Published online Nov 21, 2021. doi: 10.3748/wjg.v27.i43.7423
Peer-review started: March 30, 2021
First decision: June 26, 2021
Revised: July 5, 2021
Accepted: October 27, 2021
Article in press: October 27, 2021
Published online: November 21, 2021
Processing time: 234 Days and 4.6 Hours
Chronic pancreatitis (CP) is a complex disease associated with gene-gene or gene-environment interactions. The incidence of idiopathic CP has shown an increasing trend, withits phenotypeshaving changed considerably in the last two decades. The diseaseitself can be regulated before it reaches the stage of established CP; however, the etiopathogenesis underlying idiopathic CP remains to be established, making the condition difficult to cure. Unfortunately, there also remains a lack of consensus regarding the beneficial effects of antioxidant therapiesfor CP. It is known that antioxidant therapy does not reduce inflammatory and fibrotic cytokines, making it unlikely that they could modulate the disease process. Although antioxidants are safe, very few studies to date have reported the long-term beneficial effects in patients with CP. Thus, studies are being performed to identify drugs that can improve symptoms and alter the natural history of CP. Statins, with their numerous pleiotropic effects, may play a role in the treatment of CP, butin 2006, their use was found to be associated with the undesirable side effect of promoting pancreatitis. Latter studies showed favourable effects of statins in CP, highlighting the particular benefits of lipophilic statins, such as lovastatin and simvastatin, over the hydrophilic statins, such as rosuvastatin. Ultimately, studies to repurpose N-acetylcysteine as a CP therapy areyielding very promising results.
Core Tip: The clinical management of a majority of chronic diseases has seen a paradigm shift over the last two decades. To date, however, a well-defined standard of care has not been established for patients with chronic pancreatitis (CP). Lack of sufficient scientific evidence regarding the use of antioxidant supplementation, in particular, provides opportunities to repurpose drugs and study their efficacy and safety in clinical trials. Statins and N-acetylcysteine represent two of the most promising molecules for the treatment of CP, today.
- Citation: Mehta RM, Pandol SJ, Joshi PR. Idiopathic chronic pancreatitis: Beyond antioxidants. World J Gastroenterol 2021; 27(43): 7423-7432
- URL: https://www.wjgnet.com/1007-9327/full/v27/i43/7423.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i43.7423
Rajiv M Mehta, MD, is Professor and Head of the Department of Gastroenterology and Clinical Research at Surat Institute of Digestive Sciences (SIDS) Hospital and Research Centre in Surat, India. He received his undergraduate and postgraduate degrees from Baroda University in Vadodara, India, in 1996 and 2000, respectively. He received his advanced degree in gastroenterology from Amrita Institute of Medical Sciences (AIMS) in Kochi, India. Only 5 years later, in 2005, he was awarded the prestigious presidential gold medal from the National Board of Examination, New Delhi, in Gastroenterology. In his continued efforts to advance the overall field of gastroenterology, Dr. Mehta authored the very popular “Clinical Gastroenterology” book for undergraduate and post-graduate students, withits fourth edition published in December 2019 at the Asia Pacific Digestive Disease Week held in Kolkata, India. He has also published more than 50 articles in various prestigious journals, to date. Dr. Mehta has also been collaborating with Dr. Stephan Pandol and his team from Cedars-Sinai Medical Center in Los Angeles, CA, United States over the past 3 years, focusing on chronic pancreatitis (CP). Collectively, this group isinvolved in the development of novel biomarkers for the diagnosis of “early CP”. Additionally, Dr. Mehta has investigated the effects of simvastatin on the histology of L-arginine-induced pancreatitis in mouse models, in association with the Jay Research Foundation (JRF) in Vapi, Gujarat, India. Defining the overall role of simvastatin and N-acetylcysteine (NAC) in CPtreatment remains Dr. Mehta’s seminal work, while defining the role of genetic polymorphisms in patients with idiopathic CP is his focused area of interest. Further, he is diligently working towards the development of “New Chemical Entity” in pancreatic cancer.
CP is a fibro-inflammatory disorder of the exocrine pancreas occurring in individuals with genetic, environmental and other risk factors who develop persistent pathological responses to parenchymal injury or stress[1]. CP is characterized by acinar cell damage, ductal dysfunction, persistent inflammation, atrophy, fibrosis, and neuroimmune responses. The clinical course of CP involves significant abdominal pain, exocrine function deficiency (manifested as maldigestion), and endocrine deficiency (manifested as diabetes). The causes of CP include alcohol intake, smoking, metabolic derangements, genetic disorders, autoimmune factors, obstructive mechanisms, and idiopathic aetiologies[2]. Unfortunately, it is difficult to determine the exact prevalence of CP, since the early diagnosis of CP continues to be challenging.
Alcoholic chronic pancreatitis (ACP) is most commonly observed in Western countries, whereas idiopathic chronic pancreatitis (ICP) is observed more frequently in developing countries, like India; reportedly, ICP accounts for 57.3%–69.6% of the cases of CP in India[3]. The cause of ICP remains unknown[4]. In genetically susceptible individuals, environmental factors initiate the fibroinflammatory process (gene-gene or gene-environment interplay), which leads to the development of CP[5]. The subtle pathophysiological changes pose challenges to the early diagnosis of CP, and the parameters for early diagnosis are ill-defined. Late-stage CP is characterized by variable fibrosis and calcification in the pancreatic gland, leading to parenchymal and ductal changes[6].
Abdominal pain is a well-recognized and debilitating symptom, prompting patients with CP to seek medical assistance. Among all the complications of CP, abdominal pain was found to be strongest predictor of poor quality of life (QoL)[7]. Endoscopic and surgical treatments are often performed to relieve pain, but these methods are invasive and are beneficial only in a specific subgroup of patients with CP. The therapeutic strategies currently used for the management of CP include a combination of analgesics, pancreatic enzymes, adequate nutrition, and antioxidants[8]. However, the effect of antioxidants on providing sustained pain relief or reversing disease activity has not been established, thus far. As such, further studies are warranted to address this unmet need for an alternative therapeutic approach for CP.
The use of antioxidant therapy is based on observations that CP tissues show marked oxidative changes.
Oxidative stress is caused by an imbalance between production and accumulation of reactive oxygen species (ROS) in cells and tissues and the ability of a biological system to detoxify these reactive products[9]. Xenobiotics, such as alcohol and smoking molecules, are detoxified in the body through phase I and phase II pathways. The phase I reaction involves cleaving the parent molecule by enzymes into a less toxic molecule. The phase II reaction adds an endogenous molecule to the compound at the end of phase I to make it more polar and excretable. Increased exposure to xenobiotics may overwhelm the capacity of phase I and phase II detoxification pathways and result in oxidative stress. Production of ROS is a particularly destructive aspect of oxidative stress. ROS are mainly produced by mitochondria, during both physiological and pathological conditions[10]. When the production of ROS increases, they exert harmful effects on important cellular structures, like proteins, lipids and nucleic acids, leading to destruction of the cell membrane, depletion of cellular antioxidants, and alteration in various signalling pathways. Previous studies have indicated that oxidative stress is involved, to a varying extent, in the onset and/or progression of several diseases[9].
Pancreatic acinar cells are the main site for generation of oxidant stress and, therefore, are exposed to its detrimental effects. Intra-acinar oxidative stress leads to impairment of the transsulfuration pathway, which is required for zymogen exocytosis[11,12]. This leads to recurrent intra-acinar zymogen activation. Methionine and ascorbic acid appear to be important components in maintaining the transsulfuration pathway[13]. Several studies conducted in the 1990s showed that deficiency of essential antioxidants, such as vitamin A, ascorbic acid, methionine, vitamin E and selenium, is particularly prevalent among patients with ACP. Persistent exposure to xenobiotics via smoking and consumption of alcohol increase the levels of oxidative stress. Thus, the role of oxidative stress and micronutrient deficiency in patients with CP exposed to high levels of xenobiotics has been established. “Tropical pancreatitis” has been typically associated with protein and micronutrient deficiencies; however, the role of malnutrition in the etiopathogenesis of CP has been discarded[14].
Over the past one and a half decades, results of several studies conducted in India have shown that the phenotype of patients with ICP has changed significantly. A complex gene-environment interplay is now known to be involved in the development of ICP[5]. Our experience has revealed that the levels of antioxidant micronutrients are normal in patients with ICP who carry normal genetic polymorphisms (in press as of the writing of this paper).
In 1990, Uden et al[15] performed a double-blind, placebo controlled, crossover trial in 20 patients to determine the efficacy of the combination of 600 mcg of organic selenium, 9000 IU of b carotene, 0.54 g of vitamin C, 270 IU of vitamin E and 2 g of methionine. Results of this trial indicated that antioxidants were superior to placebo in relieving pain. Several non-randomized studies in small patient populations have also shown the benefits of antioxidant treatments in patients with CP. In a study by Bharadwaj et al[16], 147 patients were randomized to an antioxidant therapy or placebo group, and the results indicated a beneficial effect of antioxidant therapy in reducing “painful days” and in improving the markers of oxidative stress. Although the study by Bharadwaj et al[16] was conducted in a large and heterogenous patient population (including patients with ACP as well as those with ICP), validated pain scores were not used and formal analysis of QoL was not performed. Results of the ANTICIPATE study showed that administration of antioxidants to patients with painful ACP does not reduce pain or improve QoL, despite a sustained increase in blood levels of antioxidants[17]. The results of a randomized controlled trial by Singh et al[18] showed that antioxidant supplementation increased the blood antioxidant levels but produced no additional benefit on endocrine and exocrine functions, markers of fibrosis, inflammation, nutritional status, pain or QoL.
Gooshe et al[19] performed a meta-analysis to determine the efficacy and adverse effects of antioxidant therapy in patients with acute pancreatitis (AP), CP and post-endoscopic retrograde cholangiopancreatography pancreatitis (commonly known as PEP). This meta-analysis provided evidence to support the efficacy of antioxidant therapy only in AP, whereas its effects in CP and PEP were less clear. The meta-analysis by Rustagi et al[20] demonstrated a benefit of antioxidants; however, the investigators did not control for a heterogenous study population and the use of different types of antioxidants among such. The most recent Cochrane Systematic Review of 18 studies concluded that antioxidants could result in a slight reduction in pain in patients with CP but there were no conclusive data reported for analgesic requirement and QoL[21]. Rupjoyti et al[22] showed that treatment with a methionine-containing antioxidant was associated with a significant increase in the number of pain-free patients and a trend towards decreased requirement for hospital visits or admissions. Thus, methionine may help to restore the transsulfuration pathway and decrease intrapancreatic inflammation. Rupjoyti et al[22] performed a meta-analysis based upon data from January 1980 to August 2014, encompassing eight studies (six randomized controlled trials and two non-randomized trials). Although the overall results supported the efficacy of methionine supplementation, when the two non-randomized studies (by Shah et al[23] and Castasno et al[24]) were excluded, the antioxidant combination was no longer statistically significant for decreasing the pain score.
A recent meta-analysis by Mohta et al[25] showed negative results for antioxidants’ ability to reduce pain and improve QoL in patients with CP. These findings are important because all studies included in the meta-analysis had been performed using a similar type of antioxidant and were based on a combination of commercially-available antioxidants and those used in clinical practice; therefore, the findings of that meta-analysis are more relevant to clinical practice. However, the meta-analysis itself had some limitations. First, there was variation in the method of reporting of pain among the studies included and, as such, the analysis had to be performed with two different parameters, namely the visual analogue scale (VAS) score and pain-free participants. This resulted in a decrease in the number of patients that could be simultaneously included in the analysis. Second, limited information was available regarding the QoL.
Although the micronutrient antioxidant therapy was proposed for relieving the pain associated with CP more than three decades ago, this treatment has been used sporadically; moreover, the optimal formulation and duration of the antioxidant regimen has not been completely elucidated. Further, a majority of the clinical trials were not well-designed and did not include a homogenous study population. In view of the fluctuating nature of this disease, a well-defined method to determine the pain scores and measure the QoL was not developed and validated. Differences in the assessment and reporting of pain (i.e., VAS score, numeric rating score, brief pain inventory scores, vocabulary score sheet, and “painful days”) in various clinical studies make study comparisons and meta-analysis difficult. These featurescan be attributable to the inconsistent results obtained in the various meta-analysis[20-22,25,26].
The lack of alternative therapies for patients with CP warrants an urgent need for a well-designed study to evaluate the effect of antioxidant therapy in a clearly defined patient population.
Antioxidant therapy has inconsistent efficacy in patients with CP. Therefore, a reliable, effective, safe and predictable agent represents an unmet (but required) need for the treatment of CP. A statin appears to be the most appropriate candidate.
Several primary and secondary prevention trials have shown the clinical benefits of statins in coronary artery disease[27]. An increasing number of studies indicate that statins have many non-lipid-lowering effects, known as pleotropic effects[28,29]. Some of the effects include anti-inflammatory actions and improvement of endothelial function by prevention of lipid peroxidation. Statins exert antioxidant effects by increasing the bioavailability of nitric oxide[30], decreasing the production of ROS[31] and inhibiting the distinct oxidation pathways[32]. Antiproliferative and immuno
The relationship between statins and AP is controversial, considering simvastatin- induced AP was found in a previous study[34]. Statins are among the most widely prescribed medications worldwide for cardiovascular diseases. Thus, it is important to understand the relationship between statins and pancreatitis. A population-based study from Denmark found no association between the risk of AP and use of statins[35]. In contrast, a recent meta-analysis of 21 high-quality randomized controlled trials showed an overall decrease in the risk of pancreatitis among patients treated with statins compared with those treated with placebo[36]. However, since pancreatitis was not the primary outcome of the 21 trials included in that meta-analysis, these results may not be a true indicator of the protective effects. The protective effects of simvastatin and ezetimibe were shown in the Study of Heart and Renal Protection (SHARP) study[37]. The result of a recent study by Wu et al[38] also indicated that simvastatin and atorvastatin were associated with an overall decrease in the risk of AP. Moreover, subgroup analysis in the same study showed a decrease in the risk of pancreatitis in patients with chronic alcohol abuse, suggesting the possible role of simvastatin in preventing recurrent AP and subsequent progression to CP.
The effects of simvastatin pre-treatment on 10 Wistar rats was published by a group from Brazil in 2008[39]. They reported no beneficial effects on pancreatic inflammation but a trend towards improved survival rate in the simvastatin group. Of interest, the lovastatin treatment was found to successfully inhibit invitro activation of pancreatic stellate cells[40]. This is an important observation, as activated stellate cells mediate the fibroinflammatory response of CP. In a similar study of L-arginine-induced pancreatitis in rats, performed by Metalka et al[41], levels of malondialdehyde (commonly known as MDA) were significantly reduced in the pancreas tissues of the simvastatin treatment group.
Another considerable pathologic process that occurs in pancreatitis is impaired autophagy[42-44]. Autophagy is the process of removal of damaged cellular compounds, including dysfunctional mitochondria (mitophagy) during stress conditions[45]. Because dysfunctional mitochondria are a significant source of ROS generation, as indicated above, their dysfunction can be an important source of cellular stress that can promote pancreatitis. During autophagy, organelles such as mitochondria and cytoplasmic materials are sequestered by the autophagosomes (a double–membrane structure) and transported to the lysosome for digestion[46]. Autophagosome-lysosome fusion is impaired in pancreatitis, resulting in incomplete autophagy andmitophagy[47]. Piplani et al[48] showed that simvastatin restores autophagy and mitophagy, resulting in improvement in pancreatitis pathology in the cerulein-induced model of experimental AP.
Autophagosome accumulation during pancreatitis can promote pancreatic cancer[49]. CP is the strongest identified risk factor for pancreatic cancer[50]. Intriguingly, the results of a study involving 250 patients with pancreatic cancer showed an improved survival among those patients with diabetes who were subject to statin treatment[51]. Based on these results, Jeon et al[52] retrospectively analysed a cohort of elderly patients with pancreatic cancer using the Surveillance, Epidemiology and End Results (commonly known as SEER) database of patients in the United States. They studied the use of statins in patients after diagnosis of pancreatic ductal adenocarcinoma. Simvastatin was the most prescribed statin. The patients who used statins were found to live longer after their cancer diagnosis (median overall survival of 4.7 mo; inter-quartile range of 1.9-11.7 mo) as opposed to those who were not prescribed statins (median overall survival of 2.4 mo; interquartile range of 1.5-7.3 mo). There was a favourable impact of statin use on survival in those who had undergone pancreatectomy vs those who had undergone no surgery (HR = 0.80, 95%CI: 0.66, 0.97). Of interest, simvastatin treatment was associated with significantly lower hazard of death compared to no statin treatment (HR = 0.91, 95%CI: 0.84, 0.99). The results of that study were confirmed by another, showing the beneficial effect of statins in pancreatic cancer patients in a large health care system in Southern California, United States[53].
The mechanism of action of statins in pancreatic cancer remains poorly understood. Statins are known to decrease the expression of inflammatory cytokines and to modulate the expression of several genes involved in angiogenesis and inflammation, which may protect against carcinogenesis. Statins also inhibit protein prenylation. This prevents the proper functioning of guanosine triphosphatase proteins, such as Ras and Rho, thereby inhibiting downstream pathways that are involved in cell growth, proliferation, survival, motility and invasion, which leads to cell cycle arrest in G1. Further-more, statins impair cancer cell proliferation by inhibiting the synthesis of cholesterol, which is essential for new membrane formation in rapidly proliferating cells[54].
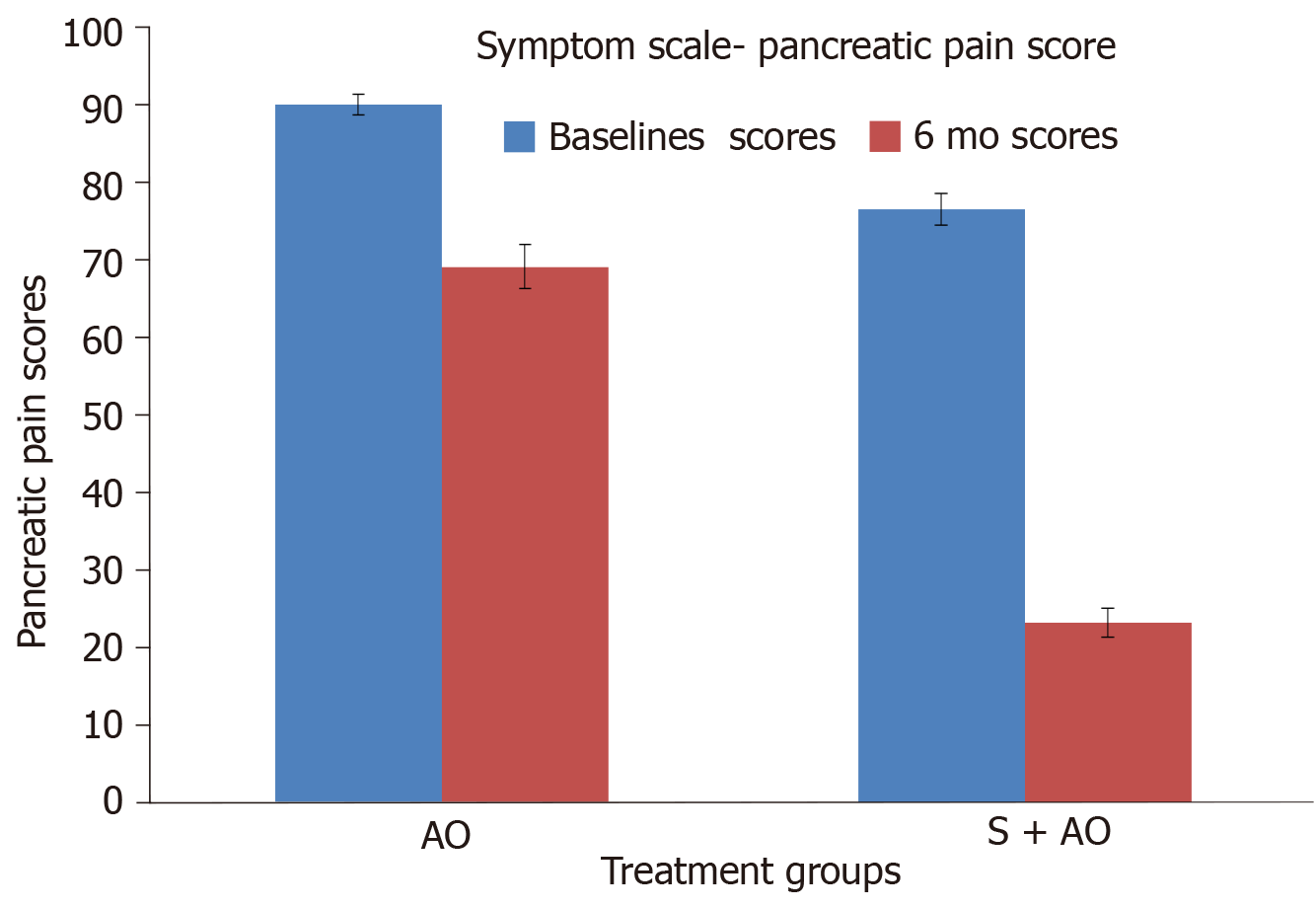
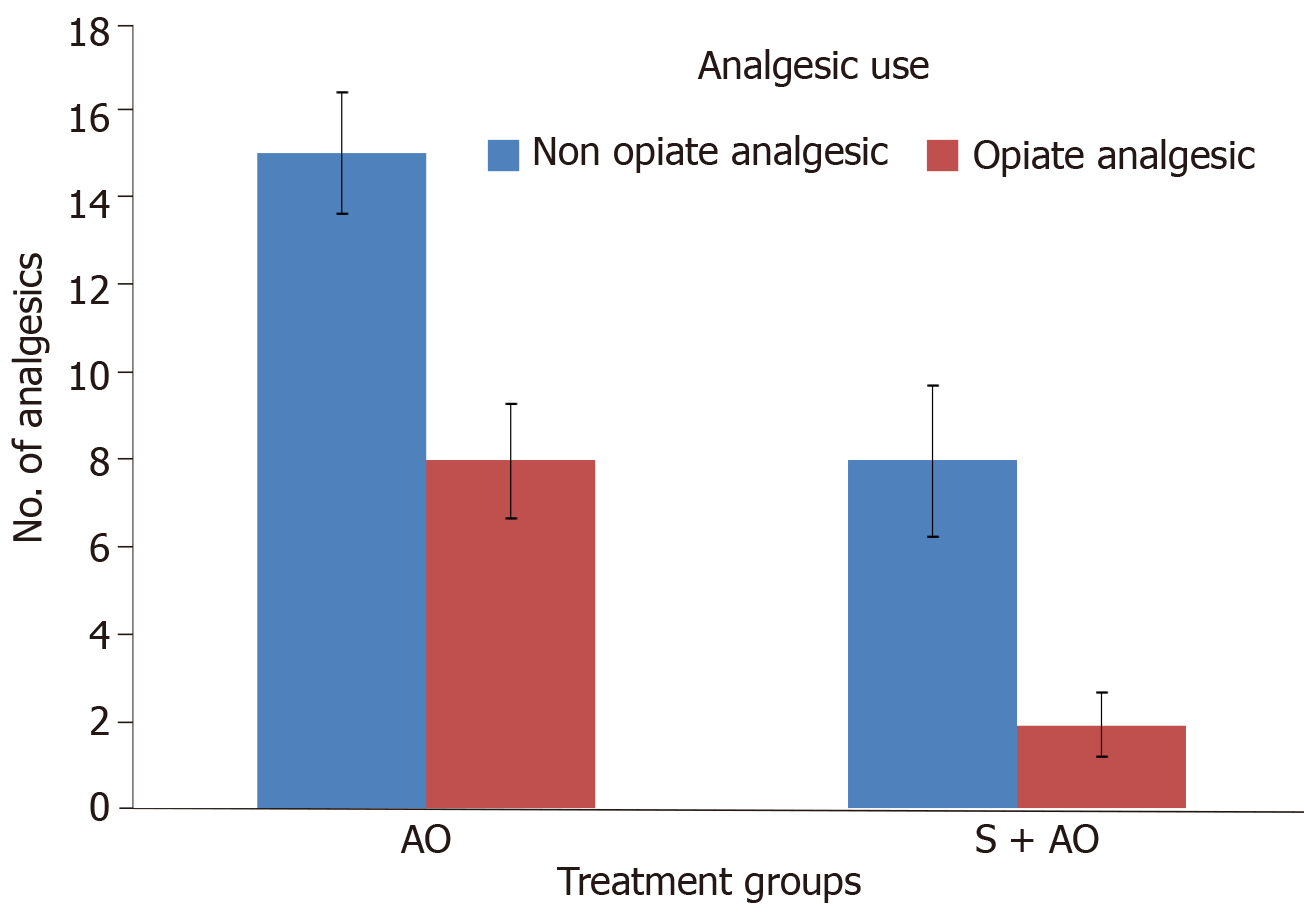
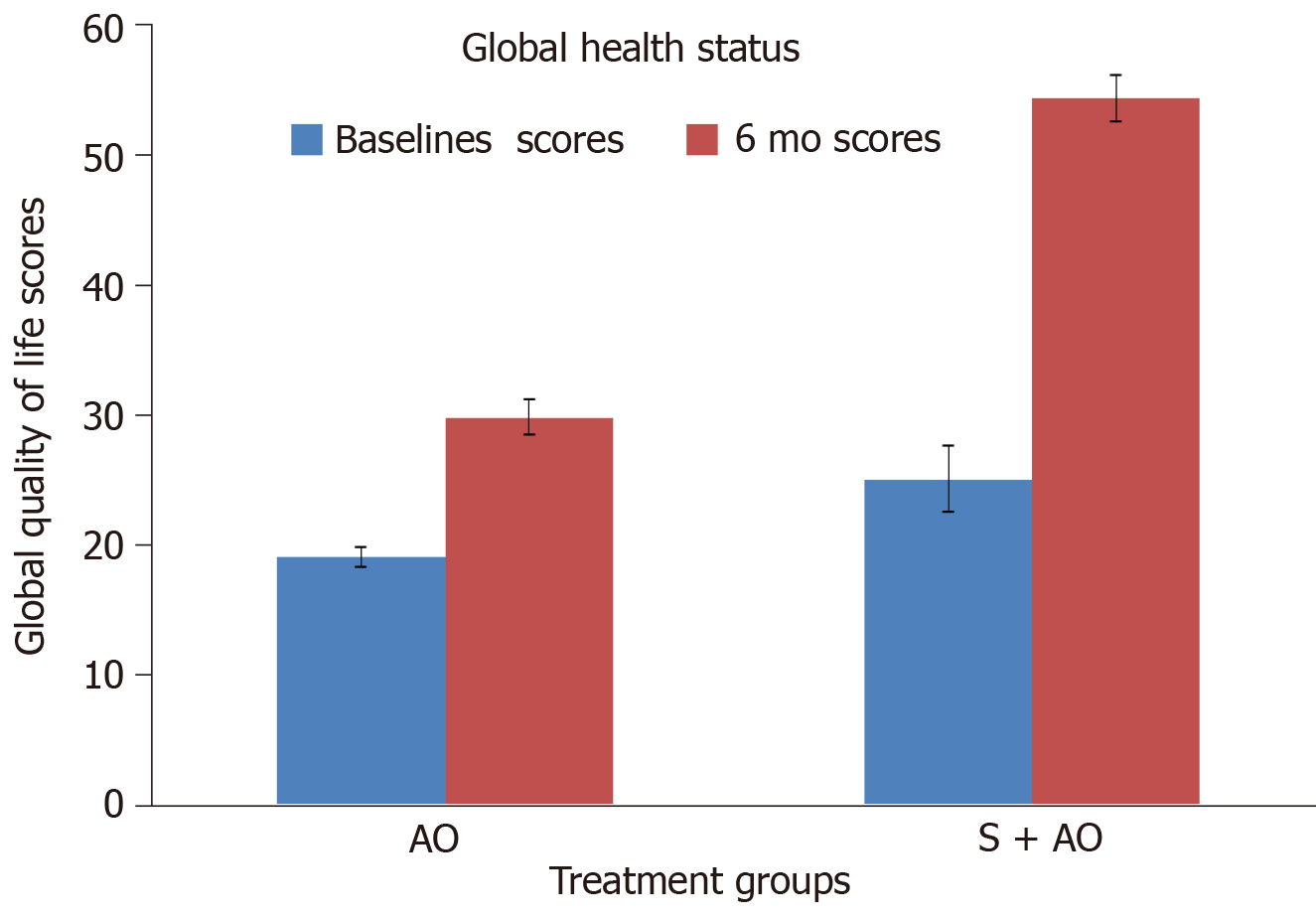
On the basis of the experimental and limited retrospective/population-based data, we performed two prospective studies to assess the role of simvastatin in patients with ICP. In the first prospective study, patients were assigned to receive either the standard antioxidant preparation (a tablet containing 3000 IU β carotene, 550 mg methionine, 200 μg selenium, 40 mg vitamin C, and 10 mg vitamin E administered thrice daily) or the combination of the standard antioxidant preparation and simvastatin (40 mg/d). Improvement in pain was assessed using the VAS. At the end of 12 mo, the decrease in the VAS score was significantly greaterin the simvastatin group (P = 0.032)[55]. In the other pilot study (under publication), health-related QoL was assessed using the European Organization for Research and Treatment of Cancer (referred to as EORTC) QLQ PAN 28 and QLQ C30 scoring. The results of the study showed that at 6 mo patients who received a combination of simvastatin and antioxidants showed a significant improvement in the pancreatic pain score compared to those who received antioxidants alone (P= 0.01) (Figure 1). Patients who received simvastatin and antioxidants also consumed fewer analgesics (P = 0.03) (Figure 2) and required less hospitalization (P = 0.04) than those who received antioxidants alone. Our findings indicate that, compared to antioxidants alone, simvastatin in combination with antioxidants significantly improves the overall QoL (Figure 3) (P = 0.01), which is consistent with the findings reported in several preclinical studies. Thus, simvastatin may emerge to modulate the disease process in CP.
Considering the complexity of CP, we have also identified NAC as another molecule that can be repurposed for CP. NAC exerts antioxidant and antifibrotic effects via inhibition of tissue growth factor signalling in fibrogenic cells[56]. In the environment of inflamed pancreatic tissue, activation of pancreatic stellate cells produces abundant extracellular matrix proteins, leading to fibrosis as well as inflammatory cytokines. Beneficial roles of the combination of simvastatin and NAC on the pathophysiology of inflammation and necrosis of the acinar cell involve inhibition of pancreatic stellate cells. Thus, we designed a prospective, randomized, open-labelled clinical trial entitled “The safety and efficacy of simvastatin plus standard of care or simvastatin plus NAC plus standard of care vs only standard of care in patients with idiopathic acute recurrent pancreatitis and chronic pancreatitis” (i.e., the SNAPstudy). This study aims to assess not only the QoL but also various biomarkers for inflammation in CP.
There is a scarcity of scientific evidence to substantiate the use of antioxidants in the treatment of CP. Simvastatin and NAC appear to be promising candidates for the treatment of CP.
The authors would like to acknowledge their consultant team of Dr. Mayank Kabrawala, Dr. Pankaj Desai, and Dr. Subhash Nandwani for their generous support during patient care and clinical trials. The authors would also like to extend their gratitude to Dr. K. Rajagopalan for his editorial assistance.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu ZL S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Lew D, Afghani E, Pandol S. Chronic Pancreatitis: Current Status and Challenges for Prevention and Treatment. Dig Dis Sci. 2017. 62:1702-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, Mayerle J, Drewes AM, Rebours V, Akisik F, Muñoz JED, Neoptolemos JP. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 309] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 3. | Hao L, Wang LS, Liu Y, Wang T, Guo HL, Pan J, Wang D, Bi YW, Ji JT, Xin L, Du TT, Lin JH, Zhang D, Zeng XP, Zou WB, Chen H, Xie T, Li BR, Liao Z, Cong ZJ, Xu ZL, Li ZS, Hu LH. The different course of alcoholic and idiopathic chronic pancreatitis: A long-term study of 2,037 patients. PLoS One. 2018;13:e0198365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Pham A, Forsmark C. Chronic pancreatitis: review and update of etiology, risk factors, and management. F1000Res. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Garg PK, Narayana D. Changing phenotype and disease behaviour of chronic pancreatitis in India: evidence for gene-environment interactions. Glob Health Epidemiol Genom. 2016;1:e17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Brock C, Nielsen LM, Lelic D, Drewes AM. Pathophysiology of chronic pancreatitis. World J Gastroenterol. 2013;19:7231-7240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Anderson MA, Akshintala V, Albers KM, Amann ST, Belfer I, Brand R, Chari S, Cote G, Davis BM, Frulloni L, Gelrud A, Guda N, Humar A, Liddle RA, Slivka A, Gupta RS, Szigethy E, Talluri J, Wassef W, Wilcox CM, Windsor J, Yadav D, Whitcomb DC. Mechanism, assessment and management of pain in chronic pancreatitis: Recommendations of a multidisciplinary study group. Pancreatology. 2016;16:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Barry K. Chronic Pancreatitis: Diagnosis and Treatment. Am Fam Physician. 2018;97:385-393. [PubMed] |
| 9. | Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:8416763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1959] [Cited by in RCA: 2332] [Article Influence: 291.5] [Reference Citation Analysis (0)] |
| 10. | Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 3803] [Article Influence: 345.7] [Reference Citation Analysis (0)] |
| 11. | Tandon RK, Garg PK. Oxidative stress in chronic pancreatitis: pathophysiological relevance and management. Antioxid Redox Signal. 2011;15:2757-2766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Braganza JM. A framework for the aetiogenesis of chronic pancreatitis. Digestion. 1998;59 Suppl 4:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Braganza JM. Micronutrient (‘antioxidant’) therapy for chronic pancreatitis: Basis and clinical experience. Pancreapedia: Exocrine Pancreas Knowledge Base. 2015;. [DOI] [Full Text] |
| 14. | Garg PK. Chronic pancreatitis in India: untying the nutritional knot. Indian J Gastroenterol. 2011;30:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Uden S, Bilton D, Nathan L, Hunt LP, Main C, Braganza JM. Antioxidant therapy for recurrent pancreatitis: placebo-controlled trial. Aliment Pharmacol Ther. 1990;4:357-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Bhardwaj P, Garg PK, Maulik SK, Saraya A, Tandon RK, Acharya SK. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology. 2009;136:149-159.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Siriwardena AK, Mason JM, Sheen AJ, Makin AJ, Shah NS. Antioxidant therapy does not reduce pain in patients with chronic pancreatitis: the ANTICIPATE study. Gastroenterology. 2012;143:655-663.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Singh N, Ahuja V, Sachdev V, Upadhyay AD, Goswami R, Ramakrishnan L, Dwivedi S, Saraya A. Antioxidants for Pancreatic Functions in Chronic Pancreatitis: A Double-blind Randomized Placebo-controlled Pilot Study. J Clin Gastroenterol. 2020;54:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Gooshe M, Abdolghaffari AH, Nikfar S, Mahdaviani P, Abdollahi M. Antioxidant therapy in acute, chronic and post-endoscopic retrograde cholangiopancreatography pancreatitis: An updated systematic review and meta-analysis. World J Gastroenterol. 2015;21:9189-9208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Rustagi T, Njei B. Antioxidant therapy for pain reduction in patients with chronic pancreatitis: a systematic review and meta-analysis. Pancreas. 2015;44:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Ahmed Ali U, Jens S, Busch OR, Keus F, van Goor H, Gooszen HG, Boermeester MA. Antioxidants for pain in chronic pancreatitis. Cochrane Database Syst Rev. 2014;CD008945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Talukdar R, Murthy HV, Reddy DN. Role of methionine containing antioxidant combination in the management of pain in chronic pancreatitis: a systematic review and meta-analysis. Pancreatology. 2015;15:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Shah NS, Makin AJ, Sheen AJ, Siriwardena AK. Quality of life assessment in patients with chronic pancreatitis receiving antioxidant therapy. World J Gastroenterol. 2010;16:4066-4071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | De las Heras Castaño G, García de la Paz A, Fernández MD, Fernández Forcelledo JL. Use of antioxidants to treat pain in chronic pancreatitis. Rev Esp Enferm Dig. 2000;92:375-385. [PubMed] |
| 25. | Mohta S, Singh N, Gunjan D, Kumar A, Saraya A. Systematic review and meta-analysis: Is there any role for antioxidant therapy for pain in chronic pancreatitis. JGH Open. 2021;5:329-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Zhou D, Wang W, Cheng X, Wei J, Zheng S. Antioxidant therapy for patients with chronic pancreatitis: A systematic review and meta-analysis. Clin Nutr. 2015;34:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5442] [Article Influence: 236.6] [Reference Citation Analysis (0)] |
| 28. | Blake GJ, Ridker PM. Are statins anti-inflammatory? Curr Control Trials Cardiovasc Med. 2000;1:161-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 899] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 30. | Scalia R, Gooszen ME, Jones SP, Hoffmeyer M, Rimmer DM 3rd, Trocha SD, Huang PL, Smith MB, Lefer AM, Lefer DJ. Simvastatin exerts both anti-inflammatory and cardioprotective effects in apolipoprotein E-deficient mice. Circulation. 2001;103:2598-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Wassmann S, Laufs U, Bäumer AT, Müller K, Ahlbory K, Linz W, Itter G, Rösen R, Böhm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 303] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 32. | Shishehbor MH, Brennan ML, Aviles RJ, Fu X, Penn MS, Sprecher DL, Hazen SL. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 33. | Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 975] [Cited by in RCA: 994] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 34. | Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5:648-61; quiz 644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 366] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 35. | Bang UC, Watanabe T, Bendtsen F. The relationship between the use of statins and mortality, severity, and pancreatic cancer in Danish patients with chronic pancreatitis. Eur J Gastroenterol Hepatol. 2018;30:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Preiss D, Tikkanen MJ, Welsh P, Ford I, Lovato LC, Elam MB, LaRosa JC, DeMicco DA, Colhoun HM, Goldenberg I, Murphy MJ, MacDonald TM, Pedersen TR, Keech AC, Ridker PM, Kjekshus J, Sattar N, McMurray JJ. Lipid-modifying therapies and risk of pancreatitis: a meta-analysis. JAMA. 2012;308:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 37. | Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1946] [Cited by in RCA: 1756] [Article Influence: 125.4] [Reference Citation Analysis (0)] |
| 38. | Wu BU, Pandol SJ, Liu IL. Simvastatin is associated with reduced risk of acute pancreatitis: findings from a regional integrated healthcare system. Gut. 2015;64:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Almeida JL, Sampietre SN, Mendonça Coelho AM, Trindade Molan NA, Machado MC, Monteiro da Cunha JE, Jukemura J. Statin pretreatment in experimental acute pancreatitis. JOP. 2008;9:431-439. [PubMed] |
| 40. | Jaster R, Brock P, Sparmann G, Emmrich J, Liebe S. Inhibition of pancreatic stellate cell activation by the hydroxymethylglutaryl coenzyme A reductase inhibitor lovastatin. Biochem Pharmacol. 2003;65:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Matalka II, Mhaidat NM, Fatlawi LA. Antioxidant activity of simvastatin prevents L-arginine-induced acute toxicity of pancreas. Int J Physiol Pathophysiol Pharmacol. 2013;5:102-108. [PubMed] |
| 42. | Barreto SG, Habtezion A, Gukovskaya A, Lugea A, Jeon C, Yadav D, Hegyi P, Venglovecz V, Sutton R, Pandol SJ. Critical thresholds: key to unlocking the door to the prevention and specific treatments for acute pancreatitis. Gut. 2021;70:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 43. | Gukovskaya AS, Gorelick FS, Groblewski GE, Mareninova OA, Lugea A, Antonucci L, Waldron RT, Habtezion A, Karin M, Pandol SJ, Gukovsky I. Recent Insights Into the Pathogenic Mechanism of Pancreatitis: Role of Acinar Cell Organelle Disorders. Pancreas. 2019;48:459-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Gukovsky I, Pandol SJ, Mareninova OA, Shalbueva N, Jia W, Gukovskaya AS. Impaired autophagy and organellar dysfunction in pancreatitis. J Gastroenterol Hepatol. 2012;27 Suppl 2:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 45. | Ryter SW, Cloonan SM, Choi AM. Autophagy: a critical regulator of cellular metabolism and homeostasis. Mol Cells. 2013;36:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 46. | Nakamura S, Yoshimori T. New insights into autophagosome-lysosome fusion. J Cell Sci. 2017;130:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 47. | Diakopoulos KN, Lesina M, Wörmann S, Song L, Aichler M, Schild L, Artati A, Römisch-Margl W, Wartmann T, Fischer R, Kabiri Y, Zischka H, Halangk W, Demir IE, Pilsak C, Walch A, Mantzoros CS, Steiner JM, Erkan M, Schmid RM, Witt H, Adamski J, Algül H. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology. 2015;148:626-638.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 48. | Piplani H, Marek-Iannucci S, Sin J, Hou J, Takahashi T, Sharma A, de Freitas Germano J, Waldron RT, Saadaeijahromi H, Song Y, Gulla A, Wu B, Lugea A, Andres AM, Gaisano HY, Gottlieb RA, Pandol SJ. Simvastatin induces autophagic flux to restore cerulein-impaired phagosome-lysosome fusion in acute pancreatitis. Biochim Biophys Acta Mol Basis Dis. 2019;1865:165530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Grazioli S, Pugin J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front Immunol. 2018;9:832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 50. | Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 433] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 51. | Nakai Y, Isayama H, Sasaki T, Mizuno S, Sasahira N, Kogure H, Kawakubo K, Yamamoto N, Hirano K, Ijichi H, Tateishi K, Tada M, Koike K. Clinical outcomes of chemotherapy for diabetic and nondiabetic patients with pancreatic cancer: better prognosis with statin use in diabetic patients. Pancreas. 2013;42:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Jeon CY, Pandol SJ, Wu B, Cook-Wiens G, Gottlieb RA, Merz CN, Goodman MT. The association of statin use after cancer diagnosis with survival in pancreatic cancer patients: a SEER-medicare analysis. PLoS One. 2015;10:e0121783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Wu BU, Chang J, Jeon CY, Pandol SJ, Huang B, Ngor EW, Difronzo AL, Cooper RM. Impact of statin use on survival in patients undergoing resection for early-stage pancreatic cancer. Am J Gastroenterol. 2015;110:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Gong J, Sachdev E, Robbins LA, Lin E, Hendifar AE, Mita MM. Statins and pancreatic cancer. Oncol Lett. 2017;13:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Mehta R, Kabrawala M, Nandwani S, Desai P, Kalra P, Prajapati R, Joshi P. Early Clinical Experience with Simvastatin for Treating Pain in Patients with Idiopathic Chronic Pancreatitis. J Clin Diagn Res. 2019;13. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Weiskirchen R. Hepatoprotective and Anti-fibrotic Agents: It's Time to Take the Next Step. Front Pharmacol. 2015;6:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |