Published online Oct 7, 2021. doi: 10.3748/wjg.v27.i37.6332
Peer-review started: February 18, 2021
First decision: April 18, 2021
Revised: April 30, 2021
Accepted: August 20, 2021
Article in press: August 20, 2021
Published online: October 7, 2021
Processing time: 222 Days and 17.2 Hours
Abdominal cocoon or “encapsulating peritoneal sclerosis” (EPS) is an uncommon and rare cause of intestinal obstruction. Only a few cases have been reported in paediatric patients. Typically, EPS is described as the primary form in young adolescent girls from tropical and subtropical countries because of viral peritonitis due to retrograde menstruation or a history of peritoneal dialysis. Most patients are asymptomatic or present with abdominal pain, which is likely to occur secondary to subacute bowel obstruction. Findings at imaging, such as ultra
We present a case of EPS in a 12-year-old boy 8 wk after primary surgery for resection of symptomatic jejunal angiodysplasia. There was no history of peritoneal dialysis or drug intake.
In this report, we sought to highlight the diagnostic, surgical, and histopathological characteristics and review the current literature on EPS in paediatric patients.
Core Tip: Abdominal cocoon syndrome is rare in children. Cases with no history of previous peritoneal dialysis are practically nonexistent. Our report of a 12-year-old boy reveals that abdominal surgery can be a trigger for the development of encapsulating peritoneal sclerosis (EPS). Indications for surgery for EPS are usually due to mechanical ileus, and the final diagnosis is made intraoperatively. Surgical therapy for EPS is the first choice, and total intestinal enterolysis of EPS seems to be the best approach. The long-term prognosis for children is good.
- Citation: Keese D, Schmedding A, Saalabian K, Lakshin G, Fiegel H, Rolle U. Abdominal cocoon in children: A case report and review of literature. World J Gastroenterol 2021; 27(37): 6332-6344
- URL: https://www.wjgnet.com/1007-9327/full/v27/i37/6332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i37.6332
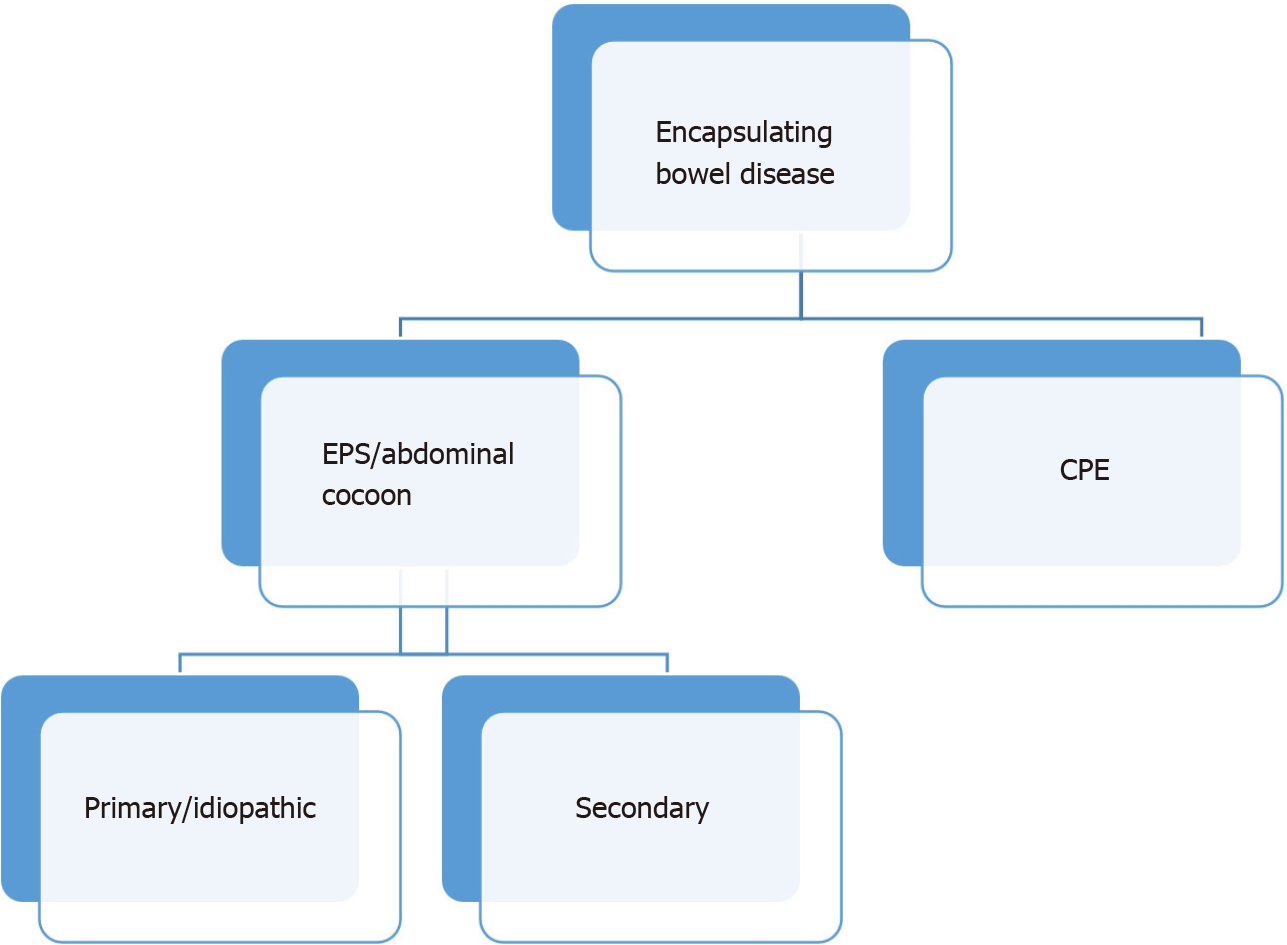
Encapsulating bowel disease is a very rare but serious condition. The differentiation between congenital peritoneal encapsulation (CPE) and encapsulating peritoneal sclerosis (EPS) is obvious. CPE, reported for the first time in 1868 by Cleland[1], is the result of an embryological intestinal malformation that occurs when the yolk sac returns to the abdominal cavity, creating an accessory membrane covering the bowel. This is to be distinguished from EPS, which is far more common than CPE and was first described in 1907 by Owtschinnikow[2] with the term “peritonitis chronica fibrosa incapsulata”. In 1987, Foo et al[3] used the term “abdominal cocoon” for primary or idiopathic EPS in cases where no established cause could be identified.
EPS has been recognized to occur in various geographically and ethnically diverse locations. In addition to various terms, such as “peritoneal sclerosis, peritoneal fibrosis, sclerosing peritonitis, sclerotic thickening of the peritoneal membrane, sclerosing obstructive peritonitis or encapsulating peritonitis”, EPS is the most common name[4]. Because inflammatory features are not always apparent, the use of the word peritonitis, as in the general terminology, is not preferred. ESP has been proposed as the appropriate term by the International Society for Peritoneal Dialysis when peritoneal dialysis is the reason for the ubiquitous inflammatory processes that cause cocoon formations. Today, most authors use the term abdominal cocoon not only for the primary or idiopathic form but also for the secondary form of EPS[5-8] (Figure 1). The primary type is classically described in young adolescent girls from tropical and subtropical countries, which is mostly idiopathic in nature. A few of the etiopathogenesis proposed for the primary form are viral peritonitis, retrograde menstruation with superimposed viral infection, and gynecological infection-inducing cell-mediated immunological tissue damage. However, these theories may not explain the etiopathogenesis in all patients, as this condition is also seen in men, premenstrual women, and children. The secondary form can occur secondary to multiple predisposing factors in addition to peritoneal dialysis, e.g., peritoneum inflammation with fibroblastic proliferation, systemic lupus erythematosus, liver cirrhosis, endometriotic cyst, abdominal trauma/surgery, abdominal tuberculosis, or malignancy[9,10].
EPS is characterized by total or partial encasement of the bowel within a thick fibrocollagenous membrane that envelopes the small intestine in the form of a cocoon as a result of chronic intraabdominal fibroinflammatory processes[11]. The result is the formation of fibrous tissue sheets that cover, fix, and ultimately constrict the gut, compromising its motility. Most patients are asymptomatic or present with (recurrent) abdominal pain, which is likely to occur secondary to partial/complete bowel obstruction. Usually, patients have had prior episodes with similar symptoms that have resolved spontaneously. With the development of complete sclerosis due to the formation of a cocoon, there are overt signs of mechanical ileus.
Clinically, the abdomen is often soft at palpation. A soft nontender mass may be palpable in the central part of the abdomen, which represents clumped-up bowel loops. Imaging findings seen on ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) are often nonspecific[7]. Other imaging modalities, such as barium studies or positron emission tomography studies, have been discussed and have not revealed any appreciable advantage in the diagnosis of EPS. Therefore, this condition is often only diagnosed intraoperatively, followed by histological analysis[12].
EPS has a clearly different histology than CPE. The thin membrane on the visceral peritoneum contributes to the formation of the intestinal encapsulation of EPS. Histologically, the membrane is comprised mainly of organized fibrin, probably derived from plasma exudation from the peritoneal microvasculature. Peritoneal fibroblasts appear swollen and exhibit an increased level of cellularity, accompanied by the expression of various activation and proliferation markers. Persistent inflammatory changes are also predictive of the onset of EPS[4].
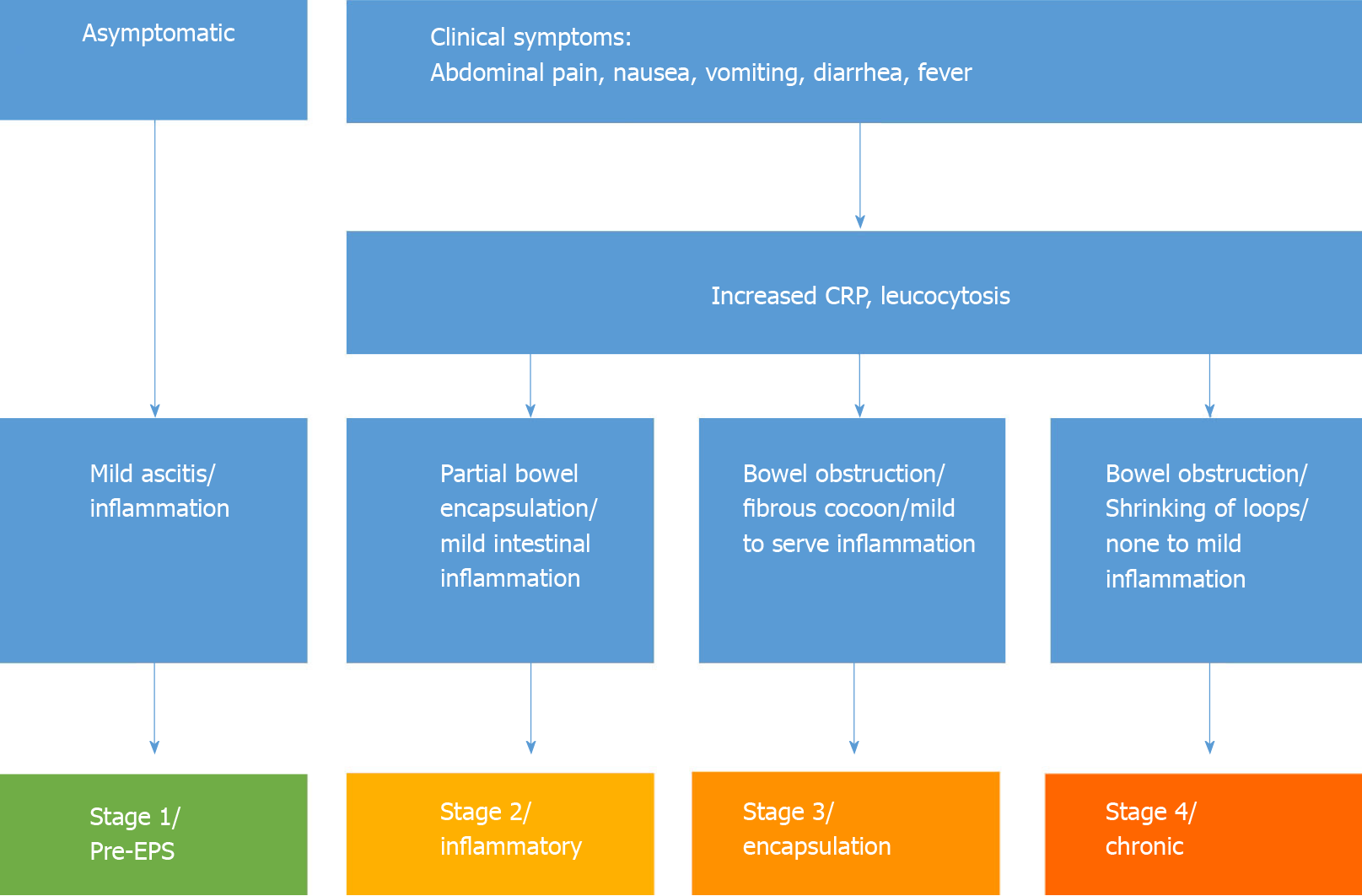
The development of EPS has been divided, taking into account clinical and pathological findings in four stages, as proposed by Nakamoto[13]. The pre-EPS stage is characterized by the presence of ultrafiltration failure and/or altered solute transport status; this is followed by inflammatory, encapsulating, and obstructive phases, the last of which is characterized by intestinal obstruction and the formation of an “abdominal cocoon”. A staging system has been proposed in the peritoneal catheter (PD)-associated EPS literature based on a combination of clinical, laboratory, and radiographic findings. Nakamoto[13] categorized patients with EPS into Stage 1 (pre-EPS), Stage 2 (inflammatory), Stage 3 (encapsulating), and Stage 4 (chronic) based on abdominal symptoms, inflammation, encapsulation, and intestinal findings (Figure 2). Different conservative therapeutic approaches have been proposed depending on the stage of the disease, which include nutritional support, immunosuppression with corticosteroids as the best studied, colchicine, azathioprine, mycophenolate mofetil, cyclosporine, and mammalian target of rapamycin. Furthermore, antifibrotics such as tamoxifen can increase the efficiency of immunosuppressive drugs.
The division into four disease stages enables stage-appropriate therapy for EPS. Table 1 shows the stage-dependent therapy of EPS according to Nakamoto[13]. For a long time, surgical treatment of EPS was not considered appropriate due to the high perioperative mortality rate of up to 70%. On the other hand, several studies showed that, independent of the therapy procedure, the overall mortality in EPS patients was 67%. In a recent study, the mortality in surgically treated patients was only 42%. This reduction in mortality can only be achieved by timely and adequate surgical therapy. However, to date, there is neither a uniform surgical therapy concept nor standardized perioperative management.
| Stage | Therapy options |
| Stage 1/pre-EPS | Discontinuation of pertioneal dialysis |
| ("Sedation" of the peritoneum) | |
| Peritoneal lavage | |
| Glucocorticoids | |
| Stage 2 | Glucocorticoids |
| Stage 3 | Glucocorticoids |
| Total parenteral nutrition | |
| Stage 4 | Operation |
To our knowledge, there has been no prior definitive, systematic review of EPS in children without a history of PD. Due to the assumption that younger patients are at greater risk for EPS, it is important to highlight the age groups of the patients.
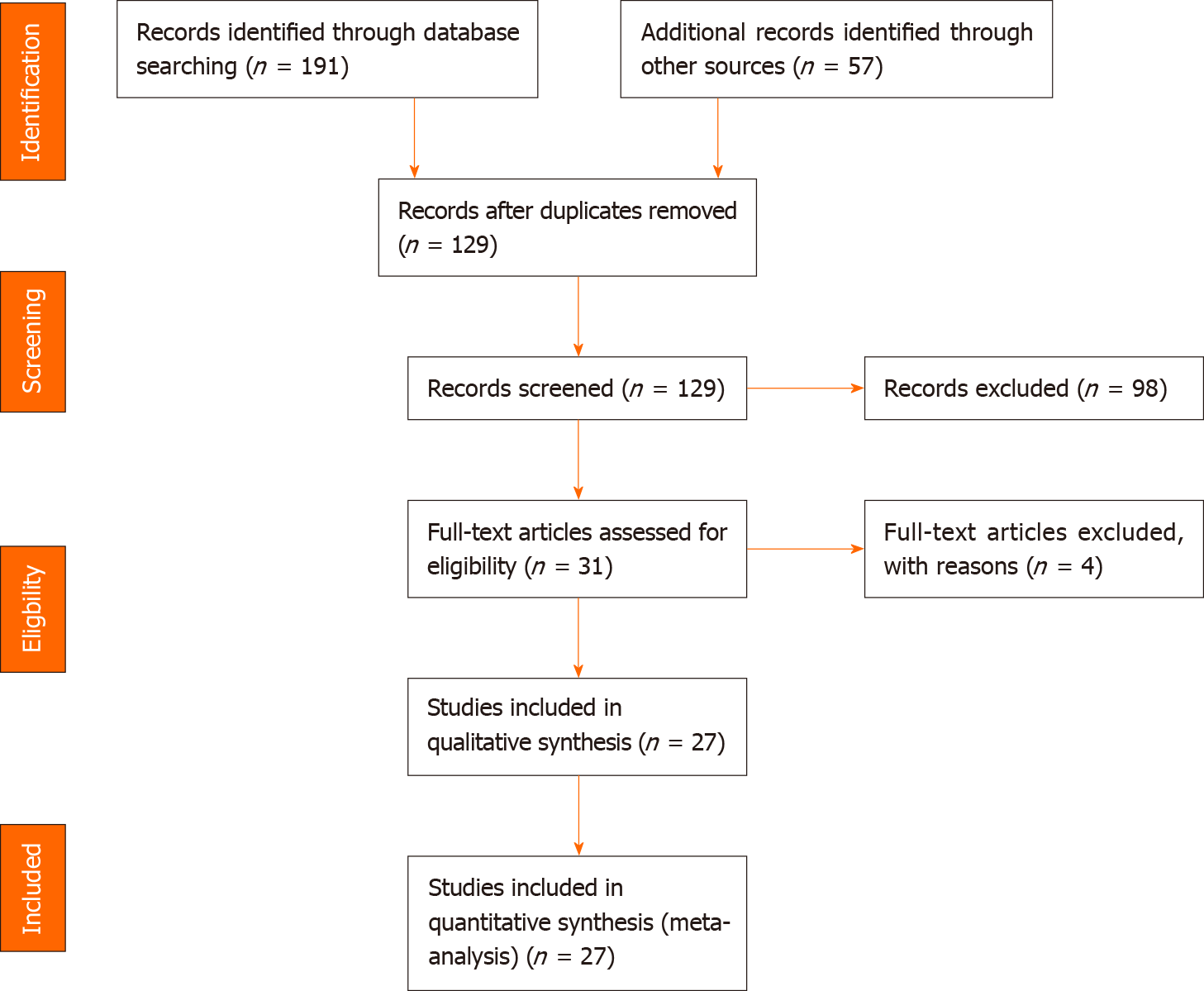
In the following, we present a case of a 12-year-old boy with EPS 8 wk after primary laparotomy. We report his medical history, clinical presentation at admission, laboratory values, preoperative diagnostic and intraoperative findings, histological results, and days until discharge (short-term outcomes). The main objective of the present study was to perform a systematic review of the literature to identify studies on EPS/abdominal cocoons in children. For review, we scanned PubMed, Medline, and Google Scholar search engines for articles containing the terms “EPS”, “abdominal cocoon”, “abdominal cocoon syndrome”, “encapsulating bowel disease”, and “SEP”, children and paediatric” published from January 1990 to January 2020. Terms were entered alone or in combinations.
We included all articles reporting case series or case reports in the English and German languages. All series and case reports reporting patients younger than 18 years were included (quantitative screening). Further eligibility criteria were a report of the clinical presentation, sex and age, operation as the treatment, and histopathological findings (qualitative screening). Data for all clinical, diagnostic, therapeutic, and etiologic findings were collected from the different databases. We included primary (idiopathic) and secondary forms [abdominal surgery, abdominal trauma, abdominal tuberculosis, peritoneal shunts, liver transplantation, recurrent peritonitis, beta-blocker treatment (practolol or propranolol), chemotherapy, gastrointestinal malignancy, systemic lupus erythaematosus, or parasitic infection] of EPS with the exception of EPS due to PD. We excluded PD-associated EPS because, as mentioned above, in these cases, ESP is the appropriate term with inflammatory processes in the cocoon, such as formation of the bowel. We then created a PRISMA flow chart showing the results of the literature search (Figure 3).
A 12-year-old boy with Xq24 deletion syndrome presented with symptoms of intermittent intestinal obstruction 8 wk after primary surgery.
The child was symptomatic with a distended abdomen, refusal of feeds, and episodes of abdominal pain. Furthermore, he presented with large gastric residuals that became bilious in the next week.
Eight weeks previously, he presented with suspected intestinal bleeding without further symptoms. US, abdominal MRI, endoscopy, and laparoscopy did not reveal the cause of the chronic bleeding. Specialized angiographic MRI was suspicious for a small intraluminal vascular tumour in the ileum. Limited laparotomy and meticulous palpation of the small bowel enabled the identification of the tumour in the lower ileum. Limited small bowel resection and primary anastomosis were performed. Further clinical investigation of the whole small and large bowel did not reveal any further pathological findings. Histopathological examination showed a venous malformation (2.5 cm × 2.0 cm) at the transition of the jejunum to the ileum. The following clinical course was uneventful. The boy was discharged on the 5th postoperative day.
Except for Xq24 deletion syndrome, there was no other personal or family history of acute or chronic disease.
On physical examination, the patient showed a distended abdomen and abdominal pain. The examination of the heart, lungs, and further organ status showed no abnormalities. Due to his Xq24 deletion syndrome, he showed psychomotor delay, intellectual disability, and a characteristic craniofacial dysmorphism.
Laboratory findings showed slightly elevated C-reactive protein (0.3 mg/dL) and normal findings across the rest of the blood panel.
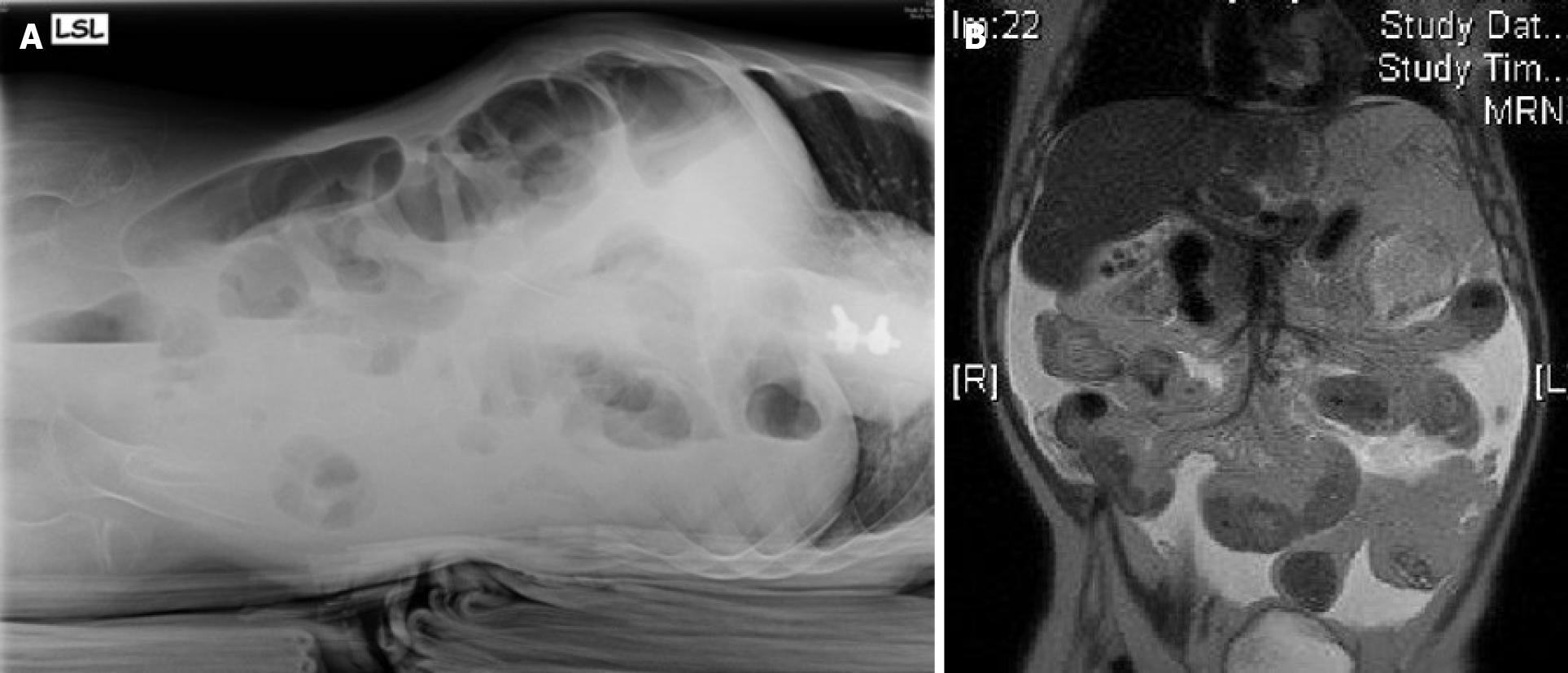
The abdominal US showed pendulum peristalsis and free fluid in the abdominal cavity, and a plain abdominal X-ray showed some air fluid levels (Figure 4A). A gastrointestinal contrast study was performed, which revealed distended small bowel loops (maximum diameter of 50 mm) and narrow segments of the large intestine. There was appropriate intestinal transit, with the presence of contrast in the jejunal and ileal loops after 1 h of administration. The contrast agent arrived at the terminal ileum and caecum at 2 h of intestinal transit. Due to the inconclusive results, we performed MRI, which revealed a distended duodenum and small bowel loops with multiple intraluminal air fluid levels and thickened walls but with no signs of obstruction (Figure 4B).
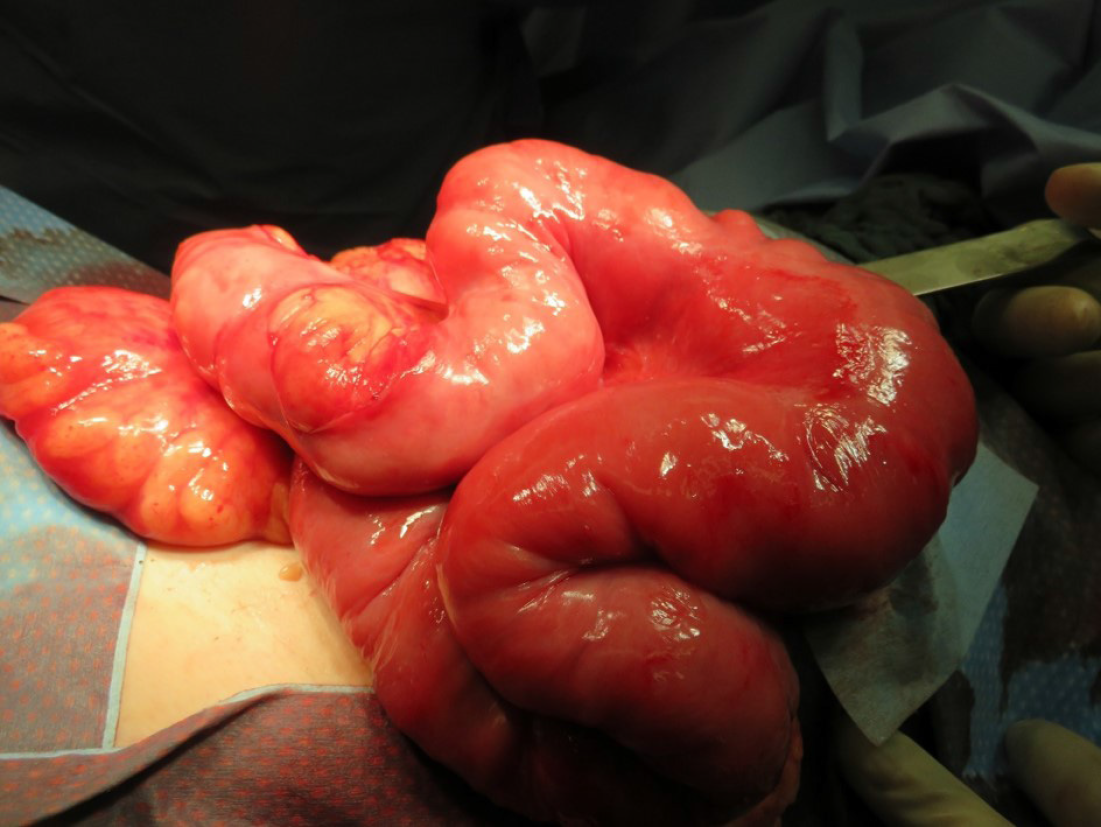
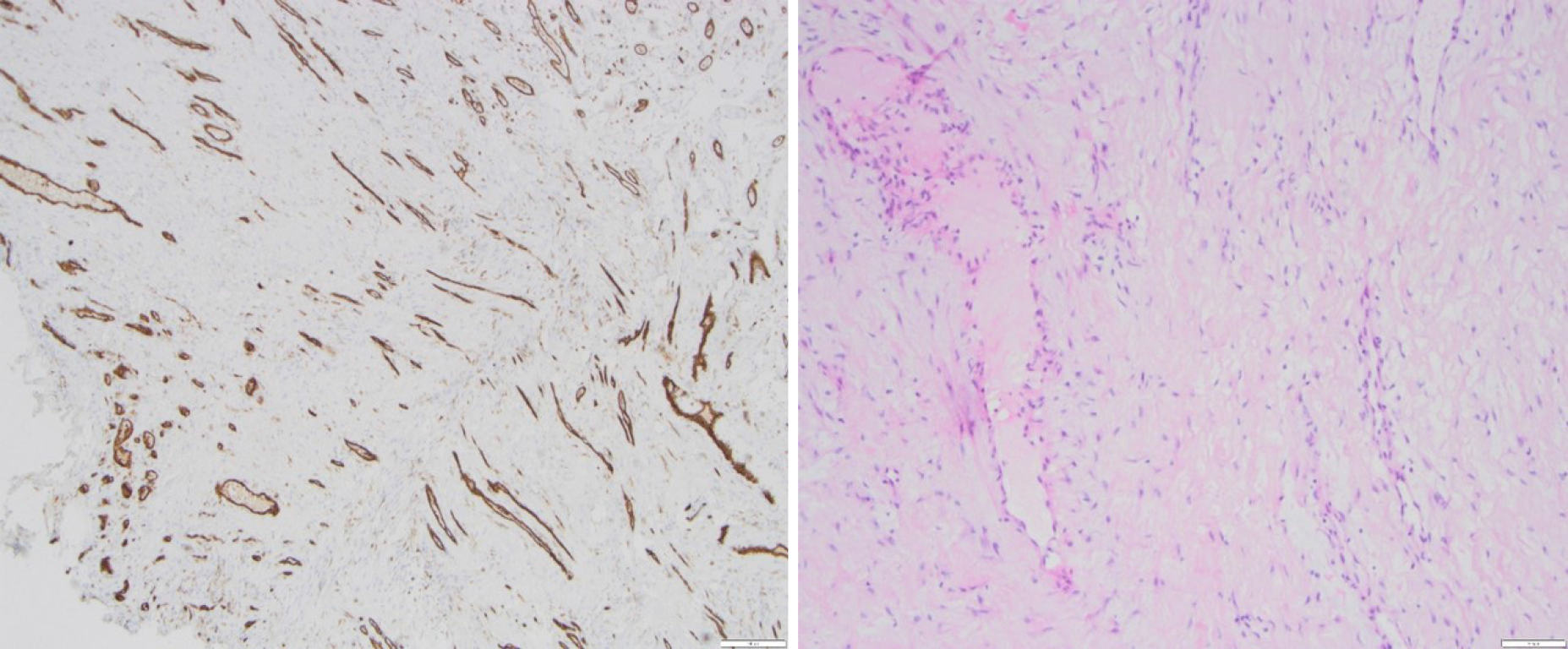
Due to the clinical symptoms of obstructive bowel disease and inconclusive radiological signs, the patient underwent relaparotomy. Intraoperatively, we found a completely encapsulated small bowel (Figure 5). The massive, fibroconnective membrane encapsulated the bowel from the ligament of Treitz to the ileocecal junction. The thick, firm, and white membrane was not attached to the parietal peritoneum. Microscopic findings showed partially mesothelium-covered, predominantly edematous loosened fibre-rich connective tissue with prominent, predominantly small vessels with a prominent endothelial lining with hyperchromatic oval nuclei, mostly positive for CD31 and CD34. No evidence of malignancy was found (Figure 6).
We performed extensive adhesiolysis by peeling the fibrous pseudocapsulae from the small bowel. This resulted in freeing of the small bowel but also in multiple serosal injuries with diffuse bleeding. No bowel perforation occurred.
The following clinical course was prolonged due to peritonitis, massive ascites, and paralytic ileus. Eventually, 2 wk after surgery, the first feeds could be established, and the child was discharged on the 23rd postoperative day. The follow-up is now 12 mo and uneventful. A summary of the most relevant aspects of the case is listed in Table 2.
| Age | Clinical symptoms | Labatory findings | Intraoperive findings | Histology |
| 11 yr | Abdominal pain; Distended abdomen; Vomiting (bilious) | CRP: 0.3 mg/dL | Complete; encapsulation of the small bowel | Fibrous connective tissue with prominent vas-cularization, predomi-nantly from the venous type |
Articles identified after reviewing and applying the inclusion criteria are listed in Table 3. No comparable case could be found in children presenting with EPS after surgery. Sahoo et al[14] reported EPS as an idiopathic form in a case series of 4 patients (3 boys, 1 girl) between 6 and 8 years of age, and Mehta et al[15] and Bassiouny et al[16] reported EPS in a 7-year-old boy without underlying disease. Ahmad et al[17] mentioned a case report of EPS in a newborn as a consequence of meconium peritonitis. In total, 27 articles could be found reporting 33 patients (Table 3). Among these patients, 24 were girls and 9 were boys. Their ages ranged from 2 d to 17 years. Especially in older patients (≥ 10 years of age), EPS was detected more often in girls (f:m = 22:2). Various radiological modalities for diagnosis (singly or in addition) were reported, such as abdominal X-rays (n = 21), US (n = 18), contrast studies (n = 8), and CT scans (n = 11). In three cases, no diagnostic tool was mentioned. Thirty-two patients underwent surgery (laparotomy), while 1 patient underwent conservative treatment with antitubercular medications. When laparotomy was performed, adhesiolysis and excision was the treatment of the choice (n = 32). Two patients underwent appendectomy simultaneously, and two patients underwent temporary enterostomy due to pronounced bowel inflammation. In 3 patients, limited bowel resection, including the ileocecal region, was performed since it was too difficult to incise and release the envelope without injuring the intestines. In all reported cases with surgical treatment, the operation and postoperative period were uneventful.
| Ref. | Age/sex | Clinical presantation | Diagnostic | Etiology | Treatment |
| Ahmad et al[17], 2013 | 2 d/m | Polyhydramnion, vomiting, abdominal distension, failure to pass meconium | US, X-ray | Meconium peritonitis | Surgery |
| Sahoo et al[14] 1996 | 6 yr/m | Abdominal pain, bilious vomiting | US, X-ray, BMFT | Idiopathic | Surgery |
| Mehta et al[15], 1994 | 7 yr/m | Colicky periumbilical pain, vomiting, constipation | US, X-ray | Idiopathic | Surgery |
| Bassiouny et al[16], 2011 | 7 yr/m | Crampy abdominal pain | X-ray, contrast enema | Idiopathic | Surgery |
| Sahoo et al[14], 1996 | 6 yr/m | Constipation, on-and-off vomiting | US, X-ray, BMFT | Idiopathic | Surgery |
| Tolstrup et al[35], 2017 | 8 yr/f | Signs of enteritis | CT | Idiopathic | Surgery |
| Sahoo et al[14], 1996 | 8 yr/f | Intermittent bilious vomiting, abdominal distension | BMFT | Idiopathic | Surgery |
| Sahoo et al[14], 1996 | 8 yr/m | Colicky abdominal pain, vomiting, constipation | X-ray | Idiopathic | Surgery |
| Kaushik et al[10], 2006 | 9 yr/m | Abdominal pain, vomiting, constipation | - | Abdominal tuberculosis | Surgery (with temp. enterostomy) |
| Okobia et al[37], 2001 | 10 yr/f | Abdominal pain, abdominal distension, weight loss | X-ray | Idiopathic | Surgery (with appendectomy) |
| Bassiouny et al[16], 2011 | 12 yr/f | Colicky abdominal pain, vomiting | US, X-ray | Idiopathic | Surgery |
| Okobia et al[37], 2001 | 12 yr/f | Colicky abdominal pain, abdominal distension | US, X-ray | Idiopathic | Surgery (with appendectomy) |
| Dehn et al[29], 1985 | 12 yr/f | Abdominal pain, bilious vomiting | US, X-ray | Idiopathic | Surgery |
| Kayastha et al[23], 2012 | 13 yr/f | Abdominal pain in tright iliac region, vomiting | US | Idiopathic | Surgery |
| Sreevathsa et al[33], 2013 | 13 yr/f | Colicky central abdominal pain | US, X-ray, BMFT | Idiopathic | Surgery (with ileocaecal resection) |
| Pillai et al[26], 2006 | 13 yr/f | Abdominal pain, vomiting | US, X-ray, CT | Idiopathic | Surgery |
| Chatura et al[40], 2012 | 14 yr/f | Colicky abdominal pain | US | Idiopathic | Surgery (with ileocolectomy) |
| Ibrahim et al[41], 2009 | 14 yr/m | Colicky abdominal pain, vomiting, constipation | X-ray | Idiopathic | Surgery (with appendectomy) |
| Calvo et al[42], 2015 | 14 yr/f | Colicky abdominal pain, bilious vomiting, weight loss | US, CT | Idiopathic | Surgery |
| Shah et al[43], 2013 | 14 yr/f | Colicky abdominal pain, vomiting | CT / BMFT | Idiopathic | Surgery |
| Sreevathsa et al[33], 2013 | 14 yr/f | Colicky central abdominal pain | US, X-ray, BMFT | Idiopathic | Surgery (with ileocaecal resection) |
| Kumar et al[44], 2012 | 14 yr/f | Colicky abdominal pain, vomiting | US, CT | Idiopathic | Conservatively |
| Yucel et al[53], 2011 | 15 yr/f | Abdominal pain | X-ray, CT | Idiopathic | Surgery |
| Raju[45], 2004 | 15 yr/f | Recurrent abdominal pain | X-ray, contrast study | Idiopathic | Surgery |
| Ndiaye et al[46], 2012 | 15 yr/f | Subocclusive obstruction, loss of weight (11kg) | CT | Idiopathic | Surgery |
| Choudhury et al[47], 2009 | 15 yr/f | Crampy abdominal pain right abdomen, weight loss | - | Idiopathic | Surgery (with partial ileocolic resection) |
| Mordehai et al[48], 2001 | 15 yr/f | Crampy abdominal pain | US, X-ray | Idiopathic | Surgery |
| Mohanty et al[49], 2009 | 15 yr/f | Colicky abdominal pain, vomiting | US, X-ray, CT | Idiopathic | Surgery |
| Kumar et al[36], 2009 | 16 yr/f | Abdominal pain in the left lower abdomen, vomiting | US, X-ray, CT | Idiopathic | Surgery |
| Jayant et al[50], 2011 | 16 yr/f | Small bowel obstruction | CT | Idiopathic | Surgery |
| Kaushik et al[10], 2006 | 17 yr/m | Abdominal pain, vomiting, constipation | - | Abdominal tuberculosis | Surgery (with temp. enterostomy) |
| Naniwadekar et al[51], 2014 | 17 yr/f | Abdominal pain, vomiting, constipation | US, X-ray, GI scopy | Idiopathic | Surgery |
| Kaur et al[52], 2012 | 17 yr/f | Recurrent abdominal pain | US, X-ray, CT | Idiopathic | Surgery |
The clinical picture of EPS is largely unknown in the medical world[18]. The embryonal condition is characterized by an accessory peritoneal sac derived from the peritoneum of the yolk sac as it is withdrawn into the abdominal cavity during the 12th week of gestation[3]. Only a few risk factors are known[9]. This disease can occur as a late consequence of long-term peritoneal dialysis[19-23]. However, even this is not necessarily always the case, since cases are known in which EPS is already visible shortly after initiation of PD therapy[11]. In these cases, the mortality depends on the duration of PD, ranging between 30% and 60%. In other cases where EPS is not associated with PD, various reports have reported beta blockers as the cause of EPS[10]. In animal models, practolol inhibits the release of surfactant from type II pneumocytes, resulting in damage to the abdominal serosa[24]. Tumours with peritoneal dissemination and infectious peritonitis are other frequently mentioned risk factors for EPS, supported by epidemiological data. Pathogens significantly increase the risk, highlighting Staphylococcus aureus, fungi, Pseudomonas spp., and Haemophilus influenza[3,4,10]. Fowler et al[25] suggested echovirus infection as an etiologic factor, and Foo et al[3] thought tuberculosis could be involved.
Several theories have been formulated about the pathogenesis of idiopathic EPS[26], for example, retrograde menstruation due to virus infection or pelvic peritonitis with tissue damage incurred by cell-mediated immunity[3,5,11]. Retrograde menstruation explains the relatively high number of adolescent girls with idiopathic abdominal cocoons (Table 3). Moreover, several tumour entities and/or abdominal operations may lead to adhesions and peritoneal fibrosis[27-29]. Therefore, overall, the most common theory is developing a membrane in the form of sclerosis secondary to peritonitis[17,18]. Prospectively controlled randomized studies are lacking, and most studies on EPS present small patient collections over a long period of time[10-13].
The presented case is very unusual because of the child’s young age, the atypical geographical region, and a very rare cause (secondary to abdominal surgery). Most of the other publications with paediatric patient collections are secondary forms after tuberculosis infection or after prolonged PD. The short time interval to the primary laparotomy in our patient is a special feature that has not been described in the literature thus far. EPS after kidney transplantation (as an abdominal surgical intervention) has been reported in case reports; however, these patients also had a history of PD or chronic kidney diseases[30]. In many cases of idiopathic EPS, no correlation can be detected with any potential risk factor[4,31-33]. Nevertheless, two considerations are important: First, in these forms, peritoneal disorders are often associated with general connective tissue damage, particularly of the serous membranes, such as abdominal trauma; this indicates a major role of immunopathogenesis in EPS. The second factor can be a genetic predisposition, which is suggested due to the high frequency of EPS in women from subtropical regions and familial forms such as multifocal family fibrosclerosis.
In reports including patients with long-term PD, pathogenesis is often described with the “two-hit”-hypothesis[3,4] when disruption of normal peritoneal/mesothelial physiology is a result of extensive PD over a period of years and predisposes the individual to a second hit that triggers the process. This second hit may take the form of an episode of peritonitis, discontinuing PD, or an acute intra-abdominal event. In our case, this hypothesis is unlikely because he was a non-PD patient, and there was a very short period between the two events. Therefore, the reason for the development of EPS in our patient must be found in the previous operation with resection of lymphangioma as an abdominal trauma and trigger for sclerosis. Another reason for developing EPS could be cystic lymphangioma of the small bowel itself as a benign vascular tumour with peritoneal dissemination. Garosi[19] and Célicout et al[34] described different tumour entities in connection with EPS in patients without PD, including histiocytic lymphoma, gastric, ovarian, pancreatic and renal cell carcinoma, and oedematous polyposis of the colon. Cystic lymphangioma as a trigger for EPS could not be found in the literature thus far. Because the lymphangioma was resected in total during the first operation, we believe that primary abdominal surgery was the trigger for EPS in our patient.
Because the early clinical features are often nonspecific[35,36], it is difficult to make a definite preoperative diagnosis of EPS. There are no specific serological markers for EPS. Ultrasonography may show dilated small bowel loops, ascites with or without parietal loculations, and a membrane covering the small bowel loops with occasional formation of a mass of bowel loops and adhesions[37]. Abdominal X-rays have a low sensitivity, showing mostly dilated small bowel loops with multiple air fluid levels. Peritoneal and bowel wall calcification has also been reported. Abdominal CT has a higher sensitivity (70%-90%) to detect the etiology of high-grade small bowel obstruction but should be used cautiously due to radiation risks in young patients. In our search of the literature, MRI has not been reported in paediatric patients with abdominal cocoons. Nevertheless, MRI has been recently reported as another useful modality in diagnosing EPS and may be a preferred technique in children.
In the international literature, two surgical therapy approaches for EPS have been described but were only reported in studies within adult patient collections. In 1998, Célicout et al[34] published a study in which 3 of 32 patients had dialysis-associated EPS. In the remaining patients, the EPS was the result of a malignancy (14 of 32) or due to b-blocker intake (5 of 32). The indication for the operation was, in all cases, the ultima ratio after long-lasting ileus disease. Japanese reports are based on studies that only included PD patients[38]. The intraoperative approach in Japan includes removal of the interenteric adhesions and the detachment of the encapsulating small intestinal membranes. The occlusive membranes on the intestine are dissected through longitudinal incisions. However, peritoneal sclerosis makes it difficult to identify the "right" layer, so special attention must be paid to serosa lesions. These are insidious because they can lead to occult perforations postoperatively with an increase in small bowel motility and the resulting increased pressure. In case of doubt, any altered segments of the small intestine are therefore resected. In Japan, in suitable cases, solitary stenoses are dilated with a Miller-Abott probe from the intraluminal side. Thus, even tandem stenoses can be corrected without resection. The balloon is deflated after completion of the dilatation and left in situ for splinting of the intestine. Usually, the probe is removed after 1 wk. In the opinion of the Japanese authors, the Noble plicature can prevent reobstruction by kinking as well as the reoccurrence of adhesions or entrapment of the small intestine in the Douglas cavity or in the excavation rectovesicalis, respectively.
The use of probes for intestinal splinting and the methods of plication have been discussed very controversially in Germany and have only historical significance. In our opinion, these methods, which carry their own risk, are not reliable enough to prevent the recurrence of ileus disease. In an international comparison, Japan has the most experience with EPS.
In addition to total intestinal enterolysis, which was performed in all cases with surgical treatment, deserosation, and decapsulation, peritonectomies, fibrin membranectomy, and omentectomies were performed in accordance with the findings. Additionally, an opening of the bursa omentalis for the decompression of fluid or for complete unleashing of the stomach may be essential.
There are hardly any reports from the Anglo-American region regarding surgical therapy for EPS. Studies from Australia[39] and Canada[8] mostly refer to con
In work published in 1998 by Célicout et al[34], the authors showed a mortality rate of 19% and an anastomosis failure rate of 12%. The total complication rate of 38% was the lowest at that time in the literature but was still considered too high[21]. Ten years later, Kawaguchi et al[5] published a retrospective study that included 130 surgically treated EPS patients. The reported mortality was 6.9%. However, the recurrence rate was very high (25.4%). Of the 130 patients, 33 had to be reoperated on for recurrence or postoperative ileus, and some patients underwent reoperations up to six times. In our reported case, as well as in the reports with paediatric patients, there were no complications after the operation, and no recurrence was described.
A few histological features, such as fibrin deposits, fibroblast swelling, angiogenesis, and mononuclear inflammatory infiltrate, differentiate peritoneal fibrosis from actual EPS. Since this means that even fewer predictive histological features are available, the German Peritoneal Biopsy Register was established at the initiative of the German Society of Nephrology. Here, we present prospective histomorphology characteristics of peritoneal biopsies with the aim of analysing the pathogenesis of the disease and producing reproducible algorithms for histological diagnosis confirmation. Therefore, the histological evaluation of EPS can now be increasingly based on standardized criteria. The Institute of Pathology at the Robert Bosch Hospital has become the reference centre for the histomorphology evaluation of peritoneal biopsies of the "European EPS Working Group”. Our histological results showed fibrous connective tissue with prominent vascularization, predominantly from the venous type, matching an early form of EPS.
The diagnosis of EPS is mainly made intraoperatively, and surgery is often indicated due to symptomatic ileus. A history of abdominal trauma, surgery, or abdominal tumour can be a trigger for developing EPS. Surgery, including adhesiolysis and excision, is the first choice of treatment. Total intestinal enterolysis of EPS is the best surgical approach. The long-term prognosis for children is good.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: German Association of Paediatric Surgery; EUPSA; WOFAPS.
Specialty type: Surgery
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mayr J, Wani I S-Editor: Liu M L-Editor: Filipodia P-Editor: Liu JH
| 1. | Cleland. On an Abnormal Arrangement of the Peritoneum, with Remarks on the Development of the Mesocolon. J Anat Physiol. 1868;2:201-206. [PubMed] |
| 2. | Owtschinnikow PJ. Peritonitis chronica fibrosa incapsulata. Arch für Klinische Chirurgie. 1907;83:623-634. |
| 3. | Foo KT, Ng KC, Rauff A, Foong WC, Sinniah R. Unusual small intestinal obstruction in adolescent girls: the abdominal cocoon. Br J Surg. 1978;65:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 205] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Allam H, Al Yahri O, Mathew S, Darweesh A, Suliman AN, Abdelaziem S, Khairat M, Toro A, Di Carlo I. The enigma of primary and secondary encapsulating peritoneal sclerosis. BMC Surg. 2016;16:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. 2000;20 Suppl 4:S43-S55. [PubMed] |
| 6. | Kirshtein B, Mizrahi S, Sinelnikov I, Lantsberg L. Abdominal cocoon as a rare cause of small bowel obstruction in an elderly man: report of a case and review of the literature. Indian J Surg. 2011;73:73-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Varun, Arunagiri. An Observational study of patients undergoing conservative Vs surgical management for adhesive small bowel obstruction. Masters thesis, Kilpauk Medical College, 2014. |
| 8. | Devabalan TK, Nadarajan AR, Roopavathana B, Chase S, Nayak S, Eapen A. Primary and secondary abdominal cocoon- diagnostic and management challenges: retrospective study. Int Surg J. 2019;6:1360-1367. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Brown MC, Simpson K, Kerssens JJ, Mactier RA; Scottish Renal Registry. Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol. 2009;4:1222-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Kaushik R, Punia RP, Mohan H, Attri AK. Tuberculous abdominal cocoon--a report of 6 cases and review of the Literature. World J Emerg Surg. 2006;1:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Mandavdhare HS, Kumar A, Sharma V, Rana SS. Abdominal cocoon: An enigmatic entity. Tropical Gastroenterol. 2016;37:156-167. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Singhal M, Krishna S, Lal A, Narayanasamy S, Bal A, Yadav TD, Kochhar R, Sinha SK, Khandelwal N, Sheikh AM. Encapsulating Peritoneal Sclerosis: The Abdominal Cocoon. Radiographics. 2019;39:62-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Nakamoto H. Encapsulating peritoneal sclerosis--a clinician's approach to diagnosis and medical treatment. Perit Dial Int. 2005;25 Suppl 4:S30-S38. [PubMed] |
| 14. | Sahoo SP, Gangopadhyay AN, Gupta DK, Gopal SC, Sharma SP, Dash RN. Abdominal cocoon in children: a report of four cases. J Pediatr Surg. 1996;31:987-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Mehta MH, Patel RV, Patel CK, Balar NN. Peritoneal encapsulation and abdominal cocoon in a male child. Pediatr Surg Int. 1994;9:415-416. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Bassiouny IE, Abbas TO. Small bowel cocoon: a distinct disease with a new developmental etiology. Case Rep Surg. 2011;2011:940515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Ahmad S, Kayastha K, Javed S, Wasti A. Abdominal cocoon secondary to meconium peritonitis in a neonate: a case report. J Neonatal Surg. 2013;2:12. [PubMed] |
| 18. | Cai J, Wang Y, Xuan Z, Hering J, Helton S, Espat NJ. The abdominal cocoon: a rare cause of intestinal obstruction in two patients. Am Surg. 2007;73:1133-1135. [PubMed] |
| 19. | Garosi G. Different aspects of peritoneal sclerosis. Contrib Nephrol. 2003;18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Nomoto Y, Kawaguchi Y, Kubo H, Hirano H, Sakai S, Kurokawa K. Sclerosing encapsulating peritonitis in patients undergoing continuous ambulatory peritoneal dialysis: a report of the Japanese Sclerosing Encapsulating Peritonitis Study Group. Am J Kidney Dis. 1996;28:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 177] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Nakayama M, Yamamoto H, Ikeda M, Hasegawa T, Kato N, Takahashi H, Otsuka Y, Yokoyama K, Yamamoto R, Kawaguchi Y, Hosoya T. Risk factors and preventive measures for encapsulating peritoneal sclerosis--Jikei experience 2002. Adv Perit Dial. 2002;18:144-148. [PubMed] |
| 22. | Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol. 2015;21:675-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (2)] |
| 23. | Kayastha K, Mirza B. Abdominal cocoon simulating acute appendicitis. APSP J Case Rep. 2012;3:8. [PubMed] |
| 24. | Xia J, Xie W, Chen L, Liu D. Abdominal cocoon with early postoperative small bowel obstruction: A case report and review of literature in China. Medicine (Baltimore). 2018;97:e11102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Fowler R. Primary peritonitis: changing aspects 1956-1970. Aust Paediatr J. 1971;7:73-83. [PubMed] |
| 26. | Pillai J, Kumar S, Praneena. Idiopathic abdominal cocoon. Indian J Radiol Imaging. 2006;16:483-485. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Marusawa H, Katsurada A, Takaya H, Kumegawa Y, Kajimura K, Yamashita Y. A case of encapsulating peritonitis associated with pancreatic ascites induced by carcinoma of the pancreas. Nihon Shokakibyo Gakkai Zasshi. 1996;93:932-936. [PubMed] |
| 28. | Stenram U. Sclerosing peritonitis in a case of benign cystic ovarian teratoma. A case report. APMIS. 1997;105:414-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Dehn TC, Lucas MG, 39Wood RF. Idiopathic sclerosing peritonitis. Postgrad Med J. 1985;61:841-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Sufrin G, Chasan S, Golio A, Murphy GP. Paraneoplastic and serologic syndromes of renal adenocarcinoma. Semin Urol. 1989;7:158-171. [PubMed] |
| 31. | Dehner LP, Coffin CM. Idiopathic fibrosclerotic disorders and other inflammatory pseudotumors. Semin Diagn Pathol. 1998;15:161-173. [PubMed] |
| 32. | Saied GA, Hassan AZ, Ossip M. Idiopathic sclerosing encapsulating peritonitis. Case report and review of literature. European Surg. 2010;42:103-106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Sreevathsa MR, Harsha AH. Chronic encapsulating peritonitis or cocoon abdomen. Trop Gastroenterol. 2013;34:204-206. [PubMed] |
| 34. | Célicout B, Levard H, Hay J, Msika S, Fingerhut A, Pelissier E. Sclerosing encapsulating peritonitis: early and late results of surgical management in 32 cases. French Associations for Surgical Research. Dig Surg. 1998;15:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Tolstrup J, Lauritsen ML. [Abdominal cocoon syndrome in an eigth-year-old girl caused acute bowel obstruction]. Ugeskr Laeger. 2017;179:V05170380. [PubMed] |
| 36. | Kumar A, Ramakrishnan TS, Sahu S, Mishra KB. Idiopathic sclerosing encapsulating peritonitis--is a preoperative diagnosis possible? Surg Today. 2009;39:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Okobia Evbuomwan NM, Osime U, Okonofua FE. The abdominal Cocoon – A report of three cases and reviews of literature. Nigerian J Clin Practice. 2001;4:100-103. |
| 38. | Kawaguchi Y, Saito A, Kawanishi H, Nakayama M, Miyazaki M, Nakamoto H, Tranaeus A. Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit Dial Int. 2005;25 Suppl 4:S83-S95. [PubMed] |
| 39. | Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant. 1998;13:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 222] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Chatura RK, Nayak VJ. Abdominal cocoon: case report of a rare cause of intestinal obstruction. Indian J Pathol Microbiol. 2012;55:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Ibrahim NA, Oludara MA. Abdominal cocoon in an adolescent male patient. Trop Doct. 2009;39:254-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Calvo PA, Marta DDS, Isnard Blanchara RM, Ojanguren Sabánb I, Castellví Gil A. Abdominal cocoon syndrome: A diagnostic and therapeutic challenge. Case report. Cir Esp. 2015;93:e61-e62. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Shah MY, Gedam BS, Sonarkar R, Gopinath KS. Abdominal cocoon: an unusual cause of subacute intestinal obstruction. Indian J Surg. 2013;75:391-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Kumar J, Garg A, Chowdhury V, Prakash A, Singh S. Abdominal cocoon--a rare case of intestinal obstruction. A report of two cases. Arab J Gastroenterol. 2012;13:188-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Raju GS. Abdominal cocoon due to primary peritonitis: barium meal is valuable in diagnosis. Trop Gastroenterol. 2004;25:80-81. [PubMed] |
| 46. | Ndiaye AR, Mbengue A, Soko TO, Diémé EP, Diagne NM, Diouf CT, Fall A, Fall F, Diop Y, Diakhaté IC. Idiopathic sclerosing encapsulating peritonitis: a case in an adolescent girl. Diagn Interv Imaging. 2012;93:629-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Choudhury T, Kamal M. Abdominal Cocoon - A Case Report with Short Review of Literature. BSMMU J. 2009;2:81-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Mordehai J, Kleiner O, Kirshtein B, Barki Y, Mares AJ. Peritoneal encapsulation: a rare cause of bowel obstruction in children. J Pediatr Surg. 2001;36:1059-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Mohanty D, Jain BK, Agrawal J, Gupta A, Agrawal V. Abdominal cocoon: clinical presentation, diagnosis, and management. J Gastrointest Surg. 2009;13:1160-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Jayant M, Kaushik R. Cocoon within an abdominal cocoon. J Surg Case Rep. 2011;2011:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Naniwadekar RG, Kulkarni SR, Bane P, Agrarwal S, Garje A. Abdominal cocoon: an unusual presentation of small bowel obstruction. J Clin Diagn Res. 2014;8:173-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Kaur R, Chauhan D, Dalal U, Khurana U. Abdominal cocoon with small bowel obstruction: two case reports. Abdom Imaging. 2012;37:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Yucel AF, Kocakusak A, Arikan S, Demirbag N, Tarlaci A, Batur S. A rare cause of acute abdomen: perforated primary sarcomatoid carcinoma of the small intestine - report of a case, with a brief review of the literature. J Cancer Res Ther. 2011;7:348-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |