Published online Oct 7, 2021. doi: 10.3748/wjg.v27.i37.6248
Peer-review started: March 2, 2021
First decision: April 17, 2021
Revised: April 29, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: October 7, 2021
Processing time: 211 Days and 1 Hours
Shigella flexneri (S. flexneri) is a major pathogen causing acute intestinal infection, but the systematic oxidative damage incurred during the course of infection has not been investigated.
To investigate the incurred systemic RNA oxidative damage and the diagnostic value of RNA oxidative metabolites during S. flexneri-induced intestinal infection.
In this study, a Sprague-Dawley rat model of acute intestinal infection was established by oral gavage with S. flexneri strains. The changes in white blood cells (WBCs) and cytokine levels in blood and the inflammatory response in the colon were investigated. We also detected the RNA and DNA oxidation in urine and tissues.
S. flexneri infection induced an increase in WBCs, C-reactive protein, interleukin (IL)-6, IL-10, IL-1β, IL-4, IL-17a, IL-10, and tumor necrosis factor α (TNF-α) in blood. Of note, a significant increase in urinary 8-oxo-7,8-dihydroguanosine (8-oxo-Gsn), an important marker of total RNA oxidation, was detected after intestinal infection (P = 0.03). The urinary 8-oxo-Gsn level returned to the baseline level after recovery from infection. In addition, the results of a correlation analysis showed that urinary 8-oxo-Gsn was positively correlated with the WBC count and the cytokines IL-6, TNF-α, IL-10, IL-1β, and IL-17α. Further detection of the oxidation in different tissues showed that S. flexneri infection induced RNA oxidative damage in the colon, ileum, liver, spleen, and brain.
Acute infection induced by S. flexneri causes increased RNA oxidative damage in various tissues (liver, spleen, and brain) and an increase of 8-oxo-Gsn, a urinary metabolite. Urinary 8-oxo-Gsn may be useful as a biomarker for evaluating the severity and prognosis of infection.
Core Tip: Shigella flexneri (S. flexneri) is a major pathogen causing acute intestinal infection, but the systematic oxidative damage incurred during the course of infection has not been investigated. By establishing a Sprague-Dawley rat model with S. flexneri infection in the intestine, we found that 8-oxo-7,8-dihydroguanosine (8-oxo-Gsn) levels were markedly increased in the colon, ileum, liver, spleen, and brain after infection, showing that S. flexneri can exacerbate systemic RNA oxidation. The changing trend in levels of urinary 8-oxo-Gsn (as the RNA oxidative metabolites) was consistent with the changes in the white blood cell count, C-reactive protein level, and cytokine levels during infection. Urinary 8-oxo-Gsn may be useful as a biomarker for evaluating the severity and prognosis of infection.
- Citation: Nie JJ, Pian YY, Hu JH, Fan GQ, Zeng LT, Ouyang QG, Gao ZX, Liu Z, Wang CC, Liu Q, Cai JP. Increased systemic RNA oxidative damage and diagnostic value of RNA oxidative metabolites during Shigella flexneri-induced intestinal infection. World J Gastroenterol 2021; 27(37): 6248-6261
- URL: https://www.wjgnet.com/1007-9327/full/v27/i37/6248.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i37.6248
Shigella infection is a major public health concern around the world, causing acute diarrhea, known as shigellosis or bacillary dysentery. There are almost 165 million cases of diarrhea caused by Shigella each year, of which 163 million occur in developing countries[1]. People of all ages can be infected by Shigella, but children under five years old are most susceptible[2].
Shigella flexneri (S. flexneri) is the most frequently isolated Shigella species worldwide[2]. The pathogenesis of S. flexneri involves invasion of the mucosa by bacteria, which replicate within and spread between the mucosal epithelial cells, resulting in a subsequent severe inflammatory response and epithelial destruction in the colon tissue. However, the oxidative damage in the colon during S. flexneri-induced infection has not been investigated. In the present study, the oxidative stress changes in the intestine during S. flexneri-induced acute intestinal infection were investigated.
Shigella infection can cause inflammatory responses in the intestine, which is also a potential source of inflammation-related cytokines[3]. Researchers have established cell models/animal models and observed the infected patients to investigate the inflammation-related cytokine production. In cell models, S. flexneri was able to cause an increase in interleukin (IL)-1, IL-10, and tumor necrosis factor α (TNF-α) in macrophage-like cells[4]. Outer membrane protein A of S. flexneri can trigger B cells to secret the cytokines IL-6 and IL-10[5]. The production of the cytokines IL-4, IL-6, and IL-8 is increased in HeLa cells with S. flexneri infection[6]. Researchers also found that the TNFα and IL-1β production is reduced in mouse macrophages with an S. flexneri msbB gene mutation[7]. In mouse models, S. flexneri infection can cause an increase in the IL-1β production[8]. In S. flexneri-infected patients, the TNF-α, IL-1, IL-4, IL-6, and interferon-γ levels increased significantly after infection[3].
In the present study, we established an S. flexneri-infected rat model to explore the changes in the levels of the above-mentioned cytokines (IL-1, IL-6, IL-10, and TNF-α) as well as IL-17α, which plays important roles in host immunity against intracellular pathogens.
Urinary 8-oxo-7,8-dihydroguanosine (8-oxo-Gsn) and 8-oxo-7,8-dihydro-2-deoxy
Accumulating evidence suggests that intestinal infection caused by Gram-negative bacteria may also influence other organs. For example, the Gram-negative bacterium Escherichia coli can form extracellular amyloid, which may play a role in causing sporadic Alzheimer’s disease[14]. S. dysenteriae, a causative agent of bloody diarrhea, may cause diarrhea-associated hemolytic uremic syndrome and neurological disorders[15]. Acute intestinal infection induced by Salmonella can cause oxidative damage to the intestine, liver, and spleen[13]. However, the effects of Shigella-induced intestinal infection on other tissues have not been reported.
We hope that the rat infection model established in the present study will help improve our understanding of how vertebrates regulate their response to S. flexneri infection. We further intended to explore whether or not S. flexneri infection in the intestine can cause oxidative damage to other organs, such as the liver, spleen, and brain.
The S. flexneri strain used in this study (CICC 21534) was purchased from the China Center of Industrial Culture Collection (CICC; Beijing, China). The source of the CICC 21534 strain was Guizhou Health and Epidemic Prevention Station, China. The serotype of CICC 21534 was F1a.
S. flexneri strain CICC 21534 was stored at -80 °C. The isolate was subcultured on blood agar plates twice and then suspended in Trypticase Soy Broth (bioMérieux SA, 69280 Marcy I’Etoile, France). The bacterial concentration was measured spectrophotometrically and confirmed by serial dilution on blood agar.
Twenty-four 4-wk-old healthy male Sprague-Dawley (SD) rats were purchased from Vital River (Beijing, China). All appropriate measures were taken to minimize the pain or discomfort of the rats. The rats were housed in a temperature-controlled room (24 ± 2 °C). Drinking water and food were provided ad libitum. There were three rats in each cage. The rats were not pretreated with any antibiotics and were all healthy before treatment, with normal white blood cell (WBC) counts and a normal flora in the feces.
The rats were divided randomly into either an infection group or a control group. In the infection group (n = 12), rats were infected by gavage with 1 dose of 1 × 109 colony-forming units (CFU) of the S. flexneri strain. In the control group (n = 12), rat gavage was performed with phosphate-buffered saline (PBS). On days 1, 5, 9, and 18, three rats per group were euthanized by intraperitoneal injection of pentobarbital sodium (200 mg/kg), and tissues were obtained.
For the establishment of the infection model, before infection, the rats were starved overnight. To neutralize the gastric acid, 400 μL of 3% NaHCO3 was administered orally 15 min before infection. The rats in the infection group were then orally administered with 1 × 109 CFU of S. flexneri in a volume of 1 mL. The establishment of infection was verified by the continuous detection of S. flexneri in feces, an increased WBC count, and obvious lymphocyte infiltration in the colon. In the control group, rats were also starved overnight and administered with 3% NaHCO3 orally 15 min before PBS administration.
We took care of and sacrificed the animals strictly according to animal welfare laws and regulations. This study was approved by the Ethical Committee of the Institute of Medical Biotechnology, Chinese Academy of Medical Sciences (approval number: IMB-201803140206).
The body temperature (rectal temperature) and body weight of the rats were measured every morning.
For the qualitative detection, conventional bacterial culture methods were used to detect S. flexneri in feces. Samples were collected and inoculated onto blood agar, China blue agar plates (Jinzhang Science and Technology Development Co. Ltd, Tianjin 300190, China), and Salmonella-Shigella (bioMérieux SA, 69280 Marcy I’Etoile, France) agar plates within 2 h of collection. S. flexneri was identified using mor
For the quantitative detection of S. flexneri, the feces and tissues were weighed after collection and then diluted in 1 mL of PBS and homogenized with a tissue homo
WBCs in blood samples were counted manually. A 20-μL blood sample was added to 380 μL of 2% ethanoic acid solution. Then, WBCs were counted in a hemacytometer using a conventional light microscope.
Blood samples were collected by the tail vein sampling method. Serum C-reactive protein (CRP) levels were detected with a Rat CRP ELISA kit (Immunology Con
The cytokines TNF-α, IL-6, IL-10, IL-1β, IL-4, and IL-17α were measured using a commercially available MILLIPLEX MAP Kit (Rat Cytokine/Chemokine Magnetic Bead Panel, 96-Well Plate Assay, Cat. #RECYTMAG-65K, RECYMAG65K27PMX, RECYMAG-65PMX27BK; Millipore Corporation, MA, United States) according to the manufacturer’s protocol. In this study, 1/2 dilution of serum was appropriate for detection. A Luminex 200 System (Luminex Corporation, TX, United States) was used for quantification.
The manual bladder expression method was used to collect the urine samples of rats. Urine samples were stored at -80 °C. Urinary 8-oxo-dGsn and 8-oxo-Gsn were detected by the liquid chromatography with tandem mass spectrometry method as reported[13]. In brief, all urine samples were analyzed using an Agilent 1290 Infinity UHPLC instrument equipped with an Agilent 6490 triple-quadrupole mass spectrometer with a Jet Stream ESI source and iFunnel (Agilent, United States; parameters are shown in Supplementary Tables 1-3). The concentrations of 8-oxo-dGsn and 8-oxo-Gsn were then normalized by urinary creatinine to correct for the impact of water intake/ excretion on urine. The results are presented as the ratio of the concentrations of nucleosides (8-oxo-dGsn and 8-oxo-Gsn) and creatinine.
Histopathological tests were performed using standard laboratory procedures. Tissue samples were fixed in 10% neutral-buffered formalin and embedded in paraffin. The paraffin-embedded tissue was then sliced into 4-μm-thick sections with a microtome and placed onto glass slides for further experiments. The tissue sections were deparaffinized with xylene, hydrated through an alcohol series, and then stained with hematoxylin and eosin (H&E). After dehydration and clearing, the mounting tissue sections were observed under a light microscope. H&E Stain kit was purchased from ZSGB-BIO (Beijing, China).
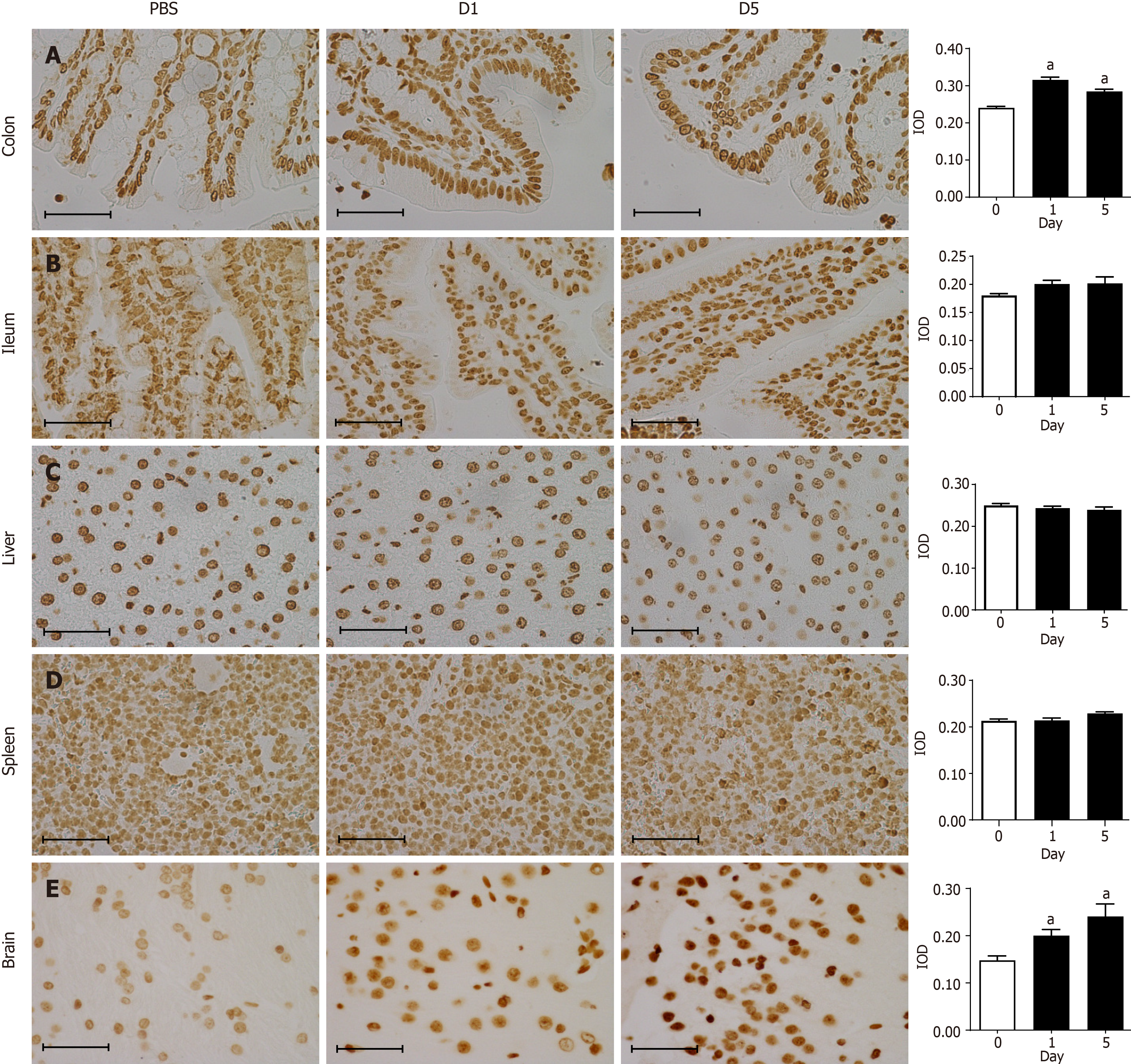
Immunohistochemical staining was performed to detect the expression of 8-oxo-Gsn in the ileum, colon, liver, spleen, and brain. In brief, paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated through a series of graded alcohols. Antigen retrieval was performed in citric acid buffer at 95 °C for 40 min. DNase I (TaKaRa Biotechnology Co., Ltd., Dalian, China) was added to slides to remove genomic DNA from samples. The slides were washed with 1 × PBS, and 3% hydrogen peroxide was added to eliminate endogenous peroxidases. Slides were then blocked with goat serum working solution. Primary monoclonal 15A3 antibody (ab62623; Abcam, Cambridge, United Kingdom) was added, and slides were incubated at 37 °C for 2 h (antibody dilutions: Colon, 1:700; ileum, 1:2000; liver, 1:700; spleen, 1:700; brain, 1:2000). The sections were incubated with HRP-conjugated secondary antibody (PV-6002; ZSGB-BIO) for 20 min. Coloration was performed with DAB (ZSGB-BIO), and the sections were observed under a microscope[16].
To detect the expression of 8-oxo-dGsn in tissue, tissue sections were treated with RNase A (TaKaRa Biotechnology Co., Ltd.) to remove RNA from samples. The primary monoclonal antibody was N45.1 (ab48508; Abcam). The dilutions of primary antibody were 1:2000 for the colon, 1:3000 for the ileum, 1:3000 for the liver, 1:500 for the spleen, and 1:2000 for the brain.
The SPSS 16.0 software program was used for the statistical analyses. Quantitative data are expressed as the mean ± SD and were analyzed by a t-test or one-way analysis of variance (ANOVA) after the normality test. Pearson's correlation coefficient (r) was used when comparing the linear relationship between the variables with a normal distribution. An area under the receiver operating characteristic (ROC) curve was created to assess the predictive value of urinary 8-oxo-Gsn and cytokines for the presence of infection. The results were considered significant only when P < 0.05.
The body temperature showed no significant changes during the infection pro
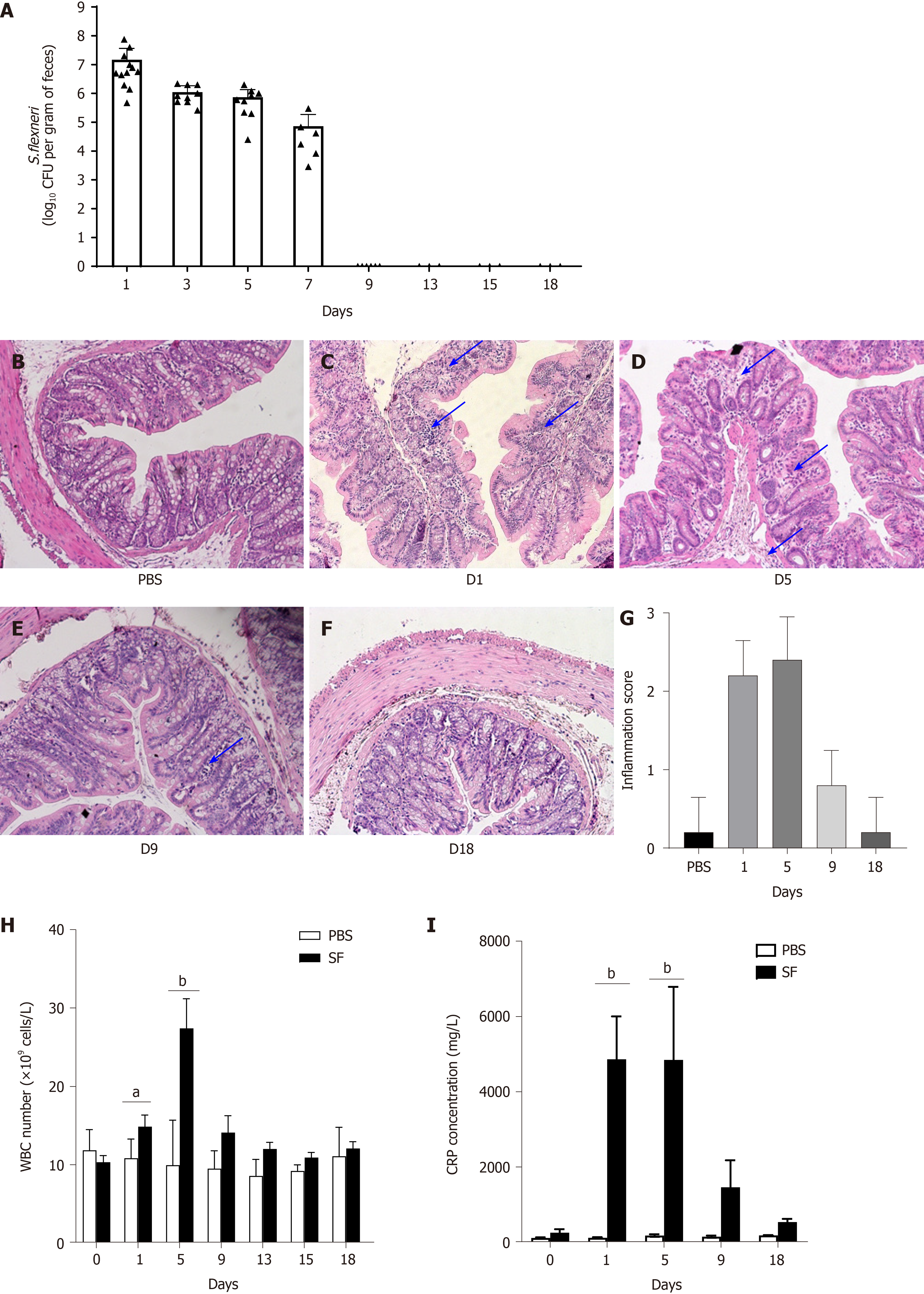
After the intragastric administration of S. flexneri, serial fecal cultures were performed at fixed time points (days 1, 3, 5, 7, 9, 13, 15, and 18) in the two groups. In the control group, S. flexneri was undetectable during the experiment. In the infection group, S. flexneri was detectable in the feces of all infected rats after infection (day 1) and remained until day 7, whereas it was undetectable on day 9 (Figure 1A). In the intestine, liver, and spleen, S. flexneri was detectable on days 1 and/or 5. S. flexneri was undetectable in the brain at all time points.
Shigella can invade the colonic epithelial cells and provoke an intense inflammatory response. Compared with the control group (Figure 1B), obvious leukocyte infiltration was observed in the colonic mucosa on day 1 (P < 0.001 vs control; Figure 1C and G) and day 5 (P < 0.001 vs control; Figure 1D and G) after infection with S. flexneri, as detected by H&E staining. Intestinal inflammation was decreased on day 9 (Figure 1E), which was accompanied by pathogen elimination. By day 18, the colonic mucosa had returned to normal (Figure 1F).
The WBC count is often used to assess bacterial infections. The changes in the WBC count after the establishment of intestinal infection are shown in Figure 1H. The WBC count increased after oral exposure to S. flexneri and peaked on day 5 (P < 0.0001, WBCday5 = 27.42 ± 3.77 × 109 cells/L). The WBC count then decreased and returned to baseline after day 13. In the control group, there were no significant changes in the WBC count during the experiment.
Furthermore, it was shown that the level of CRP, an acute-phase response protein commonly used as a marker of inflammation, was significantly increased after exposure to S. flexneri on day 1 (CRP = 4.87 ± 1.59 × 103 mg/L, P = 0.005), and the level remained high when it was detected on day 5 (CRP = 4.85 ± 1.41 × 103 mg/L, P = 0.002). The CRP level then decreased and returned to baseline on day 18 (Figure 1I).
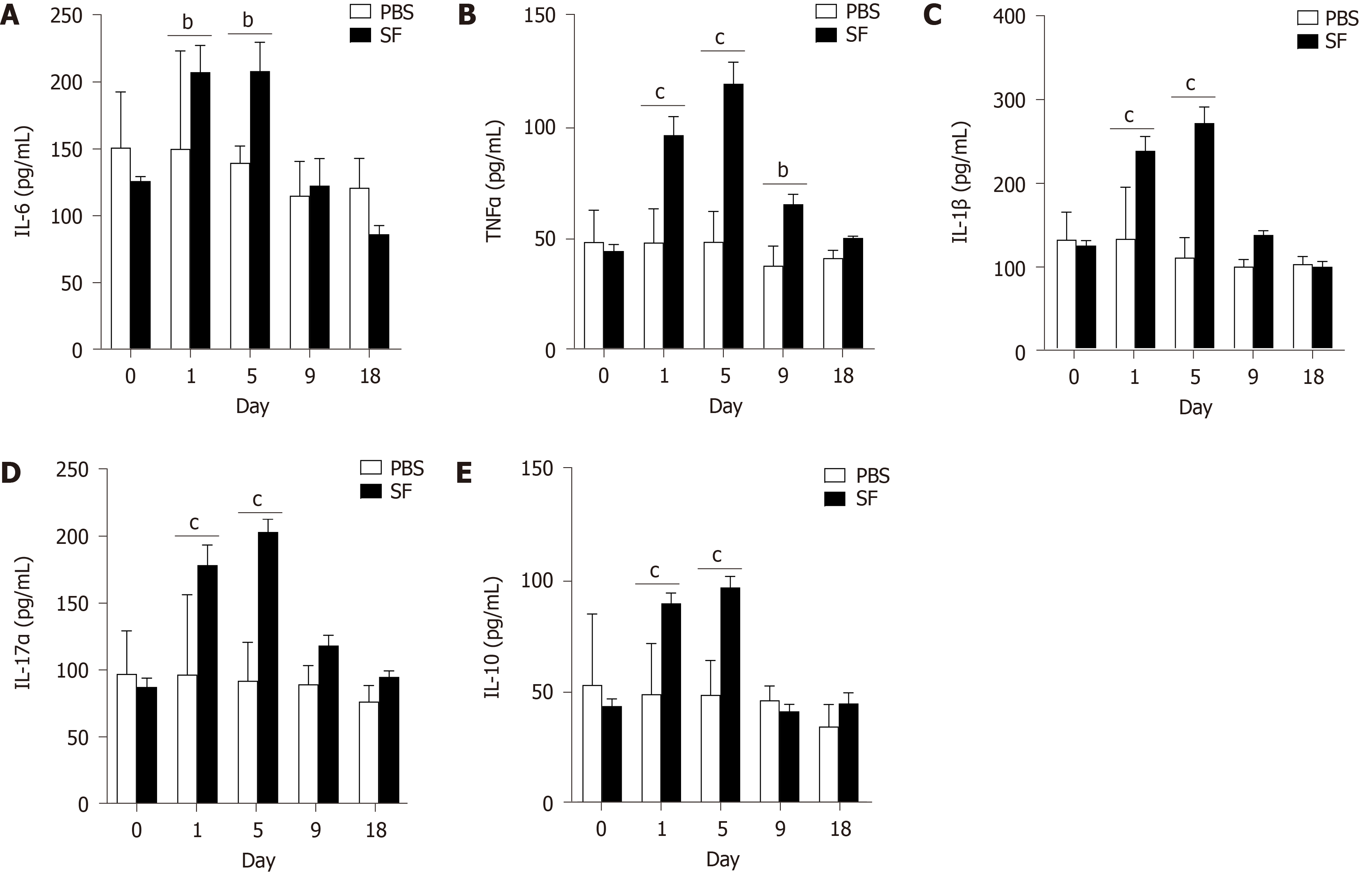
The expression of IL-6, an important mediator of the acute-phase response, was significantly increased in the infection group on day 1 (207.26 ± 19.93 pg/mL, P = 0.02) and remained high when detected on day 5 (207.99 ± 21.49 pg/mL, P = 0.01; Figure 2A). After pathogen clearance on day 9, the levels of IL-6 decreased and returned to baseline.
The expression of TNF-α, a cell-signaling protein involved in systemic inflammation and one of the cytokines that make up the acute phase reaction, was also significantly increased after infection (day 1: 96.07 ± 8.29 pg/mL, P < 0.001) and peaked on day 5 (119.11 ± 9.69 pg/mL, P < 0.001). The TNF-α level then decreased and returned to the baseline at the end of the experiment (Figure 2B).
Consistent with this trend, increased levels of proinflammatory IL-1β (Figure 2C) and IL-17α (Figure 2D) were observed after infection and peaked on day 5. IL-10, a cytokine with multiple, pleiotropic effects in immunoregulation and inflammation, was also significantly produced after bacterial infection (Figure 2E).
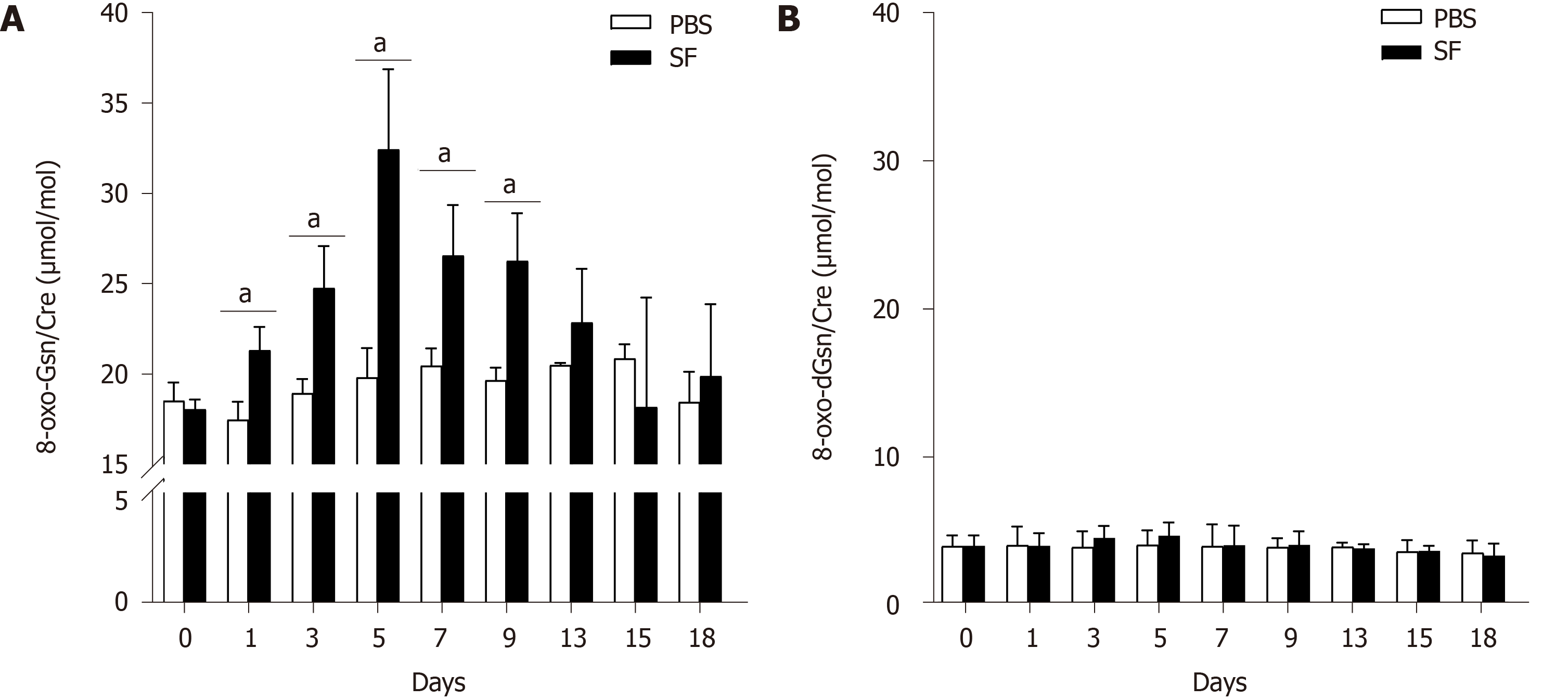
8-oxo-Gsn levels were increased after oral exposure to S. flexneri. Compared with the control group, significantly increased levels of 8-oxo-Gsn were observed from day 1 to day 9 (P < 0.05), with the level peaking on day 5 (P = 0.017). After day 5, the level of 8-oxo-Gsn began to decrease and returned to baseline on day 15 (Figure 3A). The trends in the changes in the urinary 8-oxo-Gsn level were consistent with the changes in colonic tissue inflammation, the WBC count, and the CRP level, suggesting that urinary 8-oxo-Gsn may serve as a biomarker for assessing the severity of the infection.
In the control group, no significant changes in the 8-oxo-Gsn level were observed at any time point. For 8-oxo-dGsn, no significant changes were observed at any time point (Figure 3B).
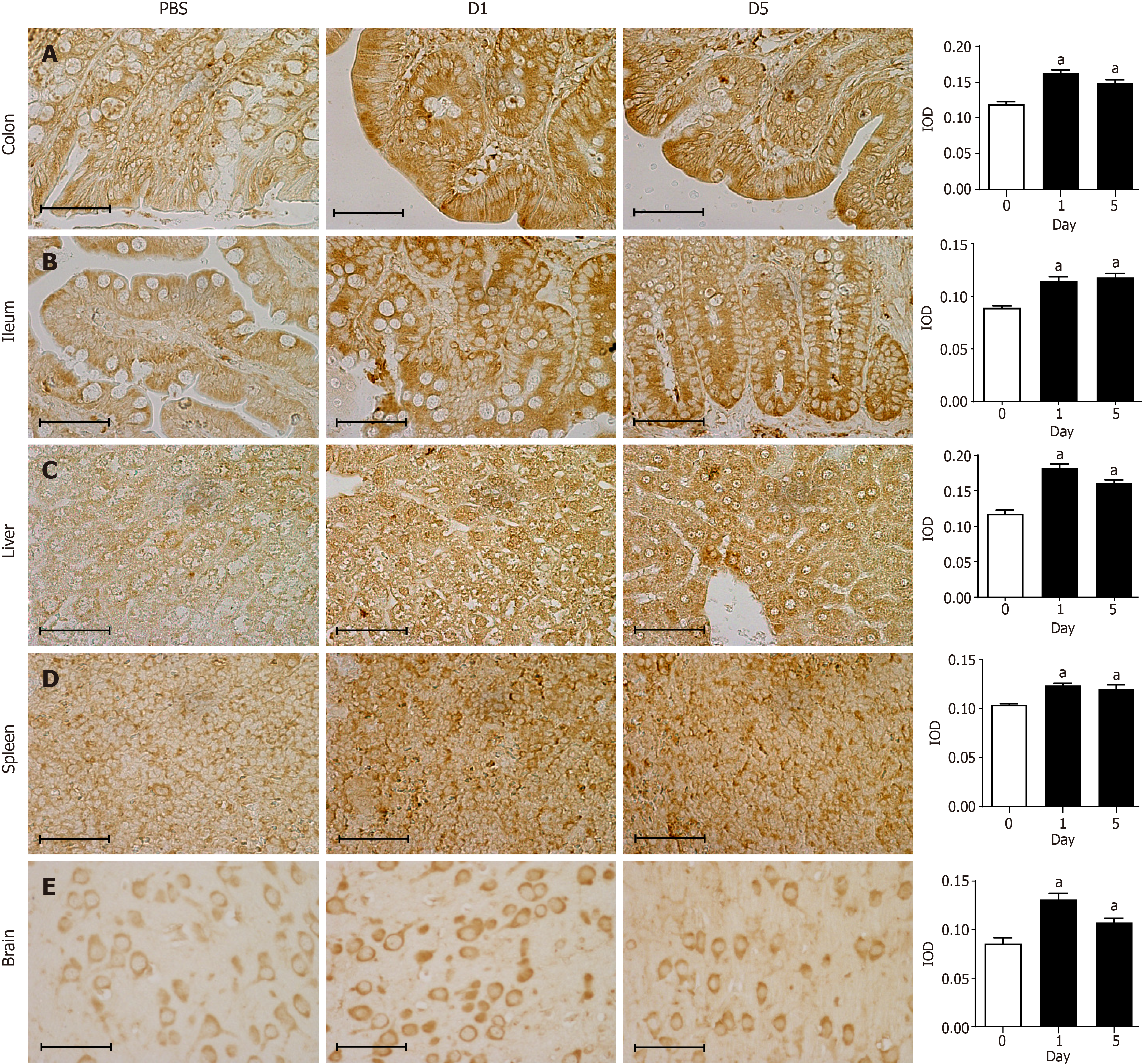
Immunohistochemical staining showed that the 8-oxo-Gsn staining intensity in the infected colon tissue was significantly altered compared with normal colon tissue (P < 0.05; Figure 4A). In infected colon tissues, antibodies directed against the RNA damage marker 8-oxo-Gsn produced more intense staining than uninfected colon tissues, thereby indicating a general increase in oxidative stress and RNA damage. Although 8-oxo-Gsn was more highly expressed on day 1 than on day 5, the difference between the two time points was not statistically significant. In the ileum (Figure 4B), liver (Figure 4C), spleen (Figure 4D), and brain tissues (Figure 4E), the expression of 8-oxo-Gsn was significantly higher on days 1 and day 5 than that in uninfected tissues.
The levels of the DNA damage marker 8-oxo-dGsn were significantly increased in the colon and brain tissues after infection (Figure 5A and E). In the infection group, there was no significant change in the 8-oxo-dGsn expression in the ileum, liver, or spleen (Figure 5B-D).
The 8-oxo-Gsn concentration in the urine of the infection group was positively correlated with the WBC count (r = 0.652, P < 0.001) and levels of IL-6 (r = 0.461, P = 0.005), TNF-α (r = 0.541, P = 0.001), IL-10 (r = 0.359, P = 0.032), IL-1β (r = 0.402, P = 0.017), and IL-17α (r = 0.431, P = 0.009). No significant correlation was found between 8-oxo-Gsn and CRP (r = 0.289, P = 0.203).
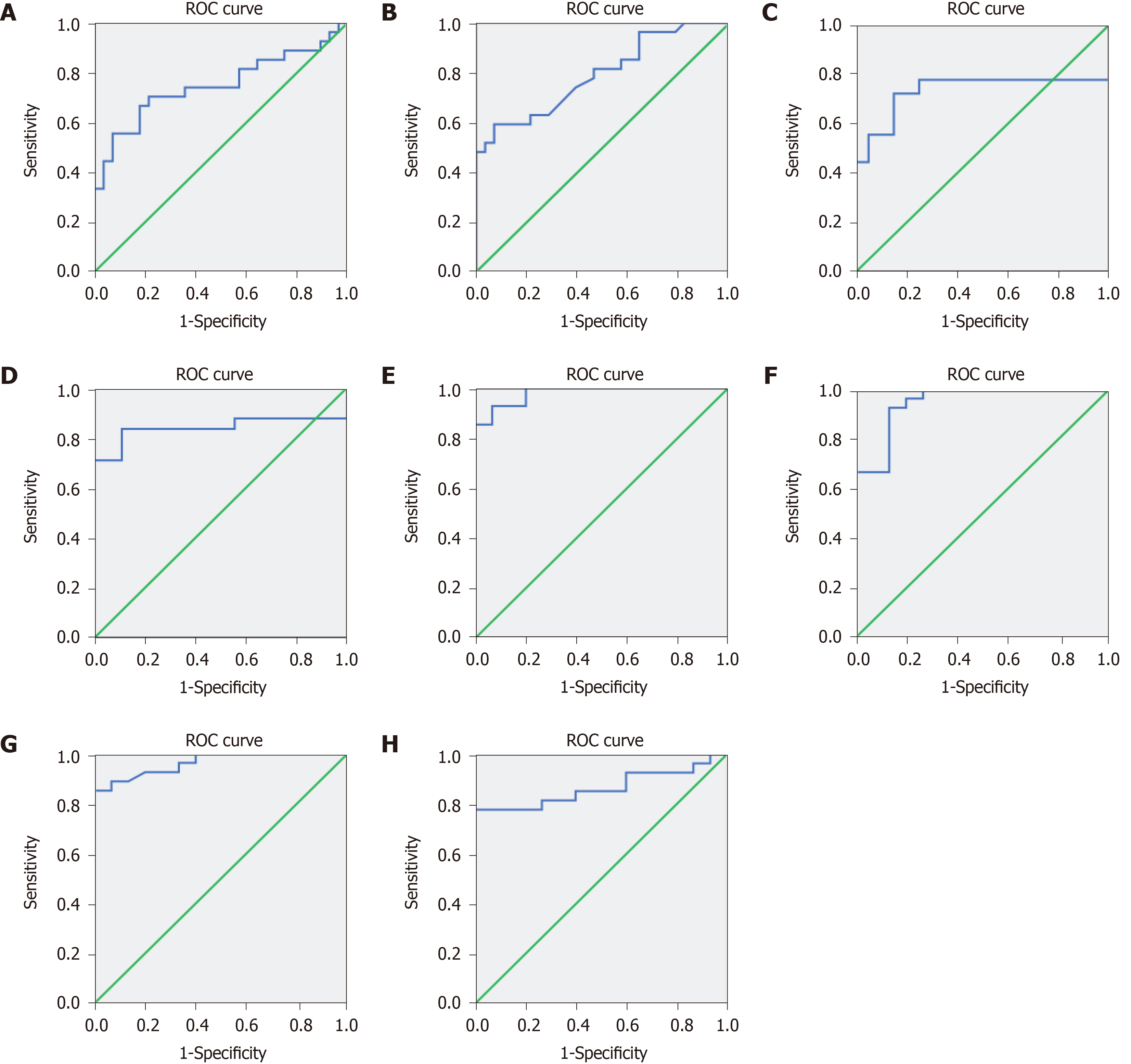
HE staining and fecal discharge are the gold standards for diagnosing the presence of infection. The predictive value of 8-oxo-Gsn in identifying the presence of intestinal infection was assessed using a ROC curve. The area under the ROC curve of urinary 8-oxo-Gsn was 0.750 (95%CI, 0.615–0.885, P = 0.001) (Figure 6A).
The predictive value of the WBC count and CRP for identifying intestinal infection was also assessed using a ROC curve. The area under the ROC of the WBC count was 0.792 (95%CI, 0.674–0.911, P < 0.001) (Figure 6B), and that for CRP was 0.733 (95%CI, 0.544–0.923, P = 0.014) (Figure 6C).
For inflammatory cytokines, the area under the ROC of IL-6 (Figure 6D), TNF-α (Figure 6E), IL-1β (Figure 6F), IL-17α (Figure 6G), and IL-10 (Figure 6H) was 0.838 (95%CI, 0.698–0.978, P = 0.003), 0.980 (95%CI, 0.948–1.012, P < 0.001), 0.948 (95%CI, 0.882–1.014, P < 0.001), 0.964 (95%CI, 0.917–1.012, P < 0.001), and 0.864 (95%CI, 0.752–0.977, P < 0.001), respectively.
The present findings suggested that urinary 8-oxo-Gsn can be used to identify the presence of infection and to assess the severity of infection. In this study, Shigella infection in the gastrointestinal tract induced significant increases in the 8-oxo-Gsn concentration, the WBC count, and the CRP level. The WBC count is widely used to evaluate the severity of bacterial infections. CRP, an acute-phase protein of hepatic origin, is a non-specific marker of inflammation and one of the most sensitive acute-phase reactants.
The levels of 8-oxo-Gsn, a marker of oxidative stress, as well as the WBC count and the CRP level, which are markers of inflammation, were all increased significantly on day 1 and peaked on day 5. Following the clearance of the pathogen, the values for these biomarkers returned to the baseline level.
The correlation analysis showed that 8-oxo-Gsn was positively correlated with the WBC count. Although no significant correlation was found between 8-oxo-Gsn and CRP, the trend of the urinary 8-oxo-Gsn concentration was consistent with the changes of the WBC count and CRP level. The sharp increase in the CRP level on day 1 (20 times higher than that on day 0) may be the reason for the absence of a significant correlation. The predictive value of the 8-oxo-Gsn concentration, WBC count, and CRP level for differentiating between infection and non-infection was assessed using the ROC curve. The results showed that 8-oxo-Gsn achieved a fair accuracy in classifying subjects as infected or uninfected. When the area under the ROC curve for the 8-oxo-Gsn concentration was compared to those of the WBC count and CRP level, all values ranged from 0.70 to 0.80, indicating that these diagnostic markers have a similar discrimination ability for the infection status[17]. Furthermore, urinary 8-oxo-Gsn is a noninvasive biomarker and can be measured more frequently than blood biomarkers, such as the WBC and CRP.
The immunohistochemistry results showed that in addition to intestinal tract tissue, 8-oxo-Gsn (RNA oxidative product) levels were increased in the liver, spleen, and brain tissues. Previous studies have shown that Shigella has a predilection to infect and colonize the large bowel[2,18]. The increased oxidative stress in other tissues might be induced by endo- or exotoxin produced by Shigella strains, according to previous studies[14,15,19]. Our study suggested that diarrhea caused by Shigella bacteria could induce a transient elevation in systemic oxidative stress.
The levels of the DNA oxidative product 8-oxo-dGsn showed no significant changes in urine, ileum, liver, or spleen tissues. The increase was only detected in the colon and brain. The results suggested that DNA oxidative damage occurred less frequently than RNA oxidative damage in the course of acute infection. This might be because DNA molecules have a double-stranded structure, which is protected by histones and the cell nucleus[11]. All of these protections reduce the DNA oxidative damage during acute infection. Previous studies have shown that, in the course of chronic infection, DNA oxidative damage occurred more frequently, which helps promote inflammation-mediated carcinogenesis[20,21].
The present study showed that the levels of the cytokines IL-6, TNF-α, IL-1β, IL-17α and IL-10 were significantly increased after infection. Previous studies also showed that S. flexneri induced the release of both inflammatory (IL6, IL-8, and IL1β) and anti-inflammatory cytokines (IL-10)[22-24]. However, changes in the levels of IL-17α in S. flexneri-related infection have not been investigated. IL-17 has emerged as a central player in the mammalian immune system[25]. To promote inflammation, IL-17 acts in concert with IL-1β and TNF-α[26], with IL-1β being required to mediate inflammation in S. flexneri infection[8]. The present results suggested that S. flexneri infection activates inflammatory cytokines. Our findings also showed that the cytokine levels were positively correlated with the concentration of 8-oxo-Gsn, a marker of RNA oxidation, which proved again that 8-oxo-Gsn was useful as a diagnostic biomarker in infection.
In summary, during the early stage of Shigella infection, tissue inflammation occurs at the site of pathogen colonization, accompanied by an increase in reactive oxygen species (ROS) and the release of inflammatory cytokines. With the clearance of Shigella, the levels of oxidative stress markers and inflammatory cytokines decline, and the tissue inflammation recovers. The results suggested that although an increase in ROS can induce RNA oxidative damage in the colon and various tissues, this is temporary and differs from DNA oxidative damage, which occurs more frequently under conditions of chronic infection[20]. Thus, the combination of oxidative stress and inflammatory cytokines promotes infection recovery. A previous study showed that the RNA oxidative nucleic acid 8-oxoGTP increased protein variation[27]. The influence of various mutated proteins in infection, especially in the course of chronic infection, should be investigated in future studies.
Shigella infection is a major public health concern around the world. Shigella flexneri (S. flexneri) is a major pathogen causing acute intestinal infection and is more frequently fatal than other Shigella species. The white blood cell (WBC) count and levels of C-reactive protein (CRP) and cytokines have always been used to evaluate the severity of Shigella-induced infection. Because RNA oxidation plays a substantial role in the progression of multiple diseases, the RNA oxidative product 8-oxo-7,8-dihydroguanosine (8-oxo-Gsn) may be useful as a biomarker for predicting infectious disease progression and severity.
There is no information regarding the diagnostic value of urinary 8-oxo-Gsn and 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxo-dGsn) in intestinal infection induced by S. flexneri. In addition, the correlation of blood inflammatory-related cytokines with RNA oxidative metabolites is unclear. Furthermore, the systematic tissue oxidative damage during Shigella-induced intestinal infection has not been investigated.
To determine the systemic RNA oxidative damage incurred in S. flexneri-induced intestinal infection and to evaluate the diagnostic value of the RNA oxidative metabolites 8-oxo-Gsn in urine.
Sprague-Dawley rats were used to establish an acute intestinal infection model by oral gavage of S. flexneri strains. The CRP level was measured by ELISA, and cytokines were detected using the MILLIPLEX MAP Kit according to the manufacturer's protocol. Hematoxylin and eosin staining was performed to detect tissue inflammation. Liquid chromatography with tandem mass spectrometry was used to determine the 8-oxo-dGsn and 8-oxo-Gsn levels in urine. Immunohistochemical staining was performed to detect the expression of 8-oxo-Gsn and 8-oxo-dGsn in the ileum, colon, liver, spleen, and brain.
Intestinal infection induced by S. flexneri caused an increase in inflammation-related markers in the blood [WBCs, CRP, tumor necrosis factor α (TNF-α), interleukin (IL)-6, IL-10, IL-1β, IL-4, and IL-17a] and RNA oxidative damage-related markers in the urine (8-oxo-Gsn). The urinary 8-oxo-Gsn level increased after infection and returned to the baseline level after recovery from infection. A correlation analysis showed that urinary 8-oxo-Gsn was positively correlated with the WBC count and the cytokines IL-6, TNF-α, IL-10, IL-1β, and IL-17α. The evaluation of oxidation in different tissues showed that intestinal infection caused by S. flexneri induced an increase in RNA oxidative damage in various tissues, including the intestine, liver, spleen, and brain.
This study demonstrated that acute intestinal infection induced by S. flexneri not only causes RNA oxidative damage in the intestine but also other tissues as well (liver, spleen, and brain). Furthermore, the urinary metabolite of RNA oxidation 8-oxo-Gsn was shown to be useful as a diagnostic index to evaluate the infection status.
Urinary 8-oxo-Gsn can be used as a noninvasive diagnostic biomarker to evaluate the severity and prognosis of infection in clinical practice.
We are grateful to the members of the Institute of Geriatrics of the Ministry of Health for advice and assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kashyap MK, Scarfì S S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Li JH
| 1. | Sheikh AF, Moosavian M, Abdi M, Heidary M, Shahi F, Jomehzadeh N, Seyed-Mohammadi S, Saki M, Khoshnood S. Prevalence and antimicrobial resistance of Shigella species isolated from diarrheal patients in Ahvaz, southwest Iran. Infect Drug Resist. 2019;12:249-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Jennison AV, Verma NK. Shigellaflexneri infection: pathogenesis and vaccine development. FEMS Microbiol Rev. 2004;28:43-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 180] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Raqib R, Lindberg AA, Wretlind B, Bardhan PK, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 120] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Bando SY, Moreno AC, Albuquerque JA, Amhaz JM, Moreira-Filho CA, Martinez MB. Expression of bacterial virulence factors and cytokines during in vitro macrophage infection by enteroinvasive Escherichia coli and Shigella flexneri: a comparative study. Mem Inst Oswaldo Cruz. 2010;105:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Bhowmick R, Pore D, Chakrabarti MK. Outer membrane protein A (OmpA) of Shigella flexneri 2a induces TLR2-mediated activation of B cells: involvement of protein tyrosine kinase, ERK and NF-κB. PLoS One. 2014;9:e109107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | García-Weber D, Dangeard AS, Cornil J, Thai L, Rytter H, Zamyatina A, Mulard LA, Arrieumerlou C. ADP-heptose is a newly identified pathogen-associated molecular pattern of Shigella flexneri. EMBO Rep. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Ranallo RT, Kaminski RW, George T, Kordis AA, Chen Q, Szabo K, Venkatesan MM. Virulence, inflammatory potential, and adaptive immunity induced by Shigella flexneri msbB mutants. Infect Immun. 2010;78:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, Zychlinsky A. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 310] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Halliwell B. Why and how should we measure oxidative DNA damage in nutritional studies? Am J Clin Nutr. 2000;72:1082-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Poulsen HE, Specht E, Broedbaek K, Henriksen T, Ellervik C, Mandrup-Poulsen T, Tonnesen M, Nielsen PE, Andersen HU, Weimann A. RNA modifications by oxidation: a novel disease mechanism? Free Radic Biol Med. 2012;52:1353-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Nie B, Gan W, Shi F, Hu GX, Chen LG, Hayakawa H, Sekiguchi M, Cai JP. Age-dependent accumulation of 8-oxoguanine in the DNA and RNA in various rat tissues. Oxid Med Cell Longev. 2013;2013:303181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Isobe C, Abe T, Terayama Y. Homocysteine may contribute to pathogenesis of RNA damage in brains with Alzheimer's disease. Neurodegener Dis. 2009;6:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Hu JH, Nie JJ, Gao ZX, Weng QH, Wang ZH, Li CB, Pian YY, Zhang R, Jiang ZL, Xia MM, Cai JP. Oxidative DNA and RNA damage and their prognostic values during Salmonella enteritidis-induced intestinal infection in rats. Free Radic Res. 2018;52:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Zhan X, Stamova B, Sharp FR. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer's Disease Brain: A Review. Front Aging Neurosci. 2018;10:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 15. | Lee MS, Tesh VL. Roles of Shiga Toxins in Immunopathology. Toxins (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Zheng JD, Hei AL, Zuo PP, Dong YL, Song XN, Takagi Y, Sekiguchi M, Cai JP. Age-related alterations in the expression of MTH2 in the hippocampus of the SAMP8 mouse with learning and memory deterioration. J Neurol Sci. 2009;287:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Li F, He H. Assessing the Accuracy of Diagnostic Tests. Shanghai Arch Psychiatry. 2018;30:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 18. | Schroeder GN, Hilbi H. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev. 2008;21:134-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 417] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 19. | Obata F, Tohyama K, Bonev AD, Kolling GL, Keepers TR, Gross LK, Nelson MT, Sato S, Obrig TG. Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons. J Infect Dis. 2008;198:1398-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Ohnishi S, Ma N, Thanan R, Pinlaor S, Hammam O, Murata M, Kawanishi S. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid Med Cell Longev. 2013;2013:387014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 21. | Tsukiyama-Kohara K. Role of oxidative stress in hepatocarcinogenesis induced by hepatitis C virus. Int J Mol Sci. 2012;13:15271-15278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Marteyn B, Gazi A, Sansonetti P. Shigella: a model of virulence regulation in vivo. Gut Microbes. 2012;3:104-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Liu G, Pilla G, Tang CM. Shigella host: Pathogen interactions: Keeping bacteria in the loop. Cell Microbiol. 2019;21:e13062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Samandari T, Kotloff KL, Losonsky GA, Picking WD, Sansonetti PJ, Levine MM, Sztein MB. Production of IFN-gamma and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J Immunol. 2000;164:2221-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 694] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 26. | Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 268] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Dai DP, Gan W, Hayakawa H, Zhu JL, Zhang XQ, Hu GX, Xu T, Jiang ZL, Zhang LQ, Hu XD, Nie B, Zhou Y, Li J, Zhou XY, Zhang TM, He Q, Liu DG, Chen HB, Yang N, Zuo PP, Zhang ZX, Yang HM, Wang Y, Wilson SH, Zeng YX, Wang JY, Sekiguchi M, Cai JP. Transcriptional mutagenesis mediated by 8-oxoG induces translational errors in mammalian cells. Proc Natl Acad Sci USA. 2018;115:4218-4222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |