Published online Jul 28, 2021. doi: 10.3748/wjg.v27.i28.4493
Peer-review started: January 28, 2021
First decision: March 29, 2021
Revised: April 11, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: July 28, 2021
Processing time: 178 Days and 12.6 Hours
Gastrointestinal cancers occur in a total of eight different locations, each of them with a different standard of care. This article is not an exhaustive review of what has been published in 2020. We have concentrated on the thirteen phase III randomized studies that are practice-changing. All these studies are oral presentations which have been given in one of the four major oncology congresses, namely American Society of Clinical Oncology (ASCO), ASCO gastrointestinal (GI), European Society of Medical Oncology (ESMO) and ESMO-GI. We provide a concise view of these major trials and their main outcomes, and put these results into context.
Core Tip: Gastrointestinal cancers are one of the most frequent cancers and a leading cause of cancer deaths worldwide. Gastroenterologists, hepatologists and visceral surgeons interact daily with these patients. Bringing the community up to speed with new treatment paradigms in gastrointestinal oncology could improve patient care. Here we provide a clear, comprehensive and short overview of the most important practice changing trials from 2020.
- Citation: Bordry N, Astaras C, Ongaro M, Goossens N, Frossard JL, Koessler T. Recent advances in gastrointestinal cancers. World J Gastroenterol 2021; 27(28): 4493-4503
- URL: https://www.wjgnet.com/1007-9327/full/v27/i28/4493.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i28.4493
Gastrointestinal cancers are the most common form of cancer, affecting both men and women. A multidisciplinary approach is critical to ensure the most effective treatment and best care for each individual patient. 2020 has been a significant year with regard to gastrointestinal cancers, with many practice-changing trials taking place. Here, we aim to provide a clear, comprehensive and brief overview of highlights in the care of gastrointestinal cancer in 2020.
Human epidermal growth factor receptor 2 (HER2) inhibition by trastuzumab and chemotherapy has shown clinically and statistically significant improvements in disease-free survival (DFS) and overall survival (OS) in HER2-positive advanced gastroesophageal cancer compared to chemotherapy alone and is now the standard of care for these patients[1].
NRG-RTOG 1010, presented at American Society of Clinical Oncology (ASCO) 2020, was an open-label randomized phase III trial including 203 patients with newly diagnosed HER2-positive (immunohistochemistry 3+ or FISH positive) esophageal cancer (EC) or gastroesophageal junction (GEJ) cancer[2]. Patients were randomized to receive trimodal standard of care (chemotherapy, radiotherapy and surgery) with or without trastuzumab. Participants in the standard of care arm received chemo-radiation therapy (CXRT) for 6 wk followed by surgery. Participants on the experimental treatment arm received weekly neoadjuvant trastuzumab during CXRT followed by adjuvant trastuzumab for 13 treatments after surgery. The primary endpoint of the trial was DFS. The median DFS was 14.2 mo in the standard of care arm and 19.6 mo in the experimental arm with a hazard ratio (HR) 0.97 (0.69, 1.36) and P = 0.85. OS was similar in both arms, 38.9 mo vs 38.5 mo in the experimental arm. Pathological complete response was equivalent in both arms: 29% vs 27% in the experimental arm.
Impact: HER2 inhibition is currently not recommended in the neoadjuvant setting for localised HER-2 amplified EC or GEJ cancer.
CXRT improves local control and survival in EC; however, local recurrence will occur in 50% of patients.
The ARTDECO trial presented at ASCO-GI 2020 was a randomised phase III multicentre study which randomised 260 patients with locally advanced unresectable EC (93%) and GEJ cancer (7%)[3]. Patients were treated with a standard dose of 50.4 Gy/1.8 Gy/5.5 wk to the tumor and regional lymph nodes vs the same dose combined with an integrated boost of 0.4 Gy per fraction (total 61.6 Gy) to the primary tumor. Chemotherapy consisted of 6 weekly concurrent carboplatin and paclitaxel in both arms. The primary endpoint was local progression-free survival (LPFS). There was no statistical difference in 3-year LPFS with 71% in the standard arm vs 73% in the experimental arm, nor was there a statistically significant difference in OS, with 41% vs 40% for the standard arm and experimental arm, respectively. LPFS was similar per histology. Overall grade 4 and 5 toxicity was 12% and 4% in the standard arm vs 14% and 10% in the experimental arm.
Impact: Radiation dose escalation in definitive CXRT treatment for locally advanced EC does not improve local control or OS.
The risk of recurrence after neoadjuvant CXRT followed by surgery remains high, > 50% during the first 2 years in EC and GEJ cancer[4]. Currently there is no established adjuvant treatment in this setting. Nivolumab — an anti-PD1 checkpoint inhibitor — demonstrated superior survival in previously treated unresectable advanced or recurrent esophageal squamous cell carcinoma (ESCC) vs chemotherapy (ATTRA
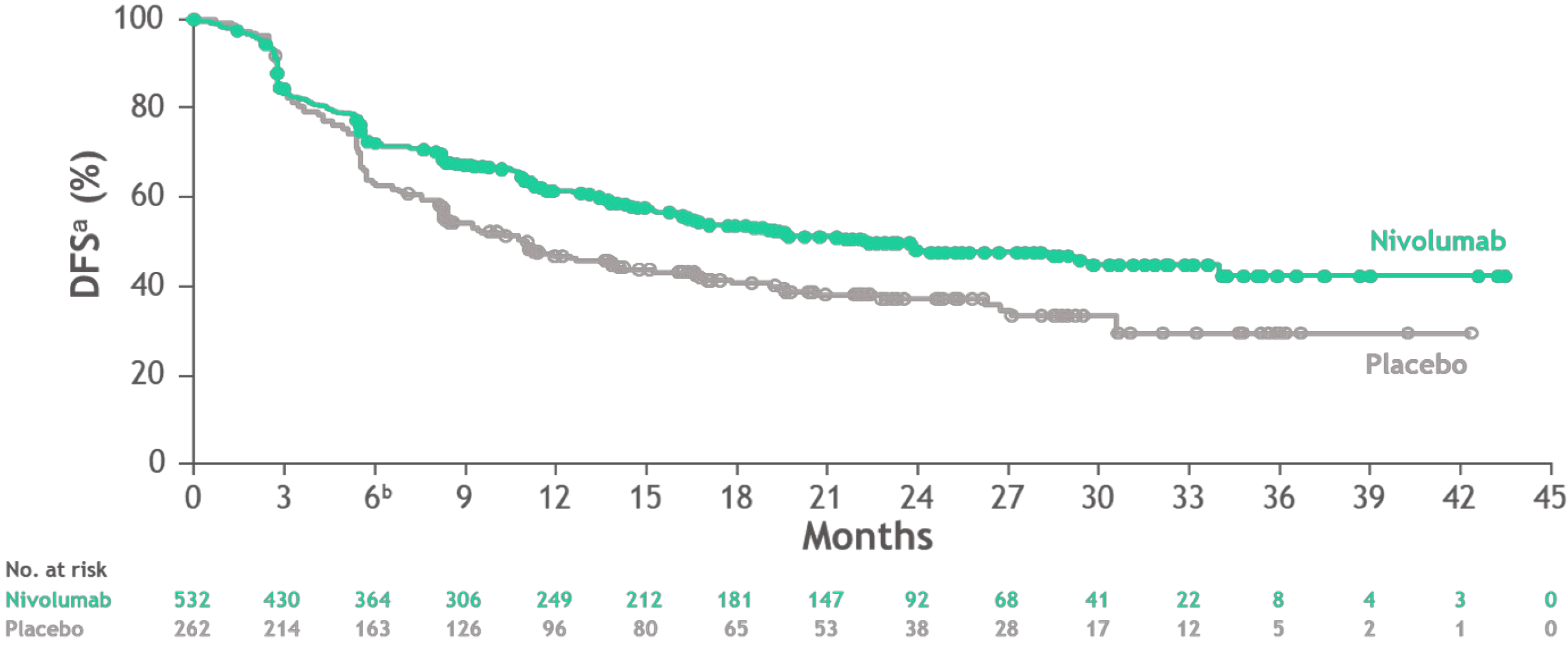
The CheckMate 577 study presented at European Society of Medical Oncology (ESMO) 2020 and recently published in the New England Journal of Medicine was a randomised, double-blind phase III study, evaluating nivolumab vs placebo in the adjuvant setting after trimodal therapy for EC and GEJ cancer[7]. Following R0 resection, 794 patients with stage II (approximately 35%) or III (approximately 65%), EC (approximately 60%) and EGJ cancer (approximately 40%) and residual pathologic disease were randomised to receive nivolumab for one year or placebo. Most patients (approximately 70%) had adenocarcinomas. The primary endpoint was DFS. At a pre-specified interim analysis, adjuvant nivolumab showed an improved DFS of 22.4 mo compared to 11 mo in the placebo arm with HR = 0.69 (0.56-0.86) and P = 0.0003. Treatment-related adverse events (TRAEs) occurred in 13% of the nivolumab arm vs 6% in the placebo arm (Figure 1), patient-reported outcomes were similar in both arms.
Impact: Nivolumab is the first adjuvant therapy to provide a reduction in the risk of recurrence or death in resected EC/GEJ cancer with residual disease after trimodal treatment. These results establish nivolumab as a new standard of care in these patients.
Patients with inoperable or metastatic GC have a median OS of less than 1 year[8,9]. Currently, these patients are treated with doublet chemotherapy which has improved OS and quality of life (QoL) compared to single-agent chemotherapy or best supportive care alone[10,11].
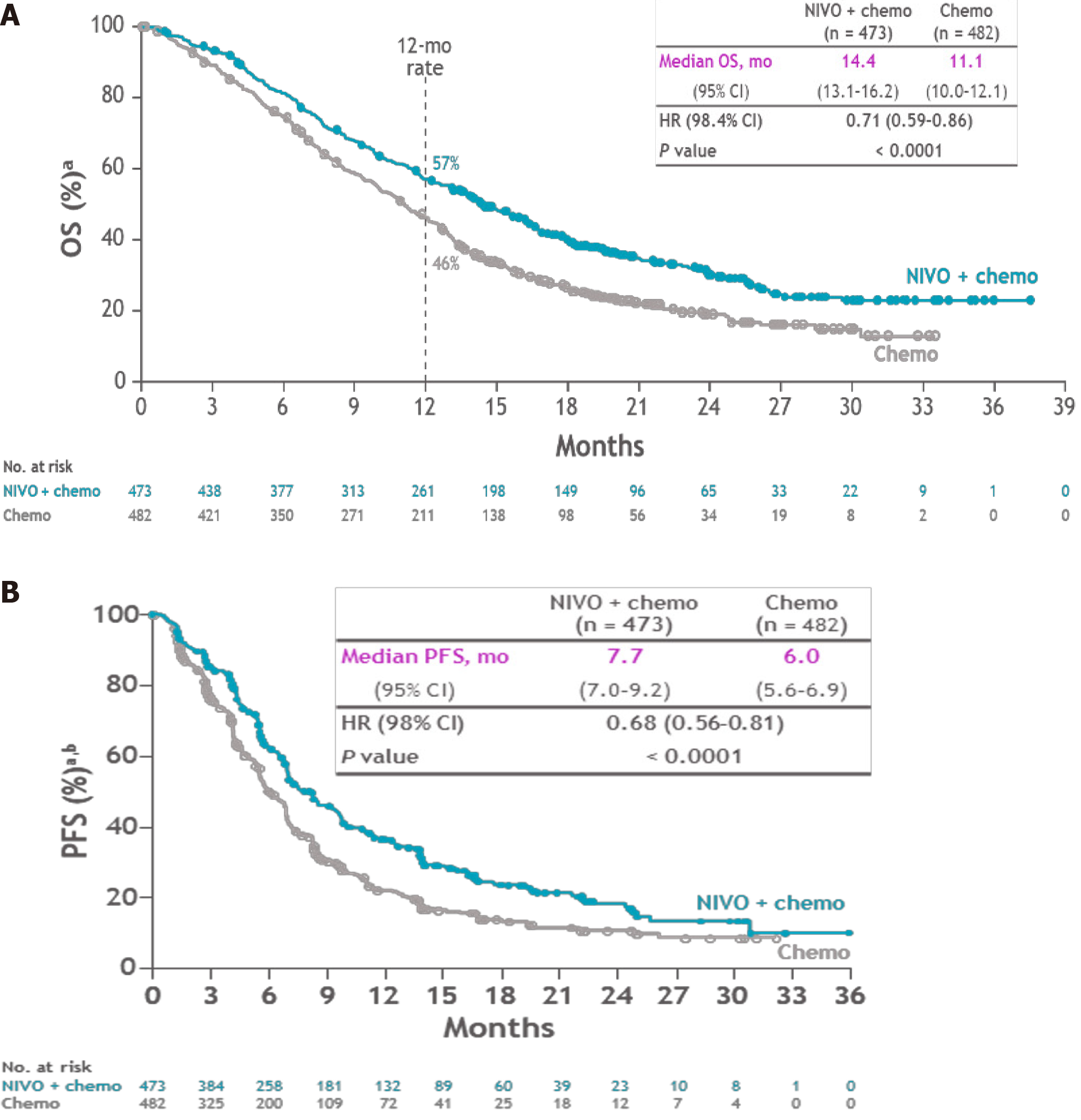
The CheckMate 649 trial, presented at ESMO 2020, evaluated in an open label, randomised phase III trial the efficacy of nivolumab in the treatment of previously untreated, unresectable advanced or metastatic GC/EGJ cancer and esophageal adenocarcinoma[12]. A total of 1581 patients were treated with nivolumab plus chemotherapy (FOLFOX or XELOX) or chemotherapy alone. Programmed cell death ligand 1 (PD-L1) protein expression in gastric or GEJ adenocarcinoma is determined by using the combined positive score (CPS), which is the number of PD-L1 staining cells (tumour cells, lymphocytes, macrophages) divided by the total number of viable tumour cells, multiplied by 100. The specimen should be considered to have PD-L1 expression if CPS ≥ 1. Primary outcomes were OS and PFS in patients with CPS > 5%. OS in CPS > 5% was 14.4 mo in the nivolumab and chemotherapy arm vs 11.1 mo in the chemotherapy arm alone with HR: 0.71 (0.59-0.86, P < 0.0001) (Figure 2A). PFS in CPS > 5% was 7.7 mo in the experimental arm vs 6 mo (P < 0.0001) in the control arm (Figure 2B). A statistically significant benefit was also seen with nivolumab when assessing OS and PFS in patients with PD-L1 CPS ≥ 1% and the overall randomised population. Serious TRAEs occurred in 22% of the experimental arm vs 12% in the control arm.
The ATTRACTION-4 trial, presented at ESMO 2020, was a double-blind, phase III randomised trial evaluating the combination of nivolumab with chemotherapy (SOX or CAPOX) vs chemotherapy alone in GC/EGJ cancer[13]. Primary outcomes were PFS and OS. At an interim-analysis, median PFS was improved in the experimental arm at 10.45 mo vs 8.34 mo, HR: 0.68 (0.51-0.90) and P = 0.0007. However, OS was comparable in both arms 17.45 mo vs 17.15 mo in the experimental arm (P = 0.257).
The Keynote 590 study, presented at ESMO 2020, was a double-blind, placebo-controlled study evaluating pembrolizumab (anti-PD-1) plus chemotherapy (cisplatin and 5FU) vs placebo plus chemotherapy as first-line treatment for locally advanced unresectable or metastatic AEC or ESCC or advanced/metastatic EGJ Siewert I adenocarcinoma[14]. Primary outcomes were OS in ESCC with CPS > 10% and whole population and PFS in the whole population. The majority of subjects (approximately 70%) had ESCC, AEC represented approximately 15% and EGJ approximately 12% of the study population. OS was significantly increased in the experimental arm in ESCC with CPS > 10% with a median OS of 13.9 mo vs 8.8 mo in the control arm (P < 0.0001) as well as in the ESCC overall population with OS of 12.4 mo vs 9.8 mo (P = 0.0006). PFS in ESCC was significantly increased in the experimental arm with 6.3 mo vs 5.8 mo (P < 0.0001).
Impact: The combination of PD-1 blockage (nivolumab or pembrolizumab) with chemotherapy could be considered a new standard first-line option for advanced or metastatic ESCC CPS > 10%, GC and EGJ CPS > 5%.
For the last 10 years the first-line treatment of unresectable hepatocellular carcinoma (HCC) was sorafenib. In 2018, lenvatinib showed its non-inferiority compared to sorafenib, offering a second option in first-line treatment[15].
The IMbrave150 trial was an open-label, phase III trial. Patients with unresectable HCC and without previously systemic treatment were randomly assigned to receive either atezolizumab (anti-PD-L1) plus bevacizumab (anti-VEGF) or sorafenib[16]. All patients were Child-Pugh A and the aetiology of underlying liver disease was viral hepatitis in approximately 70% of the study population. The co-primary endpoints were OS and PFS. At the first interim analysis, OS at 12 mo was 67.2% in the experimental arm and 54.6% in the standard arm with HR 0.58 (0.42-0.79, P < 0.001). Median PFS was 6.8 mo in the experimental arm vs 4.3 mo (P < 0.001). Grade 3 or 4 adverse events occurred in 56.5% in the experimental arm and in 55.1% in the control arm. Moreover, this combination delayed the deterioration of patient-reported functioning and QoL vs sorafenib in this patient population to a meaningful degree.
Impact: Atezolizumab + bevacizumab is now considered first-line standard of care therapy in patients with unresectable HCC.
For stage III colon cancer, the addition of oxaliplatin to a fluoropyrimidine improved DFS and OS in the adjuvant setting. Since 2004, a 6-mo regimen of FOLFOX or CAPOX is the standard adjuvant therapy in stage III disease[17-19]. However, oxaliplatin is associated with cumulative neurotoxicity that can be severe, which potentially affects patients’ activities of daily living, and can persist long beyond the actual treatment. If efficacy were maintained, shorter adjuvant therapy duration would be beneficial for patients as it could spare them toxic effects and health expenditures.
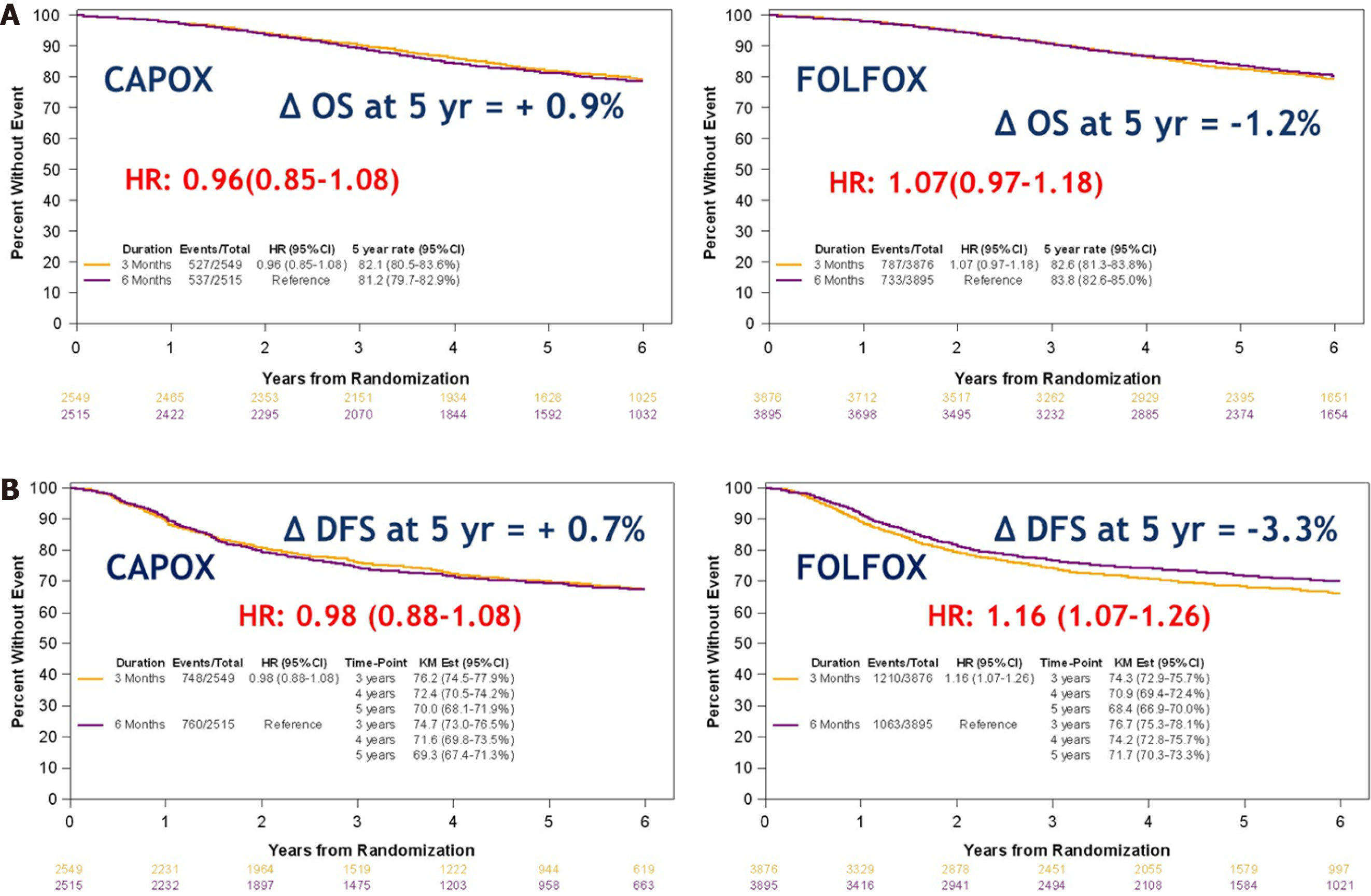
An international duration evaluation of adjuvant therapy trial investigated the non-inferiority of 3 mo of adjuvant therapy (either FOLFOX or CAPOX) compared to 6 mo of treatment[20]. The primary endpoint was the rate of DFS at 3 years. Non-inferiority of 3 mo of therapy, as compared to 6 mo, was not confirmed in the overall population. However, in patients treated with CAPOX, 3 mo of therapy was as effective as 6 mo, particularly in the lower-risk subgroup.
At this year’s ASCO, Sobrero et al[21] presented the OS (secondary outcome) and an update of the DFS (median follow-up of 6 years). Again non-inferiority of OS for 3 mo (82.4%) vs 6 mo (82.8%) treatment was not demonstrated (P = 0.0583). OS was 82.1% for patients treated with 3 mo of CAPOX compared to 81.2% in those receiving it for 6 mo. In patients treated with FOLFOX, 3 mo survival was 82.6% compared to 83.8% for 6 mo survival (Figure 3A). Five-year DFS was 70% for 3 mo CAPOX compared to 69.3% for 6 mo and 68.4% for 3 mo FOLFOX compared to 71.7 for 6 mo (Figure 3B). Finally, for low risk (T1-3, N1) cancers, OS with 3 mo treatment was 89.6% compared to 88.9% for 6 mo. In high risk (T4 / N2) cancers, OS was 72% for 3 mo and 74.1% for 6 mo.
Impact: Stage III low risk colon cancer can be treated with either 6 mo FOLFOX or 3 mo CAPOX. High-risk stage III colon cancer can be treated with 6 mo FOLFOX or 6 mo CAPOX, although the difference in clinical benefit between 3 and 6 mo CAPOX is small.
In colon cancer, 30%-50% of all patients will relapse and die of the disease. Intensive follow-up of patients after curative surgery to detect recurrence and/or metachronous cancers is recommended[22]. However, these recommendations are based on expert opinions, and clinical trials have shown contrasting results[23]. A Cochrane meta-analysis showed no survival benefit of intensive follow-up[24]. Which tests should be performed and what the optimal frequency for surveillance of cancer recurrence might be are still unknown.
The PRODIGE 13 study, presented at ESMO 2020, was a prospective multicentre phase III-controlled trial evaluating the impact of intensive follow-up on OS in 1995 resected stage II or III colorectal cancer (CRC) patients[25]. Patients were double randomised, first in the CEA assessment arm vs no assessment and then in the intensive radiological follow-up arm [computed tomography (CT)-scan every 6 mo] vs low intensity follow-up (abdominal ultrasound every 3 mo and thoracic radiography every 6 mo). The primary endpoint was 5-year OS.
The majority (77%) of patients were less than 75 years old and had CRC (16% rectal, 84% colon cancer). Half of them had stage II disease, 50% being high risk stage II. This study showed no difference in terms of OS and recurrence-free survival for any of the surveillance arms. In the sub-group of patients with recurrence, curative surgery was achieved in 40.9% of the ‘’minimum follow-up’’ group, 66.3% in the ‘’CEA’’ group and standard imaging, 50.7% in the ‘’no CEA and CT scan’’ group, and 59.5% in the maximum follow-up group. Although the differences were significant (P = 0.0035), OS was identical in all arms (P = 0.887).
Impact: A low intensity surveillance (no CEA, abdominal ultrasound and chest X-ray) could soon be the new standard for CRC surveillance after curative resection for patients not amenable to a second curative resection.
Patients with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) CRC represent 5% of all patients with metastatic CRC. The presence of MSI-H is associated with decreased survival rates and less response to conventional chemotherapy[26]. However, MSI-H or dMMR tumours respond better than microsatellite stable tumours to anti-PD1 or antiPD-L1, immunotherapy. In May 2017, the FDA approved the use of pembrolizumab (anti-PD-1), for the treatment of metastatic MSI-H/dMMR CRC patients who have progressed on chemotherapy.
The KEYNOTE-177 trial presented at the plenary session at ASCO 2020 and recently published in the New England Journal of Medicine was a randomised, phase III clinical trial that evaluated the efficacy of pembrolizumab in the first-line treatment of newly diagnosed MSI-H/dMMR metastatic CRC compared to chemotherapy (doublet +/- biologic)[27]. The co-primary outcomes were PFS and OS. At a median follow-up of 32.4 mo, the median PFS with pembrolizumab was 16.5 mo compared to 8.2 mo with chemotherapy HR: 0.60 (0.45-0.80, P = 0.0002). Adverse events grade ≥ 3 were less common (22%) in the experimental arm compared to the standard arm (66%). QOL assessed with QLQ-C30 and EQ-5D was improved with pembrolizumab compared to chemotherapy (P = 0.002).
Impact: Pembrolizumab (anti-PD1) is a new standard of care in the first-line treatment of MSI-H/dMMR metastatic CRC.
Approximately 20% of patients with CRC are diagnosed with stage IV disease. For those with an asymptomatic primary tumour, the question remains whether the primary tumour should be removed first.
The iPACS study presented at ASCO-GI 2020 was a randomised phase III trial comparing primary tumour resection (PTR) plus chemotherapy compared to chemotherapy alone in asymptomatic stage IV CRC patients[28]. The primary endpoint was OS. Investigators aimed to recruit 770 patients but due to slow accrual the sample size was decreased to 280. The Data and Safety Monitoring Committee (DSMC) recommended early termination of the trial due to futility at the first interim analysis in September 2019. With a median follow-up period of almost 2 years, there was no OS difference (P = 0.69) between PTR plus chemotherapy (OS: 25.9 mo) and the chemotherapy alone arm (26.7 mo). Median PFS was 10.4 mo for PTR plus chemotherapy vs 12.1 mo for chemotherapy alone. There were three treatment-related deaths following PTR due to postoperative complications.
Impact: Currently, PTR in asymptomatic stage IV CRC patients cannot be recom
Locally advanced rectal cancer is treated with neoadjuvant CXRT followed by surgery 8 to 12 wk later. Neoadjuvant CXRT provides a significant reduction in local recur
To ensure efficient treatment of both the local tumour and possible micro-metastases, an alternative approach would be to administer the systemic therapy preoperatively after short-course radiotherapy (5 d). During this waiting period the patient is in a good condition to receive an optimal dose of chemotherapy. Two phase III trials (RAPIDO and PRODIGE 23) hypothesized that neoadjuvant chemotherapy will result in increased survival in locally advanced rectal cancer patients.
The RAPIDO trial, presented at ASCO 2020 and recently published in The Lancet Oncology, is a phase III randomised trial comparing standard CXRT before surgery +/- adjuvant chemotherapy (standard arm) to total neoadjuvant treatment (TNT) with short-course radiotherapy (5 × 5 Gy) followed by chemotherapy (XELOX 6 cycles or FOLFOX 9 cycles) before surgery (experimental arm)[33]. The trial accrued only patients with locally advanced rectal cancers defined as cT4a/b, extramural vascular invasion, cN2, involved mesorectal fascia, or enlarged lateral lymph nodes. The primary outcome was disease-related treatment failure (DrTF) defined as distant metastasis, locoregional failure, new primary CRC or treatment-related death. The 3-year DrTF was improved in the experimental arm at 23.7% vs 30.4% for the control arm (P = 0.019). The experimental arm showed an improved pathological complete response (28% vs 14%; P < 0.001) and reduced distant metastases (20.0% vs 26.8%, P = 0.005), but no improvement in locoregional failure rates (8.7% vs 6.0%, P = 0.09), 3-year OS (89.1% vs 88.8%, P = 0.59) or QoL.
The PRODIGE 23 trial presented at ASCO was a phase III randomised trial investigating TNT with modified (m)FOLFIRINOX (leucovorin, infusional 5-FU, irinotecan, oxaliplatin) before standard CXRT, followed by surgery and adjuvant treatment in locally advanced rectal cancer patients compared to standard CXRT followed by surgery and compulsory adjuvant treatment[34]. This trial also accrued only patients with locally advanced rectal cancers defined as cT3 or cT4 M0. The primary endpoint was DFS. Surgical morbidity did not differ between the two arms. TNT significantly improved pathological complete response rate at 27.5% compared to 11.7% in the CXRT alone arm (P = 0.001). TNT significantly improved 3-year DFS (75.7% vs 68.5%, HR 0.69, P = 0.034) and 3-year metastasis-free survival (78.8% vs 71% in CXRT alone, HR 0.64, P = 0.02). Global quality-of-life scores were similar.
Impact: TNT could be an option in locally advanced rectal cancer patients.
This year has been particularly significant in gastrointestinal oncology (Table 1). Immunotherapy has finally entered the scene with numerous indications - first-line MSI-H/dMMR metastatic CRC, first-line metastatic ESCC CPS > 10%, as adjuvant treatment in GC with CPS > 5%, as adjuvant treatment in resected EC/GEJ cancer with residual disease after trimodal treatment and first-line advanced HCC. The field has reached maturity with treatment de-escalation in the adjuvant setting for CRC and in the follow-up after curative surgery for CRC. On the other hand, in locally advanced rectal cancer intensification with TNT has found its place in the treatment landscape.
| Studies | Population | Intervention | Control | Primary endpoints | Results |
| Gastroesophageal cancers | |||||
| Neoadjuvant | |||||
| NRG-RTOG 1010 | HER2-positive EC or GEJ | Trastuzumab + CXRT + surgery | CXRT + surgery | DFS | 19.6 mo vs 14.2 mo (HR = 0.97) |
| ARTDECO | Locally advanced unresectable EC and GEJ | Standard CXRT + RT boost | Standard CXRT | 3-yr LPFS | 73% vs 71% (NS) |
| Adjuvant | |||||
| Checkmate-577 | EC and EGJ and residual pathologic disease | Nivolumab | Placebo | DFS | 22.4 mo vs 11 mo (HR = 0.69) |
| Advanced/metastatic | |||||
| Checkmate-649 | Untreated, unresectable advanced or metastatic GC/EGJ | Nivolumab + CT | CT | OS; PFS | 14.4 mo vs 11.1 mo (HR = 0.71); 7.7 mo vs 6 mo (HR = 0.68) |
| ATTRACTION-4 | Untreated, unresectable advanced or metastatic GC/EGJ | Nivolumab + CT | CT + placebo | OS; PFS | 17.45 mo vs 17.15 mo (HR = 0.90); 10.45 mo vs 8.34 mo (HR = 0.68) |
| Keynote-590 | Untreated, unresectable advanced or metastatic EC/EGJ | Pembrolizumab + CT | CT + placebo | OS; PFS | 12.4 mo vs 9.8 mo (HR = 0.73); 6.3 mo vs 5.8 mo (HR = 0.65) |
| Hepatocellular carcinoma | |||||
| Advanced/metastatic | |||||
| Imbrave 150 | Untreated, unresectable HCC | Atezolizumab + bevacizumab | Sorafenib | 12-mo OS; PFS | 67.2% vs 54.6% (HR = 0.58); 6.8 mo vs 4.3 mo (HR = 0.59) |
| Colon cancer | |||||
| Adjuvant | |||||
| IDEA | Resected stage III CRC | 3 mo adj. CAPOX 3 mo adj. FOLFOX | 6 mo adj. CAPOX ; 6 mo adj. FOLFOX | 5-yr DFS | 70% vs 69.3% (HR = 0.95); 68.4% vs 71.7% (HR = 1.16) |
| Follow-up | |||||
| PRODIGE 13 | Resected stage II or III CRC | Low intensity follow-up | High intensity follow-up | 5-yr OS | NS |
| Metastatic | |||||
| Keynote-177 | Untreated, unresectable metastatic MSI-H/dMMR CRC | Pembrolizumab | CT | PFS | 16.5 mo vs 8.2 mo (HR = 0.6) |
| iPACS | Asymptomatic stage IV CRC | Tumor resection + CT | CT | OS | 25.9 mo vs 26.7 mo (HR = 1.10) |
| Rectal cancer | |||||
| Neoadjuvant | |||||
| RAPIDO | Locally advanced rectal cancer | RT + Neoadj. CT + surgery | CXRT + surgery +/-adj. CT | 3-yr DrTF | 23.7% vs 30.4% (HR = 0.76) |
| PRODIGE 23 | Locally advanced rectal cancer | Neoadj. CT + CXRT + surgery + adj. CT | CXRT + surgery +/-adj. CT | 3-yr DFS | 75.7% vs 68.5% (HR = 0.69) |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Switzerland
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lang SA S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL
| 1. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy vs chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5325] [Article Influence: 355.0] [Reference Citation Analysis (3)] |
| 2. | Safran H, Winter KA, Wigle DA, DiPetrillo TA, Haddock MG, Hong TS, Leichman LP, Rajdev L, Resnick MB, Kachnic LA, Seaward SA, Mamon HJ, Pardo DAD, Anderson CM, Shen X, Sharma AK, Katz AW, Salo JC, Leonard KL, Crane CH. Trastuzumab with trimodality treatment for esophageal adenocarcinoma with HER2 overexpression: NRG Oncology/RTOG 1010. J Clin Oncol. 2020;38:4500-4500. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Hulshof MCCM, Geijsen D, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, Nuyttens J, Sangen MVD, Jeene P, Reinders J, Henegouwen MI van B, Hooft JE van, Laarhoven HWMV, Gaast AVD. A randomized controlled phase III multicenter study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer: ARTDECO study. J Clin Oncol. 2020;38:281-281. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binquet C. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 878] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 5. | Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab vs chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 798] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 6. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1714] [Article Influence: 214.3] [Reference Citation Analysis (0)] |
| 7. | Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, Uronis H, Elimova E, Grootscholten C, Geboes K, Zafar S, Snow S, Ko AH, Feeney K, Schenker M, Kocon P, Zhang J, Zhu L, Lei M, Singh P, Kondo K, Cleary JM, Moehler M; CheckMate 577 Investigators. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med. 2021;384:1191-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 1086] [Article Influence: 271.5] [Reference Citation Analysis (0)] |
| 8. | Cunningham SC, Kamangar F, Kim MP, Hammoud S, Haque R, Maitra A, Montgomery E, Heitmiller RE, Choti MA, Lillemoe KD, Cameron JL, Yeo CJ, Schulick RD. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg. 2005;9:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Yang D, Hendifar A, Lenz C, Togawa K, Lenz F, Lurje G, Pohl A, Winder T, Ning Y, Groshen S, Lenz HJ. Survival of metastatic gastric cancer: Significance of age, sex and race/ethnicity. J Gastrointest Oncol. 2011;2:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (2)] |
| 10. | Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H, Heuman R. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 600] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 11. | Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 896] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 12. | Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Bragagnoli AC, Liu T, Schenker M, Yanez P, Tehfe M, Poulart V, Cullen D, Lei M, Kondo K, Li M, Ajani JA, Janjigian YY. Nivolumab (nivo) plus chemotherapy (chemo) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol. 2020;31:S1191. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 13. | Boku N, Ryu MH, Oh D-Y, Oh SC, Chung HC, Lee K-W, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen J-S, Bai L-Y, Chen L-T, Kang Y-K. Nivolumab plus chemotherapy vs chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann Oncol. 2020;31:S1192. [RCA] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 14. | Kato K, Sun J-M, Shah MA, Enzinger PC, Adenis A, Doi T, Kojima T, Metges J-P, Li Z, Kim S-B, Cho BCC, Mansoor W, Li S-H, Sunpaweravong P, Maqueda MA, Goekkurt E, Liu Q, Shah S, Bhagia P, Shen L. Pembrolizumab plus chemotherapy vs chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol. 2020;31:S1192-S1193. [RCA] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 15. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3826] [Article Influence: 546.6] [Reference Citation Analysis (1)] |
| 16. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4696] [Article Influence: 939.2] [Reference Citation Analysis (2)] |
| 17. | André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2653] [Cited by in RCA: 2732] [Article Influence: 130.1] [Reference Citation Analysis (0)] |
| 18. | André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1644] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 19. | Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 576] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 20. | Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, Meyerhardt JA, Vernerey D, Yamanaka T, Boukovinas I, Meyers JP, Renfro LA, Niedzwiecki D, Watanabe T, Torri V, Saunders M, Sargent DJ, Andre T, Iveson T. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018;378:1177-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 685] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 21. | Sobrero AF, Andre T, Meyerhardt JA, Grothey A, Iveson T, Yoshino T, Sougklakos I, Meyers JP, Labianca R, Saunders MP, Vernerey D, Yamanaka T, Boukovinas I, Oki E, Georgoulias V, Torri V, Harkin A, Taieb J, Shields AF, Shi Q. Overall survival (OS) and long-term disease-free survival (DFS) of three vs six months of adjuvant (adj) oxaliplatin and fluoropyrimidine-based therapy for patients (pts) with stage III colon cancer (CC): Final results from the IDEA (International Duration Evaluation of Adj chemotherapy) collaboration. J Clin Oncol. 2020;38:4004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, Martinelli E, Arnold D; ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 805] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 23. | Wille-Jørgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Petersen SH, Renehan AG, Horváth-Puhó E, Påhlman L, Sørensen HT; COLOFOL Study Group. Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients With Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial. JAMA. 2018;319:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 24. | Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2019;9:CD002200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Lepage C, Phelip JM, Cany L, Barbier E, Manfredi S, Deguiral P, Faroux R, Baconnier M, Pezet D, Duchmann J, Terrebonne E, Adenis A, Benabdelghani M, Ain J, Breysacher G, Boillot-Benedetto I, Pelaquier A, Prost P, Lievre A, Bouche O. Effect of 5 years of imaging and CEA follow-up to detect recurrence of colorectal cancer (CRC) - PRODIGE 13 a FFCD phase III trial. Ann Oncol. 2020;31:S410. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, Kaplan R, Quirke P, Seymour MT, Richman SD, Meijer GA, Ylstra B, Heideman DA, de Haan AF, Punt CJ, Koopman M. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322-5330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 582] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 27. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1810] [Article Influence: 362.0] [Reference Citation Analysis (0)] |
| 28. | Kanemitsu Y, Shitara K, Mizusawa J, Hamaguchi T, Shida D, Komori K, Ikeda S, Ojima H, Hasegawa S, Shiomi A, Watanabe J, Takii Y, Yamaguchi T, Katsumata K, Ito M, Okuda J, Hyakudomi R, Shimada Y, Katayama H, Fukuda H. A randomized phase III trial comparing primary tumor resection plus chemotherapy with chemotherapy alone in incurable stage IV colorectal cancer: JCOG1007 study (iPACS). J Clin Oncol. 2020;38:7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E, Fritz E, Hieber U, Lindemann HW, Grunewald M, Kremers S, Constantin C, Hipp M, Hartung G, Gencer D, Kienle P, Burkholder I, Hochhaus A. Chemoradiotherapy with capecitabine vs fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 30. | Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, Ledermann J, Sebag-Montefiore D. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) vs control. Ann Oncol. 2014;25:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 31. | Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, Glynne-Jones R, Counsell N, Bastiaannet E, van den Broek CB, Liefers GJ, Putter H, van de Velde CJ. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 419] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 32. | Zhao L, Liu R, Zhang Z, Li T, Li F, Liu H, Li G. Oxaliplatin/fluorouracil-based adjuvant chemotherapy for locally advanced rectal cancer after neoadjuvant chemoradiotherapy and surgery: a systematic review and meta-analysis of randomized controlled trials. Colorectal Dis. 2016;18:763-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) vs preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 962] [Article Influence: 240.5] [Reference Citation Analysis (0)] |
| 34. | Conroy T, Lamfichekh N, Etienne P-L, Rio E, FRANCOIS E, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, Fouchardiere CDL, Juzyna B, Rullier E, Marchal F, Castan F, Borg C. Total neoadjuvant therapy with mFOLFIRINOX vs preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2020;38:4007. [RCA] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |