Published online Feb 28, 2021. doi: 10.3748/wjg.v27.i8.725
Peer-review started: November 10, 2020
First decision: December 3, 2020
Revised: December 13, 2020
Accepted: January 21, 2021
Article in press: January 21, 2021
Published online: February 28, 2021
Processing time: 100 Days and 19.3 Hours
Endoscopic submucosal dissection to treat mucosal and submucosal lesions sometimes results in low rates of microscopically margin-negative (R0) resection. Endoscopic full-thickness resection (EFTR) has a high R0 resection rate and allows for the definitive diagnosis and treatment of selected mucosal and submucosal lesions that are not suitable for conventional resection techniques.
To evaluate the efficacy and safety of EFTR using an over-the-scope clip (OTSC).
This prospective, single-center, non-randomized clinical trial was conducted at the endoscopy center of Shengjing Hospital of China Medical University. The study included patients aged 18-70 years who had gastric or colorectal submucosal tumors (SMTs) (≤ 20 mm in diameter) originating from the muscularis propria based on endoscopic ultrasound (EUS) and patients who had early-stage gastric or colorectal cancer (≤ 20 mm in diameter) based on EUS and computed tomography. All lesions were treated by EFTR combined with an OTSC for wound closure between November 2014 and October 2016. We analyzed patient demographics, lesion features, histopathological diagnoses, R0 resection (negative margins) status, adverse events, and follow-up results.
A total of 68 patients (17 men and 51 women) with an average age of 52.0 ± 10.5 years (32-71 years) were enrolled in this study, which included 66 gastric or colorectal SMTs and 2 early-stage colorectal cancers. The mean tumor diameter was 12.6 ± 4.3 mm. The EFTR procedure was successful in all cases. The mean EFTR procedure time was 39.6 ± 38.0 min. The mean OTSC defect closure time was 5.0 ± 3.8 min, and the success rate of closure for defects was 100%. Histologically complete resection (R0) was achieved in 67 (98.5%) patients. Procedure-related adverse events were observed in 11 (16.2%) patients. The average post-procedure length of follow-up was 48.2 ± 15.7 mo. There was no recurrence during follow-up.
EFTR combined with an OTSC is an effective and safe technique for the removal of select subepithelial and epithelial lesions that are not amenable to conventional endoscopic resection techniques.
Core Tip: A prospective study of endoscopic full-thickness resection (EFTR) combined with an over-the-scope clip (OTSC) was conducted to assess the treatment of mucosal and submucosal lesions that are not amenable to conventional endoscopic resection techniques. The study had a long follow-up period and included a large number of cases, thus providing statistical strength. We found that EFTR combined with an OTSC was a safe and effective treatment modality for mucosal and submucosal lesions that cannot be treated using conventional endoscopic resection techniques.
- Citation: Guo JT, Zhang JJ, Wu YF, Liao Y, Wang YD, Zhang BZ, Wang S, Sun SY. Endoscopic full-thickness resection using an over-the-scope device: A prospective study. World J Gastroenterol 2021; 27(8): 725-736
- URL: https://www.wjgnet.com/1007-9327/full/v27/i8/725.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i8.725
While endoscopic submucosal dissection (ESD) is an established treatment method for gastrointestinal adenomas and early-stage cancer[1], it occasionally results in low rates of microscopically margin-negative (R0) resections. ESD remains controversial for the treatment of submucosal tumors (SMTs) arising from the muscularis propria, because this method has a higher risk of perforation[2-4]. In addition, when lesions are situated at anatomical locations that are difficult to access, ESD can result in high rates of adverse events. Therefore, endoscopic full-thickness resection (EFTR) is the preferred resection technique for this subgroup of tumors.
Closure of the gastrointestinal wall after EFTR is also important. The over-the-scope clip (OTSC) is a metal clip system introduced in Germany in 2008. It has good elasticity, a wingspan of 11-14 mm, strong fastening force and stability, and the ability to grip the full-thickness of the gastrointestinal tract to close more tissues. Previous studies have shown that OTSC can effectively close gastrointestinal wall defects caused by EFTR, but most of these studies had used retrospective data[5,6]. This study aimed to prospectively evaluate the efficacy and safety of EFTR combined with an OTSC for resection of these lesions.
A prospective, single-center, non-randomized clinical trial was conducted at the endoscopy center of Shengjing Hospital of China Medical University between November 2014 and October 2016. This study was approved by the Institutional Review Board and Ethics Committee of China Medical University. The trial registration number is ChiCTR-OPC-14005459.
Patients aged 18-70 years who had gastrointestinal SMTs ≤ 20 mm originating from the muscularis propria based on endoscopic ultrasound (EUS), or early gastric and colorectal cancer lesions ≤ 20 mm without evidence of lymph node metastasis based on EUS and computed tomography (CT) were included in the study. The largest diameter of the tumor was evaluated using EUS before the procedure.
The exclusion criteria included contraindications for endoscopy examination, coagulation disorders, and refusal to provide informed consent.
The primary endpoints included macroscopically complete resection (i.e., the endoscopist determined that there was no evidence of macroscopic residual lesions) and histologically complete resection (R0 resection defined as tumor-free lateral and deep resection margins).
The secondary endpoints were procedure-related adverse events, EFTR time, OTSC closure time, necessity of surgical therapy, evidence of residual or recurrent lesions during follow-up, and other long-term complications associated with OTSC.
A standard single-channel gastroscope (EG29-i10, Pentax, Tokyo, Japan) or colonoscope (EC38-i10M, Pentax, Tokyo, Japan) with a transparent cap was used for the endoscopic procedure. A linear array echoendoscope (EG3870UT; Pentax, Tokyo, Japan) or EUS microprobe (UM-2R, Olympus Corporation, Tokyo, Japan) was used for the evaluation of tumor size, echo characteristics, and the originating layer. A triangle-tipped knife (TT knife, Olympus Corporation, Tokyo, Japan) and insulation-tipped knife (IT knife, Olympus Corporation, Tokyo, Japan) were used for the dissection and resection of tumors, while hot coagulation forceps (FD- 410LR, Olympus Corporation, Tokyo, Japan) were used for gastric or colorectal wall hemostasis. Twin graspers (Ovesco Endoscopy GmbH, Tuebingen, Germany) were used for clamping the two sides of the gastric defect, and metal clips (Boston Resolution, Boston, United States) and the OTSC system (Ovesco Endoscopy GmbH, Tuebingen, Germany) were used to close the defect.
All EFTR procedures were performed in an inpatient setting under general anesthesia with endotracheal intubation with propofol. All patients received prophylactic medication 6 h before surgery with a single dose of an intravenous antibiotic (ceftriaxone, intravenous 1 g). Patients on anticoagulants (clopidogrel, heparin, warfarin, or direct oral anticoagulants) were instructed to discontinue these medications. All patients provided written informed consent.
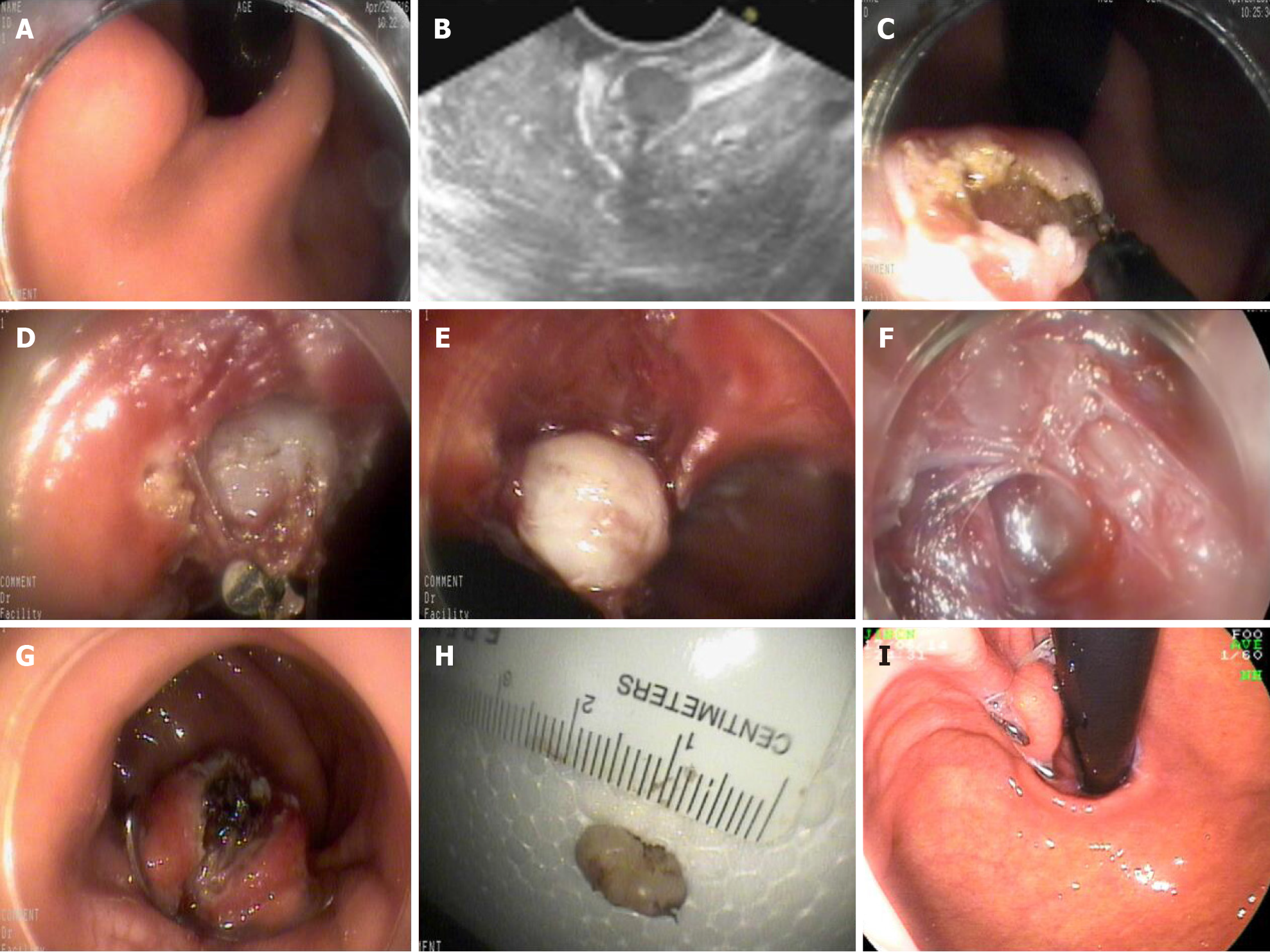
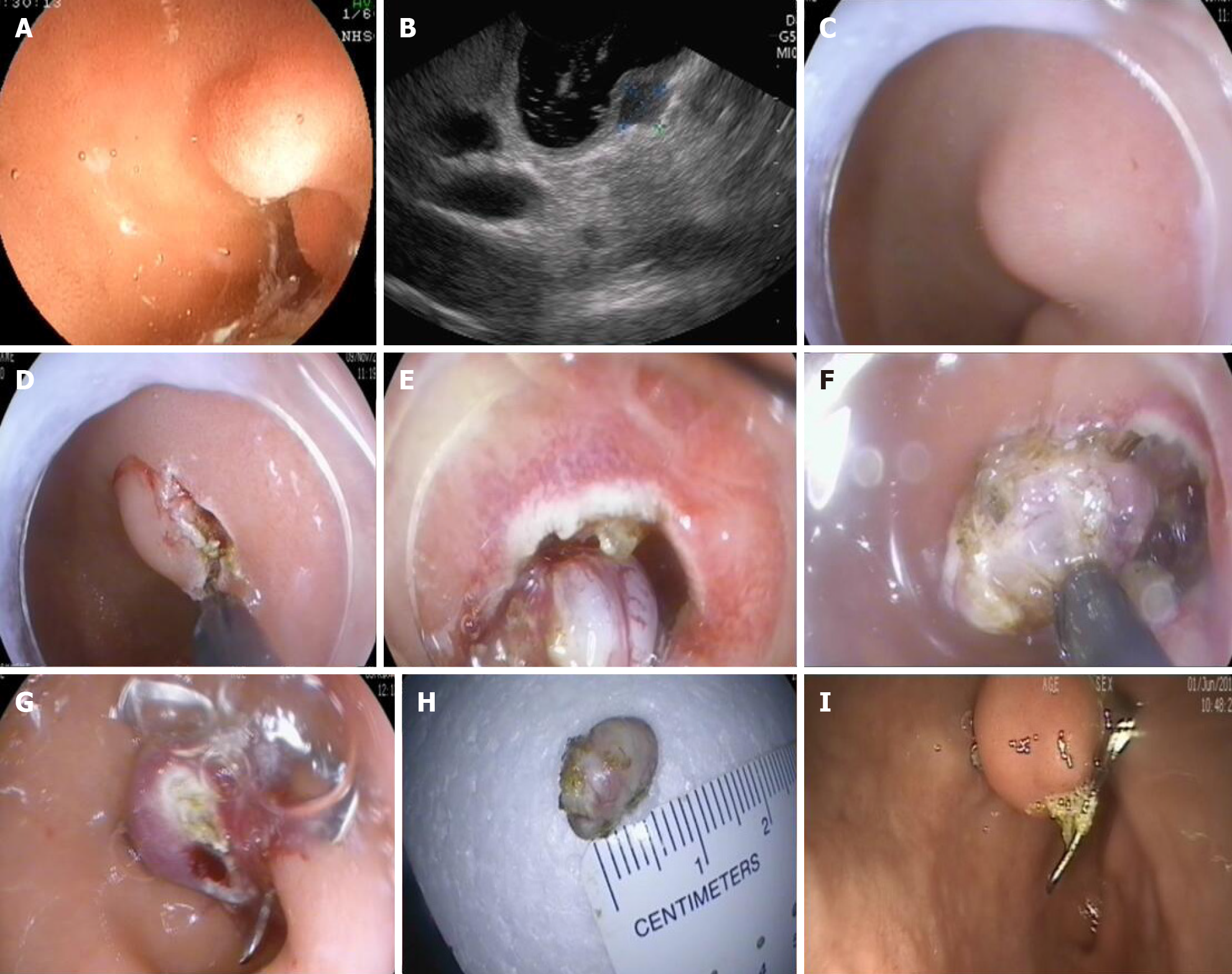
EFTR for SMTs: The gastric or colorectal mucosa and submucosa surrounding the tumor were incised with a triangle tip (TT) knife. EFTR was performed, involving the tumor and surrounding tissues, with an insulated tip (IT) knife. An iatrogenic perforation was created. Hot coagulation forceps were used to stop any bleeding.
EFTR for early cancer: Each patient first underwent a gastroscopy or colonoscopy in order to identify the lesion and to mark its lateral margins with argon plasma coagulation (APC; Erbe APC 300, 25 W; ERBE Elektromedizin GmbH; Germany). The gastric or colorectal mucosa and submucosa surrounding the tumor were incised with a TT knife. EFTR was performed, involving the tumor and surrounding tissues, with an IT knife. An iatrogenic perforation was created. Hot coagulation forceps were used to stop any bleeding.
The gastric or colorectal tissue on either side of the iatrogenic perforation was held with a double clamp or forceps and pulled into the transparent cap of the OTSC device. When both side of the defect were fully absorbed into the transparent cap, the OTSC was released to close the defect. If the defect closure was incomplete, metal clips were used to close the remainder (Figures 1 and 2).
Carbon dioxide was injected throughout the procedure. A 20-mL syringe was used to aspirate free gas from the abdomen during or after the procedure.
All patients were closely observed in the hospital. The postoperative treatment included 24 h of fasting (both food and water) as well as routine administration of antibiotics within 24 h of the procedure. Proton pump inhibitors were needed for patients with upper gastrointestinal resections. If significant hemorrhage occurred, hemostasis was obtained endoscopically or surgically. If peritonitis symptoms were observed, a gastric or intestinal decompression tube was placed. If conservative or endoscopic treatment was unsuccessful, surgical treatment was performed. On postoperative day 2, patients without postoperative bleeding or peritonitis were advanced to a liquid diet.
Patients were scheduled for endoscopy follow-up at 3 mo and at 1, 2, and 3 years after the initial EFTR, in order to observe local healing and determine if the OTSC had disintegrated. The excision site was examined for visual residual or recurrent lesions. If there was evidence of residual or recurrent lesions, a biopsy was performed.
Between November 2014 and October 2016, a total of 79 patients were screened for eligibility at our endoscopy center. Eleven patients were not included in the study. Among them, nine patients chose regular follow-up and two chose surgical operation. We enrolled 68 patients (17 men and 51 women) with an average age of 52 ± 10.5 years (range, 32-71 years). The mean diameter of the tumors was 12.6 ± 4.3 mm (range, 3-20 mm) measured by preoperative EUS. The patient and lesion characteristics are shown in Table 1.
| Patient characteristics (n = 68) | |
| Sex, n (%) | |
| Male | 17 (25) |
| Female | 51 (75) |
| Age, median (range) | 52 ± 10.5 (32-71) |
| Indication for EFTR, n (%) | |
| Submucosal tumor | 66 (97.1) |
| Early cancer | 2 (2.9) |
| Location of lesion, n (%) | |
| Cardia | 5 (7.3) |
| Gastric fundus | 41 (60.2) |
| Gastric body | 12 (17.6) |
| Antrum | 6 (8.8) |
| Duodenum | 1 (1.4) |
| Rectum | 4 (4.4) |
| Maximum diameter of lesion, mean, mm (range) | 20, 12.6 ± 4.3 (3-20) |
In our study, EFTR had a success rate of 100% (68/68). No patients were excluded due to unsuccessful advancement of the endoscope. The average EFTR procedure time was 39.6 ± 38.0 min (range, 5-236 min). The success rate of the defect closure was 100% (68/68). The average OTSC defect closure time was 5.0 ± 3.8 min (range, 2-26 mi). In 67 patients, complete closure of the defect required only one OTSC, and one patient required two OTSCs. Procedural data are shown in Table 2.
| Median procedure time, min (range) | |
| Total procedure time | 53.7 ± 41.5 (12-263) |
| EFTR time | 39.6 ± 38.0 (5-236) |
| OTSC defect closure time | 5.0 ± 3.8 (2-26) |
| Technical success, n (%) | 68 (100) |
| R0 resection, n (%) | 67 (98.5) |
Histologically complete resection (R0) was achieved in 67 (98.5%) patients. Of the 68 lesions included in the study, 66 had gastric or colorectal SMTs, and two had early colorectal cancer. Detailed histology results are shown in Table 3.
| Pathological diagnosis, n (%) | |
| GIST | 42 (61.7) |
| Leiomyoma | 14 (20.5) |
| Schwannoma | 4 (5.8) |
| Ectopic pancreas | 2 (2.9) |
| Endometriosis | 1 (1.4) |
| Fibrolipomatous hyperplasia | 1 (1.4) |
| Colorectal adenocarcinoma | 2 (2.8) |
| Inflammatory myofibroblastic tumor like hyperplasia | 1 (1.4) |
| Hyaline degeneration with calcification | 1 (1.4) |
Of the patients with SMTs, 63.6% (42/66) had gastrointestinal stromal tumors (GISTs). Of the patients with GISTs, 95.2% (40/42) exhibited a low or very low risk mitotic index. The remaining two cases had a moderate risk. Other histological findings in patients with SMTs included leiomyoma (21.2%, 14/66), schwannoma (6.1%, 4/66), and ectopic pancreas (3.0%, 2/66). R0 resection was achieved in all patients with SMTs (100%). Two patients who underwent EFTR had early colorectal cancer. One of these patients underwent additional surgery due to a diagnosis of rectal adenocarcinoma with submucosal infiltration > 2 mm.
Mild adverse events including tolerable abdominal pain, discomfort, and elevated body temperature were observed in 26 (38.2%) patients. Procedure-related adverse events were observed in 11 (16.2%) patients (Table 4). In one patient, delayed bleeding at the resection site occurred on postoperative day 2. The patient was hemodynamically stable, and a blood transfusion was not required. The patient was successfully treated by endoscopic hemostasis with standard clips. Eight patients had a fever > 37.5 °C, and all recovered after the administration of antibiotics. Two patients with upper gastrointestinal EFTR developed localized peritonitis, which was resolved after the placement of a gastric decompression tube.
The average post-procedure length of follow-up was 48.2 ± 15.7 mo. During follow-up, 24 patients (including 22 with stomach lesions and two with lesions in the colorectum) shed their OTSC; one patient developed upper gastrointestinal bleeding, which was stopped using endoscopic therapy. An OTSC of the rectum was removed during an operation in one case. The remaining patients did not shed their OTSCs. There was no recurrence during the follow-up period.
Surgical resection of gastrointestinal tumors is often associated with a high incidence of complications and mortality. The continuous innovation of endoscopic resection techniques has largely led to it replacing surgery as the primary treatment strategy for early gastrointestinal tumors. In surgically resected specimens, the risk of lymph node metastasis from intramucosal carcinoma is very small. Therefore, endoscopic resection is an option for the treatment of intramucosal carcinoma that does not require lymph node dissection.
ESD has recently become a standard therapy[7-9]. However, ESD is not always effective for specific lesions, such as some mucosal lesions with adhesion caused by pathological biopsy or repeated inflammatory stimuli and some SMTs with tight connections to the muscularis propria or exogenous SMTs. EFTR is especially beneficial in the treatment of such types of mucosal and submucosal lesions.
EFTR of early gastric cancer is mainly used for non-lifting lesions due to fibrosis or scarring as well as for lesions with challenging locations that make the ESD procedure difficult. The exposure of the lumen to the abdominal cavity during EFTR for cancerous lesions is controversial due to the potential risk of tumor cell seeding. Maehata et al[10] predicted that cancer cells, including cancer stem cells in early gastric cancers, could easily detach from the source by contact with the cancer surface. To avoid the potential risk of iatrogenic cancer cell seeding into the peritoneum, a non-exposure approach may be optimal in EFTR. The combination of laparoscopic and endoscopic approaches to neoplasia with a non-exposure technique is the most widely reported technique due to concerns about intraperitoneal metastasis[10-12]. Since ESD or laparoscopy is often used in the treatment of early gastric cancer at Shengjing Hospital of China Medical University, no case of early gastric cancer met the inclusion criteria of this study.
Current international guidelines recommend endoscopic therapy for early colorectal cancer with a low risk of lymphatic metastasis[13-15]. For lesions at difficult anatomical sites or lesions that are non-lifting due to scarring, EFTR is an alternative resection technique that expands the possibilities of endoscopic resection[16-21]. A study by Kuellmer et al[22] included 64 cases undergoing EFTR after incomplete resection of a malignant polyp (group 1) and 92 non-lifting lesions (group 2). Technical success was achieved in 144 (92.3%) out of 156 cases with an average procedural time of 42 min. R0 resection was achieved in 112 (71.8%) of 156 patients. Subgroup analysis showed an R0 resection rate of 87.5% in group 1 and 60.9% in group 2 (P < 0.001). They reported that EFTR is technically feasible and safe for the treatment of early colorectal cancers[22]. In our study, EFTR was performed in two patients with early colorectal cancer. One of these patients underwent an additional surgery due to a diagnosis of rectal adenocarcinoma with submucosal infiltration > 2 mm. However, low-risk vs high-risk lesions are difficult to discern before resection, as the criteria are based on histologic features. EFTR is also one method to remove lesions locally in order to provide further pathological diagnosis.
In our study, most cases were SMTs. SMTs originating from the muscularis propria are a good indication for EFTR. Endoscopic resection not only enables sufficient tissue to be obtained for pathological diagnosis but is also curative, as it involves resection of the tumor. EFTR is inevitable for deep or muscularis propria SMTs that are tightly connected. Ye et al[23] evaluated the safety and efficacy of EFTR (n = 51) with defect closure using clips and an endoloop for the resection of gastric subepithelial tumors of the muscularis propria. EFTR was successfully carried out in 50 (98%) patients, with a mean operation time of 52 min[23]. In our preliminary study[24], 23 cases of exposed EFTR were reported. EFTR was successfully carried out in 100% of the cases, and delayed perforation was not observed during follow-up. The average tumor size was 12.1 ± 4.7 mm (range, 6-20 mm)[24]. Li et al[25] reported 28 EFTR procedures for cases of gastric GIST originating from the muscularis propria. The average tumor size was 1.6 ± 0.4 cm, and the en bloc resection rate was 92.9%. In the study, the average EFTR time was 39.6 min (range, 5-236 min). The success rate of defect closure was 100% (68/68), and the R0 resection rate was also 100%. EFTR lasted 236 min in one case. The specific reasons are as follows: (1) The case was at the beginning of the learning curve; (2) The location of the lesion was difficult to operate; and (3) Intraoperative hemostasis took a long time.
EFTR can also be used to treat duodenal SMTs. In a study by Kappelle et al[5], EFTR was performed on 13 lesions in 12 patients: Seven gastric and six duodenal SMTs. Technical success was achieved in 11 (85%) cases. In all the 11 cases, R0 resection was achieved[5]. Ren et al[26] investigated 32 patients with non-ampullary duodenal SMTs who underwent EFTR. The complete resection rate was 100%, and neither delayed bleeding nor fistulas were observed[26]. In our study, a case of duodenal bulb SMT was included, and R0 resection was achieved. The histopathologic diagnosis of this lesion was a low-risk GIST.
There are also retrospective studies with small sample sizes that confirm the efficacy of EFTR in the treatment of colorectal SMTs originating from the muscularis propria. In a study by Albrecht et al[16], R0 resection was achieved in all colorectal SMTs, and 3-mo follow-up data showed no residual tumor. In our study, a case of rectal SMT was included. The histopathologic diagnosis of this lesion was endometriosis.
In our study, pre-procedure evaluation by EUS was necessary. EUS can assess the origin of lesions, invasion depth, size, echogenic characteristics, and the presence of enlarged lymph nodes, as well as help make a preliminary judgment about the nature of the lesion to assess the feasibility of EFTR[27-32].
To carry out EFTR safely, reliable endoscopic suturing devices are necessary to close the resected openings. There are several methods for closure after EFTR including OTSC. The defect in the wall can be closed with endoclips, clips combined with an endoloop, or a suturing device. In a study conducted by Li et al[25], the purse-string suture method with metal clips and nylon suture snares was used for the closure of gastric wall defects. The mean closure time was 54.5 ± 27.5 min[25]. In our study, the success rate of the defect closure was 100% (68/68). The average OTSC defect closure time was 5.01 ± 3.83 min (range, 2-26 min). The closure time was much shorter than that in previous reports. Several studies report OTSC-assisted EFTR, which included OTSC closure prior to EFTR[6,22,33,34]. This method is time saving, but can only be used on small lesions.
The use of an OTSC to close a 2-cm (diameter) gastric wall defect has been proven to be safe and effective. Thus, we chose the lesions with a diameter of less than 2 cm in the study.
Whether small GISTs require endoscopic resection remains controversial. The National Comprehensive Cancer Network guidelines recommend that in the case of small GISTs (≤ 2 cm) lacking high-risk EUS features [e.g., irregular border, lobulation, internal heterogeneous echogenicity, anechoic (cystic) spaces, hyperechoic foci, and tumor extraluminal growth], conservative follow-up should be performed. However, the European Society for Medical Oncology indicates that surgery should be the standard treatment for small histologically confirmed GISTs. Furthermore, some researchers have even proposed that all GISTs have malignant potential and, thus, surgical or endoscopic resection should be performed on detected GISTs.
Since endoscopic resection is a simple and minimally invasive method of obtaining histological samples, this method is recommended even for small gastric tumors originating from the muscularis propria. In this way, patients not only avoid the burden of survival and follow-up with a tumor, but also obtain an accurate diagnosis.
In our study, the average post-procedure follow-up time was 48.2 mo. During follow-up, 24 patients (including 22 with lesions in the stomach and two with lesions in the rectum) shed the OTSC. One of those 24 patients developed upper gastrointestinal bleeding due to OTSC loss. The bleeding was successfully treated using endoscopic therapy. OTSC of the rectum was removed during an operation in one case. The rest of the patients did not shed OTSC and remain in follow-up. OTSC can spontaneously fall off, but according to the current literature, the fall off time varies according to the location[35,36]. Li et al[35] found that 80% of intestinal OTSCs could spontaneously fall off within 3 mo. Shoar et al[37] reported cases of long-term attachment of OTSC in the stomach and found that 27.8% of all OTSCs would spontaneously fall off and that the possibility of falling off was significantly related to the original position of the clamp.
Long-term gastric attachment appears to be safe even if no spontaneous detachment occurs. However, removal of the clamp is necessary when complications associated with the clip (such as localized inflammation, ulcers, or luminal obstruction) occur, or when the patient strongly requests it. Several techniques can now be used to remove selective clamps, including argon arc beam, YAG laser, bipolar cutting equipment, and cold salt solution technology[36].
This study had some limitations regarding the study design. It was a single center study, and had a small sample size for early colorectal cancer. This new technique must be further investigated in larger, multicenter, randomized, controlled studies.
In conclusion, our study demonstrated that EFTR combined with OTSC is a safe and effective technique for the resection of small SMTs and early gastrointestinal cancers that are not suitable for ESD, with excellent R0 resection rates.
Endoscopic submucosal dissection to treat mucosal and submucosal lesions often results in low rates of microscopically margin-negative (R0) resection. Endoscopic full-thickness resection (EFTR) has a high R0 resection rate and allows for the definitive diagnosis and treatment of selected mucosal and submucosal lesions that are not suitable for conventional resection techniques. The aim of the study was to evaluate the efficacy and safety of EFTR using an over-the-scope clip (OTSC).
Data on the safety and efficacy of EFTR combined with OTSC in the treatment of gastrointestinal epithelial and subepithelial lesions have been lacking in high-level prospective studies.
We prospectively investigated the safety and efficacy of EFTR combined with OTSC in the treatment of epithelial and subepithelial tumors, as well as OTSC shedding during long-term follow-up, in order to provide a high level of clinical basis for the further use of this method.
A single-center prospective study was performed.
A total of 68 patients (17 men and 51 women) with an average age of 52.0 ± 10.5 years (range, 32-71 years) were enrolled in this study, which included 66 gastric or colorectal submucosal tumors and 2 early-stage colorectal cancers. The mean tumor diameter was 12.6 ± 4.3 mm. The EFTR procedure was successful in all cases. The mean EFTR procedure time was 39.6 ± 38.0 min. The mean OTSC defect closure time was 5.0 ± 3.8 min, and the success rate of closure for defects was 100%. Histologically complete resection (R0) was achieved in 67 (98.5%) patients. Procedure-related adverse events were observed in 11 (16.2%) patients. The average post-procedure length of follow-up was 48.2 ± 15.7 mo. There was no recurrence during follow-up.
EFTR combined with OTSC is an effective and safe technique for the removal of select subepithelial and epithelial lesions that are not amenable to conventional endoscopic resection techniques.
EFTR combined with OTSC is an effective and safe technique for the removal of select subepithelial and epithelial lesions that are not amenable to conventional endoscopic resection techniques.
We thank all the doctors who participated in this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imaeda H S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 476] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 2. | Chun SY, Kim KO, Park DS, Lee IJ, Park JW, Moon SH, Baek IH, Kim JH, Park CK, Kwon MJ. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc. 2013;27:3271-3279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | He Z, Sun C, Wang J, Zheng Z, Yu Q, Wang T, Chen X, Liu W, Wang B. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: a single-center study of 144 cases. Scand J Gastroenterol. 2013;48:1466-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M, Starzyńska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Kappelle WFW, Backes Y, Valk GD, Moons LMG, Vleggaar FP. Endoscopic full-thickness resection of gastric and duodenal subepithelial lesions using a new, flat-based over-the-scope clip. Surg Endosc. 2018;32:2839-2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Al-Bawardy B, Rajan E, Wong Kee Song LM. Over-the-scope clip-assisted endoscopic full-thickness resection of epithelial and subepithelial GI lesions. Gastrointest Endosc. 2017;85:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Gincul R, Ponchon T, Napoleon B, Scoazec JY, Guillaud O, Saurin JC, Ciocirlan M, Lepilliez V, Pioche M, Lefort C, Adham M, Pialat J, Chayvialle JA, Walter T. Endoscopic treatment of sporadic small duodenal and ampullary neuroendocrine tumors. Endoscopy. 2016;48:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc. 2014;6:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 9. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 928] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 10. | Maehata T, Goto O, Takeuchi H, Kitagawa Y, Yahagi N. Cutting edge of endoscopic full-thickness resection for gastric tumor. World J Gastrointest Endosc. 2015;7:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Goto O, Shimoda M, Sasaki M, Kiguchi Y, Mitsunaga Y, Akimoto T, Ochiai Y, Fujimoto A, Maehata T, Nishizawa T, Takeuchi H, Kitagawa Y, Kameyama K, Yahagi N. Potential for peritoneal cancer cell seeding in endoscopic full-thickness resection for early gastric cancer. Gastrointest Endosc. 2018;87:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Niimi K, Aikou S, Kodashima S, Yagi K, Oya S, Yamaguchi D, Yamashita H, Yamamichi N, Fujishiro M, Koike K, Seto Y. Video of the Month: A Novel Endoscopic Full-Thickness Resection for Early Gastric Cancer. Am J Gastroenterol. 2015;110:1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | ASGE Standards of Practice Committee. , Fisher DA, Shergill AK, Early DS, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Evans JA, Fanelli RD, Foley KQ, Fonkalsrud L, Hwang JH, Jue T, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD. Role of endoscopy in the staging and management of colorectal cancer. Gastrointest Endosc. 2013;78:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 766] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 15. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara KI, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 437] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 16. | Albrecht H, Raithel M, Braun A, Nagel A, Stegmaier A, Utpatel K, Schäfer C. Endoscopic full-thickness resection (EFTR) in the lower gastrointestinal tract. Tech Coloproctol. 2019;23:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Ahlenstiel G, Hourigan LF, Brown G, Zanati S, Williams SJ, Singh R, Moss A, Sonson R, Bourke MJ; Australian Colonic Endoscopic Mucosal Resection (ACE) Study Group. Actual endoscopic vs predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. 2014;80:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | Jayanna M, Burgess NG, Singh R, Hourigan LF, Brown GJ, Zanati SA, Moss A, Lim J, Sonson R, Williams SJ, Bourke MJ. Cost Analysis of Endoscopic Mucosal Resection vs Surgery for Large Laterally Spreading Colorectal Lesions. Clin Gastroenterol Hepatol 2016; 14: 271-8. e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (2)] |
| 19. | Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 407] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 20. | Zhao B, Mei D, Luo R, Lu H, Bao S, Xu H, Huang B. Clinicopathological features, risk of lymph node metastasis and survival outcome of synchronous multiple early gastric cancer. Clin Res Hepatol Gastroenterol. 2020;44:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Horiuchi Y, Ida S, Yamamoto N, Nunobe S, Ishizuka N, Yoshimizu S, Ishiyama A, Yoshio T, Hirasawa T, Tsuchida T, Kumagai K, Ohashi M, Sano T, Fujisaki J. Feasibility of further expansion of the indications for endoscopic submucosal dissection in undifferentiated-type early gastric cancer. Gastric Cancer. 2020;23:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Kuellmer A, Mueller J, Caca K, Aepli P, Albers D, Schumacher B, Glitsch A, Schäfer C, Wallstabe I, Hofmann C, Erhardt A, Meier B, Bettinger D, Thimme R, Schmidt A; FTRD study group. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc 2019; 89: 1180-1189. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Ye LP, Yu Z, Mao XL, Zhu LH, Zhou XB. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc. 2014;28:1978-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Guo J, Liu Z, Sun S, Liu X, Wang S, Ge N, Wang G, Qi Y. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc. 2015;29:3356-3362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Li J, Meng Y, Ye S, Wang P, Liu F. Usefulness of the thread-traction method in endoscopic full-thickness resection for gastric submucosal tumor: a comparative study. Surg Endosc. 2019;33:2880-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Ren Z, Lin SL, Zhou PH, Cai SL, Qi ZP, Li J, Yao LQ. Endoscopic full-thickness resection (EFTR) without laparoscopic assistance for nonampullary duodenal subepithelial lesions: our clinical experience of 32 cases. Surg Endosc. 2019;33:3605-3611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Antonini F, Delconte G, Fuccio L, De Nucci G, Fabbri C, Armellini E, Frazzoni L, Fornelli A, Magarotto A, Mandelli E, Occhipinti P, Masci E, Manes G, Macarri G. EUS-guided tissue sampling with a 20-gauge core biopsy needle for the characterization of gastrointestinal subepithelial lesions: A multicenter study. Endosc Ultrasound. 2019;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Pesenti C, Bories E, Caillol F, Ratone JP, Godat S, Monges G, Poizat F, Raoul JL, Ries P, Giovannini M. Characterization of subepithelial lesions of the stomach and esophagus by contrast-enhanced EUS: A retrospective study. Endosc Ultrasound. 2019;8:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Cazacu IM, Singh BS, Luzuriaga Chavez AA, Koduru P, Ejaz S, Weston BR, Ross WA, Lee JH, Roy-Chowdhuri S, Bhutani MS. EUS and EUS-guided FNA/core biopsies in the evaluation of subepithelial lesions of the lower gastrointestinal tract: 10-year experience. Endosc Ultrasound. 2020;9:329-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Okuwaki K, Masutani H, Kida M, Yamauchi H, Iwai T, Miyata E, Hasegawa R, Kaneko T, Imaizumi H, Watanabe M, Kurosu T, Tadehara M, Adachi K, Tamaki A, Koizumi W. Diagnostic efficacy of white core cutoff lengths obtained by EUS-guided fine-needle biopsy using a novel 22G franseen biopsy needle and sample isolation processing by stereomicroscopy for subepithelial lesions. Endosc Ultrasound. 2020;9:187-192. [PubMed] |
| 31. | Fabbri C, Fornelli A, Fuccio L, Giovanelli S, Tarantino I, Antonini F, Liotta R, Frazzoni L, Gusella P, La Marca M, Barresi L, Macarri G, Traina M, De Biase D, Fiorino S, Jovine E, Larghi A, Cennamo V. High diagnostic adequacy and accuracy of the new 20G procore needle for EUS-guided tissue acquisition: Results of a large multicentre retrospective study. Endosc Ultrasound. 2019;8:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Hu J, Ge N, Wang S, Liu X, Guo J, Wang G, Sun S. The Role of Endoscopic Ultrasound and Endoscopic Resection for Gastric Glomus: A Case Series and Literature Review. J Transl Int Med. 2019;7:149-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Andrisani G, Pizzicannella M, Martino M, Rea R, Pandolfi M, Taffon C, Caricato M, Coppola R, Crescenzi A, Costamagna G, Di Matteo FM. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: A single-centre study. Dig Liver Dis. 2017;49:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Lagoussis P, Soriani P, Tontini GE, Neumann H, Pastorelli L, de Nucci G, Vecchi M. Over-the-scope clip-assisted endoscopic full-thickness resection after incomplete resection of rectal adenocarcinoma. Endoscopy. 2016;48 Suppl 1 UCTN:E59-E60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Li P, Ma B, Gong S, Zhang X, Li W. Efficacy and safety of endoscopic full-thickness resection in the colon and rectum using an over-the-scope device: a meta-analysis. Surg Endosc. 2021;35:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Hu J, Yang Y, Ge N, Wang S, Guo J, Liu X, Wang G, Sun S. Long-term assessment of over-the-scope clip (OTSC) behavior after gastric application. Minim Invasive Ther Allied Technol. 2020;29:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Shoar S, Poliakin L, Khorgami Z, Rubenstein R, El-Matbouly M, Levin JL, Saber AA. Efficacy and Safety of the Over-the-Scope Clip (OTSC) System in the Management of Leak and Fistula After Laparoscopic Sleeve Gastrectomy: a Systematic Review. Obes Surg. 2017;27:2410-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |