Published online Dec 14, 2021. doi: 10.3748/wjg.v27.i46.7943
Peer-review started: March 16, 2021
First decision: May 1, 2021
Revised: May 12, 2021
Accepted: November 28, 2021
Article in press: November 28, 2021
Published online: December 14, 2021
Processing time: 268 Days and 16.4 Hours
Inflammatory bowel diseases (IBD) refer to a subgroup of chronic, progressive, long-term, and relapsing inflammatory disorders. IBD may spontaneously grow in the colon, and in severe cases may result in tumor lesions such as invasive carcinoma in inflamed regions of the intestine. Recent epidemiological reports indicate that old age and underlying diseases such as IBD contribute to severity and mortality in patients with coronavirus disease 2019 (COVID-19). Currently, the ongoing COVID-19 pandemic caused serious morbidity and mortality worldwide. It has also been shown that the transmembrane serine protease 2 is an essential factor for viral activation and viral engulfment. Generally, viral entry causes a 'cytokine storm' that induces excessive generation of proinflammatory cytokines/chemokines including interleukin (IL)-6, IL-2, IL-7, tumor necrosis factor-α, and interferon-γ. Future research could concentrate on developing inflammatory immunological responses that are efficient to encounter COVID-19. Current analysis elucidates the role of inflammation and immune responses during IBD infection with COVID-19 and provides a list of possible targets for IBD-regulated therapies in particular. Data from clinical, in vitro, and in vivo studies were collected in English from PubMed, Google Scholar, Scopus, and the Cochrane library until May 2021.
Core Tip: This article provides clinical evidence on synthetic or natural-based transmembrane serine protease 2 (TMPRSS2) and angiotensin-converting enzyme 2 (ACE2) inhibitors, which are able to reduce coronavirus disease 2019-induced inflammation and cytokine storms in inflammatory bowel disease patients. Hence, targeting TMPRSS2 and ACE2 could be noticed as a novel approach for inflammatory bowel diseases treatment.
- Citation: Lashgari NA, Momeni Roudsari N, Momtaz S, Abdolghaffari AH. Transmembrane serine protease 2 and angiotensin-converting enzyme 2 anti-inflammatory receptors for COVID-19/inflammatory bowel diseases treatment. World J Gastroenterol 2021; 27(46): 7943-7955
- URL: https://www.wjgnet.com/1007-9327/full/v27/i46/7943.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i46.7943
Coronavirus disease 2019 (COVID-19) is a primarily respiratory ailment that is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), additionally named as 2019 novel COVID. It is profoundly overwhelming with case casualty rates of 2%-3%. Since its appearance in December 2019 in China, COVID-19 has quickly spread and influenced populaces in virtually all areas of the world[1,2]. Old-age patients and those with chronic conditions are more prone to dreariness and mortality in COVID-19. This high mortality is implicated in misrepresented and misled invulnerable reactions that cause cytokine storms. In brief, SARS-CoV-2 infects the angiotensin-converting enzyme 2 (ACE2) expressing epithelial cells in the lung and/or the intestine, leading to a massive production of mediators that induce the immune cell activation. Overactivation of immune cells leads to severe complications including acute respiratory distress syndrome, shock, and multiorgan failure[3,4].
Inflammatory bowel diseases (IBD) include two major types: Ulcerative colitis and Crohn’s disease. IBD is characterized with persistent resistant interceded sicknesses that regularly require immunomodulatory and immunosuppressive treatments[5,6]. Therefore, patients with IBD are at high risk to different shrewd viral and bacterial contaminations. There is no solid evidence that patients with IBD are at higher risk for COVID-19 infection, although it has been indicated that patients with IBD who are pregnant are more vulnerable[7]. The current study discusses the impact of COVID-19 on IBD[8,9]. We provide evidence on mediatory effects of the transmembrane serine protease 2 (TMPRSS2) and ACE2 signaling pathways against inflammation and introduces the synthetic or natural TMPRSS2 and ACE2 inhibitors as probable approaches for IBD treatment in the COVID-19 situation[9,10].
PubMed, Google Scholar, Scopus, and Cochrane Library were searched and relevant clinical, in vivo, and in vitro articles (in English) were collected until May 2021. Search terms included "corona virus" OR "COVID-19" AND "inflammatory bowel disease" OR "IBD" OR "inflammation" AND "TMPRSS2" OR "ACE2" AND "TMPRSS2 inhibitors" OR "ACE2 inhibitors".
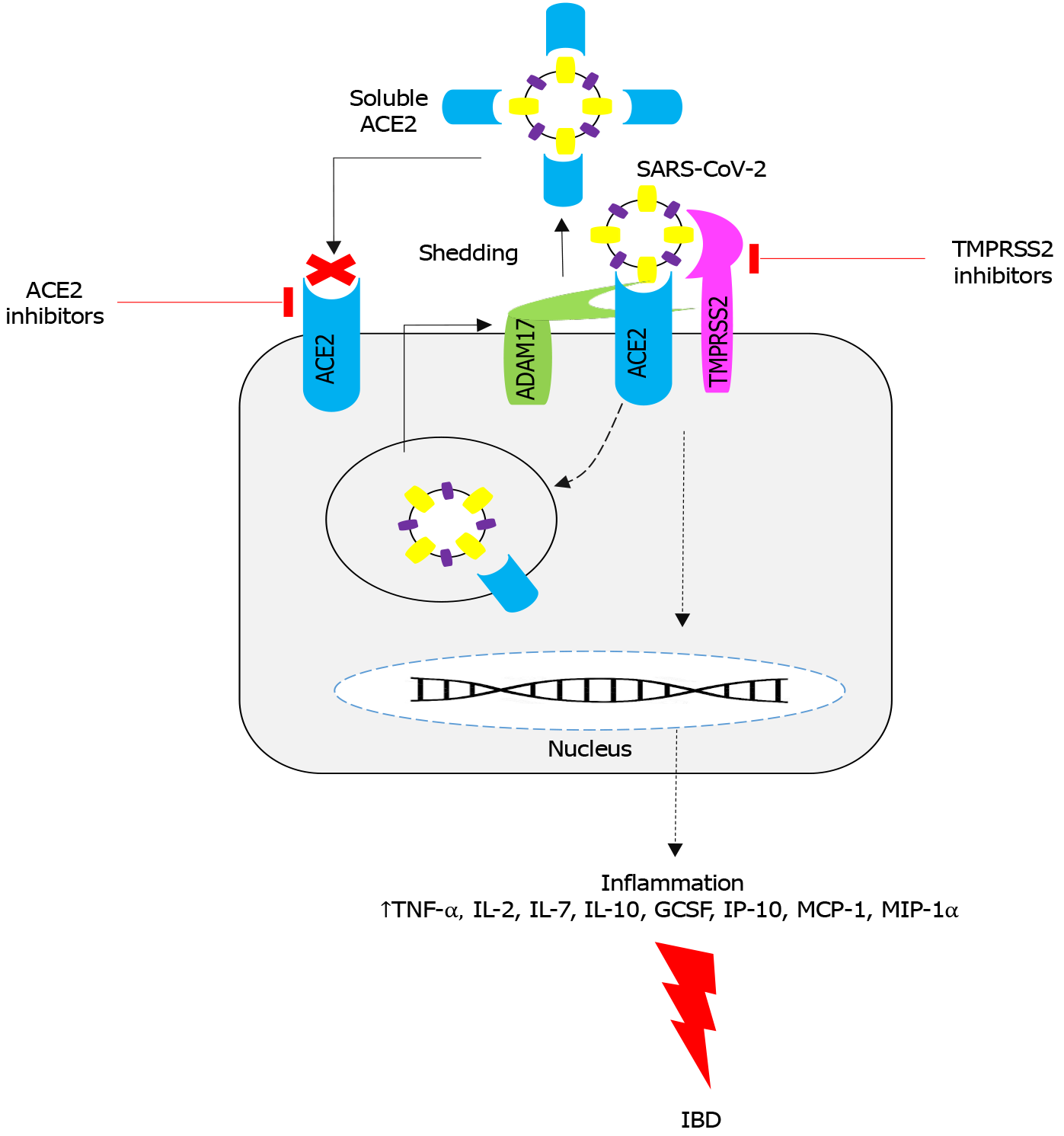
Variations in potency of the SARS-CoV-2 cell entry may account for discovering new solutions to deal with the virus. It has been reported that the entrance of SARS-CoV-2 to the human cells victimizes the SARS-CoV receptor ACE2 and TMPRSS2 for the spike (S) supermolecule priming. It is debatable whether the metallopeptidase domain seventeen [a disintegrin and metalloprotease domain 17 (ADAM17), also referred to as the tumor necrosis factor (TNF)-α-converting accelerator] located in the ACE2 ectodomain shedding may or may not counteract the virus entry by increasing the number of soluble ACE2, or it solely contributes to the ACE1/ACE2 unbalancing, inflammation, and occlusion[11]. The ACE2-receptor/S-protein interaction could be a key factor for success of virus infection and willingness. Similarly, single ester polymorphisms located inside the TMPRSS2 factor (21q22.3) can play a more important role in respiratory disorder[12]. ACE1 and ACE2 collaborate with the renin-angiotensin system to balance the native vasoconstrictor/proliferative ACE1/ angiotensin II/angiotensin II type 1/angiotensin (Ang) II/Ang type 1 receptor (ACE1/Ang-II type 1/AT1-axis), and vasodilator/antiproliferative (ACE2/Ang1-7/mitochondrial assembly-axis) actions. This ends up in the protection of organs and blood vessels by the decoagulants, medicinal drugs, anti-proliferation, anti-fibrosis, anti-alveolar vegetative cell caspase-mediated cell death, and anti-oxidative stress activities that are able to antagonize the Ang-II effects[11,13].
In a complex pathophysiological condition like COVID-19, the ACE2 cytoplasmic tail cleavage intervened by TMPRSS2 is a significant event to be considered (Figure 1). Cleavage of the ACE2 tail by TMPRSS2 increases viral load in objective cells, and TMPRSS2 could facilitate the SARS-CoV-2 passage via the SARS-S cleavage, which induces the S protein for film combination. The ACE2 cleavage may enhance viral uptake through the cathepsin L-subordinate pathway, resulting in viral integration with the endosomal layer and eventually cell contamination[11,14]. In spite of similar explicitness of TMPRSS2 and ADAM17 for ACE2, they act opposite for cleavage of ACE2. To start with, the divisions produced by the cleavage of these proteases have distinctive subatomic sizes, mainly due to various cleavage locales. Second, cleavage of ACE2 by ADAM17 forms the ACE2 ectodomain, which is shed into the extracellular medium, as the soluble ACE biologically dynamic structure[15,16]. In vitro studies have shown that the ACE2 ectodomain does not separate from the TMPRRS2-induced ACE2 cleavage. This was evidenced by a C-terminal intracellular cleavage. In this manner, the distinctions in the cleavage destinations and its organic outcomes might be basic. For sure, just the soluble ACE2 structure would have a defensive impact on prevention of viral particle aggregations[17]. Therefore, overexpression of ADAM17 and TMPRSS2 could be a primary factor in inflammation storm that is characterized by negative features such as renin-angiotensin system lopsidedness, intense irritation, and intravascular coagulation in older populations with COVID-19 comorbidities. Initiation of inflammation cycles is a key element for SARS-CoV-2 contamination[18,19].
ACE2 is the main receptor for SARS-CoV-2, providing additional insurance against the destructive impacts of viral diseases. Moreover, as referenced above, solid confirmations indicate that the outflow of ACE2 is dependent on the companion of hormonal, hereditary, and age-related systems[20,21]. Overaction of ADAM17 in both COVID-19 and the plasma level of ACE2 has been confirmed by several reports. Overexpression of the ADAM17 gene and its protein level have been implicated in several inflammatory conditions including IBD[22,23]. High levels of inflammatory cytokines and chemokines in COVID-19 patients are accounted for by more elevated levels of interleukin (IL)-2, IL-7, IL-10, granulocyte colony-stimulating factor, interferon gamma-induced protein 10, monocyte chemoattractant protein 1, macrophage inflammatory proteins-1A, and TNF-α. A significant effect of the “fiery wave” in COVID-19 indicates the cytokine storm may be firmly connected with the seriousness of the infection[24,25]. The ‘cytokine storm’ is a significant target for research about the pathogenic cycles in SARS-CoV–2 contaminations and is a way to recognize new restorative targets. On the other hand, blockade of SARS-CoV–ACE2 in the ACE2 cytoplasmic domain pathway results in upregulation of ADAM17 activity. Upre
The SARS-CoV-2 receptor ACE2 and TMPRSS2 receptor are central factors in COVID-19-induced IBD pathogenesis (Table 1 and Figure 1). These receptors are often found within the lower respiratory lot of pneumocytes and the gastrointestinal tract[7]. The ACE2 receptors are frequently located within the terminal ileum and colon. It was shown that the convergence of these receptors was higher in IBD patients, in both the energetic and calm stages of the disease[33]. The ACE2 receptors are a part of the renin angiotensin-aldosterone system that is assumed to play critical roles in controlling the provocative handle. Terminal ileum and the colon are the most affected areas in IBD[34]. IBD is also correlated with upregulation of inflammatory cytokines and the ACE2 receptors. As we discuss in this article, patients with IBD do not seem powerless against COVID-19[35]. In this context, a few theories have been proposed. For instance, the renin angiotensin-aldosterone system has two specific pathways involved in irritation course. Multiple studies confirmed that ACE2 is upregulated in IBD, and in the SARS-CoV-2 condition ACE2 exacerbates the disease symptoms. Accordingly, prevention of the ACE2 protein expression has been suggested for controlling both COVID-19 and IBD[36,37]. While the ACE-angiotensin receptor 1 pathway is favorable for inflammation, the ACE2 pathway helps in tissue security. Given the enteric inflammation in IBD, it has been suggested that the ACE2 receptors and the host cell surface proteases like TMPRSS2 may suppress SARS-CoV-2[38,39]. The ACE2 level was shown to be downregulated in colonic aggravation in animal models; thereby, some IBD drugs such as steroids and biologics were found useful for cutting down the ACE2 in infected cells. Another report declared no change in ACE2 receptors or TMPRSS2 in IBD patients when diverged from controls[40,41].
| Ref. | Clinical studies | Model of IBD | Intervention | Duration of treatment | Numbers of animals in intervention group and control group | Outcomes | Adverse effects |
| Nowak et al[25] | Clinical trial | IBD in COVID-19 | - | - | 138 treatment naïve IBD patients (cases) and 154 controls | ↑ACE2/TMPRSS2 expression; ↑Inflammation | - |
| Brenner et al[67] | 18 yr (with IBD), the Pediatric IBD Porto Group | - | TNF antagonist monotherapy (48%), followed by sulfasalazine/mesalamine (23%) | March 2020-October 2020 | Hospitalized cases (n = 14); Outpatient cases (n = 195) | Sulfasalazine/Mesalamine and steroid therapy were associated with increased hospitalization risk and TNF antagonist monotherapy was associated with decreased risk parallel those reported in adult IBD patients. PIBD patients have a relatively low risk of severe COVID-19, even when receiving biologic and/or other immune-suppressive therapies for their IBD | - |
| Norsa et al[85] | Clinical trial | Crohn disease and Ulcer colitis | Anti-inflammatory (Salicylates); thiopurines or methotrexate; biologics (Infliximab, Adalimumab, Ustekinumab, Vedolizumab, Golimumab); steroids; Other immunosuppressants (Tacrolimus, Cyclosporin, Mofetil Micofenolate) | February 2020-March 2020 | Crohn disease = 186; Ulcer colitis = 336 | IBD improvement: ↓TNF-α; ↓Inflammation; ↓ACE2/TMPRSS2 expression | - |
| Mazza et al[86] | Clinical trial | Ulcerative colitis | Methylprednisolone (40 mg/d); prednisone dosage at the time of patient’s death was 25 mg daily | December 2019-February 2020 | - | IBD improvement; Improvement in COVID-19 symptoms; ↓Inflammation | - |
| Tursi et al[87] | Clinical trial | Crohn’s disease | Adalimumab | - | - | Maintain of IBD remission during COVID-19; Managing/preventing COVID-driven pneumonia: ↓TNF-α; ↓Inflammation; ↓ACE2/TMPRSS2 expression | - |
| Bodini et al[88] | Clinical trial | IBD | Immunosuppressants/biological treatment | 3 wk | 48 patients | IBD improvement; Improvement in; COVID-19 symptoms | Increase the risk of infection |
| Tursi et al[89] | Clinical trial | Crohn’s disease | Mesalazine (3 g/d) and Adalimumab 40 mg subcutaneously | - | 74 cases | IBD improvement; Improvement in COVID-19 symptoms; ↓TNF-α; ↓Inflammation; ↓ACE2/TMPRSS2 expression | - |
| Allocca et al[90] | Clinical trial | IBD | Biological treatment | - | 162 IBD patients | IBD improvement; Improvement in COVID-19 symptoms | - |
| Jacobs et al[91] | Clinical trial | Ulcerative colitis | Tofacitinib (10 mg twice daily) | 5 mo | - | IBD improvement; Improvement in COVID-19 symptoms | Increase the risk of infection |
| Gutin et al[69] | Clinical trial | Ulcerative colitis | Biological treatment | February 2020-March 2020 | 522 patients | IBD improvement; Improvement in COVID-19 symptoms: ↓TNF-α; ↓Inflammation; ↓ACE2/TMPRSS2 expression | |
| Taxonera et al[92] | Clinical trial | Crohn’s disease | Immunomodulatory/biologics | - | n = 12 | IBD improvement; Improvement in COVID-19 symptoms; ↓TNF-α; ↓Inflammation; ↓ACE2/TMPRSS2 expression | |
| Allocca et al[93] | Clinical trial | - | Immunosuppressant or biologics | - | n = 15 | IBD improvement; Improvement in COVID-19 symptoms; ↓TNF-α; ↓Inflammation | - |
| Mak et al[94] | Clinical trial | IBD in COVID-19 | Thirty (75%) were on 5-Aminosalicylates acid, 15 (37.5%) on immunosuppressants (14 Thiopurine, one Tacrolimus), 11 (27.5%) on corticosteroids and 7 (17.5%) on biologics (3 Infliximab, 1 Adalimumab, 2 Vedolizumab and 1 Ustekinumab) | - | n = 63 | IBD improvement; Improvement in COVID-19 symptoms; ↓Inflammation | - |
| Bardasi and Alvisi[95] | Clinical trial | Crohn’s disease in COVID-19 | Subcutaneous administration of 40 mg Adalimumab | 6 mo | - | IBD improvement; Improvement in COVID-19 symptoms ↓Inflammation | - |
| Ashton et al[96] | Clinical trial | IBD in COVID-19 | Anti-TNF therapy (Infliximab or Adalimumab) | - | n = 122 | IBD improvement; Improvement in COVID-19 symptoms: ↓TNF-α; ↓Inflammation | - |
As mentioned, there are limited data on the possible impact of SARS-CoV-2 contamination on patients with IBD. Various methodologies can be utilized alone or concurrently to conquer the infection. Blockage of the ACE2 receptors and the viral S protein are the main focus of current investigations on SARS-CoV-2 regulation. So far, we discussed that blockage of the TMPRSS2 receptor and/or the ACE2/TMPRSS2 complex is likewise a plausible approach to modulate this infection. In this context, a number of synthetic or natural TMPRSS2 and ACE2 inhibitors that are able to mediate the TMPRSS2 and ACE2 signaling have been explored.
Medicinal plants are the greatest age-old wellspring of remedially valuable phyt
ACE2 is found in the outer layer of the human cell that is accounted as a likely coupling site for the S protein. A couple of experiments have shown that there is a strong link between ACE2 and the S protein. Thus, blockade of ACE2 by phyt
It was shown that hesperidin, chrysin, and emodin are also effective for COVID-19 treatment by attenuating the harmful effect of viral infection within cells[48]. Kaempferol, quercetin, and fisetin can bind with human angiotensin-converting enzyme-S-protein. In silico studies demonstrated that quercetin, quercetin 3 glucuronide-7-glucoside, quercetin 3-vicianoside, absinthin, glabridin, and gallic acid have strong affinity toward ACE2 to suppress COVID-19. Nuclear docking exa
Different studies exhibited that some other phenolic compounds such as cinnamaldehyde as well as terpenoids such as carvacrol, geraniol, anethole, L-4-terpineol, cinnamyl acidic, thymol, and pulegone possess antiviral activities through blockade of the viral S protein[52,53]. It was reported that the binding affinity of ACE2 linkage with scutellarin (a flavonoid glycoside) and glycyrrhizin (a triterpenoid) was stronger than baicalin, hesperetin, and nicotianamine[54].
Limonoids and triterpenoids also displayed similar inhibitory effects on ACE2. Another in silico study similarly demonstrated that limonin, obacunone, ursolic destructive, glycyrrhizin destructive, 7-deacetyl-7-benzoylgedunin, maslinic acid, and corosolic acid effectively target SARS-CoV-2 proteins[55]. In this line, nimbin (a triterpenoid) and curcumin exhibited high limiting proclivity on ACE2 and the S protein[56]. Epigallocatechin-3-gallate and theaflavin gallate were shown to have inhibitory effects on the S-protein central channel of SARS-CoV-2. Moreover, three alkaloids, including cepharanthine, fangchinoline, and tetrandrine, inhibited the S protein of Human coronavirus Subtype OC43 (Human-CoV-OC43) expression, while tetrandrine exhibited moderating effects on viral sicknesses. An indazole alkaloid isolated from the seeds of Nigella sativa, called nigellidine, was shown to bind the dynamic areas of SARS-CoV-2, thereby paralyzing the virus. In another study, anthraquinone emodin blocked the ACE2 and S protein conjunction[57,58].
Various classes of medications, with different powers and immunosuppressive potentials, are used for IBD treatment (Table 1 and Figure 1). At present, limited data are available for the utilization of different medications in IBD under the COVID-19 condition, henceforth the level of proof is not yet certain[59]. Current suggestions, proposed by specialists and different social orders, are overwhelmingly based on the recounted proof from the utilization of these medications during other viral pandemics like SARS and Middle East respiratory syndrome coronavirus or a few distributed case reports[60]. By and large, usage of intense immunosuppressants in IBD patients should be limited, except if totally essential. Notwithstanding, patients who are on stable upkeep portions may keep on doing as such with close contact with their physicians[61,62].
Salicylates: Salicylates are usually utilized in either oral form or as a bowel purge. They have a neighborhood activity and are improbable to influence the course of COVID-19 when are used in IBD patients, thereby they may be securely proceeded in dosages[63].
Corticosteroids: Corticosteroids are the most common drugs that are used in IBD, mainly due to their intense calming effects. Therefore, steroids may be valuable in suppression of COVID-19, particularly in conditions like intense lung injury, intense respiratory trouble disorder, and septic shock. During the SARS and Middle East respiratory syndrome pandemic, corticosteroids treatment helped to postpone viremia[64,65], while there were no general improvement in terms of septic shock or psychosis, etc.[66]. Given the absence of adequacy, the World Health Organization suggested that routine corticosteroids ought to be avoided except in explicit circumstances. Steroids are possibly kept away from the first line therapies in recently analyzed IBD patients. Notwithstanding, considering their tremendous advantages in IBD, it was suggested that the main steroids might be beneficial at low doses in patients with COVID-19 and IBD, specifically in patients that are already on treatment[67,68]. Steroids with limited site of action, for example budesonide, seem harmless to be used. Infliximab might be a therapeutic option for COVID-19 positive patients with mild respiratory symptoms[61,69].
Cyclosporin: Cyclosporin is used for serious ulcerative colitis as an option in contrast to steroids. Although, some data pointed out that cyclosporine can inhibit the coronavirus replication proteins in vitro, its prescription is controversial in patients with COVID-19 due to its strong immunosuppressive properties[70-72].
Azathioprine and methotrexate: Azathioprine is a thiopurine that is often used for IBD treatment, particularly for upkeep treatment. Curiously, past investigations have demonstrated that thiopurine analogs have both immediate and roundabout activities on smothering antiviral movement. They also hinder viral proteases once the host proteins were engaged with viral replication[73,74]. Depending on the perception of genuine viral contaminations in IBD patients who are using thiopurine, the treatment time can be estimated. Interruption in treatment up to 14 d after recuperation from COVID-19 has been suggested. Methotrexate can perhaps continue without issues[75,76].
Biologics: Current data show that infliximab and adalimumab (TNF-α inhibitors) have no unfavorable effects on the clinical course of COVID-19[62,77]. One reason speculated is the strong mitigating impact of TNF blockage, which may indeed constrict the cytokine storm in serious types of COVID-19[78,79]. Co-administration of medicines (i.e. thiopurine and infliximab) might be an option. Also, monotherapy with natural products may be considered[60,80,81]. Vedolizumab (an adversary of α4β7 integrin) is significantly explicit for movement on the gut, hence it is favorable for fundamental or pneumonic responses in COVID-19[62,82]. Ustekinumab is an approved clinical therapy for patients with IBD. Ustekinumab is a cytokine antibody and an inhibitor of IL-12 and IL-23. Currently, there are no major concerns about usage of ustekinumab in patients with IBD and COVID-19. Vedolizumab or ustekinumab might be the primary therapeutic options for individuals at higher risk of COVID-19 if biological treatments are thought of[79,83,84].
Information on the physiologic and pathophysiologic functions of ACE2/TMPRSS2 is still scant. ACE2/TMPRSS2 is very much described in the cardiovascular and renal frameworks. Yet little data exist regarding other organ frameworks, for example the gastrointestinal system. Moreover, specific function of the ACE2/TMPRSS2 axis in pathologic conditions was traditionally restricted to cardiovascular illnesses. Although, considering the ACE2/TMPRSS2 as a multifunctional protein has accomplished significance as of late.
The current COVID-19 pandemic has featured the importance of ACE2/TMPRSS2 as a receptor for SARS-CoV-2, yet research is expected to determine whether the ACE2/TMPRSS2 levels enhance the pathogenesis of COVID-19 or could benefit the course of illness by diminishing the malicious impacts of Ang II. Moreover, the relationship between ACE2/TMPRSS2, the intestinal amino corrosive vehicle, and IBD merits further consideration in patients with IBD. At last, association of ACE2/TMPRSS2 to integrins raises concerns and expectations, particularly because there were just two articles regarding the matter. Taking everything into account, investigating the multifunctional nature of ACE2/TMPRSS2 in IBD (by describing its appearance/movement in the blood, gut, as well as excrement of patients with IBD and solid control patients) will develop the knowledge on the pathophysiology of this illness. In accordance with this objective, recognizable proof of other biomarkers of infection movement, treatment reaction, and new medication target, as well as setting of the novel helpful alternatives is required to affect tolerant consideration.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moreira TMM, Soares RLS S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention? Cancer Discov. 2020;10:779-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 2. | Scaldaferri F, Pugliese D, Privitera G, Onali S, Lopetuso LR, Rizzatti G, Settanni CR, Pizzoferrato M, Schiavoni E, Turchini L, Amatucci V, Napolitano D, Bernabei T, Mora V, Laterza L, Papa A, Guidi L, Rapaccini GL, Gasbarrini A, Armuzzi A. Impact of COVID-19 pandemic on the daily management of biotechnological therapy in inflammatory bowel disease patients: Reorganisational response in a high-volume Italian inflammatory bowel disease centre. United European Gastroenterol J. 2020;8:775-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020;323:2329-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 729] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 4. | Li X, Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit Care. 2020;24:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 446] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 5. | Lashgari NA, Roudsari NM, Momtaz S, Ghanaatian N, Kohansal P, Farzaei MH, Afshari K, Sahebkar A, Abdolghaffari AH. Targeting Mammalian Target of Rapamycin: Prospects for the Treatment of Inflammatory Bowel Diseases. Curr Med Chem. 2021;28:1605-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Lashgari NA, Roudsari NM, Zandi N, Pazoki B, Rezaei A, Hashemi M, Momtaz S, Rahimi R, Shayan M, Dehpour AR, Abdolghaffari AH. Current overview of opioids in progression of inflammatory bowel disease; pharmacological and clinical considerations. Mol Biol Rep. 2021;48:855-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Neurath MF. COVID-19 and immunomodulation in IBD. Gut. 2020;69:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 8. | Reinsch S, Stallmach A, Grunert PC. The COVID-19 Pandemic: Fears and Overprotection in Pediatric Patients with Inflammatory Bowel Disease and Their Families. Pediatr Gastroenterol Hepatol Nutr. 2021;24:65-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Yang C, Xiao SY. COVID-19 and inflammatory bowel disease: A pathophysiological assessment. Biomed Pharmacother. 2021;135:111233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 740] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 11. | Suárez-Fariñas M, Tokuyama M, Wei G, Huang R, Livanos A, Jha D, Levescot A, Irizar H, Kosoy R, Cording S, Wang W, Losic B, Ungaro RC, Di'Narzo A, Martinez-Delgado G, Suprun M, Corley MJ, Stojmirovic A, Houten SM, Peters L, Curran M, Brodmerkel C, Perrigoue J, Friedman JR, Hao K, Schadt EE, Zhu J, Ko HM, Cho J, Dubinsky MC, Sands BE, Ndhlovu L, Cerf-Bensusan N, Kasarskis A, Colombel JF, Harpaz N, Argmann C, Mehandru S. Intestinal Inflammation Modulates the Expression of ACE2 and TMPRSS2 and Potentially Overlaps With the Pathogenesis of SARS-CoV-2-related Disease. Gastroenterology. 2021;160:287-301.e20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 12. | Batchu SN, Kaur H, Yerra VG, Advani SL, Kabir MG, Liu Y, Klein T, Advani A. Lung and Kidney ACE2 and TMPRSS2 in Renin-Angiotensin System Blocker-Treated Comorbid Diabetic Mice Mimicking Host Factors That Have Been Linked to Severe COVID-19. Diabetes. 2021;70:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Sakamoto A, Kawakami R, Kawai K, Gianatti A, Pellegrini D, Kutys R, Guo L, Mori M, Cornelissen A, Sato Y, Bellasi A, Faggi L, Hong C, Romero M, Guagliumi G, Virmani R, Finn AV. ACE2 (Angiotensin-Converting Enzyme 2) and TMPRSS2 (Transmembrane Serine Protease 2) Expression and Localization of SARS-CoV-2 Infection in the Human Heart. Arterioscler Thromb Vasc Biol. 2021;41:542-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Kermani NZ, Song WJ, Badi Y, Versi A, Guo Y, Sun K, Bhavsar P, Howarth P, Dahlen SE, Sterk PJ, Djukanovic R, Adcock IM, Chung KF; U-BIOPRED Consortium. Sputum ACE2, TMPRSS2 and FURIN gene expression in severe neutrophilic asthma. Respir Res. 2021;22:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Wang H, Sun X, VonCannon JL, Kon ND, Ferrario CM, Groban L. Estrogen receptors are linked to angiotensin-converting enzyme 2 (ACE2), ADAM metallopeptidase domain 17 (ADAM-17), and transmembrane protease serine 2 (TMPRSS2) expression in the human atrium: insights into COVID-19. Hypertens Res 2021; 1-3. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Calcagnile M, Forgez P, Iannelli A, Bucci C, Alifano M, Alifano P. Molecular docking simulation reveals ACE2 polymorphisms that may increase the affinity of ACE2 with the SARS-CoV-2 Spike protein. Biochimie. 2021;180:143-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Zarubin A, Stepanov V, Markov A, Kolesnikov N, Marusin A, Khitrinskaya I, Swarovskaya M, Litvinov S, Ekomasova N, Dzhaubermezov M, Maksimova N, Sukhomyasova A, Shtygasheva O, Khusnutdinova E, Radzhabov M, Kharkov V. Structural Variability, Expression Profile, and Pharmacogenetic Properties of TMPRSS2 Gene as a Potential Target for COVID-19 Therapy. Genes (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Ozono S, Zhang Y, Ode H, Sano K, Tan TS, Imai K, Miyoshi K, Kishigami S, Ueno T, Iwatani Y, Suzuki T, Tokunaga K. SARS-CoV-2 D614G spike mutation increases entry efficiency with enhanced ACE2-binding affinity. Nat Commun. 2021;12:848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 355] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 19. | Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis 2021; 1-15. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 497] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 20. | Toyonaga T, Araba KC, Kennedy MM, Keith BP, Wolber EA, Beasley C, Steinbach EC, Schaner MR, Jain A, Long MD, Barnes EL, Herfarth HH, Isaacs KL, Hansen JJ, Kapadia MR, Guillem JG, Gulati AS, Sethupathy P, Furey TS, Ehre C, Sheikh SZ. Increased colonic expression of ACE2 associates with poor prognosis in Crohn's disease. Sci Rep. 2021;11:13533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Kyrou I, Randeva HS, Spandidos DA, Karteris E. Not only ACE2-the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduct Target Ther. 2021;6:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 22. | Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY). 2020;12:10087-10098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 23. | Dong M, Zhang J, Ma X, Tan J, Chen L, Liu S, Xin Y, Zhuang L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131:110678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 24. | Xiao L, Sakagami H, Miwa N. ACE2: The key Molecule for Understanding the Pathophysiology of Severe and Critical Conditions of COVID-19: Demon or Angel? Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 25. | Nowak JK, Lindstrøm JC, Kalla R, Ricanek P, Halfvarson J, Satsangi J. Age, Inflammation, and Disease Location Are Critical Determinants of Intestinal Expression of SARS-CoV-2 Receptor ACE2 and TMPRSS2 in Inflammatory Bowel Disease. Gastroenterology. 2020;159:1151-1154.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | de Lucena TMC, da Silva Santos AF, de Lima BR, de Albuquerque Borborema ME, de Azevêdo Silva J. Mechanism of inflammatory response in associated comorbidities in COVID-19. Diabetes Metab Syndr. 2020;14:597-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 27. | Rothan HA, Byrareddy SN. The potential threat of multisystem inflammatory syndrome in children during the COVID-19 pandemic. Pediatr Allergy Immunol. 2021;32:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Zipeto D, Palmeira JDF, Argañaraz GA, Argañaraz ER. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front Immunol. 2020;11:576745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 29. | Abassi Z, Higazi AAR, Kinaneh S, Armaly Z, Skorecki K, Heyman SN. ACE2, COVID-19 Infection, Inflammation, and Coagulopathy: Missing Pieces in the Puzzle. Front Physiol. 2020;11:574753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Burgueño JF, Reich A, Hazime H, Quintero MA, Fernandez I, Fritsch J, Santander AM, Brito N, Damas OM, Deshpande A, Kerman DH, Zhang L, Gao Z, Ban Y, Wang L, Pignac-Kobinger J, Abreu MT. Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients With IBD. Inflamm Bowel Dis. 2020;26:797-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 31. | Potdar AA, Dube S, Naito T, Botwin G, Haritunians T, Li D, Yang S, Bilsborough J, Denson LA, Daly M, Targan SR, Fleshner P, Braun J, Kugathasan S, Stappenbeck TS, McGovern DPB. Reduced expression of COVID-19 host receptor, ACE2 is associated with small bowel inflammation, more severe disease, and response to anti-TNF therapy in Crohn's disease. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Azimi A. TMPRSS2 inhibitors, Bromhexine, Aprotinin, Camostat and Nafamostat as potential treatments for COVID-19. 2020. [DOI] [Full Text] |
| 33. | Queiroz NSF, Barros LL, Azevedo MFC, Oba J, Sobrado CW, Carlos AS, Milani LR, Sipahi AM, Damião AOMC. Management of inflammatory bowel disease patients in the COVID-19 pandemic era: a Brazilian tertiary referral center guidance. Clinics (Sao Paulo). 2020;75:e1909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Fragoso RP, Rodrigues M. COVID-19 and pediatric inflammatory bowel disease: How to manage it? Clinics (Sao Paulo). 2020;75:e1962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Al-Ani A, Prentice R, Rentsch C, Johnson D, Ardalan Z, Heerasing N, Garg M, Campbell S, Sasadeusz J, Macrae F. Prevention, diagnosis and management of COVID-19 in the inflammatory bowel disease patient. Aliment Pharmacol Ther 2020. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 36. | An P, Ji M, Ren H, Su J, Ding NS, Kang J, Yin A, Zhou Q, Shen L, Zhao L, Jiang X, Xiao Y, Tan W, Lv X, Li J, Liu S, Zhou J, Chen H, Xu Y, Liu J, Chen M, Cao J, Zhou Z, Tan S, Yu H, Dong W, Ding Y. Prevention of COVID-19 in patients with inflammatory bowel disease in Wuhan, China. Lancet Gastroenterol Hepatol. 2020;5:525-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 37. | Prentice RE, Tjandra D, Garg M, Lubel JS, Fourlanos S, Johnson D, Al-Ani A, Christensen B. Letter: ACE2, IBD and COVID-19-why IBD patients may be at reduced risk of COVID-19. Aliment Pharmacol Ther. 2020;52:1422-1423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Reuken PA, Wüst M, Löffler B, Bauer M, Stallmach A. Letter: SARS-CoV-2-induced gastrointestinal inflammation. Aliment Pharmacol Ther. 2020;52:1748-1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Sultan K, Mone A, Durbin L, Khuwaja S, Swaminath A. Review of inflammatory bowel disease and COVID-19. World J Gastroenterol. 2020;26:5534-5542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Viana SD, Nunes S, Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities - Role of gut microbiota dysbiosis. Ageing Res Rev. 2020;62:101123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 41. | Kavaliers M, Innes DG. Novelty-induced opioid analgesia in deer mice (Peromyscus maniculatus): sex and population differences. Behav Neural Biol. 1988;49:54-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Bhuiyan FR, Howlader S, Raihan T, Hasan M. Plants Metabolites: Possibility of Natural Therapeutics Against the COVID-19 Pandemic. Front Med (Lausanne). 2020;7:444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 43. | Panyod S, Ho CT, Sheen LY. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J Tradit Complement Med. 2020;10:420-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 44. | Huang J, Tao G, Liu J, Cai J, Huang Z, Chen JX. Current Prevention of COVID-19: Natural Products and Herbal Medicine. Front Pharmacol. 2020;11:588508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 45. | Boozari M, Hosseinzadeh H. Natural products for COVID‐19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytother Res 2020. [RCA] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 46. | Naik B, Gupta N, Ojha R, Singh S, Prajapati VK, Prusty D. High throughput virtual screening reveals SARS-CoV-2 multi-target binding natural compounds to lead instant therapy for COVID-19 treatment. Int J Biol Macromol. 2020;160:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 47. | da Silva Antonio A, Wiedemann LSM, Veiga-Junior VF. Natural products' role against COVID-19. RSC Adv 2020; 10: 23379-23393. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 48. | Chen H, Du Q. Potential natural compounds for preventing SARS-CoV-2 (2019-nCoV) infection. 2020 Preprint. Available from: 202001.0358.v3. |
| 49. | Seadawy MG, Gad AF, Shamel M, Elharty B, Mohamed MF, Elfiky AA, Ahmed A, Zekri ARN. in vitro: Natural Compounds (Thymol, Carvacrol, Hesperidine, And Thymoquinone) Against SARS-CoV2 Strain Isolated From Egyptian Patients. 2020 Preprint. Available from: 2020.11.07.367649. |
| 50. | Muchtaridi M, Fauzi M, Khairul Ikram NK, Mohd Gazzali A, Wahab HA. Natural Flavonoids as Potential Angiotensin-Converting Enzyme 2 Inhibitors for Anti-SARS-CoV-2. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 51. | Dabaghian F, Khanavi M, Zarshenas MM. Bioactive compounds with possible inhibitory activity of Angiotensin-Converting Enzyme-II; a gate to manage and prevent COVID-19. Med Hypotheses. 2020;143:109841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Kulkarni SA, Nagarajan SK, Ramesh V, Palaniyandi V, Selvam SP, Madhavan T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J Mol Struct. 2020;1221:128823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 53. | Rathinavel T, Meganathan B, Kumarasamy S, Ammashi S, Thangaswamy S, Ragunathan Y, Palanisamy S. Potential COVID-19 Drug from Natural Phenolic Compounds through In Silico Virtual Screening Approach. Biointerface Res Appl Chem 2020; 10161-10173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Solnier J, Fladerer JP. Flavonoids: A complementary approach to conventional therapy of COVID-19? Phytochem Rev 2020; 1-23. [RCA] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 55. | Luo P, Liu D, Li J. Pharmacological perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int J Antimicrob Agents. 2020;55:105995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 56. | Dowluru KS, Rao KRS. Phylogenetic analysis and In silico Screening of drug targets for ACE2 in Human and Spike Glycoprotein in Sars-CoV-2 for control of COVID19. 2020 Preprint. Available from: rs.3.rs-23862/v1. |
| 57. | Mhatre S, Naik S, Patravale V. A molecular docking study of EGCG and theaflavin digallate with the druggable targets of SARS-CoV-2. Comput Biol Med. 2021;129:104137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 58. | Jang M, Park R, Park YI, Cha YE, Yamamoto A, Lee JI, Park J. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem Biophys Res Commun. 2021;547:23-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 59. | Lichtenstein GR, Rubin DT. Coronavirus and Patients With Inflammatory Bowel Disease: Management Strategies for the Practicing Clinician. Am J Gastroenterol. 2020;115:1566-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Burke KE, Kochar B, Allegretti JR, Winter RW, Lochhead P, Khalili H, Colizzo FP, Hamilton MJ, Chan WW, Ananthakrishnan AN. Immunosuppressive Therapy and Risk of COVID-19 Infection in Patients With Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2021;27:155-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 61. | Sebastian S, Walker GJ, Kennedy NA, Conley TE, Patel KV, Subramanian S, Kent AJ, Segal JP, Brookes MJ, Bhala N, Gonzalez HA, Hicks LC, Mehta SJ, Lamb CA; PROTECT-ASUC Study Group. Assessment, endoscopy, and treatment in patients with acute severe ulcerative colitis during the COVID-19 pandemic (PROTECT-ASUC): a multicentre, observational, case-control study. Lancet Gastroenterol Hepatol. 2021;6:271-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Zingone F, Buda A, Savarino EV. Starting a biologic therapy in IBD patients amidst COVID-19: hold, careful monitoring or testing? J Crohns Colitis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Attauabi M, Seidelin J, Burisch J; Danish COVID-IBD Study Group. Association between 5-aminosalicylates in patients with IBD and risk of severe COVID-19: an artefactual result of research methodology? Gut. 2021;70:2020-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 65. | Saad AF, Chappell L, Saade GR, Pacheco LD. Corticosteroids in the Management of Pregnant Patients With Coronavirus Disease (COVID-19). Obstet Gynecol. 2020;136:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 66. | Tlayjeh H, Mhish OH, Enani MA, Alruwaili A, Tleyjeh R, Thalib L, Hassett L, Arabi YM, Kashour T, Tleyjeh IM. Association of corticosteroids use and outcomes in COVID-19 patients: A systematic review and meta-analysis. J Infect Public Health 2020. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 67. | Brenner EJ, Pigneur B, Focht G, Zhang X, Ungaro RC, Colombel JF, Turner D, Kappelman MD, Ruemmele FM. Benign Evolution of SARS-Cov2 Infections in Children With Inflammatory Bowel Disease: Results From Two International Databases. Clin Gastroenterol Hepatol. 2021;19:394-396.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 68. | El Ouali S, Rubin DT, Cohen BL, Regueiro MD, Rieder F. Optimal inflammatory bowel disease management during the global coronavirus disease 2019 pandemic. Curr Opin Gastroenterol. 2021;37:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Gutin LS, Lam AY, Velayos FS, Santos SA. Going Viral: Management of IBD in the Era of the COVID-19 Pandemic. Dig Dis Sci. 2020;65:1571-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Schuurmans MM, Hage R. Cyclosporine A and COVID-19 - The COQUIMA cohort. EClinicalMedicine. 2021;31:100680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Pathania YS. Cyclosporine: hope for severe COVID-19? BMJ Support Palliat Care 2021 . [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Al-Ani AH, Prentice RE, Christensen B. Letter to the Editor: Care of the Patient with IBD Requiring Hospitalisation During the COVID-19 Pandemic. J Crohns Colitis 2020. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 73. | Ghoshal UC, Sahu S, Biswas SN, Singh P, Chaudhary M, Ghoshal U, Tiwari P, Rai S, Mishra SK. Care of inflammatory bowel disease patients during coronavirus disease‐19 pandemic using digital health‐care technology. JGH Open 2021. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Mansouri P, Farshi S, Nickhah N, Nobari NN, Chalangari R, Nilforoushzadeh MA. Immunosuppressive/Immunomodulatory Therapies in Dermatology and COVID-19. Clin Dermatol 2021. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | D’Silva KM, Serling-Boyd N, Hsu TY, Sparks JA, Wallace ZS. SARS-CoV-2 antibody response after COVID-19 in patients with rheumatic disease. Ann Rheum Dis 2021. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Kasperkiewicz M. COVID-19 outbreak and autoimmune bullous diseases: A systematic review of published cases. J Am Acad Dermatol. 2021;84:563-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 77. | Festa S, Aratari A, De Biasio F, Fascì-Spurio F, Papi C. Screening for active COVID-19 infection prior to biologic therapy in IBD patients: Let's not increase our uncertainty without reducing our concerns. Dig Liver Dis 2020; 52: 1246-1247. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Danese S, Cecconi M, Spinelli A. Management of IBD during the COVID-19 outbreak: resetting clinical priorities. Nat Rev Gastroenterol Hepatol. 2020;17:253-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 79. | Weissman S, Aziz M, Smith WL, Elias S, Swaminath A, Feuerstein JD. Safety of biologics in inflammatory bowel disease patients with COVID-19. Int J Colorectal Dis. 2021;36:2051-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Gargallo-Puyuelo CJ, Laredo V, Gomollón F. Thiopurines in Inflammatory Bowel Disease. How to Optimize Thiopurines in the Biologic Era? Front Med (Lausanne). 2021;8:681907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Aysha AA, Rentsch C, Prentice R, Johnson D, Bryant RV, Ward MG, Costello SP, Lewindon P, Ghaly S, Connor SJ, Begun J, Christensen B. Practical management of inflammatory bowel disease patients during the COVID-19 pandemic: expert commentary from the Gastroenterological Society of Australia Inflammatory Bowel Disease faculty. Intern Med J. 2020;50:798-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Weidinger C, Hegazy AN, Glauben R, Siegmund B. COVID-19—from mucosal immunology to IBD patients. Mucosal Immunol 2021; 1-8. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Shields S, Dunlop A, Seenan JP, Macdonald J. Disease monitoring of biologic treatment in IBD: early impact and future implications of COVID-19 pandemic. BMJ 2020. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Fung M, Babik JM. COVID-19 in Immunocompromised Hosts: What We Know So Far. Clin Infect Dis. 2021;72:340-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 372] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 85. | Norsa L, Indriolo A, Sansotta N, Cosimo P, Greco S, D'Antiga L. Uneventful course in IBD patients during SARS-CoV-2 outbreak in northern Italy. Gastroenterology 2020; 159: 371-372. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 86. | Mazza S, Sorce A, Peyvandi F, Vecchi M, Caprioli F. A fatal case of COVID-19 pneumonia occurring in a patient with severe acute ulcerative colitis. Gut. 2020;69:1148-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 87. | Tursi A, Papa A. Impact of Anti-TNFα Antibodies on the Risk of Covid-19 and its Severity in Patients with Inflammatory Bowel Diseases. J Crohns Colitis. 2020;14:1646-1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Bodini G, Demarzo MG, Casagrande E, De Maria C, Kayali S, Ziola S, Giannini EG. Concerns related to COVID-19 pandemic among patients with inflammatory bowel disease and its influence on patient management. Eur J Clin Invest. 2020;50:e13233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Tursi A, Angarano G, Monno L, Saracino A, Signorile F, Ricciardi A, Papa A. COVID-19 infection in Crohn's disease under treatment with adalimumab. Gut 2020; 69: 1364-1365. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 90. | Allocca M, Fiorino G, Furfaro F, Gilardi D, Radice S, D'Amico F, Zilli A, Danese S. Maintaining the Quality Standards of Care for Inflammatory Bowel Disease Patients During the COVID-19 Pandemic. Clin Gastroenterol Hepatol. 2020;18:1882-1883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 91. | Jacobs J, Clark-Snustad K, Lee S. Case Report of a SARS-CoV-2 Infection in a Patient With Ulcerative Colitis on Tofacitinib. Inflamm Bowel Dis. 2020;26:e64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 92. | Taxonera C, Sagastagoitia I, Alba C, Mañas N, Olivares D, Rey E. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52:276-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 93. | Allocca M, Fiorino G, Zallot C, Furfaro F, Gilardi D, Radice S, Danese S, Peyrin-Biroulet L. Incidence and Patterns of COVID-19 Among Inflammatory Bowel Disease Patients From the Nancy and Milan Cohorts. Clin Gastroenterol Hepatol. 2020;18:2134-2135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 94. | Mak JWY, Weng MT, Wei SC, Ng SC. Zero COVID-19 infection in inflammatory bowel disease patients: Findings from population-based inflammatory bowel disease registries in Hong Kong and Taiwan. J Gastroenterol Hepatol. 2021;36:171-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Bardasi G, Alvisi P. SARS-CoV-2 infection in severe pediatric Crohn's disease. What about anti-tumor necrosis factor α therapy? Dig Liver Dis. 2020;52:1244-1245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Ashton JJ, Kammermeier J, Spray C, Russell RK, Hansen R, Howarth LJ, Torrente F, Deb P, Renji E, Muhammed R, Paul T, Kiparissi F, Epstein J, Lawson M, Hope B, Zamvar V, Narula P, Kadir A, Devadason D, Bhavsar H, Beattie RM. Impact of COVID-19 on diagnosis and management of paediatric inflammatory bowel disease during lockdown: a UK nationwide study. Arch Dis Child. 2020;105:1186-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |