Published online Jul 7, 2021. doi: 10.3748/wjg.v27.i25.3925
Peer-review started: February 24, 2021
First decision: April 18, 2021
Revised: April 21, 2021
Accepted: June 1, 2021
Article in press: June 1, 2021
Published online: July 7, 2021
Processing time: 131 Days and 19.3 Hours
Endoscopic submucosal dissection (ESD) has shown to be effective in manage
To compare the surgical, histological, and oncological outcomes between ESD and EMR in the treatment of colorectal polyps, with subgroup analysis comparing the efficacy of ESD and EMR between Japan and the rest of the world.
Embase and Medline databases were searched from inception to October 2020 in accordance with PRISMA guidelines for studies comparing en bloc, complete resection, margin involvement, resection time, need for additional surgery, complications, and recurrence rate of ESD with EMR.
Of 281344 colorectal polyps from 21 studies were included. When compared to EMR, the pooled analysis revealed ESD was associated with higher en bloc and complete resection rate, and lower lateral margin involvement and recurrence. ESD led to increased procedural time, need for additional surgery, and perfora
ESD resulted in better resection outcomes and lower recurrence compared to EMR. With appropriate training, ESD is preferred over EMR as the first-line therapy for resection of colorectal polyps, without restricting to lesions greater than 20 mm and those with high suspicion of submucosal invasion.
Core Tip: The present study is the most extensive meta-analysis evaluating the surgical, histological, and oncological outcomes of endoscopic submucosal dissection (ESD) in comparison to endoscopic mucosal resection (EMR) in the treatment of colorectal polyps. Our analysis also showed the increased proficiency in performing ESD and EMR in Japan as compared to the rest of the world.
- Citation: Lim XC, Nistala KRY, Ng CH, Lin SY, Tan DJH, Ho KY, Chong CS, Muthiah M. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal polyps: A meta-analysis and meta-regression with single arm analysis. World J Gastroenterol 2021; 27(25): 3925-3939
- URL: https://www.wjgnet.com/1007-9327/full/v27/i25/3925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i25.3925
In recent years, the incidence of colorectal polyps has increased drastically with the widespread implementation of national colorectal cancer (CRC) screening programs. CRC screening with colonoscopy provides the opportunity to identify and remove any precursor lesions[1] and polypectomy has been shown to reduce the incidence and mortality of CRC[1-3]. Currently, 2 major forms of polypectomy are performed, namely endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) with the latter procedure favored by western countries.
ESD was first proposed in 1999 as an endoscopic resection technique for safe en bloc removal of superficial lesions in the upper gastrointestinal tract[4]. Although colorectal ESD is widely practiced in the Asian countries, the implementation of ESD in Western countries where endoscopic mucosal resection is preferred is still debatable. According to the 2015 European Society of Gastrointestinal Endoscopy (ESGE), ESD is strongly recommended when there is high suspicion of submucosal invasion as determined by morphologic factors and advanced surface pattern, especially in lesions greater than 20 mm[5]. Despite the technical difficulties faced, ESD is designed for en bloc resection of lesions in an attempt to achieve complete resection, reliable histopathological analysis, and reduced recurrence rate.
Previous meta-analyses and systematic reviews comparing ESD with EMR mainly focused on data reported from Asian countries with limited data coming from outside of Japan, without controlling for confounders or lesions < 20 mm[6,7]. Since then, numerous studies comparing between ESD and EMR have been reported from the West and other countries[8-16]. We sought to perform an expanded meta-analysis to include lesions ≤ 20 mm, and incorporate regression analysis to control for confounders.
A search was conducted with reference to the PRISMA methodology on October 4, 2020 in Medline and Embase[17]. Keywords and Mesh terms were employed in the search strategy relating to the ‘Colorectal’, ‘ESD’ and ‘EMR’, the full list of terms included in the search can be found in the Supplementary Material 1. References were managed with Endnote X9. Two authors (XCL, KRYN) were involved in the sieving of the abstracts. Articles that compared between EMR and ESD for colorectal polyps were included in the search. Study designs included both retrospective and prospective cohorts, and case-controlled studies. Editorials, conferences abstracts, and commentaries were excluded in the review.
Based on the pre-determined inclusion criterion, article selection was performed by two authors with consensus from an independent third author should discrepancies arise. Information pertaining to the demographics (country, sample size, age, gender, polyp location, polyp size, polyp macroscopic type and depth of cancer invasion) and outcomes were extracted. Outcomes were limited to en bloc resection, complete resection, lateral and vertical margin involvement, lymphovascular invasion, mean operation time, additional surgery required, perforation, bleeding, and recurrence. En bloc resection was defined as the resection of the lesion in a single specimen, and complete resection was defined as the resection of the lesion with no marginal involvement of neoplastic tissue, as assessed by the pathologist. Additional surgical operations performed were considered in cases with intra-operative complications as a result of ESD or EMR or those with technical difficulties. Bleeding was considered both intra-operative and post-operative, and perforation was considered when diagnosed endoscopically during resection or radiologically by the presence of free air. Recurrence was defined as the detection of local or secondary primary tumors on interval colonoscopy. When estimates of mean and standard deviation (SD) were not available for continuous data, well established formulae were used to estimate mean and SD from median and range[18].
Three type of analysis were performed in this review. Firstly, a meta-analysis of proportions was used to pool results from the individual arms (ESD and EMR) using a freeman turkey double arsine transformation[19]. The freeman turkey double arsine transformation is recommended for single arm meta-analysis and necessary to stabilize the variance[20]. Next, comparative meta-analysis after a 0.5 continuity correction was to compare the difference in ESD and EMR, using risk ratios (RR) as the outcome of interest for dichotomous variables and weighted mean differences for continuous variables[21]. A 0.5 continuity correction was considered appropriate to incorporate 0 events into the meta-analysis. Regardless of heterogeneity measures (I2, tau, Cochran Q test), all analysis was performed in random effects with the Dersimonian and Laird model[22,23]. Region (Japan vs rest of the world) was defined with refences to the manuscript country of origin. Publication bias was assessed with harbord test and egger regression for dichotomous and continuous variables respectively when > 10 studies were available. A univariate meta regression was also conducted with in random effects using the restricted maximum likelihood model with Kapp variance estimator to adjust for confounders in the outcomes[24]. A subgroup analysis was considered to compare between the articles originating from Japan and outside of Japan and a sensitivity analysis was performed to only include studies that have polyps size of ≥ 20 mm. All articles were graded with the Newcastle Ottawa scale for cohort studies, grading articles on the domains of study group selection, study group comparability, and ascertainment of outcomes of interest[25]. The statistical methods of this study were reviewed by Cheng Han Ng (ORCID ID: 0000-0002-8297-1569) from Yong Loo Lin School of Medicine, National University of Singapore.
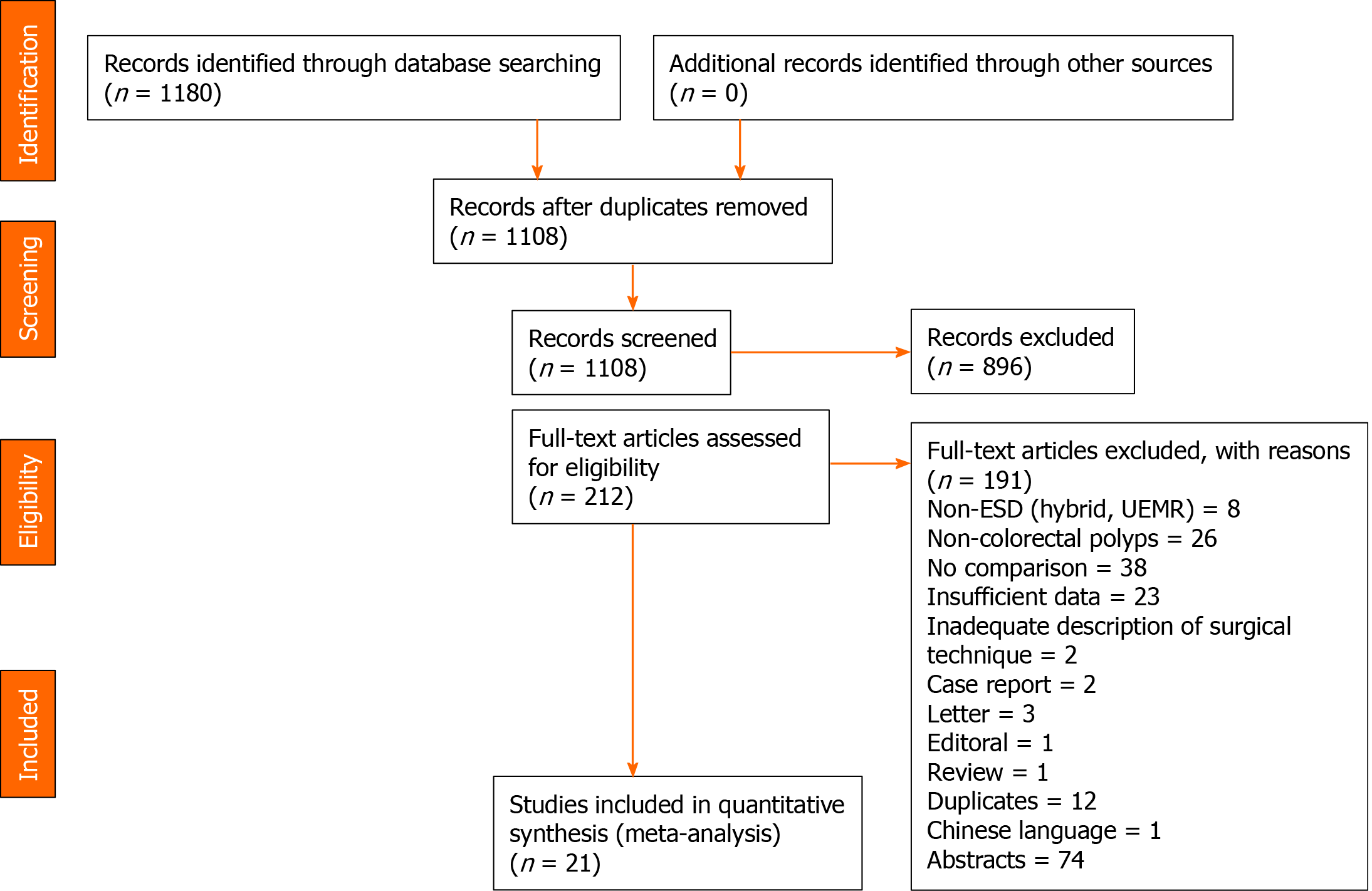
A systematic search of the literature using our search strategy yielded 1180 articles. After removal of duplicates, 895 were excluded based on study title and abstract, and 212 underwent full text review, of which 21 articles comparing ESD with EMR in resection of colorectal polyps were subsequently included in the meta-analysis (Figure 1)[8-16,26-37]. In total, 12 originated from Japan[26-37], five from European[10-13,16], three from South Korea[8,9,14] and one from China[15]. Of the 21 studies, there were 17 retrospective cohort studies, two prospective cohort studies and two retrospective case-control studies. In total, 281344 colorectal polyps were resected, of which 19573 underwent ESD while 261771 underwent EMR. Quality assessment of those included studies mostly scored 6-7. A summary of the study characteristics of the included articles can be found in the Supplementary Table 1.
The summary of the results is found in Table 1. The pooled analysis showed a higher rate of en bloc resection (RR = 1.837; 95%CI: 1.464 to 2.305; P < 0.001), and a lower frequency of positive lateral margin involvement (RR = 0.292; 95%CI: 0.089 to 0.995; P = 0.042) in the ESD group than the EMR group. Publication bias was significant for en bloc resection rate (P = 0.0025). No significant difference in the rates of positive vertical margin involvement was observed between ESD and EMR groups (RR = 4.368; 95%CI: 0.409 to 46.710; P = 0.223). However, the rate of complete resection was higher in the ESD compared to EMR groups (RR = 1.504; 95%CI: 1.041 to 2.174; P < 0.03). Significantly, the time taken for ESD was longer than EMR (RR = 72.709; 95%CI: 54.487 to 90.931; P < 0.001). ESD group required more additional surgical operations relative to that of EMR group (RR = 3.139; 95%CI: 1.360 to 7.243; P = 0.007).
| Total papers | Sample size (ESD) | Pooled proportions | Sample size (EMR) | Pooled proportions | RR (CI) | P value | Publication bias | |
| En bloc resection | 11 | 1641 | 89% (0.83-0.94) | 1411 | 47% (0.36-0.59) | 1.837 (1.464-2.305) | < 0.001 | 0.0025 |
| Positive lateral margin | 2 | 123 | 3% (0.01-0.06) | 187 | 14% (0.09-0.19) | 0.292 (0.089-0.995) | 0.042 | - |
| Positive vertical margin | 1 | 38 | 5% (0.00-0.17) | 83 | 1% (0.00-0.07) | 4.368 (0.409-46.710) | 0.223 | - |
| Complete resection | 8 | 918 | 82% (0.74-0.88) | 1012 | 56% (0.34-0.77) | 1.504 (1.041-2.174) | 0.03 | - |
| Lymphovsacular invasion | 1 | 54 | 6% (0.03-0.13) | 23 | 0% (0.00-0.04) | 4.352 (0.248-76.483) | 0.315 | - |
| Mean procedural time | 8 | 1087 | - | 838 | - | 72.709 (54.487-90.931) | < 0.001 | - |
| Additional surgery | 2 | 99 | 13% (0.07-0.21) | 153 | 5% (0.02-0.09) | 3.139 (1.360-7.243) | 0.007 | - |
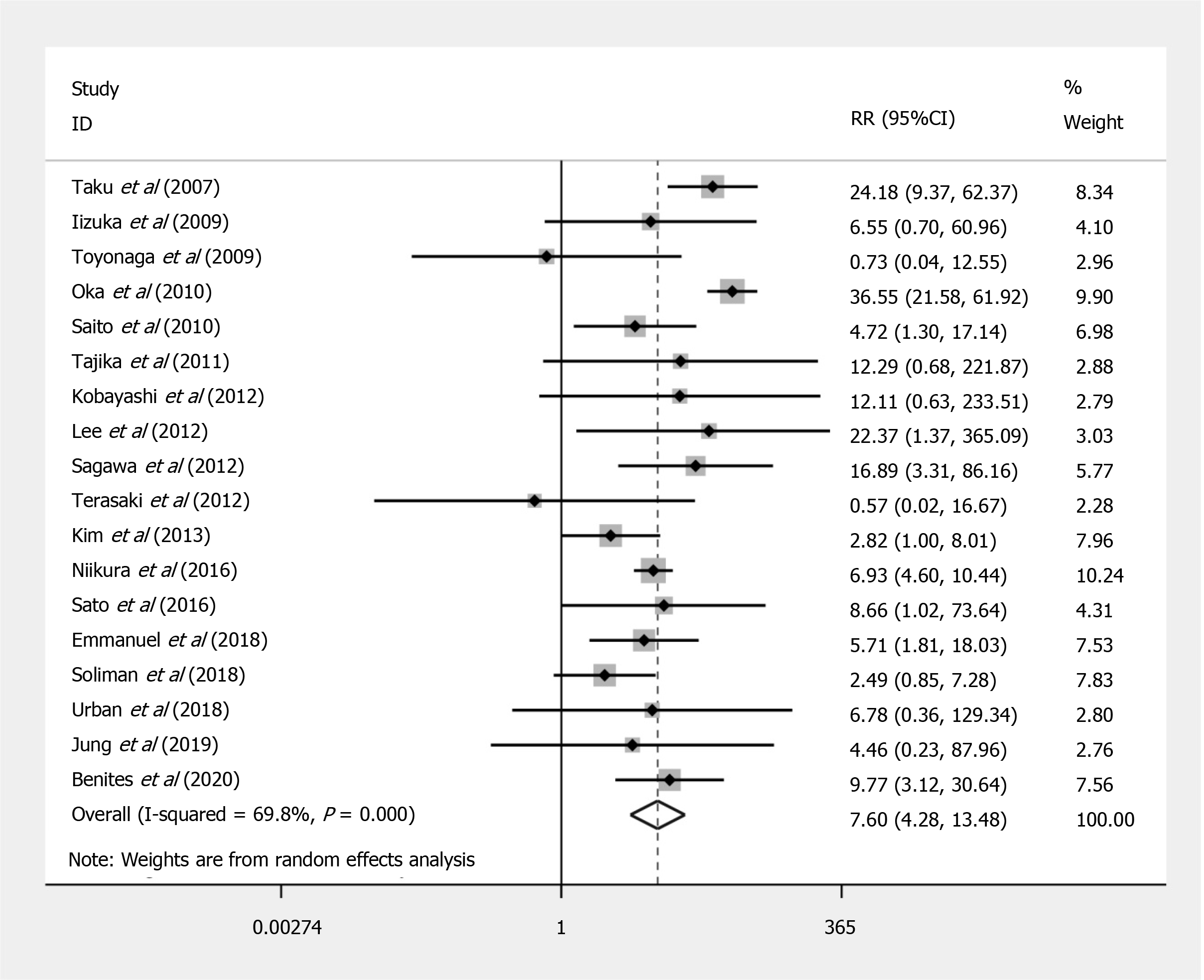
| Perforation | 18 | 19470 | 5% (0.03-0.09) | 260901 | 0% (0.00-0.01) | 7.597 (4.281-13.479) | < 0.001 | 0.301 |
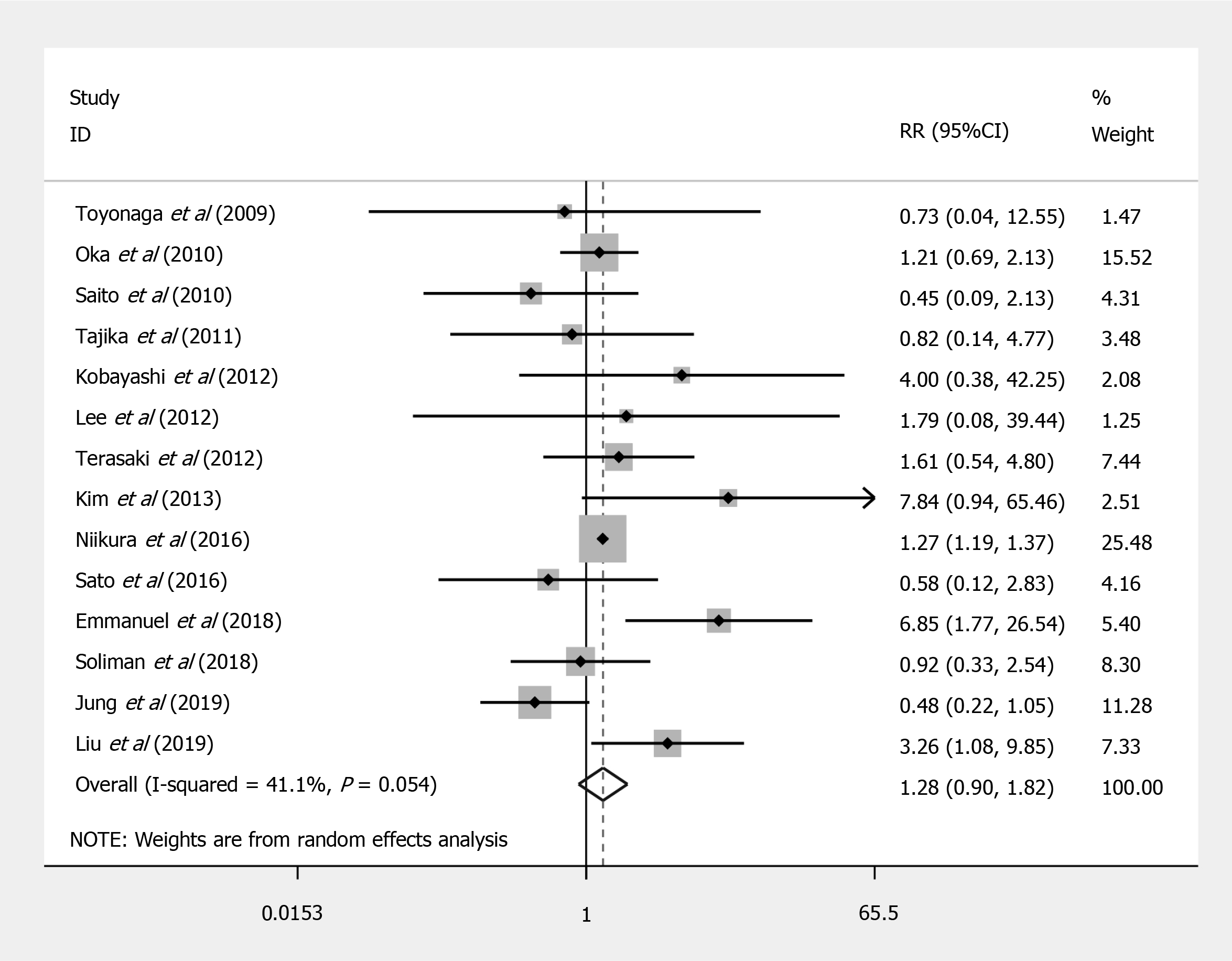
| Bleeding | 14 | 20048 | 3% (0.02-0.05) | 257065 | 3% (0.02-0.04) | 1.277 (0.896-1.820) | 0.175 | 0.139 |
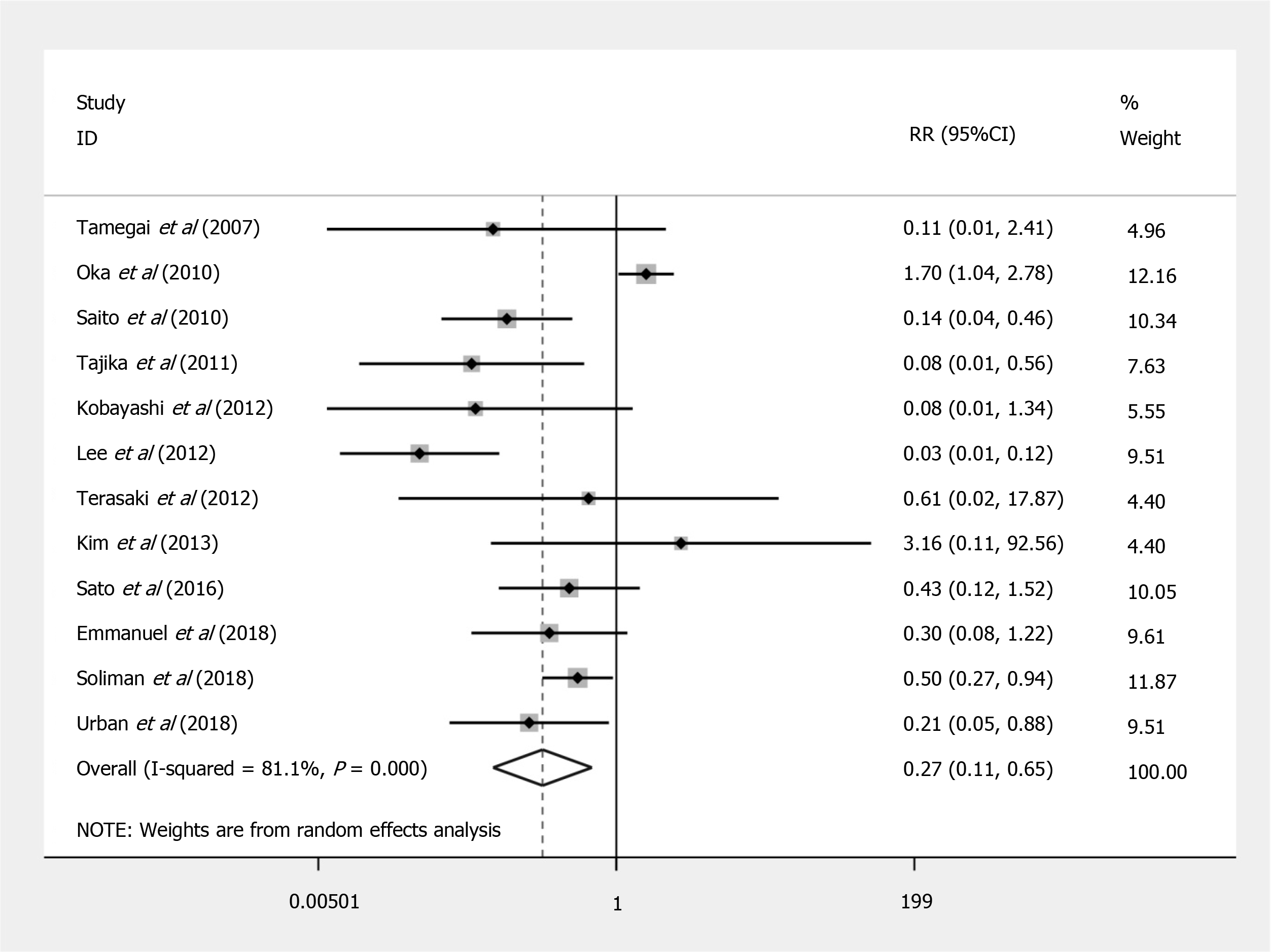
| Recurrences | 12 | 1822 | 2% (0.01-0.03) | 37721 | 10% (0.04-0.17) | 0.269 (0.112-0.648) | 0.003 | 0.725 |
Compared to EMR, ESD shows a significant increased risk of perforation (RR = 7.597; 95%CI: 4.281 to 13.479; P < 0.001) (Figure 2), but no significant difference in the bleeding risk was observed between the two groups (RR = 1.277; 95%CI: 0.896 to 1.820; P = 0.175) (Figure 3). The rate of recurrence in the ESD group was lower than that of the EMR group (RR = 0.269; 95%CI: 0.112 to 0.648; P = 0.003) (Figure 4).
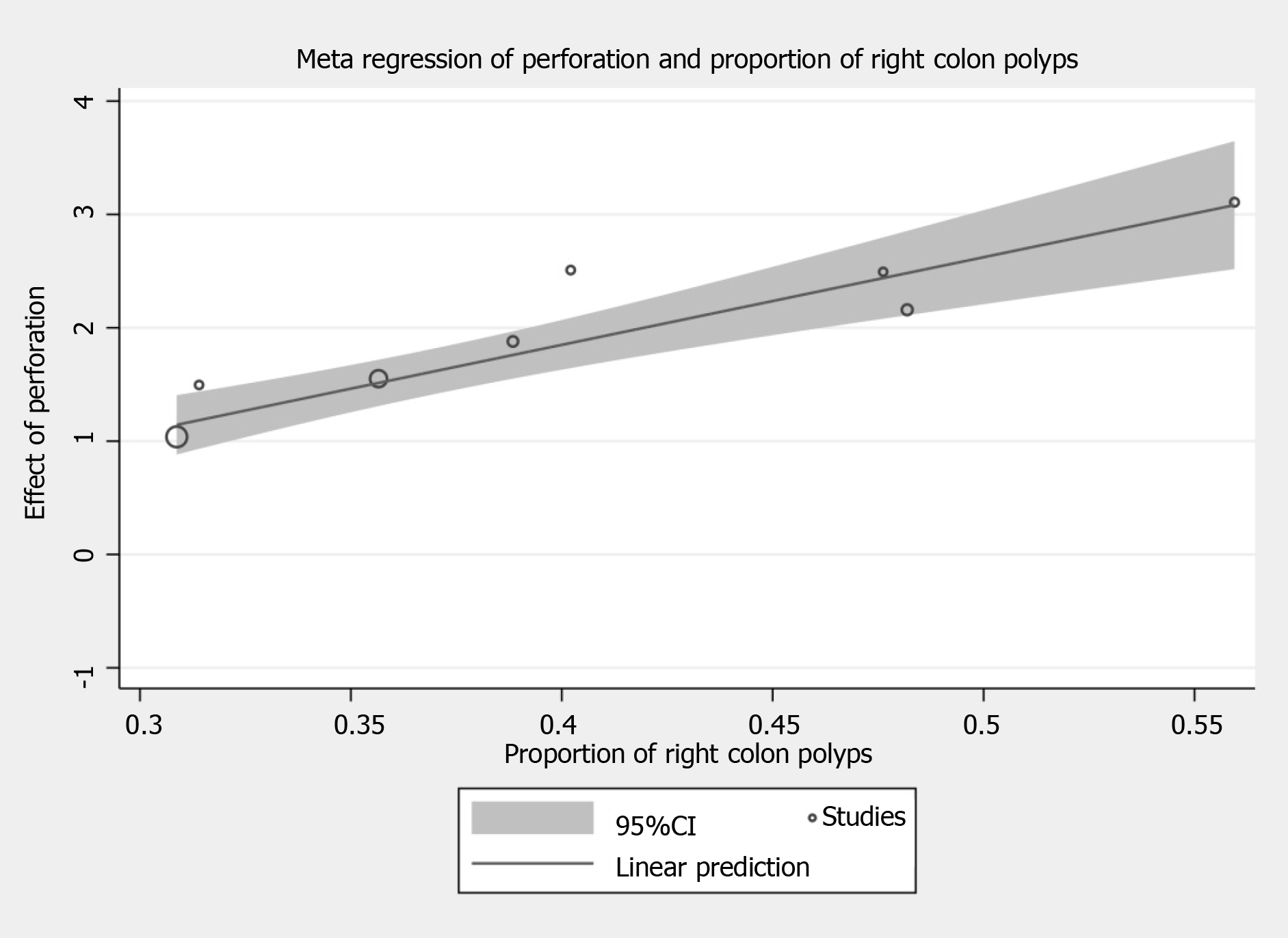
The influence of the included covariates on en bloc resection, complete resection, risk of perforation and bleeding, and rate of recurrence evaluated by meta-regression are summarized in Table 2. Meta-regression analysis for risk of perforation suggested that right colonic polyps (β = 7.731; 95%CI: 4.965 to 10.497; P < 0.001) correlated with an increased risk in perforation in ESD (Figure 5). Other factors including polyp size and depth of invasion, did not influence the en bloc resection rate, complete resection rate, the risk of bleeding, and recurrence rate.
| En bloc resection | Complete resection | Perforation | Bleeding | Recurrence | |||||||||||
| n | Beta (CI) | P value | n | Beta (CI) | P value | n | Beta (CI) | P value | n | Beta (CI) | P value | n | Beta (CI) | P value | |
| Age | 8 | -0.017 (-0.168-0.133) | 0.785 | 5 | -0.010 (-0.426-0.406) | 0.944 | 9 | 0.073 (-0.191-0.336) | 0.535 | 8 | -0.201 (-0.540-0.138) | 0.197 | 8 | 0.254 (-0.039-0.547) | 0.078 |
| Gender | 6 | -6.023 (-23.152-11.106) | 0.384 | 4 | -20.662 (-108.758-67.434) | 0.419 | 8 | -7.868 (-22.078–6.342) | 0.224 | 7 | -4.446 (-27.915-19.022) | 0.647 | 6 | -9.096 (-62.174-43.982) | 0.659 |
| Polyp size | 10 | 0.242 (-0.037-0.086) | 0.390 | 6 | -0.034 (-0.098-0.030) | 0.217 | 10 | -0.034 (-0.117-0.049) | 0.378 | 9 | -0.041 (-0.177-0.095) | 0.498 | 8 | 0.077 (-0.118-0.272) | 0.369 |
| Lateral spreading tumor | 10 | 1.262 (-1.072-3.596) | 0.248 | 7 | -0.481 (-4.655-3.693) | 0.779 | 11 | -2.324 (-5.536-0.888) | 0.136 | 9 | -0.680 (-5.586-4.226) | 0.753 | 9 | 3.827 (-2.323-9.977) | 0.185 |
| Right colon | 8 | 1.247 (-2.024-4.517) | 0.387 | 5 | 2.373 (-0.881-5.626) | 0.103 | 8 | 7.731 (4.965-10.497) | < 0.001 | 7 | 1.688 (-12.442-15.817) | 0.771 | 6 | -8.739 (-26.616-9.139) | 0.246 |
| Colon | 7 | -1.803 (-4.486-0.880) | 0.145 | 4 | -1.914 (-7.213-3.385) | 0.26 | 8 | 0.759 (-3.184-4.701) | 0.654 | 6 | 6.649 (-5.182-18.480) | 0.194 | 7 | -1.102 (-4.754-2.551) | 0.473 |
| Rectum | 7 | 1.803 (-0.880-4.486) | 0.145 | 4 | 1.914 (-3.385-7.213) | 0.26 | 8 | -0.759 (-4.701-3.184) | 0.654 | 6 | -6.649 (-18.480-5.182) | 0.194 | 7 | 1.102 (-2.551-4.754) | 0.473 |
| Submucosal invasion | 8 | -0.004 (-0.014-0.007) | 0.431 | 5 | -0.006 (-0.020-0.008) | 0.253 | 9 | 0.001 (-0.049-0.051) | 0.956 | 8 | -0.016 (-0.033-0.001) | 0.061 | 7 | -6.508 (-27.976-14.960) | 0.471 |
| Muscularis propria invasion | 8 | -5.870 (-17.738-5.998) | 0.272 | 5 | -0.157 (-21.715-21.401) | 0.983 | 9 | 6.731 (-33.772-47.234) | 0.706 | 8 | -109.836 (-1040.943-821.272) | 0.783 | - | - | - |
The results of the sensitivity analysis are summarized in Supplementary Table 2 and results were largely unchanged after a sensitivity analysis. When a sensitivity analysis was performed to include ≥ 20 mm colorectal polyps only, the pooled analysis revealed higher rate of en bloc resection (RR = 1.932; 95%CI: 1.389 to 2.688; P < 0.001), longer operation time (RR = 3.247; 95%CI: 59.249 to 87.245; P < 0.001), higher risk of perforation (RR = 4.513; 95%CI: 2.531 to 8.046; P < 0.001) and lower recurrence rate (RR = 0.191; 95%CI: 0.085 to 0.431; P < 0.001) in ESD compared to EMR groups. Furthermore, in the two studies included, more additional surgical operations were required (RR = 3.139; 95%CI: 1.360 to 7.243; P = 0.007) in the ESD than in the EMR groups.
The results of the subgroup analysis comparing outcomes of ESD with EMR in Japan vs the rest of the world are presented in Table 3.
| Japan papers | Pooled proportions (ESD) | Pooled proportions (EMR) | RR (CI) | P value | Rest of the world papers | Pooled proportions (ESD) | Pooled proportions (EMR) | RR (CI) | P value | |
| Overall results | ||||||||||
| En bloc resection | 6 | 89% (0.77-0.97) | 53% (0.38-0.67) | 1.658 (1.270-2.165) | < 0.001 | 5 | 90% (0.85-0.94) | 41% (0.22-0.61) | 2.201 (1.411-3.433) | 0.001 |
| Positive lateral margin | 2 | 3% (0.00-0.06) | 14% (0.09-0.19) | 0.292 (0.089-0.955) | 0.042 | - | - | - | - | - |
| Positive vertical margin | 1 | 5% (0.01-0.17) | 1% (0.00-0.05) | 4.368 (0.409-46.710) | 0.223 | - | - | - | - | - |
| Complete resection | 2 | 79% (0.73-0.84) | 53% (0.48-0.58) | 1.452 (1.303-1.618) | < 0.001 | 6 | 85% (0.78-0.91) | 59% (0.27-0.88) | 1.562 (0.921-2.650) | 0.098 |
| Lymphovsacular invasion | 1 | 6% (0.03-0.13) | 0% (0.00-0.04) | 4.352 (0.248-76.483) | 0.315 | - | - | - | - | - |
| Mean procedural time | 4 | - | - | 72.106 (48.831-95.382) | < 0.001 | 4 | - | - | 73.916 (36.075-111.757) | < 0.001 |
| Additional surgery | 2 | 13% (0.07-0.21) | 5% (0.02-0.09) | 3.139 (1.360-7.243) | 0.007 | - | - | - | - | - |
| Perforation | 11 | 4% (0.01-0.07) | 0.0002% (0.00-0.00081) | 9.586 (4.425-20.768) | < 0.001 | 7 | 8% (0.05-0.12) | 1% (0.00-0.02) | 4.602 (2.729-7.759) | < 0.001 |
| Bleeding | 8 | 2.4% (0.01-0.04) | 2.1% (0.01-0.03) | 1.267 (1.174-1.367) | < 0.001 | 6 | 5% (0.02- 0.11) | 3% (0.01-0.06) | 1.986 (0.716-5.508) | 0.188 |
| Recurrences | 7 | 1% (0.01-0.02) | 7% (0.02-0.15) | 0.274 (0.071-1.054) | 0.06 | 5 | 3% (0.00-0.08) | 16% (0.04-0.32) | 0.245 (0.073-0.819) | 0.022 |
| Sensitivity analysis (≥ 20 mm only) | ||||||||||
| En bloc resection | 4 | 82% (0.72-0.91) | 50% (0.33-0.67) | 1.645 (1.174-2.306) | 0.004 | 2 | 91% (0.88-0.93) | 33% (0.28-0.37) | 2.668 (1.752-4.063) | < 0.001 |
| Positive lateral margin | 2 | 3% (0.00-0.06) | 14% (0.09-0.19) | 0.292 (0.089-0.955) | 0.042 | - | - | - | - | - |
| Positive vertical margin | 1 | 5% (0.01-0.17) | 1% (0.00-0.07) | 4.368 (0.409-46.710) | 0.223 | - | - | - | - | - |
| Complete resection | 2 | 79% (0.73-0.84) | 53% (0.48-0.58) | 1.452 (1.303-1.618) | < 0.001 | 2 | 90% (0.87-0.93) | 77% (0.73-0.82) | 1.613 (0.209-12.452) | 0.647 |
| Lymphovsacular invasion | 2 | 6% (0.03-0.13) | 0% (0.00-0.04) | 4.352 (0.248-76.483) | 0.315 | - | - | - | - | - |
| Mean procedural time | 2 | - | - | 69.167 (48.446-89.888) | < 0.001 | 1 | - | - | 82.700 (67.578-97.822) | < 0.001 |
| Additional surgery | 2 | 13% (0.07-0.21) | 5% (0.02-0.09) | 3.139 (1.360-7.243) | 0.007 | - | - | - | - | - |
| Perforation | 5 | 4% (0.01-0.07) | 0% (0.00-0.01) | 5.235 (2.123-12.910) | < 0.001 | 3 | 7% (0.05-0.10) | 1% (0.00-0.03) | 4.546 (1.674-12.346) | 0.003 |
| Bleeding | 4 | 3% (0.00-0.07) | 3% (0.01-0.05) | 0.895 (0.438-1.829) | 0.762 | 3 | 3% (0.00-0.08) | 1% (0.00-0.04) | 2.233 (0.489-10.197) | 0.300 |
| Recurrences | 5 | 1% (0.00-0.02) | 7% (0.02-0.15) | 0.204 (0.097-0.429) | < 0.001 | 3 | 3% (0.00-0.10) | 27% (0.10-0.20) | 0.179 (0.032-0.990) | 0.049 |
ESD in Japan had an en bloc resection rate of 89% (95%CI: 0.77 to 0.97), perforation risk of 4% (95%CI: 0.01 to 0.07), bleeding risk of 2.4% (95%CI: 0.01 to 0.04) and recurrence rate of 1% (95%CI: 0.01 to 0.02) while its EMR had an en bloc resection rate of 53% (95%CI: 0.38 to 0.67), perforation risk of 0.0002% (95%CI: 0.00 to 0.00081), bleeding risk of 2.1% (95%CI: 0.01 to 0.03) and recurrence rate of 7% (95%CI: 0.02 to 0.15). Comparing between the two techniques in Japan, ESD had higher en bloc resection rate (RR = 1.658, 95%CI: 1.270 to 2.165, P < 0.001), perforation risk (RR = 9.586, 95%CI: 4.425 to 20.768, P < 0.001) and bleeding risk (RR = 1.267, 95%CI: 1.174 to 1.367, P < 0.001) than EMR. Following sensitivity analysis, similar results were obtained, except ESD had lower recurrence rate than EMR (RR = 0.204, 95%CI: 0.097 to 0.429, P < 0.001), and there was no difference in bleeding risk (RR = 0.895, 95%CI: 0.438 to 1.829, P = 0.762) between the two techniques.
With regards to studies from the rest of the world, ESD had an en bloc resection rate of 90% (95%CI: 0.85 to 0.94), perforation risk of 8% (95%CI: 0.05 to 0.12), bleeding risk of 5% (95%CI: 0.02 to 0.11) and recurrence rate of 3% (95%CI: 0.00 to 0.08) while EMR had an en bloc resection rate of 41% (95%CI: 0.22 to 0.61), perforation risk of 1% (95%CI: 0.00 to 0.02), bleeding risk of 3% (95%CI: 0.01 to 0.06) and recurrence rate of 16% (95%CI: 0.04 to 0.32). Comparing between the two techniques, ESD had higher en bloc resection rate (RR = 2.201, 95%CI: 1.411 to 3.433, P = 0.001), perforation rate (RR = 4.602, 95%CI: 2.729 to 7.759, P < 0.001) and lower recurrence rate (RR = 0.245, 95%CI: 0.073 to 0.819, P = 0.022) than EMR. Following sensitivity analysis, similar results were obtained.
The advancements in endoscopic resection techniques have resulted in the shift from radical surgery to minimally invasive and organ-sparing endoscopic resection techniques, such as ESD and EMR, for the treatment of colorectal lesions. With reference to 2015 ESGE guidelines, ESD should be considered for colorectal lesions larger than 20 mm, with high suspicion of submucosal invasion, or those where en bloc resection by EMR are not feasible[5]. Previous meta-analyses comparing ESD and EMR for colorectal polyps primarily reported data from Asian countries, with 72.7% of the published studies from Japan[6]. Since then, several retrospective and prospective studies comparing ESD and EMR for the treatment of colorectal polyps have been published outside of Japan. The present study is the most extensive meta-analysis evaluating the surgical, histological, and oncological outcomes of ESD in comparison to EMR in the treatment of colorectal polyps. Nine out of 21 studies (42.9%) on colorectal polyps included in this meta-analysis were conducted in countries outside of Japan. While ESD has been known to provide significantly better resection outcomes and lower recurrence rate, our analysis found that polyp size and depth of invasion did not significantly influence the en bloc and complete resection rate, bleeding and perforation risk, and recurrence rate in colorectal polyps that was not previously reported. Additionally, previous reviews were confined only to sessile lesions larger than 20 mm[6,7]. Our analysis also showed the increased proficiency in performing ESD and EMR in Japan as compared to the rest of the world.
Consistent with previous studies, ESD showed benefits in the technical, histological, and oncological outcomes. Pooled analysis showed higher rates of en bloc resection and complete resection in ESD than in EMR albeit significant publication bias (P = 0.0025). En bloc resection offers the technical advantage of removing the entire pathologic specimen, thus allowing for detailed histologic evaluation. This results in an increase in the complete resection rate which in turn reduces the recurrence rate. Therefore, en bloc resection with ESD is favored as it provides curative treatment without the need for surgery for lesions with significant likelihood of submucosal invasion[38]. However, the advantages of ESD come at the expense of longer procedural time, additional surgical operations, and increased perforation risk compared to EMR[6]. The high rate of additional surgical operations for ESD is presumed to be due to the aggressive selection of ESD for T1 Lesions. Although the perforation risk was higher with ESD, most perforations in the studies included were treated conservatively or endoscopically using endoclips[9,12,31,33,34,37].
Meta-regression was performed to assess the risk factors that affect surgical, histological, and oncological outcomes. Our present analysis showed that polyp size did not affect the risk of perforation, which was reported otherwise in studies by Kim et al[39] and Hong et al[40] Polyp size and depth of invasion were also not associated with significant change in en bloc and complete resection rate, risk of bleeding, and recurrence between ESD and EMR. Furthermore, the en bloc resection rate (before RR = 1.837, 95%CI: 1.464 to 2.305, P < 0.001; after RR = 1.932, 95%CI: 1.389 to 2.688, P < 0.001) and recurrence rate (before RR = 0.269, 95%CI: 0.112 to 0.648, P = 0.003; after RR = 0.191, 95%CI: 0.085 to 0.431; P < 0.001) appeared comparable before and after the sensitivity analysis to ≥ 20 mm polyps. Instead, the risk of perforation was increased in patients with right colonic polyps and this was consistent with previous study which identified the technical difficulty and proficient endoscopic skills required to remove polyps from right colon safely[41]. As such, training should ensure endoscopists achieve procedural proficiency in left sided lesions before proceeding to attempt right sided lesions. Other factors including polyp size and depth of invasion are less important criteria when deciding between EMR and ESD in skilled tertiary centers.
Despite the advantages of ESD, the data regarding the efficacy of colorectal ESD have been inconsistent and vary between Japan and the rest of the world. One of the reasons is the limitations to the implementation of ESD in other countries, which are in part due to the lack of expertise and training centers. To date, no meta-analysis comparing ESD and EMR between Japan and the rest of the world have been performed. Our single arm meta-analysis found that Japan performed better than the rest of the world in ESD and EMR. Significantly, perforation is a major concern in ESD. The perforation risk of ESD and EMR was 4% and 0.0002%, respectively in Japan, and 8% and 1%, respectively in the rest of the world. While there is an observed increase risk of perforation from Japan only studies (RR = 9.586) compared to the rest of the world (RR = 4.602), even after sensitivity analysis, the risk of perforation for ESD was only statistically higher in Japan only studies due to the very low risk of perforation in EMR in Japan. In addition, the challenges of doing ESD in difficult could have resulted in the higher perforation rate in ESD compared with EMR in Japan. Another important factor to consider is the recurrence rate of ESD and EMR which was 1% and 7%, respectively in Japan and 3% and 27%, respectively in the rest of the world following sensitivity analysis. The observed increase in recurrence rate from Japan only studies (RR = 0.204) compared to the rest of the world (RR = 0.179) was due to the much lower recurrence rate of EMR in Japan as compared to the rest of the world. Overall, our results seem to favor studies from Japan and are in tandem with a single arm analysis of ESD only procedures with subgroup on region efficacy[42]. However, most of the studies originating from the rest of the world should be interpreted with the understanding that these studies are mainly from tertiary centers and the results may not be generalized to non-tertiary centers.
The potential of ESD resection is limited by the difficulty in conducting the procedure as the length of procedure for ESD even when performed by experienced endoscopists can be three times longer than that for EMR[43]. However, the advancement in endoscopic resection equipment has been shown to shorten the procedure time despite a relatively short training duration[44]. Using the cumulative sum method, Miyakawa et al[44] recently reported the use of Stag-Beetle Knife Jr for ESD in a Japan single-center study generated good learning curve to achieve satisfactory resection speed (min/cm2), which allowed the acquisition of proficient and safe skills within 120 cases[44]. Other alternatives to ESD do exist, such as hybrid ESD and pre-cut EMR. This hybrid approach has been shown to have lower en bloc resection rate (68.4% vs 91.0%) and complete resection rate (60.6% vs 82.9%) than conventional ESD[42]. Currently, underwater EMR has been thought to be a safe and effective method with higher rate of en bloc resection and lower rate of recurrence[45], but no head-to-head comparisons have been done between UMER and ESD.
The inclusion of 21 studies with a total of 281344 polyps based on our search strategy and inclusion criteria represents the most extensive meta-analysis on this issue. However, as no randomized controlled trials comparing the performance between EMR and ESD have yet been conducted, this highlights the need for a randomized study to better understand the efficacy and safety of these techniques in the management of colorectal polyps. The evaluation of heterogeneity allowed us to compare the significant differences in the performance of ESD and EMR between Japan and the rest of the world.
Our meta-analysis has some limitations. While we aimed to decrease heterogeneity, the included articles used a variety of EMR techniques including standard EMR, piecemeal EMR, EMR with small incision, EMR-precutting and EMR-circumferential incision. This, however, was an acceptable confounding factor in previous meta-analysis analysis. Also, a major factor that we were unable to regress for was the procedural skills of each centers. ESD and EMR are largely operator dependent and we were only able to account for it in a subgroup analysis comparing between studies conducted in Japan and the rest of the world.
Evidence from this meta-analysis suggests that with appropriate training, ESD is preferred over EMR as the first-line therapy for resection of colorectal polyps, without restricting to lesions greater than 20 mm and those with high suspicion of submucosal invasion. Our overall findings are consistent with previous meta-analyses showing ESD is associated with higher rate of en bloc and complete resection, and lower recurrence compared to EMR, but at the cost of increased procedural time, need for additional surgical operations and perforation risk. This is coupled with the new finding that confounders including polyp size and invasion depth did not influence the rates of en bloc and complete resection, bleeding risk, and recurrence.
Endoscopic submucosal dissection (ESD) has shown to be effective in management of colorectal neoplasm in the Asian countries, while its implementation in Western countries where endoscopic mucosal resection (EMR) is preferred is still debatable.
Previous meta-analyses and systematic reviews comparing ESD with EMR included studies mainly conducted in Asia, with limited data coming from outside of Japan and did not control for confounders or lesions smaller than 20 mm.
To compare the outcomes of ESD and EMR in the treatment of colorectal polyps, with subgroup analysis comparing the efficacy of these two techniques between Japan and the rest of the world.
Embase and Medline databases were searched in accordance with PRISMA guidelines for studies comparing en bloc, complete resection, margin involvement, resection time, need for additional surgery, complications, and recurrence rate of ESD with EMR in patients with colorectal lesions.
ESD was associated with better resection outcomes and lower recurrence rate when compared to EMR. Meta-regression analysis suggested only right colonic polyps correlated with an increased perforation risk in ESD, while confounders including polyp size and invasion depth did not significantly influence the resection outcomes, bleeding risk and recurrence. Subgroup analysis showed that Japan performed better than the rest of the world in both ESD and EMR with lower perforation risk.
This meta-analysis suggests that with appropriate training, ESD is preferred over EMR as the first-line therapy for resection of colorectal polyps, without restricting to lesions greater than 20 mm and those with high suspicion of submucosal invasion. Increased proficiency in performing ESD and EMR was shown in Japan as compared with the rest of the world.
This highlights the need to establish adequate training programs for colorectal ESD to be performed effectively. A randomized controlled trial is necessary to better understand the efficacy and safety of these techniques in the management of colorectal polyps.
We would like to thank Dr. Hiroaki Ikematsu of the Division of Science and Technology for Endoscopy, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center for his advice on the writing of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Protopapas AA S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3116] [Article Influence: 97.4] [Reference Citation Analysis (1)] |
| 2. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2269] [Article Influence: 174.5] [Reference Citation Analysis (1)] |
| 3. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3231] [Article Influence: 646.2] [Reference Citation Analysis (2)] |
| 4. | Yamamoto H, Yube T, Isoda N, Sato Y, Sekine Y, Higashizawa T, Ido K, Kimura K, Kanai N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 923] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 6. | Arezzo A, Passera R, Marchese N, Galloro G, Manta R, Cirocchi R. Systematic review and meta-analysis of endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal lesions. United European Gastroenterol J. 2016;4:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, Bhandari P, Boeri F, Conio M. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol. 2016;104:138-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 8. | Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Kim YJ, Kim ES, Cho KB, Park KS, Jang BK, Chung WJ, Hwang JS. Comparison of clinical outcomes among different endoscopic resection methods for treating colorectal neoplasia. Dig Dis Sci. 2013;58:1727-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Acquistapace F, Maternini F, Snider L, Bellini O, Moglia P, Capretti P. Endoscopic treatment of superficial colorectal neoplasms. Retrospective analysis of a single center technique and results. G Chir. 2015;36:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Emmanuel A, Gulati S, Burt M, Hayee B, Haji A. Combining eastern and western practices for safe and effective endoscopic resection of large complex colorectal lesions. Eur J Gastroenterol Hepatol. 2018;30:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Soliman H, Brieau B, Guillaumot MA, Leblanc S, Barret M, Camus M, Dior M, Terris B, Coriat R, Prat F, Chaussade S. Invasive pit pattern, macronodule and depression are predictive factors of submucosal invasion in colorectal laterally spreading tumours from a Western population. United European Gastroenterol J. 2018;6:1569-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Urban O, Falt P, Fojtik P, Andelova R. Comparison of endoscopic mucosal resection and endoscopic submucosal dissection in the treatment of flat neoplastic lesions in the rectum. Gastroenterol Hepatol. 2018;72:193-198. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Jung JS, Hong JY, Oh HH, Kweon SS, Lee J, Kim SW, Seo GS, Kim HS, Joo YE. Clinical outcomes of endoscopic resection for colorectal laterally spreading tumors with advanced histology. Surg Endosc. 2019;33:2562-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Liu C, Wu R, Sun X, Tao C, Liu Z. Risk factors for delayed hemorrhage after colonoscopic postpolypectomy: Polyp size and operative modality. JGH Open. 2019;3:61-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Benites Goñi H, Palacios Salas F, Marin Calderón L, Bardalez Cruz P, Vásquez Quiroga J, Alva Alva E, Calixto Aguilar L, Alférez Andía J, Dávalos Moscol M. Closure of colonic deep mural injury and perforation with endoclips. Rev Esp Enferm Dig. 2020;112:772-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 46803] [Article Influence: 2925.2] [Reference Citation Analysis (0)] |
| 18. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 6847] [Article Influence: 622.5] [Reference Citation Analysis (0)] |
| 19. | Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1058] [Cited by in RCA: 1601] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 20. | Rousseau MJ, Evans JC. Key statistical assumptions and methods in one-arm meta-analyses with binary endpoints and low event rates, including a real-life example in the area of endoscopic colonic stenting. Cogent Med. 2017;4:1334318. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. Metan: Fixed- and random-effects meta-analysis. Stata J. 2008;8:3-28. [DOI] [Full Text] |
| 22. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30211] [Article Influence: 774.6] [Reference Citation Analysis (0)] |
| 23. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40075] [Article Influence: 1431.3] [Reference Citation Analysis (2)] |
| 25. | Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, 2012. [cited 10 January 2021]. Available from: http://www.ohri.ca/Programs/clinical_epidemiology/oxford.asp. |
| 26. | Taku K, Sano Y, Fu KI, Saito Y, Matsuda T, Uraoka T, Yoshino T, Yamaguchi Y, Fujita M, Hattori S, Ishikawa T, Saito D, Fujii T, Kaneko E, Yoshida S. Iatrogenic perforation associated with therapeutic colonoscopy: a multicenter study in Japan. J Gastroenterol Hepatol. 2007;22:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy. 2007;39:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Iizuka H, Okamura S, Onozato Y, Ishihara H, Kakizaki S, Mori M. Endoscopic submucosal dissection for colorectal tumors. Gastroenterol Clin Biol. 2009;33:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S31-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Oka S, Tanaka S, Kanao H, Ishikawa H, Watanabe T, Igarashi M, Saito Y, Ikematsu H, Kobayashi K, Inoue Y, Yahagi N, Tsuda S, Simizu S, Iishi H, Yamano H, Kudo SE, Tsuruta O, Tamura S, Cho E, Fujii T, Sano Y, Nakamura H, Sugihara K, Muto T. Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc. 2010;22:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T. Clinical outcome of endoscopic submucosal dissection vs endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 32. | Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, Hara K, Hijioka S, Imaoka H, Ogura T, Haba S, Yamao K. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol. 2011;23:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Kobayashi N, Yoshitake N, Hirahara Y, Konishi J, Saito Y, Matsuda T, Ishikawa T, Sekiguchi R, Fujimori T. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol. 2012;27:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Sagawa T, Kakizaki S, Iizuka H, Onozato Y, Sohara N, Okamura S, Mori M. Analysis of colonoscopic perforations at a local clinic and a tertiary hospital. World J Gastroenterol. 2012;18:4898-4904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Terasaki M, Tanaka S, Oka S, Nakadoi K, Takata S, Kanao H, Yoshida S, Chayama K. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol. 2012;27:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Niikura R, Yasunaga H, Yamada A, Matsui H, Fushimi K, Hirata Y, Koike K. Factors predicting adverse events associated with therapeutic colonoscopy for colorectal neoplasia: a retrospective nationwide study in Japan. Gastrointest Endosc. 2016;84:971-982.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 37. | Sato T, Fukuzawa M, Gotoda T, Moriyasu F. Comparison of clinical outcomes between colorectal EMR and ESD. J Tokyo Med Univer. 2016;74:154-162. |
| 38. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara KI, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 430] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 39. | Kim ES, Cho KB, Park KS, Lee KI, Jang BK, Chung WJ, Hwang JS. Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy. 2011;43:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Hong SN, Byeon JS, Lee BI, Yang DH, Kim J, Cho KB, Cho JW, Jang HJ, Jeon SW, Jung SA, Chang DK. Prediction model and risk score for perforation in patients undergoing colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2016;84:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 41. | Nishiyama H, Isomoto H, Yamaguchi N, Fukuda E, Ikeda K, Ohnita K, Mizuta Y, Nakamura T, Nakao K, Kohno S, Shikuwa S. Endoscopic submucosal dissection for colorectal epithelial neoplasms. Dis Colon Rectum. 2010;53:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, Frazzoni L, Bhandari P, Bellisario C, Bazzoli F, Repici A. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74-86.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 43. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 266] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 44. | Miyakawa A, Kuwai T, Sakuma Y, Kubota M, Nakamura A, Itobayashi E, Shimura H, Suzuki Y, Shimura K. Learning curve for endoscopic submucosal dissection of early colorectal neoplasms with a monopolar scissor-type knife: use of the cumulative sum method. Scand J Gastroenterol. 2020;55:1234-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Choi AY, Moosvi Z, Shah S, Roccato MK, Wang AY, Hamerski CM, Samarasena JB. Underwater vs conventional EMR for colorectal polyps: systematic review and meta-analysis. Gastrointest Endosc. 2021;93:378-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |