Published online Jul 28, 2021. doi: 10.3748/wjg.v27.i28.4687
Peer-review started: January 27, 2021
First decision: February 24, 2021
Revised: March 10, 2021
Accepted: July 13, 2021
Article in press: July 13, 2021
Published online: July 28, 2021
Processing time: 179 Days and 7 Hours
Diagnostic accuracy of various tumor markers and their combinations for hepatocellular carcinoma (HCC) was not fully investigated.
To evaluate the diagnostic accuracy of alpha-fetoprotein (AFP), the Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), and protein induced by vitamin K absence or antagonist-II (PIVKA-II) and their combination for HCC diagnosis.
Patients with newly detected liver mass or elevated serum AFP levels were considered eligible. Serum AFP level, AFP-L3 fraction, and PIVKA-II level were measured at the first visit.
In total, 622 patients were included; 355 patients (57.1%) had chronic liver disease, and 208 (33.4%) had liver cirrhosis. HCC was diagnosed in 160 patients (25.7%). The area under the receiver operating characteristics curves (AUROCs) of the serum AFP, AFP-L3 fraction, AFP-L3, and PIVKA-II levels for the diagnosis of HCC were 0.775, 0.792, 0.814, and 0.834, respectively. A novel diagnostic model was developed by classifying patients in a 1:1 ratio into training and validation sets. Using the binary regression analysis of the training cohort, the AFP, AFP-L3 fraction, and PIVKA-II (ALPs) score was calculated as follows: ALPs score = 3.8 × [serum AFP level (ng/mL) × AFP-L3 fraction (%) × 0.01] + 0.2 × PIVKA-II level (mAU/mL). The AUROC of the ALPs score for diagnosis of HCC was 0.878, significantly higher than that of serum AFP level (P < 0.001), AFP-L3 fraction (P < 0.001), PIVKA-II level (P = 0.036), and AFP-L3 level (P = 0.006). The optimal ALPs score cut-off was 5.3 (sensitivity, 85.0%, specificity 80.1%). The validation cohort showed similar results.
The ALPs score calculated using serum AFP level, AFP-L3 fraction, and PIVKA-II level showed improved accuracy in HCC diagnosis.
Core Tip: The value of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein (AFP-L3) for improving diagnostic accuracy of hepatocellular carcinoma has not been fully evaluated. We investigated performance of AFP-L3 in patients with newly detected liver mass or elevated serum AFP level. In total of 622 patients, we observed significantly higher diagnostic accuracy of AFP-L3 level than with AFP level (P < 0.001). In addition, we developed a novel diagnostic model, AFP, AFP-L3 fraction, and protein induced by vitamin K absence or antagonist-II (PIVKA-II) score, derived from AFP level, AFP-L3 fraction, and PIVKA-II level, and it showed significantly higher area under the receiver operating characteristics curves of 0.878 than those of AFP level, AFP-L3 fraction, and PIVKA-II level (all P < 0.05).
- Citation: Lee HA, Lee YR, Lee YS, Jung YK, Kim JH, An H, Yim HJ, Jeen YT, Yeon JE, Byun KS, Seo YS. Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein improves diagnostic accuracy for hepatocellular carcinoma. World J Gastroenterol 2021; 27(28): 4687-4696
- URL: https://www.wjgnet.com/1007-9327/full/v27/i28/4687.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i28.4687
Hepatocellular carcinoma (HCC) is the sixth most common tumor and the fourth significant cause of cancer-related death worldwide[1]. Although there have been many advancements in diagnosis of HCC, still, considerable number of patients are diagnosed as intermediate or advanced-stage HCC[2]. Therefore, improved diagnostics, especially for early-stage HCC where curative treatment is conceivable are warranted.
Imaging study plays a critical role in diagnosis of HCC, although limitations exist[3,4]. Furthermore, the imaging methods are not always applicable for certain patients, since the selection of imaging modality and contrast agent is subject to multiple factors, such as modality availability, scan time, throughput, technical capability of each institution, costs, radiologist expertise, patient preference, and safety considerations.
Therefore, although serum biomarkers usually have a minor role in the diagnosis of HCC, identifying biomarkers to better diagnose or predict HCC remains important priority in clinical research[5-7]. The American Association for the Study of Liver Diseases guideline suggests that alpha-fetoprotein (AFP) > 20 ng/mL and normal ultrasonography findings should alert recall procedures for possible HCC diagnosis, however, the routine use of novel biomarkers for HCC screening requires further evaluation[7-9]. The European association for the study of the liver guideline also indicate a lack of tumor markers for accurate and early detection of HCC owing to suboptimal previous data[5,9-11].
Various tumor markers including protein induced by vitamin K absence or antagonist-II (PIVKA-II), and novel blood parameter, the Lens culinaris agglutinin- reactive fraction of AFP (AFP-L3), have received considerable attention and were evaluated in several studies[12,13]. AFP-L3 is AFP-isoform reflecting changes in the carbohydrate chain. Since AFP-L3 showed high specificity (92.0%–99.4%) and low sensitivity (18.8%–37.0%) for HCC[14-16], it is considered a more specific biomarker for HCC diagnosis. Recent studies evaluated the predictive performance of the combination of AFP, AFP-L3, and PIVKA-II, which showed improved HCC diagnostic ability than either AFP or PIVKA-II[17,18]. However, those results were inconclusive because of the small sample size and the specific characteristics of study population.
Hence, we conducted this retrospective study with a large number of patients, who were referred to our tertiary clinic for newly detected liver mass or elevated serum AFP level. We investigated and compared the predictive ability of AFP, AFP-L3, and PIVKA-II as well as their combination for HCC diagnosis and developed and validated a new diagnostic model for HCC.
Patients who were referred from the primary clinic to the Korea University Medical Center (a tertiary academic teaching hospital) for newly detected liver mass or elevated serum AFP level between 2015 and 2019 were enrolled (Supplementary Figure 1). The exclusion criteria were as follows: (1) Age < 18 years; (2) Insufficient laboratory findings or imaging data; (3) History of HCC; (4) Patients with liver failure; (5) History of liver transplantation or liver resection for reasons other than HCC; and (6) History of any malignancy other than HCC. This study protocol followed the ethical guidelines of the 1975 Declaration of Helsinki, and the institutional review board of Korea University Anam Medical Center. The requirement for informed consent was waived owing to the retrospective nature of the study.
HCC diagnosis was based on non-invasive criteria or pathology or both in cirrhotic patients and pathology in non-cirrhotic patients. The non-invasive criteria were based on identification of typical hallmarks of HCC, obtained by multiphasic computed tomography or multiphase magnetic resonance imaging (nodule > 1 cm with arterial hypervascularity and portal/delayed-phase washout)[19,20]. Liver cirrhosis was diagnosed when typical ultrasonographic findings were found, with a low platelet count (< 100000/μL), varices, or overt complication of cirrhosis[21,22].
Serum AFP level, AFP-L3 fraction, and PIVKA-II level were measured in patient blood samples collected at their first visit. We measured the AFP level and AFP-L3 fraction using the mTAS assay (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and the PIVKA-II level using an enzyme immunoassay (Fujirebio Inc., Tokyo, Japan). Assay sensitivities were 0.3 ng/mL for AFP and 0.1 ng/mL for PIVKA-II. The percentage of AFP-L3 was determined in samples where both subfractions (AFP-L1 and AFP-L3) were > 0.3 ng/mL. The AFP-L3 level was calculated as follows: Serum AFP-L3 level = serum AFP level (ng/mL) × serum AFP-L3 fraction (%) × 0.01.
Data are presented as mean ± SD or numbers with percentages. The statistical significance of differences between continuous and categorical variables were compared using Student’s t-test or Mann-Whitney U test and chi-square test, respectively. The predictive accuracies for the diagnosis of HCC were assessed using area under the receiver operating characteristics curves (AUROCs) as well as 95% confidence intervals. Optimal cutoff values were chosen to maximize the sum of the sensitivity and specificity. The sensitivity, specificity, positive predictive value (PPVs), and negative predictive value (NPVs) were computed for the optimal cutoff value. The AUROCs were compared using the Hanley-McNeil test[23]. Statistical analyses were conducted using IBM SPSS Statistics software (version 23.0.0.0., IBM Corporation, Armonk, NY, United States). Two-sided P values < 0.05 were considered significant.
In total, 622 patients were finally included for the statistical analysis. The baseline characteristics of the study population are presented in Table 1. In the study population, chronic liver disease and liver cirrhosis were present in 355 (57.1%) and 208 (33.4%) patients, respectively. Among the 160 (25.7%) patients with HCC, BCLC stage was 0 in 35 (21.9%) patients, A in 50 (31.3%) patients, B in 32 (20.0%) patients, C (26.3%) in 42 patients, and D (0.6%) in 1 patients. The mean serum AFP, fraction of AFP-L3, AFP-L3 level and PIVKA-II level were significantly higher in patients with HCC than in those without HCC (all P < 0.001) (Table 1).
| Variables | All patients (n = 622) | Patients without HCC (n = 462) | Patients with HCC (n = 160) | P value |
| Demographic variables | ||||
| Age, years | 56.5 ± 13.5 | 54.1 ± 13.4 | 63.5 ± 11.3 | < 0.001 |
| Male gender | 344 (55.3) | 226 (48.9) | 118 (73.8) | < 0.001 |
| Chronic liver disease | 355 (57.1) | 198 (42.9) | 157 (98.1) | < 0.001 |
| Etiology of liver disease | < 0.001 | |||
| Hepatitis B virus | 211 (33.9) | 114 (24.7) | 97 (60.6) | |
| Hepatitis C virus | 26 (4.2) | 7 (1.5) | 19 (11.9) | |
| Alcoholic liver disease | 72 (11.6) | 39 (8.4) | 33 (20.6) | |
| Others | 46 (7.4) | 38 (8.2) | 8 (5.0) | |
| Liver cirrhosis | 208 (33.4) | 91 (19.7) | 117 (73.1) | < 0.001 |
| Laboratory variables | ||||
| Total bilirubin, mg/dL | 1.4 ± 2.7 | 1.3 ± 2.7 | 1.8 ± 2.7 | 0.072 |
| Albumin, g/dL | 4.2 ± 1.7 | 4.3 ± 1.9 | 3.8 ± 0.7 | < 0.001 |
| Prothrombin time, INR | 1.1 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | < 0.001 |
| Alanine aminotransferase, IU/L | 44.8 ± 88.3 | 43.8 ± 99.6 | 47.8 ± 42.1 | 0.624 |
| AFP level, ng/mL | 3638.6 ± 58824.2 | 18.2 ± 63.8 | 14092.6 ± 115614.1 | < 0.001 |
| AFP-L3 fraction, % | 6.7 ± 16.7 | 1.9 ± 3.9 | 20.6 ± 28.0 | < 0.001 |
| AFP-L3 level, ng/mL | 833.1 ± 8811.3 | 1.1 ± 4.7 | 3235.3 ± 17185.1 | < 0.001 |
| PIVKA-II level, mAU/mL | 1613.4 ± 10561.6 | 37.9 ± 257.3 | 6162.5 ± 20185.1 | < 0.001 |
| ALPs score | 3488.4 ± 33695.4 | 11.9 ± 54.4 | 13526.8 ± 65558.1 | < 0.001 |
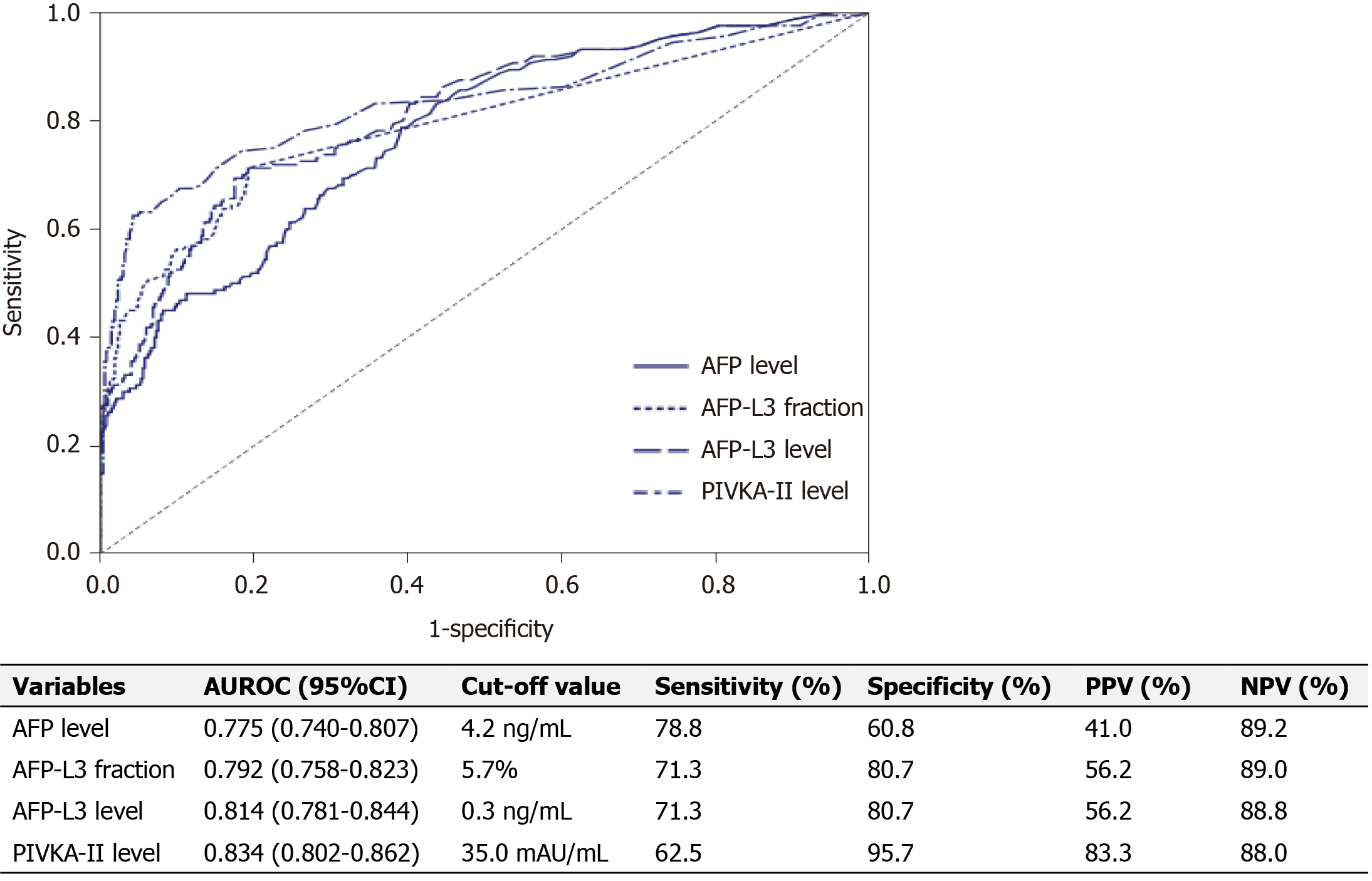
For HCC diagnosis, AFP level, fraction of AFP-L3, level of AFP-L3 and PIVKA-II level had AUROC values of 0.775, 0.792, 0.814, and 0.834, respectively (Figure 1). The AUROC of the serum AFP level was similar to that of serum AFP-L3 fraction (P = 0.321), though significantly lower than that of the serum AFP-L3 level (P < 0.001) and PIVKA-II level (P = 0.038). The AUROCs of the serum AFP-L3 fraction, AFP-L3 level, and PIVKA-II level were similar (Figure 1). For diagnosis of HCC, The optimal cutoff values of the AFP level, fraction of AFP-L3, level of AFP-L3, and PIVKA-II level were 4.2 ng/mL, 5.7%, 0.3 ng/mL, and 35.0 mAU/mL, respectively.
A novel HCC diagnostic model was developed by classifying all included patients into training and validation sets in a 1:1 ratio under stratifying them by presence or absence of HCC. The baseline characteristics did not differ significantly between the two datasets (Supplementary Table 1).
In the training set, the mean serum AFP, fraction of AFP-L3, AFP-L3 level and PIVKA-II level were significantly higher in patients with HCC than in those without HCC (all P < 0.001). The AUROCs of serum AFP level, AFP-L3 fraction, PIVKA-II level and AFP-L3 levels were 0.749, 0.786, 0.829, and 0.802, respectively. The AUROC of the serum AFP-L3 level was significantly higher than that of serum AFP level (P < 0.001), whereas no significant difference was observed in the other tumor marker comparisons (all P > 0.05).
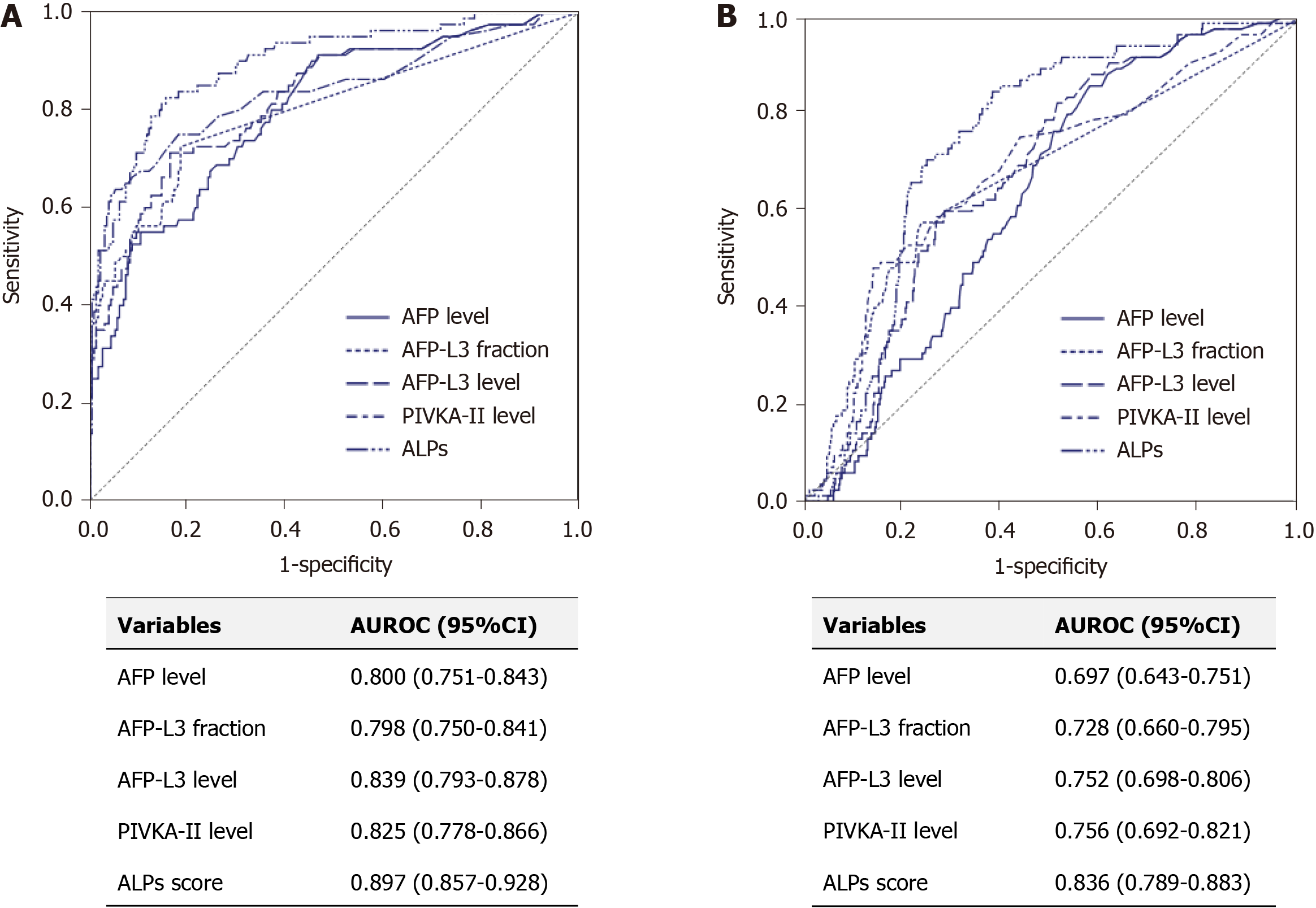
Next, we performed binary regression analysis for the presence of HCC using the levels of two tumor markers, AFP-L3 level and PIVKA-II level (Table 2). Using the beta coefficients of each marker, we developed AFP, fraction of AFP-L3, and PIVKA-II (ALPs) score: ALPs score = 3.8 × [serum AFP level (ng/mL) × fraction of AFP-L3 (%) × 0.01] + 0.2 × PIVKA-II level (mAU/mL). In training set, mean ALPs score was significantly higher in patients with HCC than in those without HCC (10657.4 vs 11.9, P < 0.001). For the HCC diagnosis, ALPs score had significantly higher AUROC of 0.878, than those of serum AFP level (P < 0.001), fraction of AFP-L3 (P < 0.001), level of PIVKA-II (P = 0.036), and level of AFP-L3 (P = 0.006) (Figure 2A). The best cutoff value of ALPs score was 5.3 (sensitivity, 85.0%, specificity 80.1%, PPV 59.6%, and NPV 93.9%).
| Beta coefficient | Standard error | P value | |
| AFP-L3 level | 0.038 | 0.017 | 0.021 |
| PIVKA-II level | 0.002 | 0.001 | 0.003 |
In the validation set, the mean serum AFP, fraction of AFP-L3, AFP-L3 level and PIVKA-II level were significantly higher in patients with HCC than in those without HCC (all P < 0.001). With an optimal ALPs score cutoff value of 5.3, the sensitivity, specificity, PPV, and NPV were 85.0%, 77.5%, 56.7%, and 93.7%, respectively. The AUROCs of the serum AFP level, AFP-L3 fraction, PIVKA-II level, AFP-L3 level, and ALPs score were 0.800, 0.798, 0.839, 0.825, and 0.897, respectively. The AUROC of serum AFP-L3 level was also significantly higher than that of serum AFP level (P > 0.001), whereas the ALPs score had higher AUROC than those of level of AFP (P < 0.001), fraction of AFP-L3 (P < 0.001), level of PIVKA-II (P = 0.024), and level of AFP-L3 (P = 0.002) (Figure 2B).
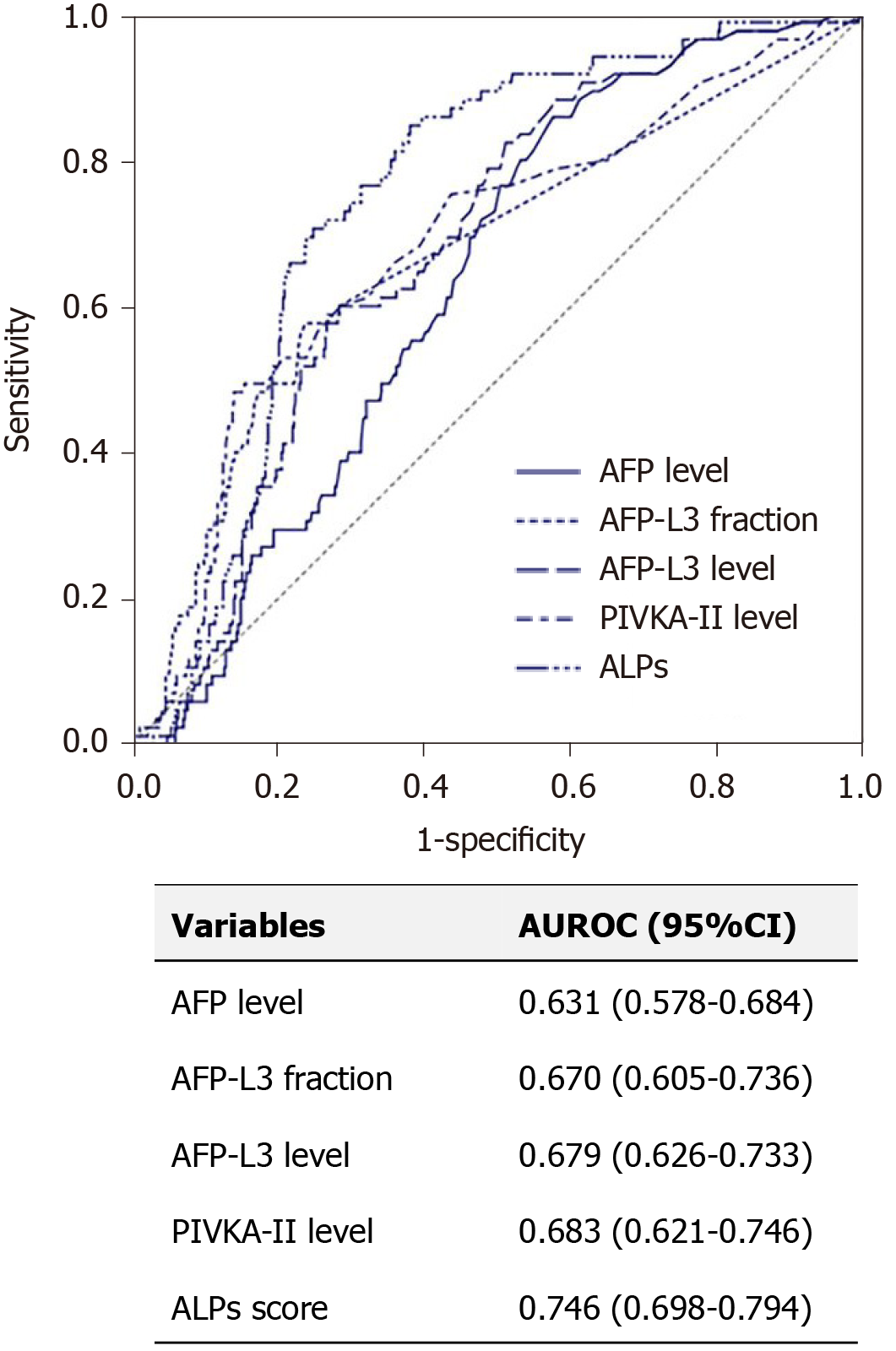
In total, 53 (8.5%) patients had very early and early-stage HCC. The baseline characteristic of these patients are presented in Supplementary Table 2. The predictive performance of the ALPs score and other tumor markers for very early and early-stage HCC was evaluated in all patients. The AUROCs of the level of AFP, fraction of AFP-L3, level of AFP-L3, level of PIVKA-II and ALP score for diagnosing very early and early-stage HCC were 0.631, 0.670, 0.679, 0.683, and 0.746, respectively. The AUROC of the serum AFP-L3 level was significantly higher than that of the serum AFP level (P < 0.001), whereas the ALPs score had higher AUROC than those of level of AFP (P < 0.001), fraction of AFP-L3 (P = 0.009), level of PIVKA-II (P = 0.015), and level of AFP-L3 (P = 0.010) (Figure 3). The optimal cutoff value of ALPs score was 6.7 (sensitivity 70.6%, specificity 85.5%, PPV 47.2%, and NPV 94.5%).
The ability of AFP-L3 alone or its combination with other markers for diagnosis of HCC has not been fully evaluated. In this large retrospective study of patients with newly detected liver mass or elevated serum AFP level, we observed a significantly higher diagnostic accuracy of AFP-L3 level than with AFP level (P < 0.001). In addition, we developed a novel diagnostic model for HCC, ALPs score, which is derived from the AFP level, AFP-L3 fraction, and PIVKA-II level. For HCC diagnosis, ALPs score had AUROC of 0.878, which was significantly higher than those of the AFP level, fraction of AFP-L3, and PIVKA-II level (all P < 0.05).
Our study has several strengths and clinical implications. First, we showed the significantly higher diagnostic accuracy of the AFP-L3 level in comparison with the AFP level for HCC diagnosis (P < 0.001). The AUROC of the serum AFP-L3 level was 0.814, and the optimal cutoff was 0.3 ng/mL with sensitivity of 71.3% and specificity of 80.7%. Although the AFP-L3 has been expected to compensate for low specificity of AFP level, previous studies showed conflicting results regarding the superiority of AFP-L3 over AFP for diagnosis of HCC[17,18,24,25]. However, in previous studies, the AFP-L3 fraction, and not the AFP-L3 level, was compared to the AFP level, thus leading to comparable results between the two variables. The present study also showed the comparable diagnostic accuracy between AFP-L3 fraction and the AFP level for HCC diagnosis (P = 0.321); however, the AFP-L3 level, calculated by multiplying the AFP level with the AFP-L3 fraction, showed consistent superiority over the AFP level for HCC diagnosis. Therefore, the use of AFP-L3 level rather than AFP-L3 fraction might be beneficial in future studies.
Second, we developed and validated a novel diagnostic model of HCC, ALPs score, derived from the combination of the AFP and PIVKA-II levels, and AFP-L3 fraction. The AUROC of the ALPs score for HCC diagnosis was 0.878, which was superior than those of the level of AFP, fraction of AFP-L3, AFP-L3 level, and PIVKA-II level (all P < 0.05) in both the training and validation sets. The optimal ALPs score cutoff for HCC diagnosis was 5.3 with sensitivity of 85.0% and specificity of 77.5%–80.1%. It seems that the high sensitivity and specificity of ALPs score, which could be easily obtained from simple blood tests has significant clinical implication. Lim et al[18] showed that the combined use of all three tumor markers improved the diagnostic accuracy compared to each marker alone in detecting HCC (AUROC of 0.877) and early-stage HCC (AUROC of 0.773). However, the specificity of the combination of three tumor markers for HCC diagnosis in Lim et al[18] was much lower than that of present study (60.1% vs 77.5%–80.1%), and this difference might be caused from the different patient population between two studies. In addition, our study derives its strength compared to the study by Lim et al[18] by developing a novel score with the diagnostic value of each marker, which was reweighted through binary regression analysis.
Recently, Best et al[17] introduced the GALAD score, derived from a combination of gender, age, AFP level, AFP-L3 fraction, and PIVKA-II level with an AUROC of 0.9242 (sensitivity of 85.6% and specificity of 93.3%), for detecting (very) early-stage HCC. However, such high AUROC for (very) early-stage HCC with only serum markers seems clinically unacceptable. In this study, the AUROC of ALPs score to predict very early and early-stage HCC was 0.746, which was significantly superior compared to other markers (all P < 0.05). Owing to the small number of patients with very early and early-stage HCC in our study (53 patients), we were unable to confirm the clinical validity of the ALPs score for the prediction of very early and early-stage HCC. Large multicenter cohort studies are required to evaluate the predictive ability of the ALPs score for diagnosing very early and early-stage HCC.
The use of AFP as a diagnostic marker for HCC has some shortcomings, including an increase in diseases other than HCC[26], large false-negatives, and missed diagnoses[27]. Although PIVKA-II is less likely to be elevated in other liver disease than AFP[26], the results of previous studies were restrictive to prove the sufficient role of PIVKA-II as a solitary biomarker for HCC diagnosis, since the superior ability of PIVKA-II over AFP was only shown in specific patient groups[14,28,29]. Therefore, recent studies investigated the ability of combined AFP and PIVKA-II in HCC diagnosis and showed the higher diagnostic ability of the combination of the two markers compared to that of either biomarker alone[10,30]. AFP and PIVKA-II complement each other’s role, and similarly, AFP-L3 could act as a complementary biomarker for AFP and PIVKA-II in diagnosing HCC while improving the sensitivity and specificity.
We are also aware of several issues that remain unresolved. First, since this was a retrospective study, potential selection bias might be present. Second, 42.9% of the enrolled patients had no underlying liver disease, hence, the clinical implications of the ALPs score in patients with chronic liver disease could not be evaluated. However, because many patients without any underlying liver disease also have elevated AFP level or liver nodule, the result of our study could be beneficial in clinical practice. Further studies including patients with specific liver disease and studies for external validation are required. Third, the cost of using combination of tumor markers is another issue, therefore, further studies evaluating the cost-effectiveness are warranted.
In conclusion, AFP-L3 level, calculated by multiplying the AFP level with the AFP-L3 fraction, exhibited a considerably higher diagnostic ability than the AFP level for HCC diagnosis. The novel diagnostic model, ALPs score may be valuable compared to individual tumor markers for HCC diagnosis in patients with and without chronic liver disease.
Identifying useful biomarkers to diagnose hepatocellular carcinoma (HCC) remains important priority in clinical research. Recently, various tumor markers including protein induced by vitamin K absence or antagonist-II (PIVKA-II), and novel blood parameter, the Lens culinaris agglutinin- reactive fraction of alpha-fetoprotein (AFP-L3), have been introduced and evaluated.
Several studies showed improved HCC diagnostic ability of the combination of AFP, AFP-L3, and PIVKA-II, than either AFP or PIVKA-II. However, because of the small sample size and the specific characteristics of the study population, conflicting results have been reported.
We aimed to investigate and compare the predictive ability of AFP, AFP-L3, and PIVKA-II as well as their combination for HCC diagnosis in this retrospective study with a large number of patients. Further, we developed and validated a new diagnostic model for HCC.
Patients referred from primary clinics to the Korea University Medical Center for newly noted space occupying lesion in liver or elevated serum AFP level between 2015 and 2019 were enrolled. Their serum AFP level, AFP-L3 fraction, and PIVKA-II level were measured in blood samples collected at their first visit.
It was observed that AFP-L3 level exhibited a considerably higher diagnostic accuracy than with AFP level (P < 0.001). In addition, a novel diagnostic model for HCC, ALPs score, derived from the AFP level, AFP-L3 fraction, and PIVKA-II level showed highest area under the receiver operating characteristics curves of 0.878 for HCC diagnosis. It was significantly higher than those of the AFP level, AFP-L3 fraction, and PIVKA-II level (all P < 0.05).
Finally, AFP-L3 level, calculated by multiplying the AFP level with the AFP-L3 fraction had a significantly higher diagnostic ability than the AFP level for HCC diagnosis. The novel diagnostic model, ALPs score was more valuable compared to individual tumor markers for HCC diagnosis.
Further studies including patients with specific liver disease and studies for external validation and cost-effectiveness are required.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: The Korean Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu M S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55828] [Article Influence: 7975.4] [Reference Citation Analysis (132)] |
| 2. | Yoon JS, Lee HA, Park JY, Kim BH, Lee IJ, Chon YE, Hong SK, Lee DH, Kong H, Won Y, Kim E, Lee J. Hepatocellular Carcinoma in Korea Between 2008 and 2011: an Analysis of Korean Nationwide Cancer Registry. J Liver Cancer. 2020;20:41-52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Tanabe M, Kanki A, Wolfson T, Costa EA, Mamidipalli A, Ferreira MP, Santillan C, Middleton MS, Gamst AC, Kono Y, Kuo A, Sirlin CB. Imaging Outcomes of Liver Imaging Reporting and Data System Version 2014 Category 2, 3, and 4 Observations Detected at CT and MR Imaging. Radiology. 2016;281:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Sofue K, Burke LMB, Nilmini V, Alagiyawanna M, Muir AJ, Choudhury KR, Jaffe TA, Semelka RC, Bashir MR. Liver imaging reporting and data system category 4 observations in MRI: Risk factors predicting upgrade to category 5. J Magn Reson Imaging. 2017;46:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 6. | Leerapun A, Suravarapu SV, Bida JP, Clark RJ, Sanders EL, Mettler TA, Stadheim LM, Aderca I, Moser CD, Nagorney DM, LaRusso NF, de Groen PC, Menon KV, Lazaridis KN, Gores GJ, Charlton MR, Roberts RO, Therneau TM, Katzmann JA, Roberts LR. The utility of Lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a United States referral population. Clin Gastroenterol Hepatol. 2007;5:394-402; quiz 267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3029] [Article Influence: 432.7] [Reference Citation Analysis (3)] |
| 8. | Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 421] [Article Influence: 10.3] [Reference Citation Analysis (16)] |
| 9. | Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D, Dalhgren J, Chia D, Lok AS, Wagner PD, Srivastava S, Schwartz M. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 571] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 10. | Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL, Dienstag JL; HALT-C Trial Group. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 11. | Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 683] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 12. | Song BC, Suh DJ, Yang SH, Lee HC, Chung YH, Sung KB, Lee YS. Lens culinaris agglutinin-reactive alpha-fetoprotein as a prognostic marker in patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization. J Clin Gastroenterol. 2002;35:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Saito Y, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Yamada S, Asanoma M. Prediction of recurrence of hepatocellular carcinoma after curative hepatectomy using preoperative Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein. Hepatol Res. 2012;42:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Chen J, Wu G, Li Y. Evaluation of Serum Des-Gamma-Carboxy Prothrombin for the Diagnosis of Hepatitis B Virus-Related Hepatocellular Carcinoma: A Meta-Analysis. Dis Markers. 2018;2018:8906023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Kudo M. Alpha-fetoprotein-L3: Useful or Useless for Hepatocellular Carcinoma? Liver Cancer. 2013;2:151-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Sterling RK, Jeffers L, Gordon F, Venook AP, Reddy KR, Satomura S, Kanke F, Schwartz ME, Sherman M. Utility of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxy prothrombin, alone or in combination, as biomarkers for hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009;7:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Best J, Bilgi H, Heider D, Schotten C, Manka P, Bedreli S, Gorray M, Ertle J, van Grunsven LA, Dechêne A. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol. 2016;54:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Lim TS, Kim DY, Han KH, Kim HS, Shin SH, Jung KS, Kim BK, Kim SU, Park JY, Ahn SH. Combined use of AFP, PIVKA-II, and AFP-L3 as tumor markers enhances diagnostic accuracy for hepatocellular carcinoma in cirrhotic patients. Scand J Gastroenterol. 2016;51:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019;25:245-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 20. | Kim YY, Park MS, Aljoqiman KS, Choi JY, Kim MJ. Gadoxetic acid-enhanced magnetic resonance imaging: Hepatocellular carcinoma and mimickers. Clin Mol Hepatol. 2019;25:223-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 22. | Jung KS, Kim SU, Ahn SH, Park YN, Kim DY, Park JY, Chon CY, Choi EH, Han KH. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5096] [Cited by in RCA: 5070] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 24. | Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, Cha SW, Kim YS, Cho YD, Kim HS, Kim BS, Park S, Bang HI. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore). 2017;96:e5811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Choi JY, Jung SW, Kim HY, Kim M, Kim Y, Kim DG, Oh EJ. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol. 2013;19:339-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 26. | Ayoub WS, Steggerda J, Yang JD, Kuo A, Sundaram V, Lu SC. Current status of hepatocellular carcinoma detection: screening strategies and novel biomarkers. Ther Adv Med Oncol. 2019;11:1758835919869120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573-10583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 294] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (7)] |
| 28. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (4)] |
| 29. | Nakamura S, Nouso K, Sakaguchi K, Ito YM, Ohashi Y, Kobayashi Y, Toshikuni N, Tanaka H, Miyake Y, Matsumoto E, Shiratori Y. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol. 2006;101:2038-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Song P, Gao J, Inagaki Y, Kokudo N, Hasegawa K, Sugawara Y, Tang W. Biomarkers: evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and china. Liver Cancer. 2013;2:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |