Published online Nov 7, 2021. doi: 10.3748/wjg.v27.i41.7144
Peer-review started: July 8, 2021
First decision: August 19, 2021
Revised: August 20, 2021
Accepted: October 18, 2021
Article in press: October 18, 2021
Published online: November 7, 2021
Processing time: 120 Days and 18.9 Hours
Different forms of pregenomic and other hepatitis B virus (HBV) RNA have been detected in patients’ sera. These circulating HBV-RNAs may be useful for monitoring covalently closed circular DNA activity, and predicting hepatitis B e-antigen seroconversion or viral rebound after nucleos(t)ide analog cessation. Data on serum HBV-RNA quasispecies, however, is scarce. It is therefore important to develop methodologies to thoroughly analyze this quasispecies, ensuring the elimination of any residual HBV-DNA. Studying circulating HBV-RNA quasispe
To establish a next-generation sequencing (NGS) methodology for analyzing serum HBV-RNA and comparing it with DNA quasispecies.
Thirteen untreated chronic hepatitis B patients, showing different HBV-genotypes and degrees of severity of liver disease were enrolled in the study and a serum sample with HBV-DNA > 5 Log10 IU/mL and HBV-RNA > 4 Log10 copies/mL was taken from each patient. HBV-RNA was treated with DNAse I to remove any residual DNA, and the region between nucleotides (nt) 1255-1611 was amplified using a 3-nested polymerase chain reaction protocol, and analyzed with NGS. Variability/conservation and complexity was compared between HBV-DNA and RNA quasispecies.
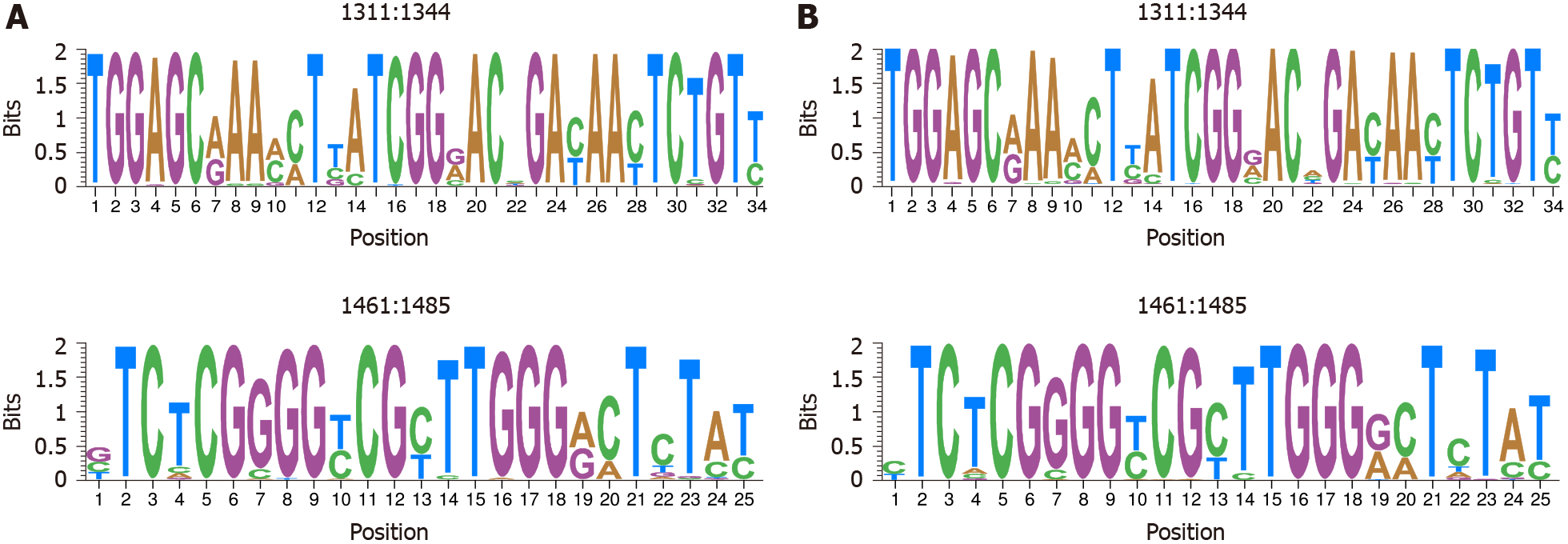
No HBV-DNA contamination was detected in cDNA samples from HBV-RNA quasispecies. HBV quasispecies complexity showed heterogeneous behavior among patients. The Rare Haplotype Load at 1% was greater in DNA than in RNA quasispecies, with no statistically significant differences (P = 0.1641). Regarding conservation, information content was equal in RNA and DNA quasispecies in most nt positions [218/357 (61.06%)]. In 102 of the remaining 139 (73.38%), HBV-RNA showed slightly higher variability. Sliding window analysis identified 4 hyper-conserved sequence fragments in each quasispecies, 3 of them coincided between the 2 quasispecies: nts 1258-1286, 1545-1573 and 1575-1604. The 2 hyper-variable sequence fragments also coincided: nts 1311-1344 and 1461-1485. Sequences between nts 1519-1543 and 1559-1587 were only hyper-conserved in HBV-DNA and RNA, respectively.
Our methodology allowed analyzing HBV-RNA quasispecies complexity and conservation without interference from HBV-DNA. Thanks to this, we have been able to compare both quasispecies in the present study.
Core Tip: Hepatitis B virus (HBV) quasispecies composition and its evolution are related to liver disease progression. HBV-RNA in serum is potentially useful for analyzing viral quasispecies, even in patients with low levels of or suppressed serum HBV-DNA. Few studies have analyzed circulating HBV-RNA quasispecies, and similarities and differences with DNA quasispecies should be assessed. We used next-generation sequencing to analyze RNA quasispecies variability/conservation and complexity, without interference of HBV-DNA, in untreated chronic hepatitis B patients. Comparison of both quasispecies showed similar results between them. DNA quasispecies tended toward greater complexity, while RNA quasispecies tended toward higher variability.
- Citation: Garcia-Garcia S, Cortese MF, Tabernero D, Gregori J, Vila M, Pacín B, Quer J, Casillas R, Castillo-Ribelles L, Ferrer-Costa R, Rando-Segura A, Trejo-Zahínos J, Pumarola T, Casis E, Esteban R, Riveiro-Barciela M, Buti M, Rodríguez-Frías F. Cross-sectional evaluation of circulating hepatitis B virus RNA and DNA: Different quasispecies? World J Gastroenterol 2021; 27(41): 7144-7158
- URL: https://www.wjgnet.com/1007-9327/full/v27/i41/7144.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i41.7144
The hepatitis B virus (HBV) is the etiological agent of hepatitis B and can cause both acute and chronic liver disease. In 2015, roughly 257 million people worldwide were estimated to suffer from chronic hepatitis B (CHB) infection, the major complications of which are cirrhosis and hepatocellular carcinoma[1].
The HBV genome is a 3.2 kb-long partially double-stranded relaxed circular DNA (rcDNA), with a complete minus-strand and an incomplete plus-strand. After cy
In a single HBV infection, viral genomes are present as a complex mutant spectrum constituted by closely related, but not identical, viral populations (genetic variants), termed viral quasispecies[9]. Those variants can occur through numerous host-virus interactions during the viral replication cycle, encompassing not only cellular factors but also viral factors, essentially the error-prone viral polymerase[10]. Serum cir
Taking this in mind, the aim of this study was to establish a methodology, based on next-generation sequencing (NGS), for thoroughly analyzing serum HBV-RNA quasispecies without interference from circulating HBV-DNA. This methodology has been tested in a group of untreated CHB patients to gain a preliminary insight into comparison of genetic variability/conservation and complexity between circulating HBV-RNA and DNA quasispecies.
The study was approved by the Ethics Committee of the Vall d’Hebron Research Institute (PR(AG)146/2020) and all patients provided written informed consent for participation. No animals were used.
In this cross-sectional study, we included a serum sample from 13 well-characterized untreated CHB patients attending the outpatient clinic of Vall d’Hebron University Hospital (Barcelona, Spain). All samples were selected taking into account HBV-DNA levels > 5 Log10 IU/mL and HBV-RNA levels > 4 Log10 copies/mL to ensure sufficient levels of both HBV-DNA and RNA to study their quasispecies. In addition, heterogeneity in terms of HBV genotypes and degrees of severity of liver disease were also taken into account in patient selection, in order to obtain an overall picture of differences between HBV-DNA and RNA quasispecies. Exclusion criteria were positive testing for hepatitis D virus, hepatitis C virus, and human immunodeficiency virus.
HBV serological markers such as HBsAg, HBeAg, anti-HBe antibodies were tested using commercial chemiluminescent immunoassays on a COBAS 8000 analyzer (Roche Diagnostics, Rotkreuz, Switzerland). HBV-DNA was quantified on a COBAS 6800 analyzer (Roche Diagnostics, Mannheim, Germany) with a lower detection limit of 10 IU/mL. HBV genotypes were determined as previously explained[13]. HBV-RNA was quantified using an in-house method. The standard RNA to create the standard curve for this quantification was obtained as follows: 1.1× HBV genome from pTRiEx1.1-HBV[15] was subcloned downstream of a T7 promoter in a pEF6/V5-His TOPO TA vector (Thermo Fisher Scientific-Life Technologies, Austin, TX, United States) following the manufacturer’s instructions, to ensure its in vitro transcription (pEF6-HBV). Once cloned, the correct orientation and insertion of HBV genome was analyzed by Sanger Sequencing, and pEF6-HBV plasmid was isolated from bacteria using the NucleoBond Xtra Midi Kit (Macherey-Nagel, Dueren, Germany). The pEF6-HBV plasmid was then linearized by NotI restriction enzyme digestion (New England Biolabs, Beverly, MA, United States), and used as template for in vitro transcription reaction using the MEGAscript T7 Transcription Kit (Thermo Fisher Scientific-Life Technologies, Austin, TX, United States), by adding the plasmid pEF6-HBV concentration of 0.5 μg/μL diluted in water. At the same time, the in vitro transcription of pTRI-Xef positive control DNA provided with the kit was also performed. The RNA resulting from transcription was then purified using the MEGAclear Transcription Clean-up Kit (Thermo Fisher Scientific-Life Technologies, Austin, TX, United States) following the manufacturer’s instructions. A DNase I treatment (Life Technologies, Austin, TX, United States) was then carried out for 15 min at room temperature, followed by heat-inactivation at 65 °C for 10 min and adding 2 μL of 25 mmol/L EDTA solution to the reaction mixture, in order to remove the template DNA. The purity of DNAse I-treated RNA was checked by measuring the absorbance of 1 μL of the sample at 260 nm using the NanoDrop spectrophotometer (Thermo Fisher Scientific-Life Technologies, Austin, TX, United States). The concentration of this RNA was quantified from 3 μL of the sample by Qubit fluorimeter using the Qubit RNA HS Assay Kit (Thermo Fisher Scientific-Life Technologies, Austin, TX, United States). The absence of DNA in DNAse I-treated RNA template was verified using the DNA Master PLUS HybProbe kit in a LightCycler 480 Instrument II system (Roche, Mannheim, Germany) using primers and probe specified in the Supplementary Table 1.
For absolute quantification analyses, the standard curve was defined using the quantified retrotranscribed RNA, taken to a concentration of 7.51 Log10 copies/mL, and serial 1:10 dilutions of this standard covering concentrations until 1.51 Log10 copies/mL. The points of the standard curve were defined as the mean Ct of a triplicate measurement of the original 7.51 Log10 copies/mL standard and its serial dilutions. The standard curve obtained was saved and imported as an external standard curve in each HBV-RNA quantification experiment. In those experiments, at least one dilution of standard RNA used to define one of the standard curve points must be included, in order to adjust the standard curve. Creation of the RNA standard curve and experiments to quantify HBV-RNA levels quantification were performed using one-step quantitative reverse transcription polymerase chain reaction (RT-qPCR) reaction, using the LightCycler 480 RNA Master Hydrolysis Probes kit (Roche, Mannheim, Germany). RT-qPCR program in the Light Cycler 480 instrument was programmed as described in the Supplementary Table 1.
In this study, we analyzed the region between nucleotides (nt) 1255 to 1611, located at the HBX 5’ end. In this region, we previously identified hyper-conserved sequence stretches in the circulating HBV-DNA quasispecies[13,14], which we aimed at analy
For each serum sample, HBV-DNA was extracted from 200 μL of serum using QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The region of interest was amplified through a 3-round polymerase chain reaction (PCR) protocol. The first-round PCR covered a 1338-bp region and was performed as previously described by our group[16] (Supplementary Table 1). The second-round and third-round PCRs, were also performed as previously detailed[13] (Supplementary Table 1). The second-round PCR amplified the region of interest between nt 1255 and 1611 of the HBV genome, yielding final products flanked by universal M13 sequences at both ends. In the third-round PCR, a specific pair of primers was used for each sample, consisting of the same M13 universal sequences (forward and reverse) at their 5’ ends and a multiplex identifier (MID) or barcode sequence at their 3’ ends. Each individual patient sample required a different MID. The PCR products obtained in this amplification, also known as amplicons, were visualized as single bands on a 1.5% agarose electrophoresis gel, stained with SybrSafe DNA gel Stain (Life Technologies, Carlsbad, CA, United States) with 1x TAE running buffer (Roche Diagnostics, Mannheim, Germany). PCR products were then purified from agarose gel using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). Amplicon quality was verified using the Agilent 2200 TapeStation System and D1000 ScreenTape kit (Agilent Technologies, Waldbronn, Germany). Purified DNA from each sample was quantified by means of fluorescence, using the Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies, Carlsbad, CA, United States) adjusted to the same concentration, and pooled.
In the same serum samples, HBV-RNA was isolated from 140 μL serum using the QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany), according to the ma
At the same time, elimination of any residual HBV-DNA after DNAse I treatment was verified by means of qPCR, as described for the DNAse I-treated RNA obtained from in vitro transcription of pEF6-HBV vector, described above. This qPCR ex
Finally, amplicon pools obtained from both HBV-DNA and RNA were sequenced using the MiSeq platform (Illumina, San Diego, CA, United States). Those purified pools were processed using the DNA library preparation kit Kapa Hyper Prep kit (Kapa Biosystems-Roche, Cape Town, South Africa), with which amplicon ends were repaired and A-tailed. Each pool was then indexed using the SeqCap Adapter Kit A (Roche Sequencing, Pleasanton, CA, United States). After index ligation pools had been purified with KAPA Pure Beads (Kapa Biosystems-Roche, Cape Town, South Africa) and re-amplified to increase indexed amplicon concentration using the KAPA HiFi HotStart ReadyMix PCR Kit (Roche Sequencing, Pleasanton, CA, United States). PCR products were repurified with another round of KAPA Pure Beads and quan
Bioinformatics processing and haplotype-centric data analysis pipeline were perfor
The HBV-DNA and RNA quasispecies complexities were evaluated by computation of the Rare Haplotype Load (RHL), a new diversity index that measures enrichment of quasispecies in minority genomes below a given threshold[17]. RHL was calculated using the haplotypes (unique sequences covering the full amplicon observed on the set of sequences) resulting from the intersection of forward and reverse strands without filtering by a minimum abundance. In this study, RHL was computed as the sum of the relative frequencies of all haplotypes obtained from HBV-DNA and RNA qua
Sequence conservation at nt level was determined by calculating the information content (IC, bits) of each position in a multiple alignment of all intersected haplotypes obtained in frequencies > 0.25%, as previously explained[13]. Nt IC ranges from 0 bits to 2 bits for a given position, where 2 bits represents the maximum IC value (100% conservation, i.e., no nt changes in a given position in any of all haplotypes analyzed). The mean IC was calculated in windows of 25 nt, moved in steps of 1 nt through nt 1255-1611 (sliding window analysis), the 5% of those windows with the highest mean IC values (the most conserved) and the 5% with the lowest mean IC values (the most variable) were selected. Afterwards, those consecutive windows were connected and their sequences were represented as sequence logos using the R language package motifStack[18]. In each position of the logos, letters representing the nts identified are stacked. The relative sizes of letters indicate the relative frequencies of the nt represented at a given position in the multiple alignments of the haplotypes, and the total height of each stack represents the IC of each position.
All statistical analyses were performed in R[19]. Qualitative parameters were ex
Clinical, virological, and serological parameters from the 13 patients at the time of obtention of samples included are summarized in Table 1. Most of those patients were HBeAg +ve men, showing clinical characteristics compatible with chronic hepatitis stage of CHB infection[12]. In terms of severity of liver disease, 8 patients were classified as patients with nonsignificant fibrosis [Ishak fibrosis stage (F) < 3 or transient elastography < 7-8.5 kPa], and 5 patients were classified as patients with significant fibrosis (liver biopsy F ≥ 3 and < 5 or transient elastography > 7-8.5 and < 11-14 kPa, n = 3) or cirrhosis (liver biopsy F5-6 or transient elastography > 11-14 kPa, n = 2), according to World Health Organization Guidelines[20].
| Total, n = 13 | |
| Age, yr, median (IQR) | 41.35 (31.7-47.2) |
| Male, n (%) | 11 (84.6) |
| Genotype, n (%) | |
| A | 3 (23.1) |
| C | 3 (23.1) |
| D | 2 (15.4) |
| E | 2 (15.4) |
| F | 3 (23.1) |
| ALT, IU/L, median (IQR) | 124 (66-160) |
| HBeAg +ve, n (%) | 11 (84.6) |
| HBV DNA, Log10 IU/mL, median (IQR) | 8 (8-8.43) |
| HBV RNA, Log10 copies/mL, median (IQR) | 5.18 (4.94-6.02) |
| Fibrosis, n (%)1 | |
| Nonsignificant | 8 (61.64) |
| Significant | 3 (23.08) |
| Cirrhosis, n (%) | 2 (15.39) |
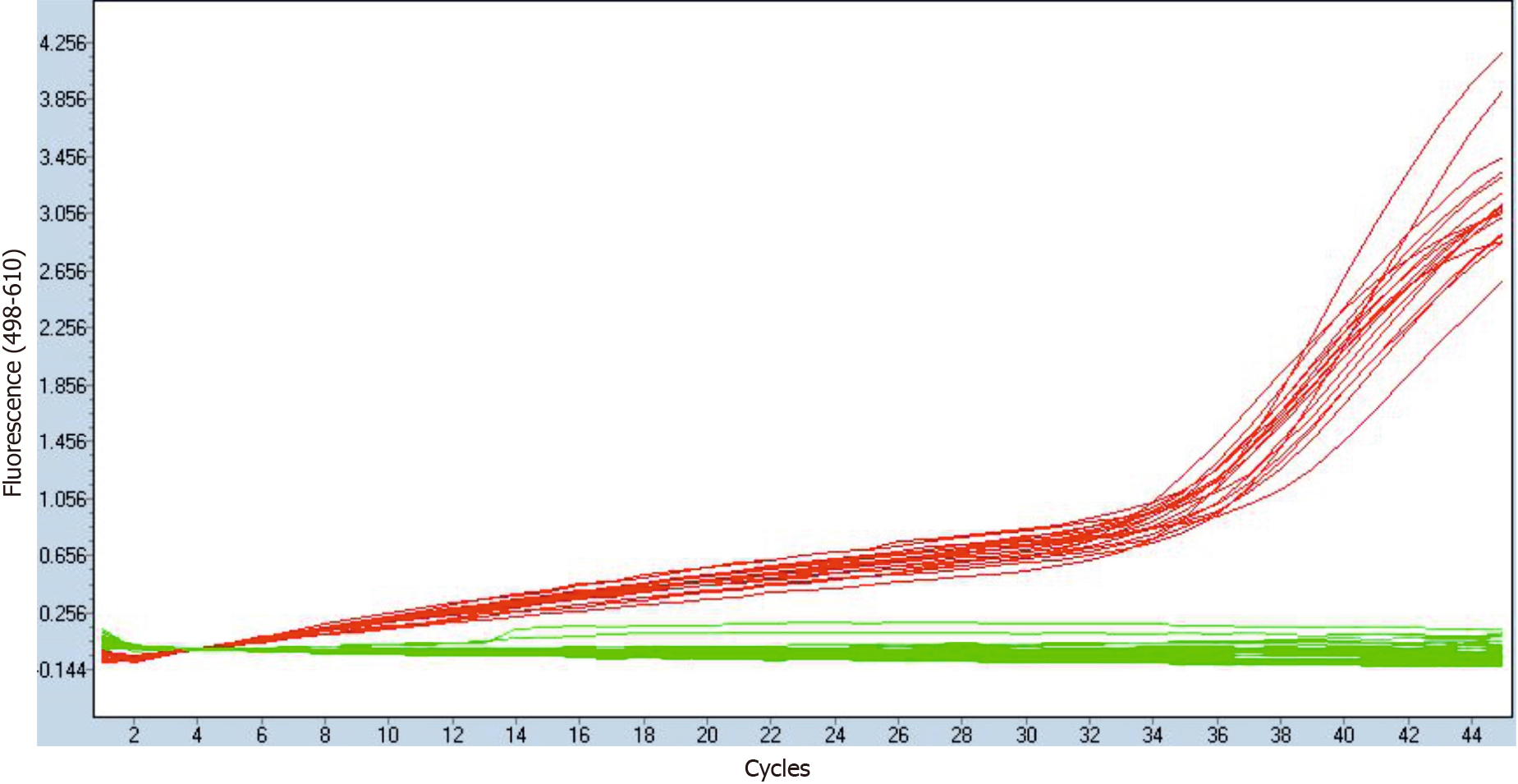
The qPCR verification of residual HBV-DNA elimination from HBV-RNA isolations confirmed the absence of HBV-DNA from DNAse I-treated RNA samples, while showing the presence of cDNA after their reverse transcription. An illustrative example is shown in Figure 1. No HBV-DNA contamination was therefore detected in cDNA samples derived from HBV-RNA quasispecies.
After NGS using the MiSeq platform, Fastq files obtained were filtered by our bioinformatics procedure to obtain the set of haplotypes common to forward and reverse strands. Before subsequent filtering by abundance, those haplotypes repre
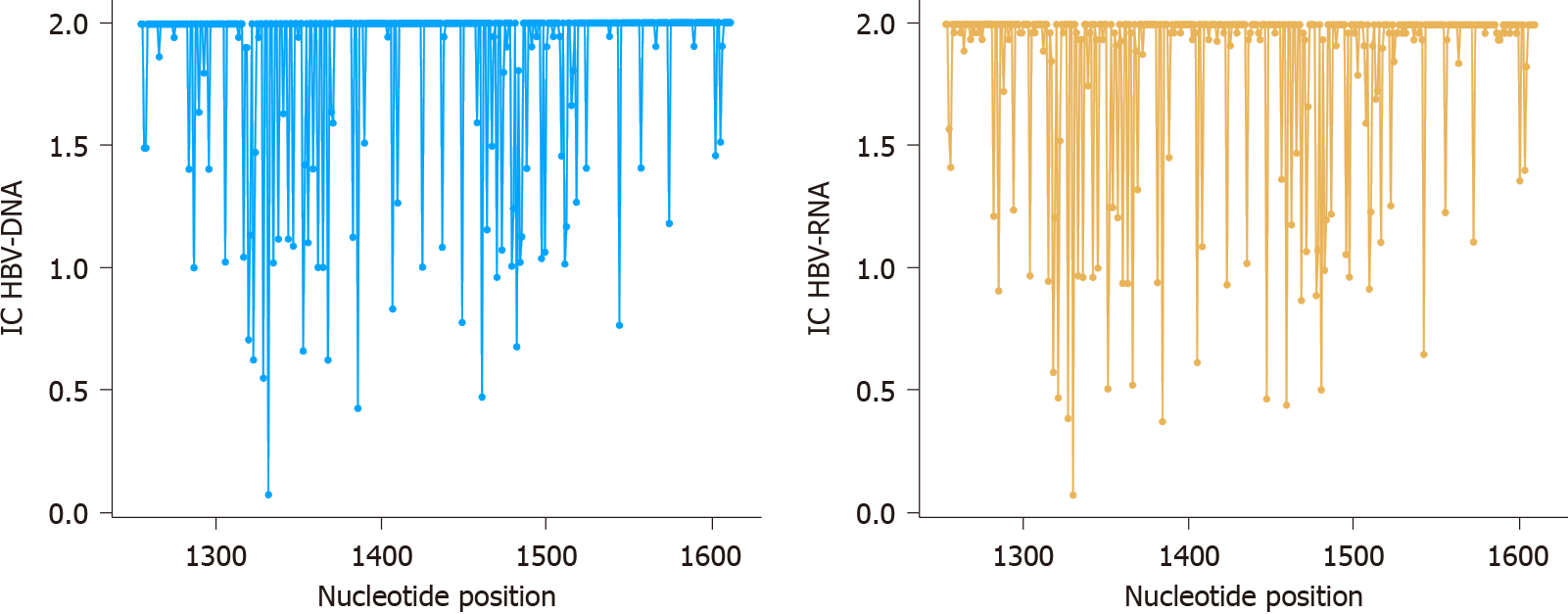
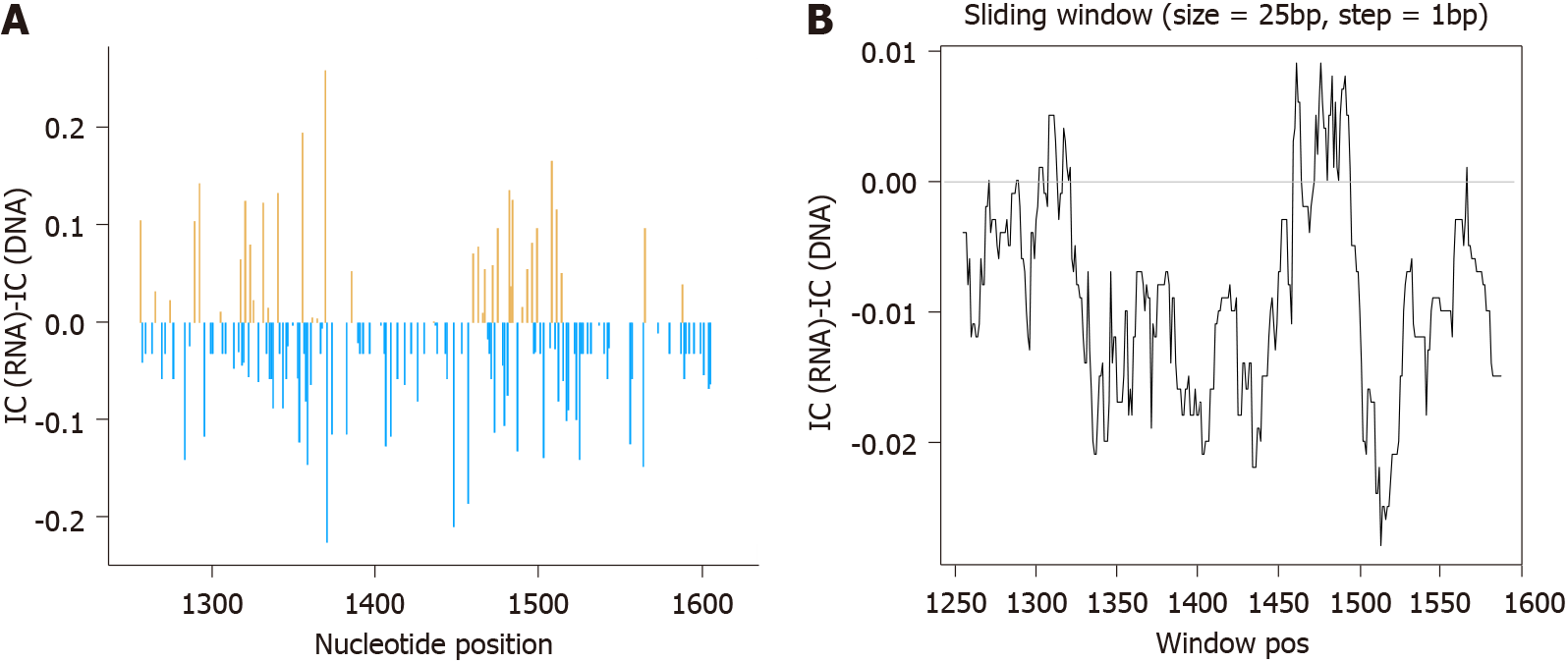
After RHL calculation, we proceeded to eliminate all haplotypes with abundances below 0.25%, obtaining 2.91 × 106 sequence reads with a median of 1.58 × 105 reads/sample (IQR, 1.29 × 105-2.28 × 105) for HBV-RNA and 5.66 × 104 reads/sample (IQR, 3.2 × 104-5.76 × 104) for HBV-DNA. These haplotypes were the basis for conservation analysis through IC calculation position by position in both HBV-RNA and DNA quasispecies (Figure 3). Differences in sequence conservation between the 2 quasispecies were determined by subtracting IC DNA values from IC RNA of 357 nt positions analyzed; between nt 1255-1611. In 218 (61.06%) of these positions, IC values of both HBV-RNA and DNA quasispecies were coincident, while in the remaining 139 (38.93%) nt positions differences between IC of both quasispecies ranged from -0.26 to 0.23, with IC DNA > IC RNA in most of them (102/139, 73.38%) (Figure 4A). These differences were also studied by sliding window analysis of the mean IC RNA-IC DNA values (calculated in windows of 25 nt positions displaced in steps of 1 position between them) confirming that, in general, the HBV-RNA quasispecies was slightly less conserved than the DNA quasispecies (Figure 4B). In keeping with this analysis, HBV-RNA displayed more positions with some variability (IC < 2 bits), 135/357 (38%), than HBV-DNA, 85/357 (24%) (P < 0.01, two-proportions z-test).
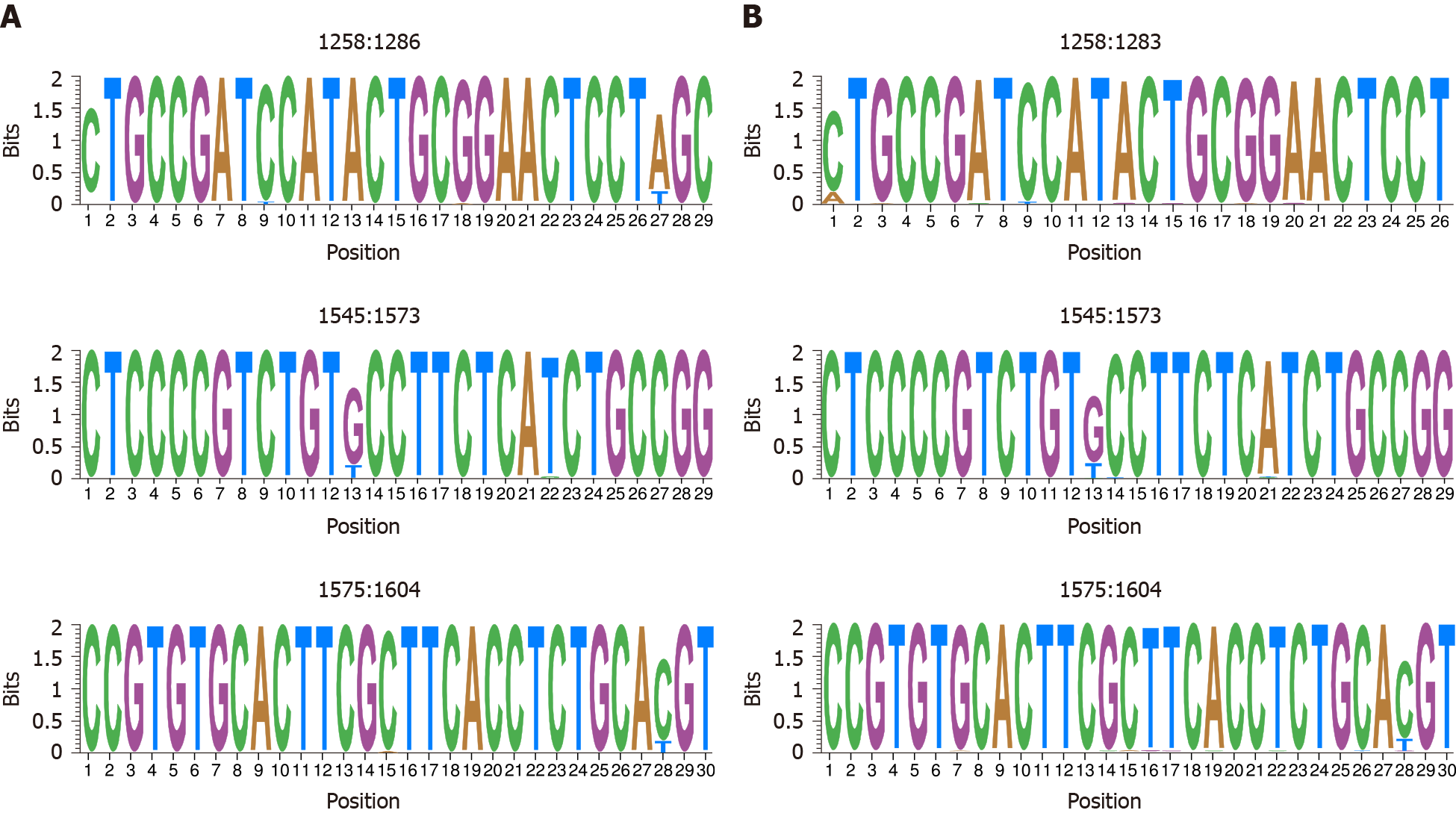
The high degree of similarity between IC values in both HBV-DNA and HBV-RNA quasispecies was also observed in the sliding window analysis of IC in the multiple alignments of haplotypes obtained in both quasispecies. Concatenation of the 5% of windows with higher mean IC values (the most conserved), yielded 4 sequence logos in both quasispecies 3 (75%) of which coincided between both quasispecies: nts 1258-1286 (1283 in RNA quasispecies), 1545-1573 and 1575-1604 (Figure 5). In addition, sliding window analysis also showed the sequence stretch 1519-1543 to be hyper-conserved only in HBV-DNA quasispecies and 1559-1587 only in HBV-RNA qua
Serum HBV RNA results from a mixture of different viral RNA species: Intact and spliced pgRNA, and truncated and polyA-free RNAs, with varying levels depending on the phase of HBV infection and antiviral treatment[21]. In addition, HBX transcripts have also been detected[3]. This serum HBV RNA has been suggested as a potential surrogate marker both to reflect intrahepatic cccDNA levels during the natural course of CHB infection[22,23] and under NA treatment[4], and to predict treatment outcome[4,21] such as HBeAg seroconversion[5-7]. During antiviral treatment, the kinetics of serum HBV-DNA and HBV-RNA seem to be dissociated. While serum HBV-DNA levels rapidly decline after starting treatment, HBV-RNA levels fall more slowly and the HBV-DNA/HBV-RNA ratio increases significantly. This allows inference in cccDNA transcription in the absence of detectable serum HBV DNA and may predict viral rebound after NA cessation[8]. In addition, the slower decline of serum HBV RNA levels may also make it possible to study circulating viral quasispecies, even when serum HBV-DNA levels are too low to do so. However, few studies to date have analyzed circulating HBV-RNA quasispecies[4,6] and little data is therefore available on their similarities and differences. Indeed, it is reasonable to think that both quasispecies may display significant differences between them, as they are subjected to different sources of genetic variability, due to the error-prone viral polymerase, contributing to an estimated error rate of approximately 1 per 104-105 bp for minus-strand DNA synthesis and approximately 1 per 3.6-15 × 104 bp for second-strand DNA synthesis[24]. Hence, reverse transcription becomes an outstanding source of varia
The first aim of this study was to establish a reliable methodology to thoroughly analyze serum HBV-RNA quasispecies, without interference from HBV-DNA qua
Since viral polymerase errors are considered an important source of genetic variability for the HBV genome, we considered RHL to be a diversity index especially suitable for comparing complexities between HBV RNA and DNA quasispecies and assessing the effect of viral polymerase activity on the latter. This is because this diversity index refers to the fraction of the quasispecies with the lowest fitness, indicative of the intensity with which replication errors accumulate. High viremia and long infection times would tend to result in high RHL values. High values may also be given to mutagen treatments[17]. In our study, HBV-DNA quasispecies showed a higher mean RHL than RNA quasispecies, which suggests an increased presence of low frequency HBV genomes in DNA quasispecies, probably due to the error-prone reverse-transcription origin of HBV-DNA. However, these differences were not statistically significant, either when including all patients or when separating them ac
Comparison of both HBV-DNA and RNA quasispecies conservation yielded similar results, with HBV-DNA slightly more conserved than HBV-RNA despite the effect of reverse-transcription over DNA quasispecies. This observation may indicate that only a fraction of packaged pgRNA is reverse-transcribed and released. In addition, in this study, we identified 3 hyper-conserved regions which coincided in both HBV-RNA and HBV-DNA quasispecies. The first hyper-conserved region identified was between nt 1258-1286, while the second hyper-conserved region consisted of 2 nt fragments (1545-1573 and 1575-1604) spanning a region between nt 1545-1604. Interestingly, these 3 conserved sequence fragments almost overlapped with hyper-conserved sequence fragments described in a previous study carried out by our group at circulating DNA quasispecies level (1255-1286, 1545-1573 and 1575-1603)[13]. Likewise, a recently published study by our group[14] also performed in HBV-DNA quasispecies, reported some of these hyper-conserved regions (1258-1286 and 1575-1605). In addition, these 2 previous studies[13,14] also reported as hyper-conserved the sequence stretch between positions 1519-1543, which we found among the 5% most conserved windows at HBV-DNA quasispecies level but not at HBV-RNA quasispecies level. Conversely, we identified the sequence stretch 1559-1587 as hyper-conserved only in HBV-RNA quasispecies. This fragment was not identified in our previous studies, although it is included in the region between nt 1519 and 1603 defined by 3 conserved nt fragments (1519-1543, 1545-1573, and 1575-1603) described in one of them[13]. Thus, despite the high degree of similarity between sequence conservation in HBV-DNA and RNA quasispecies, this may follow different trends in some sequence stretches. Finally, assessment of the most variable windows (the 5% with the lowest mean IC values) identified the fragments between nt 1311-1344 and 1461-1485 as the most variable in both DNA and RNA quasispecies, similar to the most conserved stretches identified, which confirmed the similarities between both quasispecies.
Given the important and wide role of hepatitis B X protein (HBx), a multifunctional transactivator protein encoded by the HBX gene, in the replication of HBV, particularly in intrahepatic cccDNA stability and transcription, it is an interesting target for new therapies to treat CHB-infected patients[8]. Moreover, HBx is located next to the 3’ end of all the HBV mRNAs, which means that targeting its sequence may interfere in the synthesis of all the viral proteins[13]. This concept is in line with other strategies aiming for functional cure. Thus, identifying highly conserved regions may suggest valuable targets for pan-genotypic approaches with minimum likelihood of antiviral drug resistance. With this in mind, we analyzed a region of the HBX gene where we had previously identified hyper-conserved regions in the circulating HBV-DNA quasispecies[13,14], which we intended to confirm in circulating HBV-RNA. In fact, serum HBV-RNA was found to be genetically homogenous with intrahepatic HBV-RNA[4] the main target for this kind of antiviral therapies. Therefore, given that some sequence fragments may follow different conservation trends in both HBV-DNA and RNA quasispecies, when looking for targets for directed gene therapy, it may be worth verifying their conservation not only at serum HBV-DNA level but also at RNA level. This would make it possible to ensure their effect on their main target. In this study, we confirmed that most of the hyper-conserved regions identified by our group in previous studies at DNA level[13,14] coincided with those identified in serum HBV-RNA, and may thus also be conserved at intrahepatic HBV-RNA level.
The broad range of HBV genotypes and degrees of severity of liver disease from all patients included in the present study enabled to get an overview of complexity and conservation of HBV-DNA and RNA quasispecies. Nevertheless, the main limitation of the present study was that the comparison with this small and heterogeneous group of patients just enabled to take a preliminary picture of HBV-DNA and RNA qua
In summary, we developed a methodology for analyzing serum HBV-RNA qua
Interestingly, although HBV-DNA and RNA quasispecies showed a similar degree of conservation in the HBX 5’region, HBV-RNA quasispecies tended toward higher variability than HBV-DNA. Most hyper-conserved and hyper-variable regions iden
Recent studies show that hepatitis B virus (HBV)-RNA detected in serum is mainly encapsidated pregenomic RNA (pgRNA), which could be a useful biomarker for monitoring covalently closed circular DNA activity. During nuclos(t)ide analogs (NA) therapy it is predictive of hepatitis B e-antigen seroconversion and for following viral rebound after treatment cessation. However, few studies have analyzed the serum HBV-RNA quasispecies, a complex mutant spectrum constituted by closely related, but not identical, viral populations. Analysis of serum circulating HBV-RNA qua
Composition and evolution over time of HBV quasispecies is closely linked to liver disease progression, and serum circulating HBV-RNA is potentially useful for its analysis, even in situations with low or undetectable serum level of HBV-DNA. However, HBV-RNA has not been subjected to reverse transcription, which is the main source of variability in the HBV genome and it may therefore present significant differences with respect to the serum HBV-DNA quasiespecies. Nonetheless, little data is available on the comparison of genetic variability/conservation and complexity of both quasispecies. Moreover, analysis of serum HBV-RNA quasispecies may be a very useful tool when looking for hyper-conserved targets for targeted gene therapy, due to its similarities with intrahepatic HBV-RNA, the main target of this kind of antiviral therapy. Thus, in previous studies, we identified hyper-conserved sequence stretches in the circulating HBV-DNA quasispecies, which we aimed to analyze at the cir
This study aimed to establish a methodology to achieve an in-depth analysis serum HBV-RNA quasispecies without interference from circulating HBV-DNA, using next-generation sequencing (NGS). With this methodology, we aimed to compare both serum HBV-RNA and DNA quasispecies in a group of untreated chronic hepatitis B (CHB) patients. Considering the potential of HBV-RNA for analyzing HBV quasis
Serum samples were taken from 13 untreated CHB patients attending the outpatient clinic of Vall d’Hebron University Hospital (Barcelona, Spain). HBV-DNA levels > 5 log10 IU/mL, HBV-RNA levels > 4 Log10 copies/mL and heterogeneity in terms of HBV genotypes and degrees of severity of liver disease were considered as inclusion criteria. HBV-RNA and DNA were extracted differently using specific manual isolation protocols. In addition, HBV-RNA, which was quantified by an in-house method, was treated with DNAse I (Life Technologies, Austin, United Ststes) to remove any residual DNA present. The elimination of residual DNA after DNAse I digestion was verified by means of quantitative PCR (qPCR). In parallel, these samples were retrotranscribed into cDNA, and along with DNA isolates the 5’ end region of HBX, between nucleotides 1255-1611 (the amplicon analysed), was amplified by a 3-nested PCR protocol and later sequenced using NGS (MiSeq, Illumina, United States). HBV-RNA and DNA quasispecies complexity was evaluated using Rare Haplotype Load (RHL) index, which measures enrichment of quasispecies in minority genomes. Sequence conservation and variability was determined by calculating the information content (IC) of each position by aligning all unique sequences covering the full amplicon (i.e., haplotypes) in a multiple alignment. After this analysis, the most conserved and variable sequence stretches were represented as sequence logos, which were compared between both quasispecies.
After treatment of RNA isolates with DNase I, we confirmed that no residual HBV-DNA was present in the HBV-RNA isolates and that contamination by HBV-DNA in sequences obtained from HBV-RNA was therefore unlikely. HBV quasispecies complexity showed heterogeneous behavior among patients. While RHL was greater in DNA than in RNA quasispecies, differences were not statistically significant. This tendency of HBV DNA quasispecies toward higher quasispecies complexity than HBV-RNA needs to be studied further in larger groups of patients. In general, conservation was highly coincident between HBV-RNA and DNA quasispecies; the majority of nt positions showed the same IC value in both of them. Interestingly, HBV-RNA quasispecies was slightly less conserved than DNA and displayed more positions with some variability (IC < 2 bits). Sliding window analysis in HBV-DNA and RNA quasispecies showed 4 sequence fragments as the most conserved in each of them. Of those fragments 3 coincided between both quasispecies, but 1 was found to be among the most conserved only in HBV-DNA and 1 only in HBV-RNA. The most variable sequence fragments coincided in both quasispecies.
In this study, we describe a methodology for analyzing serum HBV-RNA quasispecies without HBV-DNA interference. This methodology allowed us to compare HBV-DNA and RNA quasispecies. For quasispecies complexity, we analyzed the RHL index, a new reliable diversity index to diagnose mutant spectrum expansions, such as may have occurred with the error-prone reverse transcription of HBV-RNA into DNA. However, although we detected a tendency to greater quasispecies complexity in HBV-DNA than in RNA, the differences were statistically non-significant, which may indicate an increased presence of low-frequency HBV genomes in DNA quasispecies. For this reason, we suggest that further studies in larger groups of patients be performed to confirm this observation. We also found a highly coincident conservation between HBV-RNA and DNA quasispecies in the HBX 5’ region. Interestingly, HBV-RNA quasispecies showed slightly higher variability than HBV-DNA quasispecies, which may indicate that only a fraction of packaged pgRNA is reverse-transcribed and released. The hyper-conserved sequences identified were highly coincident to what was observed in previous studies by our group in HBV-DNA quasispecies. Neverthe
In this study, data were obtained from patients with a broad range of HBV genotypes and degrees of severity of liver disease. This allowed us to make a preliminary comparison of complexity and conservation of HBV-DNA and RNA quasispecies. However, further studies with a larger sample size are warranted to confirm our results. The main goal of the methodology for HBV-RNA quasispecies described here is to use it in groups of patients where HBV-DNA levels are usually too low for PCR amplification (e.g., patients under NA treatment, since the generation of HBV-RNA is not inhibited by NA directly), in order to be able to monitor HBV quasispecies in those patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wu W S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | World Health Organization. Hepatitis B. Fact sheet. 2015; No204. [cited 20 March 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b. |
| 2. | Caballero A, Tabernero D, Buti M, Rodriguez-Frias F. Hepatitis B virus: The challenge of an ancient virus with multiple faces and a remarkable replication strategy. Antiviral Res. 2018;158:34-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Stadelmayer B, Diederichs A, Chapus F, Rivoire M, Neveu G, Alam A, Fraisse L, Carter K, Testoni B, Zoulim F. Full-length 5'RACE identifies all major HBV transcripts in HBV-infected hepatocytes and patient serum. J Hepatol. 2020;73:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Wang J, Yu Y, Li G, Shen C, Meng Z, Zheng J, Jia Y, Chen S, Zhang X, Zhu M, Song Z, Wu J, Shao L, Qian P, Mao X, Wang X, Huang Y, Zhao C, Zhang J, Qiu C, Zhang W. Relationship between serum HBV-RNA levels and intrahepatic viral as well as histologic activity markers in entecavir-treated patients. J Hepatol. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | van Bömmel F, Bartens A, Mysickova A, Hofmann J, Krüger DH, Berg T, Edelmann A. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology. 2015;61:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 6. | Yu XQ, Wang MJ, Yu DM, Chen PZ, Zhu MY, Huang W, Han Y, Gong QM, Zhang XX. Comparison of Serum Hepatitis B Virus RNA Levels and Quasispecies Evolution Patterns between Entecavir and Pegylated-Interferon Mono-treatment in Chronic Hepatitis B Patients. J Clin Microbiol. 2020;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 7. | Ji X, Xia M, Zhou B, Liu S, Liao G, Cai S, Zhang X, Peng J. Serum Hepatitis B Virus RNA Levels Predict HBeAg Seroconversion and Virological Response in Chronic Hepatitis B Patients with High Viral Load Treated with Nucleos(t)ide Analog. Infect Drug Resist. 2020;13:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: From discovery to regulatory approval. J Hepatol. 2017;67:847-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (1)] |
| 9. | Quer J, Rodríguez-Frias F, Gregori J, Tabernero D, Soria ME, García-Cehic D, Homs M, Bosch A, Pintó RM, Esteban JI, Domingo E, Perales C. Deep sequencing in the management of hepatitis virus infections. Virus Res. 2017;239:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Revill PA, Tu T, Netter HJ, Yuen LKW, Locarnini SA, Littlejohn M. The evolution and clinical impact of hepatitis B virus genome diversity. Nat Rev Gastroenterol Hepatol. 2020;17:618-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (2)] |
| 11. | Cao J, Luo S, Xiong Y. The Variability of Amino Acid Sequences in Hepatitis B Virus. Virol Sin. 2019;34:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3768] [Article Influence: 471.0] [Reference Citation Analysis (1)] |
| 13. | González C, Tabernero D, Cortese MF, Gregori J, Casillas R, Riveiro-Barciela M, Godoy C, Sopena S, Rando A, Yll M, Lopez-Martinez R, Quer J, Esteban R, Buti M, Rodríguez-Frías F. Detection of hyper-conserved regions in hepatitis B virus X gene potentially useful for gene therapy. World J Gastroenterol. 2018;24:2095-2107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Cortese MF, González C, Gregori J, Casillas R, Carioti L, Guerrero-Murillo M, Riveiro-Barciela M, Godoy C, Sopena S, Yll M, Quer J, Rando A, Lopez-Martinez R, Pacín Ruiz B, García-García S, Esteban-Mur R, Tabernero D, Buti M, Rodríguez-Frías F. Sophisticated viral quasispecies with a genotype-related pattern of mutations in the hepatitis B X gene of HBeAg-ve chronically infected patients. Sci Rep. 2021;11:4215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Durantel D, Carrouée-Durantel S, Werle-Lapostolle B, Brunelle MN, Pichoud C, Trépo C, Zoulim F. A new strategy for studying in vitro the drug susceptibility of clinical isolates of human hepatitis B virus. Hepatology. 2004;40:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Godoy C, Tabernero D, Sopena S, Gregori J, Cortese MF, González C, Casillas R, Yll M, Rando A, López-Martínez R, Quer J, González-Aseguinolaza G, Esteban R, Riveiro-Barciela M, Buti M, Rodríguez-Frías F. Characterization of hepatitis B virus X gene quasispecies complexity in mono-infection and hepatitis delta virus superinfection. World J Gastroenterol. 2019;25:1566-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Gregori J, Soria ME, Gallego I, Guerrero-Murillo M, Esteban JI, Quer J, Perales C, Domingo E. Rare haplotype load as marker for lethal mutagenesis. PLoS One. 2018;13:e0204877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 18. | Ou J, Wolfe SA, Brodsky MH, Zhu LJ. motifStack for the analysis of transcription factor binding site evolution. Nat Methods. 2018;15:8-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | The R Foundation. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Viena, Austria. 2020. [cited 13 July 2020]. In: The R Foundation [Internet]. Available from: https://www.r-project.org/. |
| 20. | World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. [cited 15 April 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/. |
| 21. | Cornberg M, Lok AS, Terrault NA, Zoulim F; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference‡. J Hepatol. 2020;72:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 22. | Liu S, Zhou B, Valdes JD, Sun J, Guo H. Serum Hepatitis B Virus RNA: A New Potential Biomarker for Chronic Hepatitis B Virus Infection. Hepatology. 2019;69:1816-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Liu Y, Jiang M, Xue J, Yan H, Liang X. Serum HBV RNA quantification: useful for monitoring natural history of chronic hepatitis B infection. BMC Gastroenterol. 2019;19:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Park SG, Kim Y, Park E, Ryu HM, Jung G. Fidelity of hepatitis B virus polymerase. Eur J Biochem. 2003;270:2929-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Jansen L, Kootstra NA, van Dort KA, Takkenberg RB, Reesink HW, Zaaijer HL. Hepatitis B Virus Pregenomic RNA Is Present in Virions in Plasma and Is Associated With a Response to Pegylated Interferon Alfa-2a and Nucleos(t)ide Analogues. J Infect Dis. 2016;213:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, Zhang R, Chen R, Li T, Zhang T, Yuan Q, Li PC, Huang Q, Colonno R, Jia J, Hou J, McCrae MA, Gao Z, Ren H, Xia N, Zhuang H, Lu F. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol. 2016;65:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 337] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 27. | Cortese MF, Garcia-Garcia S, Casillas R, Lopez-Martinez R, Pacin Ruiz B, Sopena S, Tabernero D, Ferrer-Costa RM, Riveiro-Barciela M, Buti M, Rodriguez-Frías F. Gene Silencing by GAPMERS: A New Therapeutic Tool in HBV Infection. Hepatology. 2020;72:504A-505A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |