| Aoyama et al[16] (2019) | Japan | 2012-2016 | SR | 50 | Esophagectomy | 2- or 3-field lymphadenectomy | 13/37 | 6 |
| Chiba et al[24] (2010) | Japan | 1992-2008 | SR | 110 | Esophagectomy | Lymphadenectomy | 33/77 | 7 |
| Huh et al[25] (2018) | South Korea | 1996-2015 | SR | 275 | Esophagectomy | Lymphadenectomy | 40/235 | 6 |
| Li et al[26] (2013) | China | 2006-2011 | SR | 189 | Esophagectomy | Lymphadenectomy | 47/95 | 7 |
| Makuuchi et al[27] (1997) | Japan | 1974-1995 | SR | 133 | Esophagectomy | Lymphadenectomy | 35/98 | 6 |
| Min et al[17] (2020) | South Korea | 2001-2014 | SR | 501 | Esophagectomy | 2-field lymphadenectomy: 462; 3-field lymphadenectomy: 39 | 140/361 | 6 |
| Mitobe et al[18] (2013) | Japan | 1990-2009 | SR | 110 | Esophagectomy: 106; ER + esophagectomy: 4 | Lymphadenectomy | 37/73 | 6 |
| Moon et al[13] (2014) | South Korea | 2009-2012 | MR | 104 | Esophagectomy | Lymphadenectomy | 15/89 | 6 |
| Ozawa et al[19] (2016) | Japan | 1986-2013 | SR | 167 | Esophagectomy; VATS | 2- or 3-field lymphadenectomy | 46/121 | 6 |
| Shen et al[14] (2018) | China | 2014-2016 | MR | 221 | Esophagectomy | Lymphadenectomy | 53/168 | 7 |
| Tian et al[15] (2020) | China | 2013-2019 | MR | 243 | Esophagectomy | 3-field lymphadenectomy | 46/197 | 7 |
| Tomita et al[28] (2008) | Japan | 1998-2006 | SR | 115 | Esophagectomy | 2- or 3-field lymphadenectomy | 52/63 | 7 |
| Wang et al[29] (2016) | China | 2002-2014 | SR | 228 | Esophagectomy | 2- or 3-field lymphadenectomy | 90/138 | 6 |
| Wu et al[20] (2018) | China | 2002-2010 | SR | 240 | Esophagectomy | 2-field lymphadenectomy | 39/201 | 6 |
| Xue et al[21] (2012) | China | 1990-2004 | SR | 271 | Esophagectomy | Lymphadenectomy | 53/218 | 6 |
| Yachida et al[22] (2020) | Japan | 1986-2010 | SR | 320 | Esophagectomy | 3-field lymphadenectomy | 93/227 | 6 |
| Zhou et al[23] (2016) | China | 2008-2015 | SR | 498 | Esophagectomy | Lymphadenectomy | 87/411 | 7 |
The results for the relationship between LNM and clinicopathological factors in T1 ESCC patients after excluding studies with heterogeneity in all analyses are provided in Table 2. Galbraith plots are depicted in Supplementary Figures 1-3, plots of influence analyses in Supplementary Figure 4, forest plots in Figure 2-4, and funnel plots in Supplementary Figures 5-7.
| Factors | Studies | Patients | Heterogeneity I2 (P value) | Pooled OR (95%CI) | P value |
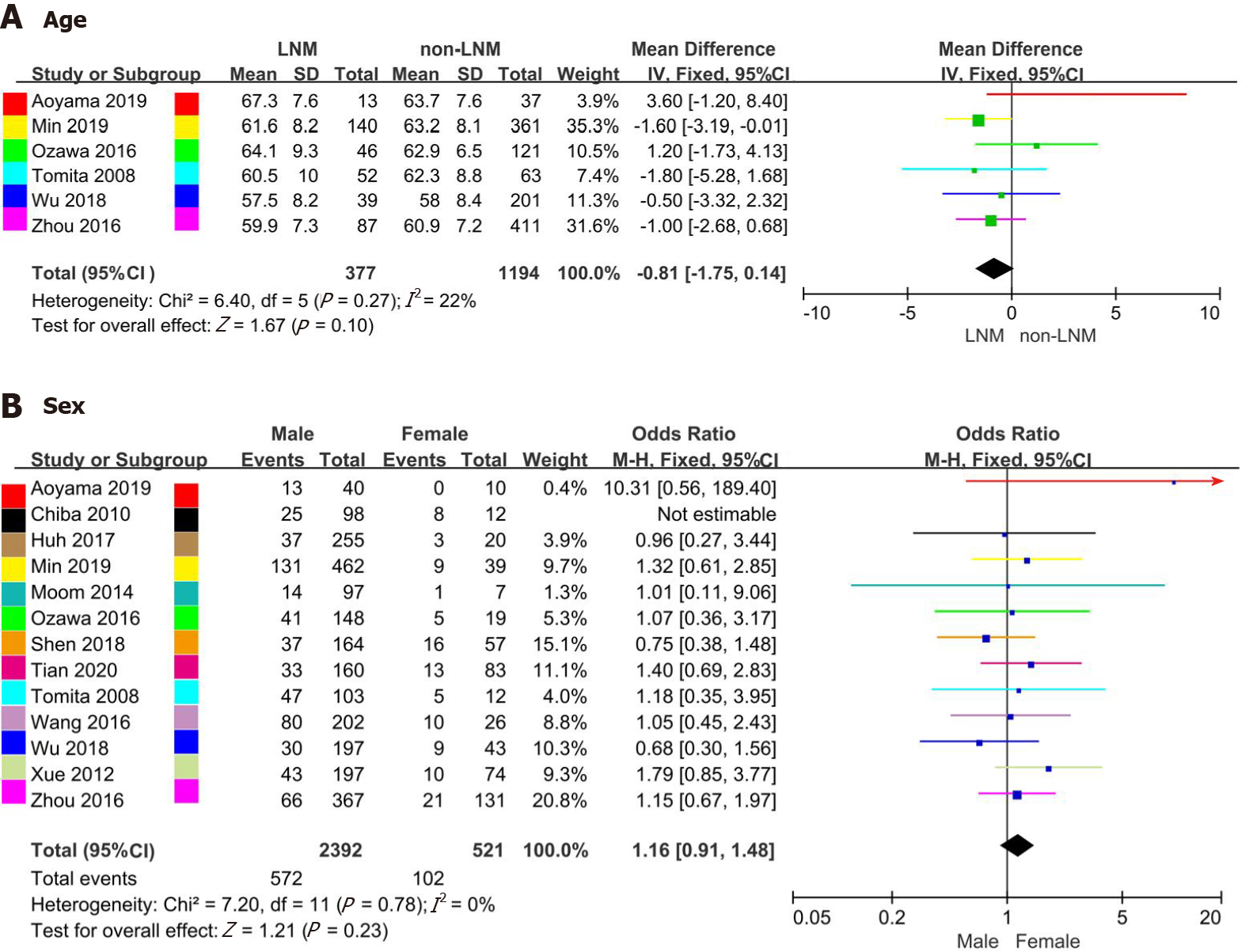
| Age (continuous) (yr) | 6 | 1571 | 22% (0.27) | -0.81 (-1.75-0.14) | 0.10 |
| Sex (male/female) | 12 | 2913 | 0% (0.78) | 1.16 (0.91-1.48) | 0.23 |
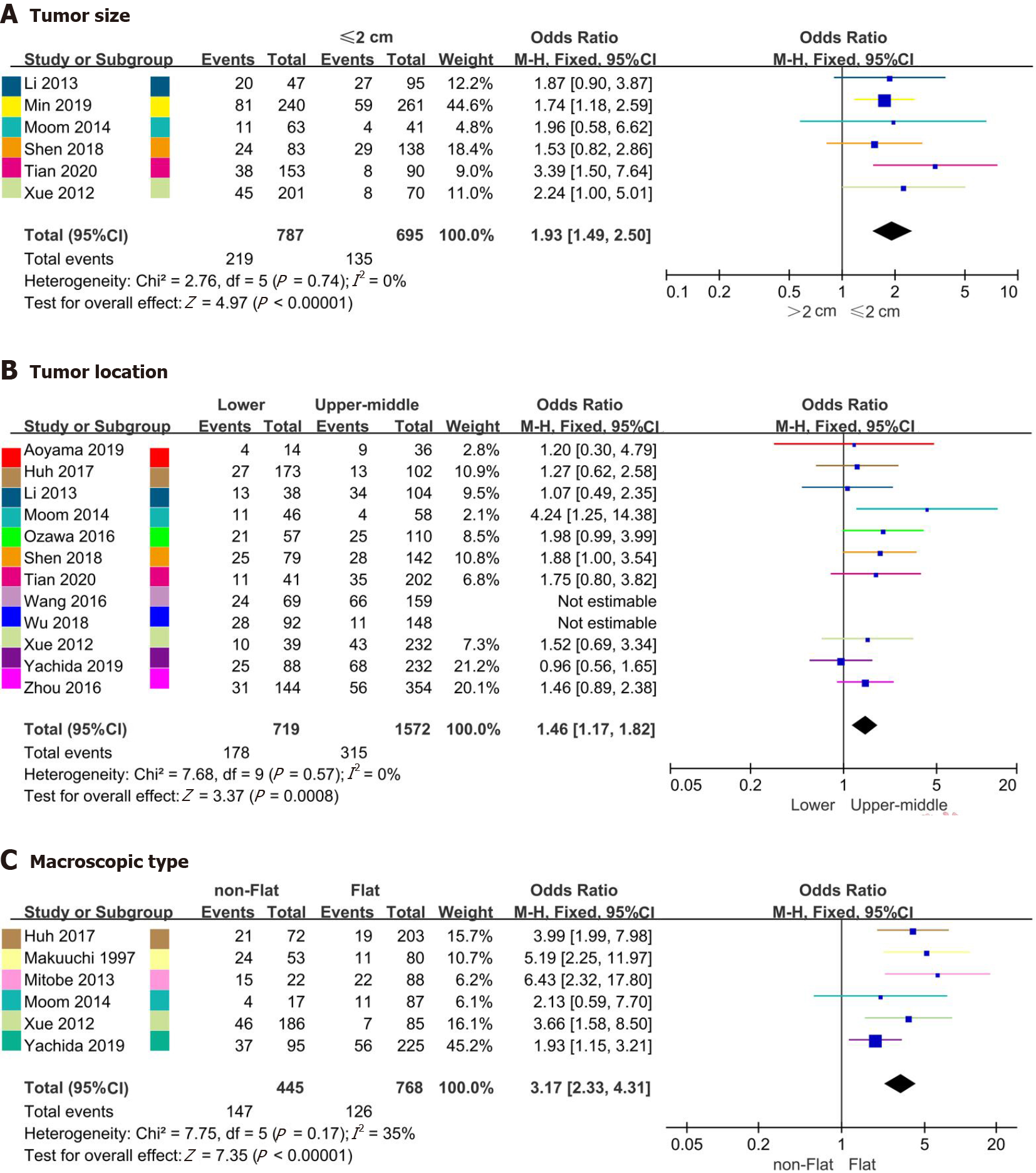
| Tumor size (> 2/≤ 2) (cm) | 6 | 1482 | 0% (0.74) | 1.93 (1.49-2.50) | < 0.001a |
| Tumor location (L/U-M) | 10 | 2291 | 0% (0.57) | 1.46 (1.17-1.82) | < 0.001a |
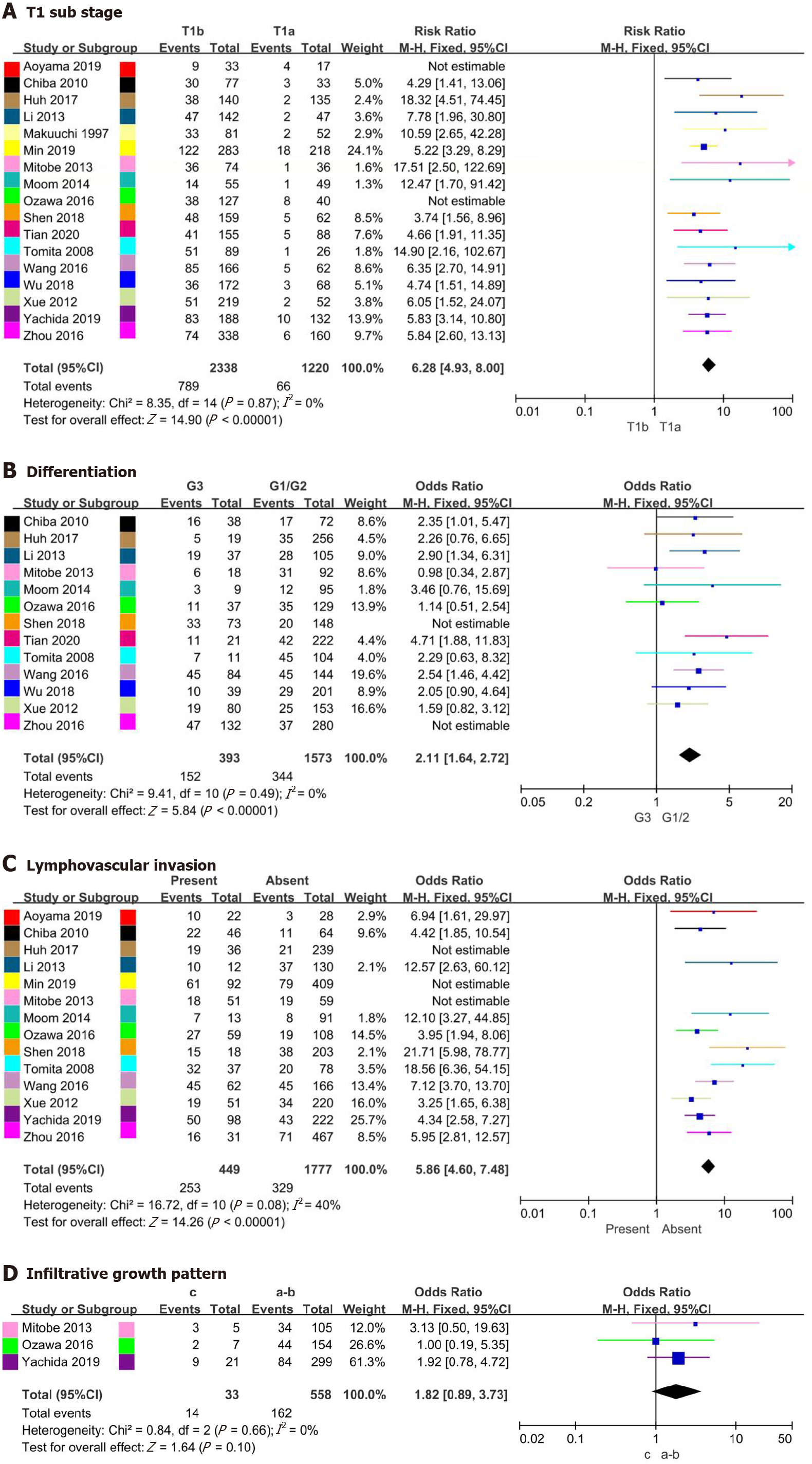
| T1 substage (T1b/T1a) | 15 | 3558 | 0% (0.87) | 6.28 (4.93-8.00) | < 0.001a |
| Differentiation (G3/G1-2) | 11 | 1966 | 0% (0.49) | 2.11 (1.64-2.72) | < 0.001a |
| LVI (present/absent) | 11 | 2226 | 40% (0.08) | 5.86 (4.60-7.48) | < 0.001a |
| Macroscopic type (nonflat/flat) | 6 | 1213 | 35% (0.17) | 3.17 (2.33-4.31) | < 0.001a |
| INF (present/absent) | 3 | 591 | 0% (0.66) | 1.82 (0.89-3.73) | 0.10 |
Six studies[16,17,19,20,23,28] reporting continuous age of patients were analyzed in this meta-analysis. The mean age in the LNM and non-LNM groups ranged from 57.5-67.3 and 58.0-63.7 years, respectively. Thirteen studies[13-17,19-21,23-25,28,29] recorded the rate of LNM in males vs females (14.4%-39.6% vs 0%-66.7%). The Galbraith plot and influence analysis indicated no heterogeneity in the studies including age analysis, though one study (Chiba et al[24]) with sex analysis exhibited strong heterogeneity (Supplementary Figures 1 and 4A). No significant difference between LNM and age was observed (OR = -0.81, 95%CI = -1.75-0.14, P = 0.10; heterogeneity: I2= 22%, P = 0.27) (Figure 2A). After excluding the study by Chiba et al[24], the results indicated that the rate of LNM according to sex was not significantly different (OR = 1.16, 95%CI = 0.91-1.48, P = 0.23; heterogeneity: I2= 0%, P = 0.78) (Figure 2B).
Six studies[13-15,17,21,26] including 1482 patients, twelve studies[13-16,19-23,25,26,29] including 2751 patients, and six studies[13,18,21,22,25,27] including 1213 patients analyzed the relationship between LNM and tumor size, location and macroscopic type, respectively. The rates of LNM for tumor size ≤ 2 cm vs tumor size > 2 cm, upper-middle ESCC vs lower ESCC and nonflat type vs flat type were 8.9%-28.4% vs 17.5%-42.6%, 6.9%-41.5% vs 15.6%-36.8% and 23.5%-68.2% vs 8.2%-25.0%, respectively. Although no heterogeneity regarding tumor size analysis or macroscopic type analysis (Supplementary Figures 2A and C) was detected, influence analysis suggested heterogeneities for tumor location in the studies of Wang et al[29] and Wu et al[20] (Supplementary Figures 2B and 4B). Moreover, the rate of LNM in patients with tumor size ≤ 2 cm was 1.93 times higher than that in patients with tumor size ≤ 2 cm (OR = 1.93, 95%CI = 1.49-2.50, P < 0.001; heterogeneity: I2= 0%, P = 0.74) (Figure 3A). Additionally, the rate of LNM differed significantly with respect to tumor location (OR = 1.46, 95%CI = 1.17-1.82, P < 0.001; heterogeneity: I2 = 0%, P = 0.57) (Figure 3B) and macroscopic type (OR = 3.17, 95%CI = 2.33-4.31, P < 0.001; heterogeneity: I2 = 35%, P = 0.17, Figure 3C).
The relationship between T1 substage and LNM was analyzed in 17 studies[13-29], involving 3775 patients, and the relationship between differentiation and LNM was analyzed in 13 studies[13-15,18-21,23-26,28,29], involving 2599 patients. The rates of LNM in T1a vs T1b and well differentiated/moderately differentiated vs poorly differentiated were 1.5%-23.5% vs 20.9%-57.3% and 12.6%-43.3% vs 23.8%-63.6%, respectively. Based on heterogeneity and influence analyses, studies with heterogeneity by Aoyama et al[16] and Ozawa et al[19] were excluded from the T1 substage analysis, and those of Shen et al[14] and Zhou et al[23] were excluded from the differentiation analysis (Supplementary Figures 3A, 3B, 4C and 4D). Nonetheless, pooled analysis suggested that the rate of LNM differed significantly for T1 substage (OR = 6.28, 95%CI = 4.93-8.00, P < 0.001; heterogeneity: I2 = 0%, P = 0.87, Figure 4A) and differentiation (OR = 2.11, 95%CI = 1.64-2.72, P < 0.001; heterogeneity: I2 = 0%, P = 0.49, Figure 4B).
Fourteen studies[13,14,16-19,21-26,28,29] involving 3112 patients reported rates of LNM in LVI present and LVI absent cases, at 35.3%-86.5% and 8.8%-32.2%, respectively. Three studies[18,19,22] involving 1213 patients reported rates of LNM in INF-c and INF-a/b cases, at 28.6%-60.0% and 28.1%-32.4%, respectively. Studies by Huh et al[25], Min et al[17] and Mitobe et al[18] with heterogeneity were excluded from the LVI analysis due to the results of heterogeneity and influence analyses (Supplementary Figures 3C and 4E). However, no heterogeneity in the analysis of INF was found (Supplementary Figure 3D). Additionally, pooled analysis showed that the rate of LNM was significantly different in LVI (OR = 5.86, 95%CI = 4.60-7.48, P < 0.001; heterogeneity: I2 = 40%, P = 0.08, Figure 4C), with no difference for INF (OR = 1.82, 95%CI = 0.89-3.73, P = 0.010; heterogeneity: I2 = 0%, P = 0.66, Figure 4D).
Funnel plots of the studies were used in the meta-analysis for age, sex, tumor size, tumor location, macroscopic type, T1 substage, differentiation, LVI and INF. According to the results, the distribution of each study was concentrated and symmetrical (Supplementary Figures 5-7). There was no evidence of publication bias.
In some institutions, ER is the preferred treatment for patients with stage T1 ESCC because of its minimally invasive nature[30,31]. However, ER can only remove local lesions, and the lymph nodes cannot be dissected. A portion of early ESCC cases with noncurative resection after ER treatment has been identified, and additional radical esophagectomy with lymph node dissection is usually recommended[32]. Wang et al[33] reported no statistical significance in recurrence-free survival and overall survival of T1 EC patients in ER + esophagectomy and esophagectomy groups; thus, ER can be accepted for patients with stage T1 cancer even if esophagectomy is eventually warranted due to LNM. Overall, lymph node status plays an important role in surgical strategy selection and prognosis[34]. The LNM rate of T1 ESCC has been reported to be 12.9%-45.0%, with a large deviation[18,28,35]. The results of this meta-analysis showed an LNM rate of 24.2%, and risk factors associated with LNM were tumor size, tumor location, T1 substage, tumor differentiation, LVI and macroscopic type.
T1 substage, tumor differentiation and tumor size have been indicated as predictors of LNM in several studies, and our study verified these results. Previous studies reported LNM rates in MM1, MM2, MM3, SM1, SM2 and SM3 of 0%, 1.5%-3.7%, 5.3%-30.8%, 8.7%-42.1%, 12.7%-40.7% and 28.4%-66.7%, respectively[14,16-18,21-23,35]. Submucosal tumors have a high risk of LNM, and there is no safe area for ER of T1b tumors[6]. Because T1a substage (MM1, MM2 and MM3) and T1b substage (SM1, SM2 and SM3) are difficult to distinguish during surgery, further staging of T1a and T1b is not necessary. In this meta-analysis, the rates of LNM in T1a and T1b were 5.4% and 33.7%, respectively. Moreover, the LNM rate increased significantly when the tumor infiltrated the submucosa, and the risk of LNM was 2.11 times higher in patients with poorly differentiated tumors than in patients with well differentiated/moderately differentiated tumors. Furthermore, well-differentiated tumor cells were closer to the mature form of the tissue, where the tumor grows slowly and has a lower risk of metastasis; poorly differentiated tumor cells were closer to the immature form of the tissue, where the tumor grows fast and is highly malignant. In the case of the lymphatic drainage network, poorly differentiated cancer cells are prone to LNM[36,37].
Tumor size > 2 cm is helpful for predicting LNM in superficial ESCC. Friedland et al[38] suggested that lesions with a size > 2 cm can be resected using endoscopic mucosal resection and if larger, by expanded endoscopic submucosal dissection. We found that the risk of metastasis was still high in larger tumors and that endoscopic submucosal dissection should be carefully selected. In addition, although tumor size is used in the tumor, node, metastasis staging system of breast, lung, and liver cancers, among others, the EC staging system does not consider the pathological features of tumor size. We believe that it is necessary to identify the cut-off value of tumor size and incorporate it into the staging system for more accurate preoperative and postoperative assessment.
In contrast to a previous meta-analysis that included both ESCC and adenocarcinoma of the esophagogastric junction, we found that tumors at a lower location had a higher risk of LNM in ESCC[39]. The relationship between tumor location and LNM mainly manifests by lymphatic drainage. Because of the network of longitudinal and transverse lymphatic vessels of the esophagus, lymphatic drainage is extensive, presenting as adjacent LNM and skip metastasis[40]. Thus, tumors in the upper esophagus mainly metastasize to the superior mediastinal lymph nodes, especially the recurrent laryngeal nerve and the superior parietal esophagus lymph nodes[30]. In contrast, LNM of middle ESCC and lower ESCC mainly occurs in the mediastinal lymph nodes and celiac lymph nodes, respectively[31]. Most studies have shown that tumor location is not a risk factor for LNM[22,25,26]. Nevertheless, Ozawa et al[19] and Wu et al[20] reported that lower ESCC had a higher risk of LNM. The middle and lower part of the esophagus stretches as the lymphatic networks develop in the submucosal layer during embryonal growth[41], which provides anatomical conditions for skip metastasis of the lymph nodes in lower esophageal carcinoma. Furthermore, the incidence of metastasis of upper mediastinal nodes can be as high as that of lower mediastinal nodes in lower ESCC[42,43]. Therefore, skip metastasis of lower ESCC is not negligible.
Macroscopic tumor type is divided into I (protruded type), II (flat type) and III (excavated type) by visual observation. Because the protruded type and excavated type have similar outcomes and prognoses, previous studies further classified the macroscopic type as a flat type (II) and nonflat type (I and III)[13,18,22,35,44,45]. It has been reported that macroscopic type correlates with invasion depth and LNM[22,27]. Additionally, tumors of type II have lower rates of LNM, malignancy and postoperative recurrence[46]. According to the present meta-analysis, the rates of LNM in nonflat ESCC and flat ESCC were 33.0% and 16.4%, respectively. Although esophagectomy is the preferred choice for tumors of macroscopic type I or III, ER is the first treatment for flat-type tumors, and excised specimens can be evaluated to decide whether esophagectomy should be performed[18].
LVI has been proven to be an independent risk factor for LNM in patients with ESCC[6,14,17,21,22,44]. Tumor cells become isolated from the tumor assembly at the primary lesion and then spread through the lymphatic or blood vessels, possibly invading lymphatic or blood vessels in the process[47], which is considered the initial step of LNM and distant metastasis[48]. Endoscopic ultrasonography and other imaging technologies can partially predict the depth of invasion and regional distribution of LNM, but LVI can only be detected after ER or surgery. If LVI is detected in a resected specimen after ER, additional surgical therapy with lymph node dissection should be considered[25,49]. Hsu et al[50] reported 5-yr overall survival rates of 28.2% and 61.1% in a positive LVI group and negative LVI group, respectively. This suggests that for patients with LVI, postoperative treatment should be the focus for improving prognosis. Additionally, hematoxylin-eosin staining and immunohistochemistry for the detection of LVI may provide a more reliable result[18].
In this meta-analysis, sex, age and INF were not risk factors for LNM in T1 ESCC patients. Nevertheless, due to the limited number of studies included (only three studies), more studies are needed to evaluate the relationship of INF with LNM. Xu et al[39] analyzed the risk factors for LNM in superficial EC with similar results. Their study included a small number of patients with esophageal adenocarcinoma, whereas all the patients in our study had ESCC. Furthermore, our inclusion criteria were stricter, and the quality of the studies was higher. For each analysis of clinicopathological features, we performed influence analysis to exclude studies with heterogeneity for more reliable results. In general, heterogeneity may be due to the number of included cases, surgical procedures, lymph node dissection and other factors.
There were some limitations in the present meta-analysis that should be considered. First, as all+ the included studies were retrospective, selection bias was inevitable. Second, the guidelines of EC staging in the included literature differed slightly and were revised several times during the study period, which may lead to inconsistent tumor staging. Third, all included studies were performed in Asian countries (China, Japan and South Korea), and the results may therefore be influenced by ethnicity.
This meta-analysis is the first to comprehensively evaluate LNM only in ESCC patients with T1 stage. We identified that patients with tumor size > 2 cm, lower tumor location, nonflat macroscopic type, T1b stage, poor differentiation and LVI had a significant risk of LNM. For these patients, radical esophagectomy may be a better choice than ER.
Endoscopic resection has been increasingly used in patients with T1 esophageal squamous cell carcinoma (ESCC). However, lymph node metastasis (LNM) has been widely reported in patients with T1 ESCC, and some studies have even found higher rates of metastasis. Endoscopic resection for T1 ESCC should be carefully considered.
Endoscopic resection is not appropriate for patients with a potential risk of LNM and the risk of metastasis must be assessed in advance to make the right decision. In addition, reports of the risk factors for LNM have been controversial.
The purpose of this meta-analysis was to assess risk factors of LNM for patients with T1 ESCC.
We conducted a comprehensive search of multiple electronic databases including PubMed, EMBASE and the Cochrane Library to select studies related to the topics. Statistical analysis was conducted via comprehensive meta-analysis software.
A total of 3775 patients with T1 ESCC from 17 studies were included. The rates of LNM in T1a and T1b were 5.4% and 33.7%, respectively. Tumor size > 2 cm, lower tumor location, nonflat macroscopic type, T1b stage, poor differentiation and lymphovascular invasion were found to be significantly associated with LNM. Conversely, sex, age and infiltrative growth pattern were not identified as risk factors for LNM.
This meta-analysis is the first to comprehensively evaluate LNM only in ESCC patients with T1 stage. For patients with a potential high risk of LNM, radical esophagectomy is superior to endoscopic resection, and potential metastatic lymph nodes can be dissected.
All included studies were performed in Asian countries (China, Japan and South Korea), and the results may therefore be influenced by ethnicity. There is still a need to include data from other continents.
Acknowledgments: We would like to thank the American Journal Experts (
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Spartalis E S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Heymach J, Krilov L, Alberg A, Baxter N, Chang SM, Corcoran RB, Dale W, DeMichele A, Magid Diefenbach CS, Dreicer R, Epstein AS, Gillison ML, Graham DL, Jones J, Ko AH, Lopez AM, Maki RG, Rodriguez-Galindo C, Schilsky RL, Sznol M, Westin SN, Burstein H. Clinical Cancer Advances 2018: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol. 2018;36:1020-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3429] [Article Influence: 489.9] [Reference Citation Analysis (1)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 4. | Plum PS, Hölscher AH, Pacheco Godoy K, Schmidt H, Berlth F, Chon SH, Alakus H, Bollschweiler E. Prognosis of patients with superficial T1 esophageal cancer who underwent endoscopic resection before esophagectomy-A propensity score-matched comparison. Surg Endosc. 2018;32:3972-3980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Naveed M, Kubiliun N. Endoscopic Treatment of Early-Stage Esophageal Cancer. Curr Oncol Rep. 2018;20:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Duan XF, Tang P, Shang XB, Jiang HJ, Yu ZT. The prevalence of lymph node metastasis for pathological T1 esophageal cancer: a retrospective study of 143 cases. Surg Oncol. 2018;27:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Weksler B, Kennedy KF, Sullivan JL. Using the National Cancer Database to create a scoring system that identifies patients with early-stage esophageal cancer at risk for nodal metastases. J Thorac Cardiovasc Surg. 2017;154:1787-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Endo M, Yoshino K, Kawano T, Nagai K, Inoue H. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus. 2000;13:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C, Portale G, Cavallin F, Rugge M. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 2008;15:3278-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Dubecz A, Kern M, Solymosi N, Schweigert M, Stein HJ. Predictors of Lymph Node Metastasis in Surgically Resected T1 Esophageal Cancer. Ann Thorac Surg. 2015;99:1879-85; discussion 1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17541] [Article Influence: 1096.3] [Reference Citation Analysis (1)] |
| 12. | Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3796] [Cited by in RCA: 4933] [Article Influence: 352.4] [Reference Citation Analysis (0)] |
| 13. | Moon JY, Kim GH, Kim JH, Kim HH, Ryu KD, Park SO, Lee BE, Song GA. Clinicopathologic factors predicting lymph node metastasis in superficial esophageal squamous cell carcinoma. Scand J Gastroenterol. 2014;49:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Shen W, Shen Y, Tan L, Jin C, Xi Y. A nomogram for predicting lymph node metastasis in surgically resected T1 esophageal squamous cell carcinoma. J Thorac Dis. 2018;10:4178-4185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Tian D, Jiang KY, Huang H, Jian SH, Zheng YB, Guo XG, Li HY, Zhang JQ, Guo KX, Wen HY. Clinical nomogram for lymph node metastasis in pathological T1 esophageal squamous cell carcinoma: a multicenter retrospective study. Ann Transl Med. 2020;8:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Aoyama J, Kawakubo H, Mayanagi S, Fukuda K, Irino T, Nakamura R, Wada N, Suzuki T, Kameyama K, Kitagawa Y. Discrepancy Between the Clinical and Final Pathological Findings of Lymph Node Metastasis in Superficial Esophageal Cancer. Ann Surg Oncol. 2019;26:2874-2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Min BH, Yang JW, Min YW, Baek SY, Kim S, Kim HK, Choi YS, Shim YM, Choi YL, Zo JI. Nomogram for prediction of lymph node metastasis in patients with superficial esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2020;35:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Mitobe J, Ikegami M, Urashima M, Takahashi H, Goda K, Tajiri H. Clinicopathological investigation of lymph node metastasis predictors in superficial esophageal squamous cell carcinoma with a focus on evaluation of lympho-vascular invasion. Scand J Gastroenterol. 2013;48:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Ozawa Y, Kamei T, Nakano T, Taniyama Y, Miyagi S, Ohuchi N. Characteristics of Postoperative Recurrence in Lymph Node-Negative Superficial Esophageal Carcinoma. World J Surg. 2016;40:1663-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Wu J, Chen QX, Shen DJ, Zhao Q. A prediction model for lymph node metastasis in T1 esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2018;155:1902-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Xue L, Ren L, Zou S, Shan L, Liu X, Xie Y, Zhang Y, Lu J, Lin D, Dawsey SM, Wang G, Lu N. Parameters predicting lymph node metastasis in patients with superficial esophageal squamous cell carcinoma. Mod Pathol. 2012;25:1364-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Yachida T, Oda I, Abe S, Sekiguchi M, Nonaka S, Suzuki H, Yoshinaga S, Taniguchi H, Sekine S, Masugata H, Masaki T, Daiko H, Saito Y. Risk of Lymph Node Metastasis in Patients with the Superficial Spreading Type of Esophageal Squamous Cell Carcinoma. Digestion. 2020;101:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Zhou Y, Du J, Li H, Luo J, Chen L, Wang W. Clinicopathologic analysis of lymph node status in superficial esophageal squamous carcinoma. World J Surg Oncol. 2016;14:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Chiba T, Kawachi H, Kawano T, Kumagai J, Kitagaki K, Sekine M, Uchida K, Kobayashi M, Sugihara K, Eishi Y. Independent histological risk factors for lymph node metastasis of superficial esophageal squamous cell carcinoma; implication of claudin-5 immunohistochemistry for expanding the indications of endoscopic resection. Dis Esophagus. 2010;23:398-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Huh CW, Jung DH, Kim JH, Ma DW, Youn YH, Park H. Clinical implication of endoscopic gross appearance in superficial esophageal squamous carcinoma: revisited. Surg Endosc. 2018;32:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Li B, Chen H, Xiang J, Zhang Y, Kong Y, Garfield DH, Li H. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2013;146:1198-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Makuuchi H, Shimada H, Mizutani K, Chino O, Nishi T, Tanaka H, Machimura T, Mitomi T, Osamura Y. Clinical pathological analysis of surgically resected superficial esophageal carcinoma to determine criteria for deciding on treatment strategy. Diagn Ther Endosc. 1997;3:211-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Tomita N, Matsumoto T, Hayashi T, Arakawa A, Sonoue H, Kajiyama Y, Tsurumaru M. Lymphatic invasion according to D2-40 immunostaining is a strong predictor of nodal metastasis in superficial squamous cell carcinoma of the esophagus: algorithm for risk of nodal metastasis based on lymphatic invasion. Pathol Int. 2008;58:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Wang S, Chen X, Fan J, Lu L. Prognostic Significance of Lymphovascular Invasion for Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2016;23:4101-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Yang AJ, Choi SH, Byun HK, Kim HJ, Choi J, Lee YC, Lee SK, Park KR, Lee CG. Management of Clinical T1N0M0 Esophageal Cancer. Gut Liver. 2019;13:315-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Chu JN, Choi J, Tramontano A, Morse C, Forcione D, Nishioka NS, Abrams JA, Rubenstein JH, Kong CY, Inadomi JM, Hur C. Surgical vs Endoscopic Management of T1 Esophageal Adenocarcinoma: A Modeling Decision Analysis. Clin Gastroenterol Hepatol 2018; 16: 392-400. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Dermine S, Leconte M, Leblanc S, Dousset B, Terris B, Berger A, Berger A, Rahmi G, Lepilliez V, Plomteux O, Leclercq P, Coriat R, Chaussade S, Prat F, Barret M. Outcomes of esophagectomy after noncurative endoscopic resection of early esophageal cancer. Therap Adv Gastroenterol. 2019;12:1756284819892556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Wang S, Huang Y, Xie J, Zhuge L, Shao L, Xiang J, Zhang Y, Sun Y, Hu H, Chen S, Lerut T, Luketich JD, Zhang J, Chen H. Does delayed esophagectomy after endoscopic resection affect outcomes in patients with stage T1 esophageal cancer? Surg Endosc. 2018;32:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Ho HJ, Chen HS, Hung WH, Hsu PK, Wu SC, Chen HC, Wang BY. Survival Impact of Total Resected Lymph Nodes in Esophageal Cancer Patients With and Without Neoadjuvant Chemoradiation. Ann Surg Oncol. 2018;25:3820-3832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Jia R, Luan Q, Wang J, Hou D, Zhao S. Analysis of Predictors for Lymph Node Metastasis in Patients with Superficial Esophageal Carcinoma. Gastroenterol Res Pract. 2016;2016:3797615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Rice TW, Ishwaran H, Hofstetter WL, Schipper PH, Kesler KA, Law S, Lerut EM, Denlinger CE, Salo JA, Scott WJ, Watson TJ, Allen MS, Chen LQ, Rusch VW, Cerfolio RJ, Luketich JD, Duranceau A, Darling GE, Pera M, Apperson-Hansen C, Blackstone EH. Esophageal Cancer: Associations With (pN+) Lymph Node Metastases. Ann Surg. 2017;265:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Ishihara R, Oyama T, Abe S, Takahashi H, Ono H, Fujisaki J, Kaise M, Goda K, Kawada K, Koike T, Takeuchi M, Matsuda R, Hirasawa D, Yamada M, Kodaira J, Tanaka M, Omae M, Matsui A, Kanesaka T, Takahashi A, Hirooka S, Saito M, Tsuji Y, Maeda Y, Yamashita H, Oda I, Tomita Y, Matsunaga T, Terai S, Ozawa S, Kawano T, Seto Y. Risk of metastasis in adenocarcinoma of the esophagus: a multicenter retrospective study in a Japanese population. J Gastroenterol. 2017;52:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Friedland S, Triadafilopoulos G. Can endoscopic resection for Barrett's dysplasia and early cancer be curative? Ann N Y Acad Sci. 2018;1434:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Xu W, Liu XB, Li SB, Yang ZH, Tong Q. Prediction of lymph node metastasis in superficial esophageal squamous cell carcinoma in Asia: a systematic review and meta-analysis. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Wang Y, Zhu L, Xia W, Wang F. Anatomy of lymphatic drainage of the esophagus and lymph node metastasis of thoracic esophageal cancer. Cancer Manag Res. 2018;10:6295-6303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 41. | Tachimori Y. Pattern of lymph node metastases of squamous cell esophageal cancer based on the anatomical lymphatic drainage system: efficacy of lymph node dissection according to tumor location. J Thorac Dis. 2017;9:S724-S730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Tachimori Y, Nagai Y, Kanamori N, Hokamura N, Igaki H. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus. 2011;24:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Tachimori Y, Ozawa S, Numasaki H, Matsubara H, Shinoda M, Toh Y, Udagawa H, Fujishiro M, Oyama T, Uno T; Registration Committee for Esophageal Cancer of the Japan Esophageal Society. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus. 2016;13:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 44. | Ma DW, Jung DH, Kim JH, Park JJ, Youn YH, Park H. Predicting lymph node metastasis for endoscopic resection of superficial esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2019; 157: 397-402. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Shimada H, Nabeya Y, Matsubara H, Okazumi S, Shiratori T, Shimizu T, Aoki T, Shuto K, Akutsu Y, Ochiai T. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg. 2006;191:250-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Xue LY, Qin XM, Liu Y, Liang J, Lin H, Xue XM, Zou SM, Zhang MY, Zhang BH, Hui ZG, Zhao ZT, Ren LQ, Zhang YM, Liu XY, Yuan YL, Ying JM, Gao SG, Song YM, Wang GQ, Dawsey SM, Lu N. Clinicopathological parameters predicting recurrence of pT1N0 esophageal squamous cell carcinoma. World J Gastroenterol. 2018;24:5154-5166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Schiefer AI, Schoppmann SF, Birner P. Lymphovascular invasion of tumor cells in lymph node metastases has a negative impact on survival in esophageal cancer. Surgery. 2016;160:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Zhao B, Zhang J, Zhang J, Luo R, Wang Z, Xu H, Huang B. Risk Factors Associated with Lymph Node Metastasis for Early Gastric Cancer Patients Who Underwent Non-curative Endoscopic Resection: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2019;23:1318-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Newton AD, Predina JD, Xia L, Roses RE, Karakousis GC, Dempsey DT, Williams NN, Kucharczuk JC, Singhal S. Surgical Management of Early-Stage Esophageal Adenocarcinoma Based on Lymph Node Metastasis Risk. Ann Surg Oncol. 2018;25:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Hsu CP, Chuang CY, Hsu PK, Chien LI, Lin CH, Yeh YC, Hsu HS, Wu YC. Lymphovascular Invasion as the Major Prognostic Factor in Node-Negative Esophageal Cancer After Primary Esophagectomy. J Gastrointest Surg. 2020;24:1459-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |