Published online Oct 7, 2021. doi: 10.3748/wjg.v27.i37.6180
Peer-review started: March 2, 2021
First decision: April 17, 2021
Revised: April 24, 2021
Accepted: August 10, 2021
Article in press: August 10, 2021
Published online: October 7, 2021
Processing time: 210 Days and 13 Hours
Surveillance for hepatocellular carcinoma (HCC) in high-risk patients with semiannual ultrasound examinations is advocated by all international guidelines. However, as long as the identification of the population to be screened and the surveillance programs are not well implemented, the real-life impact of HCC surveillance in reducing mortality for HCC cannot be known. We propose a new approach that promotes the identification of cirrhotic patients by primary care physicians (PCPs) and referral of patients to the hepatologist for surveillance. Surveillance should be incorporated, when feasible, in a hub and spoke model of comprehensive hepatology care. Training PCPs to identify cirrhotic patients and performing surveillance in a subspecialist setting are equally important to improve the effectiveness of real-life surveillance and to decrease HCC mortality over time.
Core Tip: Ultrasound surveillance for hepatocellular carcinoma is recommended by all official guidelines for high-risk patients, but its uptake at the community level is low. We discuss the obstacles hampering its implementation and propose a hub and spoke model network for the effective delivery of surveillance in the real world setting.
- Citation: Del Poggio P, Mazzoleni M, Lazzaroni S, D'Alessio A. Surveillance for hepatocellular carcinoma at the community level: Easier said than done. World J Gastroenterol 2021; 27(37): 6180-6190
- URL: https://www.wjgnet.com/1007-9327/full/v27/i37/6180.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i37.6180
Primary liver cancer is the third leading cause of cancer related mortality worldwide[1]. It includes hepatocellular carcinoma (HCC), comprising of 75%-85% cases, intrahepatic cholangiocarcinoma (10%-15%), and other rare types[1]. The HCC death toll is the highest in East Asia and Africa, with an annual mortality rate greater than 20/100000 people[2]. Mortality is lower in Western Europe and in the United States (12-12.7/100000) but it has increased rapidly in the last decade[3]. On the other hand, Eastern Europe, Scandinavia, and India have the lowest mortality rate (less than 4/100.000). The great majority of HCC cases in the Western world are diagnosed in cirrhotic patients, while in Asia and Central Africa the most significant risk factor for HCC is chronic hepatitis B virus infection with or without cirrhosis. Therefore, patients with those risk factors (cirrhosis and/or chronic hepatitis B infection) should undergo surveillance, with the goal of identifying the tumor at an early stage when curative treatments are feasible. A randomized study of chronic hepatitis B patients in China[4], several longitudinal cohort studies in patients affected by cirrhosis of any etiology[5-7], and a meta-analysis[8] have demonstrated the benefit of ultrasound surveillance (US) in improving survival. In addition, several studies have shown that US is cost effective for the surveillance of patients affected by chronic hepatitis B and of cirrhotic patients. Indeed, HCC incidence in those populations is 0.2% and 1.5% per year respectively, which is considered acceptable to define cost effectiveness[9-11]. On the other hand, the effectiveness of US is still uncertain in young Non-Asian inactive carriers of hepatitis B surface antigen (HBsAg), in patients with chronic hepatitis C infection with advanced fibrosis, and by those with nonalcoholic fatty liver disease (NAFLD) without cirrhosis. For those reasons, the three most important international guidelines advocate periodic abdominal ultrasound scans with or without alpha-fetoprotein (AFP) in all cirrhotics and chronic hepatitis B patients, irrespective of antiviral treatment[2,12,13] as a cost effective and noninvasive surveillance modality. According to the guidelines, US should be performed on a semiannual basis, with the exclusion of decompensated cirrhotics not listed for liver transplantation, in which surveillance is considered futile.
Despite the recommendations, real-life implementation of surveillance programs is far from optimal. It has recently been shown that, in addition to an increase in the proportion of HCC diagnosed at an early stage, 64% of the cases detected in the United States between 2006 and 2012 were diagnosed at an intermediate or an advanced stage[14]. That is probably the result of the low implementation of surveillance programs, with less than 20% of cirrhotics undergoing regular surveillance[15]. Surveillance is hindered by several issues, insufficient identification of patients at risk, failure to order surveillance, failure to perform surveillance with effective tools, poor patient adhe
One of the most important factors responsible for the low uptake of surveillance is the failure of primary care physicians (PCPs) to identify patients at risk to be enrolled in surveillance programs. In two web-based surveys, PCPs reported lack of knowledge of current guidelines. They could not correctly identify the at-risk categories and ordered random surveillance of chronic liver disease patients[16,17]. It is noteworthy that cirrhosis is widely under diagnosed and that increased identification and surveillance of cirrhotic patients would be very cost effective in detecting HCC at an early stage. As an example, assuming an ultrasound sensitivity of 65% for early-stage HCC[18], an increase in the detection rate of cirrhotic patients from 50% to 75% would result in a significant increase of early-stage HCC diagnosed by abdominal ultrasound. Thanks to the identification of high-risk patients, ultrasound screening would thus allow the identification of a number of early-stage HCC comparable to magnetic resonance imaging (MRI) use, which has a higher sensitivity (90%) but is more expensive and has a higher false-positive rate. Another negative consequence of the failure to identify cirrhotic patients is that the cause of liver damage is not treated, and consequently HCC would often be diagnosed in a critical patient not eligible for radical treatment anymore.
Fortunately, the performance of PCPs can be improved by training interventions[19] and by participation in specialist networks, as shown by the French experience in hepatitis C[20]. To address the problem, in 2006 we started an education program targeting 120 PCPs, aimed at the identification of cirrhotic patients and their surve
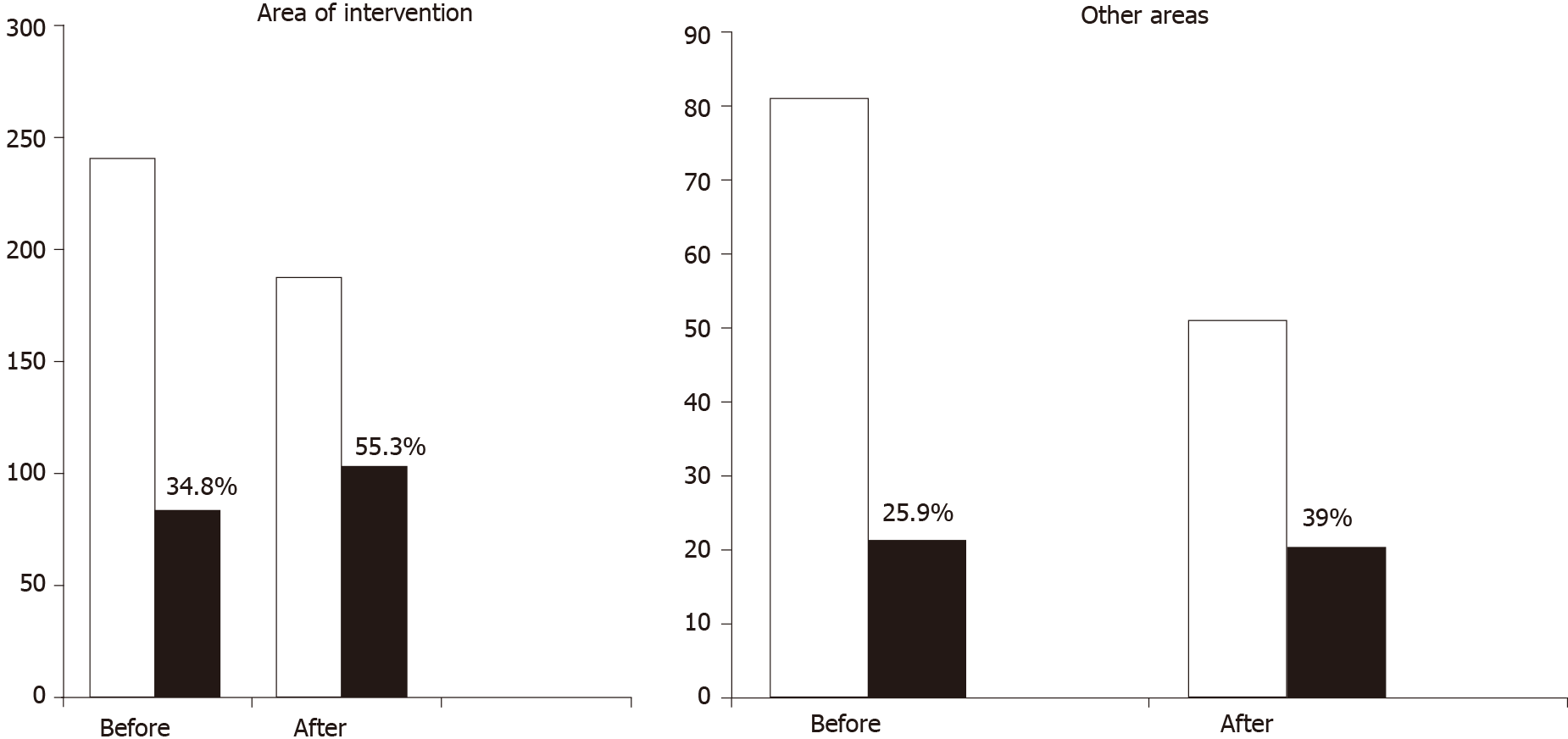
The results showed that 6 years after training PCPs, there was a significant increase in the number of HCC patients detected by US at an early stage and suitable for radical treatment (Figure 1). As a consequence, 3- and 5-year survival of HCC patients improved significantly from 35% to 48% and 20% to 40%, respectively (P < 0.05) after training. In contrast, both the proportion of HCCs detected by US, and patient survival did not change in areas where PCPs were not trained. The study has some limitations. Firstly, PCPs did not record the number of cirrhotic patients who were identified, but only the number of HCC patients who were diagnosed. Secondly, the cirrhosis criteria used by PCPs to refer the patient to the hepatologist were mainly ultrasound based using two of the following ultrasound criteria, liver surface nodularity, nonhomogeneous liver texture, left lobe or caudate lobe hypertrophy, distorted hepatic veins, spleen longitudinal diameter > 12 cm or one ultrasound criterion plus a platelet count < 130.000/mL. Currently, those criteria are obsolete, as the availability of new prop
The identification of cirrhotic patients for surveillance does not imply that surveillance will be effectively performed. In fact, surveillance is sometimes carried out by the PCP and ultrasound scans are performed by radiologists without specific expertise in liver disease. In that case, the patient is referred to the hepatologist or gastroenterologist only if a focal lesion is detected, a scenario called “population-based surveillance”. In Italy and other countries, thanks to the widespread diffusion of ultrasound equipment and the motivation of an active medical sonography society, US is directly performed by hepatologists with sonographic skills and able to perform the examinations by themselves (i.e. specialist or subspecialist surveillance). Population-based surveillance is mainly implemented in the United States, while specialist surveillance is more widespread in Europe and Asia. A systematic review and meta-analysis comparing the two US modalities reported a much higher effectiveness of surveillance in the specialist setting (73.7%) rather than in population-based cohorts (8.8 %), with the lowest effectiveness in patients with alcoholic and NASH cirrhosis[22]. There are many reasons for the difference. PCPs and radiologists without specific expertise in liver disease may have limited knowledge of the international guidelines[17,23]. Concerns of other serious comorbidities, logistic problems such as poor accessibility of ultra
Liver sonography is recommended by all existent guidelines as the standard HCC surveillance test to be used. A meta-analysis of 15 studies of cirrhotic patients showed that abdominal ultrasound had good sensitivity (84%) and excellent specificity (91%) for the detection of HCC at any stage[27]. However, the pooled sensitivity for the detection of early-stage HCC was as low as 47%, with only marginal improvement (53%) after exclusion of the studies performed before the introduction of high-density crystal probes and harmonic imaging. It is noteworthy that the sensitivity of ultrasound is characterized by a wide range of variation (from 21% to 81%), reflecting the operator dependency of US scans. In fact, in a single study performed at a university-based tertiary care center, the sensitivity of abdominal ultrasound was 82%[28]. Similarly, in a large Italian cohort study, where examinations were performed in both outside facilities and hospital wards, the sensitivity rose to 66%[18]. It is important to consider that ultrasound examinations in the United States were per
The use of CT or MRI can obviate the above-mentioned technical limitations of ultrasound, but they rarely can identify an aggressive tumor at an early stage. In one study, 20% of the ultrasound examinations were considered technically inadequate for excluding HCC[29]. In another study, 32% of HCCs were detected beyond the early stage by semiannual US, 12% of them showing biologically aggressive features, i.e., AFP > 1000 ng/mL, vascular thrombosis, distant metastasis, or infiltrative pattern[18]. Therefore, technical limitations of US to detect early-stage HCC can be deemed to be around 20%, hence it is important that ultrasound reports include a statement about the quality of the examination and the potential recommendation to perform second-level imaging (CT or MRI). MRI has a sensitivity of 83.7% for the detection of early-stage HCC[30] and should be preferred to CT, which has lower sensitivity (62.5%), similar to that of ultrasound[31]. However, it is noticeable that biologically aggressive tumors are poorly detected by MRI (16% failure to detect HCC) as well as by US (12% failure). Those tumors thus represent a hardcore of HCCs eluding both kinds of imaging surveillance. The good news is that only a small percentage of HCCs have those characteristics. However, it should be recognized that the pattern of HCC growth is heterogeneous[32] and many indolent tumors can develop an aggressive pattern with time, as shown by an increase of the percentage of biologically aggressive tumors detected by annual compared with semiannual surveillance, (i.e. 28% vs 12%)[18]. The possibility that indolent HCCs might transform into more aggressive tumors with time has raised some concerns of the common real-life practice of performing annual instead of semiannual MRI to reduce surveillance cost
Contrast-enhanced US with intravascular agents (e.g., Levovist, Sonovue) has a limited role in surveillance as it is impossible to scan the whole liver in the arterial phase when small tumors are detected[33]. On the other hand, perfluorocarbon (Sonazoid) seems to a promising agent[34], as it is taken up and retained by Kupffer cells for 60 min after bolus injection, allowing a scan of the entire liver in the late phase when HCC appears as a black hole. Sonazoid-enhanced US might therefore be useful to detect HCC developing in livers with a coarse echotexture, but it cannot be of any advantage in obese patients or severe fatty livers, where CT or MRI remain the only option to accurately exclude HCC.
The sensitivity of AFP to detect early-stage HCC is too low to be used alone as a surveillance tool[35]. Only the Asian-Pacific Guidelines recommend the use of AFP in surveillance programs, with a cutoff of 200 ng/mL[2]. This cutoff is highly specific but reduces AFP sensitivity to a level as low as 22%[12]. However, new and effective pharmacological treatment of hepatitis B and C may remove false-positive AFP results caused by necro-inflammatory activity and enable the use of lower cutoff levels, providing better AFP sensitivity without compromising its specificity. Another method to improve AFP diagnostic accuracy is to monitor its changes over time[36]. An algorithm including AFP variations has been created and was tested in the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis Trial cohort, with good results in identifying patients at increased risk of HCC[37]. A recent meta-analysis showed that the addition of AFP levels to abdominal ultrasound improved the overall sensitivity for the detection of early-stage HCC from 45% to 63%[27], but as pointed out before, the low sensitivity of US in the meta-analysis raises doubts on the quality of the ultrasound examinaions. It therefore remains an open question whether AFP can improve the performance of properly executed sonography. It is important to stress that the addition of AFP screening to abdominal sonography could adversely affect the already low level of surveillance application by PCPs because of the logistic and financial burden introduced by duplicating the surveillance tool. In our real-life practice we assess AFP levels alongside abdominal US only in patients with cured hepatitis C or well-controlled chronic hepatitis B and optimal adherence to follow-up visits. A change in AFP values over time prompts performing second-level imaging, particularly in cases with a coarse echo pattern on sonography. On the other hand, stable AFP levels permit advising the continuation of standard US, avoiding additional cost.
In recent years, new markers have been proposed for the diagnosis of early HCC. Each of them by itself does not seem to confer any benefit in diagnostic accuracy compared with US with or without AFP[35]. However, a combination of AFP-L3, AFP, and des-gamma-carboxy prothrombin was tested in a large study and proved to have a sensi
Patient adherence is key to the effectiveness of the surveillance program. Racial and ethnic minorities have poor awareness of surveillance programs, probably because of their low socioeconomic status. Patients also report difficulties scheduling surveillance ultrasound, concerns of the cost, need of off-work days, and difficulties reaching the facilities where the exam is performed[39]. The difficulties have a greater impact on subspecialty-based than on population-based surveillance, and should be addressed by appropriate interventions. In our hospital, the great majority of examinations are performed by hepatologists (PD and SL) and each appointment is directly booked by the specialist, thus making the scheduling process much easier. Visits and US examinations are reimbursed by the National Health Care System, with no additional cost for the patient. They are both performed at the same time to minimize off-work time, and if the patient is under antiviral treatment, the drug can be collected from the hospital pharmacy on the same day. Table 1 shows adherence to surveillance in 362 cirrhotic patients followed from January 2013 through December 2020 in our outpatient clinic. Adherence was defined as the performance of regular annual or semiannual ultra
| Etiology of cirrhosis | Adherent | Semiannual | Annual | Nonadherent |
| Alcoholic (103) | 55 (52%) | 31 (56%) | 24 (44%) | 48 (46%) |
| HBV (53) | 39 (73%) | 35 (90%) | 4 (10%) | 14 (27%) |
| HCV (164) | 119 (72%) | 101 (85%) | 18 (15%) | 45 (28%) |
| NAFLD (42) | 24 (57%) | 17 (71%) | 7 (29%) | 18 (43%) |
| Total: 362 | 237 (65%) | 184 (77%) | 53 (23%) | 125 (35%) |
In conclusion, despite generalized consensus of all scientific guidelines on the utility of surveillance for HCC in high-risk patients, the implementation of surveillance at the community level is far from ideal. Low access to surveillance programs is associated with a lower number of HCC diagnosed at an early stage and decreased survival. Therefore, it is important to study the obstacles hampering the adherence to surveillance of the high-risk population in order to implement appropriate inter
Adherence to surveillance programs is higher in hospital/subspecialty-based surveillance compared with population-based surveillance, in which tests are ordered by PCPs and performed by general radiologists. Subspecialty-based surveillance should therefore be the first choice when feasible. However, the geographic location of the facilities and the distance from the hospital may result in population surve
Surveillance for HCC in cirrhotic patients and chronic hepatitis B patients allows detection of the tumor at an early stage and improves survival.
International guidelines recommend semiannual ultrasound with or without AFP monitoring in these patients, but surveillance implementation at the community level is low.
Education programs targeting PCP and aiming at improving the identification and referral of patients at risk of HCC should be implemented.
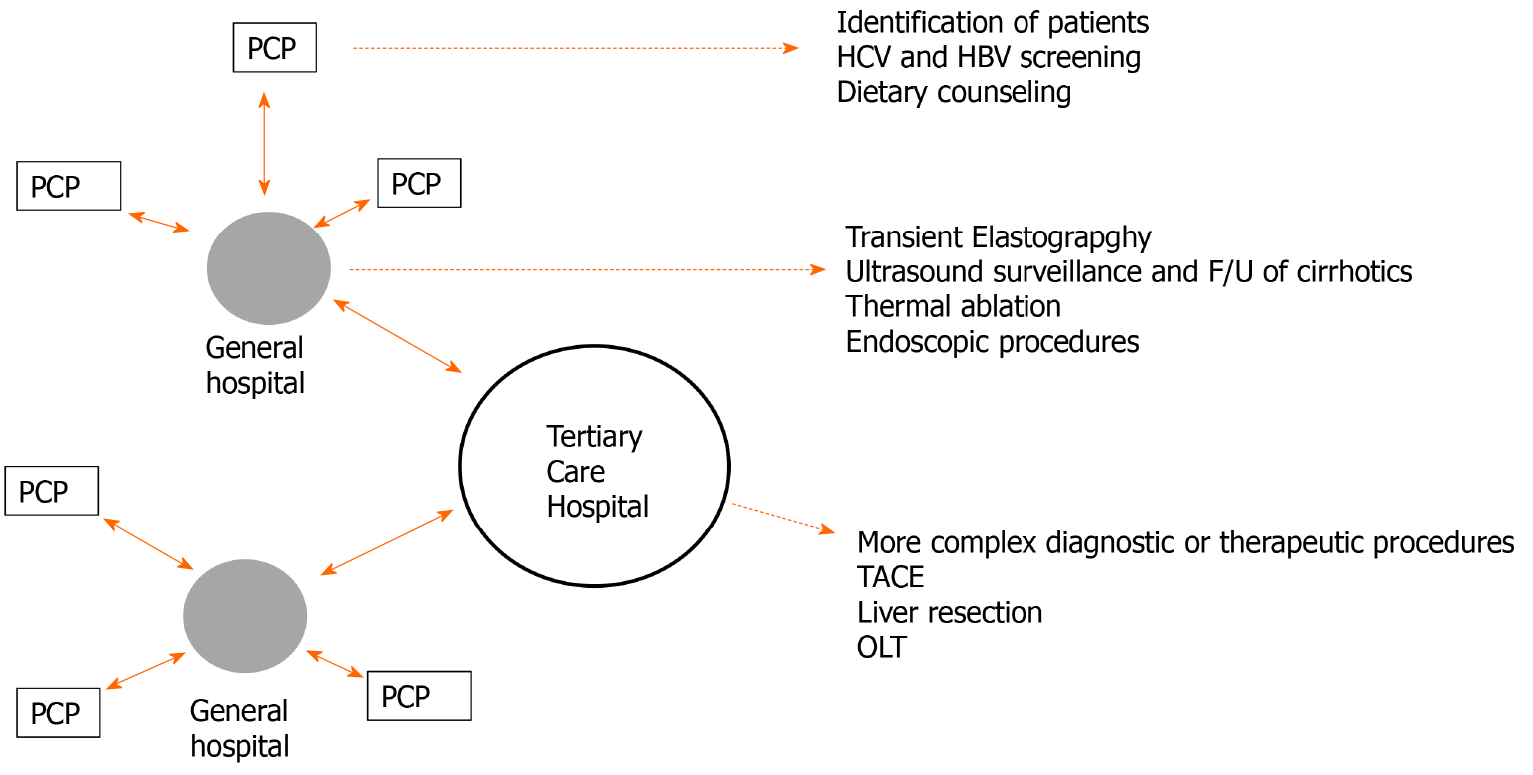
Subspecialty-based surveillance performed by the hepatologist or gastroenterologist in the setting of the liver clinic of a general hospital is the preferred model, when feasible.
Ultrasound and MRI should be used sequentially in case of inadequate sonography.
Surveillance should be incorporated into the specific care and follow-up provided by the liver clinic to enhance adherence.
The liver clinic of the general hospital should be integrated into a hub and spoke model of care alongside PCPs and tertiary referral hospitals to ensure proper access to care.
We thank Dr. Francesca De Martini for scientific and language revision of the English text.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cojocariu C, Zhang L S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wu RR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64455] [Article Influence: 16113.8] [Reference Citation Analysis (176)] |
| 2. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1637] [Article Influence: 204.6] [Reference Citation Analysis (0)] |
| 3. | Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ. 2018;362:k2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 579] [Article Influence: 82.7] [Reference Citation Analysis (1)] |
| 4. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 943] [Article Influence: 44.9] [Reference Citation Analysis (1)] |
| 5. | El-Serag HB, Kramer JR, Chen GJ, Duan Z, Richardson PA, Davila JA. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut. 2011;60:992-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Pateron D, Ganne N, Trinchet JC, Aurousseau MH, Mal F, Meicler C, Coderc E, Reboullet P, Beaugrand M. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 188] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Trevisani F, De Notariis S, Rapaccini G, Farinati F, Benvegnù L, Zoli M, Grazi GL, Del PP, Di N, Bernardi M; Italian Liver Cancer Group. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience). Am J Gastroenterol. 2002;97:734-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 602] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 9. | Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 264] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Lin OS, Keeffe EB, Sanders GD, Owens DK. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther. 2004;19:1159-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, Jackson S, Ryder S, Price A, Stein K. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess. 2007;11:1-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6039] [Article Influence: 862.7] [Reference Citation Analysis (3)] |
| 13. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3017] [Article Influence: 431.0] [Reference Citation Analysis (3)] |
| 14. | Noone A, Howlader N, Krapcho M. Cancer statistics review, 1975-2015. Bethesda, MD: National Cancer Insitute, 2018. |
| 15. | Singal AG, Yopp A, S Skinner C, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 16. | Simmons OL, Feng Y, Parikh ND, Singal AG. Primary Care Provider Practice Patterns and Barriers to Hepatocellular Carcinoma Surveillance. Clin Gastroenterol Hepatol. 2019;17:766-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Dalton-Fitzgerald E, Tiro J, Kandunoori P, Halm EA, Yopp A, Singal AG. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:791-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Del Poggio P, Olmi S, Ciccarese F, Di Marco M, Rapaccini GL, Benvegnù L, Borzio F, Farinati F, Zoli M, Giannini EG, Caturelli E, Chiaramonte M, Trevisani F; Italian Liver Cancer (ITA. LI.CA) Group. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1927-33.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Cozzolongo R, Cuppone R, Petruzzi J, Stroffolini T, Manghisi OG. Approach of primary care physicians to hepatitis C: an educational survey from a Southern Italian area. J Infect. 2005;51:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Delarocque-Astagneau E, Meffre C, Dubois F, Pioche C, Le Strat Y, Roudot-Thoraval F, Hillon P, Silvain C, Dhumeaux D, Desenclos JC; Hepatitis C Surveillance System Committee; Scientific Committee for the National Prevalence Survey of Hepatitis B and C Markers. The impact of the prevention programme of hepatitis C over more than a decade: the French experience. J Viral Hepat. 2010;17:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Del Poggio P, Olmi S, Ciccarese F, Mazzoleni M, Jazzetti M, Jamoletti C, Mattiello M, Del Poggio A, Portugali V, Stroffolini T. A training program for primary care physicians improves the effectiveness of ultrasound surveillance of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015;27:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology. 2021;73:713-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 215] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 23. | McGowan CE, Edwards TP, Luong MU, Hayashi PH. Suboptimal surveillance for and knowledge of hepatocellular carcinoma among primary care providers. Clin Gastroenterol Hepatol. 2015;13:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Farvardin S, Patel J, Khambaty M, Yerokun OA, Mok H, Tiro JA, Yopp AC, Parikh ND, Marrero JA, Singal AG. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 25. | Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol. 2015;13:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Singal AG, Tiro JA, Murphy CC, Marrero JA, McCallister K, Fullington H, Mejias C, Waljee AK, Pechero Bishop W, Santini NO, Halm EA. Mailed Outreach Invitations Significantly Improve HCC Surveillance Rates in Patients With Cirrhosis: A Randomized Clinical Trial. Hepatology. 2019;69:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 808] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 28. | Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 457] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 29. | Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, Parikh ND, Browning T, Singal AG. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 264] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 30. | Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, Won HJ, Lee SJ, Lee HC, Lee YS. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 31. | Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther. 2013;38:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 32. | Rich NE, John BV, Parikh ND, Rowe I, Mehta N, Khatri G, Thomas SM, Anis M, Mendiratta-Lala M, Hernandez C, Odewole M, Sundaram LT, Konjeti VR, Shetty S, Shah T, Zhu H, Yopp AC, Hoshida Y, Yao FY, Marrero JA, Singal AG. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology. 2020;72:1654-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 33. | Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol. 2008;48:848-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Kudo M, Hatanaka K, Kumada T, Toyoda H, Tada T. Double-contrast ultrasound: a novel surveillance tool for hepatocellular carcinoma. Am J Gastroenterol. 2011;106:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 304] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 36. | Lee E, Edward S, Singal AG, Lavieri MS, Volk M. Improving screening for hepatocellular carcinoma by incorporating data on levels of α-fetoprotein, over time. Clin Gastroenterol Hepatol. 2013;11:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Tayob N, Lok AS, Do KA, Feng Z. Improved Detection of Hepatocellular Carcinoma by Using a Longitudinal Alpha-Fetoprotein Screening Algorithm. Clin Gastroenterol Hepatol. 2016;14:469-475.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, Schweitzer N, Vogel A, Manns MP, Benckert J, Berg T, Ebker M, Best J, Dechêne A, Gerken G, Schlaak JF, Weinmann A, Wörns MA, Galle P, Yeo W, Mo F, Chan SL, Reeves H, Cox T, Johnson P. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol. 2016;14:875-886.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 39. | Wong RJ, Ahmed A; Hepatocellular Carcinoma Research Committee of the Chronic Liver Disease Foundation. Understanding Gaps in the Hepatocellular Carcinoma Cascade of Care: Opportunities to Improve Hepatocellular Carcinoma Outcomes. J Clin Gastroenterol. 2020;54:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |