Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5362
Peer-review started: March 2, 2021
First decision: June 3, 2021
Revised: July 3, 2021
Accepted: July 12, 2021
Article in press: July 12, 2021
Published online: August 28, 2021
Processing time: 175 Days and 18.3 Hours
Even though immune checkpoint inhibitors (ICIs) are effective on multiple cancer types, there are still many non-responding patients. A possible factor put forward that may influence the efficacy of ICIs is the gut microbiota. Additionally, faecal microbiota transplantation may enhance efficacy of ICIs. Nevertheless, the data available in this field are insufficient, and relevant scientific work has just commenced. As a result, the current work reviewed the latest research on the association of gut microbiota with ICI treatments based on anti-programmed cell death protein 1 antibody and anti- cytotoxic T-lymphocyte-associated protein 4 antibody and explored the therapeutic potential of faecal microbiota tran
Core Tip: Gut microbiota composition is closely associated with the efficacy of immune checkpoint inhibitors (ICIs). Specific species among the intestinal commensal bacteria may play a key role in the efficacy of ICIs against cancer. Faecal microbiota transplantation may enhance efficacy of ICIs.
- Citation: Kang YB, Cai Y. Faecal microbiota transplantation enhances efficacy of immune checkpoint inhibitors therapy against cancer. World J Gastroenterol 2021; 27(32): 5362-5375
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5362.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5362
Immune checkpoint molecules can modulate the immune system in the host via the transduction of immunosuppressive co-signals into the immunocompetent cells[1-5]. Typically, Programmed cell death protein 1 (PD-1)/programmed cell death protein ligand 1 (PD-L1) (CD274), PD-L2 (CD273), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, CD152) are the most well-known examples[6-13]. These molecules are expressed in suitable cells at the suitable timing to exert their vital parts in the prevention of over-activated immune system in the host and the maintenance of immunological tolerance and homeostasis[1,2,5]. At the same time, immune checkpoint molecules show abnormal expression within tumour tissues[3,14-16]. Therefore, a strong immunosuppressive environment will be produced within tumour tissues, leading to resistance to treatment of numerous cancers. Immune checkpoint inhibitors (ICIs) mainly function to alleviate or destroy the immunosuppression mechanisms involved in tumour microenvironment (TME) by the use of inhibitory agents targeting the immune checkpoint molecules[2,5,17]. At present, anti-CTLA-4 (like ipilimumab), anti-PD-1 (such as pembrolizumab, nivolumab, and anti-PD-L1 (such as atezolizumab, durvalumab, avelumab) antibodies have been applied in treating several cancers in the word[18-23].
At present, checkpoint blockade still shows high effectiveness on certain cases, but just about 10%-30% cancers can achieve treatment responses. The combined used of ICIs is associated with a higher response rate and greater toxicity[24], regardless of the limited research on the ICI treatment. There are several ICI resistance mechanisms related to the low response rate, which are low PD-L1 expression, low tumour mutational burden, local immunosuppression, weak tumuor cell antigenicity, tumour-infiltrating lymphocytes (TILs) functional exhaustion, no priming, and defected antigen presentation in the process of priming[25].
In addition, gut microbiome is suggested to be the potential factor that determines ICI efficacy. There are more than 100 trillion bacteria in the human gut, among which 500-1000 bacterial species have been identified to affect the mucosal immune system and exert vital parts in immune system operation under the normal or disease state[26]. Intestinal symbiotic bacteria may exert inflammatory or beneficial function while interacting with host immune system in intestinal lymphoid tissues. Therefore, faecal microbiota transplantation (FMT) can potentially improve the ICI efficacy. Nonetheless, there is only limited information on this topic, and related scientific work is merely at the beginning stage. The emergence of novel techniques has made it possible to investigate systemically the gut microbiota, which also sheds more light on the gut microbial compositions and their pathological variance. The present work aimed to review the latest research on the associations of gut microbiota with immune systems and ICI treatments based on anti-PD-1 antibody (Ab) and anti-CTLA-4 Ab and to explore the therapeutic potential of FMT combined with ICI therapy in the future.
Two steps are necessary to activate tumour-specific T cells. Firstly, the selective binding of T cell receptor (TCR) to major histocompatibility complex I that has antigen-anchoring peptides[27]. Secondly, further amplification of the activation signal of TCR/CD3 complex is performed after the synergistic effect with co-stimulatory signals like OX40, CD28, and inducible T cell co-stimulator, which finally results in T cell priming and activation[27]. By contrast, co-inhibitory signals (also known as the immune checkpoints), including PD-1, CTLA-4, T cell immunoglobulin domain, mucin domain-3, and lymphocyte activation gene-3, inhibit T cell activation via offsetting CD28- or TCR/CD3-mediated tyrosine phosphorylation through the intracellular immunoreceptor tyrosine–based inhibition motif[28-30]. Tumour cells are likely to enhance the co-inhibitory signalling pathway activity for the sake of immune escape[31,32]. ICIs can decrease the tumour antigen immune tolerance and restore the anticancer response. Anti-CTLA-4 and anti-PD-1/PD-L1 are used to treat several cancers[33-38]. Nevertheless, there is a great potential to enhance the anticancer effect of ICI.
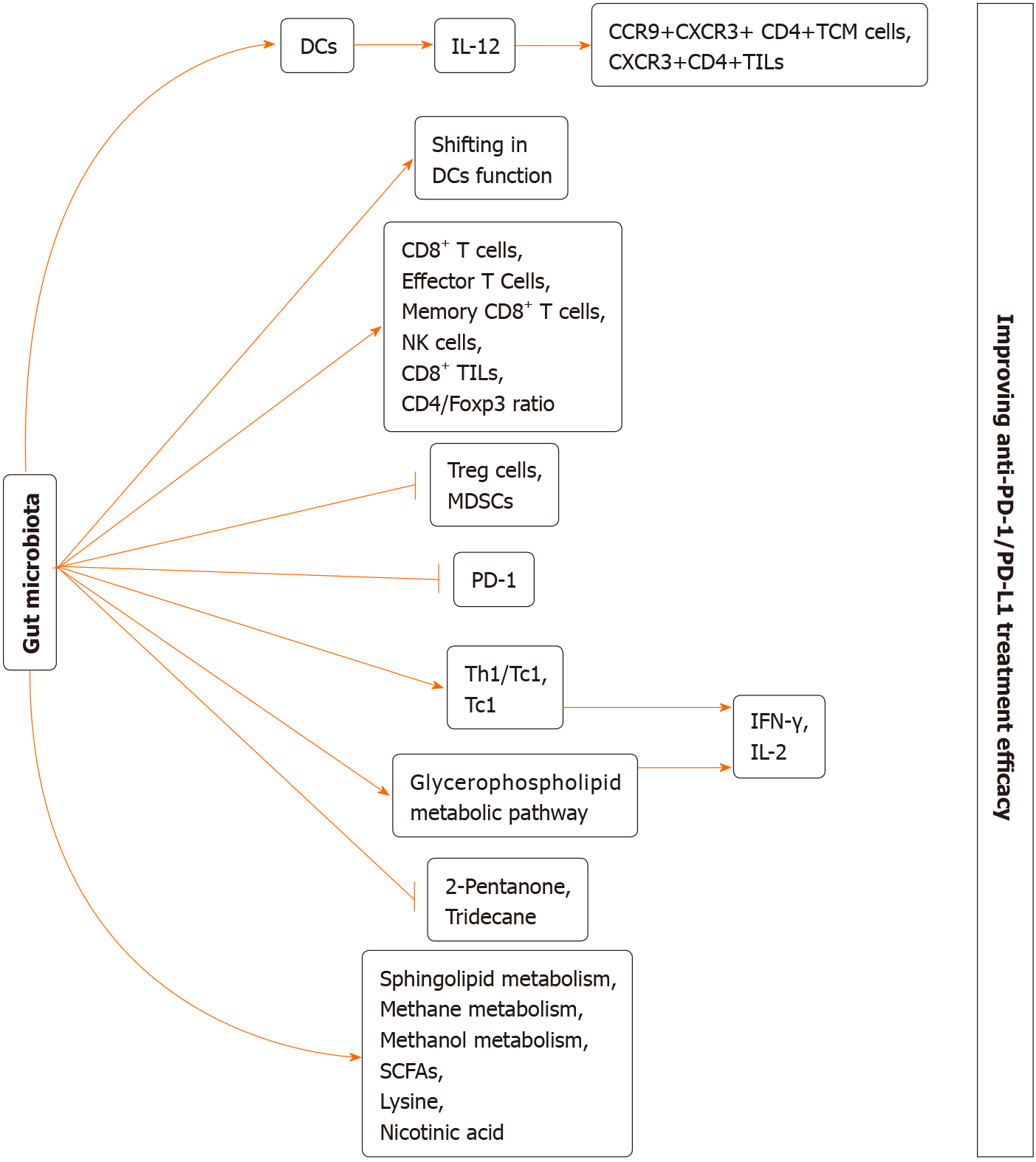
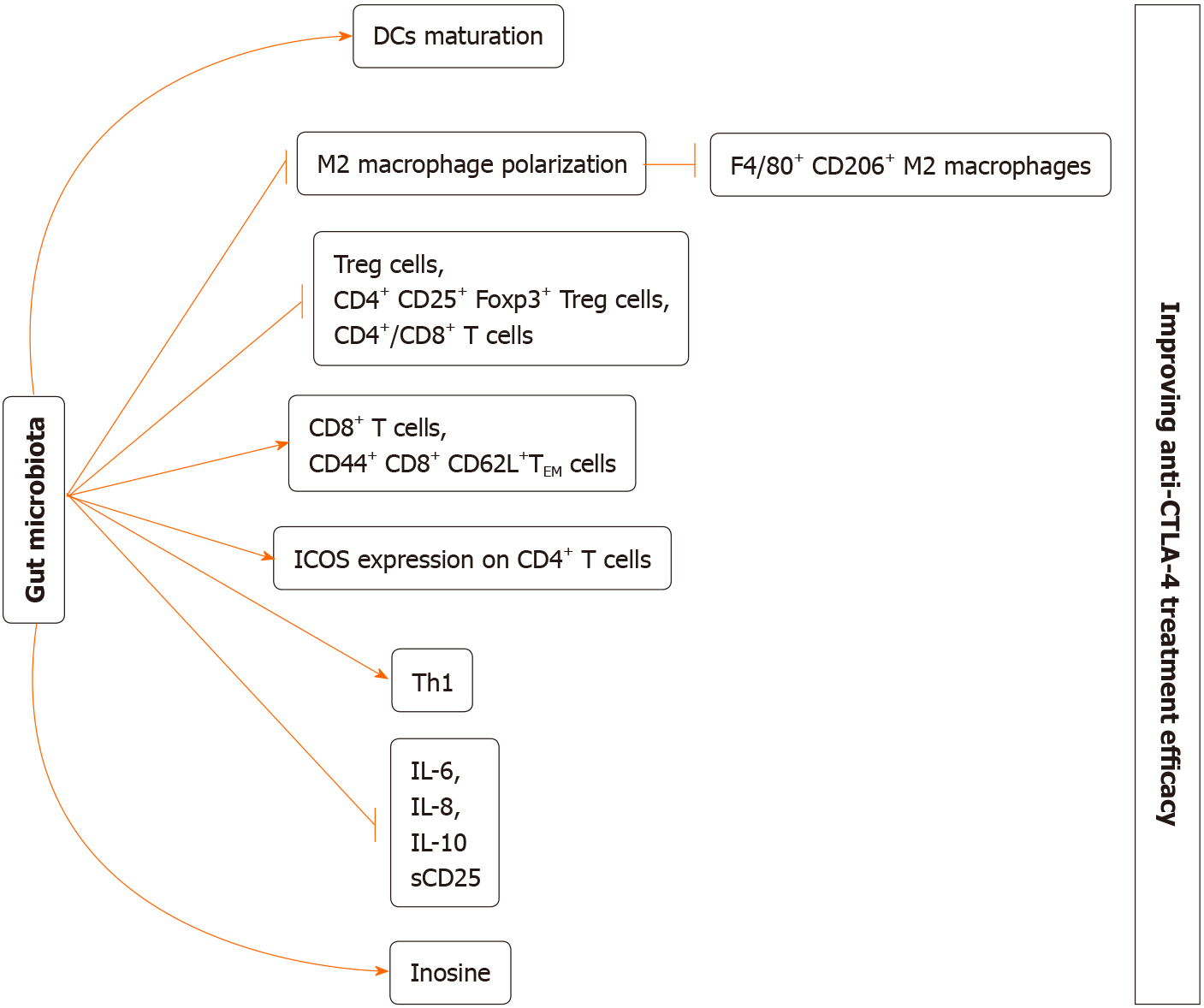
It has been recognized that gut microbiome is involved in cancer genesis and the immune surveillance that suppresses tumour progression[39-42]. Certain commensals may display the synergetic effects with treatments such as surgery, chemotherapy, radiotherapy, and immunotherapy after affecting the immune homeostasis in the intestine and immune adjustment of secondary immune organs[43-52]. ICIs can regulate tumour regression through enhancing the immune activation in the host. A series of studies suggested that gut microbiota composition shows close association with the efficacy of ICIs (Tables 1 and 2). At the same time, we revealed the potential mechanisms by which gut microbiome may be involved in the ICI efficacy (Figures 1 and 2).
| Models | Disease | Implicated microbiota | New strategies | Implicated microbiota | Ref. |
| Mice | Melanoma | Bifidobacterium↑ | (1) FMT; and (2) Commercial cocktail of Bifidobacterium including Bifidobacterium breve and B. longum | NO | Sivan et al[60], 2015 |
| Mice | CRC | Bacteroides_sp._CAG:927↑, Bacteroidales_S24-7↑, Akkermansia muciniphila↑ | NO | NO | Xu et al[61], 2020 |
| Mice | CT26 tumours | NO | GQD | s__Bacteroides acidifaciens↑, s__uncultured_organism_g__norank_f__Bacteroidales_S24-7↑ | Lv et al[62], 2019 |
| Mice | RCC | NO | (1) FMT; (2) A. muciniphila; and (3) Bacteroides salyersiae | NO | Derosa et al[66], 2020 |
| Mice | Melanoma | NO | FMT | NO | Matson et al[68], 2018 |
| Mice | MCA-205 sarcoma | NO | (1) FMT; (2) A. muciniphila; and (3) A. muciniphila with Enterococcus hirae; Alistipes indistinctus | NO | Routy et al[69], 2018 |
| Mice | (1) RET; and (2) Melanoma | NO | (1) A. muciniphila; and (2) A. muciniphila with Enterococcus hirae; | NO | Routy et al[69], 2018 |
| Human | NSCLC | Parabacteroides↑, Methanobrevibacter↑, Veillonella↓, Selenomonadales↓, Negativicutes↓ | NO | NO | Song et al[63], 2020 |
| Human | NSCLC | Gut microbial diversity↑, Alistipes putredinis↑, B. longum↑, Prevotella copri↑, Ruminococcus unclassified↓ | NO | NO | Jin et al[64], 2019 |
| Human | NSCLC | Altered gut microbiota metabolome | NO | NO | Botticelli et al[65], 2020 |
| Human | RCC | A. muciniphila ↑, Bacteroides salyersiae↑, Clostridium hathewayi↓ | NO | NO | Derosa et al[66], 2020 |
| Human | Melanoma | Gut microbial diversity↑, Clostridiales/Ruminococcaceae↑, Faecalibacterium↑, Anaerotruncus colihominis↓, Bacteroides thetaiotaomicron↓, Escherichia coli↓ | NO | NO | Gopalakrishnan et al[67], 2018 |
| Human | Melanoma | Bifidobacterium adolescentis↑, B. longum↑, Collinsella aerofaciens↑, Enterococcus faecium↑, Klebsiella pneumoniae↑, Lactobacillus species↑, Parabacteroides merdae↑, Veillonella parvula↑, Ruminococcus obeum↓, Roseburia intestinalis↓ | NO | NO | Matson et al[68], 2018 |
| Human | NSCLC and RCC | A. muciniphila ↑ | NO | NO | Routy et al[69], 2018 |
| Human | Melanoma | NO | FMT | NO | Baruch et al[83], 2021 |
| Models | Disease | Implicated microbiota | New strategies | Implicated microbiota | Ref. |
| Mice | MCA205 sarcomas | Clostridiales↑, Bacteroides thetaiotaomicron↑, B. uniformis↑, Bacteroidales↓, Burkholderiales↓, | (1) B. thetaiotaomicron; (2) B fragilis; and (3) Burkholderia cepacia | NO | Vétizou et al[70], 2015 |
| Mice | CRC | NO | Lactobacillus acidophilus cell lysates | NO | Zhuo et al[73], 2019 |
| Mice | CRC | NO | (1) Bifidobacterium pseudolongum; (2) Lactobacillus johnsonii; (3) Olsenella spp; and (4) Metabolite inosine | NO | Mager et al[74], 2020 |
| Huamn | Melanoma | Faecalibacterium genus↑, unclassified Ruminococcus↑, Lachnospiraceae genus↑, Clostridium XIVa↑, Blautia↑, Butyrate producing bacterium↑, Gemmiger formicilis↑, Bacteroides↓, B. fragilis↓, B. thetaiotaomicron↓ | NO | NO | Chaput et al[72], 2017 |
The PD-1/PD-L1 blockage treatment blocks the negative signals transduced by the PD-1 intracellular domains (like immunoreceptor tyrosine–based inhibition motif, immunoreceptor tyrosine–based switch motif)[53]. Typically, PD-1/PD-L1 blockage has been identified to promote T cell activation resulting from CD28 and TCR/CD3 while promoting T cell growth and survival by the activation of Ras-Raf-mitogen activated protein kinase and phosphatidylinositol 3 kinase-AKT signalling[54,55]. The PD-1/PD-L1 blockage treatment has been approved to treat certain malignant tumours, including non-small cell lung cancer (NSCLC), colorectal cancer (CRC), kidney cell cancer, and melanoma[56,57]. Biomarkers that contain the TIL status, PD-L1 expression, or deficiency of the mismatch repair system are tightly associated with the efficacy of PD-1/PD-L1 blockage treatment[58]. Besides those above-mentioned factors, gut microbiota contributes to difference in treatment responses as well[59].
In 2015, some investigators discover the relationship of gut microbiota with the efficacy of anti-PD-1 therapy using a mouse model[60]. Sivan et al[60] explored the therapeutic effect of anti-PD-1 therapy on C57BL/6 mice with genetic similarity, mice bearing the subcutaneous B16. SIY melanoma were obtained from two distinct mouse facilities [namely, Taconic Farms (TAC) and Jackson Laboratory (JAX)], which had markedly heterogeneous gut microbial compositions[60]. As a result, among the JAX populations, tumour growth was slower with higher sensitivity to the PD-1 blockage treatment. Such difference might be associated with the immune response. To be specific, JAX mice showed markedly enhanced CD8+ T cell aggregation within the tumour and tumour-specific T cell responses compared with the TAC counterparts. Further study suggested that the difference was abrogated by cohousing. In addition, when faecal microbiome was transferred from JAX to TAC, specific TILs increased and tumour development was suppressed. It was interesting that, in TAC, just the faecal microbiome transferred from JAX was able to suppress tumour development in the same degree with PD-1 blockage therapy, and it had synergistically regressed tumour development with PD-1 blockade therapy[60]. Gut microbiome analysis demonstrated that the abundance of Bifidobacterium was markedly increased in JAX. Meanwhile, the abundance of Bifidobacterium was significantly related to tumour specific immune cytotoxicity[60]. Administrating the commercial Bifidobacterium cocktail (namely, Bifidobacterium longum and Bifidobacterium breve) significantly suppressed tumour growth, particularly when it was used in combination with the PD-1 blockage treatment[60]. It was suggested that such increased anticancer activity was associated with the higher interferon (IFN)-γ production, greater tumour-specific CD8+ T cell proportion, and alterations of dendritic cell (DC) functions[60].
Xu et al[61] investigated the roles of gut microbiome within the MSS-type mice bearing CRC that received diverse antibiotic treatments in the response to PD-1 Ab therapy. Following PD-1 Ab therapy, injecting antibiotics offset the therapeutic effect of PD-1 Ab on suppressing tumour development relative to control group[61]. Besides, control group showed enrichment of Bacteroidales_S24-7 and Bacteroides_sp._CAG:927. At the same time, mice receiving colistin treatment showed enrichment of Bacteroides_sp._CAG:927, Bacteroides and Prevotella_sp._CAG: 1031, whereas mice receiving vancomycin treatment showed enrichment of Akkermansia_muciniphila and Prevotella_sp._CAG:485. For mice receiving vancomycin treatment, most metabolites were associated with the glycerophospholipid metabolic pathway, confirming to the metagenomic prediction pathway. Additionally, Akkermansia and Prevotella_sp._CAG:485 contributed to maintaining the therapeutic effect of PD-1 Ab through impacting glycerophospholipid metabolism[61]. Gut microbial alteration resulted in alterations of the glycerophospholipid metabolism degree, thereby affecting immune cytokine expression [such as interleukin (IL)-2 and IFN-γ] within TME, giving rise to the diverse PD-1 Ab efficacy[61]. The above results reveal that gut microbial alter
Recently, Lv et al[62] discovered that when Gegen Qinlian decoction (GQD) (one of the representative traditional Chinese medicine prescriptions) was used in conjunction with the anti-mouse PD-1 therapy in the xenograft model, it had potent effect on suppressing CT26 tumour growth. Besides, analysis on the gut microbiota also suggested that GQD used in combination with anti-mouse PD-1 therapy markedly enriched s__uncultured_organism _g__norank_f__Bacteroidales_ S24-7_and s__Bacteroides_acidifaciens group[62]. As indicated by metabolomic analysis results, metabolites with profound changes were detected in the combined treatment group[62]. Furthermore, the sphingolipid metabolism and glycerophospholipid metabolism metabolic pathways were examined[62]. Particularly, GQD combined with anti-mouse PD-1 treatment markedly promoted the fraction of CD8+ T cell subset within tumour tissue and peripheral blood samples and up-regulated IFN-γ level (an important factor of the anticancer immunotherapy)[62]. Moreover, GQD combined with anti-mouse PD-1 treatment decreased PD-1 expression while increasing IL-2 expression, revealing that such combined treatment suppressed the inhibitory checkpoints to restore efficiently T-cell functions[62]. Taken together, such findings revealed that GQD remodels gut microbiota to promote the anti-CRC efficacy of PD-1 blockade, and microsatellite stability was achieved.
Inspired by these results obtained from mouse models, many articles have been conducted to examine the association of gut microbiota with anti-PD-1 therapy among cancer cases. Song et al[63] explored the association of gut microbial structure and metabolomic features in the context of NSCLC with the anti-PD-1 therapy efficacy. According to analysis results of gut microbiome, cases from progression-free survival (PFS) ≥ 6-mo group showed markedly increased β-diversity within gut microbiota relative to that of PFS < 6-mo group[63]. Besides, those from PFS ≥ 6-mo group showed enrichment of Methanobrevibacter and Parabacteroides, whereas those from PFS < 6-mo group showed enrichment of Selenomonadales, Negativicutes, and Veillonella[63]. Furthermore, the protein families of function groups were studied using the COG, CAZy, and KO databases. As a result, 264, 859, and 390 functional groups were enriched in the above three databases, respectively, and significant differences were detected between the two groups. As revealed by analysis on bacterial metabolites, differences in the metabolic potentials of methane and methanol were significant between the two groups[63].
Jin et al[64] examined the association of gut microbiome with the clinical outcomes among the Chinese NSCLC cases receiving the anti–PD-1 therapy. Thereafter, patients were grouped as non-responder and responder groups based on the clinical response evaluated by the Response Evaluation Criteria in Solid Tumor version 1.1[64]. As a result, responders showed a greater gut microbial diversity at the beginning and stable composition in the process of treatment[64]. Besides, those showing higher microbial diversity were associated with the remarkably longer PFS in comparison with patients showing a lower diversity[64]. Differences in composition were detected between both groups, among which, Alistipes putredinis, Prevotella copri, and Bifidobacterium longum were enriched in responder group, while Ruminococcus unclassified was enriched in non-responder group[64]. In addition, the author applied multicolor flow cytometry to analyzed the systemic immune responses, which suggested that patients showing a greater gut microbial diversity were associated with higher proportions of peripheral blood natural killer cell and unique memory CD8+ T cell subsets upon anti–PD-1 treatment[64]. Botticelli and coworkers[65] also investigated the impact of gut microbial metabolome on anti-PD-1 therapy efficacy among NSCLC cases. As a result, 36% cases presented early progression, whereas the rest 64% showed progression at 12 mo later[65]. Besides, as revealed by gut microbiota metabolomic profiling, tridecane (alkane) and 2-Pentanone (ketone) were tightly related to early progression; by contrast, nicotinic acid, lysine and short chain fatty acids (namely, butyrate, propionate) were closely related to long-term benefits[65].
Recently, Derosa et al[66] assessed the significance of faecal bacterial composition in the anti-PD-1 treatment effect among patients with advanced renal cell carcinoma (RCC). Relative to RCC cases who received PD-1 blockage treatment with no use of antibiotics, RCC cases who received anti-PD-1 treatment in the presence of antibiotic treatment had evidently decreased objective response rates, which remarkably impacted the microbial composition. As a result, certain species like Clostridium hathewayi were dominant, and their abundances were higher in faecal samples of RCC cases relative to normal subjects. Tyrosine kinase inhibitors administered before nivolumab were related to the shift of microbial composition. For establishing the cause-effect relation of gut microbial composition with the anti-PD-1 therapy efficacy, some preclinical studies discovered that RCC-bearing mice receiving FMT from RCC cases developed resistance to anti–PD-1 therapy (NR-FMT). At the same time, both beneficial commensals (Bacteroides salyersiae and A. muciniphila) verified through whole genome sequencing and FMT successfully compensated the NR-FMT mice.
Conforming to the above results, Gopalakrishnan et al[67] evaluated the gut microbiota in melanoma cases who received the PD-1 blockage treatment (faecal samples from 43 cases, including 13 non-responders and 30 responders). As a result, responders exhibited a greater gut microbial diversity. Besides, in faecal samples, α-diversity showed positive correlation with PFS[67]. Further analysis indicated that the level of Clostridiales/Ruminococcaceae, Faecalibacterium (belonging to the Ruminococcaceae family, Clostridiales order) was higher in responders, while Anaerotruncus colihominis, Bacteroides thetaiotaomicron (belonging to Bacteroidales order), and Escherichia coli were significantly enriched in non-responders[67]. Additionally, the abundance of Faecalibacterium and Bacteroidales showed positive and negative relationships with tumour infiltrating CD8+ T cell level, respectively. The high abundances of Faecalibacterium, Ruminococcaceae, and Clostridiales in peripheral blood were accom
For investigating the association of antibiotic-induced dysbiosis with the reduced efficacy, investigators compared the compositions of gut microbiota in responders with those in non-responders[69]. Across the enriched bacterial species in the responders, A. muciniphila showed the highest correlation with the response rate of patients[69]. In addition, the IFN-γ production-induced immune reactions between Tc1 and Enterococcus hirae as well as between Th1/Tc1 and Akkermansia muciniphila predicted better patient survival[69]. In addition, clinical trial conducted using the mouse model suggested that mice that received FMT from responders showed superior response to the anti-PD-1 therapy and had higher proportion of CXCR3+CD4+TILs, whereas those that received FMT from non-responders, underwent antibiotic treatments and those in the GF status developed resistance to anti-PD-1 therapy[69]. Interestingly, antibiotic treatment reversed the efficacy of PD-1 blockade treatment through A. muciniphila recolonization in the presence or absence of Enterococcus hirae. Administration of E. hirae and A. muciniphila through oral gavage can increase CCR9+CXCR3+CD4+central memory T cells, promote IL-12 and IFN-γ secretion, and increase the CD4/Foxp3 ratio within tumour bed[69].
CTLA-4 is also a research hotspot apart from PD-1/PD-L1. The anti-CTLA-4 therapy can reverse the CTLA-4-hijacked activity of the co-stimulatory signal transduction pathway (CD28-CD80/86). Therefore, it is important to identify factors that modulate the anti-CTLA-4 therapy efficacy, so as to mitigate drug resistance and promote the treatment response.
Vétizou et al[70] carried out a trial for investigating the gut microbial impact on the efficacy of anti-CTLA-4 therapy[70]. In the mouse model of MCA205 sarcomas, compared with GF mice and those receiving broad-spectrum antibiotic treatment, specific pathogen-free mice showed higher efficacy in anti-CTLA-4 therapy[70], and commensal flora perturbation was observed after anti-CTLA-4 therapy. For certain species (B. uniformis and Bacteroides thetaiotaomicron), their abundances increased, while those of Burkholderiales and Bacteroidales declined[70]. Notably, Bacteroides fragilis, which was verified to be the immune-modulating bacteria, remained almost unchanged in the process of treatment[70,71]. Additionally, B. thetaiotaomicron, Burkholderia cepacian, and B fragilis recolonization in GF mice or those receiving antibiotic treatment reversed the resistance to anti-CTLA-4 therapy[70]. Moreover, it was further detected that B fragilis administered by oral gavage promoted DC maturation and elicited Th1 immune response within the tumour-draining lymph nodes[70]. Furthermore, adoptive Th1 cell transfer of cells specific to B. fragilis reversed the anti-CTLA-4 sensitivity in GF mice or those receiving antibiotic treatment to some extent[70]. In addition to the promoted anti-CTLA-4 effect, the treatment-related colitis was also alleviated by recolonizing Burkholderia cepacia and B. fragilis[70]. By FMT from melanoma cases, investigators discovered that the high abundance of B. fragilis was associated with tumour regression[70]. In addition, it was interesting to find that vancomycin treatment enhanced the ipilimumab efficacy, while alleviating side reactions that were not parallel to the promoted efficacy. To explore the reason, vancomycin might show indirect effect on promoting the abundance of Bacteroidales through suppressing Clostridiales proliferation[70].
Nonetheless, another trial examining the association of baseline gut microbiome with the clinical outcomes among the melanoma cases who developed metastasis came to different results from those obtained by Marie Vétizou[72]. Different from the results obtained from the clinical trial on mouse models, the low baseline abundances of B. thetaiotaomicron and B. fragilis but high abundance of Bacteroides were detected among the enrolled cases, which restricted the anticancer activity of CTLA-4. In addition, certain Firmicutes species, such as unclassified Ruminococcus, Faecalibacterium genus, Clostridium XIVa, Lachnospiraceae genus, Gemmiger formicilis, butyrate producing bacterium, and Blautia were associated with the increased response rates and superior clinical outcomes (prolonged overall survival and PFS). For exploring the underlying mechanisms, parameters associated with the immune status were analyzed, which suggested that cases exhibiting increased response to treatment had reduced baseline proportions of systemic proinflammatory cytokines (sCD25, IL-6, IL-8), CD4+/CD8+ T cells, and Tregs while increased inducible T cell co-stimulator level in CD4+ T cells. Different from the above-mentioned clinical trials, antibiotic treatment made no difference to the composition of predominant microbiota or bacteria that potentially affected the efficacy[72]. It was previously suggested that antibiotic treatment reduced the efficacy of ICI treatment, and such findings should be further investigated. Such different results among different trials might be associated with certain factors such as the heterogeneities between human and mouse models and the bias in FMT.
Recently, Zhuo et al[73] assessed the protection of anti-CTLA-4 blocking Ab (CTLA-4 mAb) in combination with Lactobacillus acidophilus cell lysates in the syngeneic BALB/c mouse model with CRC. Compared with CTLA-4 mAb monotherapy, the body weight loss was mitigated by L. acidophilus lysates. Meanwhile, CRC growth was suppressed in mice receiving combined administration, suggesting the effect of lysates on enhancing the anticancer effect of CTLA-4 mAb detected using the mouse model[73]. Such improved therapeutic effect was related to the higher proportions of effector memory T cells (CD44+CD8+CD62L+) and CD8+ T cells, but the lower proportions of M2 macrophages (F4/80+CD206+) and Treg (CD4+CD25+Foxp3+) cells within TME[73]. Additionally, L. acidophilus lysates showed a certain immunomodulatory activity by inhibiting IL-10 expression in lipopolysaccharide-activated Raw264.7 macrophages and M2 polarization[73]. Finally, faecal microbiota was subjected to 16S ribosomal RNA gene sequencing, demonstrating that combined administration markedly suppressed the abnormally increased proteobacteria abundance and partially offset the CRC-caused dysbiosis among the model mice[73]. Consistently, Mager et al[74] isolated three bacterial species—Bifidobacterium pseudolongum, Lactobacillus johnsonii, and Olsenella species—that significantly enhanced the efficacy of anti-CTLA-4 treatment in CRC mouse models. Based on further research, intestinal B. pseudolongum improved immunotherapy response by producing the metabolite inosine. Decreased gut barrier function induced by immunotherapy enlarged systemic translocation of inosine and activated antitumour T cells. The effect of inosine relied on T cell expression of the adenosine A2A receptor as well as the required co-stimulation.
In general, alterations of intestinal bacteria exert a significant influence in ensuring the efficacy of cancer with ICIs treatments, with specific changes of the commensal microbes standing for a potential way that can be used to improve or to weaken ICIs efficacy. As a result, manipulating gut microbiota composition may provide a direct and effective method to strengthen the therapeutic effect of cancer ICIs.
FMT refers to the process where the faecal suspension obtained from a normal subject is injected to the gastrointestinal tract of another subject for the sake of curing a certain disease. FMT is a direct and superior approach to enhance the efficacy of ICIs through modulating the gut microbiota in human beings. FMT has been adopted for more than 50 years. Faeces was initially adopted by Ge Hong in China in the 14th century to treat various conditions, such as diarrhea[75]. Eiseman et al[76] adopted faecal enemas to treat pseudomembranous colitis in 1958 [probably because of Clostridium difficile infection (CDI)], and this was also the first time to introduce FMT to the mainstream medicine. Thereafter, FMT has become more and more popular because of its simple use and effects on treating CDI. In recent years, FMT has been investigated in numerous other fields[77]. FMT has been found to be effective on certain disorders, like irritable bowel syndrome, inflammatory bowel disease, anorexia nervosa, metabolic disorders, multiple sclerosis, autoimmune disorders cancer, cardiovascular diseases, and neuropsychiatric disorders[78-82]. Similarly, FMT may represent an efficient approach to increase response rate in ICIs therapy. Currently, only a few clinical trials have studied the effects of FMT on PD-1 Ab immunotherapy response in cancer patients. Recently, Baruch et al[83] carried out one phase I trial for evaluating whether it was safe and feasible to perform FMT and re-induction of PD-1 blockage treatment among 10 melanoma cases who developed PD-1-refractory metastases (Table 2). Clinical responses were detected among 3 cases, including 2 with partial responses and 1 with complete response[83]. Obviously, treatment with FMT showed association with favorable changes in immune cell infiltrates and gene expression profiles in both the gut lamina propria and the TME[83]. Additionally, an ongoing single-center phase 2 clinical trial (NCT03341143) investigates the therapeutic effect of FMT combined with pembrolizumab on melanoma patients who develop resistance to the anti-PD-1 treatment[84]. However, results are not reported at present. In conclusion, such preliminary results shed more lights on the effect of FMT on anticancer treatment. As a result, FMT combined with ICIs has been regarded as the potential anticancer treatment.
Accumulating evidence has demonstrated the shift in gut microbiome composition influencing ICIs efficacy. Nevertheless, it obviously shows that in-depth studies on the mechanism(s) of interaction between gut microbiota and ICI efficacy need to be performed in different caner populations. Additionally, FMT combined with ICIs may serve as a new anticancer treatment that requires more investigation. Scientific research in this field is just at the beginning stage, and more relevant information is needed. To this end, first of all, it is of great importance to determine the mechanism by which FMT re-establishes the balanced gut microbiota, finally achieving the remarkable cure rate among cancer cases who receive ICI therapy. Secondly, well-designed randomized controlled trials are required to ensure the safety and efficacy of FMT for cancer patients with ICIs treatment. Besides, additional high-quality data (e.g., longitudinal study) are also necessary to explore potential adverse effects. Moreover, it is of importance to study the composition of the small intestinal and faecal microbiota before and after FMT. These studies can contribute to better understanding the mechanisms of this therapy as well as identify microbes and their products involved in the pathogenesis of cancer. Thirdly, the best gut microbiota composition to enhance ICIs efficiency need to be recognized. On this basis, it is important to choose the right donors. Finally, FMT represents a relatively simple procedure during short duration. Compared with the repeated hospitalization and conventional therapy, FMT has low costs. Thus, the most appropriate method and duration for FMT needs to be determined. For this reason, besides conventional approaches, FMT is promising as an alternative therapy for cancer in the future.
I owe my parents (Kang WL and Feng QH) a great deal because they have devoted most of their time and energy to cultivating me. I am very grateful to my wife and mother-in-law (Zheng LB) for taking good care of our family.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mazilu L S-Editor: Fan JR L-Editor: Filipodia P-Editor: Liu JH
| 1. | Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2277] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 2. | Adachi K, Tamada K. Immune checkpoint blockade opens an avenue of cancer immunotherapy with a potent clinical efficacy. Cancer Sci. 2015;106:945-950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1664] [Cited by in RCA: 2093] [Article Influence: 209.3] [Reference Citation Analysis (0)] |
| 4. | Nirschl CJ, Drake CG. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. 2013;19:4917-4924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34:539-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 705] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 6. | Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895. [PubMed] |
| 7. | Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1873] [Cited by in RCA: 1997] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 8. | Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1401] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 9. | Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1965] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 10. | Tamura H, Dong H, Zhu G, Sica GL, Flies DB, Tamada K, Chen L. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97:1809-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 320] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2245] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 13. | Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083-1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10346] [Article Influence: 795.8] [Reference Citation Analysis (34)] |
| 15. | Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2810] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 16. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3541] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 17. | Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 4617] [Article Influence: 659.6] [Reference Citation Analysis (0)] |
| 18. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 9907] [Article Influence: 762.1] [Reference Citation Analysis (0)] |
| 19. | Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2712] [Cited by in RCA: 2725] [Article Influence: 227.1] [Reference Citation Analysis (0)] |
| 20. | Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1915] [Article Influence: 191.5] [Reference Citation Analysis (0)] |
| 21. | Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M, Brownell I, Lewis KD, Lorch JH, Chin K, Mahnke L, von Heydebreck A, Cuillerot JM, Nghiem P. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 949] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 22. | Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, O'Donnell PH, Drakaki A, Tan W, Kurland JF, Rebelatto MC, Jin X, Blake-Haskins JA, Gupta A, Segal NH. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J Clin Oncol. 2016;34:3119-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 676] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 23. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11783] [Article Influence: 785.5] [Reference Citation Analysis (0)] |
| 24. | Kourie HR, Klastersky JA. Side-effects of checkpoint inhibitor-based combination therapy. Curr Opin Oncol. 2016;28:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 626] [Cited by in RCA: 1010] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 26. | Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12:496-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 304] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 27. | Kumar S, Leigh ND, Cao X. The Role of Co-stimulatory/Co-inhibitory Signals in Graft-vs.-Host Disease. Front Immunol. 2018;9:3003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, Li Y, Li G, Xiong W, Guo C, Zeng Z. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 998] [Article Influence: 166.3] [Reference Citation Analysis (0)] |
| 29. | Long J, Lin J, Wang A, Wu L, Zheng Y, Yang X, Wan X, Xu H, Chen S, Zhao H. PD-1/PD-L blockade in gastrointestinal cancers: lessons learned and the road toward precision immunotherapy. J Hematol Oncol. 2017;10:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 585] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 31. | Dyck L, Mills KHG. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur J Immunol. 2017;47:765-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 404] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 32. | Ren B, Cui M, Yang G, Wang H, Feng M, You L, Zhao Y. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 418] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 33. | Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV; IMpower133 Study Group. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379:2220-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 2373] [Article Influence: 339.0] [Reference Citation Analysis (0)] |
| 34. | Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med. 2018;379:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 980] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 35. | Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, Sandhu S, Larkin J, Puig S, Ascierto PA, Rutkowski P, Schadendorf D, Koornstra R, Hernandez-Aya L, Maio M, van den Eertwegh AJM, Grob JJ, Gutzmer R, Jamal R, Lorigan P, Ibrahim N, Marreaud S, van Akkooi ACJ, Suciu S, Robert C. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378:1789-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1111] [Cited by in RCA: 1381] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 36. | Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR, Broderick S, Battafarano RJ, Velez MJ, Rekhtman N, Olah Z, Naidoo J, Marrone KA, Verde F, Guo H, Zhang J, Caushi JX, Chan HY, Sidhom JW, Scharpf RB, White J, Gabrielson E, Wang H, Rosner GL, Rusch V, Wolchok JD, Merghoub T, Taube JM, Velculescu VE, Topalian SL, Brahmer JR, Pardoll DM. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;378:1976-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1477] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 37. | Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J, Chouaid C, Bidoli P, Wheatley-Price P, Park K, Soo RA, Huang Y, Wadsworth C, Dennis PA, Rizvi NA; ATLANTIC Investigators. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19:521-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 468] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 38. | Xue S, Hu M, Iyer V, Yu J. Blocking the PD-1/PD-L1 pathway in glioma: a potential new treatment strategy. J Hematol Oncol. 2017;10:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 39. | Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, Kroemer G. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7:271ps1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 335] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 40. | Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 607] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 41. | Brennan CA, Garrett WS. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu Rev Microbiol. 2016;70:395-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 428] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 42. | Hold GL. Gastrointestinal Microbiota and Colon Cancer. Dig Dis. 2016;34:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Bérard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Doré J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1543] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 44. | Mima K, Nakagawa S, Sawayama H, Ishimoto T, Imai K, Iwatsuki M, Hashimoto D, Baba Y, Yamashita YI, Yoshida N, Chikamoto A, Baba H. The microbiome and hepatobiliary-pancreatic cancers. Cancer Lett. 2017;402:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 45. | Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 652] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 46. | Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 664] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 47. | Vétizou M, Daillère R, Zitvogel L. [The role of intestinal microbiota in the response to anti-tumor therapies]. Med Sci (Paris). 2016;32:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Pitt JM, Vétizou M, Waldschmitt N, Kroemer G, Chamaillard M, Boneca IG, Zitvogel L. Fine-Tuning Cancer Immunotherapy: Optimizing the Gut Microbiome. Cancer Res. 2016;76:4602-4607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1270] [Article Influence: 141.1] [Reference Citation Analysis (0)] |
| 50. | Spranger S, Sivan A, Corrales L, Gajewski TF. Tumor and Host Factors Controlling Antitumor Immunity and Efficacy of Cancer Immunotherapy. Adv Immunol. 2016;130:75-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | West NR, Powrie F. Immunotherapy Not Working? Cancer Cell. 2015;28:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Hefazi M, Patnaik MM, Hogan WJ, Litzow MR, Pardi DS, Khanna S. Safety and Efficacy of Fecal Microbiota Transplant for Recurrent Clostridium difficile Infection in Patients With Cancer Treated With Cytotoxic Chemotherapy: A Single-Institution Retrospective Case Series. Mayo Clin Proc. 2017;92:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1490] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 54. | Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front Immunol. 2016;7:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 55. | Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 419] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 56. | Somasundaram A, Burns TF. The next generation of immunotherapy: keeping lung cancer in check. J Hematol Oncol. 2017;10:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 57. | Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 620] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 58. | Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, Wu K. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 567] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 59. | Yi M, Yu S, Qin S, Liu Q, Xu H, Zhao W, Chu Q, Wu K. Gut microbiome modulates efficacy of immune checkpoint inhibitors. J Hematol Oncol. 2018;11:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 60. | Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1979] [Cited by in RCA: 2838] [Article Influence: 283.8] [Reference Citation Analysis (2)] |
| 61. | Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, Wang N, Zhang C, Kong L, Liu Y, Zhang Y, Li Z. Gut Microbiome Influences the Efficacy of PD-1 Antibody Immunotherapy on MSS-Type Colorectal Cancer via Metabolic Pathway. Front Microbiol. 2020;11:814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 62. | Lv J, Jia Y, Li J, Kuai W, Li Y, Guo F, Xu X, Zhao Z, Lv J, Li Z. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10:415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 63. | Song P, Yang D, Wang H, Cui X, Si X, Zhang X, Zhang L. Relationship between intestinal flora structure and metabolite analysis and immunotherapy efficacy in Chinese NSCLC patients. Thorac Cancer. 2020;11:1621-1632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 64. | Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, Zheng H, Yao C, Wang Y, Lu S. The Diversity of Gut Microbiome is Associated With Favorable Responses to Anti-Programmed Death 1 Immunotherapy in Chinese Patients With NSCLC. J Thorac Oncol. 2019;14:1378-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 378] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 65. | Botticelli A, Vernocchi P, Marini F, Quagliariello A, Cerbelli B, Reddel S, Del Chierico F, Di Pietro F, Giusti R, Tomassini A, Giampaoli O, Miccheli A, Zizzari IG, Nuti M, Putignani L, Marchetti P. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med. 2020;18:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 66. | Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, Segata N, Desnoyer A, Pietrantonio F, Ferrere G, Fahrner JE, Le Chatellier E, Pons N, Galleron N, Roume H, Duong CPM, Mondragón L, Iribarren K, Bonvalet M, Terrisse S, Rauber C, Goubet AG, Daillère R, Lemaitre F, Reni A, Casu B, Alou MT, Alves Costa Silva C, Raoult D, Fizazi K, Escudier B, Kroemer G, Albiges L, Zitvogel L. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur Urol. 2020;78:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 67. | Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2999] [Cited by in RCA: 3304] [Article Influence: 472.0] [Reference Citation Analysis (0)] |
| 68. | Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 2129] [Article Influence: 304.1] [Reference Citation Analysis (1)] |
| 69. | Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2493] [Cited by in RCA: 3797] [Article Influence: 474.6] [Reference Citation Analysis (0)] |
| 70. | Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1834] [Cited by in RCA: 2546] [Article Influence: 254.6] [Reference Citation Analysis (0)] |
| 71. | Irrazabal T, Martin A. T Regulatory Cells Gone Bad: An Oncogenic Immune Response against Enterotoxigenic B. fragilis Infection Leads to Colon Cancer. Cancer Discov. 2015;5:1021-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, Vaysse T, Marthey L, Eggermont A, Asvatourian V, Lanoy E, Mateus C, Robert C, Carbonnel F. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 920] [Article Influence: 131.4] [Reference Citation Analysis (0)] |
| 73. | Zhuo Q, Yu B, Zhou J, Zhang J, Zhang R, Xie J, Wang Q, Zhao S. Lysates of Lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci Rep. 2019;9:20128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 74. | Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, Paik S, Stagg J, Groves RA, Gallo M, Lewis IA, Geuking MB, McCoy KD. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 845] [Article Influence: 169.0] [Reference Citation Analysis (0)] |
| 75. | Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755; author reply p.1755-1755; author reply p.1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 76. | Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854-859. [PubMed] |
| 77. | Kang Y, Cai Y. Altered Gut Microbiota in HIV Infection: Future Perspective of Fecal Microbiota Transplantation Therapy. AIDS Res Hum Retroviruses. 2019;35:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Kang Y, Cai Y. Gut microbiota and obesity: implications for fecal microbiota transplantation therapy. Hormones (Athens). 2017;16:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 79. | Kang YB, Cai Y, Zhang H. Gut microbiota and allergy/asthma: From pathogenesis to new therapeutic strategies. Allergol Immunopathol (Madr). 2017;45:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Di Bella S, Drapeau C, García-Almodóvar E, Petrosillo N. Fecal microbiota transplantation: the state of the art. Infect Dis Rep. 2013;5:e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 473] [Article Influence: 39.4] [Reference Citation Analysis (1)] |
| 82. | Vrieze A, de Groot PF, Kootte RS, Knaapen M, van Nood E, Nieuwdorp M. Fecal transplant: a safe and sustainable clinical therapy for restoring intestinal microbial balance in human disease? Best Pract Res Clin Gastroenterol. 2013;27:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 83. | Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N, Rotin D, Anafi L, Avivi C, Melnichenko J, Steinberg-Silman Y, Mamtani R, Harati H, Asher N, Shapira-Frommer R, Brosh-Nissimov T, Eshet Y, Ben-Simon S, Ziv O, Khan MAW, Amit M, Ajami NJ, Barshack I, Schachter J, Wargo JA, Koren O, Markel G, Boursi B. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 990] [Article Influence: 198.0] [Reference Citation Analysis (0)] |
| 84. | ClinicalTrials.gov. Fecal microbiota transplant (FMT) in melanoma patients. [cited 16 January 2019]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03341143. |