Published online Sep 7, 2021. doi: 10.3748/wjg.v27.i33.5555
Peer-review started: March 1, 2021
First decision: April 17, 2021
Revised: April 24, 2021
Accepted: July 23, 2021
Article in press: July 23, 2021
Published online: September 7, 2021
Processing time: 185 Days and 17.7 Hours
A significant breakthrough in the field of obesity research was the demonstration that an obese phenotype could be manipulated by modulating the gut microbiota. An important next step is to elucidate a human-relevant “map’’ of microbiota-host interactions that regulate the metabolic health of the host. An improved understanding of this crosstalk is a prerequisite for optimizing therapeutic strategies to combat obesity. Intestinal mucosal barrier dysfunction is an important contributor to metabolic diseases and has also been found to be involved in a variety of other chronic inflammatory conditions, including cancer, neurodegeneration, and aging. The mechanistic basis for intestinal barrier dysfunction accompanying metabolic disorders remains poorly understood. Understanding the molecular and cellular modulators of intestinal barrier function will help devise improved strategies to counteract the detrimental systemic consequences of gut barrier breakage. Changes in the composition and function of the gut microbiota, i.e., dysbiosis, are thought to drive obesity-related pathogenesis and may be one of the most important drivers of mucosal barrier dysfunction. Many effects of the microbiota on the host are mediated by microbiota-derived metabolites. In this review, we focus on several relatively well-studied microbial metabolites that can influence intestinal mucosal homeostasis and discuss how they might affect metabolic diseases. The design and use of microbes and their metabolites that are locally active in the gut without systemic side effects are promising novel and safe therapeutic modalities for metabolic diseases.
Core Tip: The manner in which the gut microbiota influences obesity development remains incompletely understood. Recent studies have indicated that the changes in the gut barrier functions act as an important driver of metabolic disorders. There is currently an urgent need to define molecular and cellular modulators of intestinal mucosal barrier homeostasis. Here, we discuss the current understanding of microbiota-derived metabolites in regulating mucosal homeostasis and how they contribute to the metabolic health of the host.
- Citation: Wei YX, Zheng KY, Wang YG. Gut microbiota-derived metabolites as key mucosal barrier modulators in obesity. World J Gastroenterol 2021; 27(33): 5555-5565
- URL: https://www.wjgnet.com/1007-9327/full/v27/i33/5555.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i33.5555
The gut microbiota is thought to function as an organ that can markedly influence human health and disease[1]. It plays an essential role in the progression of obesity and related metabolic syndromes[2-4]. Many effects of the microbiota on the host are mediated by microbiota-derived metabolites[5-8]. A complex interplay between the host immune system and the gut microbiota under normal physiological conditions maintains the mucosal barrier in a homeostatic state. This symbiotic relationship is often broken during the progression of obesity. Increased levels of circulating bacterial flagellin, lipopolysaccharides (LPS), and peptidoglycans are frequently observed in obesity and its complications due to mucosal barrier dysfunction[9-12]. The objective of this review is to provide an overview of the emerging field related to the role of gut microbiota-derived metabolites in regulating gut barrier function and metabolic health.
Intestinal barrier dysfunction leads to the translocation of microbial molecules into the systemic circulation, resulting in a state of so-called “metabolic endotoxemia” that is considered to be one of the most important initiators of metabolic disease in obesity[13-16]. However, the mechanisms underlying barrier dysfunction accompanying metabolic syndrome remain poorly understood. Thus, there is an urgent need to elucidate the molecular and cellular modulators of intestinal mucosal barrier homeostasis to devise improved strategies to counteract metabolic disease pro
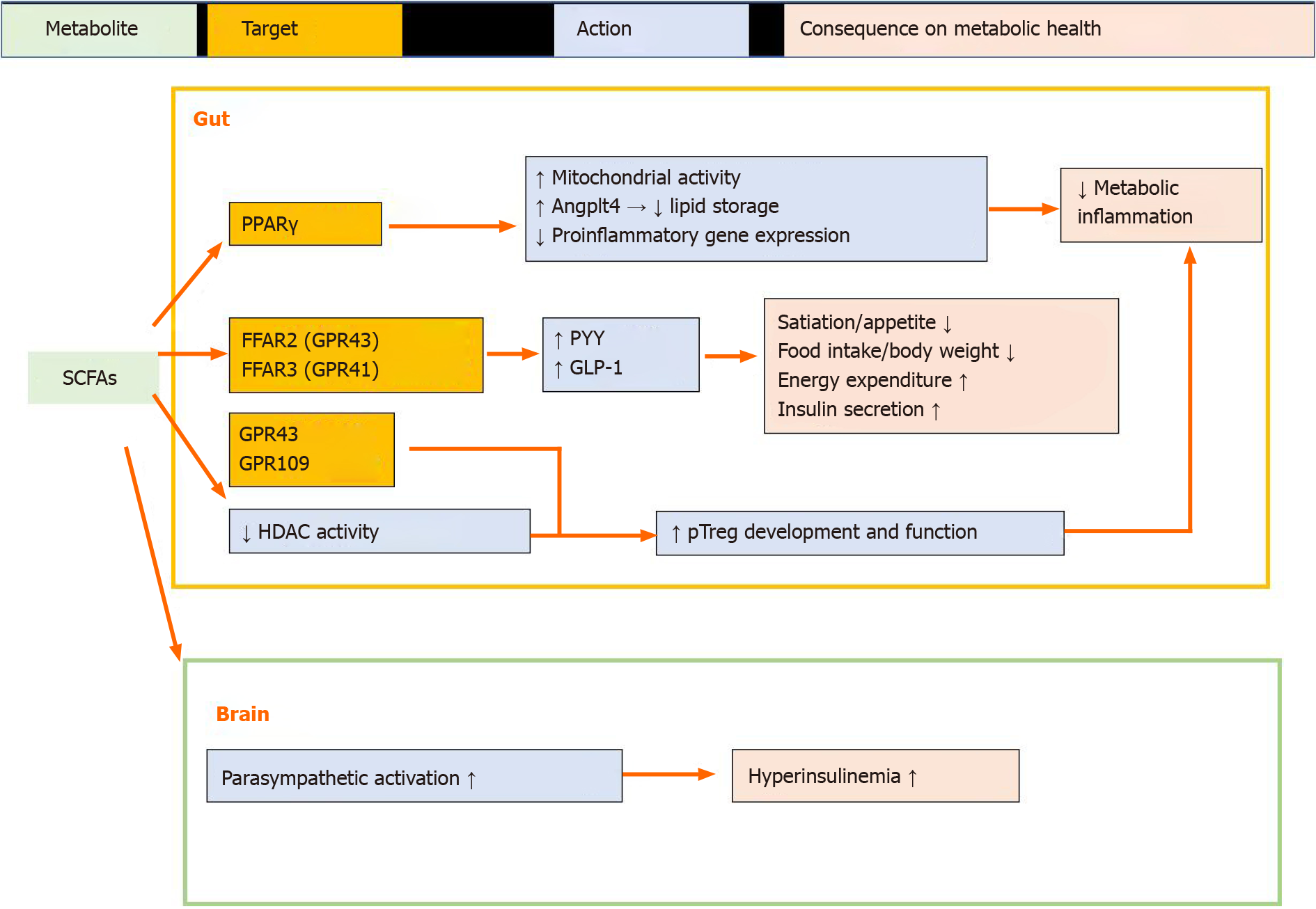
Short-chain fatty acids (SCFAs) are derived predominantly from gut microbial fermentation of otherwise indigestible dietary fiber[18]. According to a clinical study, the major SCFA-producing bacteria promoted by dietary fibers in humans were found to be Faecalibacterium prausnitzii and Eubacterium, Lactobacillus, and Bifidobacterium species[19]. The roles of SCFAs in mucosal homeostasis and metabolic health are summarized in Figure 1.
SCFAs regulate gut epithelial cell metabolic programming: Gut epithelial cells express peroxisome proliferator-activated receptor gamma (PPAR-γ)[20], which can sense microbial-derived SCFAs (e.g., butyrate). The activation of PPAR-γ by butyrate drives the energy metabolism of colonic epithelial cells toward beta-oxidation and limits the luminal bioavailability of oxygen[21-24]. This is important for maintaining the colonic surface in a physiological hypoxic state, thereby limiting the aerobic outgrowth of facultative anaerobic bacteria[25]. Short-term high-fat diet (HFD) consumption can perturb epithelial PPAR-γ signals and disrupt the microbial and physiological ecosystems within the gut[26].
PPAR-γ activation by SCFAs can also increase angiopoietin-like protein 4 (ANG
Effects of SCFAs on gut hormones: The gut is the largest endocrine organ within the body and can release more than 20 different peptide hormones that are sensitive to gut nutrient content stimulation and function to regulate a variety of physiological processes[30]. As a gut nutrient, free fatty acids (FFAs) can serve as not only cellular energy sources but also natural ligands for a group of orphan G protein-coupled receptors (GPCRs), which are known as FFA receptors (FFARs) and are expressed in the gut epithelial cells to regulate gut peptide hormone production[31-34]. In particular, FFAR3 (GPR41) and FFAR2 (GPR43) are activated by SCFAs, primarily acetate, butyrate, and propionate[32]. SCFAs can stimulate the release of the anorexigenic hormone peptide tyrosine (PYY) and glucagon-like peptide-1 (GLP-1) from colonic L cells[30] via their cognate FFARs. The release of PYY into circulation after food intake can reduce energy intake[35]. GLP-1 can act directly on pancreatic beta cells to activate insulin secretion in a glucose-dependent manner[36]. Thus, SCFA-induced release of PYY and GLP-1 from colonic L-cells may play a role in preventing the development of obesity and its related complications[37].
Role of SCFAs in maintaining mucosal immune homeostasis: The gut microbiota has co-evolved with the intestinal immune system to facilitate the maintenance of mucosal homeostasis[38]. Regulatory T cells (Tregs) are critical for limiting intestinal inflammation[39]. SCFAs play an important role in the generation of peripheral Tregs and control Treg homeostasis and function within the gut[40,41]. Butyrate, acetate, and propionate are the major bacteria-derived SCFAs that control mucosal Treg differentiation and function[40]. Several mechanisms are involved in the promotion of mucosal Treg differentiation and function by SCFAs. First, SCFAs can regulate the size and function of the colonic Treg pool through the activation of GPCRs (GPR43 and/or GPR109)[40,42]. Second, SCFAs can regulate Treg differentiation by inhibiting histone deacetylase (HDAC)[43]. HDAC activity in T cells can be suppressed by acetate, propionate, and butyrate[43]. Butyrate enhances the acetylation of histone H3 Lysine 27 at the promoter and conserved non-coding sequence regions of the Foxp3 locus, which is critical for peripheral Treg generation to promote Foxp3 gene expression[41,44]. Furthermore, the HDAC inhibitory activity of butyrate and propionate promotes the ability of dendritic cells to support Treg differentiation by repressing the expre
It should be noted that although SCFAs are generally thought to play positive roles in maintaining mucosal homeostasis, they exert negative impacts on host metabolism. In experimental studies involving mice, treatment with acetate promoted metabolic syndrome through the action of the gut–nervous system axis[50]. In a clinical trial, consumption of a propionate-containing meal resulted in the activation of catecholamine-related insulin counter-regulatory signals, thus leading to insulin resistance[51]. Approaches to avoid such negative impacts of SCFAs on host meta
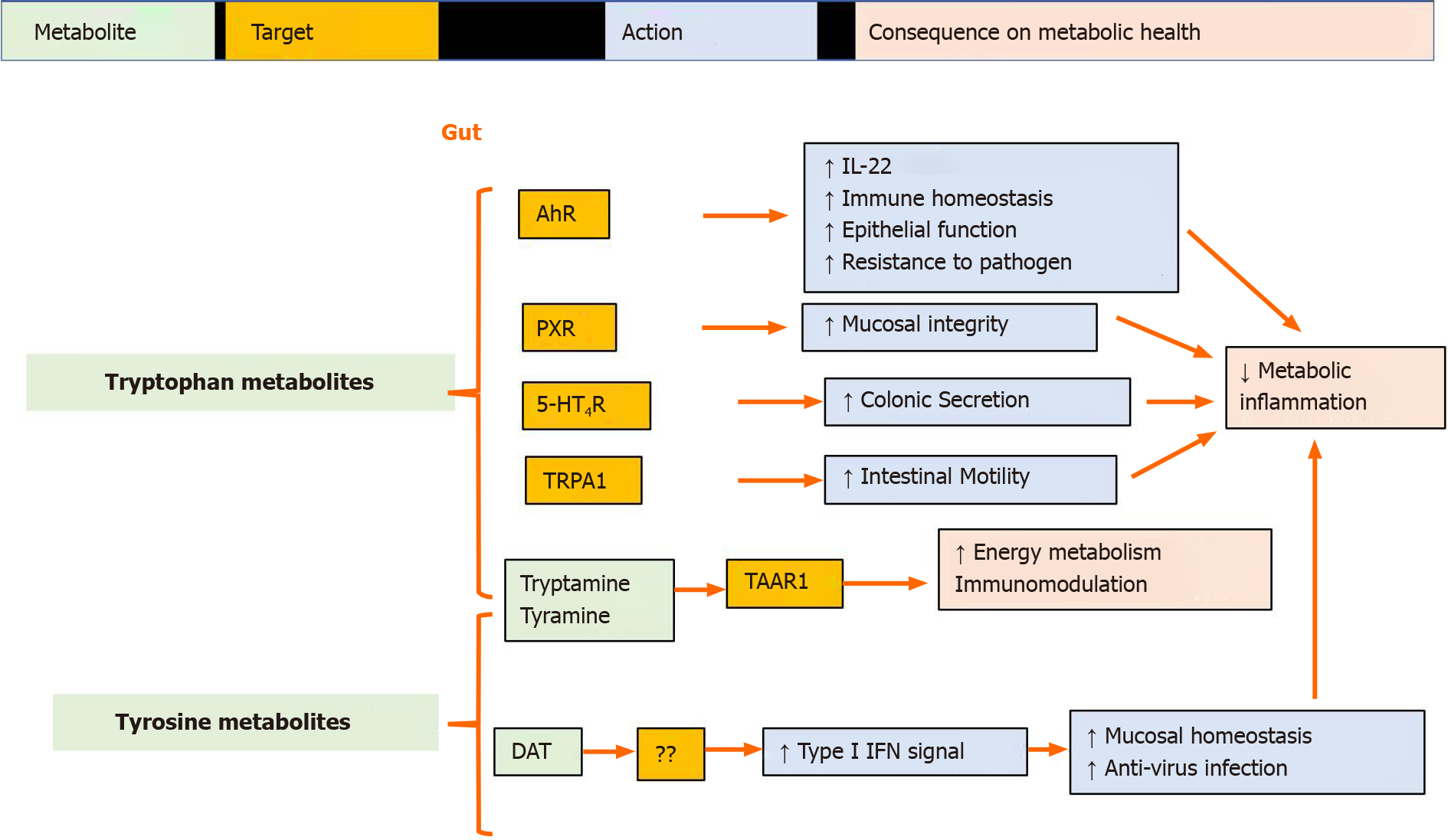
Microbiota-derived tryptophan metabolites protect the gut barrier: Recent disco
Many indole derivatives, including IPA and indoleacrylic acid (IA) are ligands for the aryl hydrocarbon receptor (AhR)[54,57,58]. AhR signaling is crucial for intestinal homeostasis because it influences epithelial renewal, barrier integrity, and immune cell activity[59]. AhR mediates the activation of type 3 innate lymphoid cells to produce IL-22[60]. IL-22 plays an important role in maintaining the mucosal barrier and regulating host metabolic functions[61,62]. IPA induces IL-10R1 by activating AhR, thus exerting anti-inflammatory effects in the intestinal mucosa[58]. IA-AhR signaling promotes barrier function and mitigates LPS-stimulated inflammatory responses[63]. Indole-3-aldehyde (IAld) promotes AhR-dependent IL22 production to provide mucosal protection against inflammation induced by the fungus Candida albicans[57]. Trp-derived AhR ligands are reduced in both HFD-induced metabolic impairments in animals and in individuals with metabolic syndrome, and supplementation with an AhR agonist or an AhR ligand-producing Lactobacillus strain leads to the improvement of metabolic disorders[64,65].
Additionally, IPA has been reported to act as a ligand for the pregnane X receptor (PXR), and in vivo, PXR signals regulate mucosal integrity through toll-like receptor 4[66]. Indole and IAld can also activate enteroendocrine cells through transient receptor potential ankyrin A1 to increase gut motility[67]. Gut microbiota-produced tryptamine activates intestinal epithelial GPCR serotonin receptor-4 (5-HT4R) to increase cAMP levels and drive colonic fluid secretion[68]. Collectively, Trp metabolites produced by the microbiota can protect gut barrier functions.
Microbiota-derived tyrosine metabolites regulate mucosal homeostasis: The aromatic amino acids phenylalanine, tyrosine (Tyr), and Trp share a decarboxylation pathway to generate trace amines, including phenylethylamine, tyramine, and tryptamine, respectively. Tyramine and tryptamine are high-affinity ligands for trace amine-associated receptor 1, which is expressed in the stomach, intestinal neuroendocrine cells, and pancreatic β cells, and regulates energy metabolism and modulates immune homeostasis[69,70].
Another common pathway involves aminotransaminase-mediated transamination, which subsequently undergoes oxidation and reduction, ultimately leading to the production of the corresponding acetic acid or propionic acid derivatives[56]. Desaminotyrosine (DAT, also known as 4-hydroxyphenylpropionic acid or p-hydroxyhydrocinnamic acid) is a degradation product derived from microbial Tyr metabolism. DAT can also be produced from microbial degradation of dietary flavonoids[71,72]. A recent study revealed that DAT protects the host from influenza virus infection by enhancing type I IFN signals[73]. Our recent data demonstrated that DAT supplementation alleviated HFD-induced fat mass accumulation and body weight increment, both of which are correlated with the anti-inflammatory effect of DAT with regard to maintaining mucosal immune homeostasis[7] (Summarized in Figure 2).
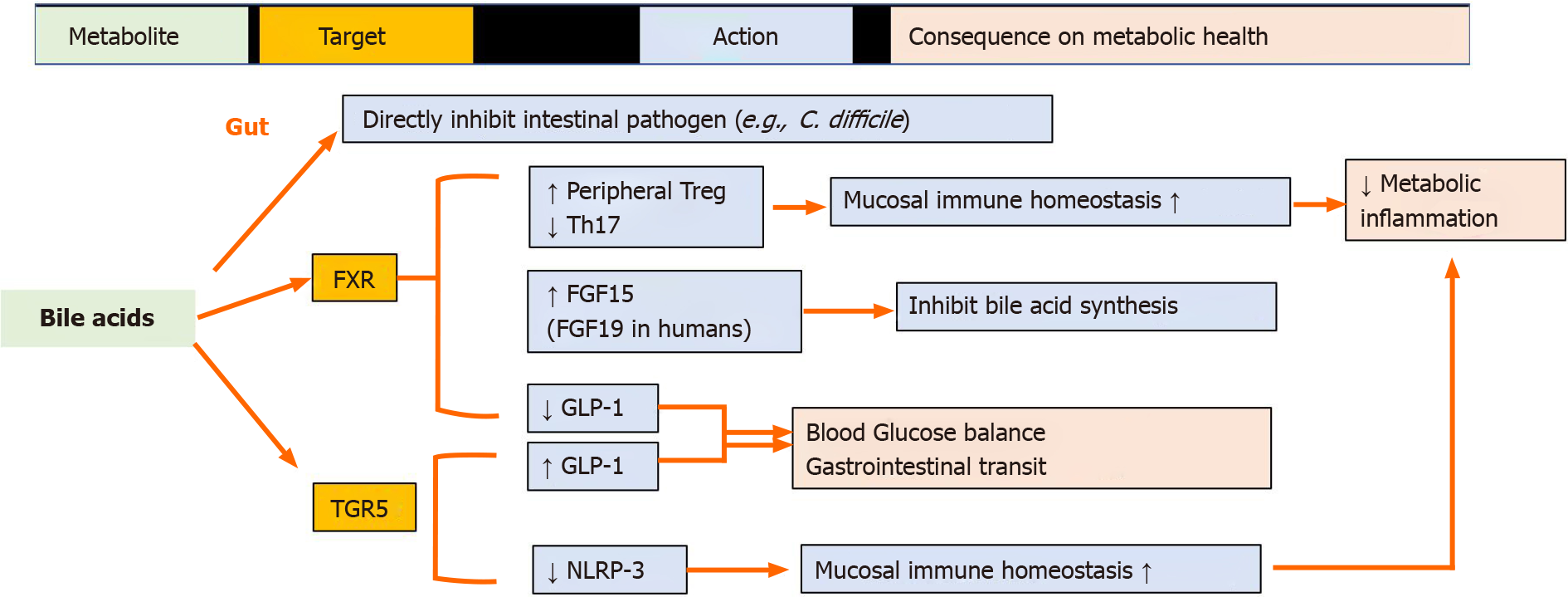
Primary bile acids (BAs) are cholesterol metabolites that are produced in the liver and converted to secondary BAs by the gut microbiota[74]. The two key microbiota-dependent processes are BA deconjugation and 7α-hydroxylation. The enzyme bile salt hydrolase, which is produced by many commensal genera such as Bacteroides, Clostridium, and Enterococcus, deconjugates primary BAs. However, only a small number of known bacteria from the Lachnospiraceae and Ruminococcaceae families perform subsequent 7α-dehydroxylation to generate secondary BAs[75]. BAs are lipid solubilizers that are crucial for dietary lipid absorption and function as signaling molecules that coordinately regulate host metabolism and inflammation by interacting with their cognate cellular receptors, including the nuclear receptors farnesoid X receptor (FXR), PXR, vitamin D receptor, membrane G protein-coupled BA receptor 1 (also known as GPCR19 or TGR5), and sphingosine-1-phosphate receptor[76,77]. Different BA profiles can produce both agonists and antagonists of their cognate receptors, thus creating a great deal of complexity regarding the beneficial or deleterious influence of BAs and their derivatives on health and disease. In the gut, BA-TGR5 signaling leads to GLP-1 expression and secretion, whereas BA-FXR signaling inhibits GLP-1 production[78]. BAs influence gut-associated inflammation by regulating gut mucosal immune cells, including T helper cells expressing IL-17a and Tregs[79-81]. The BA-TGR5–cAMP–PKA axis inhibits NLRP3 inflammasome activation in macrophages[82]. Certain BAs can exert protective effects on the gut epithelium[83] or inhibit intestinal pathogens such as Clostridium difficile[84]. In an experimental model, tauroursodeoxycholic acid inhibited intestinal inflammation and barrier disruption in mice with nonalcoholic fatty liver disease[85]. Parabacteroides distasonis, one of the core members of the gut microbiota of humans, alleviates obesity and obesity-related dysfunction in mice, at least partially, based on its ability to produce secondary BAs[86] (Summarized in Figure 3).
Polyphenols are a class of plant-derived macromolecules[87]. Approximately 90%–95% of dietary polyphenols are poorly absorbed and are partially processed by the gut microbiota[88]. Fermentation of polyphenols by the gut microbiota results in the production of bioactive metabolites that can affect host physiology. Urolithins are gut microbial metabolites derived from the polyphenol compounds, ellagitannins that are present in some fruits, nuts, and seeds (e.g., pomegranates, black raspberries, raspberries, strawberries, walnuts, and almonds)[89]. Ellagitannins are hydrolyzed to ellagic acid under physiological conditions in vivo, and ellagic acid is then gradually metabolized by the gut microbiota to produce different types of urolithins. Urolithins have been demonstrated to protect against obesity and related metabolic diseases in animal studies[90]. These effects are related to their protective role in mucosal epithelial integrity and anti-inflammatory functions[91,92].
Omega-6 polyunsaturated fatty acids (PUFAs) are abundant in the Western diet and can contribute to obesity and metabolic syndrome[93,94]. The increased ratio of omega-6/omega-3 fatty acids correlates with the incidence and prevalence of overweight individuals and obesity[94,95]. The gut microbiota confers host resistance to HFD-induced obesity by modulating dietary PUFA metabolism[96]. One of the metabolites is 10-hydroxy-cis-12-octadecenoic acid (HYA). HYA promotes GLP-1 secretion in colonic L cells and improves glucose homeostasis via the activation of GPR40 and GPR120. Additionally, HYA promotes intestinal peristalsis by acting as a low-affinity prostaglandin EP3 receptor agonist[96].
The gut microbiota plays a key role in obesity. Our current understanding of host–microbiota interactions remains in its early phase. Several challenges must be resolved before the field can be advanced. The design and use of microbes and their metabolites that are locally active in the gut without systemic side effects are promising novel and safe therapeutic modalities for metabolic diseases.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Codoñer-Franch P, Durazzo M S-Editor: Zhang H L-Editor: Webster JR P-Editor: Liu JH
| 1. | O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1785] [Article Influence: 93.9] [Reference Citation Analysis (2)] |
| 2. | Lee CJ, Sears CL, Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann N Y Acad Sci. 2020;1461:37-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 3. | Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin R. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3:559-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 560] [Cited by in RCA: 624] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 4. | Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1086] [Cited by in RCA: 1372] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 5. | Taleb S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front Immunol. 2019;10:2113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 6. | Ejtahed HS, Angoorani P, Soroush AR, Hasani-Ranjbar S, Siadat SD, Larijani B. Gut microbiota-derived metabolites in obesity: a systematic review. Biosci Microbiota Food Health. 2020;39:65-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Wei Y, Gao J, Kou Y, Liu M, Meng L, Zheng X, Xu S, Liang M, Sun H, Liu Z, Wang Y. The intestinal microbial metabolite desaminotyrosine is an anti-inflammatory molecule that modulates local and systemic immune homeostasis. FASEB J. 2020;34:16117-16128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Vallianou N, Stratigou T, Christodoulatos GS, Dalamaga M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr Obes Rep. 2019;8:317-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 9. | Tuomi K, Logomarsino JV. Bacterial Lipopolysaccharide, Lipopolysaccharide-Binding Protein, and Other Inflammatory Markers in Obesity and After Bariatric Surgery. Metab Syndr Relat Disord. 2016;14:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Pekkala S, Munukka E, Kong L, Pöllänen E, Autio R, Roos C, Wiklund P, Fischer-Posovszky P, Wabitsch M, Alen M, Huovinen P, Cheng S. Toll-like receptor 5 in obesity: the role of gut microbiota and adipose tissue inflammation. Obesity (Silver Spring). 2015;23:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Chan KL, Tam TH, Boroumand P, Prescott D, Costford SR, Escalante NK, Fine N, Tu Y, Robertson SJ, Prabaharan D, Liu Z, Bilan PJ, Salter MW, Glogauer M, Girardin SE, Philpott DJ, Klip A. Circulating NOD1 Activators and Hematopoietic NOD1 Contribute to Metabolic Inflammation and Insulin Resistance. Cell Rep. 2017;18:2415-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Hersoug LG, Møller P, Loft S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr Res Rev. 2018;31:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 13. | Winer DA, Luck H, Tsai S, Winer S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016;23:413-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 359] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 14. | Wollam J, Riopel M, Xu YJ, Johnson AMF, Ofrecio JM, Ying W, El Ouarrat D, Chan LS, Han AW, Mahmood NA, Ryan CN, Lee YS, Watrous JD, Chordia MD, Pan D, Jain M, Olefsky JM. Microbiota-Produced N-Formyl Peptide fMLF Promotes Obesity-Induced Glucose Intolerance. Diabetes. 2019;68:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 644] [Article Influence: 53.7] [Reference Citation Analysis (1)] |
| 16. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4559] [Article Influence: 253.3] [Reference Citation Analysis (1)] |
| 17. | Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 617] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 18. | Makki K, Deehan EC, Walter J, Bäckhed F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe. 2018;23:705-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 1552] [Article Influence: 221.7] [Reference Citation Analysis (0)] |
| 19. | Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1538] [Article Influence: 219.7] [Reference Citation Analysis (68)] |
| 20. | Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 611] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 21. | Lefebvre M, Paulweber B, Fajas L, Woods J, McCrary C, Colombel JF, Najib J, Fruchart JC, Datz C, Vidal H, Desreumaux P, Auwerx J. Peroxisome proliferator-activated receptor gamma is induced during differentiation of colon epithelium cells. J Endocrinol. 1999;162:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Duszka K, Oresic M, Le May C, König J, Wahli W. PPARγ Modulates Long Chain Fatty Acid Processing in the Intestinal Epithelium. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Dowdell AS, Cartwright IM, Goldberg MS, Kostelecky R, Ross T, Welch N, Glover LE, Colgan SP. The HIF target ATG9A is essential for epithelial barrier function and tight junction biogenesis. Mol Biol Cell. 2020;31:2249-2258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1348] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 25. | Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Bäumler AJ. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 807] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 26. | Tomas J, Mulet C, Saffarian A, Cavin JB, Ducroc R, Regnault B, Kun Tan C, Duszka K, Burcelin R, Wahli W, Sansonetti PJ, Pédron T. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci USA. 2016;113:E5934-E5943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 27. | Alex S, Lange K, Amolo T, Grinstead JS, Haakonsson AK, Szalowska E, Koppen A, Mudde K, Haenen D, Al-Lahham S, Roelofsen H, Houtman R, van der Burg B, Mandrup S, Bonvin AM, Kalkhoven E, Müller M, Hooiveld GJ, Kersten S. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ. Mol Cell Biol. 2013;33:1303-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 28. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4408] [Article Influence: 209.9] [Reference Citation Analysis (4)] |
| 29. | Li M, Pascual G, Glass CK. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol. 2000;20:4699-4707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 296] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 30. | Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 531] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 31. | Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free Fatty Acid Receptors in Health and Disease. Physiol Rev. 2020;100:171-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 621] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 32. | Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312-11319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1739] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 33. | Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 34. | Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, Kuwahara A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 35. | Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1704] [Cited by in RCA: 1575] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 36. | Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127:4217-4227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 37. | Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, MacDougall K, Preston T, Tedford C, Finlayson GS, Blundell JE, Bell JD, Thomas EL, Mt-Isa S, Ashby D, Gibson GR, Kolida S, Dhillo WS, Bloom SR, Morley W, Clegg S, Frost G. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744-1754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 940] [Cited by in RCA: 948] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 38. | Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768-1773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 875] [Cited by in RCA: 767] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 39. | Whibley N, Tucci A, Powrie F. Regulatory T cell adaptation in the intestine and skin. Nat Immunol. 2019;20:386-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 40. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 3944] [Article Influence: 328.7] [Reference Citation Analysis (1)] |
| 41. | Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2516] [Cited by in RCA: 3423] [Article Influence: 285.3] [Reference Citation Analysis (0)] |
| 42. | Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1687] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 43. | Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 867] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 44. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2951] [Cited by in RCA: 3779] [Article Influence: 314.9] [Reference Citation Analysis (0)] |
| 45. | Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1508] [Article Influence: 137.1] [Reference Citation Analysis (0)] |
| 46. | Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2007] [Cited by in RCA: 2396] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 47. | Li C, Spallanzani RG, Mathis D. Visceral adipose tissue Tregs and the cells that nurture them. Immunol Rev. 2020;295:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 48. | Zhao XY, Zhou L, Chen Z, Ji Y, Peng X, Qi L, Li S, Lin JD. The obesity-induced adipokine sST2 exacerbates adipose Treg and ILC2 depletion and promotes insulin resistance. Sci Adv. 2020;6:eaay6191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 49. | Chen X, Wu Y, Wang L. Fat-resident Tregs: an emerging guard protecting from obesity-associated metabolic disorders. Obes Rev. 2013;14:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 950] [Article Influence: 105.6] [Reference Citation Analysis (1)] |
| 51. | Tirosh A, Calay ES, Tuncman G, Claiborn KC, Inouye KE, Eguchi K, Alcala M, Rathaus M, Hollander KS, Ron I, Livne R, Heianza Y, Qi L, Shai I, Garg R, Hotamisligil GS. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci Transl Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 52. | Yokoyama MT, Carlson JR. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am J Clin Nutr. 1979;32:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 186] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Alkhalaf LM, Ryan KS. Biosynthetic manipulation of tryptophan in bacteria: pathways and mechanisms. Chem Biol. 2015;22:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 54. | Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos. 2015;43:1522-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 472] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 55. | Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 491] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 56. | Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 896] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 57. | Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1512] [Cited by in RCA: 1720] [Article Influence: 143.3] [Reference Citation Analysis (1)] |
| 58. | Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD, Gerich ME, Jenkins BR, Walk ST, Kominsky DJ, Colgan SP. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am J Pathol. 2018;188:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 361] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 59. | Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018;11:1024-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 60. | Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 701] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 61. | Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, Diehl L, van Bruggen N, Kolumam G, Ouyang W. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 62. | Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe. 2018;23:41-53.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 407] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 63. | Wlodarska M, Luo C, Kolde R, d'Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, Garner AL, Mohammadi S, O'Connell DJ, Abubucker S, Arthur TD, Franzosa EA, Huttenhower C, Murphy LO, Haiser HJ, Vlamakis H, Porter JA, Xavier RJ. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe. 2017;22:25-37.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 614] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 64. | Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, Michel ML, Chong-Nguyen C, Roussel R, Straube M, Jegou S, McQuitty C, Le Gall M, da Costa G, Lecornet E, Michaudel C, Modoux M, Glodt J, Bridonneau C, Sovran B, Dupraz L, Bado A, Richard ML, Langella P, Hansel B, Launay JM, Xavier RJ, Duboc H, Sokol H. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018;28:737-749.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 399] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 65. | Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018;23:1099-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 433] [Article Influence: 72.2] [Reference Citation Analysis (1)] |
| 66. | Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 731] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 67. | Ye L, Bae M, Cassilly CD, Jabba SV, Thorpe DW, Martin AM, Lu HY, Wang J, Thompson JD, Lickwar CR, Poss KD, Keating DJ, Jordt SE, Clardy J, Liddle RA, Rawls JF. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29:179-196.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 68. | Bhattarai Y, Williams BB, Battaglioli EJ, Whitaker WR, Till L, Grover M, Linden DR, Akiba Y, Kandimalla KK, Zachos NC, Kaunitz JD, Sonnenburg JL, Fischbach MA, Farrugia G, Kashyap PC. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe. 2018;23:775-785.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 289] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 69. | Liu Y, Hou Y, Wang G, Zheng X, Hao H. Gut Microbial Metabolites of Aromatic Amino Acids as Signals in Host-Microbe Interplay. Trends Endocrinol Metab. 2020;31:818-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 70. | Gainetdinov RR, Hoener MC, Berry MD. Trace Amines and Their Receptors. Pharmacol Rev. 2018;70:549-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 71. | Schoefer L, Mohan R, Schwiertz A, Braune A, Blaut M. Anaerobic degradation of flavonoids by Clostridium orbiscindens. Appl Environ Microbiol. 2003;69:5849-5854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Saura-Calixto F, Pérez-Jiménez J, Touriño S, Serrano J, Fuguet E, Torres JL, Goñi I. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol Nutr Food Res. 2010;54:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, Esaulova E, Artyomov MN, Morales DJ, Holtzman MJ, Boon ACM, Lenschow DJ, Stappenbeck TS. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 409] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 74. | Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA. Intestinal Absorption of Bile Acids in Health and Disease. Compr Physiol. 2019;10:21-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 75. | Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 737] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 76. | Parséus A, Sommer N, Sommer F, Caesar R, Molinaro A, Ståhlman M, Greiner TU, Perkins R, Bäckhed F. Microbiota-induced obesity requires farnesoid X receptor. Gut. 2017;66:429-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 352] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 77. | Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA. Bile Acid Receptors and Gastrointestinal Functions. Liver Res. 2019;3:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 78. | Shapiro H, Kolodziejczyk AA, Halstuch D, Elinav E. Bile acids in glucose metabolism in health and disease. J Exp Med. 2018;215:383-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 327] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 79. | Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, Zheng Y, Longman RS, Rastinejad F, Devlin AS, Krout MR, Fischbach MA, Littman DR, Huh JR. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576:143-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 908] [Article Influence: 151.3] [Reference Citation Analysis (0)] |
| 80. | Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C, Kasper DL. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature. 2020;577:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 696] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 81. | Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, Mai C, Jin WB, Guo CJ, Violante S, Ramos RJ, Cross JR, Kadaveru K, Hambor J, Rudensky AY. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020;581:475-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 82. | Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, Lu L, Wang D. Bile Acids Control Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome. Immunity. 2016;45:802-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 83. | Barrasa JI, Olmo N, Lizarbe MA, Turnay J. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol In Vitro. 2013;27:964-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 84. | Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1172] [Cited by in RCA: 1348] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 85. | Wang W, Zhao J, Gui W, Sun D, Dai H, Xiao L, Chu H, Du F, Zhu Q, Schnabl B, Huang K, Yang L, Hou X. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br J Pharmacol. 2018;175:469-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 86. | Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, Wang Y, Liu C, Wang W, Wang J, Liu SJ, Liu H. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019;26:222-235.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 750] [Article Influence: 150.0] [Reference Citation Analysis (1)] |
| 87. | Efenberger-Szmechtyk M, Nowak A, Czyzowska A. Plant extracts rich in polyphenols: antibacterial agents and natural preservatives for meat and meat products. Crit Rev Food Sci Nutr. 2021;61:149-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 88. | Diotallevi C, Fava F, Gobbetti M, Tuohy K. Healthy dietary patterns to reduce obesity-related metabolic disease: polyphenol-microbiome interactions unifying health effects across geography. Curr Opin Clin Nutr Metab Care. 2020;23:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 89. | Espín JC, Larrosa M, García-Conesa MT, Tomás-Barberán F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med. 2013;2013:270418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 90. | Abdulrahman AO, Kuerban A, Alshehri ZA, Abdulaal WH, Khan JA, Khan MI. Urolithins Attenuate Multiple Symptoms of Obesity in Rats Fed on a High-Fat Diet. Diabetes Metab Syndr Obes. 2020;13:3337-3348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 91. | Zhao R, Long X, Yang J, Du L, Zhang X, Li J, Hou C. Pomegranate peel polyphenols reduce chronic low-grade inflammatory responses by modulating gut microbiota and decreasing colonic tissue damage in rats fed a high-fat diet. Food Funct. 2019;10:8273-8285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 92. | Kujawska M, Jodynis-Liebert J. Potential of the ellagic acid-derived gut microbiota metabolite - Urolithin A in gastrointestinal protection. World J Gastroenterol. 2020;26:3170-3181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 93. | Galgani JE, Uauy RD, Aguirre CA, Díaz EO. Effect of the dietary fat quality on insulin sensitivity. Br J Nutr. 2008;100:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 94. | German JB, Dillard CJ. Saturated fats: what dietary intake? Am J Clin Nutr. 2004;80:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 95. | Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016;8:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1007] [Cited by in RCA: 979] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 96. | Miyamoto J, Igarashi M, Watanabe K, Karaki SI, Mukouyama H, Kishino S, Li X, Ichimura A, Irie J, Sugimoto Y, Mizutani T, Sugawara T, Miki T, Ogawa J, Drucker DJ, Arita M, Itoh H, Kimura I. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat Commun. 2019;10:4007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 277] [Article Influence: 46.2] [Reference Citation Analysis (2)] |