Published online Jul 28, 2021. doi: 10.3748/wjg.v27.i28.4710
Peer-review started: March 31, 2021
First decision: May 28, 2021
Revised: May 28, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: July 28, 2021
Processing time: 117 Days and 0.5 Hours
Gastroesophageal reflux disease (GERD) has a high prevalence worldwide, and its incidence is increasing annually. Modified Xiaochaihu Decoction (MXD) could relieve the symptoms of GERD, but the effects of MXD on GERD manifestations and relapse prevention need to be further explained. Therefore, we performed a prospective, double-blind, and double-simulation study.
To verify the efficacy of MXD for GERD and its effect on esophageal motility.
Using randomization, double-blinding, and a simulation design, 288 participants with GERD were randomized to the treatment group and control group and received herbs (MXD) plus omeprazole simulation and omeprazole plus herbs simulation, respectively, for 4 wk. The GERD-Q scale score and esophageal manometry were measured at baseline, after treatment, and at 1 mo and 3 mo follow-up visits when medication was complete to evaluate recurrence indicators.
The GERD-Q scale score in both groups decreased significantly compared to those before treatment (P < 0.01). However, no significant difference was observed between the two groups (P > 0.05). Esophageal manometry showed that participants with lower esophageal sphincter pressure reduction and the proportion of ineffective swallowing (more than 50%) improved in both groups from baseline (P < 0.01), especially in the treatment group (P < 0.05). The percentage of small intermittent contractions, large intermittent contractions, and increased pre-phase contractions in the treatment group significantly improved compared with baseline (P < 0.05) but did not improve in the control group (P > 0.05). There was no significant difference between the groups after treatment (P > 0.05). The percentage of weak esophageal contractility (distal contractile integral < 450 mmHg·s·cm), improved in both groups (P < 0.01), but no significant difference was observed between the groups after treatment (P > 0.05). The relapse rate in the treatment group was lower than that in the control group at the 1 mo (P < 0.01) and 3 mo follow-up (P < 0.05).
MXD has a similar therapeutic effect to omeprazole in mild-to-moderate GERD. The therapeutic effect may be related to increased pressure in the lower esophageal sphincter and reduced ineffective swallowing.
Core Tip: This multicenter, randomized, double-blind, double-simulation study proved that Modified Xiaochaihu Decoction has a similar therapeutic effect to omeprazole in the treatment of patients with typical symptoms of gastroesophageal reflux disease and reflux esophagitis grades A and B. Modified Xiaochaihu Decoction was superior to omeprazole in improving lower esophageal sphincter resting pressure and reducing ineffective esophagus swallowing. The recurrence rate of symptoms was significantly lower than that of omeprazole within 1 mo and 3 mo after completing treatment. Modified Xiaochaihu Decoction may be an alternative treatment to proton pump inhibitor maintenance in patients with gastroesophageal reflux disease.
- Citation: Li Z, Tao L, Zhang SS, Sun XH, Chen SN, Wu J. Modified Xiaochaihu Decoction for gastroesophageal reflux disease: A randomized double-simulation controlled trial. World J Gastroenterol 2021; 27(28): 4710-4721
- URL: https://www.wjgnet.com/1007-9327/full/v27/i28/4710.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i28.4710
Gastroesophageal reflux disease (GERD) has a high prevalence worldwide, and its incidence is increasing annually[1]. Impaired esophageal and gastric motor function as well as reduced resting pressure of the lower esophageal sphincter are considered to be involved in the pathogenesis of GERD. Chemical drugs can help relieve the symptoms and eliminate inflammation, but the relapse rate is high. It is reported that esophagitis and other symptoms are 80% and 90%, respectively, 6 mo after proton pump inhibitor (PPI) withdrawal[2]; therefore, many patients require long-term maintenance medication. Our previous observations showed that Modified Xiaochaihu Decoction (MXD) could relieve the symptoms of GERD[3]. The present study aimed to evaluate the effects of MXD on GERD manifestations and relapse prevention and to determine its underlying mechanism, from the aspect of esophageal motion, using a prospective, double-blind, and double-simulation design compared with omeprazole.
This is a prospective, double-blind, and double-simulation study, which was sponsored by Beijing Hospital of Traditional Chinese Medicine of Capital Medical University and conducted in Peking Union Medical College Hospital, Beijing Shijitan Hospital of Capital Medical University, and Shengjing Hospital of China Medical University from January 2015 to December 2019.
The study was reviewed and approved by the Ethics Committee of Beijing Hospital of Traditional Chinese Medicine of Capital Medical University. The trial registration number is ISRCTN17685397.
Participants who met the standard of the “Chinese Experts Opinion on Gastroesophageal Reflux Disease”[2] were considered for inclusion in the study.
The inclusion criteria were: (1) patients aged 18-65 years; (2) satisfied the diagnostic criteria for GERD; and (3) GERD-Q score ≥ 8, with A-B esophagitis under gastroscopy.
Exclusion criteria were: (1) a history of gastric, esophageal, and duodenal surgery; (2) presence of Zollinger-Ellison syndrome or primary esophageal motility disease; (3) suspected or confirmed to have a malignant disease or have early warning symptoms; (4) use of PPI or H2 receptor blockers within 2 wk before enrollment; (5) serious primary heart, liver, lung, kidney, pancreas, or other serious diseases that would affect their survival; (6) disabled patients (blind, deaf, dumb, mentally retarded, mentally disabled, physically disabled) as required by law; (7) suspected or confirmed history of alcohol and drug abuse; (8) allergies, such as those with a history of allergies to two or more drugs or food; or known allergies to the ingredients of MXD; and (9) pregnant and lactating women.
The sample-size calculation in this design was based on a non-inferiority test with a 1:1 comparison principle. Using the one-sided test, according to previous literature reports and research results, the formula: [n1 = n2 = 2 (Zα + Zβ) 2P (1-P)/δ2, Zα = 1.645, Zβ = 0.845, P = 0.65, δ = -0.15; n1 = n2 approximately 125] was used. In this study, the expulsion rate was designed to be 15%, the sample content required for each group was 144, and a total of 288 samples were required.
Randomization into the treatment or control group was performed with a 1:1 allocation ratio. Balanced treatment assignments were achieved by block randomization. This process was performed using SAS 9.4 software to generate a random sequence. The block length was eight. Now that there were four units and a total of 288 subjects, each unit was assigned 72 connecting consecutive codes and the corresponding allocation to treatment or control.
Participants in the treatment group were given herbal granules (2 packets/d, one packet at a time with 200 mL water, before meals) combined with omeprazole simulation tablets produced by Jiangyin Tianjiang Pharmaceutical Co., Ltd. The traditional Chinese medicine (TCM) prescriptions were mainly composed of Bupleurum 10 g, Codonopsis 15 g, fried Atractylodes 12 g, Coptis chinensis 10 g, Flos insulae 10 g, and fried Raphani 15 g. Omeprazole placebo was produced by Lunan New Times Pharmaceutical Co., Ltd.) and was taken orally, 20 mg each time, once on an empty stomach in the morning.
Participants in the control group were given omeprazole enteric-coated tablets combined with an herbal granule placebo. The herbal granule placebo was produced by Jiangyin Tianjiang Pharmaceutical Co., Ltd. The simulated MXD placebo contained 2.5% of the dose of the original formula, with a color correction agent, seasoning agent, starch, dextrin, and other auxiliary materials added. After spray drying, crushing, screening, mixing, granulation, and packaging, the granule simulation agent was placed in bags. The appearance, characteristics, and odor were the same as those of the actual herbal medicine and contained 10.3 g/bag (produced by Jiangyin Tianjiang Pharmaceutical Co., Ltd.). Omeprazole enteric-coated tablets (Lunan New Times Pharmaceutical Co., Ltd., SFDA approval No. 008140505) were taken orally, 20 mg each time, once on an empty stomach in the morning.
All medications in both groups were administered for 4 wk, and all participants had a washout period of 2 wk before taking the medication.
The blind codes were sealed separately and kept by those who were not directly involved in this clinical trial. Doctors and patients were blind to the medicine. The medicine and placebos were packaged in the same outer packaging, and their appearance, color, and characteristics were consistent. Both were made by the same manufacturer.
The ManoScan360TM gastrointestinal dynamic high-resolution esophageal manometry system (Given Imaging, United States) and a solid-state surrounding pressure measuring electrode catheter with 36 pressure measuring channels was used. Adjacent channels were spaced 1 cm apart, and each channel had 12 surround pressure points. The recorded data were analyzed with ManoView Analysis software.
The patient underwent a high-resolution manometry test after fasting for at least 8 h, and the pressure-measuring catheter was inserted through the nasal cavity. The depth of the catheter was adjusted so that the display screen showed two high-pressure areas at the proximal and distal ends of the esophagus, the upper esophageal sphincter, and the lower esophageal sphincter (LES), and the catheter was fixed at the nasal wing. During the examination, the patient was placed in the supine position and adapted for 5 min. The basal pressure level in the esophagus was recorded, and the patient swallowed 10 times with 5 mL each time. The interval between two swallows was at least 30 s, and the patient remained awake throughout the recording.
Primary endpoints: The primary endpoint of this study was the GERD-Q scale score. Positive symptom scoring may reflect the frequency of heartburn and reflux symptoms and scored 0, 1, 2, 3 points according to “0 d,” “1 d,” “2-3 d,” or “4-7 d” with a maximum score of 6 points. Negative symptom scoring may reflect the frequency of central abdominal pain and nausea and scored 3, 2, 1, 0 points according to “0 d,” “1 d,” “2-3 d,” or “4-7 d” with a maximum score of 6. Positive impact scoring may reflect the frequency of heartburn or reflux affecting sleep and the frequency of patients taking extra over-the-counter drugs such as antacids and scored 0, 1, 2, 3 points according to “0 d,” “1 d,” “2-3 d,” and “4-7 d” with a maximum score of 6 points. The highest total score of the 6 questions was 18 points. Scores were recorded before medication and at the second and fourth weeks of medication, and follow-up scores were recorded at the first and third month after medication was complete.
Secondary endpoints: Lower esophageal sphincter pressure (LESP), the percentage of ineffective swallowing > 50%, percentage of small peristaltic interruption, percentage of large peristaltic interruption, distal contractile integral (DCI), and percentage of early contractions were the secondary endpoints. As not all participants in the study had abnormal esophageal manometry indicators, only those with abnormalities at baseline were measured before and after completion of medication. Indices of esophageal manometry were recorded before and 4 wk after treatment.
Follow-up was performed at 1 mo and 3 mo after completion of medication, and the number and percentage of patients who were not on medication, on maintenance medication, intermittent medication, and on-demand medication were recorded in the treatment group and the control group.
SAS9.4 software (Beijing Hospital of TCM Version, Order Number: 9C1XJD) was used for statistical analysis. mean ± SD were used to describe the continuous variables. Frequency and percentage were used to describe the categorical variables. Statistical tests were performed using the bilateral test, and a P value < 0.05 was considered statistically significant. Measurement data were analyzed using the t-test or Wilcoxon rank-sum test. For comparisons within groups, the paired t-test or Wilcoxon signed-rank test was used. For comparisons between groups, categorical variables were analyzed using the χ2 test and Fisher’s exact test, and continuous variables were analyzed using analysis of variance if normally distributed, or using the nonparametric test if non-normally distributed. For repeated measurements at several time points between groups, the repeated measures analysis of variance or the generalized estimating equation was used.
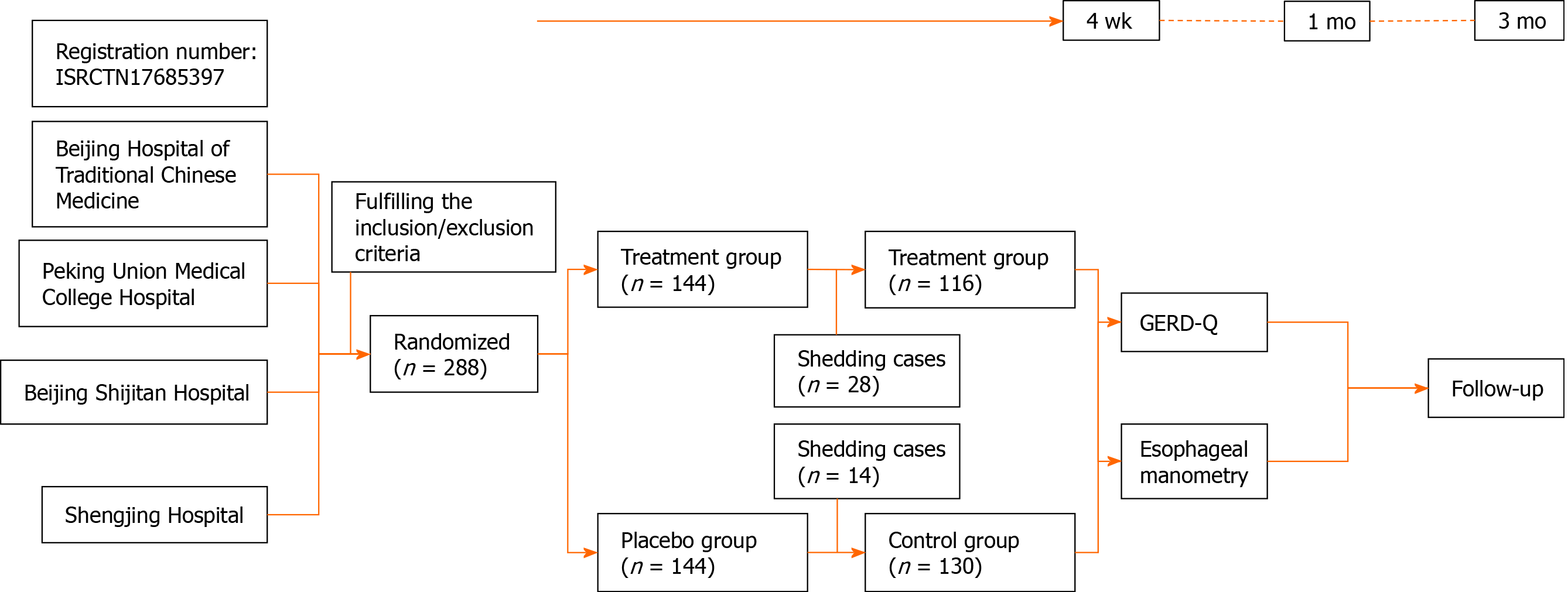
A total of 288 patients were included in this study and were divided into the treatment group and the control group, with 144 cases in each group. Twenty-eight patients in the treatment group were excluded, including 1 patient with adverse reactions (systemic pruritus after taking the medicine, and the symptoms subsided after 2 d of withdrawal), 14 cases left the study due to personal reasons, and 13 cases left due to dissatisfaction with the treatment; of the 14 patients excluded in the control group, 9 patients left the study due to personal reasons, 5 patients left due to dissatisfaction, and a total of 246 patients completed the study (Figure 1).
One hundred and sixteen patients were included in the treatment group, 50 males and 66 females, aged 18-65 years, with an average age of 50.30 ± 10.38 years. Disease course ranged from 1 mo to 36.67 years. One hundred and thirty patients were included in the control group, 64 males and 66 females, aged 24-65 years, with an average age of 50.48 ± 11.71 years. Disease course ranged from 2 mo to 20 years. There were no statistically significant differences between the two groups of patients in terms of gender, age, and duration of illness.
There were significant differences (P < 0.01) within the treatment group in relation to withdrawal of medication before treatment and treatment at 2 wk, 4 wk, 1 mo, and 3 mo. In the control group there were significant differences (P < 0.01) in relation to withdrawal of medication before treatment compared with treatment at 2 wk, 4 wk, 1 mo, and 3 mo. There were no significant differences between the treatment group and the control group during treatment and follow-up visits (Table 1).
| Treatment, n = 116 | Control, n = 130 | Mean difference (95% confidence interval) | P value | |
| Before treatment | 10.53 ± 2.01 | 10.10 ± 1.64 | 0.434 (0.067 to 0.077) | 0.077 |
| 2 wk treatment | 8.25 ± 1.85a | 7.99 ± 1.85a | 0.266 (0.100 to 0.112) | 0.108 |
| 4 wk treatment | 7.36 ± 1.71a | 7.21 ± 1.63a | 0.147 (0.463 to 0.483) | 0.469 |
| 1 mo withdrawal | 7.59 ± 1.82a | 7.59 ± 1.47a | 0.003 (0.301 to 0.309) | 0.301 |
| 3 mo withdrawal | 7.56 ± 1.78a | 7.44 ± 1.54a | 0.114 (0.826 to 0.841) | 0.837 |
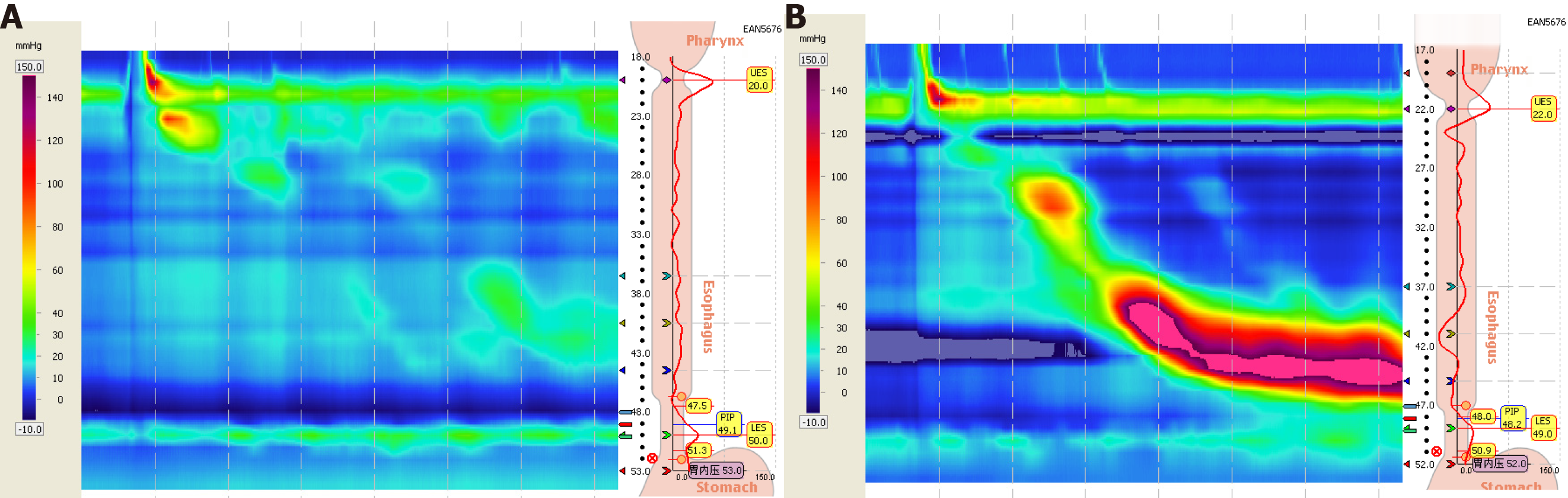
With regard to post-treatment changes in patients with reduced LESP before treatment, post-treatment LESP was higher than pre-treatment LESP in both the treatment group and control group, and there was a significant difference between the groups (P < 0.01); there was also a significant difference between the two groups after treatment (P < 0.01) (Table 2 and Figure 2).
The post-treatment proportion of small intermittent contractions in the treatment group was reduced compared with that before treatment, and the difference was statistically significant (P < 0.05). There was no significant difference within the control group before and after treatment (P > 0.05), and there was no significant difference between the two groups after treatment (P > 0.05) (Table 3).
| Treatment, n = 52 | Control, n = 49 | P value | |
| Pre-treatment, % | 30.48 ± 10.39 | 33.67 ± 9.50 | 0.077 |
| Post-treatment, % | 27.11 ± 8.47b | 29.79 ± 8.77 | 0.063 |
| Mean difference (95% confidence interval) | 3.365 (0.035 to 0.043) | 3.469 (0.057 to 0.066) | |
| P value | 0.037 | 0.051 |
The post-treatment proportion of large intermittent contractions in the treatment group was reduced compared with that before treatment, and the difference was statistically significant (P < 0.05). There was no significant difference within the control group before and after treatment (P > 0.05), and there was no significant difference between the two groups after treatment (P > 0.05) (Table 4).
The post-treatment proportion of ineffective swallowing in the treatment group and control group was reduced compared with that before treatment. The difference was statistically significant in the two group (P < 0.01), and the difference was statistically significant in the two groups after treatment (P < 0.05) (Table 5).
The post-treatment proportion of pre-phase contractions in the treatment group was reduced compared with that before treatment, and the difference was statistically significant (P < 0.05). There was no significant difference within the control group before and after treatment (P > 0.05), and there was no significant difference between the two groups after treatment (P > 0.05) (Table 6).
| Treatment, n = 21 | Control, n = 25 | P value | |
| Pre-treatment, % | 24.52 ± 8.04 | 26.00 ± 8.16 | 0.503 |
| Post-treatment, % | 19.04 ± 6.24b | 23.20 ± 7.48 | 0.052 |
| Mean difference (95% confidence interval) | 5.476 (0.025 to 0.032) | 2.800 (-1.894 to 7.494) | |
| P value | 0.035 | 0.23 |
The post-treatment value of DCI in the treatment group and control group both increased compared with the values before treatment, and the difference was statistically significant in the group (P < 0.01). There was no significant difference in the two groups after treatment (P > 0.05) (Table 7).
Patients were followed up at 1 mo after completing treatment, and a significant difference was observed between the treatment group and the control group (P < 0.01) (Table 8).
| Treatment, n = 116 | Control, n = 130 | P value | |
| Not on medication | 97 (83.6)b | 86 (66.2) | 0.002 |
| Maintenance medication | 12 (10.3) | 16 (12.3) | - |
| Intermittent medication | 1 (0.9) | 14 (10.8) | - |
| On-demand medication | 6 (5.2) | 14 (10.8) | - |
Patients were followed up at 3 mo after completing treatment, and a significant difference was observed between the treatment group and the control group (P < 0.05) (Table 9).
This multicenter, randomized, double-blind, double-simulation study proved that MXD has a similar therapeutic effect to omeprazole in the treatment of patients with typical symptoms of GERD and reflux esophagitis grades A and B. MXD was superior to omeprazole in improving LES resting pressure and reducing ineffective esophagus swallowing. The recurrence rate of symptoms was significantly lower than that of omeprazole within 1 mo and 3 mo after completing treatment. MXD may be an alternative treatment to PPI maintenance in patients with GERD.
GERD-Q scale scoring has high diagnostic accuracy in GERD and can evaluate quality of life and treatment response[4-6]. The results of this study showed that the GERD-Q scale scores in the two groups revealed significant changes before and after treatment, while there was no significant difference between the two groups indicating that MXD and the PPI omeprazole had equivalent clinical efficacy in improving the symptoms of GERD.
Esophageal dysfunction is a common cause of GERD. Abnormal dysfunction is mainly reflected in the weakened esophageal anti-reflux barrier function and esophageal clearance function. This study only compared abnormal esophageal motility indicators before and after treatment to explore the possible mechanism of MXD in the treatment of GERD. The results showed that MXD increased the LES resting pressure, enhanced the esophageal barrier effect, reduced ineffective contractions to improve esophageal clearance, reduced pre-phase contractions to adjust the coordination of esophageal movement, and improved esophageal clearance function. LES is an important component of the esophageal anti-reflux barrier, and its pressure is higher than that in the stomach to prevent the reflux of gastrointestinal content into the esophagus. It has been confirmed that LESP in GERD patients is significantly lower than in normal individuals[7]. When LESP decreases, the contents of the stomach and duodenum are more easily refluxed into the esophagus.
The research by Hu et al[8] showed that LES pressure decreases with the severity of acid reflux, and the decrease in LESP results in a significant increase in the incidence of hiatal hernia. Food in the esophagus is mainly propelled by peristalsis. When peristalsis is interrupted, the transmission and clearance functions of the esophagus in terms of the food bolus and reflux content are impaired, resulting in weakened clearance of the esophagus. Ribolsi et al[9] confirmed that esophageal peristalsis interruption in patients with GERD is associated with increased acid exposure in the supine position and increased reflux clearance time.
The Chicago esophageal dyskinesia classification released in 2015 defined swallowing with DCI < 450 mmHg·s·cm as ineffective swallowing and ineffective esophageal motility as > 50% of ineffective swallowing[10]. Frequent ineffective peristalsis of the esophagus can lead to impaired esophageal clearance and prolong the exposure duration of the food bolus and reflux content in the esophagus[11], thereby stimulating reflux symptoms. Liu et al[12] showed that GERD symptoms were closely related to DCI and ineffective esophageal peristalsis. With the widespread application of pH monitoring, the results of existing research show that ineffective esophageal peristalsis will lead to longer reflux time in both the supine and upright position and prolong average acid clearance time[8,13]. In addition, the pre-phase contractions of the esophagus will block passage of the food bolus and impair the peristaltic function of the esophagus. If the food bolus remains in the esophagus for an extended period, it will also increase stimulation of the esophageal mucosa. It can be inferred that MXD improved the patients’ symptoms via the above effects.
The difficulty in the treatment of GERD lies in its high recurrence rate. Some studies have shown that in patients with GERD who take PPIs for 4-8 wk that the recurrence rate is 26.0%-47.8%, and the recurrence rate is 30.4% during the 1 year follow-up[14-16]. The follow-up visits in the present study were conducted at 1 mo and 3 mo after completing treatment. Patients not on medication, on maintenance medication, intermittent medication, and on-demand medication were assessed. It was found that the recurrence rate following TCM was low, and its ongoing efficacy was significantly better than omeprazole. The results of this study indicated that by increasing the resting pressure of the LES, improving ineffective swallowing, and reducing pre-phase contractions, the mechanism of reflux is partially corrected, and MXD can lower the recurrence rate.
It is generally accepted that the pathophysiology of GERD mainly involves inflammation and the immune response[17-19]. Modern pharmacological studies have shown that MXD has anti-inflammatory, antioxidant, and immunomodulatory activity. Saikoside, the active component of bupleurum has significant anti-inflammatory, immunoregulatory, and neuroregulatory effects[20]. Studies have shown that saikoside can significantly reduce the expression of tumor necrosis factor-α, interleukin-1, and interleukin-6 and can inhibit the production of nitric oxide, thereby contributing to its anti-inflammatory effects, and saikoside is an effective NF-κB channel inhibitor[21,22]. Studies have shown that inulin fructan extracted from the roots of Codonopsis has anti-inflammatory and anti-oxidant effects. Li et al[23] studied the protective effect of inulin fructan on ethanol-induced acute gastric ulcers in rats, and the results showed that inulin fructan significantly increased the activity of superoxide dismutase and glutathione peroxidase in gastric tissue in a dose-dependent manner and reduced the content of malondialdehyde and nitric oxide, thereby protecting the gastric mucosa. In addition, the active component Berberine from Coptis chinensis also has similar anti-inflammatory and antioxidant effects[24], and it has been shown that Berberine has a killing effect on Helicobacter pylori[25].
This study had the following limitations: the research only compared clinical symptoms, and pH monitoring before and after treatment was not carried out. In addition, the treatment duration was 4 wk, which was insufficient. It is considered that the course of treatment should be extended to 8 wk as this would be more conducive to symptom relief and resolution of inflammation. The next phase in this research is to extend the course of treatment and carry out in-depth observations on the effect of MXD on acid reflux.
In the present study, the number of drop-outs in the TCM group was higher, most of which were due to severe acid-refractory heartburn symptoms, and the TCM did not take effect quickly. Due to the double-blind and double-simulation design of the study, we believe that the results objectively demonstrate the effects of MXD and omeprazole on clinical symptoms and esophageal dynamics in GERD patients and provide evidence-based data for GERD patients to receive further treatment. Therefore, it is recommended that patients with reflux symptoms without serious or reflux esophagitis belonging to class A-B be treated with TCM, and for patients with severe symptoms of acid reflux and heartburn, which require quick relief, both Chinese and Western medicine is suggested to obtain more satisfactory efficacy.
Gastroesophageal reflux disease (GERD) has a high prevalence worldwide, and its incidence is increasing annually. According to the preliminary experiment of the research group, Modified Xiaochaihu Decoction (MXD) could relieve the symptoms of GERD.
The effects of MXD on GERD manifestations and relapse prevention need to be further explained. Therefore, we performed a prospective, double-blind, and double-simulation study.
To verify the efficacy of MXD for GERD and its effect on esophageal motility.
Using randomization, double-blinding, and a simulation design to compare the GERD-Q scale score and esophageal manometry between patients under the treatment of MXD (treatment group) and omeprazole (control group).
In total, 288 patients were divided into the treatment group and control group. The GERD-Q scale score in both groups decreased significantly compared to those before treatment (P < 0.01). However, no significant difference was observed between the two groups (P > 0.05). Esophageal manometry showed that participants with sphincter pressure reduction and the proportion of ineffective swallowing (more than 50%) improved in both groups from baseline (P < 0.01), especially in the treatment group (P < 0.05). The percentage of small intermittent contractions, large intermittent contractions, and increased pre-phase contractions in the treatment group significantly improved compared with baseline (P < 0.05) but did not improve in the control group (P > 0.05). The percentage of weak esophageal contractility (distal contractile integral < 450 mmHg·s·cm) improved in both groups (P < 0.01). The relapse rate in the treatment group was lower than that in the control group at the 1 mo (P < 0.01) and 3 mo follow-up (P < 0.05).
MXD has a similar therapeutic effect to omeprazole in mild-to-moderate GERD. The therapeutic effect may be related to increased pressure in the lower esophageal sphincter and reduced ineffective swallowing.
Our results supported that MXD has a similar therapeutic effect to omeprazole in mild-to-moderate GERD and could improve esophageal motility.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aksionchyk M S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wang LL
| 1. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1265] [Article Influence: 115.0] [Reference Citation Analysis (2)] |
| 2. | Chen MH, Hou XH, Xiao YL, Yang YS, Yuan YZ, Zhou LY, Zou DW. Chinese expert consensus of gastroesophageal reflux disease in 2014. Wei Chang Bing Xue. 2015;3:155-168. |
| 3. | Tao L, Shen C, Zhang SS, Wu B, Zhao LQ, Deng JM. Study of Tiaoganlipihewei prescription on esophageal motility effect in patients with gastroesophageal reflux disease. Beijing Zhongyiyao. 2013;32:421-423. |
| 4. | Jonasson C, Moum B, Bang C, Andersen KR, Hatlebakk JG. Randomised clinical trial: a comparison between a GerdQ-based algorithm and an endoscopy-based approach for the diagnosis and initial treatment of GERD. Aliment Pharmacol Ther. 2012;35:1290-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Bai Y, Du Y, Zou D, Jin Z, Zhan X, Li ZS, Yang Y, Liu Y, Zhang S, Qian J, Zhou L, Hao J, Chen D, Fang D, Fan D, Yu X, Sha W, Nie Y, Zhang X, Xu H, Lv N, Jiang B, Zou X, Fang J, Fan J, Li Y, Chen W, Wang B, Zou Y, Sun M, Chen Q, Chen M, Zhao X; Chinese GerdQ Research Group. Gastroesophageal Reflux Disease Questionnaire (GerdQ) in real-world practice: a national multicenter survey on 8065 patients. J Gastroenterol Hepatol. 2013;28:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Bert F, Pompili E, Lo Moro G, Corradi A, Sagrawa Caro A, Gualano MR, Siliquini R. Prevalence of gastro-oesophageal reflux symptoms: An Italian cross-sectional survey focusing on knowledge and attitudes towards lifestyle and nutrition. Int J Clin Pract. 2021;75:e13758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | van Hoeij FB, Smout AJ, Bredenoord AJ. Predictive value of routine esophageal high-resolution manometry for gastro-esophageal reflux disease. Neurogastroenterol Motil. 2015;27:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Hu ZW, Xu H, Zhan Y, Xin RH, Sun CY, Tian SR, Zhan XL, Chen D, Wang ZG, Wu JM. [The relationship between acid reflux and esophageal motility, esophagitis and cardiac morphology in gastroesophageal reflux disease]. Zhonghua Yi Xue Za Zhi. 2019;99:3494-3499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Ribolsi M, Balestrieri P, Emerenziani S, Guarino MP, Cicala M. Weak peristalsis with large breaks is associated with higher acid exposure and delayed reflux clearance in the supine position in GERD patients. Am J Gastroenterol. 2014;109:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE; International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1373] [Cited by in RCA: 1451] [Article Influence: 145.1] [Reference Citation Analysis (0)] |
| 11. | Chugh P, Collazo T, Dworkin B, Jodorkovsky D. Ineffective Esophageal Motility Is Associated with Impaired Bolus Clearance but Does Not Correlate with Severity of Dysphagia. Dig Dis Sci. 2019;64:811-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Liu L, Li S, Zhu K, Yu W, Wang H, Guo J, Gao H. Relationship between esophageal motility and severity of gastroesophageal reflux disease according to the Los Angeles classification. Medicine (Baltimore). 2019;98:e15543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ergün M, Doğan İ, Ünal S. Ineffective esophageal motility and gastroesophageal reflux disease: a close relationship? Turk J Gastroenterol. 2012;23:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Yang JH, Kang HS, Lee SY, Kim JH, Sung IK, Park HS, Shim CS, Jin CJ. Recurrence of gastroesophageal reflux disease correlated with a short dinner-to-bedtime interval. J Gastroenterol Hepatol. 2014;29:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Min YW, Shin YW, Cheon GJ, Park KS, Kim HS, Sohn CI, Kim TN, Moon HC, Rhee PL. Recurrence and its impact on the health-related quality of life in patients with gastroesophageal reflux disease: A Prospective Follow-up Analysis. J Neurogastroenterol Motil. 2016;22:86-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Lei WY, Chang WC, Wen SH, Yi CH, Liu TT, Hung JS, Wong MW, Chen CL. Predicting factors of recurrence in patients with gastroesophageal reflux disease: a prospective follow-up analysis. Therap Adv Gastroenterol. 2019;12:1756284819864549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Winkelsett L, Malfertheiner P, Wex T, Kandulski A. Mucosal two-step pathogenesis in gastroesophageal reflux disease: repeated weakly acidic stimulation and activation of protease-activated receptor-2 on mucosal interleukin-8 secretion. Digestion. 2018;98:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Zhong C, Liu K, Wang K, Liu H, Su H, Wu J, Duan L. Developing a diagnostic understanding of GERD phenotypes through the analysis of levels of mucosal injury, immune activation, and psychological comorbidity. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Lyamina SV, Maev IV, Kalish SV, Andreev DN, Kladovikova OV, Malyshev IY. Features of the functional activity of macrophage link of immunity with gastroesophageal reflux disease depending on the type of reluctate: in vitro model. Ter Arkh. 2018;90:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Yuan B, Yang R, Ma Y, Zhou S, Zhang X, Liu Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm Biol. 2017;55:620-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 21. | Kim SO, Park JY, Jeon SY, Yang CH, Kim MR. Saikosaponin a, an active compound of Radix Bupleuri, attenuates inflammation in hypertrophied 3T3-L1 adipocytes via ERK/NF-κB signaling pathways. Int J Mol Med. 2015;35:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Park WH, Kang S, Piao Y, Pak CJ, Oh MS, Kim J, Kang MS, Pak YK. Ethanol extract of Bupleurum falcatum and saikosaponins inhibit neuroinflammation via inhibition of NF-κB. J Ethnopharmacol. 2015;174:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Li J, Wang T, Zhu Z, Yang F, Cao L, Gao J. Structure Features and Anti-Gastric Ulcer Effects of Inulin-Type Fructan CP-A from the Roots of Codonopsis pilosula (Franch.) Nannf. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Cao Y, Pan Q, Cai W, Shen F, Chen GY, Xu LM, Fan JG. Modulation of gut microbiota by berberine improves steatohepatitis in high-fat diet-fed BALB/C mice. Arch Iran Med. 2016;19:197-203. [PubMed] |
| 25. | Tan L, Li C, Chen H, Mo Z, Zhou J, Liu Y, Ma Z, Xu Y, Yang X, Xie J, Su Z. Epiberberine, a natural protoberberine alkaloid, inhibits urease of Helicobacter pylori and jack bean: Susceptibility and mechanism. Eur J Pharm Sci. 2017;110:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |