INTRODUCTION
In December 2019 a cluster of acute atypical respiratory infections were reported in the Wuhan city of Hubei province by the Chinese authorities to the World Health Organization (WHO). The responsible pathogen was identified as a new member of the family Coronaviridae, and it was called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) due to its similarity to the SARS coronavirus, previously involved in the 2002-2003 pandemic. The SARS-CoV-2-related disease was named coronavirus disease 2019 (COVID-19) and rapidly spread worldwide. Indeed, COVID-19 became a public health emergency on January 30, 2020 and, subsequently, a pandemic state was declared on March 11, 2020 by the WHO[1].
SARS-CoV-2 is a positive-sense single-stranded RNA virus, whose genome encodes for four major structural proteins: Spike (S) protein, envelope protein, membrane protein and nucleocapsid protein. The S protein mediates the entering of SARS-CoV-2 in the host cells by binding to the peptidase angiotensin receptor 2 (ACE2)[2].
COVID-19 is a contagious and highly lethal illness, especially for individuals with chronic comorbidities (such as diabetes mellitus, hypertension, cardiorespiratory disorders, chronic hepatic and renal diseases), elderly, oncological and immunosuppressed patients[2]. The infection is predominately transmitted by person to person through respiratory droplets, although many other modes of potential transmission have been postulated, which include through faecal-oral transmission. The average incubation period for COVID-19 is 5.2 d, but it can last up to 15.5 d.
The infection can have an asymptomatic course or it can present with fever, malaise and dry cough in the initial phase, during the invasion and infection of the upper respiratory tract. Patients may also experience gastrointestinal symptoms such as abdominal pain, vomiting and diarrhoea, and signs of systemic involvement (mainly neurological, cardiological, renal, and hepatological manifestations). Subsequently, the disease can involve the lower respiratory tract in approximately 20% of the cases and, in most severe situations, it can culminate in acute respiratory distress syndrome. This condition is characterized by a surge in circulatory inflammatory cytokines [mainly interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor (TNF)-α], termed ‘cytokine storm’, which is responsible for the subsequent inflammation and lung injury[1,3].
Treatments for COVID-19 change according to the disease severity: They include symptomatic and supportive therapy (such as oxygen supplementation, fluid resuscitation and vasopressors in case of septic shock), broad-spectrum antibiotics for prevention/management of secondary bacterial infections or sepsis, steroids if respiratory failure occurs, and prophylactic low molecular weight heparin in patients with moderate to severe disease because of the high risk of thromboembolism. The efficacy of antivirals (predominantly remdesivir and lopinavir/ritonavir combination), immunomodulatory drugs (including tocilizumab, chloroquine and hydroxychloroquine) and other treatments in reducing mortality and exacerbation of COVID-19 pneumonia is controversial and needs further evidence. However, these drugs are frequently used in clinical practice in the absence of any alternative[1].
As in other infectious diseases, recovered patients often continue to suffer from various long-term sequelae involving the respiratory system, as dyspnoea and cough, as well as less defined disabling manifestations; the latter include neuropsychiatric sequelae such as fatigue, anxiety, depression, post-traumatic stress disorder and insomnia[3-7]. It is still unknown whether these symptoms derive from the infection itself, from its general management (mainly medical therapies) or from the disease itself through mechanisms that have yet to be determined.
As COVID-19 affects also the gastrointestinal tract, some sequelae may derive from a disequilibrium of the intestinal homeostasis, but current evidence is almost absent[5,8].
In this review we hypothesised that the direct involvement of the gut and the one derived from COVID-19-related circumstantial conditions can predispose to the development of irritable bowel syndrome (IBS). To support this idea, we analysed the mechanism through which SARS-CoV-2 perturbs the intestinal physiology in infected individuals, went through the physiopathology of IBS and finally considered the possible factors that can subsequently trigger IBS after the COVID-19 recovery. For this aim, PubMed and Google Scholar were searched using various combinations of the terms “SARS-CoV-2”, “COVID-19”, “gastrointestinal”, “gut”, “symptoms”, “irritable bowel syndrome”, “microbiota”, and “microbiome”. Subsequently, we selected the most pertinent articles in support of reasonable common factors between COVID-19 and IBS enhancement and summarised current evidence.
GASTROINTESTINAL INVOLVEMENT OF COVID-19
Gastrointestinal manifestations of COVID-19 can be present with variable incidence (40%-50%), and include mainly diarrhoea, nausea, anorexia, vomiting, abdominal pain and belching. These symptoms may arise even in the absence of respiratory involvement or may appear after the onset of respiratory symptoms[2,9,10]. SARS-CoV-2 is also associated with other gastrointestinal symptoms. One of these include liver injury, which can manifest as increased serum aminotransferases, bilirubin and γ-glutamyl transferase[2,10]. Furthermore, elevated blood levels of amylase and lipase have been described, but a strict causality of pancreatic damage with SARS-CoV-2 infection has not been ascertained. Importantly, the drugs used to treat COVID-19 may also have impact on the gastrointestinal tract[11].
Gastrointestinal involvement in COVID-19 may be due to the capacity of SARS-CoV-2 to directly infect the intestinal tract: This hypothesis is supported by detection of the virus in enterocytes and in stool samples of affected patients, and also in faecal samples of individuals with negative nasopharyngeal tests[12-14]. As previously mentioned, SARS-CoV-2 attaches to the ACE2 to enter into human cells and to infect the host. This receptor exists in two forms: The full-length mACE2, which is located on cell membranes with a transmembrane anchor and an extracellular domain, and the sACE2, a soluble form released into blood circulation. The N-terminal domain of the mACE2 is the target of the S protein of SARS-CoV-2[15]. The S protein consists of two different subunits: The S1, which binds to the cell receptors of the host, and the S2, which mediates the fusion of the viral and cell membranes[16]. Two transmembrane protease serines, TMPRSS2 and TMPRSS4, are essential to cleave the S protein at S1/S2 and S2 sites, to enhance the S fusogenic activity, the entry and replication of the virus in mature small intestinal enterocytes[17,18].
The ACE2 is expressed in several tissues within the human body with specific localization on different cells, including enterocytes, renal tubules, gallbladder, cardiomyocytes, male reproductive cells, placental trophoblasts, ductal cells, eye, vasculature and others[15,19]. Concerning the digestive system, the expression of ACE2 gene is highest in the small intestine, but it is also present among other sites, such as colon, stomach, oesophagus, liver, biliary tract and pancreas[19-22]. Specifically, this receptor is expressed in the muscularis mucosa and mucosa of the intestine, including the epithelial cells, cholangiocytes, hepatocytes, pancreatic ductal, acinar and islet cells, and in the gastrointestinal vasculature[11,23,24]. ACE2 seems to play a key role in the intestinal homeostasis and functions. Indeed, it can regulate the blood flow perfusion by increasing the vascular resistance (primarily the mesenteric vasculature). Moreover, it is possible that ACE2 is capable of enhancing the mucosal nitric oxide production, which regulates the properties of the epithelial barrier, and of modulating the ion transport and the paracellular permeability. It can also induce duodenal secretory responses of mucosal bicarbonate against the luminal acid from the stomach and stimulate sodium and water absorption. It seems plausible that ACE2 is involved in the relaxation of the gastrointestinal wall musculature. Nonetheless, current evidence suggests that ACE2 is involved in inflammation and immunomodulation, and in the pathophysiology of IBS for contributing to enhance low-grade inflammation in the enteric nerve plexa[24,25]. The ACE2 can also regulate the intestinal amino acid homeostasis and absorption, the production of antimicrobial peptides, the intestinal motility and the gut microbiota independently of the renin-angiotensin system[24,26]. It is also reported that the deficiency of this receptor in a murine model of colitis leads to an increased susceptibility to intestinal inflammation. This effect seems to be mediated by an impaired epithelial immunity and induced dysbiosis, defined as the impairment of the diversity and function of intestinal microbes. This is suggested by the increased propensity to develop severe colitis after the faecal microbiota transplantation of an impaired intestinal microbiota from mice with genetic inactivation of ACE2 into germ-free wild-type animals[26]. Moreover, preclinical evidence indicates that ACE2 can impair the electrophysiological and synaptic functions of the neurons of the enteric nervous system, thus influencing the gastrointestinal motility, sensitivity and the pathways of inflammation[27].
Overall, it is plausible that the impairment of bowel physiology by SARS-CoV-2 may derive from a dysregulation of all these ACE2-mediated functions due to a competitive mechanism of the virus on this receptor or from a downregulation of its anti-inflammatory activity. Moreover, the gastrointestinal manifestations may arise from a direct cytopathic effect of the virus on the mucous epithelium, from a malabsorption secondary to the invasion of enterocytes, or from the triggered inflammatory response with plasma cells and lymphocytes infiltration in the intestinal lamina propria[2,28]. Accordingly, SARS-CoV-2 infection can be associated with microscopic bowel inflammation with infiltrating plasma cells and lymphocytes, and with interstitial edema in the lamina propria, as well as overt acute haemorrhagic colitis with endoscopically confirmed mucosal injury[28,29]. The hypothesis of intestinal inflammation is supported by the detection of significantly increased levels of faecal cytokines, as IL-8, in COVID-19 patients when compared to uninfected controls[30]. Additionally, a significant number of patients (approximately 30%–75%), more frequently those with gastrointestinal manifestations, has elevated values of faecal calprotectin, a protein released by neutrophils of the intestinal mucosa[31]. The occurrence of diarrhoea seems also higher among patients with higher SARS-CoV-2 RNA loads in stool samples[30]. Finally, the presence of virus-specific immunoglobulin A (IgA) in faecal samples suggests that the gastrointestinal tract may be immunologically active during SARS-CoV-2 infection[30].
It is likely that the gut homeostasis and the intestinal immunity play a major role in the pathogenesis of COVID-19 and in the enhancement of the systemic inflammation triggered by the SARS-CoV-2 infection, which is characterized by significantly higher serum IL-6, IL-8, IL-10 and TNF-α in severe cases[30]. Indeed, it is described that higher levels of faecal IL-23 correlate with more severe COVID-19 disease, as well as the finding of intestinal virus-specific IgA responses[30]. Interestingly, gut microbial alterations in COVID-19 patients can contribute to regulate systemic inflammation, as suggested by the correlation between specific changes in genera and inflammation indices[32].
IBS
IBS is the most common chronic disorder of the gut-brain interaction, and it is characterized by mild to severe recurrent abdominal pain and bloating associated to alterations in bowel habits in the absence of organic disease or biochemical abnormalities[33]. IBS is also often accompanied by other comorbidities, like psychiatric conditions, pain syndromes, overactive bladder, migraine, and visceral sensitivity[34]. The debilitating symptoms of IBS impose a significant burden on the quality of life of affected individuals, since it is associated with depression and suicidal ideation, reduces work productivity and increases the accesses to medical care[35,36]. The prevalence of IBS varies substantially between countries due to the different diagnostic criteria and survey methods used in worldwide studies, ranging from less than 1% to more than 25%, with a predominance in women in comparison to men (12.0% vs 8.6% respectively; odds ratio 1.46)[37]. Moreover, it is more frequent in lower socioeconomic groups and individuals younger than 50 years[36]. IBS is diagnosed according to the Rome criteria, a clinical classification which includes four types of IBS according to the predominant bowel habits: IBS with predominant constipation, IBS with predominant diarrhoea (IBS-D), IBS with mixed bowel habits (IBS-M) and unclassified IBS[38]. IBS-M and IBS-D are reported to be the most prevalent subtypes[37]. For an accurate diagnosis of IBS, organic underlying conditions must be excluded, with an accurate patient history, physical examination, laboratory tests and, if necessary, endoscopic assessment. Common conditions which should be ruled out include celiac disease, microscopic colitis, inflammatory bowel disease, bile acid malabsorption, colorectal cancer, and dyssynergic defecation[38,39].
The physiopathology of IBS is currently not fully understood, but it is considered a complex multifactorial disorder with a still unknown molecular pathophysiology. Indeed, it has been hypothesised that an impairment of different functions (such as central and autonomic neurophysiology, visceral nociception, bowel motility, secretory activity and psycho-somatic balance) due to perturbing factors (i.e., stress exposure, psychosocial conditions, food antigens, antibiotics and infections of various origin) leads to physiological abnormalities, which may be involved in the development and perpetuating of IBS. These include intestinal dysbiosis, increased intestinal permeability, immune cell hyper-reactivity with impaired expression and release of mucosal and immune mediators, microinflammation with altered mucosal functions, hyper-sensitivity of the enteric nervous system, dysregulation of the hypothalamus-pituitary-adrenal (HPA) axis and of the enteric nervous system. Increased levels of faecal bile acids and predisposing inheritable susceptibility are recognised as co-occurring factors as well[34,39,40]. Increasing evidence suggests that all these affected pathways are part of the microbiota-gut-brain axis, a bidirectional crosstalk between the brain, the bowel and the gut microbiota which occurs through nervous signalling, immune mediators, microbial products, tryptophan metabolites and other hormones[39,41].
Accordingly, dysbiosis may contribute to IBS by triggering the gut immune system and enhancing low-grade inflammation in susceptible individuals. This hypothesis is supported by a higher prevalence of small intestinal bacterial overgrowth and imbalances of the gut microbiota composition in patients with IBS compared with healthy controls in many recent studies, and by the benefit from the use of non-absorbable antibiotics on related symptoms. A reduction of the diversity and stability of the gut microbiota in patients with IBS has been described[42]. Increased Enterobacteriaceae, which includes several harmful genera (as Escherichia, Shigella, Campylobacter, and Salmonella), and Lactobacillaceae families, together with high levels of Bacteroides genus, reduced Faecalibacterium and Bifidobacterium genera and uncultured Clostridiales I are reported in patients with IBS in comparison with controls in a recent systematic review including 24 studies[43].
In 6%-17% of the patients suffering with IBS the onset of the symptoms occurs after a recent episode of gastrointestinal infection, which can increase up to 6-fold the risk of developing IBS. This phenomenon is characterised by the persistence of IBS-like disturbances (mainly diarrhoea and abdominal discomfort) after the resolution of the infection, and it is known as post-infectious IBS (PI-IBS). The prevalence of PI-IBS is approximately 4%-36% in patients with previous infectious gastroenteritis and is higher in females, young people, patients who experienced severe infections and individuals with psychological comorbidities. Moreover, some pathogens seem more predisposing than others; indeed, bacterial infections (particularly by Campylobacter, Shigella, Escherichia coli and Salmonella) are more likely to enhance PI-IBS than viruses and other microorganisms[34,44,45]. The pathogenesis of this condition is poorly understood, but it is hypothesized that the responsible pathogenic microorganism may trigger an immunologic and inflammatory response with low-grade inflammation and mucosal injury, which causes the prolongation of IBS symptoms in predisposed individuals. Furthermore, it is described that patients with PI-IBS may have increased macrophages and T lymphocytes in intestinal samples, together with high expression of IL-1 in rectal biopsies and elevated blood level pro-inflammatory cytokines (such as TNF-α, IL-6, IL-8, IL-10 and IL-1β)[44,46]. As in IBS, it is likely that an altered intestinal permeability, an impairment of the gut eubiosis and of the neuromuscular function are involved in PI-IBS as well[47].
COVID-19 AND ITS MANAGEMENT: WHAT ARE THE POSSIBLE TRIGGERS OF IBS?
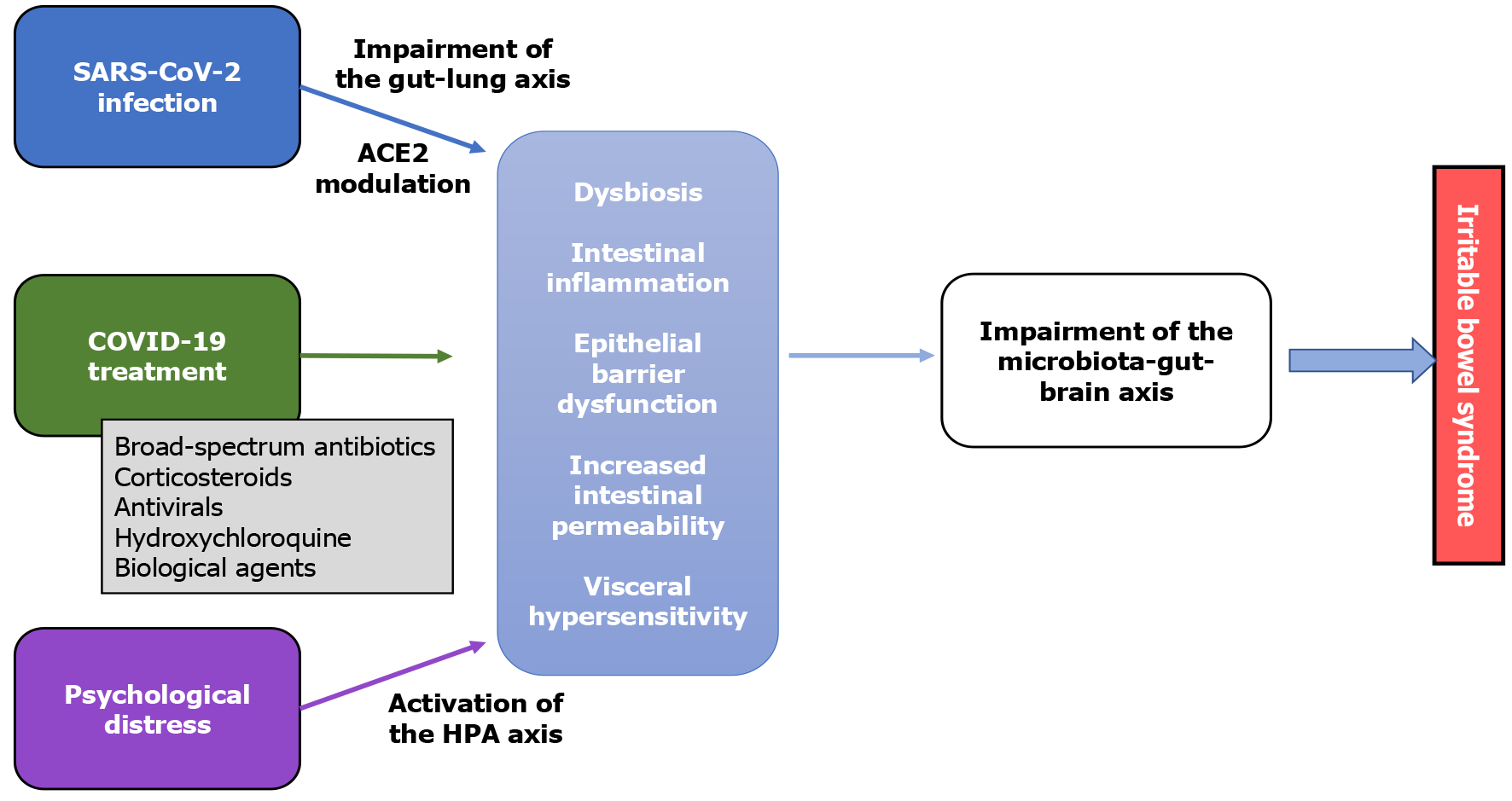
The plausible mechanisms involved in the development of IBS in individuals who experienced COVID-19 are summarised in Figure 1.
Figure 1 Possible pathophysiology of irritable bowel syndrome in coronavirus disease 2019 patients.
ACE2: Peptidase angiotensin receptor 2; COVID-19: Coronavirus disease 2019; HPA: Hypothalamus–pituitary–adrenal; SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2.
Gastrointestinal disturbances are often associated with respiratory infections or to secondary complications, and the gut-lung axis, a hypothetical bidirectional pathway which works via biochemical and immunologic systemic signalling molecules, is possibly involved in the pathophysiology. Among the perturbing factors of the gut microbial environment, respiratory viral infections, including COVID-19, can play a relevant role[48]. As previously mentioned, an impairment of the gut microbiota composition, which is frequently associated to a dysregulation of the overall intestinal homeostasis and gut-brain axis, can participate to the development and maintaining of IBS[39,41,42]. An imbalance of the gut microbiota is described in SARS-CoV-2-infected individuals. Gu et al[32] found a significant reduction in mean community richness and bacterial diversity in COVID-19 patients in comparison with healthy controls according to the Shannon diversity index and Chao diversity index. A significantly higher relative abundance of Streptococcus, Rothia, Veillonella, and Actinomyces, which are opportunistic pathogens, and a lower relative abundance of beneficial symbionts were reported. Moreover, Fusicatenibacter, Romboutsia, Intestinibacter, Actinomyces and Erysipelatoclostridium were identified as biomarkers to discriminate the COVID-19 patients from healthy individuals[32]. Zuo et al[49] reported that even antibiotics-unexposed patients with COVID-19 have a significantly changed intestinal microbiota during the hospitalization, with enrichment of opportunistic pathogens (including Clostridium hathewayi, Actinomyces viscosus, and Bacteroides nordii) and depletion of beneficial commensals when compared to healthy individuals. Moreover, a correlation between the disease severity and the baseline abundance of certain genera and strains was found, suggesting that the gut microbiota may contribute to the systemic involvement in the immune system responses; specifically, a positive relation was observed with Coprobacillus, Clostridium ramosum, and Clostridium hathewayi, while a negative association was described with Faecalibacterium prausnitzii. The loss of beneficial bacteria persisted even after a negative throat swab and the disease resolution, suggesting a persistent deleterious effect on the gut microbiota[49]. The same working group also observed that an active intestinal infection is present in approximately half of COVID-19 patients even without gastrointestinal manifestation, and persisted even after respiratory clearance of SARS-CoV-2. Of interest, stool specimen with a signature of high SARS-CoV-2 infectivity were characterised by an enrichment of opportunistic pathogens (including Collinsella aerofaciens, Collinsella tanakaei, Streptococcus infantis and Morganella morganii). On the other hand, faecal samples with a signature of “low-to-none” SARS-CoV-2 infectivity displayed higher concentration of Parabacteroides merdae, Bacteroides stercoris and Lachnospiraceae bacterium 1_1_57FAA. The latter are short-chain fatty acid producing bacteria, which play a crucial role in boosting host immunity. A longitudinal follow-up revealed relevant alterations of the faecal microbiota composition in a subset of patients[50].
More generally, intestinal and pulmonary dysbiosis are described in various acute and chronic pulmonary diseases. For example, pulmonary viral infections, such as the ones caused by influenza virus and respiratory syncytial virus, can even directly impair the gut microbiome[49]. Moreover, patients suffering from asthma have functional and structural impairment of the intestinal mucosa, and patients with chronic obstructive pulmonary disease often have leaky gut[51]. Apart from the acute COVID-19 phase, respiratory sequelae and radiological abnormalities (such as dyspnoea, chronic cough, fibrotic lung disease, bronchiectasis, and pulmonary vascular disease) may persist in recovered patients, and the optimal management is still undefined[5,52,53]. Thus, it might be plausible that an impairment of the gut homeostasis may occur in patients during the acute COVID-19 illness and persist after the disease resolution, even in those who did not experience gastrointestinal disturbances. This can be hypothetically explained by the communication between the two systems through the gut-lung axis. The existence of this connection is not entirely understood, but it is strengthened by the occurrence of lung diseases worsening as a consequence to intestinal microbial imbalances, gut inflammation and increased intestinal permeability[54]. Accordingly, it is reported that elevated values of faecal calprotectin are associated with a pathological chest X ray in COVID-19 patients[55]. Of interest, the enriched presence in faecal samples of patients with COVID-19 with high SARS-CoV-2 infectivity of Streptococcus infantis, an upper respiratory tract and oral cavity colonizer bacterial pathogen, may indicate a translocation or transmission of extraintestinal microbes into the gut during COVID-19[50]. Moreover, lung and gut are independent systems which originate from one common embryonic organ, the foregut[56]. The microbiota of these two systems develop almost simultaneously after birth and is influenced by common factors, such as diet[57]. Overall, it is possible that a COVID-19-induced dysregulation of the gut-lung axis may enhance predisposing circumstances for IBS. This is also supported by the increased occurrence of gut disturbances, like inflammatory bowel disease or IBS, in patients with chronic respiratory diseases. Moreover, a pulmonary involvement has been described in approximately 33% of patients with IBS[51].
As previously mentioned, viral enteritis is described as a risk factor for developing PI-IBS. This has been assessed for norovirus infections in particular. Porter et al[58] found a significant increase in the incidence of functional gastrointestinal disorders, including constipation, in individuals who experienced a gastroenteritis during a norovirus outbreak, suggesting that dysmotility-related disorders may arise from viral infections[58]. Previously, Marshall et al[59] described a significantly increased prevalence of PI-IBS in a small cohort of subjects after a large outbreak of acute gastroenteritis attributed to food-borne norovirus when compared to unexposed individuals (23.6% vs 3.4%, P = 0.014), with OR of 6.9 (95%CI: 1.0–48.7)[59]. Similarly, an Italian study assessed the incidence of PI-IBS and functional gastrointestinal disorders after a norovirus outbreak. At 12 mo follow up a significant greater proportion of the infected participants (13%, 40 of 186 adults) developed PI-IBS in comparison with unexposed controls (3 of 198 subjects). The mechanisms through which IBS is elicited by norovirus are unknown, but this micro-organism can lead to epithelial barrier dysfunction, increased intestinal permeability, reduction in villous surface area and villous height, and to a mucosal immune response with an increase of cytotoxic intra-epithelial T cells[47]. It is possible that these alterations may trigger a perpetual immune stimulation or a prolonged immune activation toward cross-reacting non-pathogenic antigens, which impairs the gut sensory-motor function[58]. Since SARS-CoV-2 can have an intestinal tropism and induce intestinal flogosis[13,30,31], it can be speculated that a mechanism similar to that of norovirus is involved in the enhancement of PI-IBS.
Other non-infective factors may possibly play a key role in IBS pathophysiology. To date, COVID-19 management has involved a wide range of medications, whose efficacy has yet to be rigorously proven or is still under evaluation, and that can enhance a dysbiotic state. Above all, empiric antimicrobial use is often part of the treatment of respiratory infections, including SARS-CoV-2[60]. It is well known that broad-spectrum antibiotics can cause a rapid and significant drop in taxonomic richness, diversity and evenness, that can persist even for years after the treatment interruption. Beyond taxonomical compositional alterations, the gene expression, protein activity and metabolism of the gut microbiota can be impaired by antibiotics. Overall, these changes can predispose to intestinal infections, to overgrowth and pathogenic behaviour of resident opportunistic organisms, and to impairment of the immunological equilibrium with systemic and long-term consequences[61]. Alongside with gut dysbiosis, broad-spectrum antibiotics can induce a disruption of the intestinal barrier function by altering the tight junction protein expression and localization, enhancing a pro-inflammatory state by NLRP3 inflammasome activation and promoting autophagy[62]. Hospitalised patients affected by COVID-19 are frequently treated with broad spectrum antibiotics for 5-8 d, mainly to prevent or to treat pathogens causing atypical pneumonia and staphylococci[60]. For instance, azithromycin, which is largely prescribed to COVID-19 patients, can induce a decline in the microbial richness and diversity, as well as changes in microbiota composition, with a shift in the Actinobacteria phylum, a reduction in the relative abundance of Proteobacteria and Verrucomicrobia (including Akkermansia muciniphila) and a decrease of the levels of bifidobacteria[63,64]. It is also reported that COVID-19 patients treated with vancomycin and/or ceftriaxone went through significant compositional alteration with reduced diversity of the gut microbiota[30].
Additionally, it is described that the use of corticosteroids, which is considered a treatment option in severe COVID-19 cases, may induce dysbiosis and alter intestinal homeostasis as well[57,65]. This is supported by the influence of steroid hormones on the gut bacterial communities in animal studies. In example, gonadectomy can reduce the microbiota-related sex differences observed between male and female rats. Similarly, hormone replacement to rodent females from the birth can decreased the microbial diversity in adulthood by increasing the Firmicutes:Bacteroidetes ratio[66]. As to systemic glucocorticoid therapy, there is evidence that subcutaneous prednisolone administration can alter the gut microbiota of mice, inducing significant shifts in the relative abundance of bacteria (decrease in Verrucomicobiales and Bacteriodales and increase in Clostridiales) in comparison with controls[67]. Interestingly, it is reported that individuals with glucocorticoid-induced obesity, who received prednisolone for at least three months, have significant decrease in gut microbial diversity in comparison with healthy controls, alongside with increased Firmicutes and Actinobacteria levels, and depletion in Bacteroidetes. Taxonomic analysis revealed a significantly reduced relative abundances of Bacteroides, Bifidobacterium, and Eubacterium in treated patients, whereas Streptococcus and Geobacillus displayed higher abundances. Also the faecal content of short-chain fatty acids (propionate and butyrate), which are products of carbohydrate fermentation by the gut bacteria, tended to be remarkably lower when compared to healthy control[68].
Other treatment options for the pulmonary phase of COVID-19 include antiviral drugs, mainly the adenosine nucleotide analogue prodrug remdesivir and the protease inhibitor lopinavir in combination with ritonavir[69]. Multicentre randomized controlled trials to assess the efficacy in reducing inpatient mortality, ventilation rate, and duration of hospital stay are still undergoing, although preliminary results are overall not encouraging[70]. While remdesivir seems not to affect the gut microbiota composition, antiretrovirals may somehow have a modulatory activity. The knowledge of the impact of antiretroviral drugs on the microbiome is limited by little evidence and it is predominantly focused on human immunodeficiency virus (HIV) treatment. It is still unclear whether antiretroviral treatment can consistently restore gut health of HIV-infected individuals or not, but it is likely that the initiation and prosecution of these medications can promote dysbiotic states[71,72]. Importantly, there is evidence that this drug class can promote microbiome changes independently from those induced by HIV. Indeed, a decreased alpha diversity is reported among treated patients in comparison with untreated HIV-positive ones. Moreover, protease inhibitors can directly interfere with the in vitro adherence of Candida albicans to an epithelial cell layer, which can possibly contribute to the reduction of oral candidiasis in HIV treated individuals[73].
Even if current evidence discourages its use in the prevention or treatment of COVID-19, hydroxychloroquine has been largely administered to patients due to its in-vitro capability of inhibiting SARS-CoV-2 by interfering with membrane fusion between host cell and the virus, especially in the early phase of the pandemic[70,74]. Hydroxychloroquine was initially used as an antimalarial, but, subsequently, it has been used as a disease-modifying anti-rheumatic drug to treat rheumatic disorders due to its immunomodulatory properties[75]. The impact of this medication on the human gut microbiota has been assessed by Balmant et al[48] in patients affected by systemic lupus erythematosus. They reported that the use of this drug is associated to different degrees of dysbiosis with a dose-dependent effect[48].
Severe SARS-CoV-2 infection is associated to an aberrant immune response with a massive cytokine release, mainly the IL-6 and IL-8. The elevation of inflammatory markers, such as IL-6 and C-reactive protein, has been associated with mortality and severe disease with pulmonary involvement in comparison to moderate disease. Thus, the targeted blockade of systemic inflammation has been proposed as a strategy to treat advanced acute conditions with lung lesions when contrasting the virus alone might not be sufficient. Specifically, Tocilizumab, a monoclonal antibody that inhibits the IL-6 receptor, has been proposed in patients with advanced lung injury[69]. Little evidence about the effect of this biological agent on the intestinal microbiota is available. A study of patients with rheumatoid arthritis reported that biologics, including tocilizumab, significantly reduced the total bacterial count and led to a decrease of Clostridium coccoides group, Bifidobacterium, and Lactobacillus plantarum and Lactobacillus gasseri strains after 6 mo[76]. Furthermore, it has been hypothesized that the aetiology of tocilizumab-related intestinal perforation may lie in the compositional or functional microbial changes[8]. Possibly, another IL-6 receptor inhibitor, which is currently being tested for severe COVID-19, may induce similar changes to the intestinal microbiota, but no study with this objective has been performed to date[69].
A further significant imbalance in the commensal bacterial populations may also be caused by polypharmacotherapy (e.g. proton-pump inhibitors, laxatives and metformin), which is common especially in elderly comorbid COVID-19 patients, and by the use of commonly prescribed drugs to manage mild COVID-19, as nonsteroidal anti-inflammatory drugs[63].
Finally, another considerable element involved in the IBS onset, exacerbation and relapse, is the activation of the HPA axis consequential to the secretion of the corticotropin-releasing hormone due to acute or chronic stressful conditions. This signalling pathway affects the gut functions by regulating the stimulation of the sympathetic and parasympathetic activity, the release of catecholamines, the mucosal immunity, the intestinal barrier function, the splanchnic blood flow and the composition and growth of the gut microbiota. Immune activation and intestinal micro-inflammation are described in IBS and can increase the intestinal permeability, modulate the peripheral sensitization of mucosal neuronal afferents and the recruitment of “silent” nociceptors involved in the hypersensitivity. Similarly, stress-induced dysbiosis may modulate the neuro-immune-endocrine systems and interfere with the brain-gut axis. Accordingly, the prevalence of at least one psychiatric disorder (mainly depression and anxiety) in patients with IBS ranges from 40% to 60% approximately, and the severity of IBS manifestations is remarkably correlated with the intensity co-morbid psychiatric disturbs. Moreover, early adverse life events and major traumatic experiences are frequently described before the onset of IBS[77,78]. COVID-19 is having a significant impact on mental health worldwide, since various psychological stress-associated factors are linked to the pandemic. More than 40% of patients experiences psychological distress even when the disease is under control during the acute infection phase. A considerable role is also played by anxiety, panic and fear for the isolation environment and the uncertain sequelae following resolution of a new and dangerous disease. Long-term psychological consequences (as anxiety, depression, post-traumatic stress disorder, insomnia, irritability, memory impairment, fatigue, and traumatic memories) are frequently reported among those who suffered from COVID-19, especially hospitalized ones and individuals with previous emotional dysregulation[7,79]. Overall, COVID-19-related psychological disturbances are significant and can definitely contribute to IBS occurrence.
CONCLUSION
The COVID-19 pandemic is a threat to global public health. A wide spectrum of respiratory and systemic symptoms can occur during the acute disease with different degrees of severity, and some of them can persist over time after the recovery. A large body of evidence supports the gastrointestinal involvement of SARS-CoV-2 infection during the acute phase, possibly because the intestinal ACE2 is an additional target of viral infection. Importantly, little is known about the gastrointestinal sequelae; at present, there is no study reporting the occurrence of IBS in individuals recovered from COVID-19. However, a number of considerations may be made regarding the plausible role of COVID-19, its management and global setting in the enhancement of IBS. Specifically, it can be assumed that many factors contributing to promote a dysbiotic state, epithelial barrier impairment, intestinal inflammation and gut dysfunction (like antibiotics and other treatments of the acute phase, gut-lung axis impairment, disease-related psychological stress, as well as the virus itself) can be involved in this process. Prospective cohort studies are necessary to confirm these hypotheses before clinical significance can be concluded.