Published online Jun 21, 2021. doi: 10.3748/wjg.v27.i23.3342
Peer-review started: February 4, 2021
First decision: February 24, 2021
Revised: March 5, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: June 21, 2021
Processing time: 134 Days and 2.7 Hours
Inflammatory bowel diseases (IBD) is related to uncontrolled immune response. Currently, there is no successful treatment for significant improvement in IBD. Stem cells display their therapeutic effects through their repopulating capacity or secreting factors.
To investigate the effects of conditioned mouse adipose-derived stem cells (mADSCs) secretome on colitis-induced mice.
mADSCs were isolated from adipose tissue of C57BL/6 mice. Conditioned mADSCs secrectome was obtained by culturing of mADSCs with lipopolysaccharides (LPS, 1 μg/mL) for 24 h. Acute colitis was induced by 2% dextran sulfate sodium (DSS) drinking water for 7 d and then normal drinking water for 4 d. The mice were treated with normal culture medium (NM group), conditioned mADSCs secretome (CM group) or mADSCs (SC group). The length of colon and histopatholgy of colon tissues were evaluated. The mRNA expression levels of inflammatory cytokines in colon tissue and the serum interleukin (IL)-6 levels were determined.
The isolated mADSCs maintained the mADSCs specific gene expression profiles during experiment. The conditioned mADSCs secretome released by the treatment of mADSCs with LPS contained mainly inflammatory chemokines, colony-stimulating factors and inflammatory cytokines. The loss of body weight and reduction in colon length were ameliorated in the CM group. The conditioned mADSCs secretome reduced the histological score in colon tissue. The expression of IL-1b and IL-6 mRNAs in colon tissues significantly inhibited in the CM group compared to SC group and NM group, respectively. The elevation of serum IL-6 levels was also ameliorated in the CM group. These results indicate that the conditioned mADSCs secretome suppressed the synthesis of inflammatory cytokines in damaged colon tissue and the elevation of serum IL-6 concentration in DSS-induced mice
Conditioned mADSCs secretome might play regenerative roles by the suppression of IL-6 in serum and tissue during acute colitis, and may be more effective than stem cells themselves in the regeneration of colon tissue.
Core Tip: The therapeutic ability of mesenchymal stem cells (MSCs) is mostly mediated by their paracrine effects. Cell free therapy using MSCs secretome could be more promising strategy than stem cells based therapy. The present study demonstrates that the conditioned secretome of adipose–derived stem cells (ADSCs) has more efficient effects for improving acute colitis in dextran sulfate-sodium-induced mouse model. The effects by the conditioned secretome of ADSCs were mediated by suppression of interleukin (IL)-6 mRNA synthesis in colon tissue and serum IL-6 protein levels, which suggests that the stem cell secretome may have efficient therapeutic potential for incurable inflammatory bowel diseases.
- Citation: Lee S, Heo J, Ahn EK, Kim JH, Kim YH, Chang HK, Lee SJ, Kim J, Park SJ. Conditioned secretome of adipose-derived stem cells improves dextran sulfate sodium-induced colitis in mice. World J Gastroenterol 2021; 27(23): 3342-3356
- URL: https://www.wjgnet.com/1007-9327/full/v27/i23/3342.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i23.3342
Inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn’s disease (CD), are multifactorial disorders characterized by chronic inflammation, visceral hypersensitivity and diarrhea in the gastrointestinal tract[1]. Although the etiology of IBD remains unknown, it is well accepted that IBD is related to a dysregulated immune response, genetic susceptibility, and the environment[2]. Uncontrolled production of inflammatory cytokines and chemokines by infiltrating immune cells ultimately lead to damage of colon tissue. Current treatments for IBD are intended to control the inflammatory intestinal process using immunosuppressive agents; however, these current treatments are not entirely effective to maintain remission of intestinal inflammation and can result in multiple adverse effects due to their inefficacy or toxicity[3]. The use of certolizumab perol in patients with moderate-to-severe CD triggered a mild improvement in therapeutic response rates but no significant improvement in remission rates[4]. Therefore, it is necessary to develop novel therapeutic approaches that are effective, feasible and safe for IBD patients.
Stem cells are immature tissue precursor cells that have the ability to self-renew and to differentiate into various cell lineages[5]. Due to these properties, stem cells have emerged as attractive therapeutic tools for incurable diseases such as IBD[6]. Mesenchymal stem cells (MSCs) are mesoderm-derived fibroblast-like stem cells that have been isolated from various adult tissues including adipose tissue[7], skin[8], muscle[9], and peripheral blood[10]. The characteristics of MSCs are identified by the adherence of cells to plastic dishes in standard culture conditions, the expression profiles of specific cell surface molecules and their differentiation potential into osteogenic, chondrogenic and adipogenic lineage[11,12]. Besides their differentiation potential, MSCs also regulate the immune response including in vitro suppression of T-cell proliferation, B-cell function and dendritic cell maturation[13,14]. The three main actions of MSCs with regard to therapeutic potential are their homing action for migration of MSCs into damaged sites[15], differentiation action for replacing damaged tissues[16], and paracrine actions for secreting bioactive factors[17]. Generally, the protocol of stem cell therapy requires hundreds of millions of MSCs per treatment. However, the overall quantity of MSCs in the body is scarce, and in vitro cell expansion is necessary for obtaining enough cells before implantation. In vitro manipulation of MSCs for expansion or differentiation affects the quality of the cells such as their senescence, differentiation capacity and survival of the administered MSCs in vivo, which may influence homing and engraftment of MSCs into injured sites[18,19]. Although MSCs have become a promising therapeutic strategy for IBD because of their immunosuppressive and tissue healing ability[20], MSCs transplantation in IBD patients had yield inconsistent results, which may be due to the different sources of MSCs with distinct differentiation and regenerative potential, and the variety of protocols for treatments[21].
It has been known that the therapeutic ability of MSCs is mostly mediated by their paracrine effects. MSCs secrete a variety of protective bio-active factors (it is called secretome) such as cytokines, chemokines, growth factors, lipid mediators, hormones, and exosomes[22], which play important roles in the cross-talk between cells and the surrounding tissues for tissue repair and regeneration by their paracrine actions[23]. Many studies have shown that a range of bioactive factors play an important role in the modulation of the immune response. interleukin (IL)-6 and IL-10 secreted by MSCs inhibited the differentiation of macrophage into dendritic cells[24]. Therefore, MSCs secretome has received attention for concerning its potential use in tissue repair and regeneration[25]. The use of MSCs secretome as cell-free therapy may provide several advantages over direct stem cell-based therapy such as safety associated with immunocompatibility and tumorigenicity by stem cells, evaluation for dosage in a manner analogous to conventional pharmaceutical agents, storage for a long time without loss of potency, avoidance of invasive cell collection procedures, and mass production with less time and cost[26,27]. The effectiveness of MSCs secretome as therapeutic applications has been demonstrated in a variety of diseases such as colitis, gastric mucosal injury, osteoarthritis, spinal cord injury and cardiovascular disease[28-31]. It has been suggested that the therapeutic effectiveness of MSCs secretome has been attributed to the mixture of bioactive factors with their attendant paracrine activities[32]. These studies suggest that cell free therapy using the MSCs secretome could be more promising strategy than stem cell based therapy in regenerative medicine. Therefore, the purpose of this study was to investigate whether the conditioned mouse adipose-derived stem cells (ADSCs) secretome has a regenerative effect on damaged colon tissue in an acute colitis mouse model. The results showed that the conditioned mouse ADSCs (mADSCs) secretome recovered the colon tissue damaged by acute colitis in the mouse model. Moreover, the effects of the conditioned ADSCs secretome were more potent than those of ADSCs themselves in the regeneration of damaged colon tissue in acute colitis.
C57BL/6 mice (female, 8-10 wk-old) were purchased from the ‘KOATECH’ laboratory animal company. The animals were kept in in pathogen-free facility at controlled conditions (20-23 °C, 12 h/12 h light/dark cycle, 50% humidity) with free access of sterilized regular mouse chow and drinking water. Mice were housed 2 wk before the experiment for adaptation. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee at Kosin University College of Medicine (IACUC protocol No. Kosin15-12).
mADSCs were isolated from subcutaneous, inguinal, epididymal and mesenteric fat of mice. Briefly, animals were euthanized with using CO2. Fresh adipose tissues were washed with phosphate-buffered saline (PBS; Hyclone, Logan, UT, United States) and minced for 5 min with the sterilized fine scissors. The minced adipose tissues were digested with 0.1% collagenase type I (Invitrogen, Carlsbad, CA, United States) for 40 min at 37 °C in a shaking bath and then centrifuged at 260 × g for 7 min to obtain a pellet. The pellet was incubated in a 1:1 ratio of culture medium (StemX VIVO; R&D systems, Minneapolis, MN, United States) and DMEM/F12 (Invitrogen) along with 5% FBS (Hyclone, Logan, UT, United States) and 1 × penicillin-streptomycin (Invitrogen) overnight at 37 °C and 5% CO2. The residual non-adherent red blood cells were removed by washing with the medium after 24 h. The cells grown until 80%-90% confluence were subcultured. The medium was changed every 2 d. For all experi
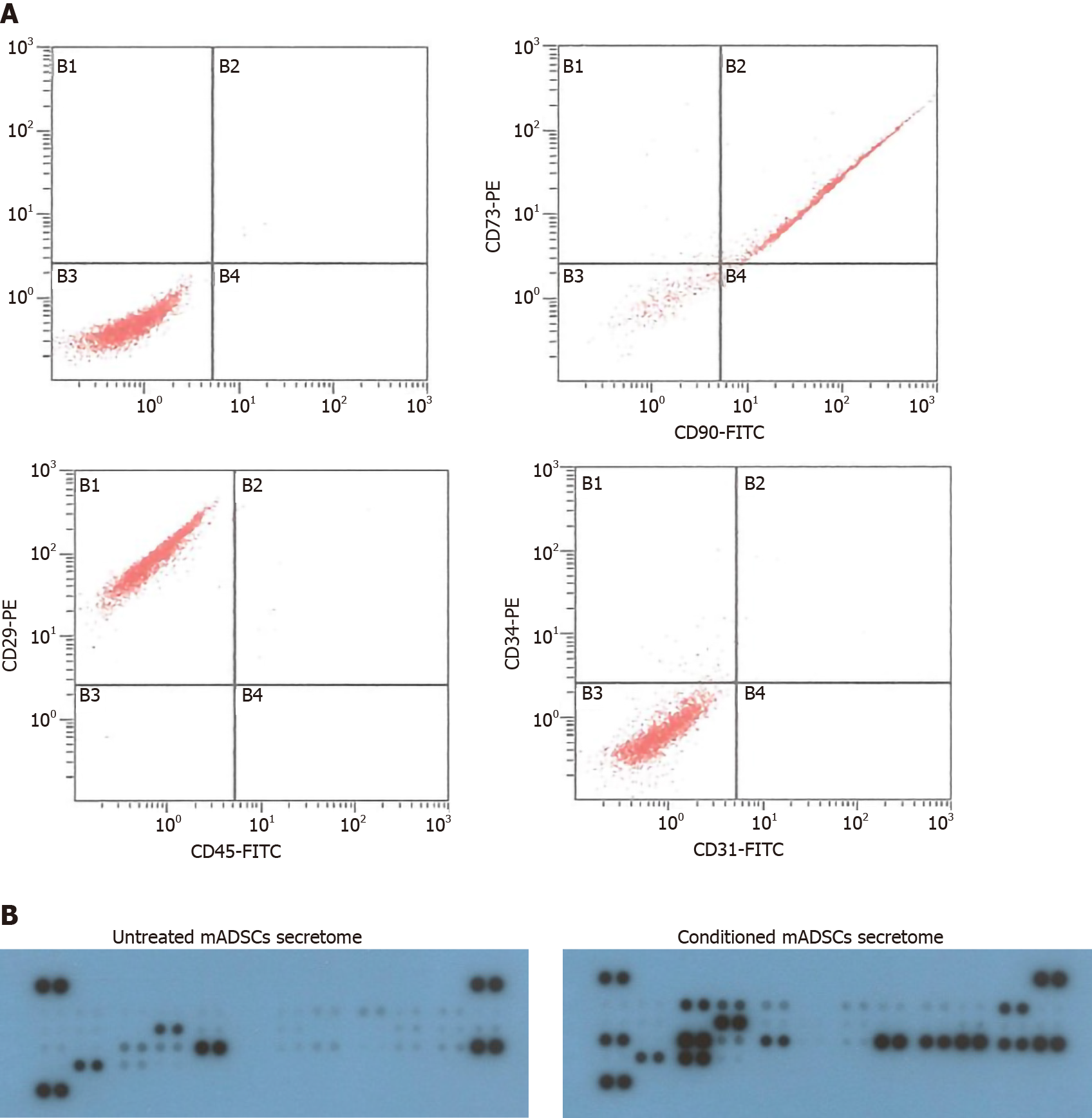
To identify the isolated mADSCs, fluorescence-activated cell sorting analysis was performed. Approximately 5 × 105 cells were incubated with the conjugated antibodies, CD29-PE, CD31-FITC, CD34-PE, CD45-FITC, CD73-PE and CD90-FITC (Beckman Coulter, Brea, CA, United States), in the dark for 30 min at room temperature. After washing with PBS twice, the cells were resuspended in PBS and analyzed using a flow cytometer (Beckman Coulter, Brea, CA, United States).
To prepare the mADSCs secretome, mADSCs (1 × 105 cells/well) were seeded onto 6-well plates in culture medium and incubated for 24 h. And then serum-free medium with or without 1 μg/mL lipopolysaccharides (LPS; Sigma, Saint. Louis, MO, United States) replaced the culture medium of the cells. After 24 h, the supernatants were filtered using a 0.45 μm sieve and frozen in -80 °C. To concentrate the supernatants, the frozen supernatants were lyophilized using the lyophilizer (SFDSM12; SamWon Freezing Engineering Co., Busan, South Korea). The lyophilized powders were resuspended in PBS solution, transferred into Amicon Ultra-15 Centrifugal Filter Devices (3000 MWCO; Millipore, Burlington, MA, United States) and then centrifuged at 4000 × g for 50 min in 4 °C condition. The aliquots of concentrated mADSCs secretome were stored in -80 °C deep freezer until use. The conditioned mADSCs secretome stimulated with LPS were used for the animal experiment. Untreated mADSCs secretome were used as a control for the analysis of cytokine antibody array.
To characterize the mADSCs secretome, cytokine antibody array was performed with a Proteome Profiler mouse cytokine array kit (R&D systems), following the manufacturer instructions. Briefly, the mADSCs secretome were mixed with reconstituted detection antibody cocktail and incubated at room temperature for 1 h. Then the mixture of sample/antibody was incubated with the supplied membrane overnight at 4 °C on a rocking platform. After washing the membrane in wash buffer for 10 min on a rocking platform, Streptavidin-horse radish peroxidase (HRP) was added to the membrane and incubated for 30 min at room temperature. After washing, the membrane was labeled with Chemi Reagent Mix and exposed to X-ray film. The intensity of each spot was analyzed using Gel Documentation System Software (FluorChem HD2; Alpha Innotech, Santa Clara, CA, United States). The intensity of each spot was normalized with the intensity of positive reference spot on each membrane. The normalized intensity values were used to compare the cytokine profiles in untreated mADSCs secretome and the conditioned mADSCs secretome used in this study.
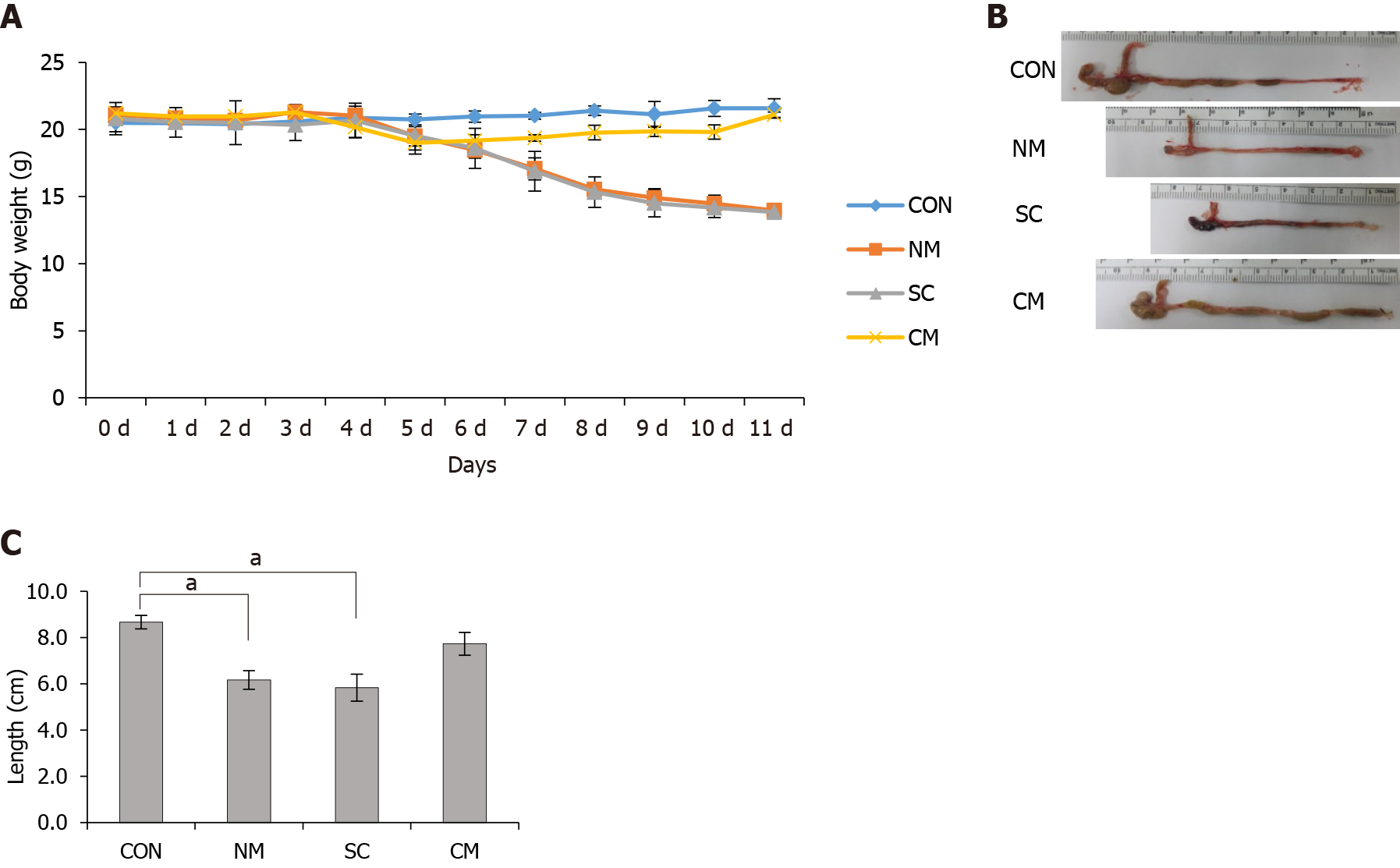
To induce acute colitis, C57BL/6 mice (female, 8 wk old) were supplied with 2% dextran sodium sulfate (DSS; MW = 36000-50000; MP Biomedicals, Solon, OH, United States) for 7 d and then with normal drinking water for 4 d. Animals were divided into 4 groups, control (CON) (n = 4), normal culture medium (NM) (n = 6), mADSCs (SC) (n = 6) and conditioned mADSCs secretome (CM) (n = 6) groups. Animals in CON group received normal drinking water during the experimental period, and animals in the NM, SC and CM groups received 2% DSS with intraperitoneal injection of 100 μL nomal medium solution three times (on 4, 6 and 8 d; NM group), 1 × 105 cells/100 μL ADSCs once (on 4 d; SC group) or 100 μL mADSCs secretome three times (on days 4, 6, and 8; CM group), and then received normal drinking water ad libitum. Animals were monitored daily for the body weight, the appearance of diarrhea, and the presence of blood in the stool. The length of the colon was measured after sacrificing animals on 11 d.
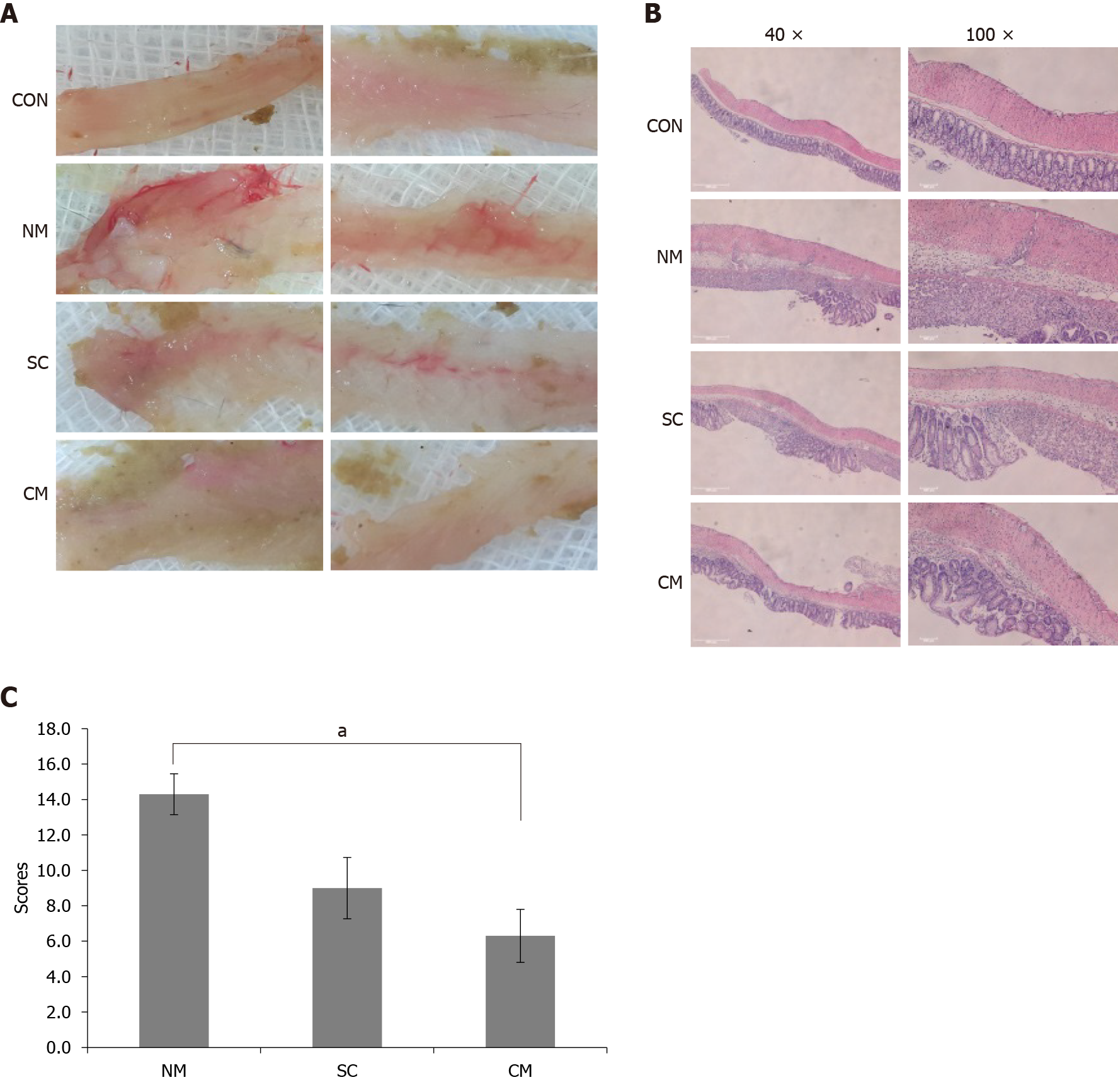
To evaluate the histopathology of colon tissues, colons were incised longitudinally, fixed with 10% buffered formalin and embedded in paraffin. The paraffin-embedded colon tissue sections (4 μm-thickness) were stained with hematoxylin-eosin (H&E) to evaluate the damage of colon tissues. The damage of colon tissues was determined by four histological scoring parameters including inflammation severity (score 0-3), inflammation extent (score 0-3), tissue injury (score 0-4) and crypt damage (score 0-4) as described previously[33]. The histological score was defined as the sum of the four parameter scores.
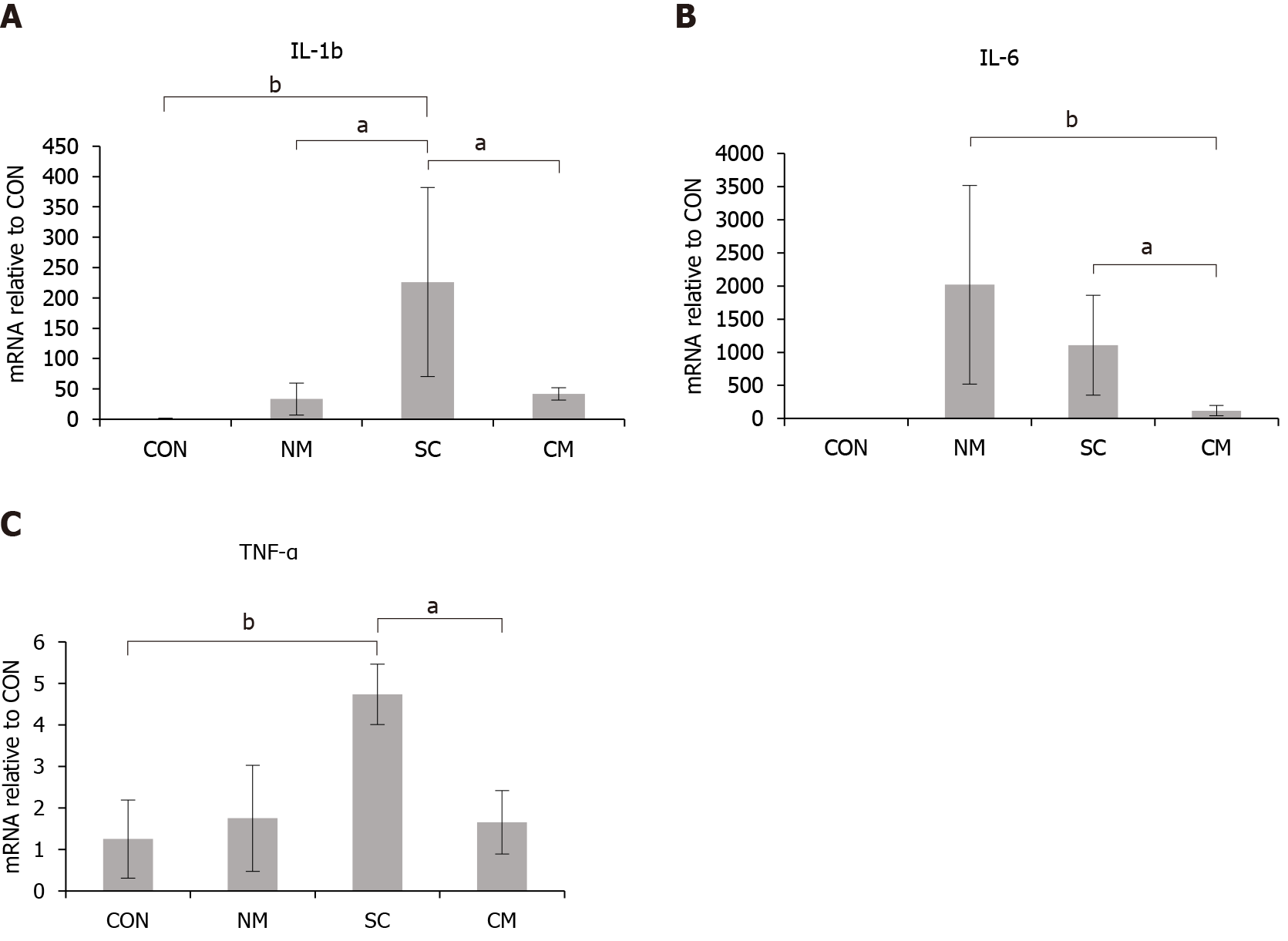
To determine the expression levels of inflammatory cytokines in colon tissue, total RNA was extracted from each colon segment using Trizol reagent (Invitrogen). After assessing the quality and concentration, 3 μg of total RNA was subjected to cDNA synthesis using the TOPscript™ cDNA synthesis Kit (Enzynomics, Daejeon, Korea). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using Power SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, United States) and 500 nM of each primer as follows: IL-1b, F: 5’-GAAATGCCACCTTTTGACAGTG-3’, R: 5’-TGGATGCTCTCATCAGGACAG-3’; IL-6, F: 5’-CTGCAAGAGACTTCCATCCAG-3’. R: 5’-AGTGGTATAGACAGGTCTGTTGG-3’; tumor necrosis factor-α (TNF-α), F: 5’-CTGAACTTCGGGGTGATCGG-3’, R: 5’-GGCTTGTCACTCGAATTTTGAGA-3’; Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), F: 5’- TGGCCTTCCGTGTTCCTAC-3’, R: 5’-GAGTTGCTGTTGAAGTCGCA-3’. The following conditions were used: 95 °C/15 min, followed by 40 cycles of 95 °C/30 s, 60 °C/30 s, and 72 °C/30 s in the RT-PCR (7300 RT-PCR System; Applied Biosystems, Foster City, CA, United States). The levels of mRNA expression were normalized according to the internal control of the housekeeping gene GAPDH and represented as the levels of mRNA expression in NM, SC and The CM groups relative to those in CON group.
To determine the concentration of serum IL-6, blood was obtained from the inferior vena cava using a 21-gauge syringe and centrifuged at 100 × g for 3 min, then serum was collected and stored at -20 °C until use. The concentration of IL-6 in serum was measured using mouse IL-6 enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go (eBiosciences, San Diego, CA, United States) according to the manufacturer's protocols. Briefly, an ELISA plate was coated with anti-mouse IL-6 and incubated overnight at 4 °C. After washing 3 times with wash buffer, the plate was blocked with diluent solution at room temperature for 1 h. Then, serum sample was added to the plate and incubated overnight at 4 °C. After washing, the plate was incubated with detection antibody at room temperature for 1 h and then with Avidin-HRP for 30 min. After incubating with TMB solution for 5 min, the reaction was stopped with stop solution. The absorbance was measured at 450 and 570 nm. The concentrations of serum IL-6 were determined with the standard curve and represented as pg/mL.
Data values were expressed as mean ± SEM for each group. Statistical analysis was performed with the Kruscal-Wallis test using SPSS version 18. Differences with P < 0.05 or P < 0.005 were considered to be statistically significant.
mADSCs were isolated from abdominal adipose tissue of 7 week-old C57BL/6 mice. To characterize the isolated mADSCs, flow cytometry analysis was performed to validate the surface antigens of mADSCs at passage 3 or 4. The isolated mADSCs were positive for CD90, CD73, and CD29, but negative for CD45, CD34. CD31 (Figure 1A). The expression profiles of the surface antigens of mADSCs were not changed during passages 3-7 (data not shown). Therefore, mADSCs at passage 3 through 5 were used for all experiments. These results indicate that the isolated mADSCs maintain the gene expression profile specific for mADSCs during the experiment.
Conditioned mADSCs secretome were prepared from the conditioned medium in which mADSCs were cultured with 1 μg/mL of LPS for 24 h and compared with mADSCs secretome cultured without LPS. Cytokine profiles in mADSCs secretome and the conditioned mADSCs secretome were determined using mouse cytokine array kit containing 40 kinds of cytokine antibody (Figure 1B). 21 antibodies out of 40 antibodies were detected as strong positive spots with more than 1000 intensity in the conditioned mADSCs secretome (Table 1). The conditioned mADSCs secretome contained a high amount of colony-stimulating factors [granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage-colony stimulating factor], inflammatory chemokines [(C-X-C motif) ligand (CXCL) 1, CXCL2, carbon tetrachloride (CCL)3, CCL5, and CCL2] and inflammatory cytokines (TNF-α and IL-6). The amount of CCL4, G-CSF, CXCL2 and CXCL10 increased more than 10-folds in the conditioned mADSCs secretome compared to mADSCs secretome. These results indicate that the conditioned mADSCs secretome by the stimulation of LPS contains high amount of colony-stimulating factors, inflammatory chemokines and cytokines.
| Cytokine | mADSCs | MADSCs w/LPS | Ratio1 |
| CCL4 | 218 | 4516 | 20.8 |
| G-CSF | 218 | 4138 | 19.0 |
| CXCL2 | 467 | 4811 | 10.3 |
| CXCL10 | 433 | 4342 | 10.0 |
| GM-CSF | 335 | 3198 | 9.5 |
| CCL3 | 629 | 4986 | 7.9 |
| TNF-α | 836 | 5863 | 7.0 |
| IL-1ra | 515 | 3610 | 7.0 |
| CXCL1 | 1583 | 6263 | 4.0 |
| CCL1 | 307 | 1178 | 3.8 |
| CCL5 | 1137 | 4194 | 3.7 |
| CD54 | 243 | 796 | 3.3 |
| CXCL9 | 399 | 1180 | 3.0 |
| M-CSF | 1530 | 3147 | 2.1 |
| IL-6 | 3197 | 4642 | 1.5 |
| IFN-γ | 512 | 559 | 1.1 |
| CXCL12 | 4711 | 4798 | 1.0 |
| TIMP-1 | 3735 | 3553 | 1.0 |
| CCL2 | 5228 | 3646 | 0.7 |
| IL-1α | 852 | 482 | 0.6 |
To investigate the effects of conditioned mADSCs secretome in the DSS-induced colitis model of mice, mice in the NM, SC, and CM groups were treated with 2% DSS in drinking water from days 0 to 7 and then with normal drinking water thereafter until sacrifice. The NM mice and CM mice were injected intraperitoneally on days 4, 6, and 8 with normal medium and conditioned mADSCs secretome, respectively. SC mice were injected with mADSCs (5 × 105 cells/100 μL/mouse) through the tail vein on day 4. In all treatment groups, body weight started to decrease on day 5 (Figure 2A). The NM and SC groups kept the loss of body weights until sacrificed on day 11. However, the body weight in the CM group started to be recovered on day 7 and similar to body weight in untreated CON group on day 11. On day 11 mice were sacrificed and colon length was measured without tension from cecum to rectum (Figure 2B). Colon length in NM and SC groups significantly (P < 0.05) decreased compared to that in the CON group, but colon length in the CM were longer than that in NM and SC groups (Figure 2C). These results indicate that the conditioned mADSCs secretome improved the recovery of body weight and colon length in DSS-induced mice.
To investigate the effects of conditioned mADSCs secretome on the damage of colon tissues in DSS-induced mice, the damage of colon tissues was assessed by histological score using H&E stained colon tissue sections. To assess the overall damage to colon tissue, macroscopic appearances of the opened whole colon tissues were examined after sacrificing the mice on day 11 (Figure 3A). Colon tissues from the NM and SC groups showed striking hyperemia and a little hyperemia, respectively. Meanwhile, colon tissues from the CM group did not show hyperemia. In H&E stained tissues (Figure 3B), the CM group showed no loss of mucosa without crypt damage whereas the NM and SC groups showed severe loss of mucosa with crypt damage. The NM and SC group also showed the significant infiltration of immune cells, but the CM group showed a little infiltration of immune cells. The damage to colon tissues was assessed using histological scores determined by the severity of inflammation, tissue injury and crypt damage (Figure 3C). Histological scores in the CM group were significantly lower than in the NM group (14.4 ± 1.2 in the NM group vs 6.3 ± 1.5 in the CM group, P < 0.05). These results indicate that the conditioned mADSCs secretome improved the recovery of damaged colon tissue in DSS-induced mice.
The mRNA expression levels of inflammatory cytokines IL-1b, IL-6, and TNF-α were evaluated in colon tissues of DSS-induced mice on day 11 after treatment using qRT-PCR (Figure 4). The expression levels of mRNAs in all groups were expressed as the relative levels compared to the CON group. The expression of IL-1b mRNAs increased in the NM group, SC group and CM group compared to the CON group. The expression of IL-1b mRNA in the CM group was significantly lower (P < 0.05) than in the SC group. The expression of IL-6 mRNA increased in the NM group and SC group. The expression levels of IL-6 in the CM group were significantly lower than in both of the NM group (P < 0.005) and SC group (P < 0.05). The expression level of TNF-α mRNAs was significantly higher in the SC group than in the CON group (P < 0.005) and the CM group (P < 0.05). However, the expression level of TNF-α mRNAs in the NM and CM groups did not differ from that in the CON group. These results indicate that the conditioned mADSCs secretome suppressed the upregulation of IL-6 mRNA expression in damaged colon tissue of DSS-induced mice.
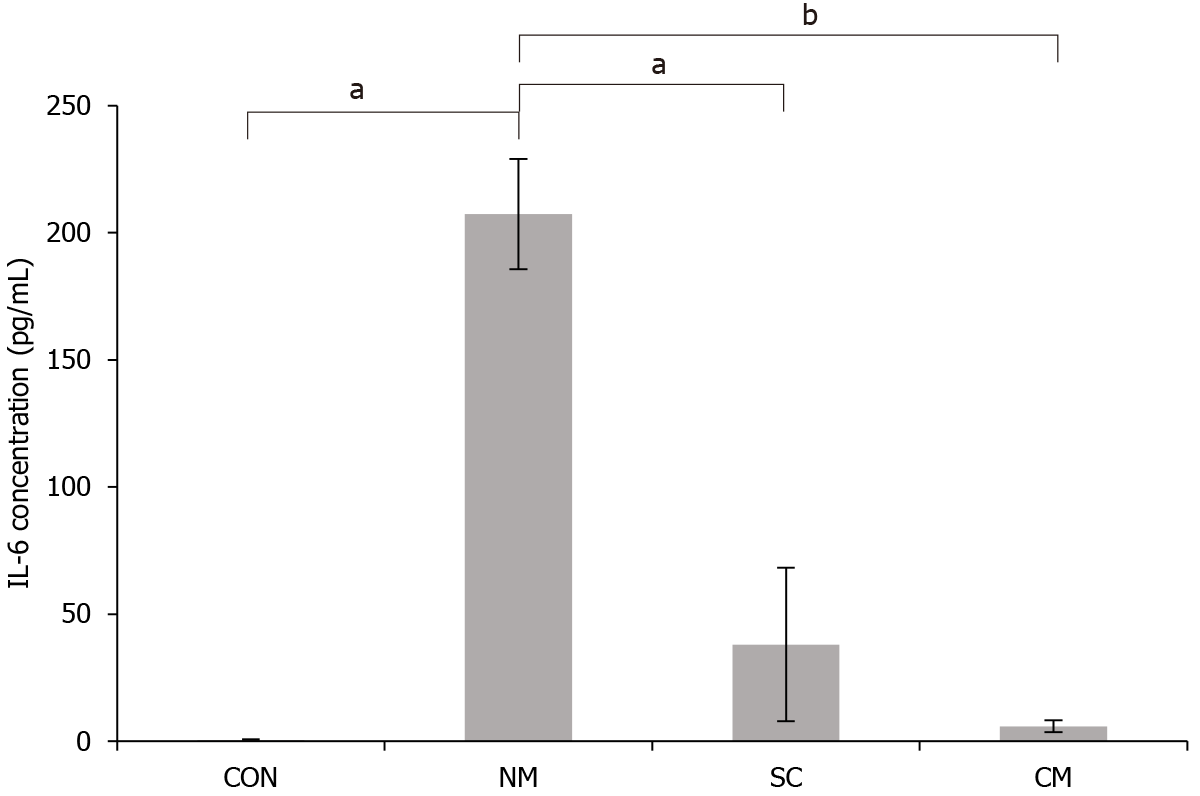
To investigate the effects of conditioned mADSCs secretome on serum IL-6 concentration of DSS-induced mice, the DSS-induced mice were sacrificed on 11 d after DSS treatment. The concentration of IL-6 protein in serum was determined by ELISA (Figure 5). The concentration of serum IL-6 in DSS-induced mice was significantly higher in NM group (207.40 pg/mL) than in the SC group (38.01 pg/mL, P < 0.05) and the CM group (5.94 pg/mL, P < 0.05). The concentration of serum IL-6 in the CM group was not much different from that in the CON group compared to the NM group and SC group. These results indicated that the conditioned mADSCs secretome suppressed the elevation of serum IL-6 concentration in DSS-induced mice.
ADSCs have emerged as a promising therapeutic tool for tissue inflammation and injury. The majority of clinical trials for MSC therapy have used ADSCs than other type of MSCs because of their availability, less invasiveness and cell yield from adipose tissue[34,35]. Although the regenerative effects of ADSCs may be the result of their ability to migrate and engraft into injured sites, their immune-modulatory activity is also mediated by soluble paracrine factors secreted from ADSCs. However, it is remains unclear whether the therapeutic efficacy of soluble paracrine factors is better than ADSCs themselves in inflammation-related disease. This study showed that the conditioned ADSCs secretome had more potent therapeutic effects than ADSCs themselves in the regeneration of damaged colon tissue in acute colitis through the suppression of IL-6 production
In this study, mADSCs isolated from abdominal adipose tissue of 7 w-old C57BL/6 mice were used for the transplantation and preparation of conditioned mADSCs secretome. The mADSCs expressed the stromal-associated markers (CD90, CD73 and CD29) known as positive markers of mADSCs, and did not expressed hematopoiectic markers (CD34 and CD45) or endothelial cell marker (CD31), which are known as negative markers for mADSCs. The results of this study are consistent with the result of the previous study[36], which confirms the characteristics of the isolated mADSCs. It has been shown that ADSCs secretome consists of the growth factors for wound healing, cytokines for modulation of immune system, and chemokines for cell migration and engraftment[26,37]. A variety of culture conditions for ADSC culture affects the compositions of ADSCs secretome, which may influence the biological functions for tissue repair and regeneration[38]. It has been reported that the MSC secretome contains pro-inflammatory cytokines (IL-1b, IL-6, IL-8 and IL-9) as well as anti-inflammatory cytokines (IL-10, IL-13, IL-17 and IL-1ra), and the balance between these pro-inflammatory and anti-inflammatory cytokines may influence the final effect[26]. Moreover, the exposure of human ADSCs to LPS for 24 h increase the secretion of hematopoietic factors (G-CSF, GM-CSF and M-CSF) and the pro-inflammatory cytokines (IL-6, IL-8, IL-11 and TNF-α)[39]. In this study, we treated mADSCs with 1 μg/mL LPS to prepare the conditioned mADSCs secretome. The exposure of mADSCs to LPS for 24 h increased the secretion of pro-inflammatory cytokines (IL-6, IL-17 and TNF-α), anti-inflammatory cytokines (IL-1ra and IL-5), hematopoietic factors (G-CSF, GM-CSF and M-CSF), cell recruitment-related chemokines (CCL1, CCL3, CCL4 and CCL5) and inflammatory-related chemkines (CXCL1 and CXCL2), which is consistent with the previous studies[37,39]. Taken together, these results show that the conditioned mADSCs secretome used in this study contains various components involved in the immunomodulation and regeneration, which suggests that the conditioned mADSCs secretome may display more efficient regenerative effects in the recovery of damaged tissues.
Many studies have shown the therapeutic potent of ADSCs in several inflammatory diseases such as acute colitis, thyroiditis, and arthritis[36,40,41]. The injection of mADSCs reduced the histopathologic severity of colitis, body weight loss, diarrhea and inflammation in trinitrobenzene sulfonic acid (TNBS)- or DSS-induced colitis mouse models[42]. However, the clinical results of ADSCs therapy for inflammatory diseases are inconsistent, which may be due to the different cell sources and cell preparation methods[43,44]. Moreover, MSCs therapy requires a large number of cells and is influenced by uncertain factors such as administration routes and culture conditions, which indicates that there is a difficulty in standardizing MSCs therapy protocols. Therefore, recent studies have focused on the secretome from MSCS as an alternative therapeutic option for tissue regeneration[26]. In this study, we compared the therapeutic effects of conditioned mADSCs secretome with mADSCs in DSS-induced acute colitis mouse model. The injection of conditioned mADSCs secretome intraperitoneally into mice significantly improved the recovery of body weight, colon length and histopathological scores, but the intraperitoneal injection of mADSCs did not show any improvement in the recovery of mice. In a previous study, the intraperitoneal injection of mADSCs recovered body weight and significantly reduced histopathological signs in mice with TNBS-induced colitis[42]. Another study, however, showed that the intraperitoneal injection of mADSC did not improve the recovery of body weight, colon length and histological score in mice with DSS-induce colitis[45]. The inconsistent results in the effect of mADSCs on acute colitis may results from the different ADSCs injection protocols including variation in cell dose, injection timing and cell preparation method. However, our results showing the achievement of significant improvement by the treatment of mADSCs secretome in DSS-induced colitis is consistent with the results of a previous study reporting a reduction in DSS-induced colitis by the intraperitoneal injection of umbilical cord-derived MSC extract[46]. Therefore, the present study indicates that mADSCs secretome might be a more efficient and manageable therapeutic tool in regenerative medicine than the direct injection of mADSCs themselves.
Cytokines are key regulators in the intestinal immune response including inflammation. It has been known that IL-1b, IL-6 and TNF-α are important pro-inflammatory cytokines promoting inflammation in the innate immune response[47]. Previous studies have reported that ADSC or BMSC decreased the expression of IL-1b, IL-6 and TNF-α mRNA in colon tissues of mice treated with TNBS or DSS in comparison with untreated mice[41,48]. In our results, mADSC treatment decreased the expression of IL-6 mRNA in colon tissue, but did not decrease the expression of IL-1b and TNF-α mRNA in colon tissue of DSS-treated mice. However, treatment with mADSC secretome decreased the expression of IL-1b, IL-6 and TNF-α mRNAs in colon tissue of DSS-treated mice. Moreover, the serum levels of IL-6 protein were much less with treatment of the conditioned mADSC secretome than with mADSCs themselves. Our results indicate that treatment using conditioned mADSCs secretome is more effective in suppressing inflammation than in treatment with mADSCs themselves, which is mediated through the inhibition of pro-inflammatory cytokine synthesis in colon tissue as well as the reduction of serum IL-6 levels.
In conclusion, conditioned mADSCs secretome could inhibit the synthesis of pro-inflammatory cytokines in colon tissue and reduce serum IL-6 levels more effectively than mADSCs themselves. This effective suppression of proinflammatory cytokines in colon tissue and serum might mainly contributes to the recovery of damaged colon tissue in a DSS-induced acute colitis model. Further studies are needed to identify the factors in conditioned mADSCs secretome involved in the suppression of pro-inflammatory cytokines in damaged colon tissue.
Inflammatory bowel diseases (IBD) causing chronic and destructive inflammation of the gastrointestinal tract is mainly related to uncontrolled immune response. Current treatment for IBD using immunosuppressive agents is not successful for significant improvement in remission rates. It is necessary to develop effective, feasible and safe therapeutic strategy for IBD. Stem cells having the ability to regulate immune response have emerged as attractive therapeutic tools for incurable IBD.
Mesenchymal stem cells (MSCs) including adipose-derived stem cells (ADSCs) has been known as a promising therapeutic for IBD. However, the transplantation of MACSs in IBD patients showed inconsistent results because of their distinct differentiation and regenerative potential, and the variety of protocols for treatment. In addition, the therapeutic ability of MSCs is mostly mediated by their secretome including cytokines, chemokines, growth factor, and hormones. Therefore, it has been suggested that the MSCs secretome may have therapeutic potential for IBD treatment.
Although the immune-modulatory activity of ADSC is mediated by soluble paracrine factors, it is still unclear whether the therapeutic efficacy of soluble factors secreted from ADSCs is better than ADSCs themselves in inflammation-related diseases. Therefore, the purpose of this study was to investigate the effects of conditioned mouse ADSCs (mADSCs) secretome on colitis-induced mice.
mADSCs were isolated from C57BL/6 mice (female, 8-10 wk-old) and identified using fluorescence-activated cell sorting. The conditioned mADSCs secretome was obtained by culturing mADSCs in serum-free medium with lipopolysaccharide (1 μg/mL) for 24 h, and characterized using a Proteome Profiler mouse cytokine array kit. For induction of mouse acute colitis, mice (C57BL/6. female, 8-wk-old) were supplied with 2% dextran sodium sulfate for 7 d and then normal drinking water for 4 d. Animals were divided into 4 groups, control (CON) group receiving normal drinking water during the experimental period, normal culture medium (NM) group receiving 100 μL normal culture medium three times (on 4, 6 and 8 d), mADSCs (SC) group receiving 1 × 105 cells/100 μL ADSCs once (on 4 d) and conditioned mADSCs secretome (CM) group receiving 100 μL mADSCs secretome three times (on days 4, 6, and 8). The length of the colon and histopatholgy of colon tissues were evaluated after sacrificing animals on 11 d. The mRNA expression levels of inflammatory cytokines in colon tissue were determined by quantitative real-time polymerase chain reaction assay, and the serum interleukin (IL)-6 levels were determined by enzyme-linked immunosorbent assay.
The isolated mADSCs maintained the mADSCs specific gene expression profiles during experiment. The conditioned mADSCs secretome obtained by culturing mADSCs with 1 μg/mL of lipopolysaccharides for 24 h contained high amounts of colony-stimulating factors, inflammatory chemokines and cytokines. The conditioned mADSCs secretome in dextran sulfate sodium (DSS)-induced mice ameliorated the loss of body weight and reduction of colon length, and improved the recovery of damaged colon tissue. The expression of IL-1b mRNA and IL-6 mRNA in colon tissues was significantly lower (P < 0.05) in the CM group than in the SC group (P < 0.05) and/or NM group (P < 0. 005). However, the expression level of tumor necrosis factor-α mRNAs in the NM and CM groups did not differ from that in the CON group. The concentration of serum IL-6 in DSS-induced mice was significantly lower in the CM group (5.94 pg/mL, P < 0.005) and the SC group (38.01 pg/mL, P < 0.05) than in NM group (207.40 pg/mL), which indicates that the conditioned mADSCs secretome suppressed the elevation of serum IL-6 concentration in DSS-induced mice.
This study suggests that conditioned mADSCs secretome may inhibit the synthesis of pro-inflammatory cytokines in the damaged colon tissue, and reduce serum IL-6 Levels more effectively than mADSCs themselves in DSS-induced mice. This effective suppression of proinflammatory cytokines in colon tissue and serum might mainly contributes to the recovery of damaged colon tissue in DSS-induced mice, which suggests the therapeutic potential of the conditioned mADSCs secretome for IBD treatment.
The conditioned mADSCs secretome may serve as a novel therapeutic tool for IBD. Therefore, it is needed to investigate the factors in conditioned mADSCs secretome involved in the effective regeneration of damaged colon tissue.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sitkin S S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1346] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 2. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1532] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 3. | Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011;63:629-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 4. | Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 803] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 5. | Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1167] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 6. | Leiman DA, Lichtenstein GR. Therapy of inflammatory bowel disease: what to expect in the next decade. Curr Opin Gastroenterol. 2014;30:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, Benhaim P, Hedrick MH, Fraser JK. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 324] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 8. | Orciani M, Di Primio R. Skin-derived mesenchymal stem cells: isolation, culture, and characterization. Methods Mol Biol. 2013;989:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, Yap S, Pollett JB, Drowley L, Cassino T, Gharaibeh B, Deasy BM, Huard J, Péault B. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Villaron EM, Almeida J, López-Holgado N, Alcoceba M, Sánchez-Abarca LI, Sanchez-Guijo FM, Alberca M, Pérez-Simon JA, San Miguel JF, Del Cañizo MC. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1421-1427. [PubMed] |
| 11. | Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 743] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 12. | Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 664] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 13. | Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120-4126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 982] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 14. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1333] [Article Influence: 74.1] [Reference Citation Analysis (1)] |
| 15. | Teo GS, Ankrum JA, Martinelli R, Boetto SE, Simms K, Sciuto TE, Dvorak AM, Karp JM, Carman CV. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells. 2012;30:2472-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1014] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 17. | Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1645] [Cited by in RCA: 1540] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 18. | Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 573] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 19. | Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 584] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 20. | Griffin MD, Elliman SJ, Cahill E, English K, Ceredig R, Ritter T. Concise review: adult mesenchymal stromal cell therapy for inflammatory diseases: how well are we joining the dots? Stem Cells. 2013;31:2033-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Flores AI, Gómez-Gómez GJ, Masedo-González Á, Martínez-Montiel MP. Stem cell therapy in inflammatory bowel disease: A promising therapeutic strategy? World J Stem Cells. 2015;7:343-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | L PK, Kandoi S, Misra R, S V, K R, Verma RS. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 315] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 23. | Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 24. | English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 25. | González-González A, García-Sánchez D, Dotta M, Rodríguez-Rey JC, Pérez-Campo FM. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J Stem Cells. 2020;12:1529-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (5)] |
| 26. | Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 868] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 27. | Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A. 2012;18:1479-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 280] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 28. | Pouya S, Heidari M, Baghaei K, Asadzadeh Aghdaei H, Moradi A, Namaki S, Zali MR, Hashemi SM. Study the effects of mesenchymal stem cell conditioned medium injection in mouse model of acute colitis. Int Immunopharmacol. 2018;54:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Xia X, Chiu PWY, Lam PK, Chin WC, Ng EKW, Lau JYW. Secretome from hypoxia-conditioned adipose-derived mesenchymal stem cells promotes the healing of gastric mucosal injury in a rodent model. Biochim Biophys Acta Mol Basis Dis. 2018;1864:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | van Buul GM, Villafuertes E, Bos PK, Waarsing JH, Kops N, Narcisi R, Weinans H, Verhaar JA, Bernsen MR, van Osch GJ. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthritis Cartilage. 2012;20:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 31. | Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 645] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 32. | Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95:2196-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 33. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 918] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 34. | Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? vs. 21:2724-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 600] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 35. | Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 740] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 36. | Choi EW, Shin IS, Park SY, Yoon EJ, Kang SK, Ra JC, Hong SH. Characteristics of mouse adipose tissue-derived stem cells and therapeutic comparisons between syngeneic and allogeneic adipose tissue-derived stem cell transplantation in experimental autoimmune thyroiditis. Cell Transplant. 2014;23:873-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 38. | Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie. 2013;95:2222-2228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 39. | Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A, Ludlow JW, Stricker DM, Potiny S, Green P, Halvorsen YD, Cheatham B, Storms RW, Gimble JM. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 461] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 40. | Stavely R, Robinson AM, Miller S, Boyd R, Sakkal S, Nurgali K. Human adult stem cells derived from adipose tissue and bone marrow attenuate enteric neuropathy in the guinea-pig model of acute colitis. Stem Cell Res Ther. 2015;6:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 386] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 42. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 483] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 43. | García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 571] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 44. | Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, Liu B, Sun L. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012;61:468-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Gonçalves Fda C, Schneider N, Pinto FO, Meyer FS, Visioli F, Pfaffenseller B, Lopez PL, Passos EP, Cirne-Lima EO, Meurer L, Paz AH. Intravenous vs intraperitoneal mesenchymal stem cells administration: what is the best route for treating experimental colitis? World J Gastroenterol. 2014;20:18228-18239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Song JY, Kang HJ, Hong JS, Kim CJ, Shim JY, Lee CW, Choi J. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci Rep. 2017;7:9412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2215] [Cited by in RCA: 1938] [Article Influence: 107.7] [Reference Citation Analysis (0)] |
| 48. | Chen Z, He X, Chen X, Lin X, Zou Y, Wu X, Lan P. Bone marrow mesenchymal stem cells ameliorate colitis-associated tumorigenesis in mice. Biochem Biophys Res Commun. 2014;450:1402-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |