Published online Dec 28, 2021. doi: 10.3748/wjg.v27.i48.8374
Peer-review started: July 29, 2021
First decision: October 16, 2021
Revised: October 25, 2021
Accepted: December 16, 2021
Article in press: December 16, 2021
Published online: December 28, 2021
Processing time: 147 Days and 9.9 Hours
In a recent systematic review and meta-analysis of observational studies, the author found potential errors in the selection and extraction processes. The recalculated summary relative risks and the results of a dose-response meta-analysis showed that oral contraceptive use may not be associated with the risk of pancreatic cancer in women.
Core Tip: A systematic review and meta-analysis of observational studies conducted recently concluded that oral contraceptive use was associated with a decreased risk of pancreatic cancer in women. However, the author found potential errors in the selection and extraction processes. The recalculated summary relative risks and the results of a dose-response meta-analysis showed that oral contraceptive use may not be associated with the risk of pancreatic cancer in women. As this conclusion contradicted that reported recently, it is necessary to re-evaluate the direction and statistical significance of this risk through an updated meta-analysis in the future.
- Citation: Bae JM. Use of oral contraceptives and risk of pancreatic cancer in women: A recalculated meta-analysis of prospective cohort studies. World J Gastroenterol 2021; 27(48): 8374-8377
- URL: https://www.wjgnet.com/1007-9327/full/v27/i48/8374.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i48.8374
I recently read the systematic review and meta-analysis conducted by Ilic et al[1] comprising 10 case-control studies and 11 cohort studies, which concluded that the use of oral contraceptives (OCU) was associated with a decreased risk of pancreatic cancer in women (PCW) [summary relative risk (sRR) = 0.85; 95% confidence intervals (CI) = 0.73-0.98; P = 0.03]. Interestingly, the subgroup analysis according to the study design showed no statistical significance in case-control studies but showed borderline statistical significance in cohort studies (sRR = 0.84; 95%CI = 0.70-1.00; P = 0.05).
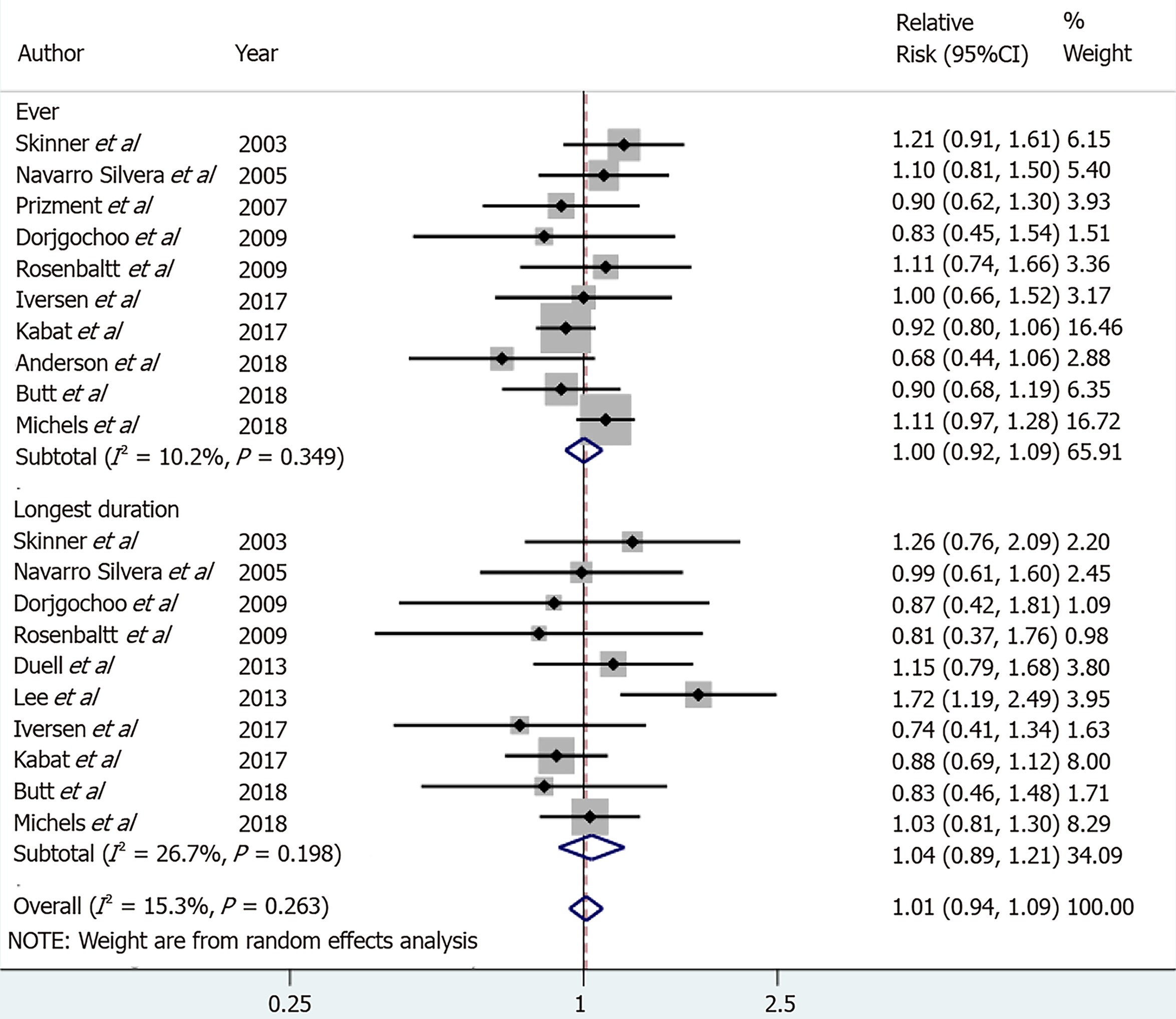
However, while reviewing the results of the 11 selected cohort studies, I found the following potential errors. First, among the 11 selected studies, the study by Teras et al[2] was a cohort study that analyzed the mortality of PCW; therefore, excluding this study would be valid based on the research hypothesis; second, it would be necessary to include the two cohort studies[3,4] that were considered in other studies on the risk of various cancers associated with OCU[5,6]; finally, in the two studies that did not provide an RR for the ever group[7,8], the RR's direction was opposite to that of the forest plot shown in the study by Ilic et al[1].
Considering these issues, I recalculated the sRR of the longest duration (LD) group as well as the ever group. The statistical significance disappeared in both groups, and the sRRs were 1 or higher (Figure 1). Egger’s test was performed to evaluate publication bias, and no statistical significance was noted in either group (P = 0.439 and 0.817 in the ever group and LD group, respectively).
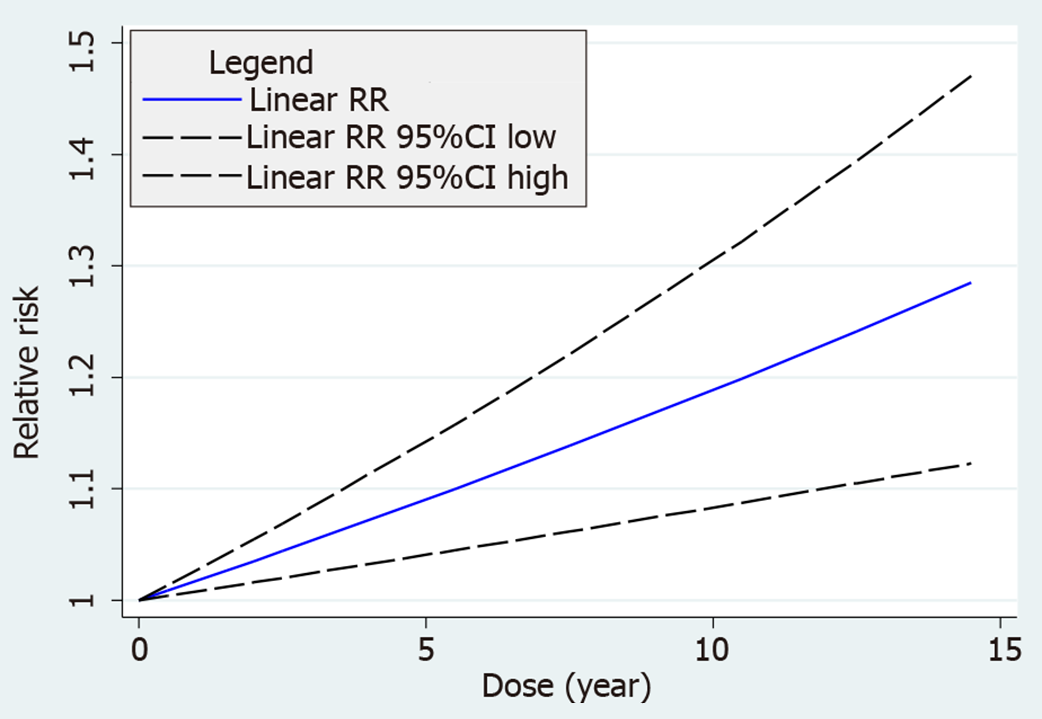
Eight of the 12 selected cohorts[3,7-13] provided the information necessary for performing a dose-response meta-analysis. A two-stage random-effects dose-response model was used with a dosing unit of 1 year (P of goodness-of-fit = 0.041). The results showed borderline statistical significance with a linear dose-response relationship between OCU duration and PCW risk (sRR = 1.015; 95%CI = 0.999-1.030; P = 0.057) (Figure 2).
Based on the results of the recalculated sRRs and DRMA, the OCU may not be associated with the risk of PCW. Because my conclusion contradicts that reported by Ilic et al[1], it is necessary to re-evaluate the direction and statistical significance of risk through an updated meta-analysis in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tung TH S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Ilic M, Milicic B, Ilic I. Association between oral contraceptive use and pancreatic cancer risk: A systematic review and meta-analysis. World J Gastroenterol. 2021;27:2643-2656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 2. | Teras LR, Patel AV, Rodriguez C, Thun MJ, Calle EE. Parity, other reproductive factors, and risk of pancreatic cancer mortality in a large cohort of U.S. women (United States). Cancer Causes Control. 2005;16:1035-40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Rosenblatt KA, Gao DL, Ray RM, Nelson ZC, Wernli KJ, Li W, Thomas DB. Oral contraceptives and the risk of all cancers combined and site-specific cancers in Shanghai. Cancer Causes Control. 2009;20:27-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners' Oral Contraception Study. Am J Obstet Gynecol. 2017;216:580.e1-580.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 5. | Wu L, Zhu J. Linear reduction in thyroid cancer risk by oral contraceptive use: a dose-response meta-analysis of prospective cohort studies. Hum Reprod. 2015;30:2234-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Rodriguez-Lara V, Avila-Costa MR. An Overview of Lung Cancer in Women and the Impact of Estrogen in Lung Carcinogenesis and Lung Cancer Treatment. Front Med (Lausanne). 2021;8:600121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Duell EJ, Travier N, Lujan-Barroso L, Dossus L, Boutron-Ruault MC, Clavel-Chapelon F, Tumino R, Masala G, Krogh V, Panico S, Ricceri F, Redondo ML, Dorronsoro M, Molina-Montes E, Huerta JM, Barricarte A, Khaw KT, Wareham NJ, Allen NE, Travis R, Siersema PD, Peeters PH, Trichopoulou A, Fragogeorgi E, Oikonomou E, Boeing H, Schuetze M, Canzian F, Lukanova A, Tjønneland A, Roswall N, Overvad K, Weiderpass E, Gram IT, Lund E, Lindkvist B, Johansen D, Ye W, Sund M, Fedirko V, Jenab M, Michaud DS, Riboli E, Bueno-de-Mesquita HB. Menstrual and reproductive factors in women, genetic variation in CYP17A1, and pancreatic cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int J Cancer. 2013;132:2164-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Lee E, Horn-Ross PL, Rull RP, Neuhausen SL, Anton-Culver H, Ursin G, Henderson KD, Bernstein L. Reproductive factors, exogenous hormones, and pancreatic cancer risk in the CTS. Am J Epidemiol. 2013;178:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Skinner HG, Michaud DS, Colditz GA, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev. 2003;12:433-438. [PubMed] |
| 10. | Navarro Silvera SA, Miller AB, Rohan TE. Hormonal and reproductive factors and pancreatic cancer risk: a prospective cohort study. Pancreas. 2005;30:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Kabat GC, Kamensky V, Rohan TE. Reproductive factors, exogenous hormone use, and risk of pancreatic cancer in postmenopausal women. Cancer Epidemiol. 2017;49:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Butt SA, Lidegaardi Ø, Skovlund C, Hannaford PC, Iversen L, Fielding S, Mørch LS. Hormonal contraceptive use and risk of pancreatic cancer-A cohort study among premenopausal women. PLoS One. 2018;13:e0206358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Michels KA, Brinton LA, Pfeiffer RM, Trabert B. Oral Contraceptive Use and Risks of Cancer in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2018;187:1630-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |