Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5404
Peer-review started: April 24, 2021
First decision: June 3, 2021
Revised: June 17, 2021
Accepted: July 30, 2021
Article in press: July 30, 2021
Published online: August 28, 2021
Processing time: 122 Days and 19.2 Hours
Intestinal barrier breakdown, a frequent complication of intestinal ischemia-reperfusion (I/R) including dysfunction and the structure changes of the intestine, is characterized by a loss of tight junction and enhanced permeability of the intestinal barrier and increased mortality. To develop effective and novel therapeutics is important for the improvement of outcome of patients with intestinal barrier deterioration. Recombinant human angiopoietin-like protein 4 (rhANGPTL4) is reported to protect the blood-brain barrier when administered exogenously, and endogenous ANGPTL4 deficiency deteriorates radiation-induced intestinal injury.
To identify whether rhANGPTL4 may protect intestinal barrier breakdown induced by I/R.
Intestinal I/R injury was elicited through clamping the superior mesenteric artery for 60 min followed by 240 min reperfusion. Intestinal epithelial (Caco-2) cells and human umbilical vein endothelial cells were challenged by hypoxia/ reoxygenation to mimic I/R in vitro.
Indicators including fluorescein isothiocyanate-conjugated dextran (4 kilodaltons; FD-4) clearance, ratio of phosphorylated myosin light chain/total myosin light chain, myosin light chain kinase and loss of zonula occludens-1, claudin-2 and VE-cadherin were significantly increased after intestinal I/R or cell hypoxia/reoxygenation. rhANGPTL4 treatment significantly reversed these indicators, which were associated with inhibiting the inflammatory and oxidative cascade, excessive activation of cellular autophagy and apoptosis and improvement of survival rate. Similar results were observed in vitro when cells were challenged by hypoxia/reoxygenation, whereas rhANGPTL4 reversed the indicators close to normal level in Caco-2 cells and human umbilical vein endothelial cells significantly.
rhANGPTL4 can function as a protective agent against intestinal injury induced by intestinal I/R and improve survival via maintenance of intestinal barrier structure and functions.
Core Tip: Improving therapy on intestinal barrier dysfunction induced by ischemia/ reperfusion is still challenging. Most recently, studies indicated that patients who suffered from coronavirus disease 2019 are associated with intestinal hypoperfusion. We investigated the effect of recombinant human angiopoietin-like protein 4 on intestinal barrier structure and function deterioration using intestinal ischemia/ reperfusion models in rats as well as hypoxia/reoxygenation models in cells. Intestinal barrier indicators associated with the inflammatory and oxidative cascade, excessive activation of cellular autophagy and apoptosis were compared. Our results indicated that recombinant human angiopoietin-like protein 4 behaved as a promising therapeutic agent for intestinal ischemia/reperfusion-induced intestine injury.
- Citation: Wang ZY, Lin JY, Feng YR, Liu DS, Zhao XZ, Li T, Li SY, Sun JC, Li SF, Jia WY, Jing HR. Recombinant angiopoietin-like protein 4 attenuates intestinal barrier structure and function injury after ischemia/reperfusion. World J Gastroenterol 2021; 27(32): 5404-5423
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5404.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5404
Intestinal barrier dysfunction induced by ischemia/reperfusion (I/R) and atopic dermatitis is frequently observed in clinical practice. Hospital mortality due to acute mesenteric ischemia remains high, ranging from 60% to 90% over the past few decades[1]. Reperfusion following intestinal ischemia promotes extensive activation of inflammatory response and oxidative stress, autophagy and apoptosis, leading to a dreadful illness, such as multiple organ dysfunction syndromes (MODS), especially breakdown of the intestinal barrier[2,3]. Recent studies have indicated that coronavirus disease 2019 associated with intestinal hypoperfusion may further deteriorate barrier dysfunction and acute lung injury via a positive feedback mechanism[4]. Although remarkable progress has been made, there is no widely accepted therapy to prevent I/R associated intestinal barrier dysfunction.
Integrity of the intestinal mucosal barrier is retained by the critical factors of tight junctions and intercellular junctions, including transmembrane proteins such as zonula occludens (ZO), claudins (CLDN) and junctional adhesion molecule. These consist of a complex that forms a selectively permeable seal between adjacent epithelial cells, which is a key element in the paradigm of gut-origin MODS[5,6]. Myosin light chain (MLC) phosphorylation via MLC kinase (MLCK) is critical in pathophysiological modulation of intestinal mucosal barrier breakdown. ZO-1 and CLDN-2 behaved specifically as loss and redistribution in intestinal mucosal barrier breakdown and may be regulated via an MLCK-dependent MLC phosphorylation signaling pathway[6-8]. Another critical factor is vascular leakage, which contributes to the intestinal barrier dysfunction after I/R. Microvascular endothelial cell layer including VE-cadherin (VE-cad) creates a semi-permeable barrier between tissue and blood for the transport of lipids, proteins and electrolytes. Microvascular barrier dysfunction is crucial in the initiation and progression of vascular barrier disruption after I/R[5,9,10].
Angiopoietin-like 4 (ANGPTL4) was identified by three independent research groups simultaneously and named ANGPTL4 by The HUGO Gene Nomenclature Committee in 2000[11]. Extensive investigations have been carried out over the past decades and found that this molecule is a remarkably dynamic entity that participates in a variety of pathophysiological conditions. The functions of ANGPTL4 have been involved in energy homoeostasis, wound healing, anoikis resistance, retinopathy angiogenesis and corneal epithelium repair as well as ischemic and barrier regulation[12]. Recent studies have reported that ANGPTL4 is a therapeutic agent in vasculoprotection and counteracting permeability of the blood-brain barrier in ischemic stroke[13]. Others also reported that ANGPTL4 blocked reactive oxygen species (ROS)-induced anoikis in cholangiocarcinoma cells[14]. It is postulated that ANGPTL4 may play a role in intestinal barrier integrity after intestinal I/R.
Here, we show that treatment with recombinant human ANGPTL4 (rhANGPTL4), in a rat model of intestinal I/R and intestinal epithelial (Caco-2) cells and human umbilical vein endothelial cells (HUVECs) with hypoxia/reoxygenation (H/R) in vitro, improves intestinal histopathology injury and attenuates the intestinal mucosa inflammatory and oxidant response. rhANGPTL4 counteracts the autophagy and apoptosis of the intestine and consequently diminishes the increase of permeability and loss of tight junction proteins of the intestine. Similar effects were also observed in intestinal epithelial cells and vascular endothelial cells. We provide the first empirical evidence that ANGPTL4 protects intestinal mucosa barrier integrity and confers intestine protection. ANGPTL4 might, therefore, constitute a relevant novel therapeutic approach for intestinal mucosa after I/R.
Male Wistar rats weighing 180-220 g were purchased from Dalian Medical University Animal Experimental Center (Dalian, China, institutional protocol number: SCXK 2008-0002) and preserved under qualified experimental conditions with laboratory water and food. They were accommodated with an equipment shelter and kept at 25 °C and humidity of 40%-50% with a 12 h light-12 h dark cycle. Rats were acclimatized for 1 wk prior to experimentation. All steps were executed in line with the institutional animal care guiding principles and approved by the Institutional Ethics Committee.
Intestinal I/R models were established according to the methods reported previously[15]. In brief, superior mesenteric artery was isolated following laparotomy and blocked softly using a non-invasive microvascular clip for 60 min. Occlusion was affirmed while the vessel beats paused and intestinal color faded, then clips were removed, and the procedure lasted 240 min. Sham animals received laparotomy and superior mesenteric artery identification but without clamping.
Animals were randomly divided into 3 groups with 8 rats in each: (1) sham group; (2) vehicle (normal saline) + I/R group; and (3) I/R + rhANGPTL4 group. In group 3, rats were treated (via intravenous injection through tail vein) with rhANGPTL4 (28 mg/kg body weight in 0.5 mL normal saline) at the beginning of reperfusion. Rats in sham and I/R groups received normal saline with the same volume. All rats were sacrificed, and samples were collected for further investigation.
Blood samples of 5-6 mL were collected from the abdominal aorta at the end of reperfusion. They were preserved for 30 min at room temperature and centrifuged at 4 ºC (3000 rpm for 15 min). The isolated serum was frozen at -80 ºC. The supernatant was placed in a sterilized Eppendorf tube (Eppendorf Company, Limited, Shanghai, China) at -80 ºC. Three centimeter proximal jejunum and distal ileum was separated after animals were sacrificed and then placed at -80 ºC for further investigation.
Intestinal mucosal barrier function was assessed by mucosal-to-serosal clearance of fluorescein isothiocyanate-conjugated dextran (4 kilodaltons; FD-4) in everted ileal sacs incubated ex vivo as previously described[16]. Intestinal microvascular permea
Serum lactate dehydrogenase (LDH) was determined by a kinetic assay as previously described[16].
The jejunum segments were collected and embedded in 4% paraformaldehyde for histological evaluation. Tissues were dehydrated in graded ethanol, paraffin-embedded and sliced. Slices were prepared and stained using hematoxylin-eosin. The intestinal mucosal injury was assessed using a light microscope following the criteria described by Chiu et al[17].
TUNEL staining was performed following the manufacturer’s instructions (In Situ Cell Death Detection Kit; Roche, Germany) and previously described method[18]. TUNEL positive cells were counted under a microscope (× 400), and the apoptotic cells in each slice were counted in seven independent areas followed by averaging.
Caco-2 cells were acquired from the American Type Culture Collection (ATCC, Rockville, MD, United States). Cells were cultured in a humidified atmosphere of 5% CO2 at 37 °C in Dulbecco’s Modified Eagle Medium added with 1% nonessential amino acids, 10% fetal bovine serum and 1% glutamide (Gibco, CA, United States). In order to mimic intestinal I/R in vivo, Caco-2 cells were subjected to hypoxia for 1 h and reoxygenation for 4 h on passages 25–35 using microaerophilic equipment (Thermo Fisher Scientific, United States). Both small interfering RNA (siRNA) control and siRNA ANGPTL4 cells treated with H/R and vehicle were included. After H/R, supernatants were obtained from the monolayers and centrifuged at 1300 rpm for 10 min at 4 °C. The apoptosis of Caco-2 monolayers was determined instantly. Supernatants were preserved at -80 °C for further enzyme-linked immunosorbent assay. HUVECs (Lonza; passages 3–6) were seeded and grown in endothelial growth medium (EGM bullet kit; Thermo fisher, Shanghai, China) containing 10% (vol/vol) fetal bovine serum at 37 °C. H/R was performed as that for intestinal epithelium cells.
For siRNA transfection, 1 × 105 Caco-2 cells were seeded on six-well plates and transfected at the time of 70%-80% confluence with an ANGPTL4 siRNA or non-binding control siRNA using Lipofectamin 2000 (Invitrogen, Karlsru-he, Germany): siRNA ANGPTL4 sense 5’-AAAGCTGCAAGATGACCTCAGATGGAGGCTG-3’; anti-sense 5’-AAAAGGCTTAAGAAGGGAATCTTCTGGAAGAC-3’; siRNA control sense 5’-AAAGCTGTCTTCAAGATTGATATCGAAGACTA-3’; and anti-sense 5’-AAAATAGTCTTCGATATCAAGCTTGAAGAC A-3’. After 24 h incubation, the cells were washed, and fresh Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum was supplemented.
Full-length rhANGPTL4 was purchased from ProSpec-Tany TechnoGene Ltd. (Rehovot, Israel).
Fluorescent probe DCFH-DA (Sigma Aldrich, United States) was used to observe the intracellular accumulation of ROS. After exchanging the proper medium, cells (5 × 105 cells/mL) were incubated in 10 μM DCFH-DA solution at 37 °C for 30 min and were examined using a confocal microscope outfitted with an argon laser (488 nm, 200 mW) as previously studied[19].
Interleukin (IL)-6, tumor necrosis factor α (TNF-α) and IL-1β were quantified using rat enzyme-linked immunosorbent assay kits (BOSTER Bioengineering Co. Ltd., Wuhan, China) in intestinal tissues. Intestinal tissues were collected and coagulated on ice for 2 h. Intestinal samples were acquired and homogenized using a tissue homogenizer (IKA T10 Basic, Staufen, Germany) in 1 mL lysis buffer containing 150 mmol/L NaCl, 0.5% Triton × 100, 1 mmol/L CaCl2, 15 mmol/L Tris and 1 mmol/L MgCl2, pH 7.4. Homogenates were centrifuged at 10000 × g for 10 min. Supernatants were preserved at -80 ºC for further investigation.
Intestinal epithelial cells were stimulated with H/R in vitro and treated with or without rhANGPTL4 as described above. Supernatants of cell homogenates were harvested after treatment and transferred to a -80 ºC refrigerator for further analysis. TNF-α, IL-6 and IL-1β of the cell homogenate supernatants were determined using the enzyme-linked immunosorbent assay (Bender Med Systems, San Diego, CA, United States) and quantified according to the standard curve method.
For detecting ANGPTL4, MLCK, phosphorylated MLC (p-MLC), MLC, ZO-1, CLDN-2, VE-cad, microtubule-associated protein light chain 3 (LC3)-I, LC3-II, p62, beclin-1 and caspase-3 content in the intestine and Caco-2 cells, 30 μg protein was extracted using 12% and 15% SDS-PAGE (Bio-Rad, Hercules, CA, United States), and moved to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, United States). Membranes were incubated with primary anti-ANGPTL4 antibody (Abcam Ltd., Cambridge, United Kingdom), anti-MLCK antibody (Abcam Ltd., Hong Kong, United Kingdom), p-MLC and MLC antibody (Cell Signaling Technology, Beverly, MA, United States), anti-ZO-1 and CLDN-2 antibody and anti-VE-cad (Santa Cruz Biotechnology, United States), anti-LC3-I, anti-LC3-II, anti-p62, anti-Beclin-1 antibody (Santa Cruz Biotechnology, United States), anti-caspase-3 and anti-cleaved-caspase-3 antibody (Bioworld, Minneapolis, MN, United States) or anti-β-actin antibody in Tris-phosphate-buffered solution overnight at 4 ºC in 5% skimmed milk. The membranes were co-incubated with biotinylated secondary antibody and diluted 1:1000 in PBST containing 5% skimmed milk at 37 ºC for 2 h after washing 3 times in TBS containing 0.1% Tween 20. Then membranes underwent large-scale elution with TBS containing 0.1% Tween and exposed using an enhanced chemiluminescence kit (Beyotime Institute of Biotechnology, Hangzhou, China).
Total RNA was collected from animals with Trizol kits (Invitrogen, Carlsbad, CA, United States) in line with the manufacturer’s instructions. Reverse transcription into complementary DNA was executed using a TaKaRa RNA polymerase chain reaction (PCR) Kit (avian myeloblastosis virus) Version 3.0 (TaKaRa, Dalian, China) for PCR analysis; primers were described as follows: ANGPTL4 sense: 5’- AGACCCGAAGGATAGAGTCCC-3’ (forward), antisense: 5’-CCTTCTGGAACAGTTGCTGG-3’ (reverse); β-actin sense: 5’-AGAGGGAAATCGTGCGTGAC-3’ (forward), antisense: 5’-CAATAGTGATGACCTGGCCGT-3’ (reverse). SYBR Premix Ex Taq kit (Takara, China) was used to perform quantitative real-time PCR, and ABI 7500 Fast Real-Time PCR System (Applied Biosystems) was used for fluorescence determination during amplification. PCR cycling was performed as follows: Initial annealing at 95 for 30 s, and 40 cycles at 95 ºC for 5 s, 60 ºC for 34 s and 72 ºC for 30 s.
All measurements were calculated using mean ± SD. A one-way analysis of variance was used to compare the average values of all groups. The Student-Newman-Keuls/least significant difference test was used to compare the mean of all pairs. Student’s t test was used for the comparison of two groups. Kaplan-Meier log-rank test was applied to analyze the survival. A P value less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 18.0 statistical software package (SPSS, Chicago, IL, United States).
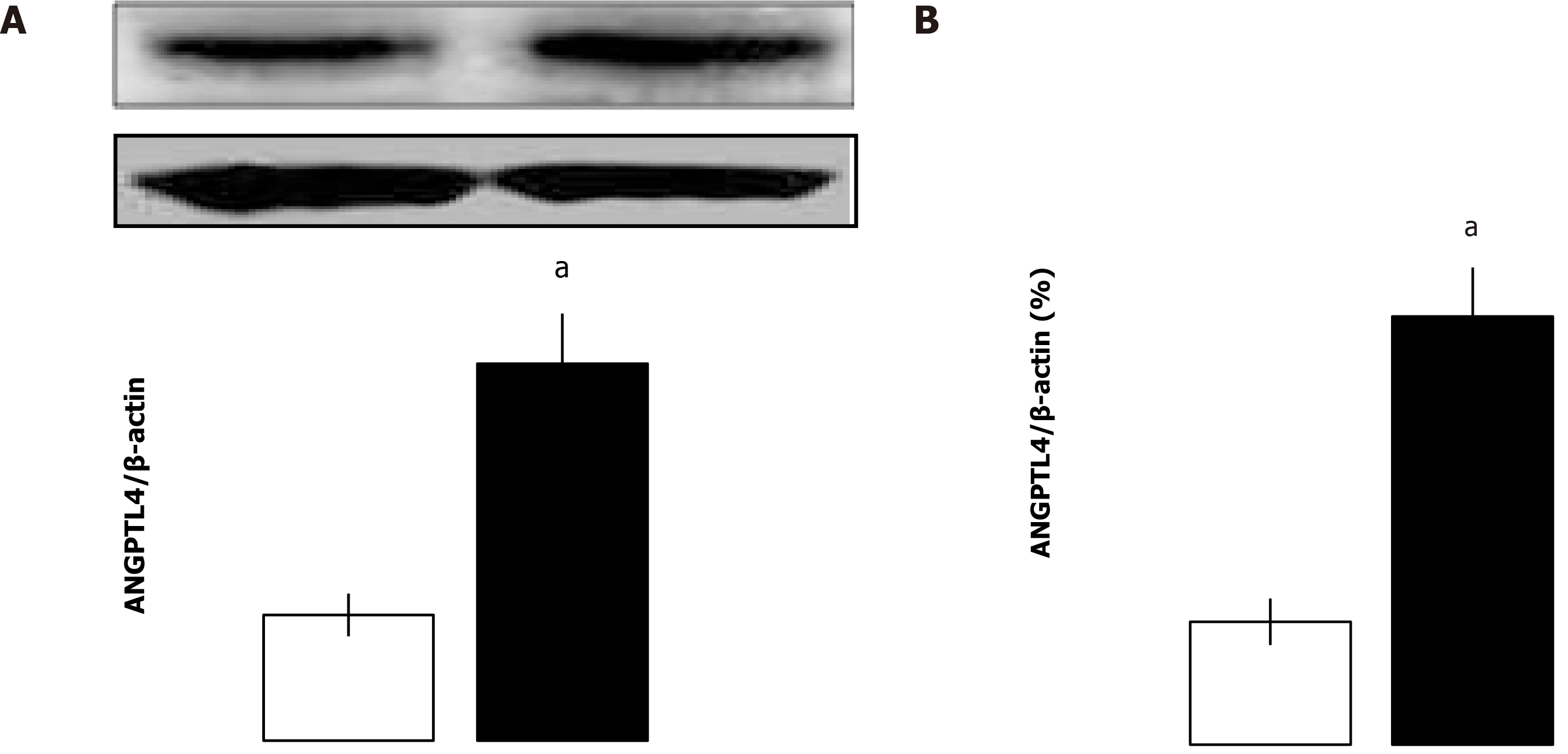
It has been reported that ANGPTL4 is induced in a mouse model of ischemic stroke and severe intestinal inflammation. We wondered whether ANGPTL4 might be expressed in the intestine after I/R in rats. Intestinal I/R injury markedly promoted the mRNA and protein expression of endogenous ANGPTL4 (P < 0.05; Figure 1). These findings indicate that endogenous ANGPTL4 is involved in intestinal I/R. Having shown that ANGPTL4 is expressed upon intestinal I/R, we then sought to investigate its effect on intestinal I/R.
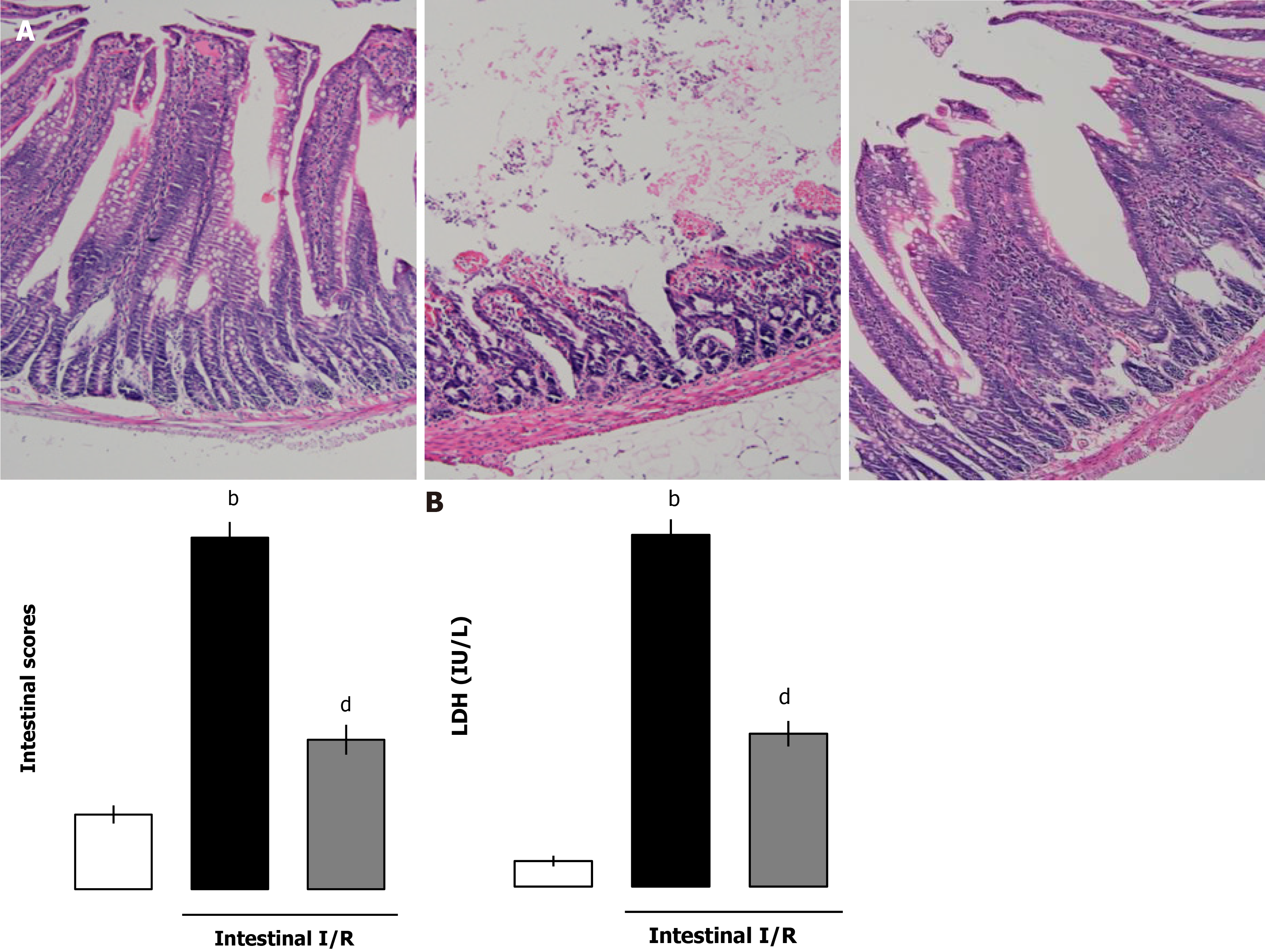
To illustrate the effect of extrinsic administration of ANGPTL4 on intestinal I/R injury, rhANGPTL4 (28 mg/kg body weight) was administered via the tail vein at the initiation of reperfusion. Intestinal I/R caused generalized and macroscopic necrosis with severe intestinal mucosal injury in intestinal areas paratactic to the macroscopically ischemic intestine (P < 0.01; Figure 2A). Similarly, serum LDH, a biochemical marker of tissue damage, was significantly elevated vs the sham group (P < 0.01; Figure 2B), suggesting the generalized tissue injury induced during this pathological process. Administration of rhANGPTL4 alleviated intestinal I/R injury significantly. Histopathologically, most parts of the intestine were free of secondary mucosal injury after treatment (P < 0.01; Figure 2A). Furthermore, LDH levels in rhANGPTL4-treated rats were significantly lower than in the sham group after I/R (P < 0.01; Figure 2B). This indicates that ANGPTL4 treatment alleviates the severity of reperfusion.
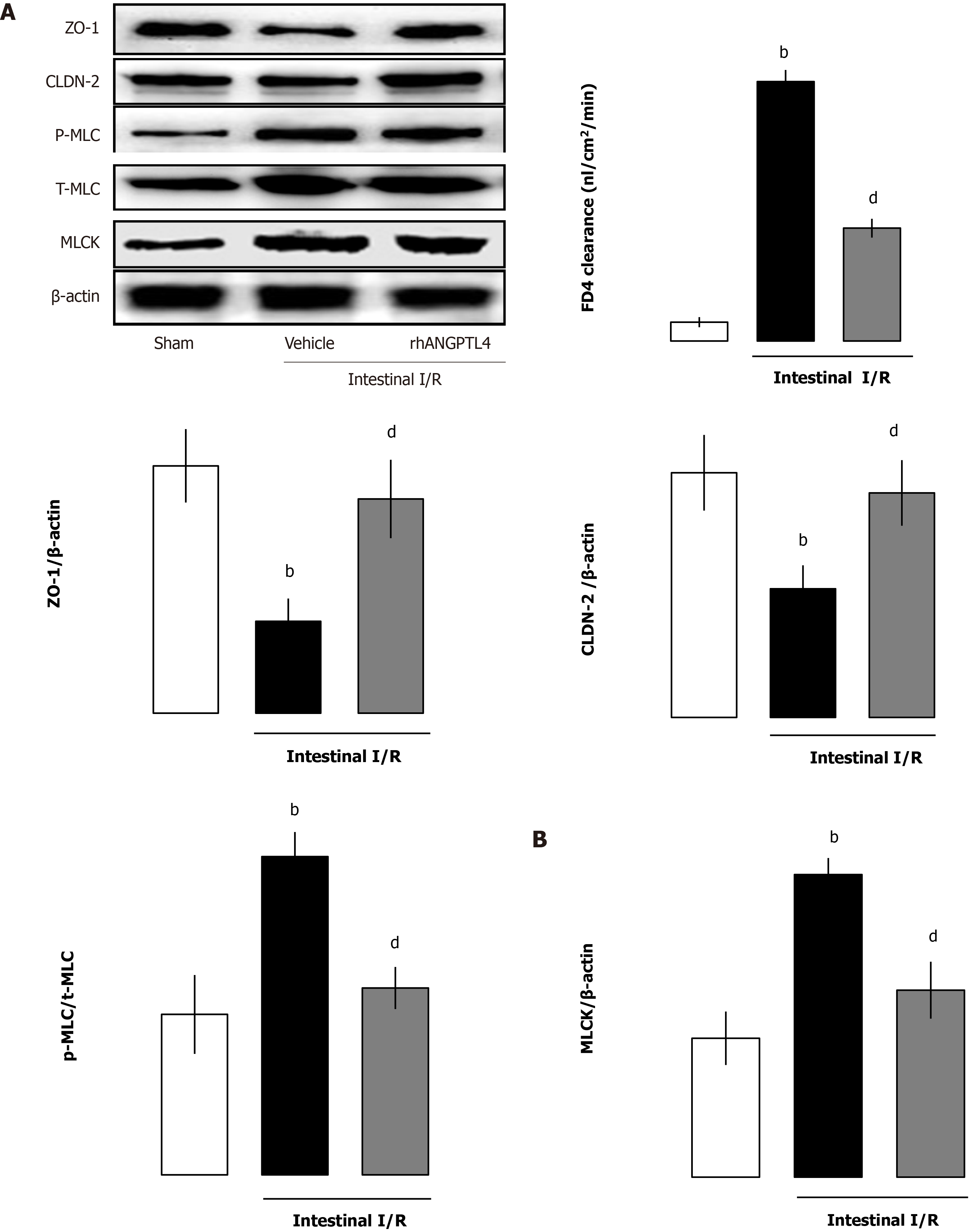
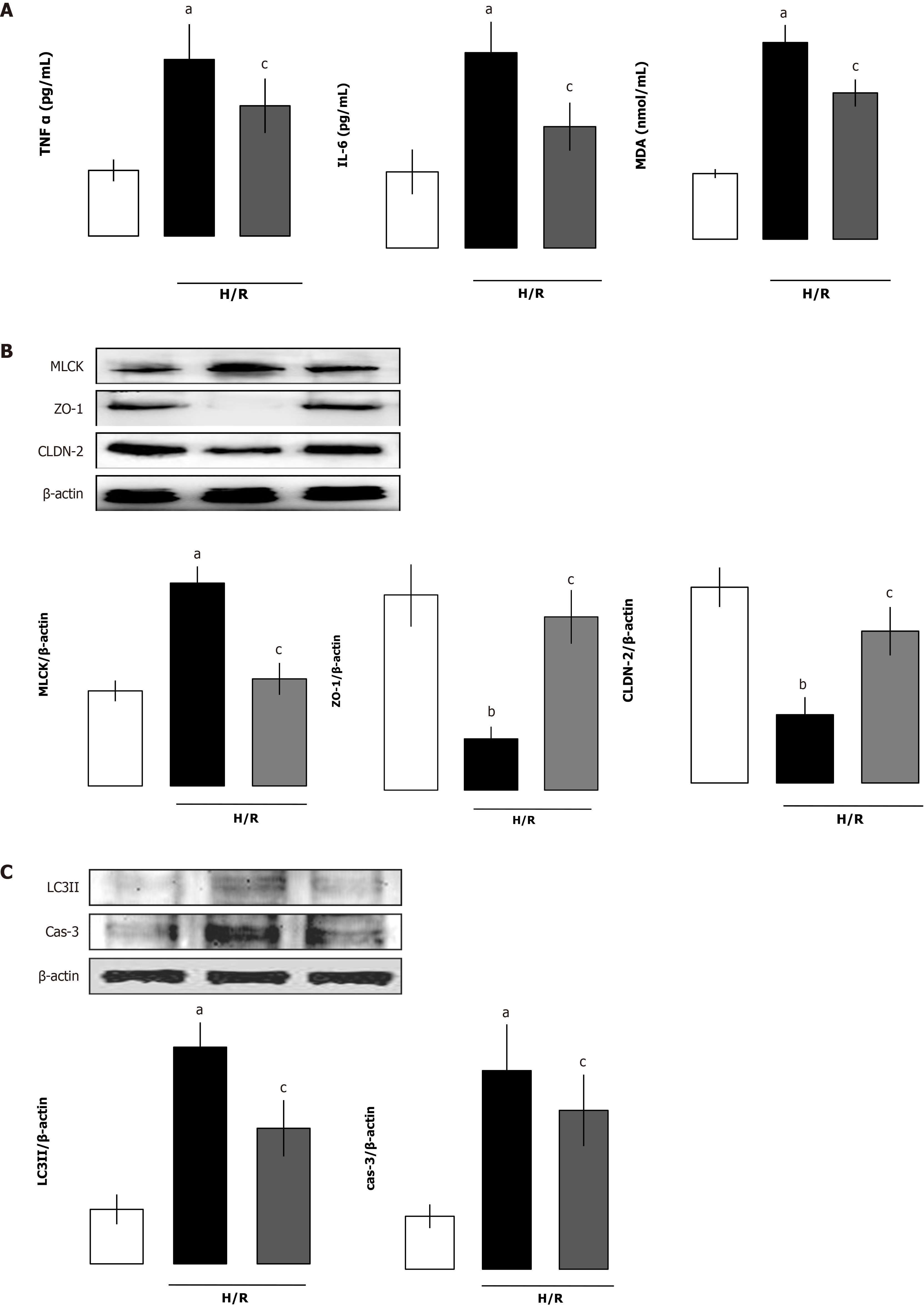
To elucidate the role of rhANGPTL4 in intestinal barrier structure and function, we evaluated intestinal permeability of FD-4, MLCK, p-MLC/MLC, ZO-1 and CLDN-2 protein expression in intestinal mucosa, and the well-established and the recently confirmed methods were used for evaluation of intestinal barrier function. In vehicle-treated animals, I/R caused obvious destruction of intestinal barrier function manifested as increased FD-4 clearance vs the sham group (P < 0.01; Figure 3A). Meanwhile, MLCK and p-MLC/total MLC were also increased coupled with a decrease of ZO-1 and CLDN-2 in vehicle-treated I/R animals. rhANGPTL4 treatment resulted in marked recovery of FD-4 clearance and changes of MLCK, p-MLC/total MLC, ZO-1 and CLDN-2 protein levels 4 h after I/R, close to normal levels compared with vehicle animals (P < 0.01; Figure 3B). This indicates that rhANGPTL4 administration improves both structural and functional repair of the intestinal barrier dysfunction induced by I/R. In addition, ANGPTL4 is involved in the changes of intestinal I/R-induced mucosal barrier structure (MLCK-tight junction protein signaling pathway) and function (FD4 clearance rate in intestinal permeability). rhANGPTL4 confers intestinal mucosal barrier protection by altering the structure and function of the intestinal mucosal barrier. In this process, the MLCK-regulated tight junction signaling pathway may be a potential mechanism. We further investigated the possible mechanism that is involved in the process of intestinal mucosa barrier function regulation.
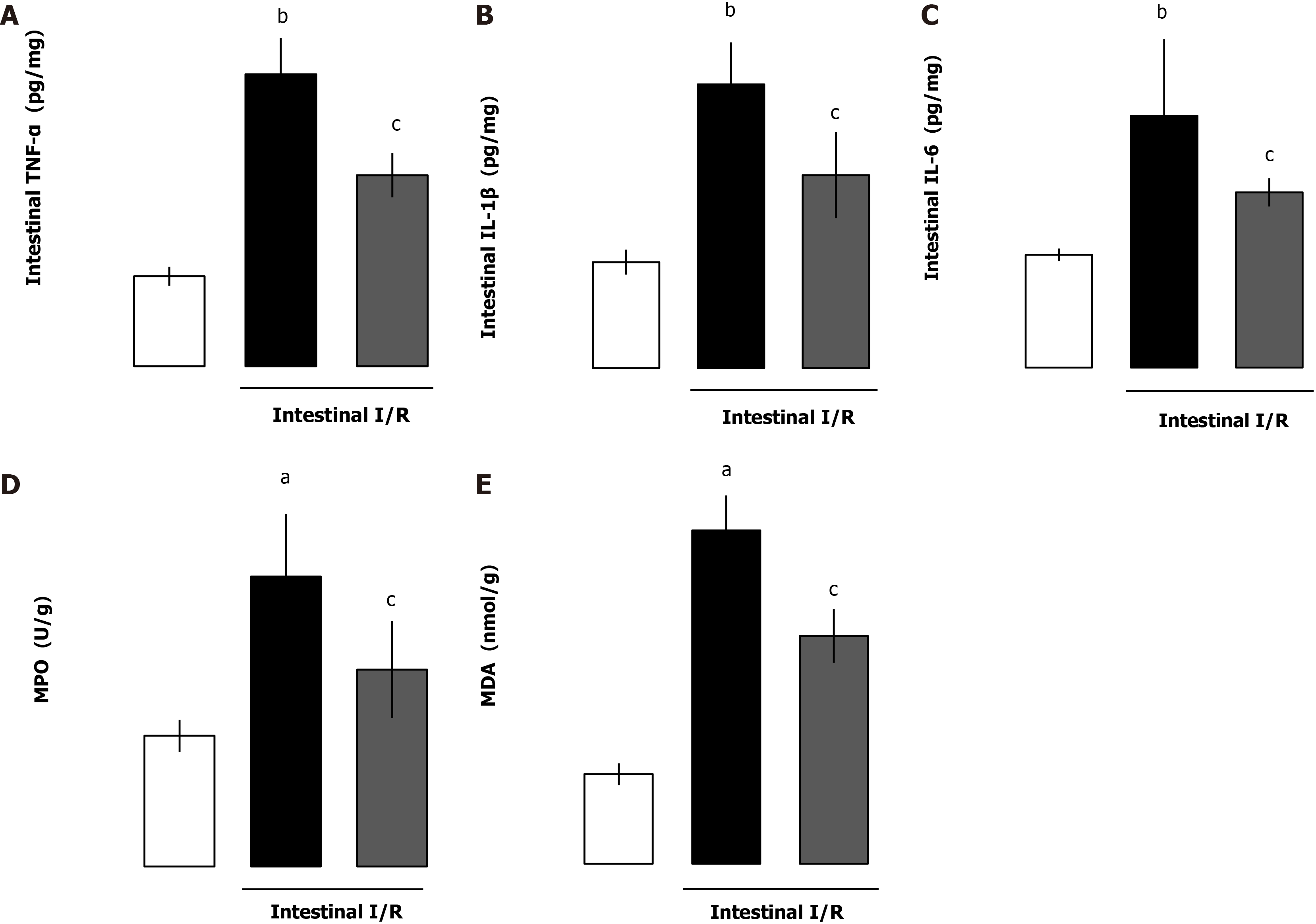
Proinflammatory cytokines are main contributing factors for the disruption of intestinal barrier dysfunction after I/R. We evaluated whether the intestinal cytokines TNF-α, IL-1β, IL-6 and myeloperoxidase (MPO) levels were affected by administration of rhANGPTL4. Although the cytokines and MPO increased markedly after I/R, rhANGPTL4 dramatically reduced the proinflammatory response (P < 0.01, P < 0.05; Figure 4A-C) and MPO level (P < 0.05; Figure 4D). To investigate whether rhANGPTL4 influences oxidative damage to tissues, we further determined intestinal malondialdehyde (MDA). Intestinal MDA levels reduced significantly after rhANGPTL4 treatment (P < 0.05; Figure 4E). Changes of these indicators were associated with intestinal barrier dysfunction. This suggests that inflammatory cytokines and the oxidative stress cascade are contributing factors in destructed intestinal barrier.
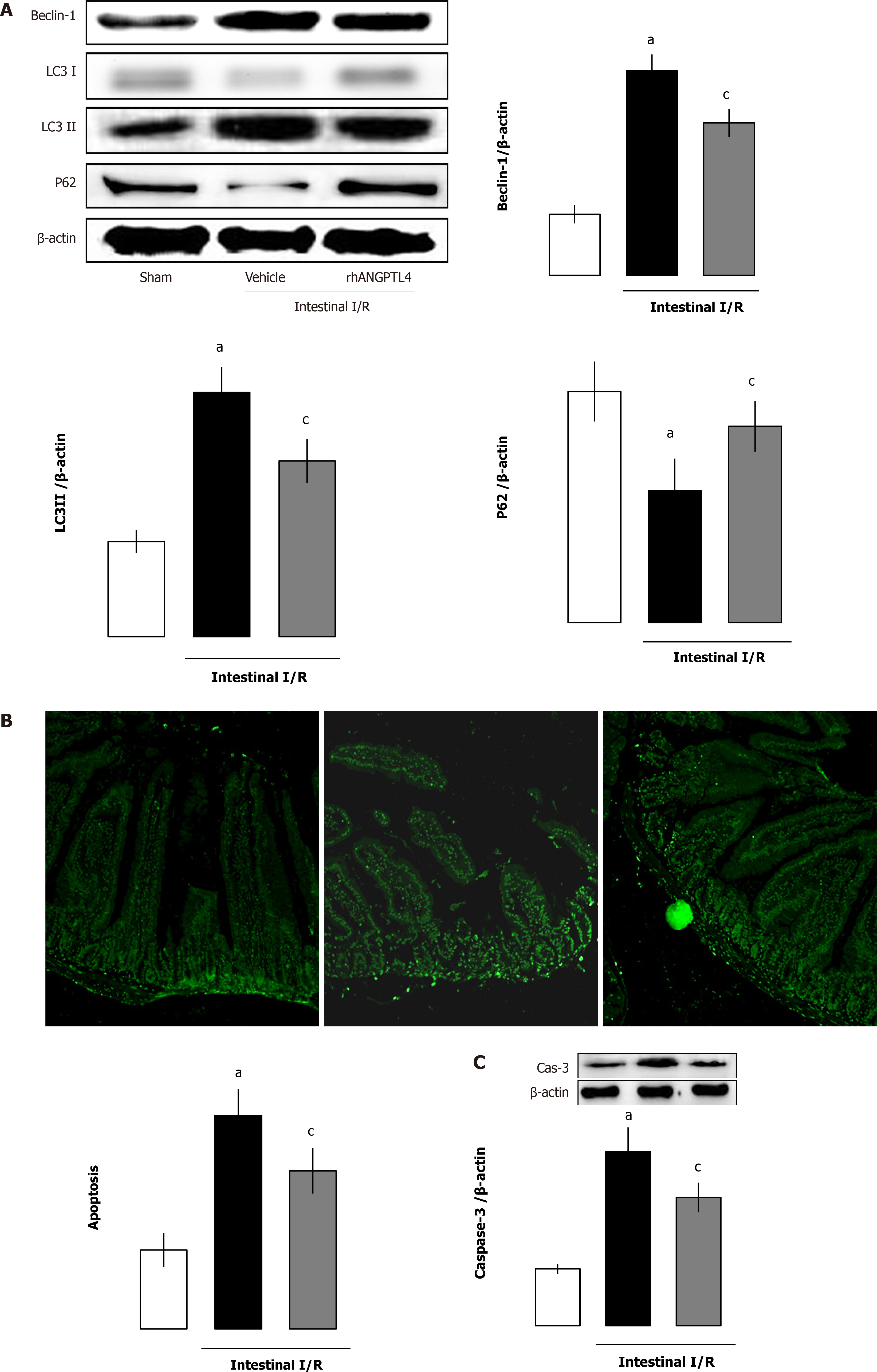
Intestinal I/R increased intestinal autophagy-related protein levels (beclin-1, LC3-I and LC3-II) but decreased the substrate p62 protein levels (P < 0.05; Figure 5A). As a classical apoptotic marker, the cleaved caspase-3 protein expression and numbers of apoptotic cells by TUNEL staining in the intestine was markedly increased after I/R. Treatment with rhANGPTL4 decreased intestinal cleaved caspase-3 levels and apoptotic cell numbers remarkably to those in the sham group (P < 0.05; Figure 5B and 5C). At the same time, we found that the autophagy–related proteins (beclin-1, LC3-I, and LC3-II) and the substrate p62 in the intestine also recovered after treatment with rhANGPTL4 to those in sham animals (P < 0.05; Figure 5A). This indicates that excessive activated autophagy and apoptosis are crucial in the destruction of intestinal barrier integrity, and rhANGPTL4 is an effective agent to inhibit autophagy and apoptosis in intestinal I/R.
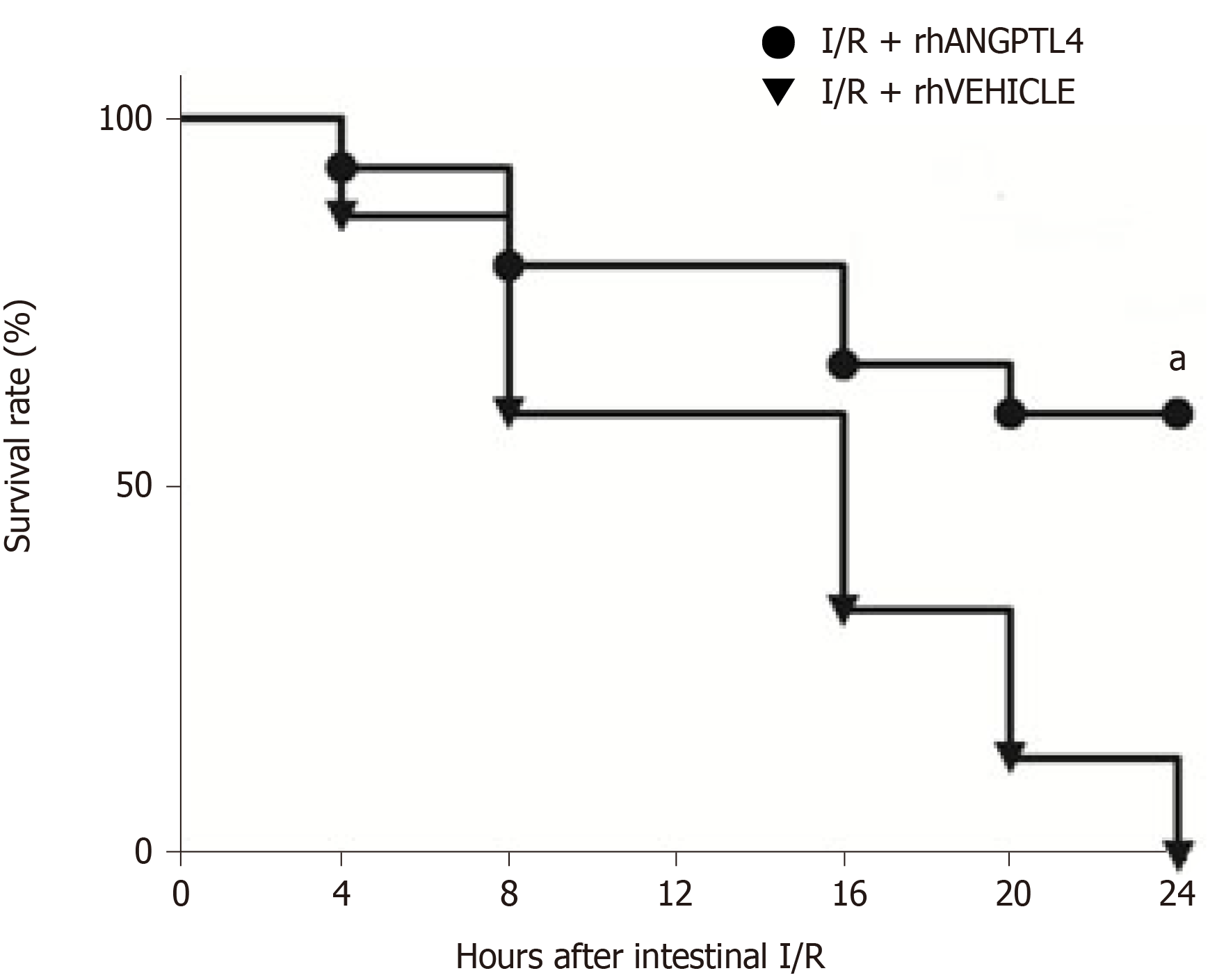
Animals in the vehicle group were sacrificed < 24 h following intestinal I/R injury (P < 0.05; Figure 6). However, rhANGPTL4-treated rats exhibited significant longer survival. The 24 h survival rate of rhANGPTL4 group was increased markedly compared to vehicle animals.
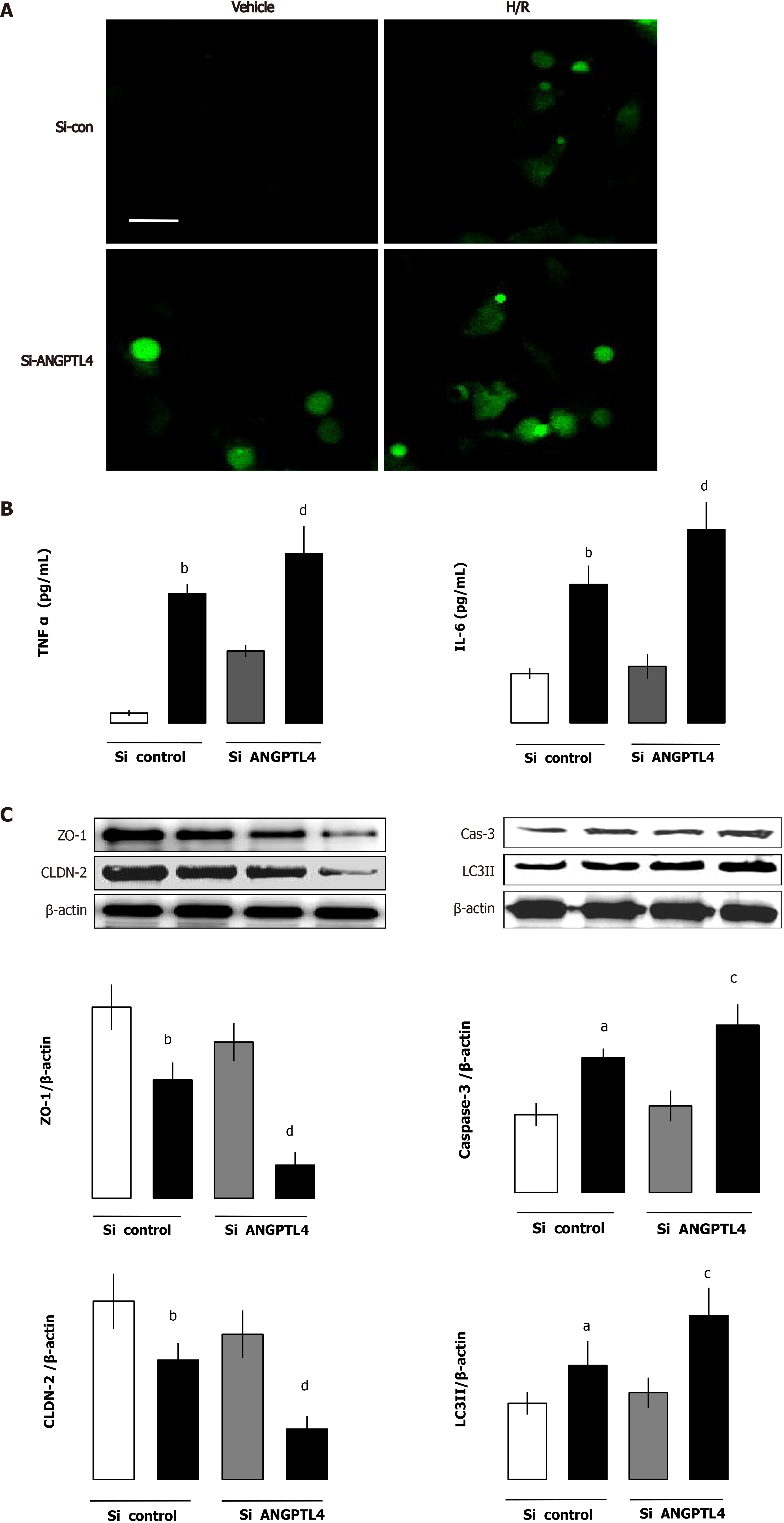
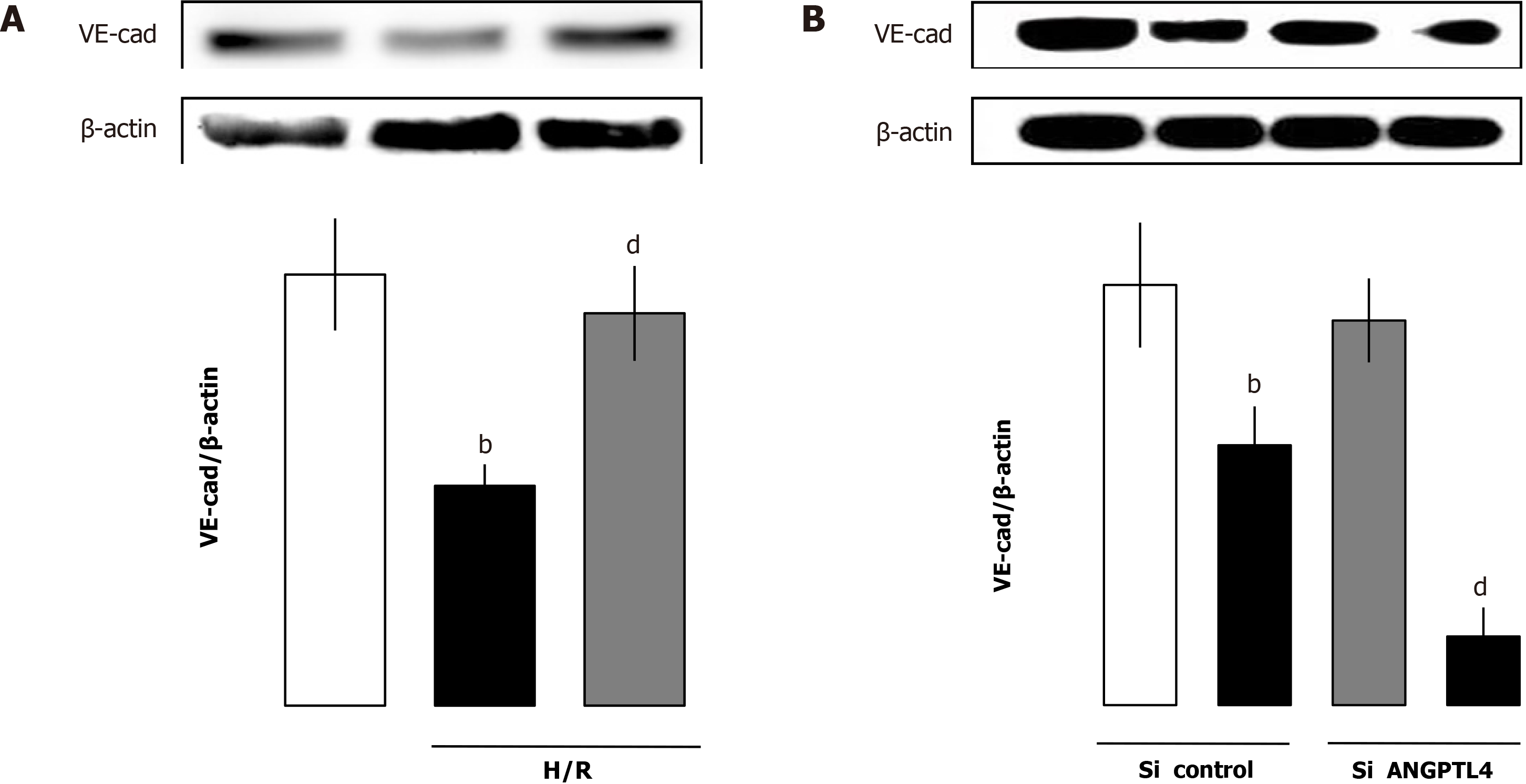
To illustrate the effect of ANGPTL4 in intestinal I/R-induced barrier dysfunction, intestinal epithelial cells were used and siANGPTL4 was challenged by H/R to imitate intestinal I/R. We investigated the inflammatory response, ROS level, autophagy and apoptosis, and intestinal barrier function was measured using the MLCK-tight junction pathway-related protein in the intestinal epithelial cells. Compared with the vehicle group TNF-α and IL-6 levels associated with ROS levels upregulated significantly in both the siANGPTL4 control and siANGPTL4 groups after H/R. The same trends of LC3-II and cleaved caspase-3 but contrary expression of ZO-1 and CLDN-2 were observed correspondingly (P < 0.01; Figure 7). These indicate that ANGPTL4 is essential for maintaining the integrity of intestinal epithelial cell barrier function when challenged by H/R.
To assess the effect of rhANGPTL4 on injury induced by H/R in siRNA ANGPTL4 (siANGPTL4) Caco-2 cells, rhANGPTL4 (10 μg/mL) or vehicle was injected before the H/R as previously described. Cell samples were harvested after reoxygenation for 4 h. Proinflammatory cytokines (including TNF-α and IL-6), MDA, cleaved caspase-3 and LC3-II levels and intestinal barrier-regulated protein expression of MLCK, ZO-1 and CLDN-2 were detected. Administration of rhANGPTL4 decreased proinflammatory cytokine (TNF-α and IL-6) levels and oxidative damage (MDA levels), inhibited autophagy (LC3-II levels) and apoptosis (cleaved caspase-3 levels) and reduced MLCK but restored the levels of ZO-1 and CLDN-2 after H/R in siANGPTL4 cells (P < 0.05, P < 0.01; Figure 8).
To investigate the role of rhANGPTL4 in vascular leakage induced by H/R in siANGPTL4 HUVECs, VE-cad was used as an indicator that plays a crucial role in the integrity of vascular endothelial function. rhANGPTL4 (10 μg/mL) or vehicle was injected before H/R. Cell samples were collected at 4 h after reoxygenation. VE-cad protein expression was detected. Administration of rhANGPTL4 restored VE-cad protein expression levels significantly. Both ANGPTL4 vicious and ANGPTL4 control HUVECs exhibited low levels of VE-cad after H/R compared with vehicle cells (P < 0.01; Figure 9).
In the present study, we provide evidence and a potential mechanism that rhANGPTL4 alleviates intestinal I/R induced injury. In vivo rhANGPTL4 was found to (1) ablate intestinal tissue damage after I/R; (2) improve survival rate; (3) relieve the inflammatory response through inhibition of proinflammatory cytokines and restrict MPO, MDA and LDH; (4) lessen the autophagy and apoptosis molecules; and (5) maintain epithelial barrier function by restoration of tight junction (ZO-1 and CLDN2), inhibiting MLCK and phosphorylation of MLC and intestinal barrier permeability (FD-4 clearance rate). In vitro studies showed that (1) ANGPTL4 deficiency aggravated the inflammatory and oxidative response, apoptosis and autophagy, associated with the loss of intestinal barrier tight junction; (2) rhANGPTL4 had the ability to inhibit inflammation and oxidation, autophagy and apoptosis and preserve the tighten junctions, and accordingly it maintained the endothelial barrier integrity under ANGPTL4 vicious circumstances; and (3) rhANGPTL4 inhibited vascular leakage via limiting the endothelial dysfunction (loss of VE-cad) in HUVECs challenged by H/R.
Intestinal I/R injury is accompanied by enhanced inflammatory cytokines and ROS cascade, augmented apoptosis and autophagy, vascular leakage and aggravated mucosal lesions[1-4,9]. Intestinal barrier breakdown has been implicated as an important turning point in intestinal I/R injury[7]. The intestinal barrier is the largest defender between the intestine and distant organs including lung and liver, which is critical in the development of MODS[13,18]. As a filter with selective permeability, this interface allows absorption of essential nutrients and eliminates the invasion of deteriorating products, including proinflammatory factors, ROS, etc. Exacerbating the inflammatory response and oxidative stress can induce intestinal epithelial cell apoptosis and autophagy, consequently destroying the intestinal mucosal barrier. In addition, intestinal mucosal breakdown facilitates the inflammatory mediators and ROS associated with the bacteria- derived products to translocate to extraintestinal organs[1-3,15,20]. Until now, the underlying mechanisms/factors of I/R inducing intestinal barrier breakdown are still unclear. Recent studies reported that MLC phosphorylation mediated by upregulated MLCK is crucial to intestinal barrier breakdown induced by hypoxia or proinflammatory cytokines[7]. Tight junctions ZO-1 and CLDN-2 regulated by the MLCK pathway are the two crucial factors regulating the intestinal barrier in I/R[5].
ANGPTL4 is normally secreted from the intestinal villus epithelium and modulated by the intestinal microbiota. Studies have shown that ANGPTL4 is essential to resist the loss of villus endothelial and lymphocyte populations to radiation-induced apoptosis[21]. As it is reported that ANGPTL4 is crucial in the postnatal lymphatic partitioning and disease, it is not unexpected that mice missing ANGPTL4 may die during the suckling period with dilated intestinal lymphatic vessels[21,22]. It is noteworthy that injection of rhANGPTL4 in vivo blocked VEGF-driven separation of the VEGFR2/VE-cad complexes, reduced myocardial infarct area and the no-reflow degree in animals. ANGPTL4 exhibited a therapeutic vasculoprotection on no-reflow and exerted cardioprotection[23-26].
However, the role of ANGPTL4 in various types of cells has not been evaluated in detail. We investigated the effects of ANGPTL4 and rhANGPTL4 in maintaining the epithelial barrier through various mechanisms under basal and I/R (H/R) conditions. This preliminary analysis indicated an assumed effect of ANGPTL4 in the circumstance of intestinal I/R-associated endothelial and epithelium barrier damage. In this study, we demonstrate that after I/R injury, intestinal barrier breakdown is associated with the up-regulation of both MLCK pathway-related protein expression and permeability of intestinal mucosa. An associated loss of tight junction protein ZO-1 and CLDN-2 as well as increased histological damage of mucosa were observed following I/R. However, treatment with rhANGPTL4 not only blocked the upregulation of MLCK pathway- related protein and intestinal mucosal permeability but also restored the tight junction proteins ZO-1 and CLDN-2 associated with a lower intestinal histological damage of mucosa. Similar results were also observed in ANGPTL4-deficient intestinal epithelial cells when challenged by H/R. However, this was not observed in the intestinal epithelial vehicle cells without H/R. This indicates that the role of ANGPTL4 is silent in normal circumstances but essential in the cellular homeostasis upon H/R stress stimulation.
It is widely accepted that the oxidative stress and inflammation cascade may contribute to the intestinal barrier destruction through various mechanisms. Activated and influxed immune cells including neutrophils and macrophages will release amplified proinflammatory cytokines (including TNF-α, IL-6 and IL-1β) associated with oxygen free radicals. Higher levels of circulating proinflammatory cytokines and oxygen free radical activation lead to a loss of tight junction, eventually causing intestinal barrier breakdown induced by intestinal I/R[2,16]. Thus, a large number of studies have confirmed that the anti-inflammatory and antioxidative strategies are protective against breakdown of the barrier induced by I/R.
A previous study implicated that ANGPTL4 deficiency can aggravate the severe intestinal inflammation induced by high dietary saturated fat in rats[27]. Further studies showed that rhANGPTL4 protected ischemic stroke and exhibited antioxidative and anti-inflammatory effects[13]. We investigated the effect of ANGPTL4 on oxidative stress and inflammation during intestinal I/R. We found an enhanced expression of ANGPTL4 in rats with intestinal I/R and human intestinal epithelial cells stimulated with H/R, which indicated that ANGPTL4 exerted an effect on modulating the inflammatory milieu following intestinal I/R for triggering the progression of intestinal barrier dysfunction. Our results showed the antioxidative and anti-inflammatory effects of rhANGPTL4 on intestinal barrier dysfunction after I/R, and rhANGPTL4 alleviated the inflammatory and oxidative cascade both in vivo and in vitro. ANGPTL4-mediated barrier protection is due to, at least partially, the inhibition of the inflammatory and oxidative cascade.
In addition to the inflammatory and oxidative cascade, apoptosis and autophagy are mutual connection pathways, and both have direct disrupting roles in intestinal epithelial cells and the intestinal mucosal barrier in response to I/R[3,4,28]. An essential level of autophagy and apoptosis is important for maintaining intestinal epithelial cellular homeostasis under normal conditions. However, if the stress persists, autophagy will not afford cell surviving anymore. Instead exorbitant autophagy and copious activation of apoptosis will cause dramatic disruption of intestinal mucosa and barrier breakdown[28]. We first explored whether increased autophagy and apoptosis were related with ANGPTL4 in intestinal I/R of a rat model and the inhibition effects of rhANGPTL4 on autophagy and apoptosis both in vivo and in vitro challenged by I/R (H/R).
Autophagy is a lysosome-mediated degradative pathway of cellular mechanisms for degrading misfolded protein in addition to the ubiquitin-proteasome system of ubiquitinated proteins[29]. The implementation of autophagy involves a series of autophagy-related proteins that are essential for induction of autophagy and recycling of autophagosomes. Microtubule-associated protein LC3 exists in two forms including LC3-I and its proteolytic derivative LC3-II. LC3-1 is conjugated with phosphatidylethanolamine to become LC3-II, which can be used as a hallmark of autophagy because its population correlates with the number of autophagosomes. P62/SQSTM1 is selectively incorporated into autophagosomes through binding to LC3 and is affirmed as a specific substrate that degrades via the autophagy-lysosomal pathway. Therefore, the p62 contents are oppositely parallel with autophagic activity. Beclin 1 (ATG6) is the first identified mammalian gene to mediate autophagy and is implicated in the nucleation of the autophagic vesicle, which is another marker of autophagy[30].
Recent studies have shown that excessive autophagy that is active in necrotizing enterocolitis and inhibiting autophagy is protective against focal cerebral ischemia in rats[4,31]. Intestinal mucosal barrier disruption is associated with excessive autophagy activation. Our results suggested that ANGPTL4 conferred intestinal mucosal barrier preservation and inhibited the excessive activated autophagy in intestinal I/R. In the present study, intestinal autophagy activated massively in rats with intestinal I/R. Upregulated expression of required autophagy markers (beclin 1 and LC3-II) and the loss of p62 protein are found in intestinal epithelium both in vivo and in vitro challenged by I/R or H/R. Consequentially activated autophagy caused accumulation of autophagic vacuoles and disrupted the majority of the cytosol and organelles, eventually causing the collapse of vital cell functions and cell death. The attenuated epithelial cell homeostasis makes intestine mucosal injury more vulnerable upon I/R. However, rhANGPTL4 reversed I/R-induced changes of autophagy-related regulators (LC3-II, beclin 1 and p62) both in vivo and in vitro. This suggested that ANGPTL4 limited intestinal mucosal exorbitant autophagy to maintain the integrity of intestinal barrier.
Apoptosis was demonstrated to be the main type of cell death in intestinal I/R. Apoptosis disrupts the epithelial cell layer directly when the intestine is subjected to I/R[4]. Studies have shown that “anoikis” exerts a crucial effect in inducing apoptosis during intestine I/R. “Anoikis” manifested as contact disrupted cell death occurring between extracellular matrix materials and cells[32]. Ikeda et al[3] reported that affected epithelial cells lost their link with the villous mesenchyma, and thus apoptosis induced by I/R could represent “anoikis”. They also revealed that distribution of epithelial cell-matrix interactions (“anoikis”) exhibited a crucial effect on initiating apoptosis in detached enterocytes[4]. It is worth noting that ANGPTL4 has been demonstrated to promote anoikis resistance in cholangiocarcinoma and hepatoma cells grown in a disassociated status. These cells try to shape a synoikis-like multi-cellular assemblage that resists apoptosis occurrence. The inhibition of ANGPTL4 by RNA interference increased detachment-related apoptosis and made tumor cells sensitized to antitumor drugs[14]. This implicates that intestinal epithelial cells may drive a similar mechanism to resist I/R-related apoptosis in intestinal mucosa. Our current results show that rhANGPTL4 resisted apoptosis and limited the increased levels of cleaved casepase-3 both in vivo and in vitro. Cells lack of ANGPTL4 showed increased apoptosis and cleaved casepase-3 levels when challenged by H/R. It can be concluded that ANGPTL4 may protect against intestinal mucosal barrier dysfunction due to I/R through anoikis resistance.
Another key factor for the intestinal barrier preservation that cannot be ignored during intestinal I/R is the vascular permeability[9]. Patients exhibited loss of plasma proteins and other macromolecules induced by increased permeability in the colonic microvasculature. During the perpetuation of intestinal I/R, microvascular injury is ubiquitously present. Intestinal microvessels manifested apparently as endothelial dysfunction. Microvascular endothelial cell layer consists of closely arranged ECs that shape a semi-permeable barrier between tissue and blood for the transport of lipids, proteins and electrolytes[33]. Therefore, microvascular barrier dysfunction exerts an important effect in the beginning and progressive phase of I/R injury. Consequently, endothelium reformation induced microvascular permeability changes and exerted a crucial effect on intestinal barrier dysfunction. The bidirectional regulation role of ANGPTL4 in vascular permeability has been extensively studied in various diseases[34-36]. However, the association of ANGPTL4 with vascular leakage induced by I/R remains obscure. In the present study, HUVECs with RNA interference and H/R exhibited a significant loss of the key regulator VE-cad. In addition, rhANGPTL4 inhibited the vascular permeability significantly, manifested as a change of VE-cad in HUVECs challenged by H/R. It is concluded that ANGPTL4 protects the intestinal barrier at least partially via inhibiting vascular leakage.
Intestinal I/R could still be an important cause of MODS, and it is of significance to note that besides the reduction of intestinal structure, ANGPTL4 treatment also significantly improved the intestinal barrier function.
This study demonstrates that rhANGPTL4 can lessen I/R injury by maintaining intestinal barrier structure and function. ANGPTL4 showed a conspicuous effect on MLCK-related proteins, tight junction and vascular permeability as well as on the inflammatory and oxidative response, autophagy and apoptosis. This implies that ANGPTL4 plays a significant role in modulating intestinal barrier integrity. It is speculated that rhANGPTL4 is important in intestinal barrier function maintenance and may have clinical advantages in I/R-related clinical circumstances. Further studies are needed to investigate the protective role of ANGPTL4 on remote organ dysfunction induced by intestinal I/R and to clarify whether this therapeutic agent targeting protection against the intestinal barrier dysfunction could be applied to patients in intensive care units.
Intestinal barrier breakdown remains frequently complicated in critical care patients of intestinal ischemia-reperfusion (I/R), severe acute pancreatitis and sepsis. Although vigorous experiments are performed in this field, the application of an instant effective agent or therapy in clinical has not yet been discovered. Recombinant human angiopoietin-like protein 4 (rhANGPTL4) is known to be protective to the blood-brain barrier when administered exogenously, and endogenous ANGPTL4 deficiency deteriorates intestinal injury.
We intend to explore and discover a novel and promising agent to confer intestinal barrier protection induced by I/R. Our results indicated recombinant agents that exhibit intestinal barrier protective characteristics. The agents may be safer and facilitated to translate into clinical use.
Understanding and regulating ANGPTL4 as a therapeutic target and recombinant human ANGPTL4 will be an application in clinical use in patients suffering from intestinal barrier dysfunction.
This research was executed using Wistar rats treated with intestinal I/R and intestinal epithelial (Caco-2) cells and human umbilical vein endothelial cells stimulated with H/R to intimate the I/R pathogenesis in vivo. Further, RNA interference was performed, and recombinant ANGPTL4 has been evaluated.
A loss of crypt epithelium and myocytes were observed in the muscularis propria. Intraluminal microdialysis were changed, as well as the biochemistry indicators were remarkably enhanced following intestinal I/R. Recombinant human ANGPTL4 treatment significantly reversed indicators above associated with inhibiting the inflammatory and oxidative cascade, excessive activation of cellular autophagy and apoptosis and was associated with an improvement of survival rate. In vitro studies showed similar results in Caco-2 and human umbilical vein endothelial cells.
Recombinant human ANGPTL4 achieved an optimal therapeutic effect for intestinal I/R-induced intestinal barrier injury. Using this model, the intestinal barrier structure and functions indicators were maintained, thus providing a promising therapeutic potential.
ANGPTL4 may be a valuable predictor, and similar research in patients who suffered critical care conditions could be evaluated.
The authors wish to thank for Chen DP and Gao J for their excellent technical support and Han CC for gifting antibodies and cells.
Manuscript source: Unsolicited manuscript
Corresponding Author’s Membership in Professional Societies: Chinese Anti-Cancer Association, No. M164006948S.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Delgado-Gallegos JL, Katada K S-Editor: Zhang H L-Editor: Filipodia P-Editor: Li JH
| 1. | Pastores SM, Katz DP, Kvetan V. Splanchnic ischemia and gut mucosal injury in sepsis and the multiple organ dysfunction syndrome. Am J Gastroenterol. 1996;91:1697-1710. [PubMed] |
| 2. | Mainous MR, Ertel W, Chaudry IH, Deitch EA. The gut: a cytokine-generating organ in systemic inflammation? Shock. 1995;4:193-199. [PubMed] |
| 3. | Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, Nomura M, Itoh G. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 235] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Norsa L, Bonaffini PA, Indriolo A, Valle C, Sonzogni A, Sironi S. Poor Outcome of Intestinal Ischemic Manifestations of COVID-19. Gastroenterology. 2020;159:1595-1597.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Jiang S, Fan Q, Xu M, Cheng F, Li Z, Ding G, Geng L, Fu T. Hydrogen-rich saline protects intestinal epithelial tight junction barrier in rats with intestinal ischemia-reperfusion injury by inhibiting endoplasmic reticulum stress-induced apoptosis pathway. J Pediatr Surg. 2020;55:2811-2819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Søfteland JM, Bagge J, Padma AM, Casselbrant A, Zhu C, Wang Y, Hellström M, Olausson M, Oltean M. Luminal polyethylene glycol solution delays the onset of preservation injury in the human intestine. Am J Transplant. 2021;21:2220-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Graham WV, He W, Marchiando AM, Zha J, Singh G, Li HS, Biswas A, Ong MLDM, Jiang ZH, Choi W, Zuccola H, Wang Y, Griffith J, Wu J, Rosenberg HJ, Snapper SB, Ostrov D, Meredith SC, Miller LW, Turner JR. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nat Med. 2019;25:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Nalle SC, Zuo L, Ong MLDM, Singh G, Worthylake AM, Choi W, Manresa MC, Southworth AP, Edelblum KL, Baker GJ, Joseph NE, Savage PA, Turner JR. Graft-versus-host disease propagation depends on increased intestinal epithelial tight junction permeability. J Clin Invest. 2019;129:902-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Wang X, Li M, Yang Z, Li H, Wang Y, Tang W, Wu Y, Xiao P, Jiang S, Shi Q, Lu Y. Comparison of the Protective Effect of Different Mild Therapeutic Hypothermia Temperatures on Intestinal Injury after Cardiopulmonary Resuscitation in Rats. Shock. 2021;56:450-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Barbaro MR, Cremon C, Morselli-Labate AM, Di Sabatino A, Giuffrida P, Corazza GR, Di Stefano M, Caio G, Latella G, Ciacci C, Fuschi D, Mastroroberto M, Bellacosa L, Stanghellini V, Volta U, Barbara G. Serum zonulin and its diagnostic performance in non-coeliac gluten sensitivity. Gut. 2020;69:1966-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Babapoor-Farrokhran S, Jee K, Puchner B, Hassan SJ, Xin X, Rodrigues M, Kashiwabuchi F, Ma T, Hu K, Deshpande M, Daoud Y, Solomon S, Wenick A, Lutty GA, Semenza GL, Montaner S, Sodhi A. Angiopoietin-like 4 is a potent angiogenic factor and a novel therapeutic target for patients with proliferative diabetic retinopathy. Proc Natl Acad Sci U S A. 2015;112:E3030-E3039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Wen L, Zhang Y, Yang B, Han F, Ebadi AG, Toughani M. Knockdown of Angiopoietin-like protein 4 suppresses the development of colorectal cancer. Cell Mol Biol (Noisy-le-grand). 2020;66:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Leth-Espensen KZ, Kristensen KK, Kumari A, Winther AL, Young SG, Jørgensen TJD, Ploug M. The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc Natl Acad Sci USA. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | San TT, Khaenam P, Prachayasittikul V, Sripa B, Kunkeaw N, Chan-On W. Curcumin enhances chemotherapeutic effects and suppresses ANGPTL4 in anoikis-resistant cholangiocarcinoma cells. Heliyon. 2020;6:e03255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Fan Z, Jing H, Yao J, Li Y, Hu X, Shao H, Shen G, Pan J, Luo F, Tian X. The protective effects of curcumin on experimental acute liver lesion induced by intestinal ischemia-reperfusion through inhibiting the pathway of NF-κB in a rat model. Oxid Med Cell Longev. 2014;2014:191624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1426] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 18. | Qian J, Li G, Jin X, Ma C, Cai W, Jiang N, Zheng J. Emodin protects against intestinal and lung injury induced by acute intestinal injury by modulating SP-A and TLR4/NF-κB pathway. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Zhai X, Lin M, Zhang F, Hu Y, Xu X, Li Y, Liu K, Ma X, Tian X, Yao J. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol Nutr Food Res. 2013;57:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Koike Y, Li B, Ganji N, Zhu H, Miyake H, Chen Y, Lee C, Janssen Lok M, Zozaya C, Lau E, Lee D, Chusilp S, Zhang Z, Yamoto M, Wu RY, Inoue M, Uchida K, Kusunoki M, Delgado-Olguin P, Mertens L, Daneman A, Eaton S, Sherman PM, Pierro A. Remote ischemic conditioning counteracts the intestinal damage of necrotizing enterocolitis by improving intestinal microcirculation. Nat Commun. 2020;11:4950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A. 2005;102:13254-13259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Bäckhed F, Crawford PA, O'Donnell D, Gordon JI. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc Natl Acad Sci U S A. 2007;104:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 23. | Gur-Cohen S, Yang H, Baksh SC, Miao Y, Levorse J, Kataru RP, Liu X, de la Cruz-Racelis J, Mehrara BJ, Fuchs E. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science. 2019;366:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 24. | Cho DI, Kang HJ, Jeon JH, Eom GH, Cho HH, Kim MR, Cho M, Jeong HY, Cho HC, Hong MH, Kim YS, Ahn Y. Antiinflammatory activity of ANGPTL4 facilitates macrophage polarization to induce cardiac repair. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK, Lam CR, Sng MK, Leong DT, Tan SM, Kersten S, Ding JL, Li HY, Tan NS. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood. 2011;118:3990-4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Ziegler AL, Pridgen TA, Blikslager AT. Environmental stressors affect intestinal permeability and repair responses in a pig intestinal ischemia model. Tissue Barriers. 2020;8:1832421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, van der Meer R, He Y, Qi L, Köster A, Tamsma JT, Tan NS, Müller M, Kersten S. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 2010;12:580-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Chen QS, Shen A, Dai JW, Li TT, Huang WF, Shi K, Deng Y, Pan L, Wei XF, Wu ZJ. IL37 overexpression inhibits autophagy and apoptosis induced by hepatic ischemia reperfusion injury via modulating AMPK/mTOR/ULLK1 signalling pathways. Life Sci. 2021;276:119424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 954] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 30. | Filali-Mouncef Y, Hunter C, Roccio F, Zagkou S, Dupont N, Primard C, Proikas-Cezanne T, Reggiori F. The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy. 2021;1-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 204] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 31. | Luo HC, Yi TZ, Huang FG, Wei Y, Luo XP, Luo QS. Role of long noncoding RNA MEG3/miR-378/GRB2 axis in neuronal autophagy and neurological functional impairment in ischemic stroke. J Biol Chem. 2020;295:14125-14139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2335] [Cited by in RCA: 2440] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 33. | Spain DA, Wilson MA, Krysztopik RJ, Matheson PJ, Garrison RN. Differential intestinal microvascular dysfunction occurs during bacteremia. J Surg Res. 1997;67:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Schucht JE, Matheson PJ, Harbrecht BG, Bond L, Jones S, Alkhateeb KJM, Ashkettle GR, Smith JW. Plasma resuscitation with adjunctive peritoneal resuscitation reduces ischemic intestinal injury following hemorrhagic shock. J Trauma Acute Care Surg. 2020;89:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Sodhi A, Ma T, Menon D, Deshpande M, Jee K, Dinabandhu A, Vancel J, Lu D, Montaner S. Angiopoietin-like 4 binds neuropilins and cooperates with VEGF to induce diabetic macular edema. J Clin Invest. 2019;129:4593-4608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 36. | Fernández-Hernando C, Suárez Y. ANGPTL4: a multifunctional protein involved in metabolism and vascular homeostasis. Curr Opin Hematol. 2020;27:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |