Published online Dec 28, 2021. doi: 10.3748/wjg.v27.i48.8357
Peer-review started: July 12, 2021
First decision: October 3, 2021
Revised: October 9, 2021
Accepted: November 30, 2021
Article in press: November 30, 2021
Published online: December 28, 2021
Processing time: 164 Days and 19.7 Hours
New prognostic factors have been reported in patients with metastatic or recurrent gastric cancer (MRGC), necessitating modifications to the previous prognostic model.
To develop a new model, MRGC patients who received fluoropyrimidines/ platinum doublet chemotherapy between 2008 and 2015 were analyzed.
A total of 1883 patients was divided into a training set (n = 937) and an independent validation set (n = 946).
Multivariate analysis showed that the following six factors were associated with poor overall survival (OS) in the training set: Eastern Cooperative Oncology Group performance score ≥ 2 and bone metastasis (2 points each), peritoneal metastasis, high alkaline phosphatase level, low albumin level, and high neutrophil-lymphocyte ratio (1 point each). A prognostic model was developed by stratifying patients into good (0-1 point), moderate (2-3 points), and poor (≥ 4 points) risk groups. In the validation set, the median OS of the three risk groups was 15.8, 10.1, and 5.7 mo, respectively, and those differences were significant (P < 0.001).
We identified six factors readily measured in clinical practice that are predictive of poor prognosis in patients with MRGC. The new model is simpler than the old and more easily predicts OS.
Core Tip: A new prognostic model for patients with metastatic or recurrent gastric cancer was developed using six clinicopathological elements (poor Eastern Cooperative Oncology Group performance score, bone metastasis, peritoneal metastasis, high alkaline phosphatase level, low albumin level, and high neutrophil-lymphocyte ratio).
- Citation: Koo DH, Ryu MH, Lee MY, Moon MS, Kang YK. New prognostic model for patients with advanced gastric cancer: Fluoropyrimidine/platinum doublet for first-line chemotherapy. World J Gastroenterol 2021; 27(48): 8357-8369
- URL: https://www.wjgnet.com/1007-9327/full/v27/i48/8357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i48.8357
Gastric cancer is one of the most common causes of cancer-related mortality worldwide and the fifth-ranked cancer in terms of associated mortality in Korea[1,2]. When gastric cancer is diagnosed at an advanced stage or in recurrent status, systemic therapy is considered the primary treatment; however, its outcome often is unsatisfactory[1,3].
Many novel agents that inhibit several pathways, combination strategies, and strict patient selection criteria are being evaluated in clinical trials to improve patient response to systemic therapies and to achieve better clinical outcomes[4]. It is necessary to allocate evenly patients with similar clinical characteristics and expected survival times to derive reliable results from clinical trials. Therefore, many investigators have attempted to develop prognostic models to predict accurate overall survival (OS). Nonetheless, existing prognostic models have certain limitations, such as lack of validation[5] or enrolling patients who do not represent patients in real practice[6]. In addition, some patients were included regardless of type of chemotherapy (e.g., single, doublet, or triplet chemotherapy with/without trastuzumab)[7].
Systemic chemotherapy for metastatic or recurrent gastric cancer (MRGC) has undergone significant changes in terms of standard treatment. Although various kinds of drugs have been trialed for use as first-line chemotherapy[8], the fluoropyrimidines plus platinum combination doublet has become the standard of care[9]. Second-line chemotherapy has emerged as another standard treatment[10]. The use of immuno-oncology agents has been accepted as a standard of care during third-line treatment and is emerging as a standard of care in the first-line setting based on positive results[11,12]. Furthermore, the use of human epidermal growth factor receptor 2 (HER2)-targeted therapies in select patients has shown excellent therapeutic efficacy and prolonged survival[13,14]. Overall, patient prognosis varies according to type of treatment[9]. Therefore, prognostic factors should be investigated in each treatment group, particularly patients who receive fluoropyrimidine/platinum doublet chemotherapy, which is considered the standard first-line treatment for HER2-negative MRGC.
Early in the 2000s, we developed a prognostic model for MRGC[7]. That model used a scoring system with eight prognostic factors [Eastern Cooperative Oncology Group (ECOG) performance score (PS) ≥ 2, bone metastasis (2 points each), no gastrectomy, peritoneal metastasis, lung metastasis, alkaline phosphatase (ALP) > 120 IU/L, albumin < 3.3 g/dL, and total bilirubin > 1.2 mg/dL (1 point each)], and patients were divided into good (0-1 point), moderate (2-3 points), and poor (≥ 4 points) risk groups. However, those factors were identified when few active chemotherapeutic agents were available and no standard chemotherapy had been established. Furthermore, those eight factors might need to be reduced to enable easier prognostic model application in clinical practice.
The neutrophil-lymphocyte ratio (NLR) is a representative blood marker of the systemic inflammatory response that reflects tumor progression, invasion, and metastasis in cancer patients[15]. The NLR is a relatively new prognostic factor that has been applied to several solid tumors[16]. Recent studies have demonstrated a close relationship between NLR status and poor prognosis in MRGC; even NLR changes during immuno-oncologic therapy can predict poor outcomes[17]. In addition, a recent meta-analysis reported that histologic type was a significant variable for OS in the first-line treatment setting[18].
Therefore, we modified our previous prognostic model by introducing NLR and histology using a cohort of MRGC patients who received first-line fluoropyrimidine/ platinum doublet chemotherapy, and we validated our new model in a different cohort.
We previously reported trends in chemotherapy patterns and survival in MRGC patients during the 16 years from 2000-2015, separated into four-year intervals[9]. During the last two of those intervals (2008-2015), more than 60% of MRGC patients received doublet treatment, and more than 55% underwent second-/third-line anticancer therapies. We developed our new model from those recent cohorts. The Stomach Cancer Registry was examined to identify all patients who received first-line palliative chemotherapy for advanced gastric cancer at Asan Medical Center (Seoul, South Korea) between January 2008 and December 2015. Patients aged 18 years or older with histologically confirmed adenocarcinoma of the stomach who received at least one palliative chemotherapy cycle were included. Patients were excluded if they received treatment other than doublet chemotherapy (such as single, triplet, or doublet with trastuzumab) or a novel agent in clinical trials, if they had a history of other malignancies, if they started first-line chemotherapy at another hospital, or if they underwent R1 resection for microscopic residual tumors just before chemotherapy. Of the 2931 patients screened, 1883 met our criteria. Patients’ medical records, stored in a prospectively collected registry, were reviewed for demographic data, tumor characteristics, treatment types, treatment responses, and survival. Patients were followed until the date of death or cessation of follow-up in October 2018. The Institutional Review Board of Asan Medical Center approved the study protocol (2020-0574). Our analysis was a retrospective design using fully anonymized data, so the IRB waived the requirement for informed consent.
Model development and validation were based on a split-sample method according to time period. During the last four-year period (2012-2015), trastuzumab in HER2-positive MRGC had been accepted as a standard of care in Korea, and ramucirumab and immunotherapy had been introduced as second-/third-line anticancer therapies. Therefore, study participants were separated by treatment period and assigned to a training set (2012-2015; n = 937) or an independent validation set (2008-2011; n = 946). The prognostic model was developed using the training set. OS was measured from the date of first-line chemotherapy until death from any cause. Progression-free survival (PFS) was measured from the date of first-line chemotherapy until tumor progression or death from any cause other than the cancer. The Kaplan–Meier method was used to estimate OS and PFS. Laboratory variables were dichotomized, using the normal value for each as the cutoff point, and survival rates were compared using the log-rank test. NLR was defined as the neutrophil count divided by the lymphocyte count. The sensitivity and specificity values of NLR were evaluated in the training set using receiver operating characteristic (ROC) curve analysis [area under the ROC curve (AUC): 0.651; 95% confidence interval (CI): 0.60-0.71]. The optimal value of NLR was 3.11 (sensitivity: 41.2%; specificity: 83.1%) according to Youden's J statistic. We selected 3.0 as the cutoff value, which had sensitivity and specificity values of 42.6% and 80.9%, respectively, for all further analyses (Supplementary Figure 1). We developed a new prognostic model by adding and deleting variables from our previous model, analyzing those variables through univariate analyses, and performing multivariate analysis using a Cox proportional hazards regression model. A risk score based on the hazard ratio (HR) was developed from the final multivariate model and validated using the validation set. A nomogram to predict OS probability was established in the training set, and its calibration was accomplished by comparing the predicted and observed probabilities. The prediction accuracy of the old and new prognostic models was compared using Harrell’s C-index; an ROC curve analysis; and a decision curve analysis (DCA), which is a method for evaluating prognostic strategies that can visualize the clinical effectiveness of a prediction model[19]. A two-sided P value < 0.05 was considered statistically significant, and 95%CIs were calculated. All statistical analyses were performed using R language (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) and the Statistical Package for the Social Sciences version 25.0 (IBM Corporation, Armonk, NY, United States).
A total of 1883 patients received palliative doublet chemotherapy as first-line treatment for MRGC between 2008 and 2015. Overall, 1746 patients (92.7%) died, and the median survival time was 11.9 mo (95%CI: 11.3-12.5). The median follow-up duration of the 137 surviving patients was 54.6 mo (interquartile range: 35.7-84.3 mo). When we compared patient characteristics between training and validation sets, proportion of men, histology findings, and occurrence of liver metastasis differed significantly between the two groups (Table 1).
| Clinical characteristics | Training set (2012-2015), n = 937 | Validation set (2008-2011), n = 946 | P value |
| Sex, male, n (%) | 583 (62.2) | 637 (67.3) | 0.020 |
| Age | |||
| Median, range | 56 (19-91) | 57 (20-85) | 0.785 |
| ≥ 65 yr, n (%) | 257 (27.4) | 259 (27.4) | 0.981 |
| ECOG PS, n (%) | |||
| 0/1 | 799 (85.6) | 817 (86.6) | 0.531 |
| 2/3 | 134 (14.4) | 126 (13.4) | |
| Prior gastrectomy performed | 389 (41.5) | 412 (43.6) | 0.372 |
| Histology, n (%) | |||
| WD/MD | 212 (22.6) | 256 (27.1) | < 0.001 |
| PD/SRC/undifferentiated | 691 (73.7) | 590 (62.4) | |
| Unclassified | 34 (3.6) | 100 (10.6) | |
| Status, n (%) | |||
| Recurrent | 318 (33.9) | 334 (35.3) | 0.533 |
| Initial metastatic | 619 (66.1) | 612 (64.7) | |
| Metastasis No., 2 or more | 385 (41.5) | 363 (38.9) | 0.249 |
| Peritoneal metastasis | 518 (55.6) | 524 (55.9) | 0.902 |
| Liver metastasis | 160 (17.2) | 226 (24.1) | < 0.001 |
| Lung metastasis | 45 (4.9) | 43 (4.6) | 0.795 |
| PALN metastasis | 346 (37.3) | 352 (37.5) | 0.942 |
| Bone metastasis | 93 (10.0) | 70 (7.5) | 0.051 |
| ALP > 120 IU/L, n (%) | 201 (21.5) | 197 (21.2) | 0.868 |
| Albumin < 3.3 g/dL, n (%) | 279 (29.8) | 249 (26.8) | 0.150 |
| Total bilirubin > 1.2 mg/dL, n (%) | 62 (6.6) | 77 (8.3) | 0.177 |
| NLR ≥ 3, n (%) | 381 (40.7) | 375 (40.3) | 0.881 |
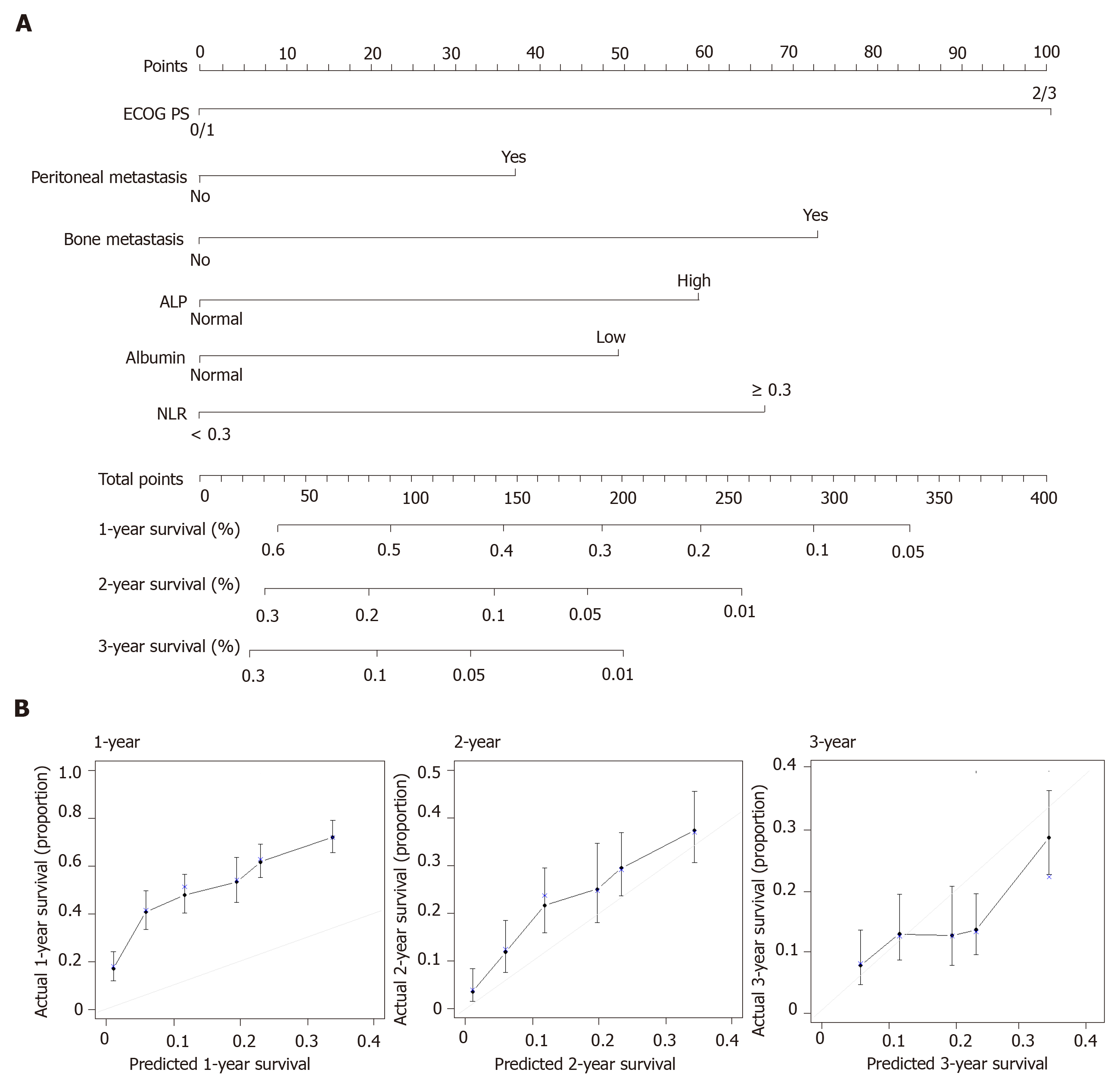
In the training set of 937 patients, 848 (90.5%) died. Univariate analyses for OS were performed for NLR (≥ 3 vs < 3), histologic type (poorly differentiated/signet-ring cell/undifferentiated vs well or moderately differentiated), and the eight factors in the previous model. A high NLR was statistically significant in the training set, but poor histology, no prior gastrectomy, lung metastases, and high total bilirubin were not. Multivariate analysis confirmed that six factors were significantly associated with poor OS (Table 2): Poor ECOG PS, peritoneal metastasis, bone metastasis, high ALP level, low albumin level, and high NLR. Risk scores were assigned based on HRs from the final multivariate model, with two points awarded for HR > 1.5 and one point awarded for HR < 1.5. Based on the resulting scores, patients were assigned to three risk categories: good (0-1 point), moderate (2-3 points), and poor (≥ 4 points). The C-index for the new model was 0.657 (95%CI: 0.637-0.677). In addition, we built a nomogram using those six factors to establish a more convenient and accurate method for survival prediction and used calibration plots to verify it (Figure 1).
| Factors | Old model | Univariate analysis | Multivariate analysis | New model | ||
| Score | HR | P value | HR | P value | Score | |
| Poor PS | 2 | 1.983 | < 0.001 | 2.005 | < 0.001 | 2 |
| No gastrectomy | 1 | 1.046 | 0.542 | - | - | - |
| Peritoneal metastasis | 1 | 1.355 | < 0.001 | 1.355 | < 0.001 | 1 |
| Bone metastasis | 2 | 1.605 | < 0.001 | 1.651 | < 0.001 | 2 |
| Lung metastasis | 1 | 1.249 | 0.188 | - | - | - |
| High ALP | 1 | 1.435 | < 0.001 | 1.406 | < 0.001 | 1 |
| Low albumin | 1 | 1.410 | < 0.001 | 1.447 | < 0.001 | 1 |
| High total bilirubin | 1 | 0.965 | 0.806 | - | - | - |
| High NLR | - | 1.445 | < 0.001 | 1.461 | < 0.001 | 1 |
| Poor histology | - | 1.104 | 0.253 | - | - | - |
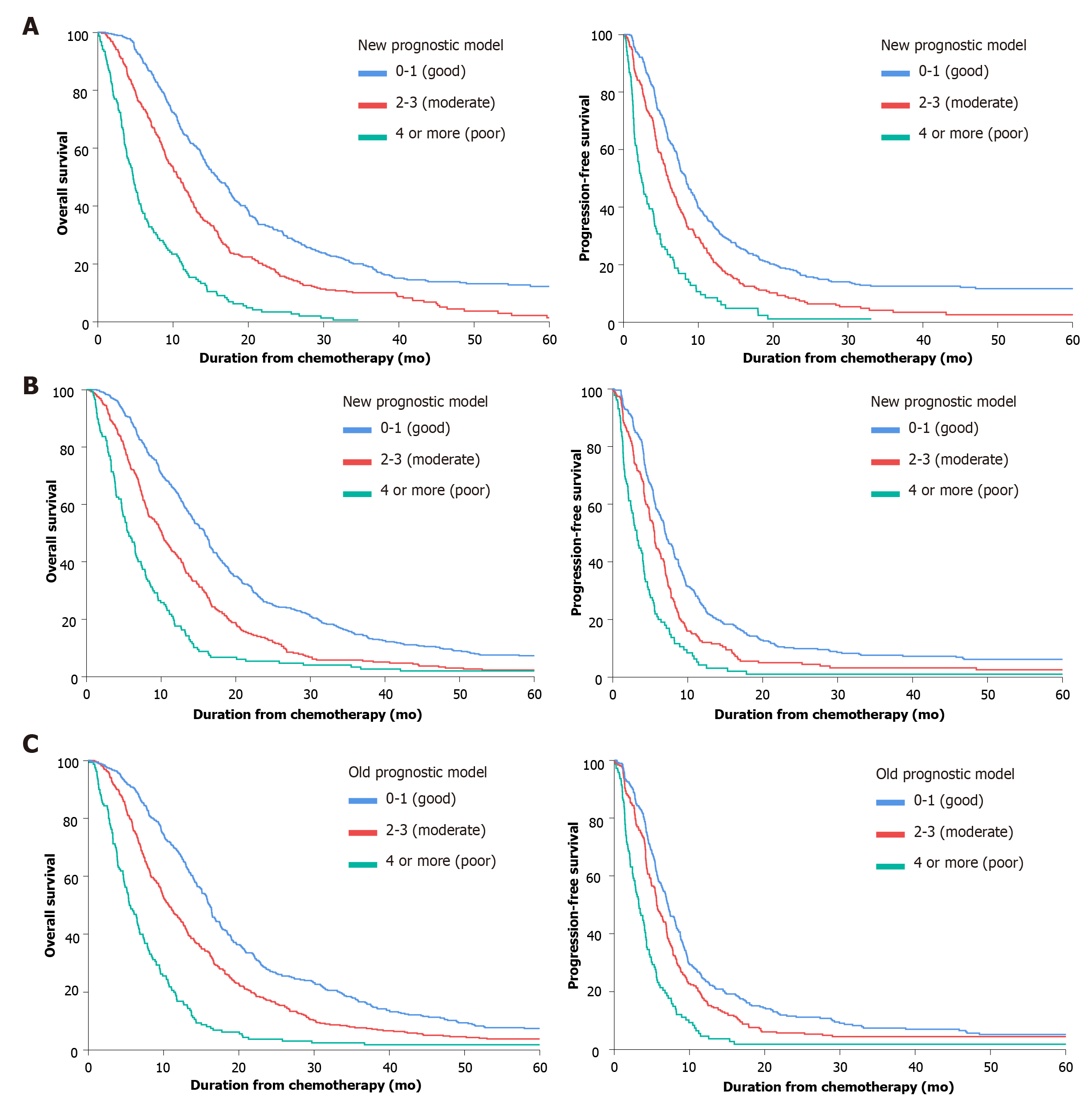
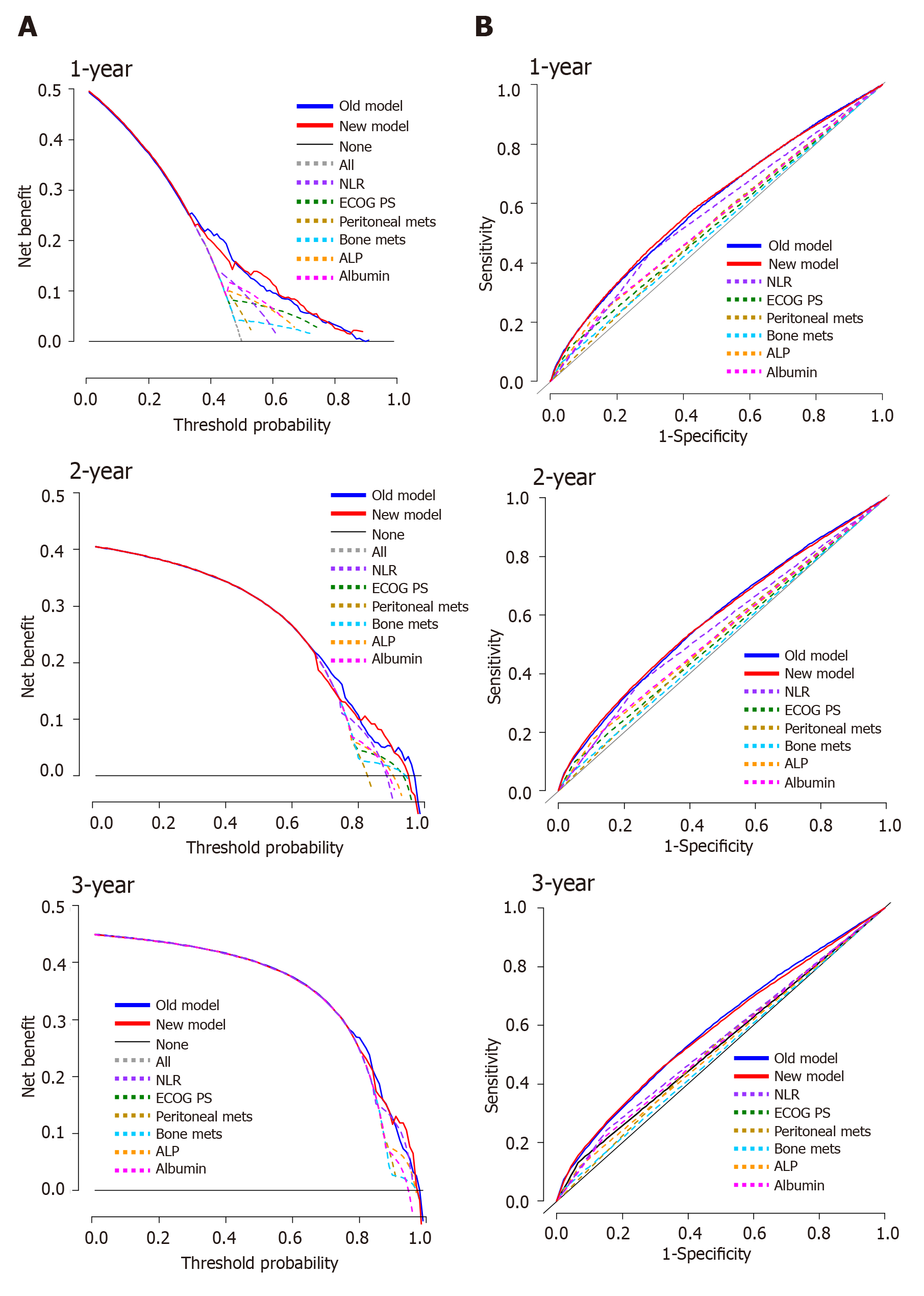
We validated the new model using a separate validation set of 946 patients (2008-2011). Among them, 898 patients (94.9%) died. The proportions of patients classified into each risk category were similar. The observed OS and PFS curves in patients in each risk category showed significant differences in both the training and validation sets (P < 0.001, log-rank test) (Table 3 and Figure 2). The old prognostic model using eight factors also had significantly different OS and PFS outcomes in each risk category. When we compared the OS predictions of the new and old models using the validation set, the C-indexes of the two models were similar [0.638 (95%CI: 0.618-0.658) and 0.635 (95%CI: 0.615-0.655), respectively]. DCA and ROC curve analyses were performed to compare the prediction accuracies of each of the six prognostic factors and the old and new models. The DCA curve showed that the old and new models both had stronger predictive accuracy than the individual prognostic factors, and the performance of the two models was similar (Figure 3). The ROC curve analysis also showed that the two models had similar AUCs at one year [0.598 (95%CI: 0.581-0.617) and 0.600 (95%CI: 0.582-0.620), respectively]. Interestingly, NLR had the largest AUC at one year (0.567; 95%CI: 0.552-0.582) among the six prognostic factors. Although the explanatory power of the two models did not differ, the new model uses two fewer factors and might be more feasible for use in clinical trials or real practice.
| Risk group | Good risk, 0-1 point(s) | Moderate risk, 2-3 points | Poor risk, ≥ 4 points | P value |
| Training set (2012-2015) | ||||
| No. of patients | 449 (48.8%) | 319 (34.7%) | 152 (16.5%) | |
| Hazard ratio (95%CI) | Reference | 1.628 (1.40-1.90) | 4.013 (3.30-4.88) | < 0.001 |
| Median OS, mo (95%CI) | 15.9 (14.5-17.4) | 10.6 (9.3-11.9) | 4.7 (4.0-5.5) | < 0.001 |
| Median PFS, mo (95%CI) | 8.3 (7.4-9.1) | 5.9 (5.1-6.6) | 2.4 (1.8-2.9) | < 0.001 |
| Survival rate (%) | ||||
| At 6 mo | 90.0% (87.2-92.8) | 74.0% (69.2-78.8) | 37.5% (29.8-45.2) | |
| At 12 mo | 63.2% (58.7-67.7) | 44.0% (38.6-49.4) | 16.1% (10.3-21.9) | |
| At 18 mo | 42.9% (38.3-47.5) | 23.4% (18.8-28.0) | 6.3% (2.4-10.2) | |
| At 24 mo | 31.2% (26.9-35.5) | 16.4% (12.3-20.5) | 2.8% (0.2-5.4) | |
| Validation set (2008-2011) | ||||
| No. of patients | 474 (52.0%) | 291 (31.9%) | 147 (16.1%) | |
| Hazard ratio (95%CI) | Reference | 1.634 (1.41-1.90) | 2.963 (2.45-3.59) | < 0.001 |
| Median OS, mo (95%CI) | 15.8 (14.8-16.9) | 10.1 (8.7-11.5) | 5.7 (4.7-6.6) | < 0.001 |
| Median PFS, mo (95%CI) | 7.0 (6.3-7.7) | 5.6 (5.1-6.1) | 3.2 (2.5-3.9) | < 0.001 |
| Survival rate (%) | ||||
| At 6 mo | 88.6% (85.7-91.5) | 72.2% (67.1-77.3) | 47.6% (39.6-55.7) | |
| At 12 mo | 64.3% (60.0-68.6) | 42.3% (36.6-48.0) | 17.0% (10.9-23.1) | |
| At 18 mo | 40.1% (35.7-44.5) | 22.0% (17.2-26.8) | 6.1% (2.2-10.0) | |
| At 24 mo | 25.9% (22.0-29.8) | 13.1% (9.2-17.0) | 4.8% (1.3-8.3) | |
| Validation set (2008-2011) according to old model | ||||
| No. of patients | 393 (41.7%) | 390 (41.4%) | 160 (16.9%) | |
| Hazard ratio (95%CI) | Reference | 1.493 (1.29-1.73) | 3.281 (2.71-3.98) | < 0.001 |
| Median OS, mo (95%CI) | 16.2 (15.3-17.1) | 10.7 (9.5-12.0) | 5.5 (4.5-6.5) | < 0.001 |
| Median PFS, mo (95%CI) | 7.1 (6.3-7.9) | 5.6 (5.1-6.2) | 3.3 (2.5-4.0) | < 0.001 |
| Survival rate (%) | ||||
| At 6 mo | 90.3% (87.4-93.2) | 75.8% (71.5-80.1) | 47.5% (39.8-55.2) | |
| At 12 mo | 68.2% (63.6-72.8) | 45.5% (40.6-50.4) | 16.3% (10.6-22.0) | |
| At 18 mo | 41.3% (36.4-46.2) | 26.5% (22.1-30.9) | 5.6% (2.0-9.2) | |
| At 24 mo | 27.4% (23.0-31.8) | 17.0% (13.3-20.7) | 3.1% (0.4-5.8) |
When we compared how the new and old models assigned the patients in the validation set to risk groups, we found that most patients were classified similarly (Supplementary Table 1). However, 35% of the moderate risk group in the old model (15% of the total patients) was reclassified into the good risk group in the new model, and the median predicted OS of those patients increased to 14.1 mo from 10.6 mo (Supplementary Figure 2).
This study evaluated several clinicopathological factors associated with the prognosis of patients with MRGC. We developed a new prognostic model using six clinicopathological elements with a nomogram in a training set and validated its appropriateness using C-index, DCA, and ROC curve analyses in a different cohort. The six factors were poor ECOG PS, bone metastasis, peritoneal metastasis, high ALP level, low albumin level, and high NLR. Combining those factors into a simple prognostic model enabled MRGC patients to be classified into three risk groups. Our old and new models showed similar prediction performance in the validation set; however, the new model is simpler and easier to apply than the old because it uses two fewer factors.
Previous prognostic models were developed based on heterogeneous treatment groups, but first-line fluoropyrimidine/platinum doublet chemotherapy has become a standard of care. Although 5-fluorouracil (5-FU) is one of the cytotoxic agents most commonly used for MRGC, randomized phase III studies have demonstrated that the oral fluoropyrimidines capecitabine[20] and S-1[21] are just as effective. Therefore, oral fluoropyrimidines (capecitabine or S-1) could be used instead of 5-FU in therapeutic combination with platinum compounds. Also, oxaliplatin-based regimens were suggested to be noninferior to cisplatin-based regimens in terms of OS in the REAL-2 study[22]. Further randomized trials have suggested that oxaliplatin is as effective for prolonging survival and generally better tolerated than cisplatin[23]. Cisplatin-free regimens in combination with oral fluoropyrimidine could offer more convenience by preventing hyperhydration, central catheterization, and hospitalization. On the other hand, triplet chemotherapy, which includes taxane to maximize efficacy, carries a limited survival benefit and increases the risk of grade 3/4 toxicities[24]. Patients treated with a single agent, either fluoropyrimidine or taxane, were considered to be intolerant of combination chemotherapy or to have recurrent disease resistant to prior adjuvant chemotherapy with fluoropyrimidine ± platinum; therefore, those patients receive less frequent subsequent chemotherapy, resulting in poor prognosis[9]. Prognostic factor analyses should be performed in patients receiving the same treatment course because the prognosis varies according to first-line chemotherapy regimen.
High NLR status, a well-known biomarker of cancer-associated inflammation, has shown a significant correlation with poor prognosis in many solid tumors[16]. NLR can be considered a surrogate of the balance between activation of the protumor inflammatory pathway and antitumor immune function.
Neutrophilia increases the number of inflammatory markers, including proangiogenic factors such as vascular endothelial growth factor, growth factors such as interleukin-8, proteases such as tissue inhibitors of metalloproteinase, and antiapoptotic markers such as nuclear factor kappa B, that support tumor growth and progression[25]. Lymphopenia represents a significant decline in the cell-mediated immune system, which is demonstrated by marked decrease in T4 helper and T8 suppressor lymphocytes. Although no exact NLR cutoff point has been defined, we chose an NLR cutoff value of 3.0 based on our ROC curve analysis. The patients in the validation set who had high NLRs had significantly worse OS and PFS (median: 8.4 and 4.8 mo) than those with low NLRs (14.4 and 6.9 mo; P < 0.001) (Supplementary Figure 3). NLR status might be a key factor in predicting the survival outcomes of MRGC patients because it is a surrogate of immune status and is convenient, inexpensive, and reproducible in practice. It also might help clinicians discern when to expect a response to further chemotherapy and immunotherapy in patients with MRGC[26].
Gastrectomy in this study refers to upfront gastrectomy performed before first-line chemotherapy or prior gastrectomy before recurrence. Several retrospective studies have reported that primary tumor resection in advanced gastric cancer could lessen the tumor burden, or so-called resected metastatic status, and result in a survival benefit[27]. However, most of those studies included patients treated in the early 2000s, when active chemotherapeutic agents were limited, and sequential chemotherapy was not established. Also, most included patients underwent both upfront gastrectomy and conversion surgery after palliative chemotherapy. In a recent prospective randomized study (the REGATTA trial), incurable gastrectomy before chemotherapy failed to show a survival benefit, and so it is no longer recommended[28]. A retrospective comparison study between an initially metastatic group and a recurrent metastatic group reported that prior gastrectomy did not affect prognosis[29]. Because our old model was developed from a cohort treated in the early 2000s, prior gastrectomy might have been a significant favorable prognostic factor. In this study, however, patients in the training set received chemotherapy between 2012 and 2015, when many more active chemotherapeutic agents were available. Therefore, prior gastrectomy would not be expected to significantly affect the prognosis of those patients.
The new model described herein has several advantages. First, it was derived by analyzing a homogeneous population treated with recent doublet first-line chemotherapy. Second, prognostic factors such as bone metastasis, which are difficult to obtain from electronic medical records, were evaluated based on clinical data sourced from a prospectively collected registry. Third, we validated our new model in a separate cohort of about 1000 patients and found that its performance was as good in the validation set as it was in the training set.
Our study also has several limitations. First, despite a large number of patients, the generalizability of this study is limited by its single-center, retrospective design and the single ethnicity of its population. Second, our new prognostic model does not apply to patients who received treatment other than doublet chemotherapy, such as single, triplet, or doublet with trastuzumab. Third, this study does not include other critical factors that affect treatment or prognosis, such as molecular biomarkers.
In conclusion, we identified six factors readily measured in clinical practice and predictive of poor prognosis in patients with MRGC. Our new prognostic model uses a scoring system that incorporates those six factors and could be used to classify patients into three groups with significantly different survival outcomes. This model performed well with a validation set and could help to predict life expectancy, guide treatment plans, analyze the findings of clinical studies, and support the design of future clinical trials.
Since systemic chemotherapy for metastatic or recurrent gastric cancer (MRGC) has become standardized, prognostic factors for MRGC patients should be investigated in patients who receive fluoropyrimidine/platinum doublet chemotherapy, which is considered the standard first-line treatment for human epidermal growth factor receptor 2-negative MRGC.
The neutrophil-lymphocyte ratio (NLR) is a representative blood marker of the systemic inflammatory response that reflects tumor progression, invasion, and metastasis in cancer patients. This is a relatively new prognostic factor in MRGC, and its change was reported to predict poor outcomes during immuno-oncologic therapy.
We modified our previous prognostic model by introducing NLR and histology using a cohort of MRGC patients, and we validated our new model in a different cohort.
Model development and validation were based on a split-sample method according to time period. Patients were separated by treatment period and assigned to a training set (2012-2015; n = 937) or an independent validation set (2008-2011; n = 946). The prognostic model was developed using the training set.
Multivariate analysis confirmed that six factors were significantly associated with poor overall survival as follow: poor performance, peritoneal metastasis, bone metastasis, high alkaline phosphatase level, low albumin level, and high NLR. The observed overall survival and progression-free survival curves in patients in each risk category showed significant differences in both the training and validation sets (P < 0.001, log-rank test).
We identified six factors readily measured in clinical practice and predictive of poor prognosis in patients with MRGC. Our new prognostic model uses a scoring system that incorporates those six factors and could be used to classify patients into three groups with significantly different survival outcomes.
Our model could help to predict life expectancy, guide treatment plans, analyze the findings of clinical studies, and support the design of future clinical trials in MRGC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Endo S, Moradi L S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1468] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 2. | Jung KW, Won YJ, Hong S, Kong HJ, Lee ES. Prediction of Cancer Incidence and Mortality in Korea, 2020. Cancer Res Treat. 2020;52:351-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 3. | Guideline Committee of the Korean Gastric Cancer Association (KGCA); Development Working Group & Review Panel. . Korean Practice Guideline for Gastric Cancer 2018: an Evidence-based, Multi-disciplinary Approach. J Gastric Cancer. 2019;19:1-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 313] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 4. | Arai H, Nakajima TE. Recent Developments of Systemic Chemotherapy for Gastric Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, Park JO, Park YS, Lim HY, Sohn TS, Noh JH, Heo JS, Park CK, Kim S, Kang WK. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18:886-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Takahari D, Boku N, Mizusawa J, Takashima A, Yamada Y, Yoshino T, Yamazaki K, Koizumi W, Fukase K, Yamaguchi K, Goto M, Nishina T, Tamura T, Tsuji A, Ohtsu A. Determination of prognostic factors in Japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912. Oncologist. 2014;19:358-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Koo DH, Ryoo BY, Kim HJ, Ryu MH, Lee SS, Moon JH, Chang HM, Lee JL, Kim TW, Kang YK. A prognostic model in patients who receive chemotherapy for metastatic or recurrent gastric cancer: validation and comparison with previous models. Cancer Chemother Pharmacol. 2011;68:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Koo DH, Ryu MH, Ryoo BY, Seo J, Lee MY, Chang HM, Lee JL, Lee SS, Kim TW, Kang YK. Improving trends in survival of patients who receive chemotherapy for metastatic or recurrent gastric cancer: 12 years of experience at a single institution. Gastric Cancer. 2015;18:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Koo DH, Ryu MH, Lee MY, Chae H, Kim EJ, Moon MS, Kang YK. Trends in Chemotherapy Patterns and Survival of Patients with Advanced Gastric Cancer over a 16-Year Period: Impact of Anti-HER2-Targeted Agent in the Real-World Setting. Cancer Res Treat. 2021;53:436-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, Park JO, Park YS, Lim HY, Kang WK, Park SH. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 11. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1901] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 12. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1715] [Article Influence: 214.4] [Reference Citation Analysis (0)] |
| 13. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5327] [Article Influence: 355.1] [Reference Citation Analysis (3)] |
| 14. | Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K; DESTINY-Gastric01 Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020;382:2419-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 830] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 15. | Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1626] [Article Influence: 162.6] [Reference Citation Analysis (0)] |
| 16. | Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 1091] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 17. | Grenader T, Waddell T, Peckitt C, Oates J, Starling N, Cunningham D, Bridgewater J. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol. 2016;27:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Ter Veer E, van Kleef JJ, Schokker S, van der Woude SO, Laarman M, Haj Mohammad N, Sprangers MAG, van Oijen MGH, van Laarhoven HWM. Prognostic and predictive factors for overall survival in metastatic oesophagogastric cancer: A systematic review and meta-analysis. Eur J Cancer. 2018;103:214-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3515] [Cited by in RCA: 3481] [Article Influence: 183.2] [Reference Citation Analysis (1)] |
| 20. | Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, Lichinitser M, Guan Z, Khasanov R, Zheng L, Philco-Salas M, Suarez T, Santamaria J, Forster G, McCloud PI. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 21. | Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 431] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 22. | Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1693] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 23. | Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, Rethwisch V, Seipelt G, Homann N, Wilhelm G, Schuch G, Stoehlmacher J, Derigs HG, Hegewisch-Becker S, Grossmann J, Pauligk C, Atmaca A, Bokemeyer C, Knuth A, Jäger E; Arbeitsgemeinschaft Internistische Onkologie. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 575] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 24. | Mohammad NH, ter Veer E, Ngai L, Mali R, van Oijen MG, van Laarhoven HW. Optimal first-line chemotherapeutic treatment in patients with locally advanced or metastatic esophagogastric carcinoma: triplet versus doublet chemotherapy: a systematic literature review and meta-analysis. Cancer Metastasis Rev. 2015;34:429-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 26. | Ota Y, Takahari D, Suzuki T, Osumi H, Nakayama I, Oki A, Wakatsuki T, Ichimura T, Ogura M, Shinozaki E, Suenaga M, Chin K, Yamaguchi K. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol. 2020;85:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Lee SS, Lee JL, Ryu MH, Chang HM, Kim TW, Kang HJ, Kim WK, Lee JS, Kang YK. Combination chemotherapy with capecitabine (X) and Cisplatin (P) as first line treatment in advanced gastric cancer: experience of 223 patients with prognostic factor analysis. Jpn J Clin Oncol. 2007;37:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, Yoshikawa T, Hahn S, Nakamura K, Park CH, Kurokawa Y, Bang YJ, Park BJ, Sasako M, Tsujinaka T; REGATTA study investigators. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 508] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 29. | Lee CM, Choi IK, Kim JH, Park DW, Kim JS, Park SH. Is noncurative gastrectomy always a beneficial strategy for stage IV gastric cancer? Ann Surg Treat Res. 2017;92:23-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |