Published online Nov 14, 2021. doi: 10.3748/wjg.v27.i42.7387
Peer-review started: July 5, 2021
First decision: July 13, 2021
Revised: July 26, 2021
Accepted: October 27, 2021
Article in press: October 27, 2021
Published online: November 14, 2021
Processing time: 128 Days and 5.2 Hours
Image-guided radiotherapy (IGRT) has significantly improved the precision in which radiotherapy is delivered in cancer treatment. Typically, IGRT uses bony landmarks and key anatomical structures to locate the tumor. Recent studies have demonstrated the feasibility of peri-tumor fiducials in enabling even more accurate delineation of target and normal tissue. The use of gold coils as fiducials in gastrointestinal tumors has been extensively studied. However, placement requires expertise and specialized endoscopic ultrasound equipment. This article reports the long-term outcomes of using a standard gastroscopy to inject liquid fiducials for the treatment of oesophageal and gastric tumors with IGRT.
To assess the long-term outcomes of liquid fiducial-guided IGRT in a cohort of oesophageal and gastric cancer patients.
A retrospective cohort study of consecutive adults with Oesophagogastric cancers referred for liquid fiducial placement before definitive/neo-adjuvant or palliative IGRT between 2013 and 2021 at a tertiary hospital in Melbourne, Australia was conducted. Up to four liquid fiducials were inserted per patient, each injection consisting of 0.2-0.5mL of a 1:1 mixture of iodized oil (Lipiodol; Aspen Pharmacare) and n-butyl 2-cyanoacrylate (Histoacryl®; B. Braun). A 23-gauge injector (Cook Medical) was used for the injection. All procedures were performed by or under the supervision of a gastroenterologist. Liquid fiducial-based IGRT (LF-IGRT) consisted of computer-assisted direct matching of the fiducial region on cone-beam computerised tomography at the time of radiotherapy. Patients received standard-IGRT (S-IGRT) if fiducial visibility was insufficient, consisting of bone match as a surrogate for tumor position. Radiotherapy was delivered to 54Gy in 30 fractions for curative patients and up to 45Gy in 15 fractions for palliative treatments.
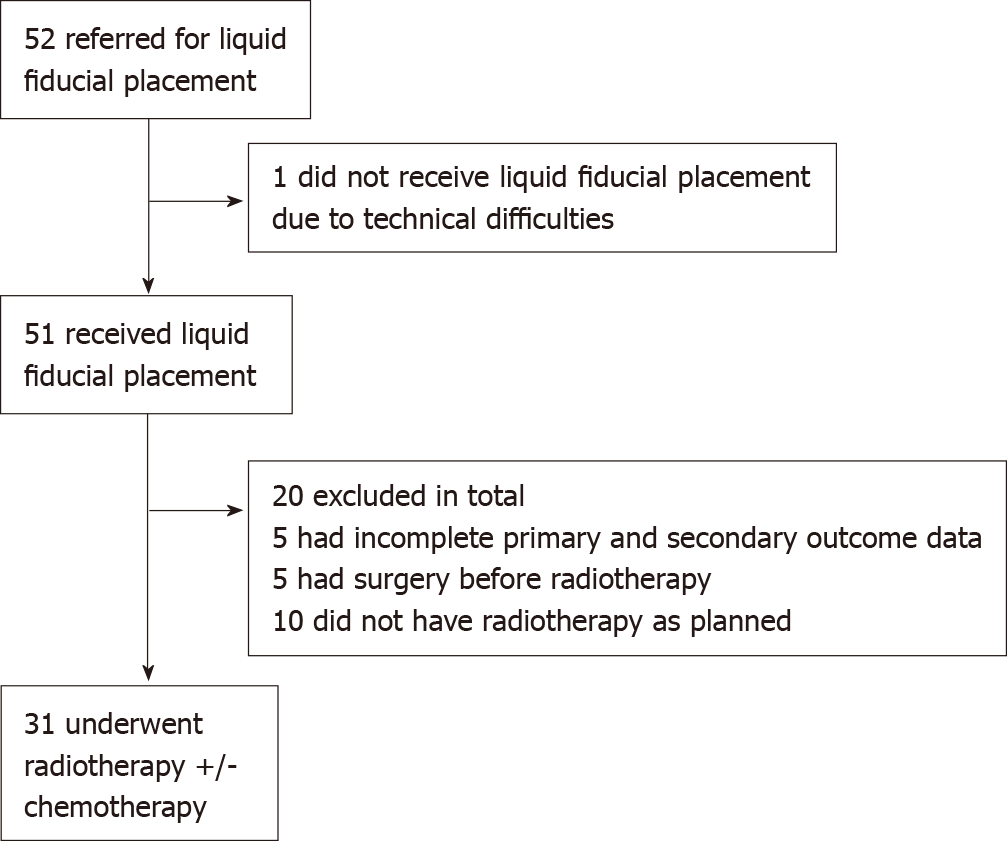
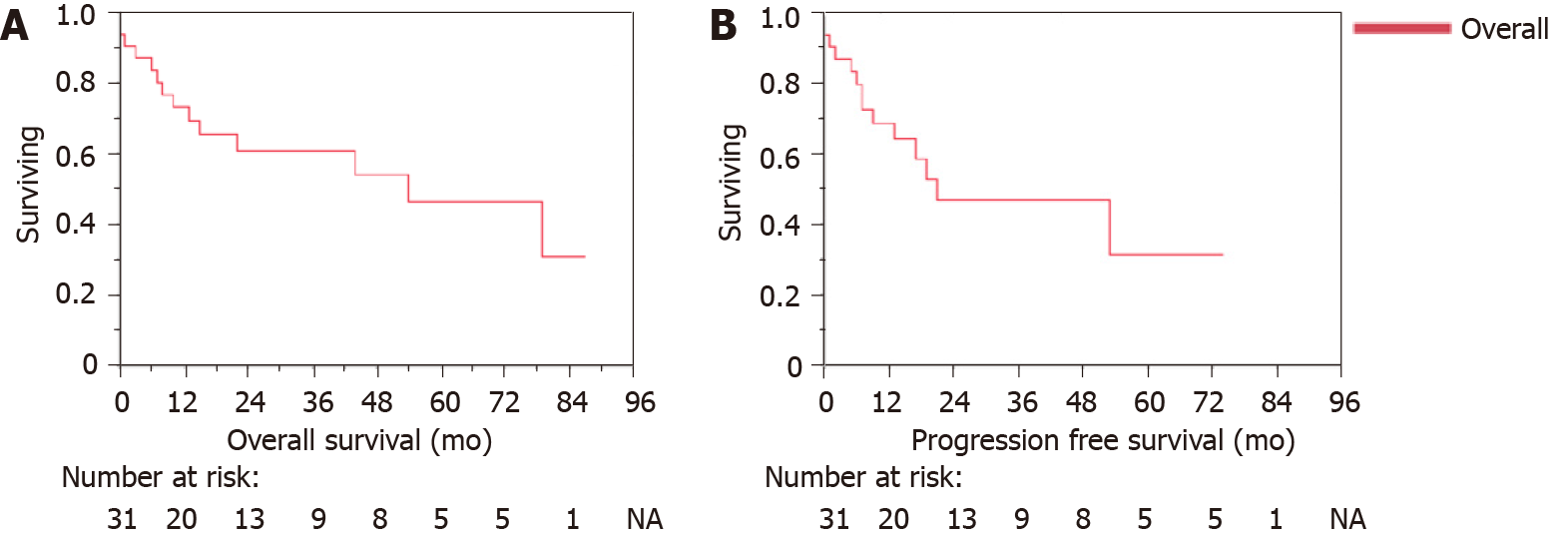
52 patients were referred for liquid fiducial placement within the study period. A total of 51 patients underwent liquid fiducial implantation. Of these a total of 31 patients received radiotherapy. Among these, the median age was 77.4 years with a range between 57.5 and 88.8, and 64.5% were male. Twenty-seven out of the 31 patients were able to have LF-IGRT while four had S-IGRT. There were no complications after endoscopic implantation of liquid fiducials in our cohort. The cohort overall survival (OS) post-radiotherapy was 19 mo (range 0 to 87 mo). Whilst the progression-free survival (PFS) post-radiotherapy was 13 mo (range 0 to 74 mo). For those treated with curative intent, the median OS was 22.0 mo (range 0 to 87 mo) with a PFS median of 14.0 mo (range 0 to 74 mo). Grade 3 complication rate post-radiotherapy was 29%.
LF-IGRT is feasible in 87.1% of patients undergoing liquid fiducial placement through standard gastroscopy injection technique. Our cohort has an overall survival of 19 mo and PFS of 13 mo. Further studies are warranted to determine the long-term outcomes of liquid-fiducial based IGRT.
Core Tip: Based on a cohort of 31 patients who had undergone lipiodol fiducial implantation through standard gastroscopy and received radiotherapy, fiducial-based image-guided radiotherapy (IGRT) was possible in 87.1%. Our cohort had an overall survival of 19 mo and progression-free survival of 13 mo. Further studies are warranted to determine the long-term outcomes of liquid-fiducial based IGRT.
- Citation: Be KH, Khor R, Lim Joon D, Starvaggi B, Chao M, Ng SP, Ng M, Zorron Cheng Tao Pu L, Efthymiou M, Vaughan R, Chandran S. Long-term clinical outcomes of lipiodol marking using standard gastroscopy for image-guided radiotherapy of upper gastrointestinal cancers. World J Gastroenterol 2021; 27(42): 7387-7401
- URL: https://www.wjgnet.com/1007-9327/full/v27/i42/7387.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i42.7387
In 2020, an estimated 1.69 million cases of oesophageal and gastric cancers were diagnosed worldwide, equating to nearly one in every twelve new diagnoses of cancer. Together esophageal and gastric cancers were responsible for approximately 1.31 million cases of cancer-related mortality[1]. In Australia, the projected age-standardised cancer-related death rates of oesophageal and gastric cancers were 7.8 in 2020, ranking in the top 5 cancer-related death[2]. As with all cancers, patients with earlier-stage tumors display a better prognosis. Unfortunately, most gastrooesophageal cancers are diagnosed at later stages, often warranting chemoradiotherapy with or without surgery[3].
Radiotherapy is one of the main treatment modality in the neoadjuvant and definitive treatment of oesophageal cancers[4-6]. Currently, its use in gastric cancer is largely limited to the post-operatively setting, however more evidence in the neoadjuvant setting is emerging with the near completion of a randomized control trial[7,8]. As such, radiotherapy either as the primary therapy or as an adjunct continues to have an important role in the treatment of patients with oesophageal and gastric cancer[9,10].
A known limitation to the delivery of radiotherapy to patients with upper gastrointestinal malignancies is that early tumors can be difficult to identify on computerized tomography (CT) scans, impairing the delineation of the tumor for radiotherapy treatment design. F-18 Fluorodeoxyglucose positron emission tomography (FDG-PET) can assist in defining the metabolically active tumor volume, but some studies indicate that up to 20% of oesophageal carcinomas are not FDG-PET avid[11]. Furthermore, the mobility of the oesophagus and stomach particularly with respiratory motion and its different physiological states such as fasting and fed states need to be considered during radiotherapy[12,13]. Fiducial placements of markers potentially allow a more accurate definition of the radiotherapy field. The use of fiducial markers to help guide radiotherapy has been demonstrated as feasible in many solid cancer treatments including the prostate, pancreas and hollow viscus of the gastrointestinal tract[12,14,15]. Fiducial-based image-guided radiotherapy (F-IGRT) in the treatment of prostate cancer has been demonstrated to have advantages of optimising radiation dosage to the tumor, whilst minimising radiation exposure to normal tissue[16,17]. Comparatively, the data for the gastrointestinal tract is less robust. A recent meta-analysis, although showing high efficacy and safety of endoscopic ultrasound (EUS)-guided fiducials for pancreatic cancer radiotherapy, highlights that more information is needed regarding the impact of fiducials on long-term clinical outcomes[18]. The insertion of fiducials within the gastrointestinal tract using EUS guidance, although attaining high technical success rates, has substantial limitations with its broad adoption in clinical practice. These include high costs, the necessity of a highly trained endoscopist (who can perform EUS) and a cumbersome solid fiducial loading method[19].
A new type of fiducial was introduced by our group to address these limitations of solid fiducials implantation. Iodized oil-based liquid fiducials are less expensive and can be inserted by an endoscopist who perform gastroscopies. This was described for the first time in our pilot study in 2016[20], in which we demonstrated that similar results could be achieved compared to solid fiducials. Although the data from this study has enabled conclusions on the safety and technical efficacy of the use of liquid fiducials, long-term efficacy data focused on the patients that received LF-IGRT was not available. Hence, this study presents data on long-term follow-up of patients with gastroesophageal cancers who received F-IGRT based on liquid fiducials placed with standard gastroscopy injection technique.
This is a retrospective cohort study of consecutive adults with oesophageal and gastric cancers referred to the endoscopy unit at Austin Health, Melbourne, Australia for liquid fiducial placement before IGRT between January 2013 and January 2021. A database of all patients referred to the endoscopy unit for liquid fiducial placement before IGRT was prospectively maintained. The study was approved after institutional board review (Austin Research Ethics Committee: H2013/04975). Informed consent was waived; patient confidentiality was maintained and protected.
Inclusion criteria were patients with: (1) A management plan discussed in a multi-disciplinary team meeting for radiotherapy for oesophageal (squamous carcinoma or adenocarcinoma) or gastric cancer; and (2) Referred to the endoscopy team for placement of fiducial markers.
Exclusion criteria were patients that did not have liquid fiducials inserted (e.g., deemed as unfit for endoscopic procedure); patients that did not have radiotherapy after placement of fiducials (e.g., declining clinical status) and patients that had surgery before radiotherapy after fiducial placement. In addition, patients who had incomplete treatment and outcome data (e.g., due to loss of follow up) were excluded from our analysis.
Patient clinical data including diagnosis, functional performance status as defined by Eastern Cooperative Oncology Group (ECOG)[21], the American Joint Committee on Cancer (AJCC) 8th edition staging[22], and treatment outcomes were retrospectively collected from patient medical records, endoscopy, radiology, surgical and histopathology reports. The national health database (©Australian Digital Health Agency) was used to assess whether the patient was still alive.
Information for patients treated at other centers was requested from their treating radiation oncologists. When information on the measured primary and secondary outcomes was available, these patients were included in the analysis.
Progression-free survival (PFS) was assessed based on patient disposition at their latest oncological follow-up appointment.
The endoscopic procedure aims to insert a total of four fiducials per patient (2 proximal and 2 distal edges of the tumor), each injection consisting of 0.2-0.5mL of a 1:1 mixture of iodized oil (Lipiodol; Aspen Pharmacare) and n-butyl 2-cyanoacrylate (Histoacryl®; B. Braun). If a tumor was obstructing the passage of the gastroscope, only fiducials on the proximal edge were placed. In addition, if a tumor was extending to the level of the cricopharyngeal, it was not technically possible to insert fiducials at the proximal edge and only distal edges were placed. A 23-gauge injector (Cook Medical®) was used for these injections. All procedures were performed by or under the supervision of a gastroenterologists. All procedures were done under sedation, which was performed by an accredited anaesthetist. The endoscope used for all procedures was a standard gastroscope [(GIF-H1180 and H190; Olympus©), Melbourne, Victoria, Australia], and did not require the aid of fluoroscopy. Patients were routinely observed after the procedure for 1 h after which they were discharged if there were no significant adverse events.
Three 2-mL syringes with a Luer LockTM that can securely be locked onto the end of the injector needle are required; two are filled with iodized oil and only one will contain the iodised oil/n-butyl 2-cyanoacrylate mixture (1 mL: 1 mL).
Step 1: The 23-gauge injector is primed with the iodised oil only outside the patient.
Step 2: When the endoscopist is ready to inject, the injector is passed down the accessory channel of the gastroscope and further primed with the iodized oil/n-butyl 2-cyanoacrylate mixture, ideally within the stomach.
Step 3: A total of four-point injections (0.2-0.5 mL each) are made into the edges of the tumor. Two injections are placed proximally and another two placed distally into the edges of the tumor, when possible.
Step 4: Once marking is completed, the injector should be flushed with the syringe containing iodised oil only to prevent accidental gluing of the accessory channel, again ideally within the stomach.
Step 5: The gastroscope is then withdrawn with the needle retracted but the injector tip itself is slightly out of the distal tip of the gastroscope.
Step 6: Once the gastroscope is removed from the patient, the injector is cut at the port end so that it can be pulled through from the distal tip.
Step 7: The gastroscope accessory channel is subsequently flushed with water. Images and a video of these steps can be found in a previous publication[20].
Following insertion of liquid fiducial markers, the patient underwent CT simulation a minimum of 24 h after insertion. The gross target volume (GTV) was defined using the fiducial markers, endoscopic report and correlative imaging (e.g., diagnostic FDG-PET/CT and CT).
For patients who received definitive treatment, a high dose clinical target volume (CTV) included the GTV plus a 1 cm margin, clipped at anatomic boundaries. A low dose CTV included the GTV plus a 3 cm margin in the cranio-caudal (C-C) axis and 1 cm in other planes (clipped to anatomic boundaries), plus regional lymph nodes at the discretion of the radiation oncologist. A 1 cm planning target volume (PTV) margin was used. The high dose PTV was treated to 54Gy in 30 fractions, and the low dose PTV was assigned 46Gy in 30 fractions. Treatments were planned using the Monaco treatment planning system (Elekta, Stockholm).
For patients who had palliative treatments, the CTV was defined using a 1 cm margin, clipped at anatomical boundaries, with a 1 cm PTV margin. A range of prescription doses were used depending on patient disposition, ranging from 30Gy in 10 fractions to 45Gy in 15 fractions.
Liquid fiducial-based IGRT (LF-IGRT) was performed when the implanted liquid fiducials could be adequately visualized at the time of radiotherapy treatment on cone-beam CT (CBCT). LF-IGRT was performed each fraction using the Elekta XVI software (Elekta Synergy, XVI version 5, Elekta AB, Stockholm, Sweden) online image verification software and an Elekta linear accelerator. A grey value match was performed on an area including the fiducial markers. If liquid fiducials could not be located on the CBCT scan, then patients were treated with S-IGRT. S-IGRT utilized a grey value match on the vertebrae only.
Postprocedural follow-up was assessed through outpatient radiation oncology appointments. Data on patients seen at our center were retrieved from electronic medical records. Patients who had the fiducials implanted at our center but had their follow-up elsewhere had their treating radiation oncologist contacted for information. Data on PFS, late complications from fiducial placement and radiotherapy complications (as per the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.[23]) were assessed.
Primary Outcome: The primary outcome was the overall survival (OS) and PFS of patients who received IGRT after liquid fiducial placement for the treatment of a gastroesophageal tumor. The OS and PFS were referenced to the time of radiotherapy completion. Progression was defined as radiographic or histologic progression (e.g., from recurrence detected on gastroscopy), coded as either local or distant in location.
Secondary outcomes: Secondary outcomes included the technical success of liquid fiducial guiding radiotherapy, adverse events rate and subgroup analyses based on radiotherapy treatment (LF-IGRT and S-IGRT), treatment intent (curative and palliative), tumor type (oesophageal and gastric) and oesophageal histology.
Key definitions: Technical success was defined as the successful delivery of LF-IGRT after the placement of liquid fiducial(s) through standard gastroscopy technique.
Gastroesophageal junction cancers classified as Siewert types I and II were analyzed as oesophageal cancers in accordance with the 8th edition of the AJCC staging guidelines[22]. Gastroesophageal junction cancers classified as Siewert type III were analyzed as gastric cancers.
Functional performance status was defined by the ECOG performance status[21].
Data are summarized as median and ranges for continuous data, and as frequency and percentages for categorical data. For continuous data, comparisons were done using the Mann-Whitney U test or Independent Samples Kruskal-Wallis test based on the normality assumption. For categorical data, Fisher’s Exact test and Likelihood Ratio Chi-Squared test were used as per high prevalence of expected cells with a count less than 5. P value of < 0.05 was considered significant. Survival rates were estimated using the Kaplan-Meier method. Statistical analyzes were performed with SPSS statistical software (IBM Corp. 2020. IBM SPSS Statistics, Version 26.0. Armonk, NY) and JMP v16.0 (SAS Institute Inc).
A total of 52 patients were referred to the endoscopy unit for liquid fiducial placement for IGRT over eight years between January 2013 and January 2021.
1 patient did not have liquid fiducial placement after endoscopic assessment as the tumor was obstructive and extended up to the level of the oropharynx and deemed unsafe to proceed. The majority of patients (98.0%) were able to have at least one edge marked with liquid fiducials, with a large proportion (77.4%) having both at distal and proximal edges marked.
51 patients had liquid fiducials inserted during the study period. Of these, 20 were excluded from our analysis. Five underwent radiotherapy at other centers and their clinical data were not available for analysis. Five had surgery prior to radiotherapy and ten did not have radiotherapy as anticipated. For instance, some patients were treated on the TOPGEAR trial and were randomized to no adjuvant therapy[8]. Therefore, data on the use of endoscopically-placed liquid fiducials during radiotherapy was available for 31 patients (Figure 1).
Our cohort of 31 patients had a median age of 77 years (range 57.5 to 88.8). The majority (71.0%) had oesophageal cancers, with a significant subset (72.7%) of these with squamous cell carcinoma (SCC). Only three patients in our cohort had gastroesophageal junction cancer of which two were classified as Siewert type III. Most patients (67.7%) had locally advanced disease without lymph node involvement or metastatic disease. Most of the cohort had an ECOG score of 0 or 1.
Three (9.6%) of patients received neoadjuvant chemoradiotherapy prior to oesophagectomy for oesophageal SCC. A large proportion (38.7%) of our cohort received definitive chemoradiotherapy, whilst a further 25.8% received definitive radiotherapy only. Detailed demographics and treatments are summarized in Table 1.
| Variables | Median/n | Range/% | |
| Age | 77.4 | 57.5-88.0 | |
| Male | 20 | 64.5 | |
| Site of cancer | Oesophageal | 21 | 67.7 |
| GOJ (Siewert I/II) | 1 | 3.2 | |
| GOJ (Siewert III) | 2 | 6.5 | |
| Gastric | 7 | 22.6 | |
| Type of cancer | Oesophageal SCC | 16 | 51.6 |
| Oesophageal adenocarcinoma | 6 | 19.4 | |
| Gastric adenocarcinoma | 9 | 29.0 | |
| Endoscopy data | Patients with both proximal and distal fiducial placed | 24 | 77.4 |
| Patients with solely proximal or distal fiducials placed | 7 | 22.6 | |
| ECOG | 0 | 13 | 41.9 |
| 1 | 11 | 35.5 | |
| 2 | 7 | 22.6 | |
| Stages1 | LN negative without distant metastasis | 21 | 67.7 |
| LN positive without distant metastasis | 6 | 19.4 | |
| Distant metastasis present | 3 | 9.6 | |
| Radiotherapy | Fiducial seen on CBCT | 29 | 93.5 |
| Fiducial-based IGRT | 27 | 87.1 | |
| Treatment intent | Neoadjuvant chemotherapy with oesophagectomy | 3 | 9.7 |
| Definitive chemoradiotherapy | 12 | 38.7 | |
| Palliative chemoradiotherapy | 3 | 9.7 | |
| Definitive radiotherapy | 8 | 25.8 | |
| Palliative radiotherapy | 5 | 16.1 |
Twenty-seven out of the 31 patients (87.1%) received LF-IGRT. Patients commenced radiotherapy after a median period of 18 d (range 9 to 44) of fiducial placement. The cohort median duration of radiotherapy was 30 d (range 14 to 47). Details on F-IGRT and S-IGRT subgroups are shown in Table 2.
| Variables | F-IGRT | S-IGRT | |
| Median/n, range/% | Median/n, range/% | ||
| Age | 77.4, 57.5-88.0 | 77.3, 64.8-85.4 | |
| Male | 19, 70.4 | 1, 25.0 | |
| Site of cancer | Oesophageal | 18, 62.1 | 3, 75.0 |
| GOJ (Siewert I/II) | 0, 0.0 | 1, 25.0 | |
| GOJ (Siewert III) | 2, 7.4 | 0, 0.0 | |
| Gastric | 7, 25.9 | 0, 0.0 | |
| Type of cancer | Oesophageal SCC | 13, 48.1 | 3, 75.0 |
| Oesophageal adenocarcinoma | 5, 18.5 | 1, 25.0 | |
| Gastric adenocarcinoma | 9, 33.3 | 0, 0.0 | |
| Endoscopy data | Patients with both proximal and distal fiducial placed | 22, 81.5 | 2, 50.0 |
| Patients with solely proximal or distal fiducials placed | 5, 18.5 | 2, 50.0 | |
| ECOG | 0 | 11, 40.7 | 2, 50.0 |
| 1 | 9, 33.3 | 2, 50.0 | |
| 2 | 7, 25.9 | 0, 0.0 | |
| Stages1 | LN negative without distant metastasis | 18, 66.7 | 4, 100.0 |
| LN positive without distant metastasis | 6, 22.2 | 0, 0.0 | |
| Distant metastasis present | 3, 11.1 | 0, 0.0 | |
| Treatment details | Time to treatment (d) | 19.0, 9.0-44 | 14 .0, 9.0-17.0 |
| Curative intent | 20, 74.1 | 4, 100.0 | |
| Palliative intent | 7, 25.9 | 0, 0.0 | |
| Dose (Grays) | 50, 25.2-55 | 47.7, 41.4-54 | |
| Fraction | 25, 10-30 | 26.5, 23-30 | |
| Duration (d) | 30, 14-47 | 37, 30-39 | |
| Chemotherapy | 14, 51.9 | 4, 100.0 | |
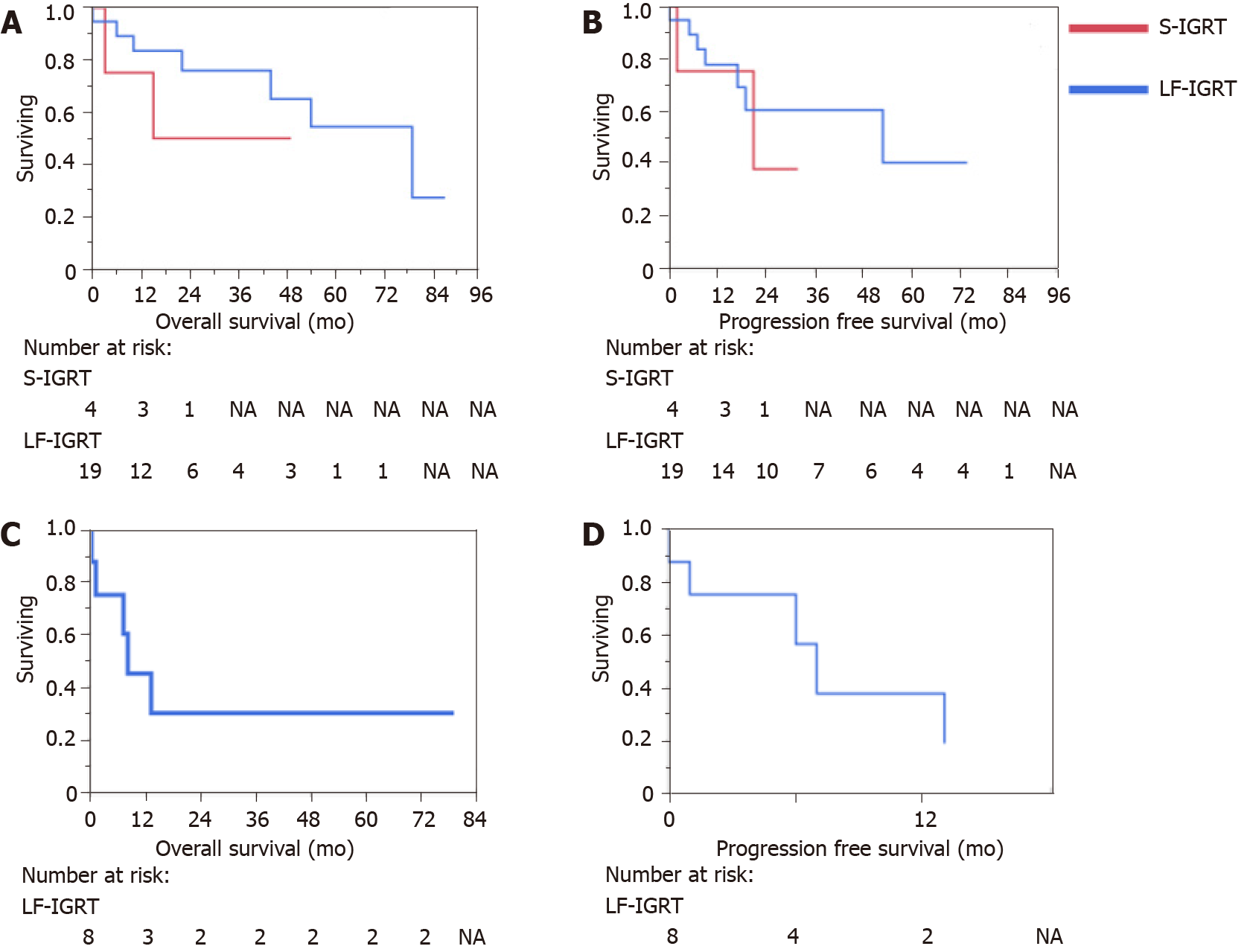
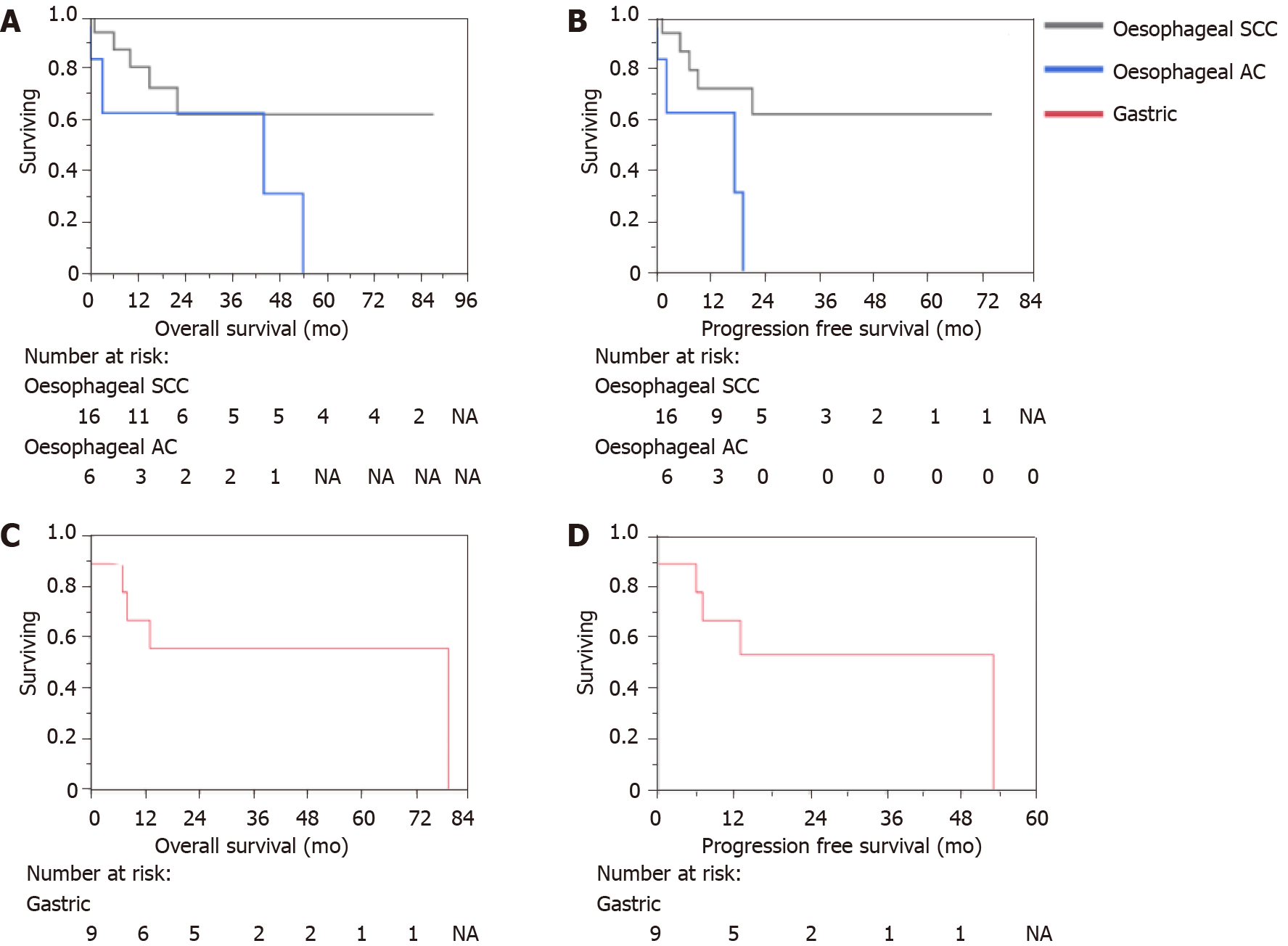
On the close-out date of 24/06/2021, 54.8% of patients were alive. The cohort OS post-radiotherapy was 19 mo (range 0 to 87 mo). Whilst the PFS post-radiotherapy was 13 mo (range 0 to 74 mo). For those treated with curative intent, the median OS was 22.0 mo (range 0 to 87 mo) with a PFS median of 14.0 mo (range 0 to 74). Nineteen patients were alive at 5 years. The 5-year survival rate for oesophageal cancer was 42.5% and for gastric cancer was 55.6% for this cohort. Kaplan-Meier curves for the overall cohort OS and PFS, treatment intent and type of cancer and histology are described in Figures 2, 3 and 4 respectively. Details on OS, PFS and 5-year survival for each subgroup analysis for the type of radiotherapy treatment (LF-IGRT and S-IGRT), treatment intent (curative and palliative), tumor type (oesophageal and gastric) and oesophageal histology are described in Table 3.
| Subgroups | OS (mo) | PFS (mo) | 5-yr survival (%) | |
| Median/n, range/% | Median/n, range/% | |||
| Treatment | F-IGRT (n = 27) | 21.0, 0-87 | 12.0, 0-74 | 47.2 |
| S-IGRT (n = 4) | 17.0, 3-49 | 18.0, 2-32 | 0.0 | |
| Intent | Curative (n = 23) | 22.0, 0-87 | 14.0, 0-74 | 51.5 |
| Palliative (n = 8) | 8.0, 0-79 | 4, 0-13 | 30.0 | |
| Location | Oesophageal (n = 22) | 18, 0-87 | 13, 0-74 | 42.8 |
| SCC (n = 16) | 20, 1-87 | 14, 1-74 | 62.0 | |
| AC (n = 6) | 10, 0-54 | 7, 0-19 | 0.0 | |
| Gastric (n=9) | 25, 0-79 | 13, 0-53 | 55.6 |
Of note, 12 patients had disease progression during the study period. Seven of these patients had local progression whilst five had distal disease progression.
No early or late adverse events occurred following the insertion of the fiducials as assessed prior to discharge on the day of the procedure and on subsequent radiation oncology follow-ups, respectively. Nine patients experienced grade three adverse events which were odynographia, dysphagia, nausea, dehydration, febrile neutropenia and lung infection during their treatment. No patient experienced grade four or five adverse events. Adverse events from their treatment are summarized in Table 4.
| LF-IGRT only | LF-IGRT with chemotherapy | S-IGRT with chemotherapy | Overall | |
| Hospitalization | 3 | 4 | 2 | 9 |
| Fatigue (G1/G2/G3) | 1/2/0 | 3/1/0 | 2/0/0 | 6/3/0 |
| Odynophagia G1/G2/G3 | 3/1/1 | 2/4/1 | 0/4/0 | 5/9/2 |
| Dysphagia (G1/G2/G3) | 2/0/0 | 0/1/0 | 0/1/1 | 2/2/1 |
| Oesophageal stricture (G1/G2/G3) | 1/0/0 | 0/0/1 | 0/0/1 | 1/0/2 |
| Nausea (G1/G2/G3) | 0/2/1 | - | - | 0/2/1 |
| Diarrhoea (G1/G2/G3) | - | 1/0/0 | - | 1/0/0 |
| Lung infection (G2, G3) | - | 0/1 | - | 0/1 |
| Dermatitis (G1/G2/G3) | - | 1/0/0 | - | 1/0/0 |
| Febrile neutropenia (G3) | - | 1 | - | 1 |
| Dehydration (G1/G2/G3) | 0/0/1 | - | - | 0/0/1 |
In this study, we describe the OS and PFS of patients with gastroesophageal tumors that underwent LF-IGRT with liquid fiducials inserted through standard gastroscopy injection technique. This report is a follow-up to our initial study which first described this technique[20]. We believe this to be the largest observational cohort study of its kind, adding to the limited body of knowledge on the long-term outcomes of F-IGRT for gastrooesophageal tumors using liquid fiducials.
Our cohort consisted mainly of patients with locally advanced oesophageal cancer with SCC. The majority (90.3%) of our cohort received chemoradiotherapy or radiotherapy alone as palliative and definitive treatment. The median OS was 19.0 mo, with the longest OS of 87 mo. Our results, albeit a small cohort, compare favorably to what is available in the literature, the 5-year survival rates for oesophageal and gastric cancers were 42.8% and 55.6%, respectively[24]. In the context of gastric cancer in an inoperable population undergoing chemoradiotherapy, the reported median survival was 25.0 mo[25]. We recognize that this is possibly due to judicious patient selection, or the relatively small numbers of patients included compared with larger randomized trials.
Also, we acknowledge that in Australia access to EUS for endoscopic staging is variable, and at our center is not routinely performed. Thus, limiting our accurate reporting of tumor staging as per AJCC 8th Edition. This further adds to our argument that the use of EUS-guided solid fiducials for marking tumors has limitations of which most can be mitigated with the use of liquid fiducials[19,20]. Additionally, the technical aspects of the injection technique required for placement of liquid fiducials is, in essence, an adaptation of a skillset that most gastroenterologists would already have in the management of gastrooesophageal variceal bleeding[26]. As such, this technique of liquid fiducials can be more easily adopted. Furthermore, our group has previous described the potential cost-saving of liquid fiducials amounting to approximately AU$1150 to AU$1750 per procedure when compared to EUS-guided guided solid fiducials insertion[20]. Since the description of lipiodol as a fiducial for gastroesophageal tumors, similar techniques have been described with similar technical success and safety profiles[27].
The use of F-IGRT has many potential benefits over S-IGRT, including facilitating a higher dose focused on the tumor with a lower dose delivered to cover the submucosal spread, and more accurate treatment delivery (matching to the tumor rather than surrounding bony structures)[17,28]. There are conflicting data regarding the efficacy of increased radiation dose in treating oesophageal cancer[29,30]. While some retrospective studies demonstrated a dose-response, recent randomized control trials failed to find a difference in outcomes[31-33]. The effect of dose escalation in optimizing cure rate may be more evident in those with early-stage SCC where the tumor is radiosensitive and the rate of distant metastasis is low[34]. Higher doses can be associated with increased normal tissue toxicity and hence focusing radiotherapy as much as possible to the tumor area is essential. F-IGRT allows a higher dose to be assigned to the tumor while simultaneously allowing for more confident identification of submucosal spread, and therefore facilitating lower doses to the adjacent esophagus.
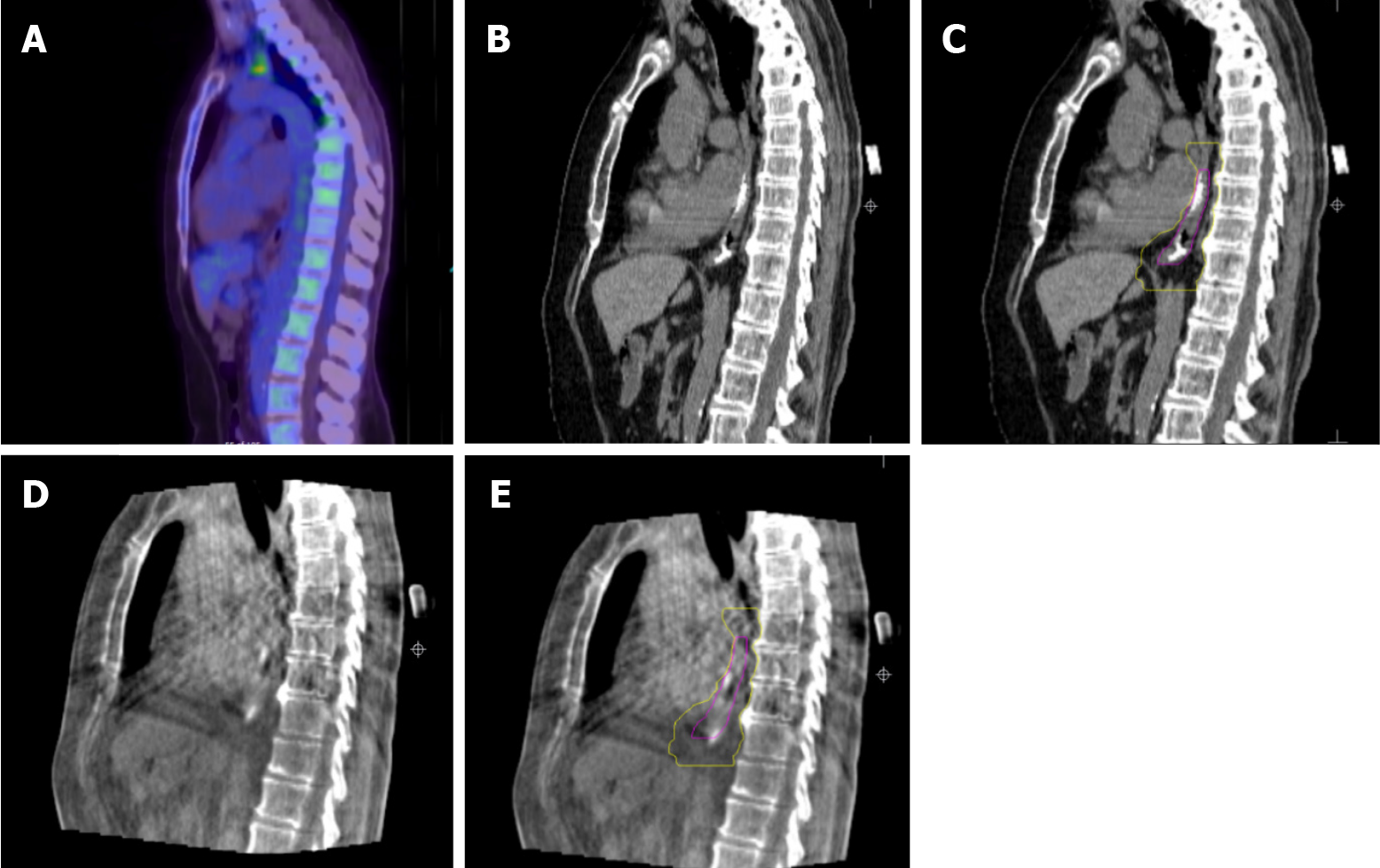
The improvement in the delivery of radiotherapy for patients having endoscopically inserted liquid fiducials is illustrated in Figure 5.
In our cohort, 87.1% successfully underwent LF-IGRT after liquid fiducial placement. In two of our patients, the liquid fiducials were not visible at the time of radiation treatment planning. We hypothesis that this could be due to extravasation or diffusion of the submucosal bleb after the procedure. For two patients, the liquid fiducials were visible but were not sufficient for F-IGRT due to the fiducials not being reliably seen. This highlights a difference compared to metallic fiducials, in that liquid fiducials can have variable shapes and distribution. In addition, we consider our definition of technical success to be more clinically relevant relative to previous definitions reported in the literature. This would account for our slightly lower success rates. However, applying the same technical definition, our rates of successful placement of liquid fiducials would be 98.1%, compared to 96.3%[18] and 98.0%[35].
Toxicity rates reported in the literature include grade 3 seen in 42% and grade 4 in 7%; mainly hematology, gastrointestinal and mouth ulceration[24]. Our lower complication rate from F-IGRT may be related to advances in treatment or the use of fiducials per se. Prospective randomized studies are needed to ascertain the utility of LF-IGRT in reducing complications.
Despite these potential benefits, prospective randomized trials are required as observational studies have failed to show differences in long-term outcomes such as OS for oesophageal cancers using IGRT[36]. Nevertheless, F-IGRT has been deemed by specialists as a promising IGRT modality for the future[37].
The limitations of our study are mainly the small numbers, the heterogeneity of the cohort, the retrospective design and the lack of a direct comparison with S-IGRT. Regarding the certainty of the delivered dose, one other study demonstrated that soft tissue (diaphragm) or bone matching on CBCT resulted in a larger margin to cover the tumor 95% of the time[38]. Direct soft tissue matching of locally advanced tumors would be possible if visible on CBCT but would be not feasible if the tumor is too small to be seen. Secondarily, although we present data on few patients, this is to date the largest cohort of F-IGRT for gastroesophageal tumors utilizing the liquid fiducial technique. The retrospective design and lack of a robust comparison between F- and S-IGRT could not be addressed in the present study but is a promising subject for future research. Furthermore, due to the small number of patients, a multivariate analysis was not performed to address potential bias in this study.
F-IGRT was considered feasible in 87.1% of patients undergoing liquid fiducial placement through standard gastroscopy injection technique. Our cohort had an OS of 19 mo and PFS of 13 mo. Further studies are warranted to determine the long-term outcomes of liquid-fiducial based IGRT.
Further studies are warranted to determine the long-term outcomes of liquid fiducial-based image-guided radiotherapy (IGRT) in the treatment of oesophagogastric cancers.
Based on a cohort of 31 patients who had undergone lipiodol fiducial implantation through standard gastroscopy and received radiotherapy, fiducial-based IGRT was possible in 87.1%. Our cohort had an overall survival (OS) of 19 mo and progression-free survival (PFS) of 13 mo.
52 patients were referred for liquid fiducial placement within the study period. A total of 51 patients underwent liquid fiducial implantation. Of these a total of 31 patients received radiotherapy. Twenty-seven out of the 31 patients were able to have liquid fiducial-based IGRT (LF-IGRT) while four had standard-IGRT (S-IGRT). There were no complications after endoscopic implantation of liquid fiducials in our cohort. The cohort OS post-radiotherapy was 19 mo (range 0 to 87 mo). Whilst the PFS post-adiotherapy was 13 mo (range 0 to 74 mo).
A retrospective cohort study of consecutive adults with oesophagogastric cancers referred for liquid fiducial placement before definitive/neo-adjuvant or palliative IGRT between 2013 and 2021 at a tertiary hospital in Melbourne, Australia was conducted. Up to four liquid fiducials were inserted per patient, each injection consisting of 0.2-0.5mL of a 1:1 mixture of iodized oil (Lipiodol; Aspen Pharmacare) and n-butyl 2-cyanoacrylate (Histoacryl®; B. Braun). A 23-gauge injector (Cook Medical) was used for the injection. All procedures were performed by or under the supervision of a gastroenterologist. LF-IGRT consisted of computer-assisted direct matching of the fiducial region on cone-beam computerized tomography (CBCT) at the time of radiotherapy. Patients received S-IGRT if fiducial visibility was insufficient, consisting of bone match as a surrogate for tumor position. Radiotherapy was delivered to 54Gy in 30 fractions for curative patients and up to 45Gy in 15 fractions for palliative treatments.
To assess the long-term outcomes of liquid fiducial-guided IGRT in a cohort of oesophageal and gastric cancer patients.
We believe this to be the largest observational cohort study of its kind, adding to the limited body of knowledge on the long-term outcomes of F-IGRT for gastrooesophageal tumors using liquid fiducials.
IGRT has significantly improved the precision in which radiotherapy is delivered in cancer treatment. Typically, IGRT uses bony landmarks and key anatomical structures to locate the tumor. Recent studies have demonstrated the feasibility of peri-tumor fiducials in enabling even more accurate delineation of target and normal tissue. The use of gold coils as fiducials in gastrointestinal tumors has been extensively studied. However, placement requires expertise and specialized endoscopic ultrasound equipment. This article reports the long-term outcomes of using a standard gastroscopy to inject liquid fiducials for the treatment of oesophageal and gastric tumors with IGRT.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: An T, Chien CR, Link A S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64611] [Article Influence: 16152.8] [Reference Citation Analysis (176)] |
| 2. | Australian Institute of Health and Welfare. Cancer data in Australia [Internet]. Canberra: Australian Institute of Health and Welfare. [cited 16 October 2021]. Available from: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia. |
| 3. | Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 365] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 4. | Chan KKW, Saluja R, Delos Santos K, Lien K, Shah K, Cramarossa G, Zhu X, Wong RKS. Neoadjuvant treatments for locally advanced, resectable esophageal cancer: A network meta-analysis. Int J Cancer. 2018;143:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Zhu LL, Yuan L, Wang H, Ye L, Yao GY, Liu C, Sun NN, Li XJ, Zhai SC, Niu LJ, Zhang JB, Ji HL, Li XM. A Meta-Analysis of Concurrent Chemoradiotherapy for Advanced Esophageal Cancer. PLoS One. 2015;10:e0128616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Noordman BJ, Verdam MGE, Lagarde SM, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch OR, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A, Sprangers MAG, van Lanschot JJB. Effect of Neoadjuvant Chemoradiotherapy on Health-Related Quality of Life in Esophageal or Junctional Cancer: Results From the Randomized CROSS Trial. J Clin Oncol. 2018;36:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M, Wilke H, Budach W. Preoperative chemotherapy vs chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 8. | Leong T, Smithers BM, Haustermans K, Michael M, Gebski V, Miller D, Zalcberg J, Boussioutas A, Findlay M, O'Connell RL, Verghis J, Willis D, Kron T, Crain M, Murray WK, Lordick F, Swallow C, Darling G, Simes J, Wong R. TOPGEAR: A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. 2017;24:2252-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 9. | Ng SP, Leong T. Role of Radiation Therapy in Gastric Cancer. Ann Surg Oncol. 2021;28:4151-4157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Yuan M, Bao Y, Ma Z, Men Y, Wang Y, Hui Z. The Optimal Treatment for Resectable Esophageal Cancer: A Network Meta-Analysis of 6168 Patients. Front Oncol. 2021;11:628706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H, Faried A, Sohda M, Fukai Y, Masuda N, Fukuchi M, Manda R, Ojima H, Tsukada K, Kuwano H, Oriuchi N, Endo K. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005;103:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Hashimoto T, Shirato H, Kato M, Yamazaki K, Kurauchi N, Morikawa T, Shimizu S, Ahn YC, Akine Y, Miyasaka K. Real-time monitoring of a digestive tract marker to reduce adverse effects of moving organs at risk (OAR) in radiotherapy for thoracic and abdominal tumors. Int J Radiat Oncol Biol Phys. 2005;61:1559-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Abbas H, Chang B, Chen ZJ. Motion management in gastrointestinal cancers. J Gastrointest Oncol. 2014;5:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 14. | Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Shirato H, Harada T, Harabayashi T, Hida K, Endo H, Kitamura K, Onimaru R, Yamazaki K, Kurauchi N, Shimizu T, Shinohara N, Matsushita M, Dosaka-Akita H, Miyasaka K. Feasibility of insertion/implantation of 2.0-mm-diameter gold internal fiducial markers for precise setup and real-time tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Nicosia L, Sicignano G, Rigo M, Figlia V, Cuccia F, De Simone A, Giaj-Levra N, Mazzola R, Naccarato S, Ricchetti F, Vitale C, Ruggieri R, Alongi F. Daily dosimetric variation between image-guided volumetric modulated arc radiotherapy and MR-guided daily adaptive radiotherapy for prostate cancer stereotactic body radiotherapy. Acta Oncol. 2021;60:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Takei Y, Monzen H, Tamura M, Doi H, Nishimura Y. Dose reduction potential of using gold fiducial markers for kilovoltage image-guided radiotherapy. J Appl Clin Med Phys. 2020;21:151-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Patel JB, Revanur V, Forcione DG, Bechtold ML, Puli SR. Endoscopic ultrasound-guided fiducial marker placement in pancreatic cancer: A systematic review and meta-analysis. World J Gastrointest Endosc. 2020;12:231-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Chandran S, Vaughan R, Efthymiou M, Sia J, Hamilton C. A pilot study of EUS-guided fiducial insertion for the multidisciplinary management of gastric cancer. Endosc Int Open. 2014;2:E153-E159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Chandran S, Vaughan R, Jacob A, Hamilton C, Joon DL, Lim K, Tog C, Bhatia K, Aly A, Sweeney T, Efthymiou M. A novel endoscopic marker for radiological localization and image-guided radiotherapy in esophageal and gastric cancers (with video). Gastrointest Endosc. 2016;83:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] |
| 22. | Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 529] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 23. | National Cancer Institute. Common terminology criteria for adverse events v4.0 (CTCAE) [Internet]. [cited 16 October 2021]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. |
| 24. | Gwynne S, Hurt C, Evans M, Holden C, Vout L, Crosby T. Definitive chemoradiation for oesophageal cancer--a standard of care in patients with non-metastatic oesophageal cancer. Clin Oncol (R Coll Radiol). 2011;23:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Saikawa Y, Kubota T, Kumagai K, Nakamura R, Kumai K, Shigematsu N, Kubo A, Kitajima M, Kitagawa Y. Phase II study of chemoradiotherapy with S-1 and low-dose cisplatin for inoperable advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2008;71:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Mahmoudi N, Whittaker JS. Glueing of fundal varices. Can J Gastroenterol. 2006;20:691-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | de Blanck SR, Scherman-Rydhög J, Siemsen M, Christensen M, Baeksgaard L, Irming Jølck R, Specht L, Andresen TL, Persson GF. Feasibility of a novel liquid fiducial marker for use in image guided radiotherapy of oesophageal cancer. Br J Radiol. 2018;91:20180236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Yu S, Lawrenson L, Wei R, Sehgal V, Hanna N, Kuo J, Daroui P, Ramsinghani N, Al-Ghazi M. The dosimetric impact of image guided radiation therapy by intratumoral fiducial markers. Pract Radiat Oncol. 2016;6:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Hulshof MCCM, Geijsen ED, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, Nuyttens JJME, van der Sangen MJC, Jeene PM, Reinders JG, van Berge Henegouwen MI, Thano A, van Hooft JE, van Laarhoven HWM, van der Gaast A. Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients With Locally Advanced Esophageal Cancer (ARTDECO Study). J Clin Oncol. 2021;39:2816-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 30. | Xu YJ, Zhu WG, Liao ZX, Kong Y, Wang WW, Li JC, Huang R, He H, Yang XM, Liu LP, Sun ZW, He HJ, Bao Y, Zeng M, Pu J, Hu WY, Ma J, Jiang H, Liu ZG, Zhuang TT, Tan BX, Du XH, Qiu GQ, Zhou X, Ji YL, Hu X, Wang J, Ma HL, Zheng X, Huang J, Liu AW, Liang XD, Tao H, Zhou JY, Liu Y, Chen M. [A multicenter randomized prospective study of concurrent chemoradiation with 60 Gy vs 50 Gy for inoperable esophageal squamous cell carcinoma]. Zhonghua Yi Xue Za Zhi. 2020;100:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Innocente R, Navarria F, Petri R, Palazzari E, Vecchiato M, Polesel J, Ziccarelli A, Martino A, Ubiali P, Tonin D, Lauretta A, Belluco C, Foltran L, Buonadonna A, Lleshi A, Colombo CB, Barresi L, Gigante M, Franchin G, De Paoli A. Feasibility and Oncological Outcome of Preoperative Chemoradiation With IMRT Dose Intensification for Locally Advanced Esophageal and Gastroesophageal Cancer. Front Oncol. 2021;11:626275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Xiao L, Czito BG, Pang Q, Hui Z, Jing S, Shan B, Wang J. Do Higher Radiation Doses with Concurrent Chemotherapy in the Definitive Treatment of Esophageal Cancer Improve Outcomes? J Cancer. 2020;11:4605-4613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Li R, Shinde A, Glaser S, Chao J, Kim J, Karam SD, Goodman K, Chen YJ, Amini A. Analyzing the impact of neoadjuvant radiation dose on pathologic response and survival outcomes in esophageal and gastroesophageal cancers. J Gastrointest Oncol. 2019;10:712-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Effeney R, Shaw T, Burmeister BH, Burmeister E, Harvey J, Mai GT, Thomas J, Barbour AP, Smithers BM, Pryor DI. Patterns of Failure Following Dose-escalated Chemoradiotherapy for Fluorodeoxyglucose Positron Emission Tomography Staged Squamous Cell Carcinoma of the Oesophagus. Clin Oncol (R Coll Radiol). 2018;30:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Coronel E, Cazacu IM, Sakuraba A, Luzuriaga Chavez AA, Uberoi A, Geng Y, Tomizawa Y, Saftoiu A, Shin EJ, Taniguchi CM, Koong AC, Herman JM, Bhutani MS. EUS-guided fiducial placement for GI malignancies: a systematic review and meta-analysis. Gastrointest Endosc. 2019;89:659-670.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Kuo YH, Fang HY, Lin YS, Lein MY, Yang CY, Ho SC, Li CC, Chien CR. Effectiveness of image-guided radiotherapy for locally advanced esophageal squamous cell carcinoma patients treated with definitive concurrent chemoradiotherapy. Thorac Cancer. 2020;11:113-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Ng J, Lee P. The Role of Radiotherapy in Localized Esophageal and Gastric Cancer. Hematol Oncol Clin North Am. 2017;31:453-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Voncken FEM, Nakhaee S, Stam B, Wiersema L, Vollenbrock SE, van Dieren JM, van Leerdam ME, Sonke JJ, Aleman BMP, Remeijer P. Quantification of Esophageal Tumor Motion and Investigation of Different Image-Guided Correction Strategies. Pract Radiat Oncol. 2020;10:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |