Published online Sep 7, 2021. doi: 10.3748/wjg.v27.i33.5566
Peer-review started: March 9, 2021
First decision: May 1, 2021
Revised: May 12, 2021
Accepted: August 12, 2021
Article in press: August 12, 2021
Published online: September 7, 2021
Processing time: 178 Days and 10.8 Hours
Oral intake is dependent on the gastric ability to accommodate the food bolus. Comparatively, neonates have a smaller gastric capacity than adults and this may limit the volume of their milk intake. Yet, we previously reported that the newborn rat gastric milk volume is greatest after birth and, when normalized to body weight, decreases with postnatal age. Such age-dependent changes are not the result of intake differences, but greater gastric accommodation and reduced emptying rate.
Hypothesizing that breastmilk-derived adiponectin is the factor regulating gastric accommodation in neonates, we comparatively evaluated its effects on the rat fundic muscle tone at different postnatal ages.
In freshly dispersed smooth muscle cells (SMC), we measured the adiponectin effect on the carbachol-induced length changes.
Adiponectin significantly reduced the carbachol-stimulated SMC shortening independently of age. In the presence of the inhibitor iberiotoxin, the adiponectin effect on SMC shortening was suppressed, suggesting that it is mediated via large-conductance Ca2+ sensitive K+ channel activation. Lastly, we comparatively measured the newborn rat gastric milk curd adiponectin content in one- and two-week-old rats and found a 50% lower value in the latter.
Adiponectin, a major component of breastmilk, downregulates fundic smooth muscle contraction potential, thus facilitating gastric volume accommodation. This rodent’s adaptive response maximizes breastmilk intake volume after birth.
Core Tip: Gastric accommodation regulates the stomach content volume. Lactating rats continuously breastfeed to keep a full gastric milk volume and their gastric emptying time is directly related to the gastric content volume. Little is known about the gastric fundic accommodation regulatory factors early in life. In this study, breastmilk-derived adiponectin is shown to promote gastric fundic relaxation, thus playing an important regulatory role during the lactating period.
- Citation: Wang H, Esemu-Ezewu P, Pan J, Ivanovska J, Gauda EB, Belik J. Adiponectin and the regulation of gastric content volume in the newborn rat. World J Gastroenterol 2021; 27(33): 5566-5574
- URL: https://www.wjgnet.com/1007-9327/full/v27/i33/5566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i33.5566
Among other factors, oral intake depends on the gastric muscle accommodation to its content. The estimated receptive gastric milk volume is 20 mL for a full-term infant[1]. For an average full-term infant weighing 3.5 kg, this amounts to 5.7 mL/kg receptive gastric volume. The comparative adult gastric receptive volume has not been ade
Comparative studies addressing the nutritional benefit and risks associated with lower (150-180 mL/kg/d) and higher (> 200 mL/kg/d) total enteral feed intake for preterm infants have been conducted. A recent metanalysis of such studies reported limited data and concluded that insufficient evidence is available[2]. Yet, at least one recent review on nutritional support strategies for preterm infants advised that more gestationally mature neonates can tolerate and benefit from a daily milk intake as high as 240 mL/kg/d or 20 mL/kg/feed[3].
Since the recommended maximum daily intake of milk exceeds the estimated stomach receptive volume, adaptive mechanisms must be present to overcome the reduced gastric functional capacity early in life. In adult life, the gastric accom
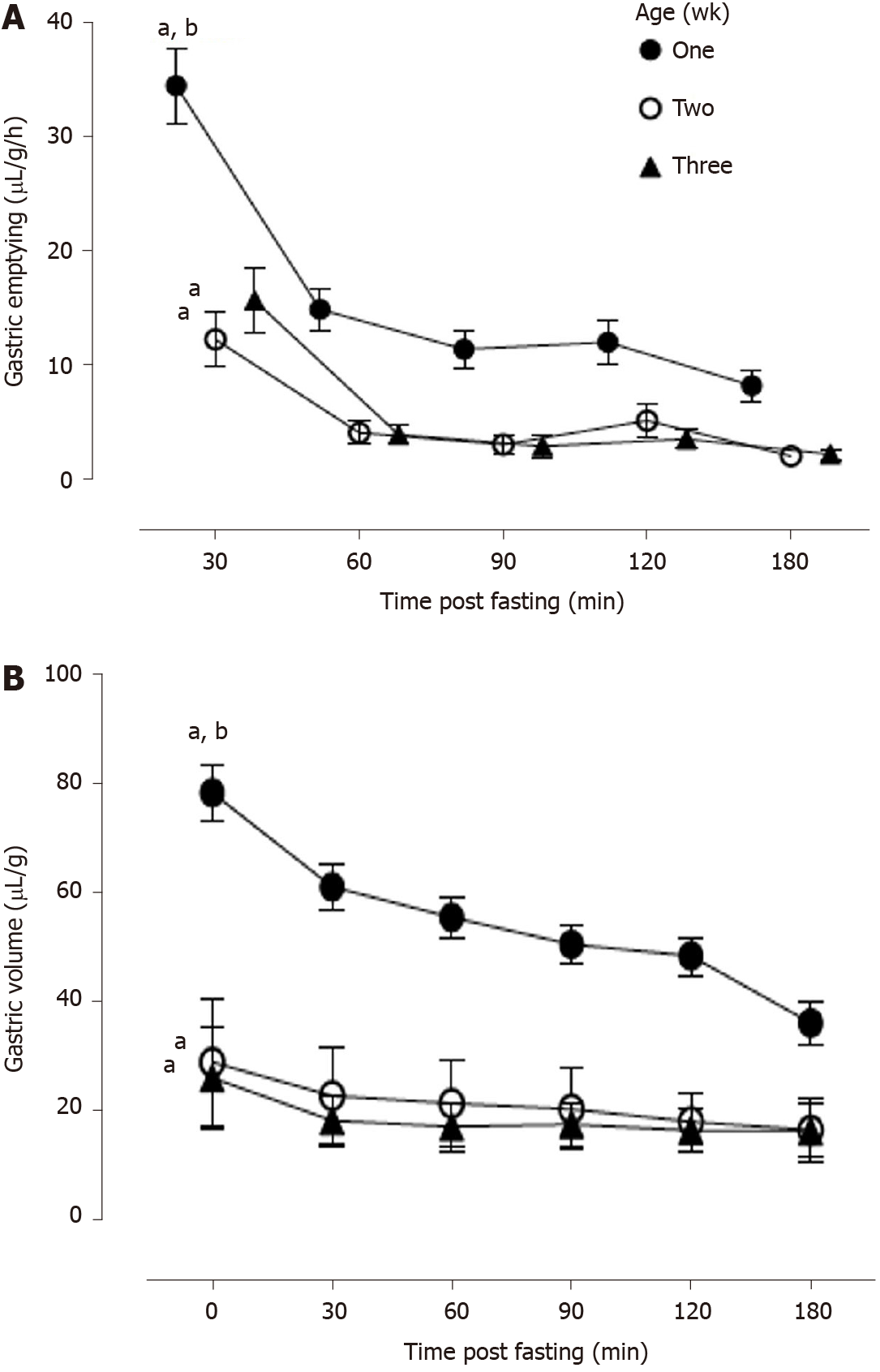
Aside from increasing total enteral intake, gastric accommodation may also have an important role in maximizing its content emptying potential. We have previously reported that the rat gastric functional capacity is increased during the immediate neonatal period and this facilitates its content emptying rate[5]. Figure 1 shows our previously reported data on the rat gastric content volume and emptying rates early in life[5]. The younger the rat, the higher is the gastric milk content, such that in the one-week pup the volume after breastfeeding, is two-fold greater, when compared with measurements obtained in the second week of life[5]. The mechanism accounting for the age-related gastric accommodation changes, early in life, is poorly understood and the main goal of the present study.
Adiponectin is an adipose tissue-derived adipokine that plays a major role in appetite regulation and other important physiological mechanisms[6]. The adiponectin concentration in human and rodent breastmilk, is more than 40 folds greater, when compared with the other major adipokines present[7,8]. The available evidence suggest that breast-derived adiponectin may play a functional role in the regulation of gastric milk volume accommodation. Adiponectin receptors are present in fetal rodents’ gastrointestinal tract[9] and in the adult rat, adiponectin modulates gastric tone[10].
A study comparatively evaluating the maternal breastmilk adiponectin content and their infants’ gastric emptying time showed an inverse relationship between these parameters[11]. That is to say that the higher the maternal adiponectin breastmilk content, the slower is their infant’s gastric emptying time. This suggest that adiponectin plays a facilitating role in the stomach receptive potential for milk volume by increasing gastric accommodation. Such regulatory role has not been previously evaluated in the newborn.
Therefore, the main goal of this study was to evaluate the adiponectin effect on the newborn rat gastric fundic muscle response to an agonist-induced contraction. We hypothesized that adiponectin downregulates the newborn fundic muscle tone thus allowing for greater accommodation to a larger milk volume, early in life.
Studies were conducted in a laboratory setting utilizing newborn and adult rat gastric tissue.
Sprague-Dawley (Charles River, Montreal, QC, Canada) newborn (3-7 d old; n = 9; female n = 5, male n = 4), juvenile (8-14 d old; n = 4 female n = 2, male n = 2) and adult (> 60 d old; n = 7 female n = 2, male n = 5) rats were studied. The adult rats were fed a regular rodent diet composed of animal and vegetable protein (Certified Rodent Diet 5002, LabDiet, St. Louis, MO, United States) and housed under standard lighting and temperature conditions.
The animals were sacrificed with pentobarbital sodium injection (60 mg/kg i.p). Immediately after death, the gastric tissue was removed fresh for functional studies. The gastric milk curd was completely removed intact, snap-frozen in liquid nitrogen and stored at -80℃ for less than one month, for later processing. The rationale for studying this stomach anatomical segment relates to the fact that accommodation of the food bolus occurs in the gastric fundus.
To evaluate the adiponectin effect on the gastric muscle contraction independently of its innervation, and other tissue factors, as well as under the more physiologic isotonic conditions, we measured the individual smooth muscle cells (SMC) shortening response to this metabolite. In summary, the gastric tissue was digested in 1 mL/mg collagenase and 0.01% Soybean Trypsin Inhibitor (ThermoFisher, Burlington, Ontario, Canada) and the SMCs were dispersed in DMEM cell culture media (Wisent, St. Bruno, Quebec, Canada). One hour after dispersion the SMCs were exposed to carbachol-at a final media concentration of 10-4 for 15 min, while maintained at 37℃ and 5% CO2 in a conventional incubator in the absence or presence of adiponectin (4 µl/mL). To study the mechanism by which adiponectin downregulates smooth muscle contraction, we evaluated the iberiotoxin (10-7 M) pre-incubation effect on the carbachol-induced response.
Following this, the cells were immediately fixed with 1% acrolein. Cells maintained for a similar duration in Calcium-free PBS media served as control. Light microscopy images at 20 × magnification was obtained using Leica DM IRE2 microscope (Wetzlar, Germany) and the cells’ length were measured using ImageJ (NIH, Bethesda, MA, United States). H2O2-induced cells length changes (shortening) were expressed as percentage of average control SMC length. The cells were obtained from seven newborns and five adult animals. A minimum of 50 H2O2-exposed and control cells from age group were evaluated.
Milk curds were obtained from newborn (3-7 d) and juvenile (8-14 d) rats. The curd proteins were extracted, and their adiponectin content quantified by utilizing a commercially available ELISA kit (RRP300, R&D systems) according to the manufacturer’s recommendations. Briefly, the pup’s milk curd was carefully extracted from the gastric cavity at once, after they were euthanized. The curd was weighed and sonicated in RIPA buffer for 5-10 s three times. The mixture was centrifuged at 17949 g. The separated middle layer (skim milk) was used for adiponectin measurement and protein quantification.
Data were first evaluated to determine Gaussian distribution by Skewness, Kurtosis and Omnibus testing. Normally distributed data were analyzed by parametric tests. Group differences were statistically evaluated by unpaired Student’s t-test or two-way ANOVA with multiple comparisons obtained by the Tukey-Kramer test. Statistical significance was determined at P < 0.05. All statistical analyses were performed with the Number Cruncher Statistical System software (NCSS, Kaysville, Utah). Data are presented as mean ± SE.
The sample size utilized for each experiment is listed in the Figure legends and refers to SMC number. Given their high yield, we utilized large sample sizes for freshly dispersed SMCs, to minimize the possibility of a type I error.
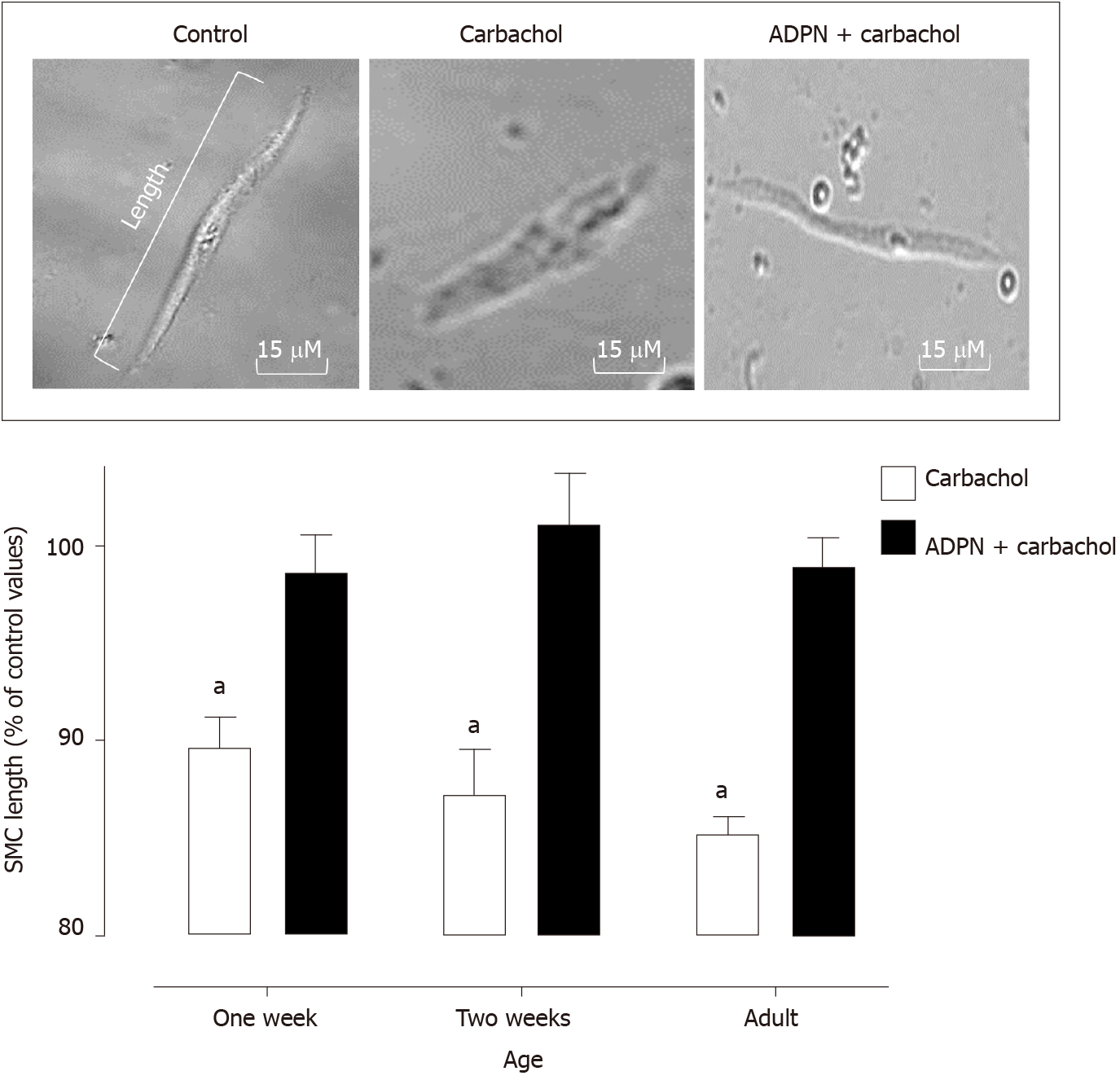
SMCs derived from rat’s gastric fundus significantly shortened in response to carbachol stimulation. One-, two-weeks and adult SMC showed a similar response. Pre-incubation with adiponectin suppressed the carbachol-induced SMC shortening at all ages (Figure 2).
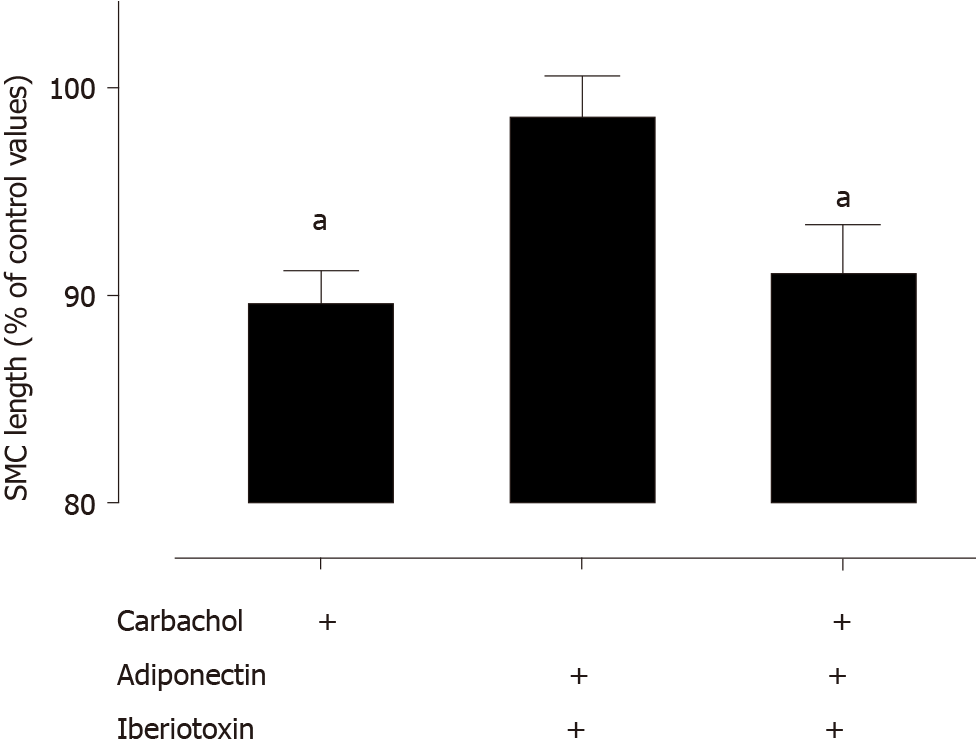
To investigate the mechanism accounting for the adiponectin-dependent effect on the agonist-induced newborn fundic SMC shortening, we tested the effect of iberiotoxin on the response (Figure 3). This large-conductance Ca2+ sensitive K+ channel inhibitor was shown, by others, to be involved in the adiponectin-induced vascular smooth muscle relaxation[12]. Following pre-incubation with iberiotoxin the carbachol-induced SMC shortening was not altered by the presence of adiponectin. This observation suggests that the adiponectin downregulation of agonist induced fundic SMC shortening is mediated via modulation of large-conductance Ca2+ sensitive K+ channels.
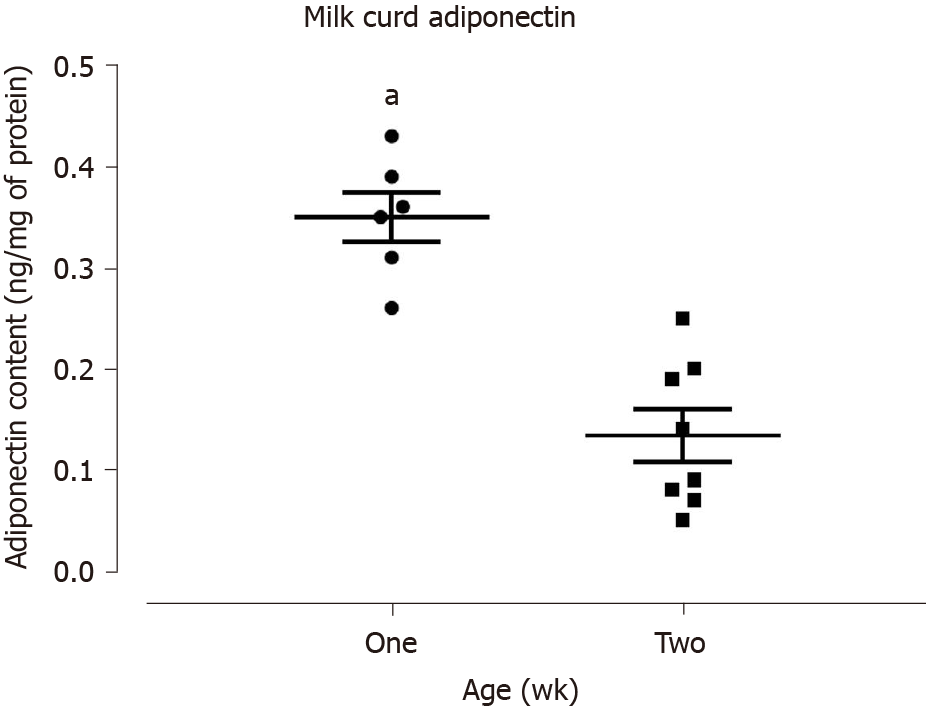
Lastly, as a surrogate measurement of the maternal breastmilk concentration, we evaluated the newborn rat milk curd adiponectin content at one- and two-weeks of age. Adiponectin was present at both ages, but the one-week milk curd adiponectin content was two-fold higher, when compared with values obtained from two-week old rats (Figure 4).
The findings of the present study suggest that breastmilk-derived adiponectin is the key regulator of gastric milk volume and clearance rate in the neonatal rat. Activation of large-conductance Ca2+ sensitive K+ channels in fundic smooth muscle cells by adiponectin mediates this response.
Whereas in humans, the newborn enteral intake is dependent on milk offer, rodent pups can independently breastfeed on demand. This leads to the well-known observation of a consistently milk-filled stomach in suckling newborn rats. Experiments conducted decades ago showed that the regulation of gastric content in the newborn rat is not based on satiety, but stomach distention[13]. In a recently published study, we took advantage of this unique neonatal behavior to evaluate in vivo, the developmental pattern of gastric content volume and emptying time.
We showed that the rat gastric content volume, normalized to body weight, is highest at one-week, when compared with the later breastfeeding period[5]. In addition, the newborn rat’s gastric milk emptying rate is directly proportional to the stomach’s curd volume[5]. We further documented that a similar positive correlation between gastric milk volume and its emptying rate is present in preterm neonates[14].
Such observations imply that, in the immediate neonatal period, an operative physiological mechanism is present and geared at maximizing enteral intake to enhance gastric accommodation to the milk volume. The present study findings suggest that breastmilk-derived adiponectin is the factor responsible for this gastric accommodation response in newborn rats.
By downregulating the fundic smooth muscle agonist-induced contraction potential, adiponectin increases gastric accommodation thus maximizing the stomach’s milk volume retention. As shown in this study, the developmental pattern of this response is mediated not by age differences in the gastric SMC properties, but by the post
Adiponectin is an adipokine primarily secreted by adipose tissue that has important physiological modulatory roles[15]. These include anti-inflammatory, antioxidant, antiatherosclerosis, appetite regulation and insulin-sensitizing effects. Yet, its presence in breastmilk raises provocative questions about its function in neonates.
Adiponectin is secreted by the breast adipose tissue and is the most abundant adipokine in human breast milk[7]. In humans, the breastmilk adiponectin content is highest in the early postpartum period and decreases overtime[16]. Similar pattern of breastmilk adiponectin content and postpartum changes were previously reported in rats[17], and the finding confirmed in the present study. Most importantly, adiponectin is rapidly taken-up by the gastrointestinal tract resulting in peak serum concentrations two hours after enteral administration in newborn mice[8]. This suggest that breastmilk-derived adiponectin plays a functional role early in life.
AdipoR1 and AdipoR2, the two adiponectin receptors are expressed during embryogenesis in mice, with the former being the predominant one in most tissues[9]. These receptors are also present in adult rodents’ gastric tissue and the electrical field stimulation contractile potential of its muscle is reduced in the presence of exogenous adiponectin[10,18]. Whether adiponectin modulates the nerve stimulus conduction, or the gastric smooth muscle itself if unclear since one study in adult mice showed an effect on the vagal nerve [18]. In adult mice, adiponectin downregulates gastric muscle cell excitability[19], but this report is the first to study fundic SMCs from a newborn rodent.
Adiponectin not only reduces gastric muscle tone but has a similar effect on other smooth muscle dependent organs. In vascular tissue, adiponectin induces vasodilation[20] and reduces uterine contractions[21]. In the present study we evaluated the adiponectin effect on freshly dispersed rat SMCs from newborn, juvenile and adult animals. At all ages we documented a similar reduction in agonist-stimulated SMC shortening showing that adiponectin acts directly on the fundic muscle.
The signal-transduction pathway responsible for the adiponectin modulation of smooth muscle contraction remains unclear. In vascular tissue, adiponectin promotes endothelial-derived nitric oxide generation and it enhances its biological activity, both of which mediate the adiponectin vasodilatory response[22-24]. In addition, adiponectin has a direct effect on vascular smooth muscle large-conductance Ca2+ sensitive K+ channels that also result in reduced vasomotor tone[12,25].
The present data show that large-conductance Ca2+ sensitive K+ channels modulate the newborn rat gastric SMC contraction potential. Iberiotoxin, a large-conductance Ca2+ sensitive K+ channels inhibitor of rodent fundic muscle relaxation[26], suppressed the adiponectin-mediated reduction in the carbachol-induced shortening. Our findings are in keeping with the adult mice gastric fundus, where adiponectin hyperpolarized the SMC resting membrane potential resulting in reduced voltage-dependent Ca2+ currents[19].
In support of breastmilk being a major source of adiponectin to the newborn, we confirmed its presence in newborn and juvenile rats’ milk curds. As previously shown to be the case for human breastmilk[16], we documented that the rat milk curds’ adiponectin content was inversely proportional to postpartum age and two-fold lower in the second, when compared with first postnatal week.
Together our findings suggest that breastmilk-derived adiponectin plays an important role in the newborn rat milk volume intake. Human derived data support the current findings. An evaluation of mothers’ breastmilk adiponectin content and their infants’ gastric emptying time showed an inverse relationship between these parameters. The higher the breastmilk adiponectin the longer was the infant’s gastric milk clearance[11].
This study has obvious limitations. Rat fundic SMCs agonist-induced shortening was used as a surrogate marker of gastric milk accommodation and the breastmilk adiponectin content was obtained from the newborn milk curds. Yet, it is well known that gastric accommodation to the food bolus depends on the fundic muscle relaxation[27] and thus the fundic SMC shortening response allows for the study of the adiponectin direct effect its muscle tone. Although the adiponectin content of the newborn gastric curd is probably not similar to the one from fresh breastmilk, if anything the former value is likely lower, when compared with breastmilk.
In conclusion, adiponectin reduces the gastric fundus motor tone allowing for greater accommodation of the food bolus. This response is present in newborn-derived smooth muscle cells and mediated via large conductance Ca2+ sensitive K+ channels. Breastmilk has a high adiponectin content and this adipokine likely enhances volume intake by increasing the gastric functional volume capacity (accommodation). These observations have translational importance since preterm infants are often fed either processed breastmilk or formula, both of which may not contain sufficient adiponectin to promote gastric fundic relaxation.
Oral intake depends on the gastric ability to accommodate the food bolus. The preterm infant has a lower gastric capacity, normalized to body weight, when compared with adults, thus potentially limiting their milk intake. Yet, we previously shown that one-week rat pups milk intake is greater than observed, as they mature.
The main rationale for the study experiments was to understand the mechanism accounting for greater food accommodation early in life.
The main objective was to evaluate the hypothesis that the adiponectin in breast milk increases the newborn rat ability to accommodate the food bolus by reducing the fundic muscle tone.
Rat freshly dispersed smooth muscle cells were used to measure the adiponectin effect on carbachol-induced fundic muscle shortening.
Adiponectin significantly reduced the carbachol-stimulated smooth muscle cells shortening independently of age, via large-conductance Ca2+ sensitive K+ channel activation.
Breast milk containing adiponectin regulates the newborn rat milk intake by increasing the gastric fundic accommodation potential.
Maternal-neonatal interaction via breast milk components content provides a novel and likely important regulatory role on intake volume early in life.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM S-Editor: Wu YXJ L-Editor: A P-Editor: Liu JH
| 1. | Bergman NJ. Neonatal stomach volume and physiology suggest feeding at 1-h intervals. Acta Paediatr. 2013;102:773-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Abiramalatha T, Thomas N, Gupta V, Viswanathan A, McGuire W. High vs standard volume enteral feeds to promote growth in preterm or low birth weight infants. Cochrane Database Syst Rev. 2017;9:CD012413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Hay WW Jr. Nutritional Support Strategies for the Preterm Infant in the Neonatal Intensive Care Unit. Pediatr Gastroenterol Hepatol Nutr. 2018;21:234-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Vanden Berghe P, Janssen P, Kindt S, Vos R, Tack J. Contribution of different triggers to the gastric accommodation reflex in humans. Am J Physiol Gastrointest Liver Physiol. 2009;297:G902-G906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Ferreira CHF, Shifrin Y, Pan J, Ivanovska J, McNamara PJ, Belik J. The newborn rat gastric emptying rate is volume and not developmentally dependent. Neurogastroenterol Motil. 2018;30:e13233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Gauda EB, Master Z. Contribution of relative leptin and adiponectin deficiencies in premature infants to chronic intermittent hypoxia: Exploring a new hypothesis. Respir Physiol Neurobiol. 2018;256:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kratzsch J, Bae YJ, Kiess W. Adipokines in human breast milk. Best Pract Res Clin Endocrinol Metab. 2018;32:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Newburg DS, Woo JG, Morrow AL. Characteristics and potential functions of human milk adiponectin. J Pediatr. 2010;156:S41-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Zhou Y, Sun X, Jin L, Stringfield T, Lin L, Chen Y. Expression profiles of adiponectin receptors in mouse embryos. Gene Expr Patterns. 2005;5:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Idrizaj E, Garella R, Castellini G, Mohr H, Pellegata NS, Francini F, Ricca V, Squecco R, Baccari MC. Adiponectin affects the mechanical responses in strips from the mouse gastric fundus. World J Gastroenterol. 2018;24:4028-4035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Gridneva Z, Kugananthan S, Hepworth AR, Tie WJ, Lai CT, Ward LC, Hartmann PE, Geddes DT. Effect of Human Milk Appetite Hormones, Macronutrients, and Infant Characteristics on Gastric Emptying and Breastfeeding Patterns of Term Fully Breastfed Infants. Nutrients. 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Weston AH, Egner I, Dong Y, Porter EL, Heagerty AM, Edwards G. Stimulated release of a hyperpolarizing factor (ADHF) from mesenteric artery perivascular adipose tissue: involvement of myocyte BKCa channels and adiponectin. Br J Pharmacol. 2013;169:1500-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Lorenz DN. Gastric emptying of milk in rat pups. Am J Physiol. 1985;248:R732-R738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Ferreira CHF, Martinez FE, Crott GC, Belik J. Gavage Feed Volume Determines the Gastric Emptying Rate in Preterm Infants. J Pediatr Gastroenterol Nutr. 2018;67:e43-e46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Cuerq C, Morineau G, Dufour-Rainfray D, Vatier C, Fellahi S, Vigouroux C, Genoux A, Lacorte JM, Charchour R, Fève B, Capeau J, Collet C, Bastard JP; groupe de travail RIHN Adipokines. [Mutltifaceted biological roles of adiponectin]. Ann Biol Clin (Paris). 2020;78:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 16. | Ley SH, Hanley AJ, Sermer M, Zinman B, O'Connor DL. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. Am J Clin Nutr. 2012;95:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Nozhenko Y, Asnani-Kishnani M, Rodríguez AM, Palou A. Milk Leptin Surge and Biological Rhythms of Leptin and Other Regulatory Proteins in Breastmilk. PLoS One. 2015;10:e0145376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Kentish SJ, Ratcliff K, Li H, Wittert GA, Page AJ. High fat diet induced changes in gastric vagal afferent response to adiponectin. Physiol Behav. 2015;152:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Idrizaj E, Garella R, Castellini G, Francini F, Ricca V, Baccari MC, Squecco R. Adiponectin Decreases Gastric Smooth Muscle Cell Excitability in Mice. Front Physiol. 2019;10:1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Fésüs G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Vyas V, Guerra DD, Bok R, Powell T, Jansson T, Hurt KJ. Adiponectin links maternal metabolism to uterine contractility. FASEB J. 2019;33:14588-14601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Sena CM, Pereira A, Fernandes R, Letra L, Seiça RM. Adiponectin improves endothelial function in mesenteric arteries of rats fed a high-fat diet: role of perivascular adipose tissue. Br J Pharmacol. 2017;174:3514-3526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ Res. 2016;119:375-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 293] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 24. | Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, Antoniades C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127:2209-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 276] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 25. | Löhn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 356] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 26. | Kim M, Han IS, Koh SD, Perrino BA. Roles of CaM kinase II and phospholamban in SNP-induced relaxation of murine gastric fundus smooth muscles. Am J Physiol Cell Physiol. 2006;291:C337-C347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Currò D, Ipavec V, Preziosi P. Neurotransmitters of the non-adrenergic non-cholinergic relaxation of proximal stomach. Eur Rev Med Pharmacol Sci. 2008;12:53-62. [PubMed] |