Published online Nov 21, 2021. doi: 10.3748/wjg.v27.i43.7563
Peer-review started: April 19, 2021
First decision: June 23, 2021
Revised: July 20, 2021
Accepted: November 2, 2021
Article in press: November 2, 2021
Published online: November 21, 2021
Processing time: 213 Days and 23.6 Hours
Autoimmune markers including plasma cells (PC), anti-smooth-muscle antibody (ASMA), anti-nuclear antibody (ANA), and raised immunoglobulin G (IgG) are commonly observed in non-alcoholic steatohepatitis (NASH), however their clinical significance is unknown.
To determine if autoimmune markers in NASH patients are independently associated with poorer clinical outcomes.
Consecutive patients with biopsy proven NASH from Christchurch Hospital, New Zealand and Singapore General Hospital (SGH) were included between 2005 to 2016 in a prospective multi-centre cohort study. Patients with other causes of chronic liver disease were excluded. IgG > 14 g/L or globulin fraction > 50%, ANA ≥ 1:40, SMA ≥ 1:40 were considered positive. Multivariate analysis was performed to assess which markers were independently associated with mortality and hepatic decompensation.
Total 261 patients were included of which 201 were from SGH. The median age was 53 and 51.9% were male. Advanced fibrosis was present in 31.4% at diagnosis. PC, ASMA, ANA and raised IgG were observed in 13.1%, 4.9%, 27.8% and 30.1% of patients respectively. After multivariate analysis, elevated IgG [Hazard Ratio (HR) 6.79, 95%CI: 2.93-17.15] and fibrosis stage (HR 1.37, 95%CI: 1.03-1.87) were found to be independently associated with increased risk of liver decompensation. Age (HR 1.06, 95%CI: 1.02-1.10) and elevated IgG (HR 3.79, 95%CI: 1.90-7.68) were independent factors associated with higher mortality risk.
Elevated IgG, rather than ANA, ASMA or plasma cells, is independently associated with increased risk of hepatic decompensation and mortality in NASH. It could hence be important for prognostication.
Core Tip: Autoantibodies such as anti-nuclear antibody (ANA) and anti-smooth-muscle antibody (ASMA) can be present in up to 20%-30% of patients with non-alcoholic steatohepatitis (NASH). However, clinical significance is not well studied and there is no published data on the impact of immunoglobulin G (IgG) and plasma cells on hepatic decompensation and mortality outcomes. Our study found that elevated IgG but not ANA, ASMA or plasma cells is associated with higher risk of mortality, including liver related death, as well as increased risk of hepatic decompensation events. Patients with IgG positive NASH should hence be identified early and monitored closely as they are at higher risk of poorer clinical outcomes.
- Citation: De Roza MA, Lamba M, Goh GBB, Lum JHM, Cheah MCC, Ngu JHJ. Immunoglobulin G in non-alcoholic steatohepatitis predicts clinical outcome: A prospective multi-centre cohort study. World J Gastroenterol 2021; 27(43): 7563-7571
- URL: https://www.wjgnet.com/1007-9327/full/v27/i43/7563.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i43.7563
Non-alcoholic fatty liver disease (NAFLD) is a growing phenomenon with an estimated global prevalence of 25%. Non-alcoholic steatohepatitis (NASH) in particular is a progressive form of NAFLD and is associated with poorer clinical outcomes and higher liver related mortality[1]. Independent predictors for poor outcomes include fibrosis[2], obesity and metabolic syndrome, diabetes mellitus (DM)[3], as well as genetic polymorphisms such as PNPLA3[4]. NASH is characterized histologically by hepatic steatosis, inflammation, hepatocellular injury and varying degrees of fibrosis[5]. The inflammatory process in NASH is a complex and heterogeneous “multi-hit” pathway in which the innate immune system plays a critical role, driving the progression of liver fibrosis and leading to cirrhosis, liver failure, the need for liver transplantation and death[6-8]. Less is known, however, about the role of the adaptive immune system and autoantibodies. Autoantibodies are produced by humoral immune responses against self-cellular proteins and nucleic acids and can be physiological or pathological[9]. When used in tandem with clinical findings, they are serological hallmarks for inflammatory autoimmune liver diseases. However, their significance in NAFLD is not well studied despite autoantibodies being present in 25%-35% of patients with NAFLD[10,11].
Adams et al[10] reported a higher grade of inflammation in NAFLD patients with positive antinuclear antibodies (ANA) and/or anti smooth muscle antibodies (ASMA) but the difference was slight (1.0 vs 1.2, P = 0.02) and there was no correlation to clinical significance or outcomes. More recent data from McPherson et al[12] looking specifically at serum immunoglobulins in NAFLD, showed that elevated serum immunoglobulin A was significantly associated with advanced fibrosis. Similarly, there was no correlation to immunoglobulins being a predictor for mortality or hepatic events independently from fibrosis and other factors. Despite evidence showing association of autoantibodies and immunoglobulins with higher histological grades of inflammation or fibrosis[10,12,13], other studies dispute these findings[14,15], and correlation to clinical outcomes is still not established in NASH. Ravi et al[16] reported no significant difference on liver disease outcomes in steatohepatitis patients with positive ANA or ASMA , however, the major limitation of the study was that overall follow-up was short (median 3 years) and alcoholic and non-alcoholic hepatitis were grouped together.
Overall, autoimmune markers such as ANA, ASMA, plasma cells and immunoglobulins with immunoglobulin G (IgG) in particular, are not well studied in NASH and their clinical significance is unknown in this population. Hence, the aim of this study is to determine if autoimmune markers in patients with biopsy proven NASH are independently associated with poorer outcomes. The outcomes measured being all cause mortality and liver decompensation events.
Consecutive patients who underwent liver biopsy at Christchurch Hospital, New Zealand (CH) and Singapore General Hospital, Singapore (SGH) were assessed for inclusion in the study. CH cohort consisted of patients with liver biopsy performed from 2008 to 2016 and the SGH cohort from 2005 to 2015. Patients with chronic liver diseases of other aetiologies such as viral hepatitis, alcohol, toxins or drugs, autoimmune including IgG4 related disease, vascular, metabolic and hereditary causes were excluded. All patients, as per local hospital protocol underwent non-invasive liver testing including serology analysis and imaging to exclude other causes of chronic liver disease. Patients were included in the study if the final histological diagnosis was NASH with NAFLD activity score (NAS) ≥ 3 on biopsy with scores for steatosis, lobular inflammation and hepatocyte ballooning[17].
ANA and ASMA were considered positive if titres were observed to be ≥ 1:40. IgG was considered elevated if > 14g/L. Additionally, if quantitative values were not available, globulin fraction (GF) was calculated by the following equation: total protein/(total protein - albumin). Since IgG is the commonest globulin type[18], individuals with GF > 50% were defined as elevated IgG. To assess presence of plasma cells, histology reports were reviewed. Plasma cells were scored as positive if any plasma cells were identified on histology specimens by the pathologist. This study conforms to ethical guidelines and was approved by our respective institutional review boards.
Patients from CH and SGH were followed up for clinical events of liver decompensation and all-cause mortality. Liver decompensation event was defined as the development of any of the following: ascites, gastrointestinal haemorrhage secondary to portal hypertension, hepatic encephalopathy, hepatorenal syndrome. Patients were followed up till 31st December 2017. Follow-up was censored at development of first liver decompensation event, death, liver transplantation, or last clinical contact in case of patients lost to follow-up.
Continuous variables were expressed as mean ± SD or median (interquartile range, IQR) and were compared using unpaired t-test. Categorical variables were compared using χ2 test. The associations of putative risk factors and outcomes were analyzed using Cox proportional hazards regression and are summarized as hazard ratios (HR) with 95%CI. The time to event outcomes were also summarized using Kaplan-Meier curves. A two-tailed P value of < 0.05 was taken to indicate a statistical significance. All analyses were undertaken using statistical software SPSS version 20.
A total of 261 patients met the study criteria. Of these, 201 patients were recruited from SGH and 60 patients from CH. Baseline characteristics of patients are listed in Table 1. Majority of patients from CH were of European origin (91.7%) while 97.5% patients from SGH were of Asian origin, reflecting the local population demographic. Median follow-up per patient was 5.1 years (IQR 3.5-7.5). Median age at inclusion in the study was 53 years (± 12.9) and 51.9% were male. The median NAS score at diagnosis was 4 (IQR 3-5) and the mean Metavir fibrosis score was 1.7 (± 1.4). 77% of patients had data available for body mass index (BMI), and comorbidities including presence of DM, of which the mean BMI was 30.61 and DM was present in 45.02% of patients. There were no significant differences in baseline characteristics between patients from SGH and CH (Table 1).
| Overall | CH | SGH | P value | |
| Patients | 261 | 60 | 201 | - |
| Regions | 0.07 | |||
| European | 60 (23.0) | 55 (91.7) | 5 (2.5) | |
| Asian | 197 (75.5) | 2 (3.3) | 195 (97.5) | |
| Others | 4 (1.5) | 3 (5.5) | 1 (0.5) | |
| Age, mean ± SD | 53 ± 12.9 | 52.9 ± 16.9 | 53 ± 11.7 | 0.30 |
| Male | 51.9% | 53.3% | 51.7% | 0.83 |
| ALT, mean ± SD | 112.3 ± 373.9 | 80.2 ± 66.6 | 121.9 ± 425.5 | 0.45 |
| AST, mean ± SD | 80.3 ± 216.7 | 60.8 ± 42.3 | 86.1 ± 246.1 | 0.43 |
| NAS, Median (IQR) | 4 (3-5) | 4 (3-5) | 4 (3-5) | 0.54 |
| Metavir Fibrosis score, mean ± SD | 1.7 ± 1.4 | 1.6 ± 1.4 | 1.7 ± 1.3 | 0.58 |
| F0 | 53 (20.3) | 13 (21.7) | 40 (19.9) | |
| F1 | 95 (36.3) | 25 (41.7) | 70 (34.8) | |
| F2 | 31 (11.9) | 5 (8.3) | 26 (12.9) | |
| F3 | 48 (18.4) | 7 (11.7) | 41 (20.4) | |
| F4 | 34 (13.1) | 10 (16.6) | 24 (11.9) |
ANA was the most common positive autoimmune marker present in 27.8% patients followed by elevated IgG observed in 23.4%. Plasma cells were found on histological examination in 13% of patients with NASH. ASMA was the least common autoimmune marker and was positive in only 4.9% of NASH patients.
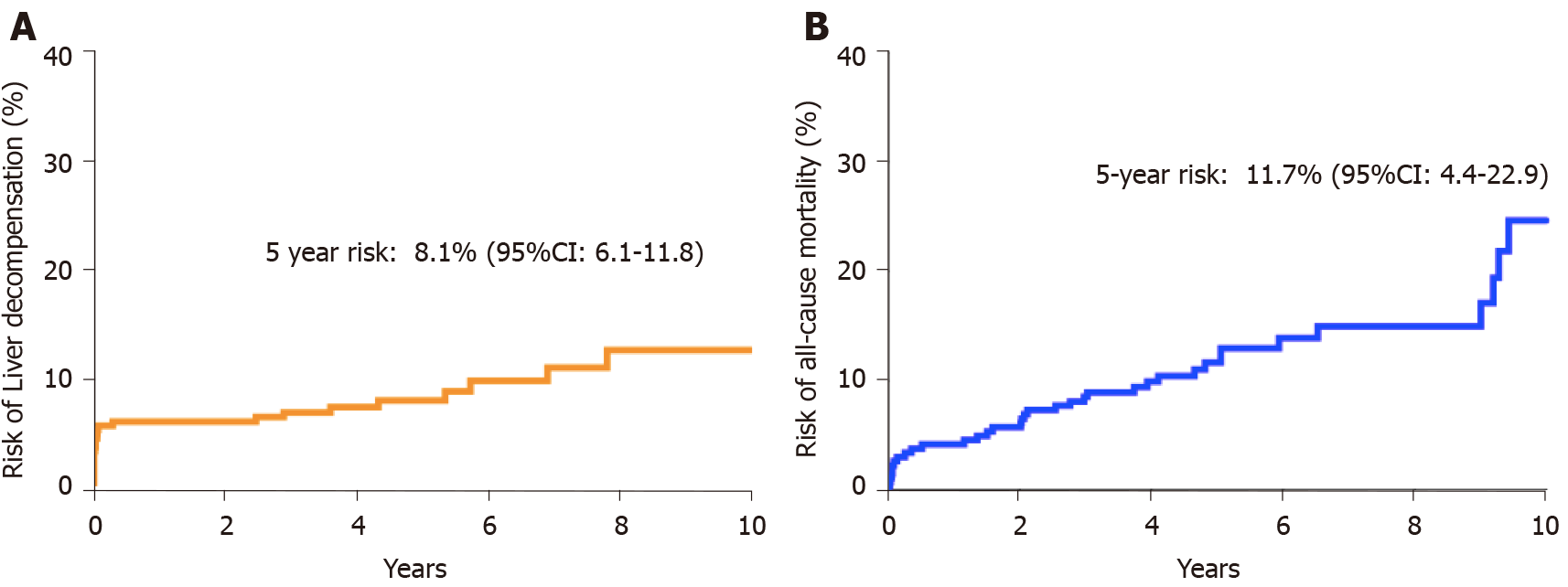
During a cumulative follow-up of 1464 person years, 25 patients developed liver decompensation. There was no significant difference between risk of liver decompensation or all-cause mortality between patients from CH and SGH. The 5-year risk of developing liver decompensation after diagnosis of NASH was 8.1% (95%CI: 6.1-11.8) and the 5-year mortality risk was 11.7% (95%CI: 4.4-22.9) (Figure 1). Ten patients developed hepatocellular carcinoma of which 7 were male. Median age at diagnosis of HCC was 65.7 years. Advanced fibrosis or cirrhosis was present in 6 of 10 patients at diagnosis of HCC. During the follow-up period, 36 patients died. Data on cause of death were available for 30 (83.3%) patients. Liver related causes of death were observed in 12 cases (40%), followed by malignancy (30%), septicaemia (17%) and cardiovascular causes of death (13%). Overall, 5-year risk of all-cause mortality was 11.7% (95%CI: 4.4-22.9) (Figure 1).
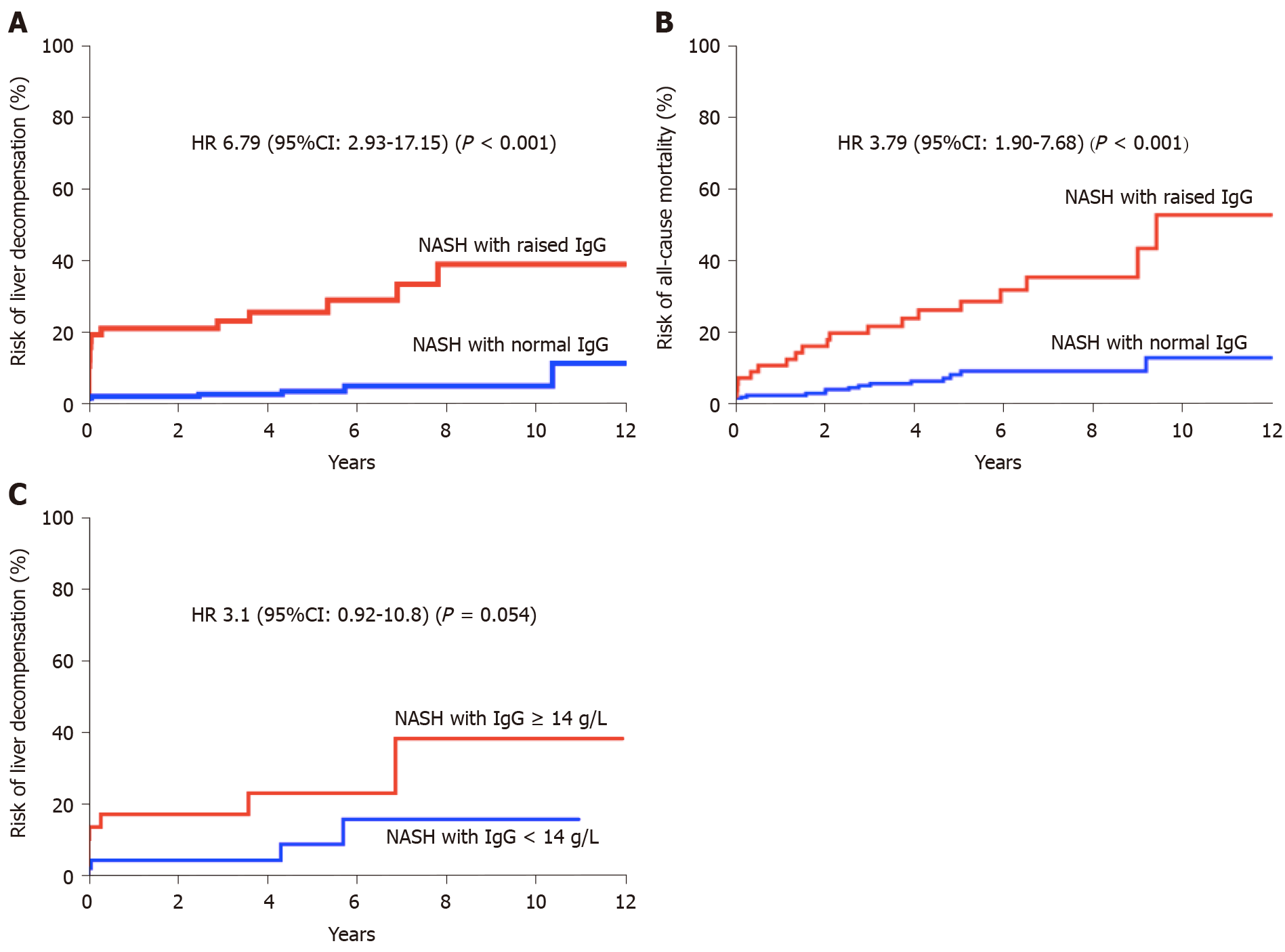
Liver decompensation: In univariate analysis (Table 2), factors associated with increased risk of liver decompensation were increasing age (HR 1.04, 95%CI: 1.00-1.08), stage of fibrosis (HR 1.67, 95%CI: 1.25-2.26) and elevated IgG (HR 8.20, 95%CI: 3.61-20.30). Other autoimmune markers (ANA, ASMA or Plasma cells) were not found to be associated with risk of liver decompensation (Table 2; Figure 1). Multivariate model was constructed with variables found to be significant in univariate analysis. In multivariate analysis, elevated IgG (HR 6.79, 95%CI: 2.93-17.15) and stage of fibrosis (HR 1.37, 95%CI: 1.03-1.87) were found to be independently associated with increased risk of liver decompensation during follow-up (Table 2).
| Predictors | Liver decompensation | All-cause mortality | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age1 (per 1 yr) | 1.04 (1.00-1.08) | 0.04 | 1.01 (0.98-1.05) | 0.46 | 1.06 (1.03-1.10) | < 0.0001 | 1.06 (1.02-1.10) | 0.001 |
| Sex | 0.99 (0.44-2.19) | 0.98 | - | - | 1.16 (0.60-2.27) | 0.65 | - | - |
| Fibrosis2 | 1.67 (1.25-2.26) | < 0.001 | 1.37 (1.03-1.87) | 0.03 | 1.27 (1.00-1.61) | 0.05 | 1.01 (0.80-1.29) | 0.91 |
| NAS3 | 0.96 (0.67-1.32) | 0.79 | - | - | 0.88 (0.66-1.16) | 0.37 | - | - |
| IgG | 8.20 (3.61-20.30) | < 0.0001 | 6.79 (2.93-17.15) | < 0.0001 | 4.50 (2.29-9.00) | < 0.0001 | 3.79 (1.90-7.68) | 0.0001 |
| ANA | 1.15 (0.41-2.86) | 0.78 | - | - | 1.14 (0.47-2.51) | 0.76 | - | - |
| ASMA | 0 | - | - | 0.94 (0.05-4.53) | 0.95 | - | - | |
| Plasma cells | 0.97 (0.23-2.81) | 0.96 | - | - | 1.20 (0.41-2.83) | 0.71 | - | - |
In a sub-group analysis where only patients with quantitative IgG values (> 14 g/L) were included (n = 43), a trend of association was observed between elevated IgG and increased risk of liver-decompensation during follow-up. (HR 3.1, 95%CI: 0.92-10.8, P = 0.054) (Figure 2).
Mortality: In univariate analysis, predictors of all-cause mortality included: increasing age (HR 1.06, 95%CI: 1.03-1.10), stage of fibrosis (HR 1.27, 95%CI: 1.00-1.61) and elevated IgG (HR 4.5, 95%CI: 2.29-9.00) (Table 2). In multivariate analysis, age (HR 1.06, 95%CI: 1.02-1.10) and elevated IgG (HR 3.79, 95%CI: 1.90-7.68) were found to be independent factors associated with increased risk of mortality (Table 2; Figure 2). Median survival in patients with elevated IgG at baseline was 9.4 years.
In this multicentre cohort study, we examined the association between the presence of autoimmune markers such as ANA, ASMA, elevated IgG and plasma cells on histology with clinical outcomes in patients with NASH. The most pertinent finding of our study is that elevated IgG at diagnosis of NASH was associated with increased risk of liver decompensation and reduced overall survival.
Autoimmune markers are commonly encountered in patients with NASH, however their clinical significance is not well defined. In a study of 225 patients with histologically confirmed NAFLD, 20% and 3% respectively were found to have the presence of ANA and ASMA[10]. Similarly, in another cohort study of NASH patients, the presence of ANA and ASMA was observed in 34% and 6% of all patients respectively[15]. The findings of our study are consistent with the reported prevalence estimates. While inflammation involving plasma cells is typically observed in AIH, the prevalence of plasma cell infiltration in NASH is not known. In the present study, plasma cell infiltration was observed in 13% of patients with histological diagnosis of NASH.
Association of ANA and/or ASMA with histological severity of NASH has been examined previously in multiple cohort studies and yielded conflicting results[10,12-15,19]. None of the studies, to our knowledge, have assessed association of autoimmune markers with long-term clinical outcomes. We found that the risk of liver decompensation or all-cause mortality were not associated with the presence of either ANA, ASMA or plasma cells, thereby suggesting that these are non-specific markers of inflammation and unlikely to be pathogenically relevant.
On the contrary, elevated IgG at diagnosis of NASH was independently associated with increased risk of liver decompensation (HR 6.79, 95%CI: 2.93-17.15, P < 0.0001) and all-cause mortality (HR 3.79, 95%CI: 1.9-7.68, P = 0.0001) during follow-up. Majority of the excess mortality in the elevated IgG cohort were liver-related. It needs to be highlighted that diagnosis of probable or definite AIH was conclusively ruled out in all patients based on the international standardised criteria and no patients were treated with immunosuppression except in cases of organ transplantation.
We do not have a concrete biological explanation for the observed pathogenic role of IgG in NASH, however several mechanisms are plausible. Firstly, oxidative stress in NASH may induce production of IgG by deployment of adaptive humoral response[5,20]. Animal and human based studies have shown that IgG directed against products of lipid peroxidation such as Malondialdehyde and 4-hydroxynonenal appears to be elevated in NASH and correlates with disease severity[20]. Secondly, anti-endotoxins IgG levels were observed to be higher in patients with NASH compared to controls (48 vs 10 GMU/mL), and IgG levels corelated with severity of NASH[20,21]. Endotoxins are generally derived from the gut microbiota[21] and are potential triggers for inflammation and insulin resistance, driving oxidative stress in NASH. Therefore, elevated IgG may be representative of high endotoxemic burden leading to rapid progression of NASH. Lastly, it is possible that elevated IgG in patients with NASH may represent an overlap with a mild degree of autoimmune hepatitis. However, currently no diagnostic criteria exist to define NASH-AIH overlap syndrome.
Our study has several limitations which ought to be acknowledged. Quantitative immunoglobulin values were not available for all patients and globulin fraction was used as a surrogate marker of elevated IgG. While globulin fraction has previously been utilised as an effective surrogate marker of hyper/hypogammaglobulinemia[22] it is possible that we may have under or overestimated the IgG effect on clinical outcomes. However, upon restricting analysis to those patients with quantitative IgG values, a similar effect was observed (although statistically non-significant), suggesting that the observed association between IgG and poor clinical outcomes is true rather than a type-1 error. Data for pre-existing medical comorbidities and current medications were only available for 77% of patients, which may have confounded overall results. However, all patients were on follow up with a specialist and would have received standard of care for hepatic decompensation, regardless of compliance to treatment. Lastly, the diagnosis of NASH was based on unblinded histology interpretation by the local pathology team, consequently an element of inter-observer bias cannot be ruled out. Our study design involved two different population cohorts from two large tertiary centres. Despite having different ethnic compositions, there were no significant differences in baseline-characteristics at inclusion in the study between the two centres suggesting that our results are generalizable. Importantly, both centres have national electronic records available making complete data ascertainment of clinical events possible.
In conclusion, we report that ANA, ASMA and plasma cells are commonly present in patients with NASH but carry no prognostic significance. On the contrary, elevated IgG is an independent predictor of increased risk of liver decompensation and reduced overall survival in patients with NASH. Presence of elevated IgG therefore represents a more aggressive NASH phenotype. Identification and close monitoring of these patients is prudent to improve overall clinical outcomes.
Autoimmune markers such as immunoglobulin G (IgG), anti-nuclear antibody (ANA), anti-smooth-muscle antibody (ASMA) can be present in patients with Non-alcoholic steatohepatitis (NASH) but their clinical significance is not well studied.
Knowing the clinical significance of autoimmune markers in patients with biopsy proven NASH can pave the way for future research to better understand why certain sub-groups of patients with NASH deteriorate more rapidly.
This study aimed to determine if any of the autoimmune markers were independently associated with worse outcomes such as mortality and hepatic decompensation. This is important as such patients can be identified for closer monitoring.
This is a prospective, multi-center study. Patients with biopsy proven NASH were included and multivariate analysis was performed to determine if any of the autoimmune markers (IgG, ANA, ASMA) were independent risk factors for mortality and hepatic decompensation
Elevated IgG was an independent risk factor for both mortality and liver decompensation after multivariate analysis with adjustment for age and fibrosis stage. The exact pathophysiology is unknown but IgG levels could possibly correlate to disease severity due to anti-endotoxins IgG and oxidative stress.
Elevated IgG is an independent predictor of increased risk of liver decompensation and reduced survival in patients with NASH. It could represent a more aggressive NASH phenotype.
Further research is needed to validate and reproduce this finding and to also establish the pathophysiology and underlying biochemical mechanisms for this observation.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: New Zealand
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khiatah B S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 2. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-97.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2231] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 3. | Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 713] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 4. | Xu R, Tao A, Zhang S, Deng Y, Chen G. Association between patatin-like phospholipase domain containing 3 gene (PNPLA3) polymorphisms and nonalcoholic fatty liver disease: a HuGE review and meta-analysis. Sci Rep. 2015;5:9284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15:349-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 665] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 6. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-54.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1240] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 7. | Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 8. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 9. | Czaja AJ, Homburger HA. Autoantibodies in liver disease. Gastroenterology. 2001;120:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Adams LA, Lindor KD, Angulo P. The prevalence of autoantibodies and autoimmune hepatitis in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2004;99:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Loria P, Lonardo A, Leonardi F, Fontana C, Carulli L, Verrone AM, Borsatti A, Bertolotti M, Cassani F, Bagni A, Muratori P, Ganazzi D, Bianchi FB, Carulli N. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. Dig Dis Sci. 2003;48:2173-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | McPherson S, Henderson E, Burt AD, Day CP, Anstee QM. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Niwa H, Sasaki M, Haratake J, Kasai T, Katayanagi K, Kurumaya H, Masuda S, Minato H, Zen Y, Uchiyama A, Miwa A, Saito K, Sudo Y, Nakanuma Y. Clinicopathological significance of antinuclear antibodies in non-alcoholic steatohepatitis. Hepatol Res. 2007;37:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Vuppalanchi R, Gould RJ, Wilson LA, Unalp-Arida A, Cummings OW, Chalasani N, Kowdley KV; Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). Clinical significance of serum autoantibodies in patients with NAFLD: results from the nonalcoholic steatohepatitis clinical research network. Hepatol Int. 2012;6:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Cotler SJ, Kanji K, Keshavarzian A, Jensen DM, Jakate S. Prevalence and significance of autoantibodies in patients with non-alcoholic steatohepatitis. J Clin Gastroenterol. 2004;38:801-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Ravi S, Shoreibah M, Raff E, Bloomer J, Kakati D, Rasheed K, Singal AK. Autoimmune Markers Do Not Impact Clinical Presentation or Natural History of Steatohepatitis-Related Liver Disease. Dig Dis Sci. 2015;60:3788-3793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8249] [Article Influence: 412.5] [Reference Citation Analysis (5)] |
| 18. | Volume 151, number 2 (1998), in article no. TO988472, "Biopersistence of synthetic vitreous fibers and amosite asbestos in the rat lung following Inhalation," by T. W. Hesterberg, G. Chase, C. Axten, W. C. Miller, R. P. Musselman, O. Kamstrup, J. Hadley, C. Morscheidt, D. M. Bernstein, and P. Thevenaz, pages 262-275. Toxicol Appl Pharmacol. 1999;155:292. [PubMed] |
| 19. | Yatsuji S, Hashimoto E, Kaneda H, Taniai M, Tokushige K, Shiratori K. Diagnosing autoimmune hepatitis in nonalcoholic fatty liver disease: is the International Autoimmune Hepatitis Group scoring system useful? J Gastroenterol. 2005;40:1130-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, Albano E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014;59:886-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 21. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4564] [Article Influence: 253.6] [Reference Citation Analysis (1)] |
| 22. | Holding S, Khan S, Sewell WA, Jolles S, Dore PC. Using calculated globulin fraction to reduce diagnostic delay in primary and secondary hypogammaglobulinaemias: results of a demonstration project. Ann Clin Biochem. 2015;52:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |