Published online Jun 7, 2021. doi: 10.3748/wjg.v27.i21.2895
Peer-review started: January 26, 2021
First decision: April 5, 2021
Revised: April 14, 2021
Accepted: May 7, 2021
Article in press: May 7, 2021
Published online: June 7, 2021
Processing time: 120 Days and 22.8 Hours
Poorly differentiated gastric neuroendocrine neoplasms (PDGNENs) include gastric neuroendocrine carcinoma (NEC) and mixed adenoneuroendocrine carcinoma, which are highly malignant and rare tumors, and their incidence has increased over the past few decades. However, the clinicopathological features and outcomes of patients with PDGNENs have not been completely elucidated.
To investigate the clinicopathological characteristics and prognostic factors of patients with PDGNENs.
The data from seven centers in China from March 2007 to November 2019 were analyzed retrospectively.
Among the 232 patients with PDGNENs, 191 (82.3%) were male, with an average age of 62.83 ± 9.11 years. One hundred and thirteen (49.34%) of 229 patients had a stage III disease and 86 (37.55%) had stage IV disease. Three (1.58%) of 190 patients had no clinical symptoms, while 187 (98.42%) patients presented clinical symptoms. The tumors were mainly (89.17%) solitary and located in the upper third of the stomach (cardia and fundus of stomach: 115/215, 53.49%). Most lesions were ulcers (157/232, 67.67%), with an average diameter of 4.66 ± 2.77 cm. In terms of tumor invasion, the majority of tumors invaded the serosa (116/198, 58.58%). The median survival time of the 232 patients was 13.50 mo (7, 31 mo), and the overall 1-year, 3-year, and 5-year survival rates were 49%, 19%, and 5%, respectively. According to univariate analysis, tumor number, tumor diameter, gastric invasion status, American Joint Committee on Cancer (AJCC) stage, and distant metastasis status were prognostic factors for patients with PDGNENs. Multivariate analysis showed that tumor number, tumor diameter, AJCC stage, and distant metastasis status were independent prognostic factors for patients with PDGNENs.
The overall prognosis of patients with PDGNENs is poor. The outcomes of patients with a tumor diameter > 5 cm, multiple tumors, and stage IV tumors are worse than those of other patients.
Core Tip: Most patients with poorly differentiated gastric neuroendocrine neoplasms (PDGNENs) present with lymph node metastasis or distant metastasis at the time of diagnosis, and the 5-year overall survival rate of patients with PDGNENs is only 5%. The malignancy of PDGNENs is very high, and the onset is relatively unclear. Routine gastroscopy may help detect PDGNENs as early as possible. Patients with a tumor diameter > 5 cm, multiple tumors, and with American Joint Committee on Cancer stage III or IV have a poor prognosis.
- Citation: Han D, Li YL, Zhou ZW, Yin F, Chen J, Liu F, Shi YF, Wang W, Zhang Y, Yu XJ, Xu JM, Yang RX, Tian C, Luo J, Tan HY. Clinicopathological characteristics and prognosis of 232 patients with poorly differentiated gastric neuroendocrine neoplasms. World J Gastroenterol 2021; 27(21): 2895-2909
- URL: https://www.wjgnet.com/1007-9327/full/v27/i21/2895.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i21.2895
Gastric neuroendocrine neoplasms (G-NENs) are a group of heterogeneous and rare malignant tumors originating from peptidergic neurons and neuroendocrine cells. Neuroendocrine neoplasms can occur throughout the body, such as in the gastrointestinal tract, pancreas, liver and gallbladder, thymus, and lung, but the gastrointestinal tract is the most commonly affected site[1]. According to the Surveillance, Epidemiology, and End Results report, the incidence of G-NENs has increased 15-fold in recent decades and reached 4.85/1000000 in 2014, mainly due to the wide application of gastroscopy and the improvement of pathological diagnosis techniques[2,3]. The incidence of poorly differentiated gastric neuroendocrine neoplasms (PDGNENs) is approximately 1/1000000 people, accounting for 16.4% of G-NENs, while the incidence of other tumors in the stomach shows a decreasing trend compared with G-NENs[4].
PDGNENs are divided into functional and nonfunctional tumors according to whether the tumor secretes active hormones and causes characteristic clinical manifestations, and the most common clinical tumors are nonfunctional tumors. The clinical symptoms of nonfunctional PDGNENs lack specificity, and early diagnosis is difficult. Generally, the clinical symptoms are caused by the tumor size or metastasis, mainly including abdominal pain and abdominal distension. Few functional PDGNENs secrete bioactive amines that cause carcinoid syndrome, including skin flushing, diarrhea, wheezing, etc. PDGNENs have a worse prognosis than neuroendocrine tumors (NETs), and tumor diameter, stage, location, and treatment are significantly correlated with the prognosis[4]. The 5-year survival rate of patients with well-differentiated G-NENs presenting with distant metastases may reach 35%, while the 5-year survival rate of patients with PDGNENs accompanied by distant metastasis is only 4%[5].
Relatively few large-cohort studies have assessed PDGNENs and limited data is available on their clinicopathological features and outcomes. Therefore, we collected the data of 232 patients with PDGNENs from multiple centers in China and analyzed the clinicopathological characteristics and prognostic factors of these patients, aiming to provide a reference for clinical work on PDGNENs.
From March 2007 to November 2019, a total of 232 patients with PDGNENs from seven centers in China were enrolled (China-Japan Friendship Hospital, n = 71; Sun Yat-Sen University Cancer Center, n = 54; The Fourth Affiliated Hospital of Hebei Medical University, n = 49; The First Affiliated Hospital Sun Yat-Sen University, n = 39; Fudan University Shanghai Cancer Center, n = 10; The Fifth Medical Center of the PLA General Hospital, n = 8; and Yunnan Tumor Hospital, n = 1). The inclusion criteria were as follows: (1) All patients were confirmed to have neuroendocrine carcinoma (NEC) or mixed adenoneuroendocrine carcinoma (MANEC) based on pathology; (2) The clinical data of the patients were relatively complete; (3) The patients underwent regular follow-up; and (4) The patients had no other tumors. This study was conducted in accordance with the provisions of the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of China-Japan Friendship Hospital (No. 2019-24-K18-1).
The pathological grading standard used in this study adopted the 2019 World Health Organization classification and grading criteria for gastrointestinal pancreatic neuroendocrine tumors[6] and updated the pathological classification and grading of gastroenteropancreatic neuroendocrine tumors. Well-differentiated gastric neuroendocrine tumors were classified into three types. NEC was no longer graded and was divided into only two subtypes: Large cell NEC (LCNEC) and small cell NEC (SCNEC). In addition, MANEC has also been replaced by mixed neuroendocrine and nonneuroendocrine tumors (MiNENs), which contain a wider range of contents. Most of the NEN components in MiNEN are NEC and may also be NETs; in addition to adenocarcinoma, other components, such as squamous cell carcinoma, may also occur in non-NEN components, but each component must account for more than 30% and be classified when reporting[7]. The 8th edition gastric cancer tumor-node-metastasis staging system was used for staging[8].
The patients were followed regularly by an outpatient review, inpatient medical record review, and telephone interview. The starting point was the time when the patient’s histopathology yielded a diagnosis of PDGNENs. The end point of the follow-up was the time of death.
Measurement data are presented as the mean ± SD, and the follow-up time is reported as the median (interquartile range). Count data are presented as numbers of cases (percentages). The Kaplan-Meier method was used for the survival analysis, and comparisons were performed using the log-rank test. Multivariable survival analyses were also performed to exclude dependent variables using Cox proportional hazards regression models. When the two-tailed P value was less than 0.05, the difference was considered statistically significant. Data were analyzed using SPSS 25.0 statistical analysis software (IBM, Chicago, IL, United States).
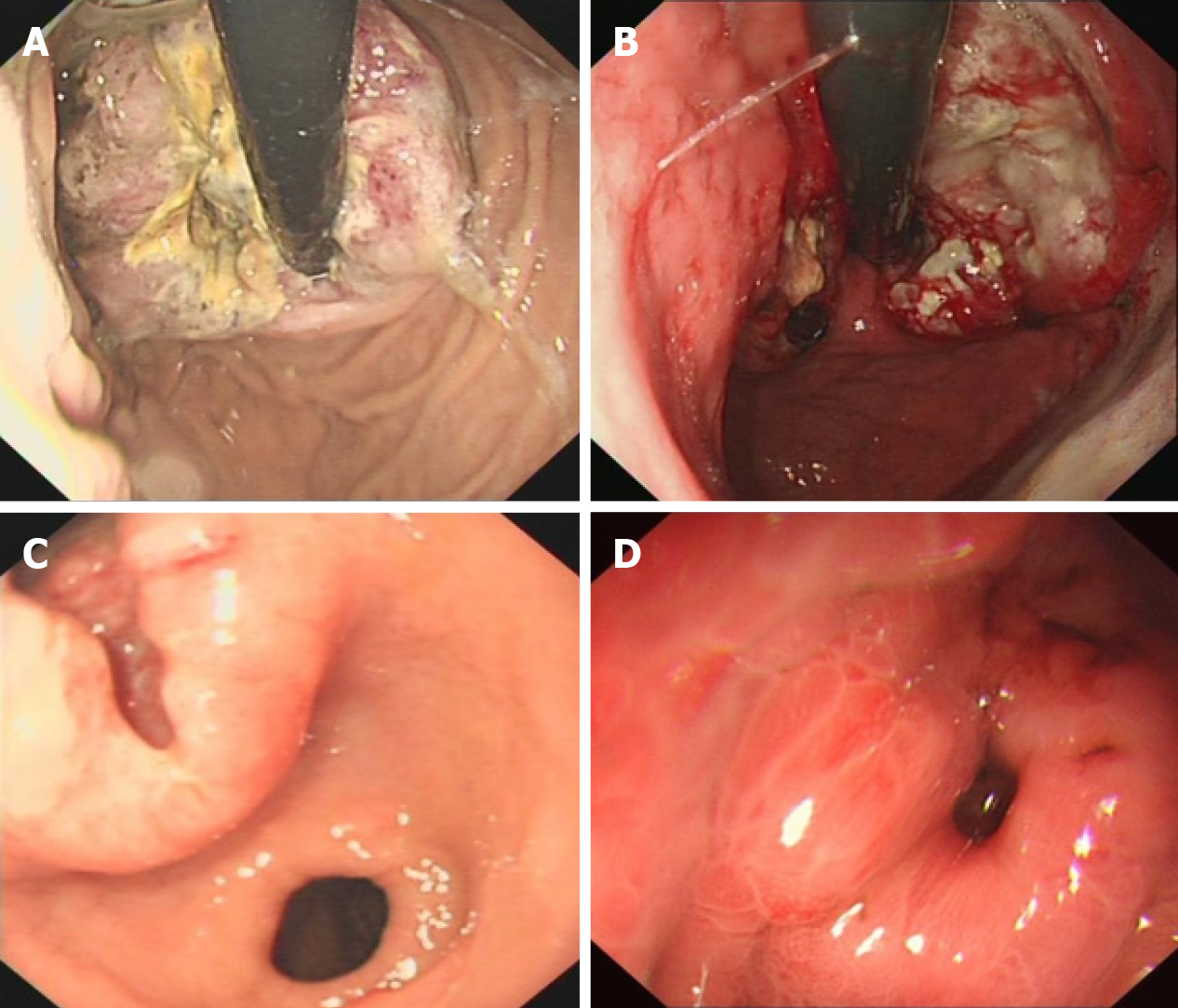
Among the 232 patients with PDGNENs, 191 (82.3%) were male, with an average age of 62.83 years (range: 30-85 years), and the elderly accounted for 65.5% of the patients (Table 1). The average diameter of the tumor was 4.66 ± 2.77 cm, and the lesions mainly invaded the serosa (116/198, 58.58%). The lymph nodes were positive in 175 (79.78%) of 225 patients, and 86 (37.39%) of 230 patients exhibited distant metastases. In addition, 113 (49.34%) patients presented with a stage II disease and 86 (37.55%) presented with stage IV disease. In terms of endoscopic performance, most of the tumors were mainly located in the cardia (65/215, 30.23%). The majority of tumors were solitary (215/219, 89.17%). In addition, the lesions were mainly ulcers (157/232, 67.67%). Typical gastroscopic findings are shown in Figure 1.
| Classification | n | Median survival time/mo | P value |
| Age (yr) | 0.26 | ||
| < 60 | 80 | 12.56 (9.42-14.57) | |
| ≥ 60 | 152 | 14.85 (11.80-18.11) | |
| Gender | 0.32 | ||
| Male | 191 | 14.50 (11.78-16.21) | |
| Female | 41 | 11.50 (7.94-12.05) | |
| Tumor number | 0.01 | ||
| Solitary | 5 | 14.02 (11.93-16.06) | |
| Multiple | 214 | 5.98 (3.40-6.60) | |
| NR | 13 | NR | NR |
| Tumor diameter (cm) | 0.01 | ||
| 0-5 | 105 | 18.10 (9.35-20.64) | |
| > 5 | 99 | 11.50 (9.41-16.58) | |
| NR | 28 | NR | NR |
| Invasion | 0.02 | ||
| M1/S/MP2 | 82 | 16.27 (12.51-17.49) | |
| Serosa | 116 | 11.54 (7.54-14.54) | |
| NR | 34 | NR | NR |
| Tumor site | 0.09 | ||
| Cardia | 65 | 16.12 (11.53-18.46) | |
| Fundus | 15 | 19.88 (3.99-28.00) | |
| Body | 33 | 15.98 (9.46-20.53) | |
| Antrum | 27 | 11.80 (8.73-17.26) | |
| C + F | 35 | 12.50 (7.96-16.03) | |
| Other | 40 | 15.23 (8.59-23.40) | |
| NR | 17 | NR | NR |
| Pathological type | 0.68 | ||
| Large cell NEC | 41 | 12.96 (12.11-13.84) | |
| Small cell NEC | 26 | 16.33 (11.30-20.69) | |
| MANEC | 38 | 12.33 (8.80-15.19) | |
| NR | 127 | 14.31 (10.40-17.59) | |
| Ki-67 index | 0.61 | ||
| 20%-50% | 60 | 12.69 (7.50-16.49) | |
| 50%-70% | 114 | 14.10 (11.74-16.25) | |
| > 70% | 38 | 13.23 (0.00-26.80) | |
| NR | 20 | NR | NR |
| Endoscopic performance | 0.90 | ||
| Polypoid | 7 | 15.00 (11.85-16.14) | |
| Mucosal elevation | 28 | 17.19 (6.99-25.00) | |
| Ulcer | 157 | 13.69 (10.66-15.33) | |
| Other | 40 | 11.00 (9.09-10.90) | |
| Tumor N staging | 0.16 | ||
| N0 | 50 | 20.00 (5.30-30.70) | |
| N1-N3 | 174 | 13.11 (9.95-16.00) | |
| NR | 8 | NR | NR |
| Distant metastasis | < 0.001 | ||
| No | 157 | 17.43 (12.94-21.05) | |
| Yes | 73 | 11.64 (9.04-12.95) | |
| NR | 2 | NR | NR |
| AJCC stage | < 0.001 | ||
| I | 5 | 88.26 (36.06-139.94) | |
| II | 25 | 31.34 (13.56-48.43) | |
| III | 113 | 16.12 (13.43-18.56) | |
| IV | 86 | 11.52 (9.05-12.94) | |
| NR | 3 | NR | NR |
| Treatment | 0.01 | ||
| Surgery | 86 | 15.47 (10.87-19.12) | |
| Chemotherapy | 40 | 12.10 (10.24-13.75) | |
| Surgery + chemotherapy | 92 | 15.70 (9.99-20.00) | |
| Other | 14 | 11.00 (0.00-21.68) |
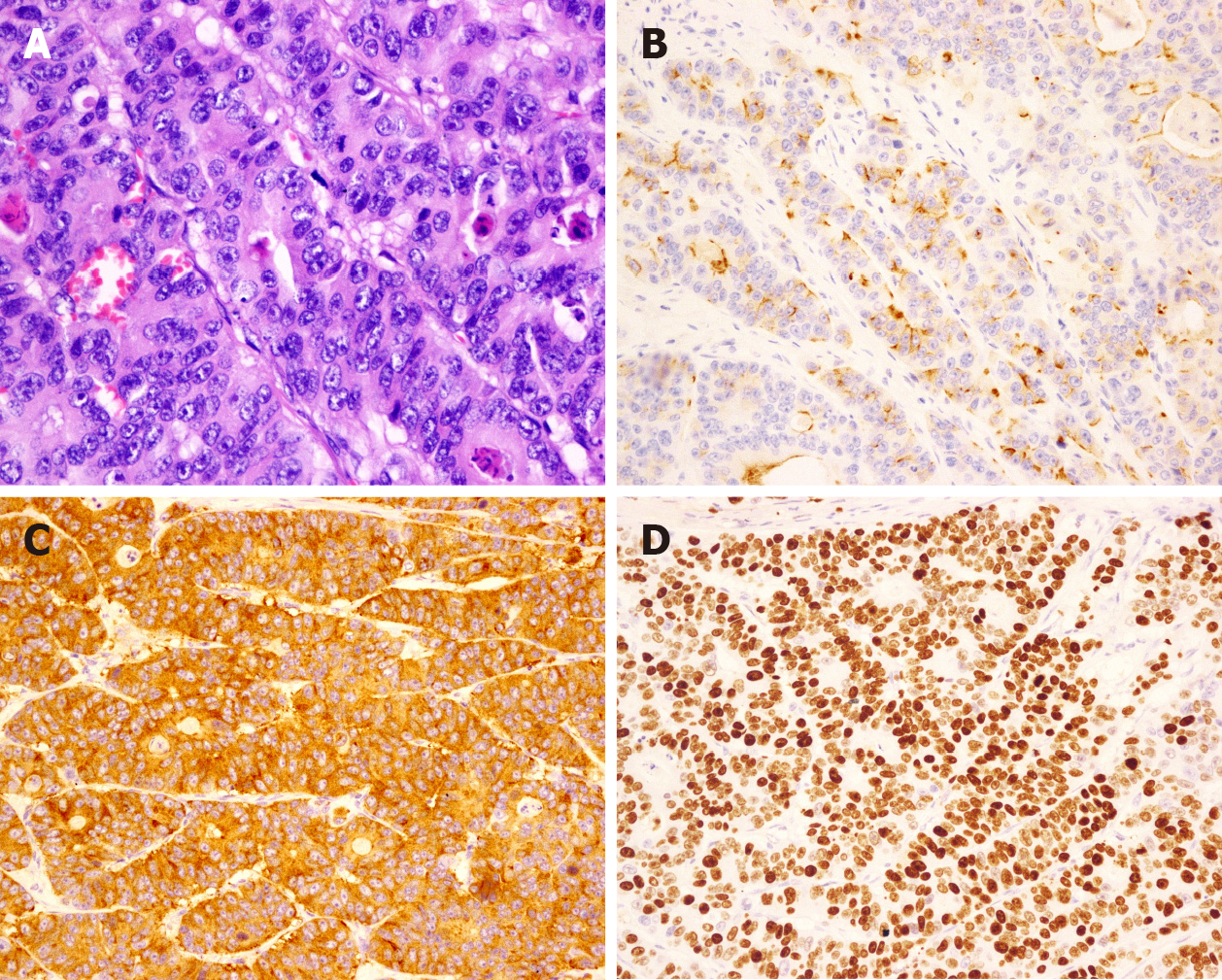
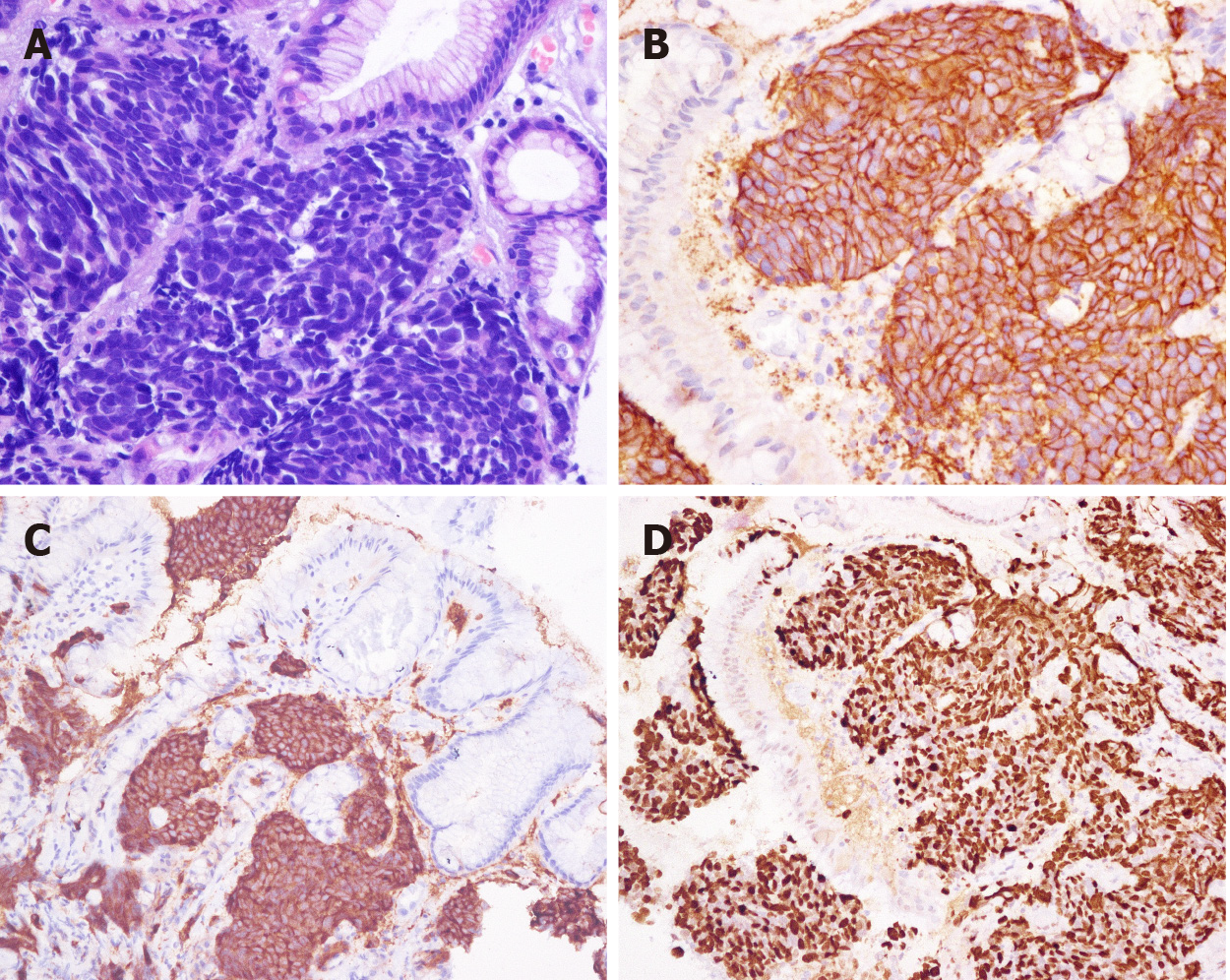
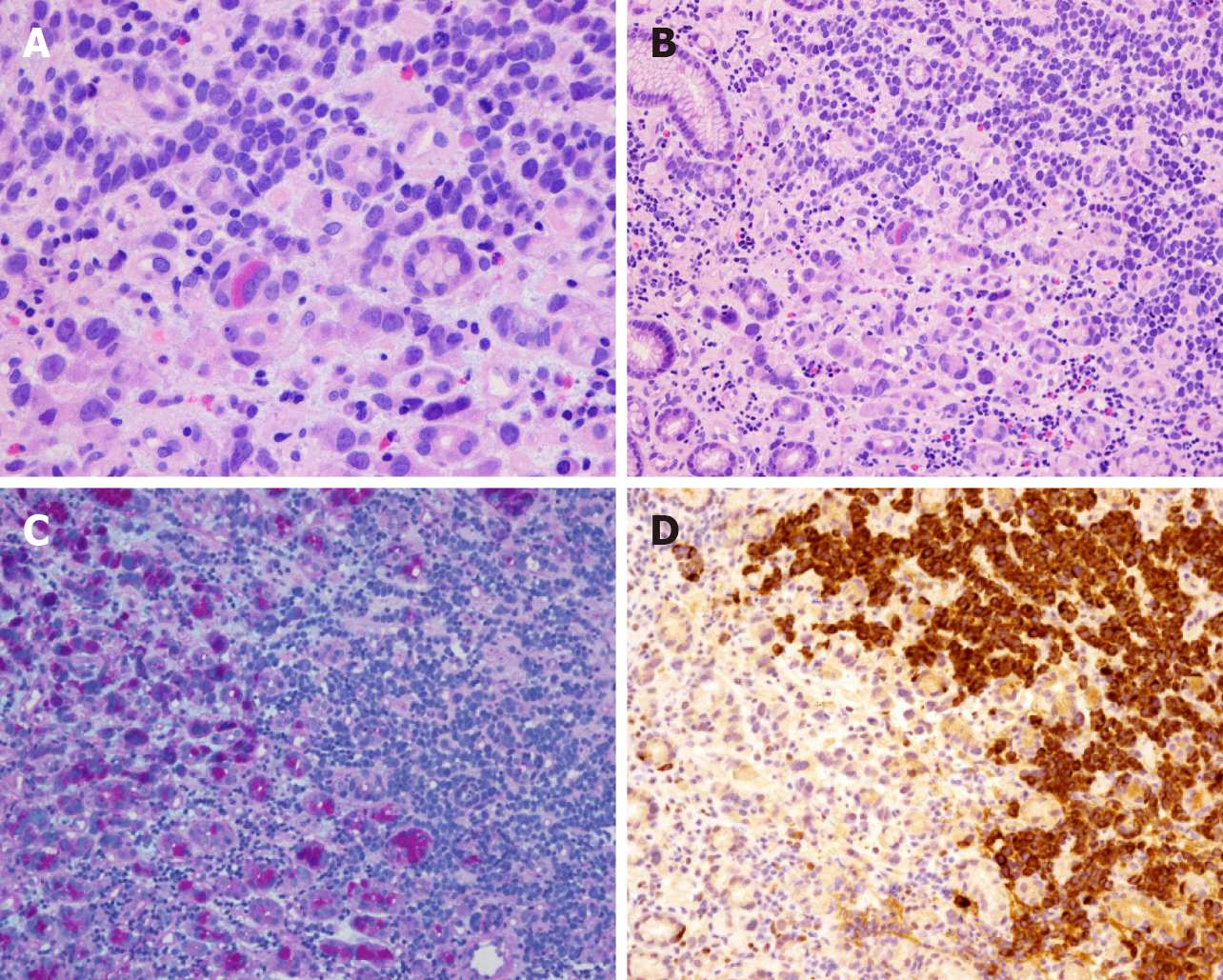
The pathological classification of 232 patients was G3. Of these patients, 41 had LCNEC (17.67%) (Figure 2), 26 had SCNEC (12.21%) (Figure 3), and 38 had MANEC (16.38%) (Figure 4); the subtype was not reported for 127 (54.74%) patients. The average Ki-67 index was 65.34% (Table 1).
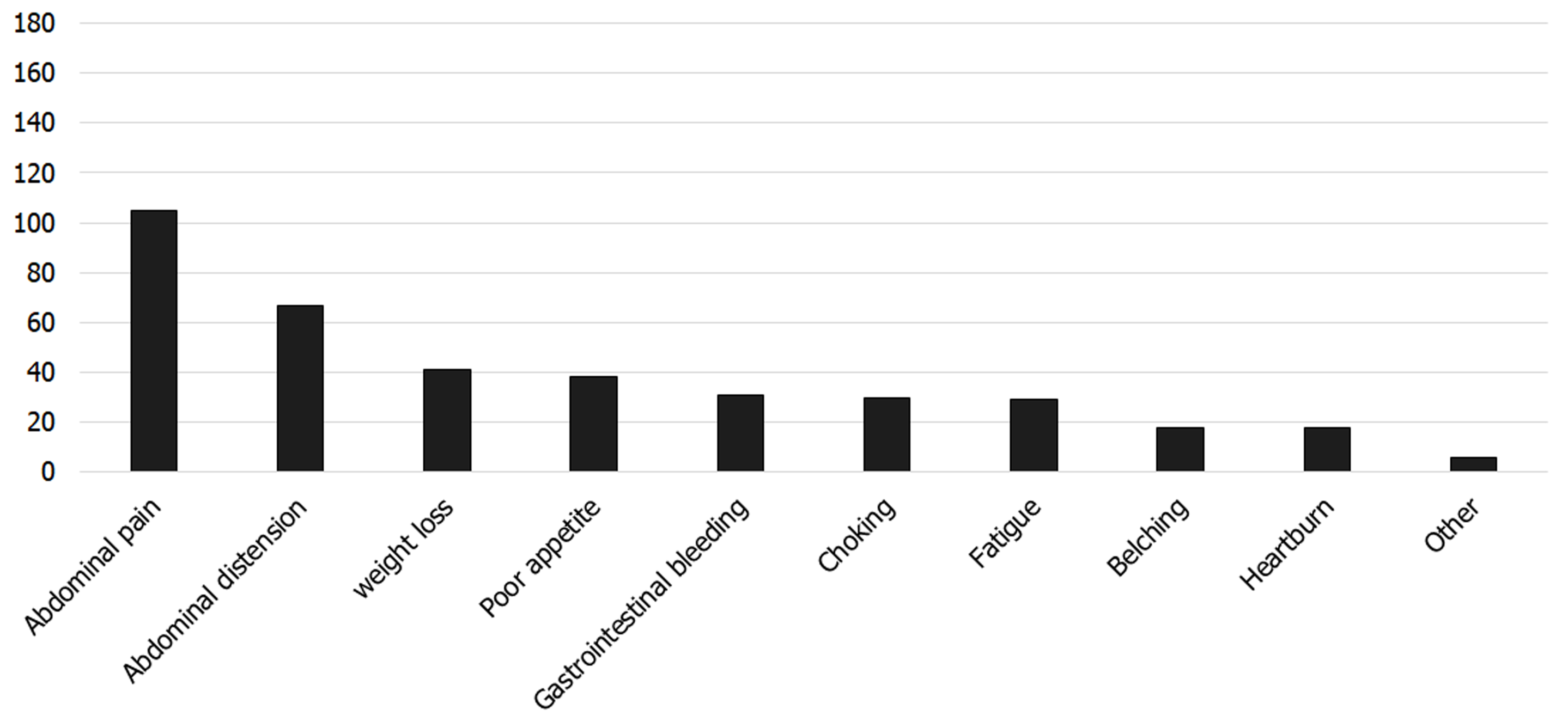
The clinical symptoms of 190 patients were recorded. One hundred and eighty-seven (98.42%) patients experienced symptoms, while three (1.58%) patients had no clinical symptoms. The main symptoms were abdominal pain (105/190, 55.26%), abdominal distension (67/190, 35.26%), weight loss (41/190, 21.58%), poor appetite (38/190, 20.00%), and gastrointestinal bleeding (31/190, 16.32%) (Figure 5).
Among the 232 patients with PDGNENs, 86 (37.07%) were treated by surgery, 40 (17.24%) were treated with chemotherapy, 92 (38.79%) were treated by surgery plus chemotherapy, and 14 (6.03%) were treated with other treatments (somatostatin analogs, targeted therapy, immunotherapy, traditional Chinese medicine treatment, etc.). One hundred and forty-three patients had no distant metastasis or resectable tumors (tumor stage I: 5 cases; stage II: 25 cases; and stage III: 113 cases), of whom 75 were treated by surgery, 6 were treated by chemotherapy, 55 were treated by surgery combined with chemotherapy, and 7 were treated with other treatments. This study analyzed patients without distant metastases and found that the median survival time of the surgery alone group was 18 mo, while the median survival time of the chemotherapy alone group was 11 mo and that of the surgery combined with chemo
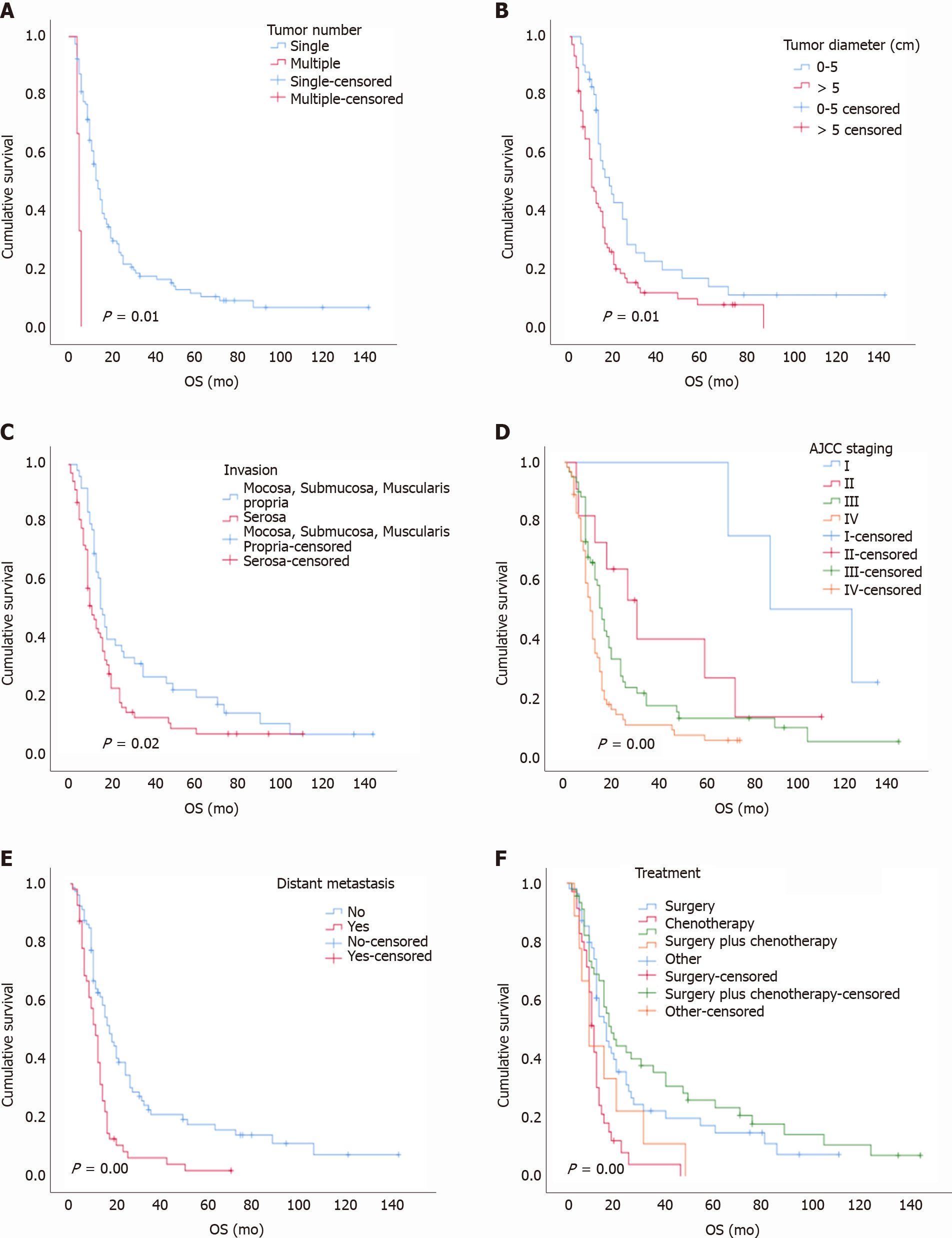
With a median follow-up time of 13.50 mo (range, 7-31 mo), the overall 1-year, 3-year, and 5-year survival rates were 47%, 15%, and 5%, respectively. The median survival time was 14 mo. The univariate analysis showed that tumor number, tumor diameter, gastric invasion status, American Joint Committee on Cancer (AJCC) stage, and distant metastasis status were correlated with the prognosis (log-rank test P < 0.05; Table 1), while age, sex, lymph node metastasis, pathological type, Ki-67 index, and tumor site were not related to the prognosis (log-rank test P > 0.05; Table 1 and Figure 6). In the multivariate analysis, the tumor number {multiple vs solitary, hazard ratio (HR) [95% confidence interval (CI)]: 3.89 (1.66-9.11), P < 0.001}, tumor diameter [5 cm vs 0-5 cm, HR (95%CI): 1.56 (1.01-2.41), P = 0.04], tumor stage [IV vs I, HR (95%CI): 5.98 (1.78-20.60), P < 0.001; III vs I, HR (95%CI): 3.582 (1.07-11.88), P = 0.03], and distant metastasis status [yes vs no, HR (95%CI): 2.16 (1.41-3.31), P < 0.001] were independent risk factors affecting the prognosis (Table 2). The overall 1-year, 3-year, and 5-year survival rates of the 232 patients with PDGNENs are shown in Table 3.
| Parameter | n | HR (95%CI) | P value |
| Tumor number | |||
| Solitary | 5 | 1 | |
| Multiple | 214 | 3.89 (1.66-9.11) | < 0.001 |
| Tumor diameter | |||
| 0-5 | 105 | 1 | |
| > 5 | 99 | 1.56 (1.01-2.41) | 0.04 |
| AJCC stage | |||
| I | 5 | 1 | |
| II | 25 | 2.71 (0.56-8.44) | 0.26 |
| III | 113 | 3.582 (1.07-11.88) | 0.03 |
| IV | 86 | 5.98 (1.78-20.06) | < 0.001 |
| Distant metastasis | |||
| No | 157 | 1 | |
| Yes | 73 | 2.16 (1.41-3.31) | < 0.001 |
| Classification | Median survival time (mo) | 1-yr survival rate (%) | 3-yr survival rate (%) | 5-yr survival rate (%) |
| Age (yr) | ||||
| < 60 | 12 | 37 | 11 | 5 |
| ≥ 60 | 15 | 52 | 18 | 5 |
| Gender | ||||
| Male | 14 | 52 | 16 | 6 |
| Female | 10 | 29 | 13 | 3 |
| Tumor number | ||||
| Solitary | 14 | 49 | 17 | 6 |
| Multiple | 5 | 33 | 0 | 0 |
| Tumor diameter (cm) | ||||
| 0-5 | 19 | 58 | 23 | 9 |
| > 5 | 12 | 40 | 13 | 5 |
| Invasion | ||||
| M1/S/MP2 | 17 | 60 | 23 | 6 |
| Serosal | 12 | 42 | 13 | 5 |
| Tumor site | ||||
| Cardia | 16 | 58 | 9 | 0 |
| Fundus | 20 | 67 | 17 | 17 |
| Body | 15 | 57 | 30 | 11 |
| Antrum | 12 | 33 | 0 | 0 |
| C + F | 13 | 41 | 6 | 0 |
| Other | 15 | 57 | 26 | 13 |
| Pathological type | ||||
| Large cell NEC | 16 | 54 | 18 | 9 |
| Small cell NEC | 13 | 34 | 19 | 9 |
| MANEC | 12 | 37 | 11 | 0 |
| NR | 14 | 52 | 16 | 5 |
| Ki-67 index | ||||
| 20%-50% | 12 | 47 | 26 | 6 |
| 50%-70% | 14 | 57 | 12 | 1 |
| > 70% | 13 | 29 | 7 | 0 |
| Endoscopic | ||||
| Performance | 14 | 67 | 17 | 0 |
| Polypoid | 16 | 54 | 25 | 8 |
| Mucosal elevation | ||||
| Ulcer | 13 | 48 | 15 | 6 |
| Other | 10 | 36 | 14 | 3 |
| LN metastasis | ||||
| No | 18 | 57 | 18 | 6 |
| Yes | 13 | 47 | 15 | 5 |
| Distant metastasis | ||||
| No | 17 | 62 | 22 | 7 |
| Yes | 11 | 28 | 7 | 2 |
| AJCC stage | ||||
| I | 60 | 100 | 100 | 14 |
| II | 31 | 73 | 41 | 8 |
| III | 16 | 58 | 17 | 5 |
| IV | 11 | 33 | 11 | 4 |
| Treatment | ||||
| Surgery | 15 | 55 | 17 | 9 |
| Chemotherapy | 12 | 29 | 4 | 0 |
| S + C | 16 | 57 | 23 | 6 |
| Other | 10 | 33 | 11 | 0 |
PDGNENs are rare tumors that are highly malignant, accounting for 6.9% of gastroenteropancreatic neuroendocrine tumors and 0.3%-1.8% of all malignant gastric tumors[9,10]. According to the Korean literature, PDGNENs account for 2.84% of all NENs and 40% of G-NENs[11]. In this article, the male-to-female ratio reached 4.66:1, and significantly more male patients were identified than female patients. Kim et al[12] reported that among 63 patients with G-NECs, 48 were male and 15 were female. Other studies[13-15] have indicated that G-NECs have a sex bias favoring males, but the reason has not been clarified.
PDGNENs are mostly nonfunctional and often detected incidentally. Among the 232 patients with PDGNENs analyzed in our study, all patients had nonfunctional lesions. The main symptoms were similar to those reported in several other studies[15-17]. Early PDGNENs are asymptomatic or have no specific symptoms, such as anemia, abdominal pain, and dyspepsia, and they are unintentionally identified through routine upper gastrointestinal endoscopy. Indeed, regular upper gastroin
Most patients with PDGNENs exhibit advanced tumors at the time of diagnosis, and patients with advanced tumors have worse outcomes than those with early-stage tumors. In our study, the majority of patients had advanced stage tumors at the time of diagnosis; 199 (85.78%) patients exhibited lymph node metastasis or distant metastasis, and these values are similar to those of several other studies[18]. Additionally, the survival analysis showed significant differences between patients with stages I-IV. The median survival time of patients with stage I disease was 88 mo, while that of stage IV patients was only 11 mo, and the 3-year overall survival rates were 100% and 11%, respectively. Patients with PDGNENs often benefit if the disease is detected at an early stage. Ishida et al[18] reported that the 5-year survival rates of 51 patients with stage I, II, III, or IV G-NECs were 66.7%, 49.3%, 64.3%, and 7.7%, respectively. Tierney et al[19] found that the median survival times of patients with stages I-II, III, and IV tumors were 40 mo, 31 mo, and 6 mo, respectively[19]. Furthermore, our study suggested that distant metastasis is an independent prognostic factor, which is consistent with relevant reports[18-21].
Tumor diameter may be relevant to the outcomes in our study. According to the study conducted by Liang et al[20], a gastric neuroendocrine tumor diameter greater than 4.2 cm was a poor prognostic factor for patients. Fang et al[22] analyzed 156 patients with G-NECs. Univariate analysis revealed a significant difference between patients with a tumor diameter less than 4.5 cm and those with a tumor diameter greater than 4.5 cm, and the 5-year survival rates were 57.9% and 29.3%, respectively. Our data suggest that a tumor diameter greater than 5 cm was a risk factor affecting the prognosis. In clinical practice, the tumor diameter of PDGNENs may be useful to predict the outcome, and patients with a tumor diameter greater than 5 cm should receive more attention.
Tumor site may also be related to the prognosis. In our study, PDGNENs were mainly located in the upper third of the stomach (total: 53.95%), while only 27 (12.56%) PDGNENs were found in the antrum. The values are similar to those of other reports[23-25]. In addition, our data showed a longer median survival time of patients with lesions in the cardia (15.97 mo) and fundus of the stomach (20.00 mo) than that of patients with lesions in the gastric antrum (12.50 mo). Hu et al[4] reported a median survival time of patients with G-NECs in the cardia and fundus of 20 mo, which was longer than that of patients with tumors in the antrum (13 mo). Bukhari et al[14] observed a better prognosis for patients with tumors in the cardia region than that of patients with tumors in the gastric antrum (median survival: 48.0 mo vs 19.0 mo)[14]. To a certain extent, we postulate that the prognosis of PDGNENs in the upper part of the stomach is better than that of tumors in the lower part.
The European Neuroendocrine Tumor Society proposed guidelines for the treatment of NEC in 2016. For patients with no distant metastasis or resectable tumors, surgical treatment can be selected, and adjuvant chemotherapy (AC) or radiotherapy can be selected after surgery. Surgery is the only curative treatment for resectable PDGNENs, but the prognosis of patients who undergo surgery alone remains very poor[26]. This study analyzed patients without distant metastases and found that the median survival time of patients treated by surgery combined with chemotherapy (23 mo) was longer than that of patients who were treated by surgery alone (18 mo) or chemotherapy alone (11 mo). Their 3-year overall survival rates were 35%, 22%, and 4%, respectively. Patients with early-stage tumors should choose the proper treatment method, which may improve the quality of life and prolong the survival time. According to a retrospective study including 69 patients with G-NECs in China, the overall 3-year survival rate of patients receiving surgery combined with chemotherapy was 68.8%, while that of patients who received surgery alone was only 3.8%[27]. Bukhari et al[14] assessed 43 patients with G-NECs. Five patients did not undergo postoperative chemotherapy, and the median survival time of these patients was 15 mo. The median survival time of the remaining 34 patients who received postoperative chemotherapy was 44 mo[14]. Mao et al[28] analyzed 806 patients diagnosed with nonmetastatic poorly differentiated colorectal NECs, and 394 (48.9%) of these patients received AC. Kaplan-Meier curves showed that the median overall survival (OS) was significantly longer for patients treated with AC vs observation (57.4 mo vs 38.2 mo; P = 0.007). The Cox proportional hazards regression analysis showed that AC was associated with a significant OS benefit [HR = 0.73, P < 0.001][28]. Surgery combined with chemotherapy has advantages and may improve the prognosis of patients compared with treatment with either approach alone.
As a retrospective study, this study provides novel insights into the diagnosis and treatment of PDGNENs and the risk factors related to the prognosis. However, some limitations should be noted. First, some patients had incomplete basic information. Second, some patients had poor compliance, and a certain number of patients were lost to follow-up (30%). In the future, prospective, multicenter, large-scale trials are still needed to identify independent risk factors that affect the prognosis of patients with PDGNENs.
In summary, the majority of patients with PDGNENs had already developed lymph node or distant metastasis at the time of diagnosis, and the prognosis was poor, with a 5-year survival rate of 5% and a median survival time of 13.50 mo. Electronic gastroscopy and pathological diagnostic technology have been widely popularized. When people experience the aforementioned symptoms, gastroscopy should be performed in a timely manner. If a lesion is detected, pathology should be performed to determine a clear diagnosis. Clinicians should pay more attention to patients with lesions greater than 5 cm because their prognosis is the worst, with a 5-year survival rate of 5%. Patients are recommended to undergo AC after surgery. In addition, tumor number, tumor diameter, AJCC stage, and distant metastasis are independent factors affecting the prognosis of patients with PDGNENs.
Poorly differentiated gastric neuroendocrine neoplasm (PDGNEN) is a rare tumor, but its incidence is gradually increasing. The clinical understanding of PDGNENs is limited, and a completely unified conclusion is difficult to determine. This study included 232 patients with PDGNENs from multiple centers in China and statistically analyzed the clinicopathological characteristics and prognostic factors of the patients, aiming to further standardize and systemize the diagnosis and treatment of PDGNENs.
This study aimed to explore the clinicopathological characteristics and prognostic factors for patients with PDGNENs, improve clinicians’ awareness of this disease, reduce the misdiagnosis and missed diagnosis of PDGNENs, and achieve early detection, early diagnosis, and early treatment.
This study analyzed the clinicopathological characteristics and prognostic factors of patients with PDGNENs to identify independent risk factors that potentially affect prognosis. By interfering with independent prognostic risk factors for PDGNENs, the quality of life of patients can be further improved and the survival period be prolonged.
This study was a retrospective study and included 232 patients with PDGNENs treated at seven centers in China from March 2007 to November 2019. The data were analyzed using the Kaplan-Meier method to evaluate the survival of patients. Single-factor analysis was performed using the log-rank test, and the Cox proportional hazard regression model was used to explore the risk factors that affect patient prognosis.
Among the 232 patients with PDGNENs, 113 (49.34%) patients had stage III tumors and 86 (37.55%) had stage IV tumors. The tumors were mainly (89.17%) solitary and located in the upper third of the stomach (cardia and fundus of stomach: 115/215, 53.49%). Most lesions were ulcers (157/232, 67.67%), with an average diameter of 4.66 cm. In terms of tumor invasion, the majority of tumors invaded the serosa (116/198, 58.58%). The median survival time of the 232 patients was 13.50 mo (range, 7-31 mo), and the overall 1-year, 3-year, and 5-year survival rates were 49%, 19%, and 5%, respectively.
According to univariate analysis, tumor number, tumor diameter, gastric invasion status, American Joint Committee on Cancer (AJCC) stage, and distant metastasis status were prognostic factors for patients with PDGNENs. Multivariate analysis showed that tumor number, tumor diameter, AJCC stage, and distant metastasis status were independent prognostic factors for patients with PDGNENs.
This study provides novel insights into the diagnosis and treatment of PDGNENs and the risk factors related to the prognosis. In the future, prospective, multicenter, large-scale research is still needed.
We are grateful to all patients and all relevant medical workers who participated in this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ezenkwa US S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Andreasi V, Partelli S, Muffatti F, Manzoni MF, Capurso G, Falconi M. Update on gastroenteropancreatic neuroendocrine tumors. Dig Liver Dis. 2021;53:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2491] [Article Influence: 311.4] [Reference Citation Analysis (4)] |
| 3. | Yang Z, Wang W, Lu J, Pan G, Pan Z, Chen Q, Liu W, Zhao Y. Gastric Neuroendocrine Tumors (G-Nets): Incidence, Prognosis and Recent Trend Toward Improved Survival. Cell Physiol Biochem. 2018;45:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Hu P, Bai J, Liu M, Xue J, Chen T, Li R, Kuai X, Zhao H, Li X, Tian Y, Sun W, Xiong Y, Tang Q. Trends of incidence and prognosis of gastric neuroendocrine neoplasms: a study based on SEER and our multicenter research. Gastric Cancer. 2020;23:591-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia. 2017;19:991-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 482] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 6. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 7. | Assarzadegan N, Montgomery E. What is New in 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch Pathol Lab Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 8. | Doescher J, Veit JA, Hoffmann TK. [The 8th edition of the AJCC Cancer Staging Manual : Updates in otorhinolaryngology, head and neck surgery]. HNO. 2017;65:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Tian FX, Cai YQ, Zhuang LP, Chen MF, Xiu ZB, Zhang Y, Liu H, Liu ZH, Liu GP, Zeng C, Lin FL, Liu J, Huang ST, Zhang LZ, Lin HY. Clinicopathological features and prognosis of patients with gastric neuroendocrine tumors: A population-based study. Cancer Med. 2018;7:5359-5369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 11. | Gastrointestinal Pathology Study Group of Korean Society of Pathologists. Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, Kim H, Kook MC, Park DY, Lee JH, Chang H, Jung ES, Kim HK, Jin SY, Choi JH, Gu MJ, Kim S, Kang MS, Cho CH, Park MI, Kang YK, Kim YW, Yoon SO, Bae HI, Joo M, Moon WS, Kang DY, Chang SJ. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res Treat. 2012;44:157-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Kim BS, Park YS, Yook JH, Kim BS. Comparison of relapse-free survival in gastric neuroendocrine carcinoma (WHO grade 3) and gastric carcinoma. Therap Adv Gastroenterol. 2017;10:407-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Liu DJ, Fu XL, Liu W, Zheng LY, Zhang JF, Huo YM, Li J, Hua R, Liu Q, Sun YW. Clinicopathological, treatment, and prognosis study of 43 gastric neuroendocrine carcinomas. World J Gastroenterol. 2017;23:516-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Bukhari MH, Coppola D, Nasir A. Clinicopathologic analysis of primary gastroenteropancreatic poorly differentiated neuroendocrine carcinoma; A ten year retrospective study of 68 cases at Moffit Cancer Center. Pak J Med Sci. 2020;36:265-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Sekerci A, Turk HM, Demir T, Seker M, Akcakaya A, Arici DS. Clinicopathological Features of Gastroenteropancreatic Neuroendocrine Neoplasms. J Coll Physicians Surg Pak. 2020;30:863-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Xie J, Zhao Y, Zhou Y, He Q, Hao H, Qiu X, Zhao G, Xu Y, Xue F, Chen J, Su G, Li P, Zheng CH, Huang CM. Predictive Value of Combined Preoperative Carcinoembryonic Antigen Level and Ki-67 Index in Patients With Gastric Neuroendocrine Carcinoma After Radical Surgery. Front Oncol. 2021;11:533039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Ma F, Wang B, Xue L, Kang W, Li Y, Li W, Liu H, Ma S, Tian Y. Neoadjuvant chemotherapy improves the survival of patients with neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma of the stomach. J Cancer Res Clin Oncol. 2020;146:2135-2142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Ishida M, Sekine S, Fukagawa T, Ohashi M, Morita S, Taniguchi H, Katai H, Tsuda H, Kushima R. Neuroendocrine carcinoma of the stomach: morphologic and immunohistochemical characteristics and prognosis. Am J Surg Pathol. 2013;37:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Tierney JF, Poirier J, Chivukula S, Pappas SG, Hertl M, Schadde E, Keutgen X. Primary Tumor Site Affects Survival in Patients with Gastroenteropancreatic and Neuroendocrine Liver Metastases. Int J Endocrinol. 2019;2019:9871319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Liang WQ, Zhang W, Qiao S, Wang BH, Wang C, Zhuang ZW, Xi HQ, Cai AZ, Wei B, Chen L. [Clinicopathologic features and prognostic analysis of 240 patients with gastric neuroendocrine neoplasms]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Zhang P, Wang W, Lu M, Zeng C, Chen J, Li E, Tan H, Yu X, Tang Q, Zhao J, Shen L, Li J. Clinicopathological features and outcome for neuroendocrine neoplasms of gastroesophageal junction: A population-based study. Cancer Med. 2018;7:4361-4370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Fang C, Wang W, Zhang Y, Feng X, Zeng Y, Li Y, Chen J, Chen Y, Zhou Z. [Clinicopathological features and prognosis of patients with gastric neuroendocrine carcinoma from multiple centers in southern China]. Zhonghua Wei Chang Wai Ke Za Zhi. 2016;19:1230-1234. [PubMed] |
| 23. | Li Y, Wang YF, Tan BB, Er LM, Zhao Q, Fan LQ, Zhang ZD, Liu Y. [Pathological characteristics and survival analysis of 355 patients with gastroenteropancreatic neuroendocrine neoplasms]. Zhonghua Zhong Liu Za Zhi. 2020;42:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Morita M, Taguchi K, Kagawa M, Nakanoko T, Uehara H, Sugiyama M, Ota M, Ikebe M, Sugimachi K, Esaki T, Toh Y. Treatment strategies for neuroendocrine carcinoma of the upper digestive tract. Int J Clin Oncol. 2020;25:842-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Lu J, Zhao YJ, Zhou Y, He Q, Tian Y, Hao H, Qiu X, Jiang L, Zhao G, Huang CM. On behalf of the Study Group for Gastric Neuroendocrine Tumours. Modified staging system for gastric neuroendocrine carcinoma based on American Joint Committee on Cancer and European Neuroendocrine Tumor Society systems. Br J Surg. 2020;107:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, Caplin M, O'Toole D, Perren A; Vienna Consensus Conference participants. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 419] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 27. | Shen C, Chen H, Yin Y, Han L, Chen J, Tang S, Yin X, Zhou Z, Zhang B, Chen Z. Surgical treatment and prognosis of gastric neuroendocrine neoplasms: a single-center experience. BMC Gastroenterol. 2016;16:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Mao R, Li K, Cai JQ, Luo S, Turner M, Blazer D 3rd, Zhao H. Adjuvant Chemotherapy Versus Observation Following Resection for Patients With Nonmetastatic Poorly Differentiated Colorectal Neuroendocrine Carcinomas. Ann Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |