Published online Nov 21, 2021. doi: 10.3748/wjg.v27.i43.7462
Peer-review started: April 23, 2021
First decision: June 23, 2021
Revised: July 9, 2021
Accepted: October 27, 2021
Article in press: October 27, 2021
Published online: November 21, 2021
Processing time: 210 Days and 10.3 Hours
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver and has an overall five-year survival rate of less than twenty percent. For patients with unresectable disease, evolving liver-directed locoregional therapies provide efficacious treatment across the spectrum of disease stages and via a variety of catheter-directed and percutaneous techniques. Goals of locoregional therapies in HCC may include curative intent in early-stage disease, bridging or downstaging to surgical resection or transplantation for early or intermediate-stage disease, and local disease control and palliation in advanced-stage disease. This review explores the outcomes of chemoembolization, bland embolization, radioembolization, and percutaneous ablative therapies. Attention is also given to prognostic factors related to each of the respective techniques, as well as future directions of locoregional therapies for HCC.
Core Tip: This article reviews prognostic factors and outcomes of current locoregional therapies for hepatocellular carcinoma, as well as future directions and promising new techniques. Therapies including transarterial bland embolization, chemoembolization, and radioembolization, as well as percutaneous ablation are reviewed. Prognostic considerations vary by indication but generally follow baseline disease staging and tumor quantification. Outcomes data reveal survival benefits in appropriately selected patients. New advances in precision medicine, combination therapy, and immunotherapy are being investigated.
- Citation: Makary MS, Ramsell S, Miller E, Beal EW, Dowell JD. Hepatocellular carcinoma locoregional therapies: Outcomes and future horizons. World J Gastroenterol 2021; 27(43): 7462-7479
- URL: https://www.wjgnet.com/1007-9327/full/v27/i43/7462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i43.7462
Hepatocellular carcinoma (HCC) is the fifth most common cancer globally[1] and the most common primary liver malignancy[2,3], comprising over 90% of liver tumors[4,5]. The overall prognosis of HCC involves a complex interplay of baseline clinical staging, underlying liver function, and demographic factors[6,7]. Nonetheless, the 5-year relative survival rate for primary liver cancer is estimated to be 19.6%[8], with a mean survival being reported between 6-20 mo[5]. The unfavorable prognosis of HCC highlights the importance of treatment innovation and improvement. Surgical therapy has been the traditional definitive management in eligible patients; however, fewer than 20% of HCC patients are candidates for surgical resection based on a variety of tumor and disease characteristics. For the remainder of HCC patients, liver-directed locoregional therapies form the mainstay of treatment.
Locoregional therapies play a vital role in HCC therapy across a vast range of disease stages[9]. Image-guided techniques with locoregional delivery of chemotherapeutic, radiotherapeutic, or ablative therapy are flourishing[10,11]. Minimally-invasive approaches, such as transarterial chemoembolization (TACE), transarterial embolization (TAE), transarterial radioembolization (TARE), and ablation may be indicated based on patient clinical status and tumor characteristics. Treatment goals may include bridging to or downstaging for transplant eligibility, inducing parenchymal hypertrophy to enhance function following resection, disease control and palliation, and, in some instances, cure[10]. In general, locoregional liver-directed treatments provide less morbidity than traditional surgical options while also improving outcomes compared to traditional systemic therapies[12]. This paper reviews prognostic factors and outcomes of locoregional therapies for HCC. We discuss how prognostic factors overlay the clinical staging systems most commonly used, the existent data regarding survival and treatment response, and future directions of locoregional HCC therapy.
TACE relies on a combination of targeted chemotherapeutic and embolic agents[13]. Transarterial therapies make use of a mismatch in blood flow between healthy liver parenchymal tissue and hepatocellular tumors. Unlike normal liver parenchyma, which derives most of its blood supply from the portal venous system, hepatocellular tumors are primarily perfused via the hepatic arterial system[14]. Thus, normal tissue is preferentially spared when therapies are targeted at tumor tissue through the hepatic arterial tree. Conventional TACE (cTACE) utilizes hepatic arterial administration of a chemotherapeutic agent emulsed with lipiodol oil to increase chemotherapeutic concentration and decrease pharmacologic washout. Chemotherapeutic and embolic agent administration via drug-eluting beads (DEB-TACE) has been shown to provide less systemic chemotherapy uptake, an increased ischemic effect, and a more homogenous drug distribution due to decreased variability in delivery technique[15-19]. Use of small drug-eluting microspheres (DEM-TACE) and balloon occlusion catheters (B-TACE) represent newer approaches to chemoembolization, albeit with less comparative data in HCC treatment at this stage[20-22]. A summary of these TACE approaches, as well as approaches, clinical strengths, and risks of the other locoregional therapies discussed here can be seen in Table 1.
| Locoregional modality | Techniques | Clinical advantages | Clinical risks |
| TACE | Drug-eluting beads or conventional delivery | Provides both local embolic and chemotherapeutic effect | PES, biloma, liver abscess, liver failure |
| TAE | Particulate or other embolic agents | Avoids radio and chemotoxicity; less expensive than other embolotherapies | PES, biloma, liver abscess, liver failure |
| TARE | Y90 microspheres | May be used in early disease with curative intent; intermediate disease can be used to increase FLV to qualify for curative intent surgery; best QoL scores of all options | PRS, RILD, radiation-induced pneumonitis, biloma, liver abscess, liver failure |
| Ablation | Radiofrequency current, microwaves, or cryoablation | Efficacious as monotherapy for early-stage disease; less morbidity than transarterial therapies | PAS, iatrogenic injury, bleeding |
The staging of HCC is particularly complex due to the varying presence of accompanying liver dysfunction. Prognostic factors for HCC patients undergoing non-surgical treatment have coalesced into several existing clinical staging systems to predict survival and adverse events. Examples include the Okuda staging system, Cancer of the Liver Italian Program staging system, Hong Kong Liver Cancer staging system, and Barcelona Clinic Liver Cancer (BCLC) classification scheme[23-25]. Other clinical indices which must be considered for prognosis include Albumin-Bilirubin and Model for End-stage Liver Disease[26]. Overall survival (OS) in HCC patients is most strongly related to performance status, tumor burden, hepatic reserve, and extrahepatic spread[23,27,28].
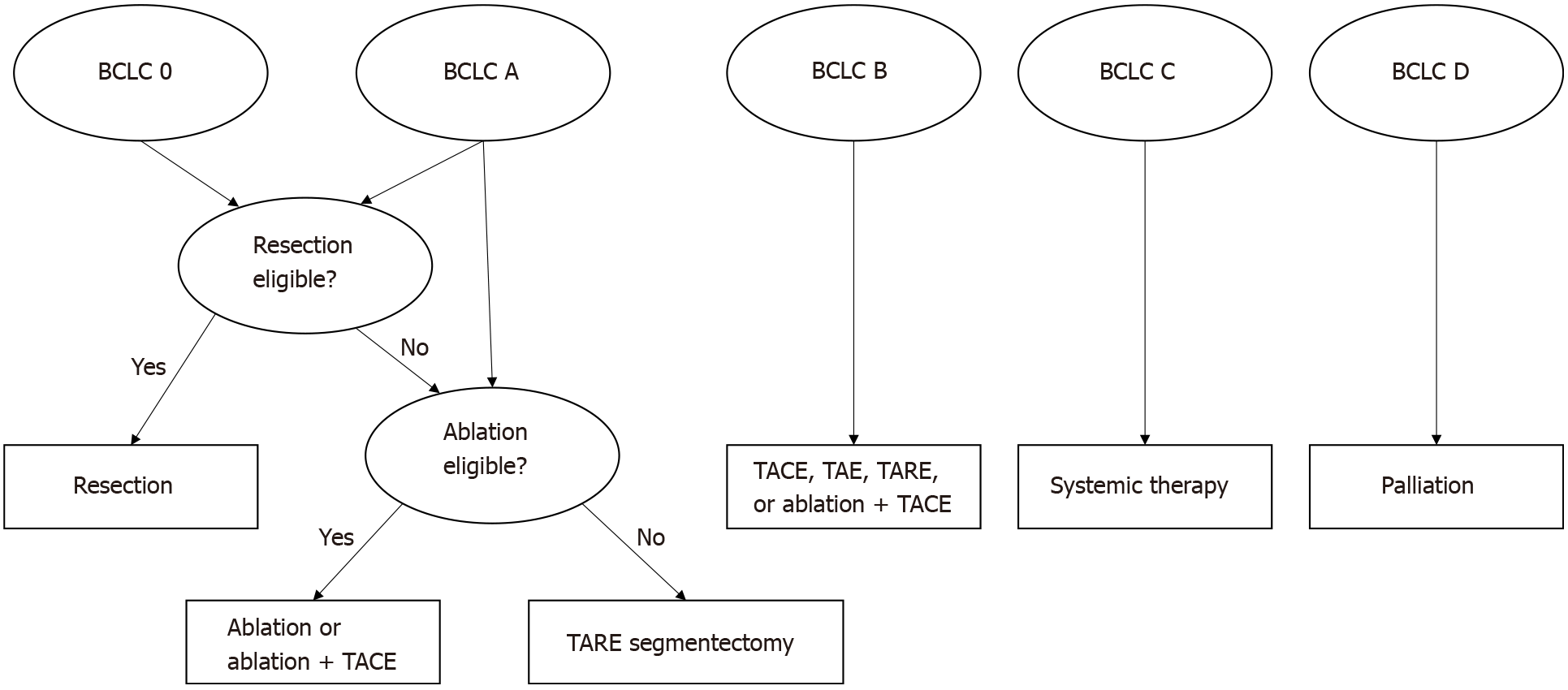
The most widely used prognostic tool in HCC is the BCLC[29,30], which has garnered international consensus endorsement for patient treatment stratification[31]. A treatment schematic for HCC based on BCLC classification is shown in Figure 1. The BCLC staging system matches liver dysfunction, tumor burden, and performance status to a recommended therapy[27,32]. Specifically, the BCLC utilizes Child-Pugh score and Eastern Cooperative Oncology Group (ECOG) status in addition to indicators of tumor burden. BCLC stratifies patients into five stages, categorized from stage 0 for “very early detection” to stage D for the most advanced disease cases.
Patients in BCLC stage 0 and BCLC stage A should generally undergo surgical resection if they are otherwise strong surgical candidates. In certain circumstances, TACE may be used as a bridge to surgery or as primary therapy when patients in these stages are non-candidates for surgery or ablation[12,33]. TACE is a first-line therapy recommendation for intermediate, unresectable HCC (BCLC stage B)[34]. Advanced disease (BCLC stage C) patients typically require systemic therapy, traditionally in the form of sorafenib or Lenvatinib[35]. More recently, combination atezolizumab and bevacizumab has gained endorsement as first-line therapy in the American Society of Clinical Oncology’s 2020 clinical practice guideline on systemic therapy[36]. When local disease control is needed for advanced disease, TACE may be indicated for use alone or in combination with systemic therapy. A current phase III study is evaluating TACE in combination with Lenvatinib and Pembrolizumab for advanced, non-metastatic disease[37]. In addition to being useful in stratifying patients to appropriate treatment, BCLC class is a useful tool in prognosticating survival following TACE, with many studies stratifying survival based on these categories.
Despite the prevalent adoption of BCLC as the gold standard staging and management decision tool for HCC, other staging tools have been investigated and are utilized in certain situations. The AFP, BCLC, Child-Pugh, and Response (ABCR) score also predict retreatment success. Specifically, ABCR uses a baseline AFP over 200, more advanced BCLC stage, increase in Child-Pugh score of at least 2 from baseline, and absence of radiologic response to create a score range of -3 to +6, correlating with survival post-TACE retreatment. A score greater than or equal to 4 prior to a second TACE treatment prognosticates poor outcomes.
Prognostic factors may also indicate risks related to post-procedural complications. Post-embolization syndrome (PES) consists of post-procedural fever in the absence of infection, transaminitis, right upper quadrant pain, and nausea or vomiting. PES is a risk common to each of the transarterial therapies. Up to 80% of patients may experience a component of PES following TACE, however rates of serious clinical sequela of PES are much lower[38]. Most cases of PES resolve within 72 h. Features predictive of an increased risk of PES following TACE are tumor > 5 cm, multiple tumors, and technical considerations relating to procedure performance[39]. Other complications of TACE include acute hepatic failure, abscess, biloma, iatrogenic dissection, and acute cholecystitis. These more serious complications are reported at a combined rate of approximately 5% of cases[40,41].
In addition to typical outcomes measures in oncology - such as OS, progression-free survival (PFS), and time-to-progression (TTP) - tumor response outcomes are especially important in HCC due to the complexity of such patients who often undergo multiple therapies that can confound long-term outcomes. The most widely used tool for measuring tumoral response to treatment in HCC is the 2010 modified Response Evaluation Criteria in Solid Tumors (mRECIST)[42,43]. The mRECIST treatment response tool builds on the traditional RECIST model of evaluating reduction in tumor size; however, because locoregional therapies induce devascularization and necrosis—and not always a reduction in size[44] - the American Association for the Study of Liver Diseases proposed mRECIST and the utilization of arterial enhancement measurements[45]. The effectiveness of these tools has been validated by a meta-analysis from Vincenzi et al[46]. More recently, three-dimensional imaging techniques have brought about more quantitative versions of these clinical response tools, namely volumetric RECIST and quantitative EASL (qEASL), which are being evaluated for efficacy and specific indications[47]. However, mRECIST remains the most widely used treatment response tool in practice.
Outcomes for both cTACE and DEB-TACE have consistently proven superior to conservative therapy[48,49]. Thus, as previously mentioned, TACE is to be considered for patients with advanced, unresectable disease who may not tolerate side effects of systemic therapy and who have acceptable hepatic function. This becomes especially important because systemic sorafenib has considerable toxicity, including diarrhea, weight loss, dermatitis, and hypophosphatemia[50]. Combination therapy using TACE with systemic sorafenib for both advanced and intermediate disease has been investigated. TACE induces ischemia which leads to the production of neoplastic angiogenic growth factors. The anti-angiogenic actions of sorafenib block these angiogenic factors. The GIDEON study[51] demonstrated through global observational data that patients given TACE concomitantly with sorafenib achieved better OS (21.6 mo) compared to patients treated with sequential TACE then sorafenib (12.7 mo) and compared to patients treated with only sorafenib (9.7 mo). The concomitant treatment group’s survival superiority was present across all BCLC stages. Importantly, the study calls attention to the need for further standardization of TACE technique as many centers reported logistical differences in treatment plans. The phase II SPACE trial[52] randomized intermediate stage HCC patients to DEB-TACE with sorafenib vs DEB-TACE with placebo treatment arms. Time to progression was similar in both treatment groups, and both options demonstrated adequate safety profiles. More recently, the phase II TACTICS trial showed a significant difference in PFS of TACE and sorafenib vs TACE alone (25.2 mo and 13.5 mo, respectively)[53]. Combination TACE and sorafenib also displayed significantly prolonged TTP.
TACE is also implicated in strategies for early-stage patients who need adjunctive procedures prior to surgery. In combination with portal vein embolization (PVE), TACE may induce contralateral liver hypertrophy to allow for tumor resection in patients with inadequate predicted future liver remnant (FLR). The utility of TACE in this setting is to decrease the risk of tumor progression during the period of time it takes PVE to induce FLR hypertrophy. A systematic review and meta-analysis concluded that TACE combined with PVE provided higher OS than PVE, portal vein ligation (PVL), or radioembolization of the portal vein alone[54]. Of patients receiving both TACE and PVE, 90% went on to receive resection. These strategies may be further explored to increase liver resection eligibility rates in the future. Bridging or downstaging patients for liver transplantation is another use for TACE in early-stage patients[5].
Several prospective studies comparing cTACE vs DEB-TACE have found no significant difference in OS, including one meta-analysis examining results from four randomized clinical trials and eight observational studies[48,49,55]. Beyond survival, the PRECISION-V trial demonstrated that in a subgroup of advanced HCC patients with Child-Pugh B, ECOG 1, bilobar, and recurrent disease, patients receiving DEB-TACE had higher rates of complete response, objective response, and disease control at 6 mo compared to patients receiving cTACE[48]. Concerning safety endpoints, DEB-TACE was originally theorized to provide fewer adverse events and a lower risk of post-embolization syndrome characteristics; however, the PRECISION-V trial found comparable 30-day adverse event incidence between the two groups[48]. Comparable safety profiles between cTACE and DEB-TACE were upheld through meta-analysis as well[55]. Observationally, DEB-TACE has displayed higher localized biliary injury rates and global hepatic damage[56]. In another randomized trial, Golfieri et al[49] found that DEB-TACE patients suffered less post-operative pain. The overall comparative safety and efficacy of cTACE vs DEB-TACE needs further exploration and likely has significant situational considerations which must be applied. Other areas of TACE outcomes that warrant further investigation include more rigorous standardization of cTACE protocols[57], the efficacy and considerations of TACE in portal vein thrombosis, and TACE for larger (> 5 cm) or multifocal lesions[58]. Primary outcomes for TACE, as well as the other forms of locoregional therapy discussed here, can be viewed in Table 2.
| Locoregional technique | Primary outcomes |
| TACE | TACE provides a survival benefit compared to supportive care in unresectable disease[34]. Concomitant TACE and sorafenib is superior to standalone therapy for unresectable disease[51-53]. Comparisons of DEB-TACE versus cTACE have yet to reveal significant differences in OS and short and long-term complication rates. Further studies are needed for considerations in more specific circumstances[48,49]. When combined with PVE, TACE provides more robust FLR increase and results in better survival compared to monotherapy strategies to enhance FLR[54]. |
| TAE | TAE provides a survival benefit compared to supportive care in unresectable disease[34]. Early data of chemoembolization has shown little survival benefit over TAE, but superior proximate outcomes such as TTP and tumor response compared to TAE[63,64]. |
| TARE | TARE shows similar complication and survival rates to TACE, while producing higher QoL scores and longer TTP[77,78]. TARE segmentectomy for early-stage disease (tumors < 3 cm) results in a 5-year survival of 75%, which is comparable to curative intent therapies such as transplantation and surgical resection[81]. TARE lobectomy provides a significant increase in FLR and is a safe mechanism to treat tumor while inducing contralateral hypertrophy[82-86]. |
| Ablation | In early-stage patients, standalone percutaneous ablation produces comparable survival outcomes to surgical resection[113-116]. RFA and MWA techniques show similar outcomes in early-stage disease (tumor < 3 cm)[108,119]. Combination therapy using TACE and ablation (particularly MWA) provide the best outcomes for large tumors (tumor 3-5 cm)[120]. |
TAE is commonly referred to as “bland” embolization because the embolic particles lack additional chemical or radiation components. With this therapy, endovascular arterial occlusion induces hypoxia and subsequent death of tumor cells[59]. As with other transarterial therapies, optimal vascular catheter placement results in a maximally selective effect on tumor cells. Care must be taken to avoid arterial-venous shunts which could cause pulmonary arterial embolization with smaller particles. Embolic agents used have historically included gel foam, polyvinyl alcohol, and various drug-eluting beads[45]. The lack of chemotoxicity and radiotoxicity associated with this therapy means that tumoral response is primarily derived from a hypoxic cell death mechanism. It also means that the therapy may hold advantages in certain patients who have an especially high need to spare healthy liver parenchyma.
BCLC class B patients receive the most disease-control benefit from TAE, followed by class C patients[48]. Additionally, BCLC class A patients may undergo TAE to maintain eligibility for transplantation per the Milan criteria, a prognostic tool shown to improve 4-year OS in liver transplant when used for patient selection[60]. The Milan criteria consists of one lesion less than 5 cm or up to three lesions less than 3 cm, no evidence of extrahepatic manifestation, and no evidence of vascular invasion. Similarly, TAE is an option for downstaging of BCLC class B patients for trans
Prognostically, a key advantage of TAE relative to other transarterial options is its gentler impact on short-term adverse events, possibly due to the avoidance of chemotherapy toxicity. As with other embolotherapies, bland embolization poses risk for PES. Agrawal et al[62] reported a higher incidence of PES among patients undergoing TACE (74.7%) compared to TAE (68.7%). PES following TACE resulted in a significantly longer hospital stay than PES following TAE (1.47 d vs 1.12 d). This observational study further identified that, in addition to the PES risk factors mentioned in the previous section, more patients who were female or who had alcohol-related HCC developed PES.
Like other embolotherapies, TAE offers a survival benefit compared to conservative treatment. Llovet et al[34] found that compared to best supportive care, repeated administration of either TACE or TAE showed a survival benefit in patients with unresectable HCC. While TACE provided even higher survival probabilities than TAE in the study, both therapies performed better than conservative treatment. Comparison of bland embolization and chemoembolization is an ongoing focus of research. Despite some data suggesting TACE's superiority to TAE, multiple studies investigating bland embolization compared to either cTACE or DEB-TACE have failed to demonstrate significant differences in OS between the two[63-65]. Importantly, much of the early data accumulated on the comparative efficacy of TACE and TAE was collected during a period of evolving indications and chemotherapeutic protocols for TACE. Furthermore, the development of DEB-TACE may continue to influence overall comparisons of bland embolization and chemoembolization.
Some studies have shown benefits to TACE compared to TAE in more proximate outcomes such as TTP, tumor recurrence, and local response. For example, a trial by Meyer et al[65] found insignificant differences in median OS and PFS among TAE and cTACE, but found a significantly greater mRECIST treatment response in cTACE vs TAE (47.3% vs 67.4% respectively). However, because several studies have shown TAE performing comparably to TACE in terms of survival, and because TAE lacks utilization of chemotherapy particles, TAE may be better tolerated in HCC patients with borderline liver function[45].
TARE, also referred to as selective internal radiotherapy (SIRT) and Y90, uses a radioisotope form of yttrium to selectively target tumor cells via the hepatic arterial tree[66]. The Y90 radioisotope is delivered using microspheres and, once reaching target tissue, undergoes beta decay to locally irradiate the tumor in a continuous, low-dose fashion over a fourteen-day period. TARE is considered a two-stage treatment process because a planning arteriography must be performed one to two weeks before the radiation-delivering procedure[67]. This planning stage helps to differentiate tumor and hepatic arterial supply, isolate the future path of radiation delivery via embolization of extrahepatic vessels at risk of nontarget microsphere delivery, and identify the degree of hepatopulmonary shunting[68]. Technetium-99m labeled macroaggregated albumin is combined with single photon emission computed tomography imaging technology to provide imaging for this stage[69]. After TARE, there is a longer wait time compared to other embolization techniques until the treatment effect is fully realized, with therapy response imaging taking place 3-6 mo following the procedure[70].
Characteristics most predictive of post-TARE prognosis are extrahepatic disease, baseline BCLC stage, ECOG performance status, and tumor burden[71]. In a multi-center study, Sangro et al[72] analyzed a cohort of 325 patients undergoing TARE. Median OS was strongly influenced by BCLC staging (BCLC A 24.4 mo; BCLC B 16.9 mo; BCLC C 10.0 mo). Other significant predictors of superior survival following TARE were ECOG performance status (ECOG 0), Child-Pugh class (A), absence of ascites, baseline total bilirubin (< 1.5), number of tumor nodules (< 5), alpha-fetoprotein level (< 400), patent portal vein, single lobe disease, and absence of extrahepatic disease.
Understanding the influence of the pre-procedural disease stage on post-TARE survival is important because TARE maintains indications across the spectrum of HCC severity. Patients with advanced disease may benefit from the local tumor control and palliative effects of TARE. TARE is an acceptable treatment alternative to TACE for first-line therapy for BCLC class B patients[73]. Early-stage patients in BCLC classes 0 and A may benefit from TARE radiation segmentectomy. Early-stage patients may also benefit from TARE lobectomy in an attempt to either downstage for trans
Common complications of TARE are well characterized. Radiation-induced liver disease (RILD) is an adverse event unique to TARE among the locoregional therapies. RILD involves an extensive array of local vascular, fibroblastic, and parenchymal change[74]. Risk of RILD may be increased by gemcitabine, which must be held for four weeks prior to the procedure[75]. Padia et al[76] report the overall risk of RILD following TARE to be 1%-4%. They also report rates of other common adverse events, including GI ulcers (0%-5%), PES requiring extended hospitalization or readmission (1%-2%), iatrogenic dissection (1%), and death within 30 d (2%). There was a less than 1% reported rate for radiation-induced skin-injury, radiation pneumonitis, radiation-induced pulmonary fibrosis, biloma requiring drainage, and abscess. A hepatopulmonary fraction above 20% predicts an increased likelihood of radiation pneumonitis. Relative contraindications to TARE include an elevated baseline bilirubin level (> 2 mg/dL), an elevated hepatopulmonary fraction (> 20%), Child-Pugh class C, ECOG score over 2, significant transaminitis (ALT or AST > 5x upper limit of normal), and total tumor burden over 70% of the liver or total tumor burden over 50% with a high number of nodules[12]. Of these variables, elevated baseline bilirubin and increased tumor burden have been shown to decrease OS[18].
TARE appears to have comparable complication and survival rates to TACE. The SIRTACE trial compared TARE vs TACE in unresectable HCC and found that a single TARE session was as safe and produced a better quality of life (QoL) change than multiple TACE sessions[77]. More recently, the PREMIERE trial revealed a significantly longer median TTP for patients receiving TARE (> 26 mo) compared to cTACE (6.8 mo). A smaller randomized trial compared QoL measures between TACE and TARE, finding that patients treated with TARE had improvements in QoL despite being treated for more severe disease than the TACE cohort. In contrast, the TACE cohort had worsened QoL post-procedurally[78]. Both TACE and TARE are being investigated to identify the optimal transarterial therapy for downstaging tumors for transplantation eligibility. Lewandowski et al[70] compared triple-drug cTACE to TARE in their ability to downstage tumor size from UNOS T3 to UNOS T2 to achieve eligibility. TARE significantly outperformed cTACE in rates of T2 achievement, event-free survival, and OS.
In addition to sustaining or achieving transplantation eligibility, TARE is also useful in early-stage disease for its ability to act as primary therapy in certain circumstances. TARE’s effectiveness in early-stage disease is in part due to its evolution into more selective indications for earlier tumors via radiation segmentectomy[79]. First described in 2011[80], TARE segmentectomy is an alternative option in non-surgical candidates whose tumor anatomy discourages ablative techniques due to nearby high-risk structures[33]. For example, in a retrospective study, Lewandowski et al[81] analyzed 70 patients with similar inclusion criteria with the additional exclusion of patients who received secondary surgery. Median OS in this cohort was found to be 6.7 years with a median TTP of 2.4 years. The cohort had comparable five-year OS (75%) and response rates to other curative-intent treatments like ablation, resection, and transplantation.
Similar to segmentectomy, radiation lobectomy is a relatively novel application of TARE. Its primary use is to treat the tumor-occupied lobe while inducing hypertrophy of the contralateral lobe, thus increasing the FLR in patients who were deemed unresectable due to low FLR[12]. Scarring of the treated lobe slowly creates a shunting of blood to the contralateral portal vein and, over time, induces hypertrophy of that lobe[82]. Multiple observational studies show that TARE lobectomy increases the FLR by an approximate average of 30% from baseline[83-86]. In contrast to TACE treatment to increase FLR, TARE lobectomy does not require concomitant PVE. A comparison of standalone PVE and TARE lobectomy by Garlipp et al[84] has proven that, while both display significant increases in FLR, PVE does it more effectively at the 6-wk mark (61.5% vs 29%). Issues have been raised with this measurement, however, as PVE has been shown to increase FLR quicker than radiation lobectomy[86], and as some evidence suggests that PVE may actually induce mild growth of existing tumor tissue[87]. The safety of using radiation lobectomy as a strategy to qualify for and subsequently undergo resection was demonstrated in a prospective cohort studied by Gabr et al[88]. Among 25 patients receiving major hepatic resection and 6 patients receiving partial hepatectomy, a range of perioperative outcomes following resection were comparable to resection-only outcomes. Survival rates at one and three years was reported at 96% and 86%, respectively.
TARE is also being compared to sorafenib as monotherapy in advanced disease. The phase III SARAH trial[89] randomized 467 patients with intermediate-stage, unresectable HCC to either sorafenib or TARE. Median OS and median PFS were comparable; however, TARE showed significantly fewer treatment-related adverse events, higher QoL scores, and a higher treatment response rate than sorafenib. The SIRveNIB trial[90] was another phase III study that failed to show significant differences in survival between TARE and sorafenib, but which also demonstrated the improved toxicity profile of TARE. Further trials are needed to better power subgroup analyses of TARE vs sorafenib and define specific patients who may see the improved tumor response of TARE translated into improved survival over sorafenib[91]. TARE is also useful in advanced disease because it has relatively less embolic activity compared to other transarterial therapies. This becomes useful in the setting of portal vein thrombus (PVT) of unresectable HCC patients. A retrospective study of HCC patients with PVT compared OS between those treated with TARE and those treated with sorafenib. TARE led to significantly longer median OS (26.2 mo) than sorafenib (8.7 mo)[92].
TARE and sorafenib combination therapy for patients ineligible for TACE but with BCLC classes B and C disease was investigated in the SORAMIC trial[93]. Again, no significant differences in median OS were found; however, survival benefit was found with combination therapy among patients without cirrhosis, with cirrhosis of nonalcoholic etiology, and in patients younger than 65 years of age. The phase III STOP-HCC trial is a larger study. It is expected to evaluate further what specific subset of patients may benefit most from combination therapy with TARE and systemic therapy. It is expected to be complete in September 2022[94]. Further, with the development of additional effective systemic therapies for HCC including immunotherapy and most recently, atezolizumab/bevacizumab, the combination of TARE with these agents is a potential area of synergy and an active area of clinical investigation[95,96].
Generally, ablation is recommended for early-stage, small tumors (up to 3 cm) in patients who would otherwise qualify for resection but are considered unsuitable candidates for surgery[73]. Percutaneous ablative techniques were originally centered around ethanol injection (PEI), however this has fallen out of favor when a patient is a strong candidate for more contemporary ablative techniques[10]. Today, commonly used ablative techniques in the setting of HCC include microwave ablation (MWA), radiofrequency ablation (RFA), and cryoablation (CA). RFA utilizes a radiofrequency electrode to deliver an alternating electric current (460-500kHz) to the target lesion. Frictional heating, necrosis, and cell death ensue. MWA utilizes a common final cell death pathway involving local heating and eventual cell death, however MWA heats tissue via an oscillating microwave field (915/2450 MHz). The properties of microwaves result in reduced heat sink effect compared to RFA. RFA has the ability to decrease unwanted energy delivery to nearby structures compared to MWA[97]. However, due to less heat-sink, MWA may perform better near large vessels, in patients with comparatively larger tumors (between 3 cm and 5 cm), and in patients with multiple nodule disease[98]. CA relies on argon and helium gasses to rapidly alternate freezing and thawing of local tissue and vascular structures[99]. CA is not as commonly used due to the complication profile[100]. Laser ablation and irreversible electroporation are two examples of newer therapies still under investigation[10].
Prognostic factors for ablative therapy follow general prognostic patterns for HCC. Across multiple studies examining prognostic factors of RFA, survival has been consistently and independently predicted by Child-Pugh classification, tumor size, and tumor number[101-104]. Long-term survival following MWA is predicted by similar factors. Three-year PFS following MWA can range from 27% to 91.7%, with heavy influence from the above clinical characteristics[105]. Prognostic factors for combination therapy of MWA with TACE were well characterized by Ni et al[106] Predictably, adjusted prognostic factors associated with better OS rates of MWA with TACE combination therapy were earlier BCLC stage, smaller tumor size, lack of portal vein thrombus, MWA therapy times, and targeted drug usage.
Complication risk of biloma following percutaneous ablation may be predicted by comparatively large lesions situated closer to major bile ducts or near the hilum[107]. Additional characteristics prognostic of increased complication risk include tumor volume, ablated tissue volume, multiple tumors, and Child-Pugh class B or above[108]. In general, however, ablation carries less morbidity than other curative therapies due to its less invasive nature, coagulative properties related to heating tissue, liver preservation, and shorter hospital stay[109,110]. Bertot et al[111] found a pooled major complication rate of 3.29% for RFA, PEI, and MWA across 34 randomized trials and observational studies.
A complication unique to ablation among the locoregional therapies is post-ablation syndrome (PAS). PAS is a transient, flu-like illness which may occur about three days following ablation and lasts an average of five days. PAS occurs in roughly 25%-35% of patients undergoing ablation and is correlated with the volume of liver tissue treated. Pre-procedural tumor volume and post-procedural rise in AST are predictive of an increased likelihood of PAS[112].
Studies have demonstrated comparable survival via ablation vs surgery in early-stage HCC, despite ablative patients usually having poorer baseline hepatic function. In 2006, percutaneous thermal ablation was compared to resection in a randomized trial of 105 patients[113]. Results showed nonsignificant differences in complete tumor elimination rates, time to first recurrence, and disease-free and OS rates at 1, 2, and 3-year follow-ups. More recently, Fang et al[114] showed through a randomized trial of 120 patients that RFA had a similar complete remission rate (95%) to surgical resection (96.7%) and similar disease-free and OS rates at years 1, 2, and 3 follow-ups. In addition, RFA demonstrated significantly better hepatic function at day-7 post-treatment and fewer post-operative complications. The trend of comparable ablative survivability does not always extend to patients meeting Milan criteria. A trial that randomized HCC patients meeting Milan criteria to either RFA or surgical resection found significantly lower OS and recurrence-free survival along with higher overall recurrence among RFA patients[115]. As shown by the STORM trial, curative-intent ablation is best as a standalone therapy, without the addition of adjuvant sorafenib following the procedure[116]. CA has largely fallen out of favor due to the severity of complications despite similar performance to other ablative techniques. A meta-analysis including a total of 433 total HCC patients revealed significantly fewer complications and less local tumor recurrence in RFA compared to CA[117].
As mentioned previously, the two ablative techniques most common in current practice are RFA and MWA, which are a source of ongoing outcomes comparison[118] and which feature similar curative-intent indications in early-stage disease (up to three tumor nodules smaller than 3 cm with the absence of extrahepatic disease) and similar complication rates[108]. A meta-analysis looking at seven studies comparing RFA and MWA found comparable rates of complete response, local recurrence, major complications, and 3-year survival[119].
HCC tumors between 3-5 cm fall outside the purview of curative-intent ablation but may still be addressed by combination therapy with TACE and ablation[9]. Comparison of the best combination therapies for specific indications in this population is ongoing. For example, Sheta et al[120] compared cTACE alone, combined MWA with cTACE, and combined RFA with cTACE in a clinical trial of 50 patients with nonresectable, single-lesion HCC greater than 4 cm. They found the highest success rates in the combined MWA with the cTACE group and the lowest success rates in the cTACE alone group. Whereas combination therapy of TACE with MWA may be indicated for 3-5 cm HCC, combination therapy of TACE with RFA may serve a role in the treatment of early-stage HCC. Kim et al[121] found that combined cTACE with RFA provided decreased local tumor progression and better PFS at 1, 3, and 5-year follow-ups. OS at follow-up intervals, however, was similar.
The future of HCC therapy will likely rely on a combination of the current proven standards of care and several other promising areas of innovation. One such promising area of growing evidence for HCC is immunotherapy. In addition to its use as a second-line monotherapy agent, immunotherapy may augment the effects of sorafenib and locoregional therapy in HCC. Locoregional therapies produce an immune response that can be augmented via immune checkpoint inhibition. Given that prognosis in HCC is correlated with T-cell tumoral infiltration[122], potentiation of both tumoral and locoregional therapy-induced T-cell response could improve outcomes. In an early-phase trial, Duffy et al[123] safely treated HCC with tremelimumab (anti-CTLA4) combined with ablation and showed that the post-procedural immune response could be enhanced. The effects of combined anti-PD1 inhibitors and TARE or TACE are also being evaluated[124]. The ongoing phase II DEMAND trial evaluates first-line combination therapy of systemic anti-angiogenic and immunotherapy while reserving TACE as second-line therapy[125].
As with immunotherapy, precision medicine has potential to create a paradigm shift in the way HCC patients are treated. By relying on big data and genomics to personalize clinical care, precision medicine will allow further customization of HCC treatment plans across the spectrum of therapeutic modalities, based on an individual’s genetic mutations, local tumor environment, and further stratification of many clinical factors already in use today. Precision medicine will likely feature small molecule inhibitors, epigenetic regulators, and monoclonal antibodies specific to an individual’s disease. A number of these agents are currently being evaluated for both safety and efficacy in advanced disease[7]. The success of these agents in treating HCC is reliant upon accurate characterization of multiple carcinogenic molecular pathways - including mutations to TERT, Wnt/ß-catenin, P53, Akt/mTOR, VEGFR, and EGFR genetic pathways. Ideally, molecular therapy will target multiple genetic pathways within the same patient and will be combined with other therapies such as locoregional treatment to optimize OS.
As treatments such as immunotherapy and molecular therapy evolve and become integrated with current standards of care, further prognostic sophistication is a priority for immediate improvement of care. This growth is already underway. In some cases, pre-procedural evaluation of inflammatory markers may provide prognostic information for HCC patients treated with locoregional therapy. A meta-analysis of 22 studies showed poorer OS following TACE in HCC patients with higher C-reactive protein levels, neutrophil to lymphocyte ratio, and platelet to lymphocyte ratio[126]. This is in line with the general understanding of inflammation being tumor-protective. More research is needed to routinely integrate inflammatory markers into the larger prognostic landscape of clinical staging systems for locoregional HCC treatments.
With the numerous advancements within HCC treatment, outcomes research must continue to be robust. With the need for increased outcomes research related to locoregional therapies comes a call for increased reporting of randomized controlled trial data. Grégory et al[127] found that nearly two-thirds of RCTs conducted regarding HCC treatment with TACE did not yield public results. This highlights the importance of increased data reporting as evidence and indications behind various locoregional therapies for HCC continue to mature. For the care of HCC patients to continue to improve, and for future directions of care such as personalized medicine and immunotherapy to flourish, high-quality outcomes data must be generated and distributed throughout the field.
HCC is the most common primary liver malignancy[2] and carries a 5-year survival rate under 20%. Organ transplant availability and eligibility is limited, and fewer than 20% of HCC patients are candidates for surgical resection. For the remainder of patients with HCC, liver-directed, locoregional therapies serve a growing purpose across a spectrum of disease stages. Transarterial and ablative procedures are involved in treatment for curative-intent, disease control, bridging to surgery, downstaging for future treatment, and palliation. In addition to bland embolization, TAE techniques with locoregional delivery of radioactive or chemotherapeutic microspheres offer survival benefits in appropriately selected patients. Microwave and radiofrequency ablative techniques offer comparatively less morbidity and curative results in select early-stage patients. Multiple indications exist for various locoregional therapies in the adjunctive realm of transplantation, resection, and systemic therapy. Prognostic considerations for locoregional therapies vary by indication but generally follow baseline disease staging and tumor quantification. Outcomes data reveal that locoregional therapies provide survival benefits in appropriately selected patients. New advances in precision medicine, combination therapy, and immunotherapy are being investigated and have potential to augment available treatment strategies.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Levi Sandri GB S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Abdelsalam ME, Murthy R, Avritscher R, Mahvash A, Wallace MJ, Kaseb AO, Odisio BC. Minimally invasive image-guided therapies for hepatocellular carcinoma. J Hepatocell Carcinoma. 2016;3:55-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Kim HS, El-Serag HB. The Epidemiology of Hepatocellular Carcinoma in the USA. Curr Gastroenterol Rep. 2019;21:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World J Gastroenterol. 2010;16:3603-3615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Wong MC, Jiang JY, Goggins WB, Liang M, Fang Y, Fung FD, Leung C, Wang HH, Wong GL, Wong VW, Chan HL. International incidence and mortality trends of liver cancer: a global profile. Sci Rep. 2017;7:45846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | Byam J, Renz J, Millis JM. Liver transplantation for hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2013;2:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 6. | Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, Yopp AC, Singal AG. Racial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2019;17:551-559.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 7. | Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 379] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 8. | Lewis DR, Chen HS, Cockburn MG, Wu XC, Stroup AM, Midthune DN, Zou Z, Krapcho MF, Miller DG, Feuer EJ. Early estimates of SEER cancer incidence, 2014. Cancer. 2017;123:2524-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Inchingolo R, Posa A, Mariappan M, Spiliopoulos S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J Gastroenterol. 2019;25:4614-4628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Mokdad AA, Singal AG, Yopp AC. Advances in Local and Systemic Therapies for Hepatocellular Cancer. Curr Oncol Rep. 2016;18:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Kis B, El-Haddad G, Sheth RA, Parikh NS, Ganguli S, Shyn PB, Choi J, Brown KT. Liver-Directed Therapies for Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Haste P, Johnson, MS. Transarterial Chemoembolization. In: Keefe NA, Haskal ZJ, Park AW, Angle JF, editors. IR Playbook: A Comprehensive Introduction to Interventional Radiology. Cham: Springer International Publishing, 2018: 381-387. |
| 14. | Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969-977. [PubMed] |
| 15. | Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, Delis S, Gouliamos A, Kelekis D. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, Fan ST. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Woo HY, Heo J. Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: Now and future. Clin Mol Hepatol. 2015;21:344-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Spreafico C, Cascella T, Facciorusso A, Sposito C, Rodolfo L, Morosi C, Civelli EM, Vaiani M, Bhoori S, Pellegrinelli A, Marchianò A, Mazzaferro V. Transarterial chemoembolization for hepatocellular carcinoma with a new generation of beads: clinical-radiological outcomes and safety profile. Cardiovasc Intervent Radiol. 2015;38:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Makary MS, Kapke J, Yildiz V, Pan X, Dowell JD. Conventional versus Drug-Eluting Bead Transarterial Chemoembolization for Neuroendocrine Tumor Liver Metastases. J Vasc Interv Radiol. 2016;27:1298-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Lucatelli P, De Rubeis G, Rocco B, Basilico F, Cannavale A, Abbatecola A, Nardis PG, Corona M, Brozzetti S, Catalano C, Bezzi M. Balloon occluded TACE (B-TACE) vs DEM-TACE for HCC: a single center retrospective case control study. BMC Gastroenterol. 2021;21:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Hatanaka T, Arai H, Kakizaki S. Balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2018;10:485-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 22. | de Baere T, Guiu B, Ronot M, Chevallier P, Sergent G, Tancredi I, Tselikas L, Dioguardi Burgio M, Raynaud L, Deschamps F, Verset G. Real Life Prospective Evaluation of New Drug-Eluting Platform for Chemoembolization of Patients with Hepatocellular Carcinoma: PARIS Registry. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Grieco A, Pompili M, Caminiti G, Miele L, Covino M, Alfei B, Rapaccini GL, Gasbarrini G. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut. 2005;54:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 24. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146:1691-700.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 25. | Levy I, Sherman M; Liver Cancer Study Group of the University of Toronto. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Kim KM, Shim SG, Sinn DH, Song JE, Kim BS, Kim HG. Child-Pugh, MELD, MELD-Na, and ALBI scores: which liver function models best predicts prognosis for HCC patient with ascites? Scand J Gastroenterol. 2020;55:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 28. | Ni JY, Kong J, Sun HL, Chen YT, Luo JH, Wang WD, Chen D, Jiang XY, Xu LF. Prognostic Factors for Survival After Transarterial Chemoembolization Combined with Sorafenib in the Treatment of BCLC Stage B and C Hepatocellular Carcinomas. Acad Radiol. 2018;25:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1218] [Article Influence: 203.0] [Reference Citation Analysis (1)] |
| 30. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1275] [Article Influence: 141.7] [Reference Citation Analysis (2)] |
| 31. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 32. | Kumar Y, Sharma P, Bhatt N, Hooda K. Transarterial Therapies for Hepatocellular Carcinoma: a Comprehensive Review with Current Updates and Future Directions. Asian Pac J Cancer Prev. 2016;17:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Kishore SA, Bajwa R, Madoff DC. Embolotherapeutic Strategies for Hepatocellular Carcinoma: 2020 Update. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 34. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 35. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3828] [Article Influence: 546.9] [Reference Citation Analysis (1)] |
| 36. | Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A, Remak WM, Shroff RT, Sohal DPS, Taddei TH, Venepalli NK, Wilson A, Zhu AX, Rose MG. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38:4317-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 417] [Article Influence: 83.4] [Reference Citation Analysis (1)] |
| 37. | Taylor MH, Schmidt EV, Dutcus C, Pinheiro EM, Funahashi Y, Lubiniecki G, Rasco D. The LEAP program: lenvatinib plus pembrolizumab for the treatment of advanced solid tumors. Future Oncol. 2021;17:637-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 38. | Blackburn H, West S. Management of Postembolization Syndrome Following Hepatic Transarterial Chemoembolization for Primary or Metastatic Liver Cancer. Cancer Nurs. 2016;39:E1-E18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 39. | Arslan M, Degirmencioglu S. Risk Factors for Postembolization Syndrome After Transcatheter Arterial Chemoembolization. Curr Med Imaging Rev. 2019;15:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol. 2006;23:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 41. | Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, Iwanaga S, Mori M, Morikawa M, Fukuda T, Hayashi K, Matsunaga N. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Kim MN, Kim BK, Han KH, Kim SU. Evolution from WHO to EASL and mRECIST for hepatocellular carcinoma: considerations for tumor response assessment. Expert Rev Gastroenterol Hepatol. 2015;9:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3303] [Article Influence: 220.2] [Reference Citation Analysis (36)] |
| 44. | Forner A, Ayuso C, Varela M, Rimola J, Hessheimer AJ, de Lope CR, Reig M, Bianchi L, Llovet JM, Bruix J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 339] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 45. | Tsochatzis EA, Fatourou E, O'Beirne J, Meyer T, Burroughs AK. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol. 2014;20:3069-3077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 46. | Vincenzi B, Di Maio M, Silletta M, D'Onofrio L, Spoto C, Piccirillo MC, Daniele G, Comito F, Maci E, Bronte G, Russo A, Santini D, Perrone F, Tonini G. Prognostic Relevance of Objective Response According to EASL Criteria and mRECIST Criteria in Hepatocellular Carcinoma Patients Treated with Loco-Regional Therapies: A Literature-Based Meta-Analysis. PLoS One. 2015;10:e0133488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 47. | Tacher V, Lin M, Duran R, Yarmohammadi H, Lee H, Chapiro J, Chao M, Wang Z, Frangakis C, Sohn JH, Maltenfort MG, Pawlik T, Geschwind JF. Comparison of Existing Response Criteria in Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization Using a 3D Quantitative Approach. Radiology. 2016;278:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 48. | Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y, Avajon Y, Gruenberger T, Pomoni M, Langenberger H, Schuchmann M, Dumortier J, Mueller C, Chevallier P, Lencioni R; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1063] [Cited by in RCA: 1208] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 49. | Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, Cucchetti A, Bolondi L, Trevisani F; PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 485] [Article Influence: 44.1] [Reference Citation Analysis (1)] |
| 50. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10270] [Article Influence: 604.1] [Reference Citation Analysis (2)] |
| 51. | Geschwind JF, Kudo M, Marrero JA, Venook AP, Chen XP, Bronowicki JP, Dagher L, Furuse J, Ladrón de Guevara L, Papandreou C, Sanyal AJ, Takayama T, Ye SL, Yoon SK, Nakajima K, Lehr R, Heldner S, Lencioni R. TACE Treatment in Patients with Sorafenib-treated Unresectable Hepatocellular Carcinoma in Clinical Practice: Final Analysis of GIDEON. Radiology. 2016;279:630-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 52. | Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, Paik SW, Reig M, Kim DY, Chau GY, Luca A, Del Arbol LR, Leberre MA, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. 2016;64:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 53. | Kudo M, Ueshima K, Torimura T, Tanabe N, Ikeda M, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Isoda N, Yasui K, Kuzuya T, Okusaka T, Furuse J, Kokudo N, Okita K, Yoshimura K, Arai Y. Randomized, open label, multicenter, phase II trial of transcatheter arterial chemoembolization (TACE) therapy in combination with sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. J Clin Oncol. 2018;36 15_suppl:4017-4017. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Tustumi F, Ernani L, Coelho FF, Bernardo WM, Junior SS, Kruger JAP, Fonseca GM, Jeismann VB, Cecconello I, Herman P. Preoperative strategies to improve resectability for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2018;20:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 55. | Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, Morosi C, Cascella T, Marchianò A, Camerini T, Bhoori S, Brunero F, Barone M, Mazzaferro V. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Monier A, Guiu B, Duran R, Aho S, Bize P, Deltenre P, Dunet V, Denys A. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol. 2017;27:1431-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 57. | Renzulli M, Peta G, Vasuri F, Marasco G, Caretti D, Bartalena L, Spinelli D, Giampalma E, D'Errico A, Golfieri R. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann Hepatol. 2021;22:100278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Mukund A, Bhardwaj K, Choudhury A, Sarin SK. Survival and outcome in patients receiving Drug Eluting Beads Transarterial Chemoembolization (DEB-TACE) for large HCC (> 5cm). J Clin Exp Hepatol. 2021;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Shah RP, Brown KT, Sofocleous CT. Arterially directed therapies for hepatocellular carcinoma. AJR Am J Roentgenol. 2011;197:W590-W602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5312] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 61. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 62. | Agrawal R, Majeed M, Aqeel SB, Wang Y, Haque Z, Omar YA, Upadhyay SB, Gast T, Attar BM, Gandhi S. Identifying predictors and evaluating the role of steroids in the prevention of post-embolization syndrome after transarterial chemoembolization and bland embolization. Ann Gastroenterol. 2021;34:241-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Kawai S, Okamura J, Ogawa M, Ohashi Y, Tani M, Inoue J, Kawarada Y, Kusano M, Kubo Y, Kuroda C. Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma--a comparison of lipiodol-transcatheter arterial embolization with and without adriamycin (first cooperative study). The Cooperative Study Group for Liver Cancer Treatment of Japan. Cancer Chemother Pharmacol. 1992;31 Suppl:S1-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Chang JM, Tzeng WS, Pan HB, Yang CF, Lai KH. Transcatheter arterial embolization with or without cisplatin treatment of hepatocellular carcinoma. A randomized controlled study. Cancer. 1994;74:2449-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 65. | Meyer T, Kirkwood A, Roughton M, Beare S, Tsochatzis E, Yu D, Davies N, Williams E, Pereira SP, Hochhauser D, Mayer A, Gillmore R, O'Beirne J, Patch D, Burroughs AK. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer. 2013;108:1252-1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 66. | Hickey R, Lewandowski RJ, Salem R. Transarterial Radioembolization (TARE). In: Keefe NA, Haskal ZJ, Park AW, Angle JF, editors. IR Playbook: A Comprehensive Introduction to Interventional Radiology. Cham: Springer International Publishing, 2018: 389-396. [DOI] [Full Text] |
| 67. | Makary MS, Krishner LS, Wuthrick EJ, Bloomston MP, Dowell JD. Yttrium-90 microsphere selective internal radiation therapy for liver metastases following systemic chemotherapy and surgical resection for metastatic adrenocortical carcinoma. World J Clin Oncol. 2018;9:20-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Gaba RC. Planning Arteriography for Yttrium-90 Microsphere Radioembolization. Semin Intervent Radiol. 2015;32:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Salem R, Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Ibrahim S, Nemcek AA Jr, Omary RA, Madoff DC, Murthy R. Technical aspects of radioembolization with 90Y microspheres. Tech Vasc Interv Radiol. 2007;10:12-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 71. | Gbolahan OB, Schacht MA, Beckley EW, LaRoche TP, O'Neil BH, Pyko M. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol. 2017;8:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 72. | Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, Ettorre GM, Salvatori R, Giampalma E, Geatti O, Wilhelm K, Hoffmann RT, Izzo F, Iñarrairaegui M, Maini CL, Urigo C, Cappelli A, Vit A, Ahmadzadehfar H, Jakobs TF, Lastoria S; European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY). Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 505] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 73. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3031] [Article Influence: 433.0] [Reference Citation Analysis (3)] |
| 74. | Kim J, Jung Y. Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med. 2017;49:e359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 75. | Murphy JD, Lucas DR, Somnay YR, Hamstra DA, Ray ME. Gemcitabine-mediated radiosensitization of human soft tissue sarcoma. Transl Oncol. 2008;1:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 76. | Padia SA, Lewandowski RJ, Johnson GE, Sze DY, Ward TJ, Gaba RC, Baerlocher MO, Gates VL, Riaz A, Brown DB, Siddiqi NH, Walker TG, Silberzweig JE, Mitchell JW, Nikolic B, Salem R; Society of Interventional Radiology Standards of Practice Committee. Radioembolization of Hepatic Malignancies: Background, Quality Improvement Guidelines, and Future Directions. J Vasc Interv Radiol. 2017;28:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 77. | Kolligs FT, Bilbao JI, Jakobs T, Iñarrairaegui M, Nagel JM, Rodriguez M, Haug A, D'Avola D, op den Winkel M, Martinez-Cuesta A, Trumm C, Benito A, Tatsch K, Zech CJ, Hoffmann RT, Sangro B. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. 2015;35:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 78. | Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, Baker T, Abecassis MM, Atassi R, Riaz A, Cella D, Burns JL, Ganger D, Benson AB 3rd, Mulcahy MF, Kulik L, Lewandowski R. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. 2013;11:1358-1365.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 79. | Malhotra A, Liu DM, Talenfeld AD. Radiation Segmentectomy and Radiation Lobectomy: A Practical Review of Techniques. Tech Vasc Interv Radiol. 2019;22:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Riaz A, Gates VL, Atassi B, Lewandowski RJ, Mulcahy MF, Ryu RK, Sato KT, Baker T, Kulik L, Gupta R, Abecassis M, Benson AB 3rd, Omary R, Millender L, Kennedy A, Salem R. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 81. | Lewandowski RJ, Gabr A, Abouchaleh N, Ali R, Al Asadi A, Mora RA, Kulik L, Ganger D, Desai K, Thornburg B, Mouli S, Hickey R, Caicedo JC, Abecassis M, Riaz A, Salem R. Radiation Segmentectomy: Potential Curative Therapy for Early Hepatocellular Carcinoma. Radiology. 2018;287:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 82. | Gabr A, Polineni P, Mouli SK, Riaz A, Lewandowski RJ, Salem R. Neoadjuvant Radiation Lobectomy As an Alternative to Portal Vein Embolization in Hepatocellular Carcinoma. Semin Nucl Med. 2019;49:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, Ryu RK, Sato KT, Gates V, Abecassis MM, Omary RA, Baker TB, Salem R. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 84. | Garlipp B, de Baere T, Damm R, Irmscher R, van Buskirk M, Stübs P, Deschamps F, Meyer F, Seidensticker R, Mohnike K, Pech M, Amthauer H, Lippert H, Ricke J, Seidensticker M. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology. 2014;59:1864-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 85. | Teo JY, Goh BK, Cheah FK, Allen JC, Lo RH, Ng DC, Goh AS, Khor AY, Sim HS, Ng JJ, Chow PK. Underlying liver disease influences volumetric changes in the spared hemiliver after selective internal radiation therapy with 90Y in patients with hepatocellular carcinoma. J Dig Dis. 2014;15:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 86. | Theysohn JM, Ertle J, Müller S, Schlaak JF, Nensa F, Sipilae S, Bockisch A, Lauenstein TC. Hepatic volume changes after lobar selective internal radiation therapy (SIRT) of hepatocellular carcinoma. Clin Radiol. 2014;69:172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 87. | de Graaf W, van den Esschert JW, van Lienden KP, van Gulik TM. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009;16:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 88. | Gabr A, Abouchaleh N, Ali R, Baker T, Caicedo J, Katariya N, Abecassis M, Riaz A, Lewandowski RJ, Salem R. Outcomes of Surgical Resection after Radioembolization for Hepatocellular Carcinoma. J Vasc Interv Radiol. 2018;29:1502-1510.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 89. | Vilgrain V, Pereira H, Assenat E, Guiu B, Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W, Barraud H, Laurent V, Mathias E, Bronowicki JP, Tasu JP, Perdrisot R, Silvain C, Gerolami R, Mundler O, Seitz JF, Vidal V, Aubé C, Oberti F, Couturier O, Brenot-Rossi I, Raoul JL, Sarran A, Costentin C, Itti E, Luciani A, Adam R, Lewin M, Samuel D, Ronot M, Dinut A, Castera L, Chatellier G; SARAH Trial Group. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1624-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 594] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 90. | Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, Choo SP, Cheow PC, Chotipanich C, Lim K, Lesmana LA, Manuaba TW, Yoong BK, Raj A, Law CS, Cua IHY, Lobo RR, Teh CSC, Kim YH, Jong YW, Han HS, Bae SH, Yoon HK, Lee RC, Hung CF, Peng CY, Liang PC, Bartlett A, Kok KYY, Thng CH, Low AS, Goh ASW, Tay KH, Lo RHG, Goh BKP, Ng DCE, Lekurwale G, Liew WM, Gebski V, Mak KSW, Soo KC; Asia-Pacific Hepatocellular Carcinoma Trials Group. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol. 2018;36:1913-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 473] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 91. | Sposito C, Mazzaferro V. The SIRveNIB and SARAH trials, radioembolization vs. sorafenib in advanced HCC patients: reasons for a failure, and perspectives for the future. Hepatobiliary Surg Nutr. 2018;7:487-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 92. | Edeline J, Crouzet L, Campillo-Gimenez B, Rolland Y, Pracht M, Guillygomarc'h A, Boudjema K, Lenoir L, Adhoute X, Rohou T, Boucher E, Clément B, Blanc JF, Garin E. Selective internal radiation therapy compared with sorafenib for hepatocellular carcinoma with portal vein thrombosis. Eur J Nucl Med Mol Imaging. 2016;43:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, Gasbarrini A, Pech M, Peck-Radosavljevic M, Popovič P, Rosmorduc O, Schott E, Seidensticker M, Verslype C, Sangro B, Malfertheiner P. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:1164-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 94. | Chauhan N, Bukovcan J, Boucher E, Cosgrove D, Edeline J, Hamilton B, Kulik L, Master F, Salem R. Intra-Arterial TheraSphere Yttrium-90 Glass Microspheres in the Treatment of Patients With Unresectable Hepatocellular Carcinoma: Protocol for the STOP-HCC Phase 3 Randomized Controlled Trial. JMIR Res Protoc. 2018;7:e11234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 95. | McRee A. Pembrolizumab Plus Y90 Radioembolization in HCC Subjects. [accessed 2021 Apr 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/show/NCT03099564 ClinicalTrials.gov Identifier: NCT03099564. |
| 96. | He AR. A Randomized Phase II Study of Atezolizumab and Bevacizumab With Y-90 TARE in Patients With Unresectable Hepatocellular Carcinoma (HCC). [accessed 2021 Apr 23]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/show/NCT04541173 ClinicalTrials.gov Identifier: NCT04541173. |
| 97. | Yang W, Yan K, Wu GX, Wu W, Fu Y, Lee JC, Zhang ZY, Wang S, Chen MH. Radiofrequency ablation of hepatocellular carcinoma in difficult locations: Strategies and long-term outcomes. World J Gastroenterol. 2015;21:1554-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 98. | Xu Z, Xie H, Zhou L, Chen X, Zheng S. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal Cell Pathol (Amst). 2019;2019:8619096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 99. | Song KD. Percutaneous cryoablation for hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:509-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 100. | Seifert JK, Junginger T, Morris DL. A collective review of the world literature on hepatic cryotherapy. J R Coll Surg Edinb. 1998;43:141-154. [PubMed] |
| 101. | Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, Xing BC, Huang XF. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 102. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-77; quiz 578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 577] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 103. | Brunello F, Cantamessa A, Gaia S, Carucci P, Rolle E, Castiglione A, Ciccone G, Rizzetto M. Radiofrequency ablation: technical and clinical long-term outcomes for single hepatocellular carcinoma up to 30 mm. Eur J Gastroenterol Hepatol. 2013;25:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Francica G, Saviano A, De Sio I, De Matthaeis N, Brunello F, Cantamessa A, Giorgio A, Scognamiglio U, Fornari F, Giangregorio F, Piscaglia F, Gualandi S, Caturelli E, Roselli P, Rapaccini GL, Pompili M. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Dig Liver Dis. 2013;45:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Vogl TJ, Nour-Eldin NA, Hammerstingl RM, Panahi B, Naguib NNN. Microwave Ablation (MWA): Basics, Technique and Results in Primary and Metastatic Liver Neoplasms - Review Article. Rofo. 2017;189:1055-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 106. | Ni JY, Sun HL, Chen YT, Luo JH, Chen D, Jiang XY, Xu LF. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol. 2014;20:17483-17490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 107. | Cloyd JM, Ejaz A, Konda B, Makary MS, Pawlik TM. Neuroendocrine liver metastases: a contemporary review of treatment strategies. Hepatobiliary Surg Nutr. 2020;9:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 108. | Ding J, Jing X, Liu J, Wang Y, Wang F, Du Z. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol. 2013;68:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 109. | Tombesi P, Di Vece F, Sartori S. Resection vs thermal ablation of small hepatocellular carcinoma: What's the first choice? World J Radiol. 2013;5:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 110. | Shiina S, Sato K, Tateishi R, Shimizu M, Ohama H, Hatanaka T, Takawa M, Nagamatsu H, Imai Y. Percutaneous Ablation for Hepatocellular Carcinoma: Comparison of Various Ablation Techniques and Surgery. Can J Gastroenterol Hepatol. 2018;2018:4756147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 111. | Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 112. | Dodd GD 3rd, Napier D, Schoolfield JD, Hubbard L. Percutaneous radiofrequency ablation of hepatic tumors: postablation syndrome. AJR Am J Roentgenol. 2005;185:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 113. | Lü MD, Kuang M, Liang LJ, Xie XY, Peng BG, Liu GJ, Li DM, Lai JM, Li SQ. [Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial]. Zhonghua Yi Xue Za Zhi. 2006;86:801-805. [PubMed] |
| 114. | Fang Y, Chen W, Liang X, Li D, Lou H, Chen R, Wang K, Pan H. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 115. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 642] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 116. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 788] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 117. | Huang YZ, Zhou SC, Zhou H, Tong M. Radiofrequency ablation versus cryosurgery ablation for hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60:1131-1135. [PubMed] |
| 118. | Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (2)] |
| 119. | Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia. 2016;32:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 120. | Sheta E, El-Kalla F, El-Gharib M, Kobtan A, Elhendawy M, Abd-Elsalam S, Mansour L, Amer I. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur J Gastroenterol Hepatol. 2016;28:1198-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 121. | Kim JW, Kim JH, Won HJ, Shin YM, Yoon HK, Sung KB, Kim PN. Hepatocellular carcinomas 2-3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs. radiofrequency ablation alone. Eur J Radiol. 2012;81:e189-e193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 122. | Gabrielson A, Wu Y, Wang H, Jiang J, Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L, Island E, Satoskar R, Banovac F, Jha R, Kachhela J, Feng P, Zhang T, Tesfaye A, Prins P, Loffredo C, Marshall J, Weiner L, Atkins M, He AR. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer Immunol Res. 2016;4:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 123. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 124. | Dendy MS, Ludwig JM, Stein SM, Kim HS. Locoregional Therapy, Immunotherapy and the Combination in Hepatocellular Carcinoma: Future Directions. Liver Cancer. 2019;8:326-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 125. | De Toni EN. Immune checkpoint inhibitors: use them early, combined and instead of TACE? Gut. 2020;69:1887-1888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 126. | Li S, Feng X, Cao G, Wang Q, Wang L. Prognostic significance of inflammatory indices in hepatocellular carcinoma treated with transarterial chemoembolization: A systematic review and meta-analysis. PLoS One. 2020;15:e0230879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 127. | Grégory J, Créquit P, Vilgrain V, Ronot M, Boutron I. Results of trials assessing transarterial chemoembolization for treating hepatocellular carcinoma are critically underreported. Eur Radiol. 2020;30:5633-5640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |