Published online Jun 7, 2021. doi: 10.3748/wjg.v27.i21.2727
Peer-review started: January 20, 2021
First decision: February 9, 2021
Revised: February 23, 2021
Accepted: April 13, 2021
Article in press: April 13, 2021
Published online: June 7, 2021
Processing time: 126 Days and 23.6 Hours
Hepatitis B virus (HBV) infection, although preventable by vaccination, remains a global health problem and a major cause of chronic liver disease. Although current treatment strategies suppress viral replication very efficiently, the optimal endpoint of hepatitis B surface antigen (HBsAg) clearance is rarely achieved. Moreover, the thorny problems of persistent chromatin-like covalently closed circular DNA and the presence of integrated HBV DNA in the host genome are ignored. Therefore, the scientific community has focused on developing innovative therapeutic approaches to achieve a functional cure of HBV, defined as undetectable HBV DNA and HBsAg loss over a limited treatment period. A deeper understanding of the HBV life cycle has led to the introduction of novel direct-acting antivirals that exert their function through multiple mechanisms, including inhibition of viral entry, transcriptional silencing, epigenetic manipulation, interference with capsid assembly, and disruption of HBsAg release. In parallel, another category of new drugs aims to restore dysregulated immune function in chronic hepatitis B accompanied by lethargic cellular and humoral responses. Stimulation of innate immunity by pattern-recognition receptor agonists leads to upregulation of antiviral cytokine expression and appears to contribute to HBV containment. Immune checkpoint inhibitors and adoptive transfer of genetically engineered T cells are breakthrough technologies currently being explored that may elicit potent HBV-specific T-cell responses. In addition, several clinical trials are attempting to clarify the role of therapeutic vaccination in this setting. Ultimately, it is increasingly recognized that elimination of HBV requires a treatment regimen based on a combination of multiple drugs. This review describes the rationale for progressive therapeutic interventions and discusses the latest findings in the field of HBV therapeutics.
Core Tip: Despite preventive vaccination and effective antiviral drugs, approximately 300 million people worldwide are at risk of liver-related morbidity and mortality from hepatitis B virus (HBV) infection. In the search for more effective strategies, research has focused on two broad categories of therapeutic intervention. First, direct-acting antivirals that interrupt various stages of the HBV life cycle are yielding promising results in clinical trials that show suppression of the HBV antigen load. Second, the need to address depleted immune responses and revive the tolerogenic liver microenvironment has brought immunotherapies to the forefront. It is likely that an upcoming treatment that combines agents from both classes will achieve HBV elimination.
- Citation: Tsounis EP, Tourkochristou E, Mouzaki A, Triantos C. Toward a new era of hepatitis B virus therapeutics: The pursuit of a functional cure. World J Gastroenterol 2021; 27(21): 2727-2757
- URL: https://www.wjgnet.com/1007-9327/full/v27/i21/2727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i21.2727
Despite the proliferation of effective vaccine programs and the introduction of well tolerated antiviral drugs, hepatitis B virus (HBV) infection remains a public health problem of international concern. The global hepatitis B surface antigen (HBsAg) seroprevalence is estimated to be 3.9%, corresponding to approximately 300 million infections[1]. HBV is a noncytopathic hepatotropic virus that causes acute or chronic infection leading to hepatic complications such as cirrhosis or hepatocellular carcinoma (HCC) and is responsible for more than 884000 deaths annually[2]. Halting the progression of end-stage liver disease and preventing the resulting liver-related mortality is the fundamental goal of HBV treatment. Current therapeutic agents, namely nucleoside or nucleotide drugs (NUCs) and interferon-α (IFN-α), have as their main goal the sustained suppression of viral replication and consequently the regression of hepatic necroinflammatory activity. Achieving undetectable hepatitis B e antigen (HBeAg) levels, with or without hepatitis B e antibody seroconversion, is an important goal that indicates partial immune control of chronic infection. Sustained loss of HBsAg is an optimal endpoint demonstrating profound suppression of viral transcription[3]. NUCs inhibit reverse transcription and are highly effective in suppressing serum HBV DNA levels, even below the limit of detection, providing a high barrier to viral resistance. However, HBsAg clearance is rarely observed even after long-term treatment (10%), and treatment discontinuation is associated with significant rates of virological or clinical relapse[4]. On the other hand, alternative IFN-based monotherapy, although providing a more durable response after treatment, is fraught with significant side effects, making it suitable only for some patient subgroups[5]. Obviously, a functional cure, defined as undetectable HBV DNA levels and loss of HBsAg over a finite treatment period, is the exception rather than the rule of the current standard of care[6].
The inefficiency of NUCs and IFN-α in eradicating HBV infection is primarily a result of their inability to eliminate episomal covalently closed circular DNA (cccDNA) and the integrated viral genome[7]. Notably, intrahepatic HBV DNA synthesis has been reported to persist despite the administration of NUCs, thus maintaining the cccDNA pool either by intracellular replenishment or by new rounds of infection caused by residual viremia[8,9]. Apparently, the propensity of HBV to chronify is closely related to the stability and persistence of cccDNA, which serves as a transcriptional template for viral replication and viral antigen production[10]. Moreover, integrated HBV DNA has been confirmed as the dominant source of HBsAg in HBe-negative individuals, revealing an additional viral strategy to maintain infectivity[11]. In parallel, HBV exploits the tolerogenic microenvironment of the liver to secrete large amounts of antigens that cause CD4+ and CD8+ T-cell and B-cell anergy or exhaustion. The HBV-induced immunosuppressive cascade and associated dysregulation of innate and adaptive immune responses contribute not only to the establishment of chronic infection but also to fibrosis progression and HCC development[12].
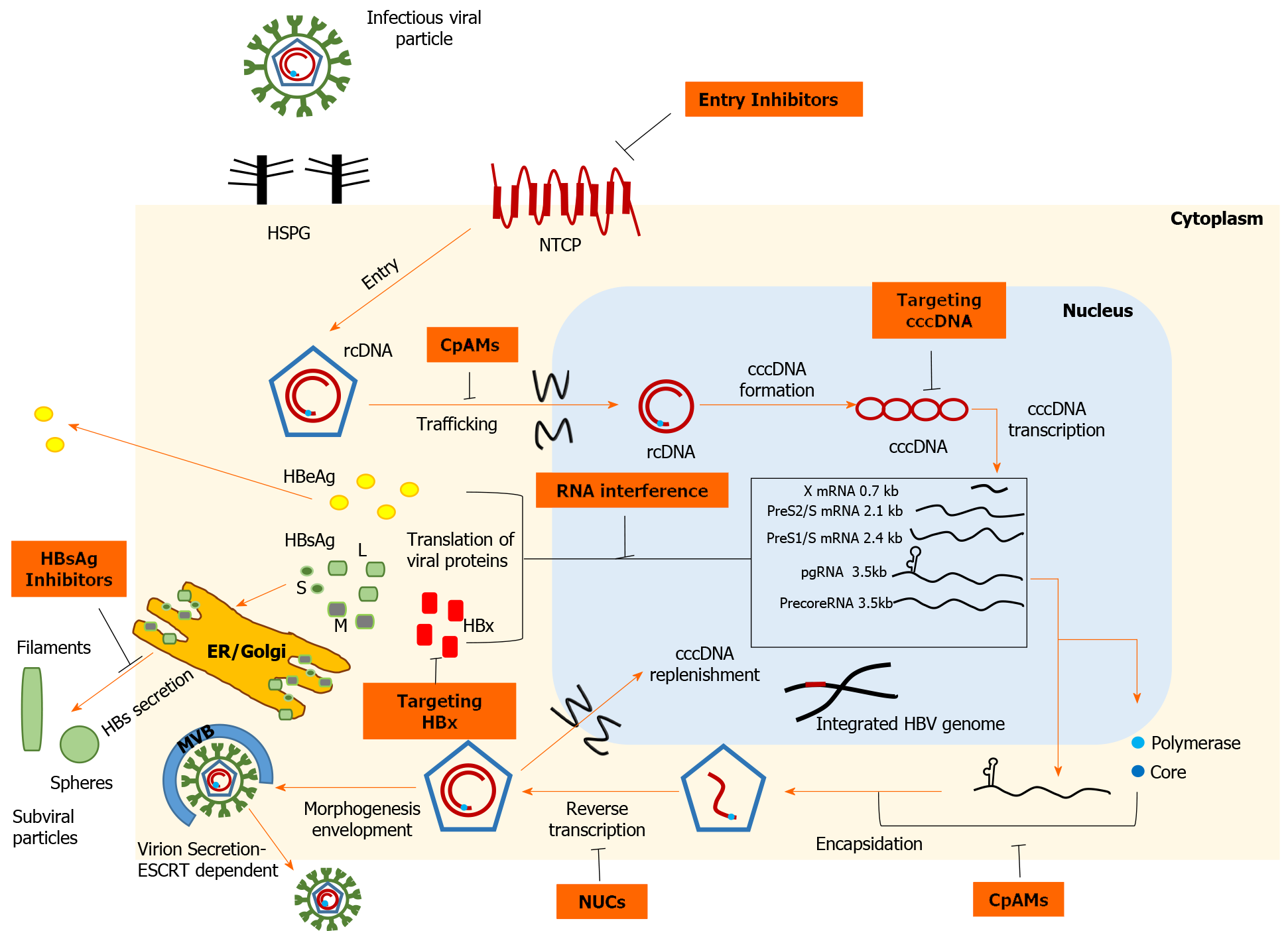
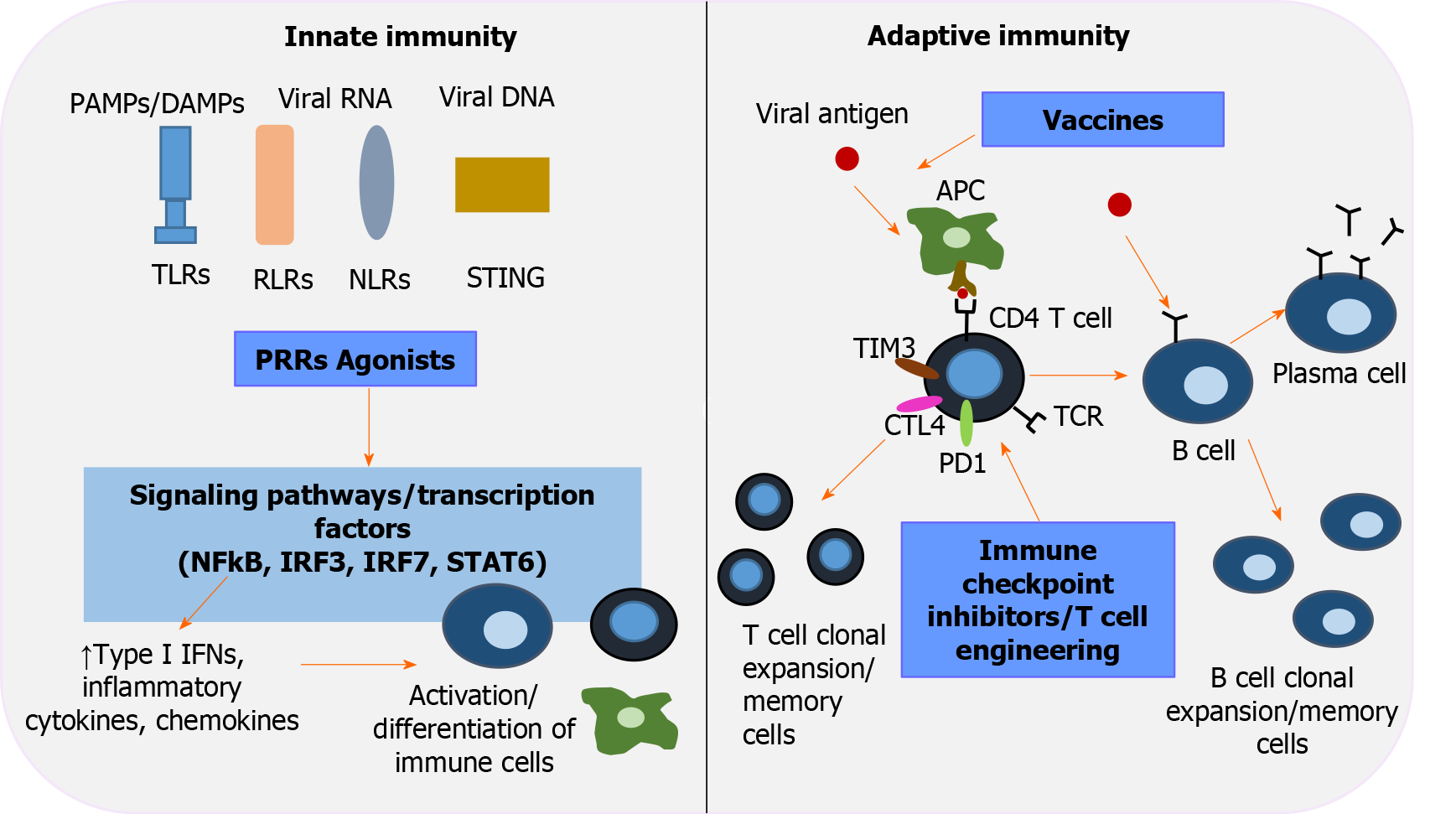
The track record in the field of anti-HCV therapy has reinvigorated research directed toward development of novel anti-HBV drugs aimed at improving cure rates by direct action on cccDNA. In this review, we briefly examine the HBV life cycle (Figure 1) to elucidate the mechanisms of action of emerging direct-acting antivirals (DAAs), which are then further analyzed. We also discuss the other major categories of agents in development that constitute immunotherapies to restore HBV-specific host responses (Figure 2).
We conducted a comprehensive review of all drug classes in development for chronic hepatitis B (CHB) treatment. The Medline database was searched for English articles published up to December 2020. The search words and strategy were “chronic hepatitis B” AND (“therapy” OR “treatment”) AND (“investigational” OR “experimental” OR “pilot”). The database search yielded 942 articles. The Drug Watch List on the Hepatitis B Foundation website (https://www.hepb.org) was accessed to identify developing therapies in that setting. Abstracts presented at major hepatology conferences through December 2020 were reviewed and summarized. In addition, the reference lists of all the above materials were reviewed for relevant articles. The Clinicaltrial.gov database was accessed to identify clinical trials investigating novel anti-HBV therapeutics. Electronic and manual searches were performed independently by two of the authors (Tsounis EP and Tourkochristou E). Beyond an all-inclusive search, emphasis was placed on drug candidates investigated in late-stage clinical trials or on agents showing potential for functional cure.
HBV is a relatively small (3.2 kb) DNA virus characterized by a compact organization of its genome, which contains four partially or completely overlapping open reading frames (ORFs). The HBV genome encodes seven proteins; L-, M-, S-HBsAg, core, polymerase and the non-structural precore/HBeAg and HBV X protein (HBx). The infectious virion or Dane particle consists of an outer shell of HBsAg floating in a lipid bilayer around the viral nucleocapsid that encases the partially double-stranded, relaxed, circular HBV DNA (rcDNA) with a copy of its polymerase. Initially, the interaction of HBV particles with the carbohydrate side chains of hepatocyte heparan sulfate proteoglycans (HSPGs) appears to initiate the attachment process[13]. The role of glypican-5, which is an additional factor facilitating viral attachment to the host cell, has also been described[14]. Virus entry into the host cell is mediated by human sodium taurocholate co-transport peptide (NTCP), a bile acid transporter specifically expressed in human liver cells, thus conferring HBV its hepatotropic property[15]. The N-terminal end of the PreS1 domain of L-HBsAg is essential for docking to the NTCP receptor[16]. The aforementioned domain also interacts with the clathrin heavy chain and the corresponding adaptor protein 2, suggesting clathrin-mediated endocytosis of the NTCP-HBV complex[17]. After fusion has occurred, the nucleocapsid, detached from its outer shell, floats in the cytoplasm and is transferred to the perinuclear region by mechanisms that remain to be elucidated. Upon approaching the nuclear pore complex, the interaction of the nucleocapsid with nucleoporin 153, an integral component of transport via importin β, enables translocation of the viral genome across the nuclear membrane[18]. After the capsid disintegrates at the nuclear pore, the rcDNA and attached polymerase are released into the nucleoplasm[19]. The host repair apparatus is then recruited to convert rcDNA into cccDNA, which resembles a nucleosomally bound mini-chromosome[20]. In this structure, the viral genome is characterized by a long half-life and serves as a transcriptional template. Several epigenetic factors that modify histone acetylation and methylation, together with HBx and core proteins (Cp), appear to orchestrate transcriptional regulation[21]. The host RNA polymerase II and supporting hepatocyte-specific transcription factors [hepatocyte nuclear factor (HNF) 1α, HNF4α] use cccDNA to synthesize the 5'-capped and 3'-polyadenylated viral transcripts, which are subsequently exported to the cytoplasm for translation[22]. The largest ORF generates greater-than-genome-length pregenomic RNA (pgRNA), which not only encodes polymerase and Cp but is also reverse transcribed to generate HBV DNA copies. The encapsidation signal ε, located at the 5'-end of pgRNA, recruits RNA polymerase and triggers encapsidation of the duplex, a process realized by the 5'-cap arrangement, the arginine-rich C-terminal domain of Cp, and host eIF4E and heat shock proteins[22-24]. After rcDNA synthesis by reverse transcription, the mature nucleocapsid can follow two different pathways. In early stages of infection, nucleocapsids return to the nuclear basket to amplify the cccDNA pool[25]. However, when a sufficient amount of HBsAg has been synthesized, the nucleocapsids bud through the endoplasmic reticulum (ER) membrane, where envelope glycoproteins settle to acquire their outer envelope[22,26]. Unlike nonin
Recent data suggest that in the absence of reinfection, proliferating hepatocytes significantly decrease the intranuclear cccDNA reservoir even without the use of cytolytic mechanisms[28]. In this setting, efficient prevention of HBV entry into the host cell could significantly curb HBV infection. Currently, the only approved agent that inhibits HBV entry is hepatitis B immunoglobulin (HBIG), a mixture of polyclonal antibodies derived from pooled human plasma from immunized donors. Antibody binding to infectivity regions within the envelope proteins, i.e. the antigenic loop of the S domain and the receptor binding site of the preS1 domain, causes antibody-mediated neutralization of virions and prevents infection of hepatocytes[29]. Nevertheless, HBIG is produced only in limited quantities by an expensive and time-consuming purification process, limiting its clinical use only for prophylaxis. In an attempt to overcome these barriers, research has focused on the development of several monoclonal antibodies (HzKR359-1, HzKR127-3.2) targeting specific epitopes of surface proteins, and preclinical studies show promising results[30]. GC1102 is a recombinant anti-HBs monoclonal antibody that is being studied in combination with NUC in a Phase II trial (NCT03801798)[31]. The early interaction of a virion with low affinity for HSPGs represents a potential point for therapeutic intervention. Indeed, heparin and suramin bind to HBV envelope proteins and disrupt viral binding to HSPGs, whereas synthetic antilipopolysaccharide peptides bind directly to HSPGs and block them[29,32,33]. Interestingly, an alternative pathway has recently been described for IFN-α to attenuate viral infectivity by secretion of soluble factors that alter HSPG function[34]. Proanthocyanidin and its oligomeric flavonoid analogs may have a therapeutic role by targeting the preS1 region of the large surface protein and inhibiting HBV uptake from the NTCP receptor without exerting serious cytotoxic effects[35]. Agents that block the NTCP receptor represent realistic and achievable treatment options and can be divided into two groups, NTCP substrates and agents that bind to NTCP without being transported[29]. Indeed, conjugated bile acids, including taurochol, ursodeoxychol, and tauroursodeoxychol, have shown antiviral activity in cell culture systems by limiting HBV/hepatitis delta virus (HDV) infection[36]. However, efficient inhibition requires bile acid concentrations well beyond the physiological range, so long-term administration could be associated with side effects. Moreover, in vitro experiments have shown that NTCP-mediated HBV entry into hepatocytes could be limited by already approved therapeutic agents, namely the antihypertensive drug irbesartan and the hypolipidemic agent ezetimibe[37,38]. On the other hand, cyclosporine A analogs and peptides derived from the HBV preS1 region constitute a second group of nonsubstrate ligands that inhibit NTCP. Although cyclosporine A, a commonly used immunosuppressant, has significant anti-HBV activity, it was associated with severe dysregulation of bile acid uptake in hepatocytes[39]. Nevertheless, the antiviral effect of cyclosporine A derivatives (SCY450, SCY995) has been demonstrated, which may be functionally separate from bile acid transport[40]. Bulevirtide, formerly known as Myrcludex B, is a synthetic myristoylated peptide derived from the preS1 domain of L-HBsAg that irreversibly blocks NTCP at nonsaturating concentrations[29]. Bulevirtide induced HBV DNA decline and contributed to alanine aminotransferase (ALT) normalization in an open-label, randomized, Phase Ib/IIa study including 48 HBeAg-negative patients with CHB (NCT02881008). However, HBsAg responses were weak, so research has primarily focused on investigating its value regarding HBV/HDV coinfection; a Phase III clinical trial of bulevirtide in this setting is ongoing (NCT03852719). A recent study found that administration of high-dose bulevirtide in patients with HBV/HDV coinfection was safe and well tolerated. Thirty patients were randomized 1:1 to receive 10 mg of bulevirtide plus pegylated interferon-alpha (Peg-IFN-α) or tenofovir for 48 wk. At week 48, remarkable HDV RNA suppression was observed in both treatment arms (median reduction of 4 log10 IU/mL); 86.7% of patients in the Peg-IFN group had undetectable HDV RNA levels and 40% of participants receiving tenofovir achieved ALT normalization[41].
The plasmid-like intranuclear cccDNA plays a central role in the HBV life cycle, serving as a template for the production of viral antigens and thus for the progeny of virions. Therefore, treatment strategies that impede cccDNA formation, promote its degradation, or support transcriptional silencing may be of great importance[42]. IFN-α and lymphotoxin-β receptor agonists upregulate the nuclear deaminases apolipoprotein B mRNA-editing enzyme and catalytic polypeptide-like (APOBEC) 3A/3B, thus causing noncytolytic degradation of cccDNA, but without complete depletion of the reservoir[43]. A similar mechanism of action has been reported for various interferons and antiviral cytokines (IFN-γ, IFN-β, IFN-λ1/λ2, TNF-α)[44,45]. Moreover, cccDNA degradation can be enhanced by destabilizing molecules. In that regard, oral administration of agent ccc_R08, derived from small molecule library research, achieved sustained declines of HBsAg and cccDNA by cccDNA destabilization in a mouse model of HBV infection[46]. Genetic editing technology provides new tools to inactivate or eliminate cccDNA. Meganucleases, zinc finger nucleases, transcription activator-like effector nucleases, and CRISPR-associated (Cas) nucleases are cleaving enzymes that target specific DNA sequences. Subsequent misrepair of break sites leads to an increased mutation rate and undermines cccDNA stability. In experimental models, cleavage proteins have shown significant antiviral activity[47-49]. In vitro experiments of the CRISPR/Cas9 system show significant functional inactivation of cccDNA by mutagenesis, inducing suppression of viral replication, and potent synergy with NUCs[50,51]. Recent evidence suggests that additive modulation of host DNA repair pathways enhances the antiviral activity of CRISPR/Cas9 and causes profound reduction of intracellular pgRNA[52]. However, fragments of viral DNA appear to be repaired in transcriptionally active "small cccDNA" formations[51]. In addition, identifying conserved cccDNA regions, ensuring effective transfer into hepatocytes, and minimizing the risk of host genetic damage from HBV DNA integration are important hurdles that must be overcome before this technology can be applied to humans. The chromatin-based structure of cccDNA is amenable to epigenetic manipulations that allow intervention at the transcriptional level. Indeed, IFN-α triggers histone hypoacetylation of cccDNA, inhibits binding of the transcription factor signal transducer and activator of transcription (STAT) 1 and STAT2 to active cccDNA, and collects corepressors, suggesting that IFN-associated antiviral activity is exerted to some extent by epigenetic mechanisms[53]. Similarly, suppression of cccDNA transcription has been observed in cell culture models following administration of the small molecule C646, which is a selective inhibitor of the histone acetyltransferases CREB binding protein and p300[54]. GS-5801 is a prodrug of a lysine demethylase-5 inhibitor and has shown antiviral activity in HBV-infected primary human hepatocytes by remodeling the chromatin methylation pattern[55]. In addition, the selective synthetic farnesoid X receptor agonist EYP001, originally developed as a therapeutic for nonalcoholic steatohepatitis, inhibits cccDNA transcription in vitro and had a satisfactory safety profile when given to CHB patients[56]. A Phase II trial of EYP001 in combination with NUCs against CHB(NCT04465916) is currently recruiting participants.
The HBx protein is essential for HBV replication by causing epigenetic regulation of the extrachromosomal viral genome, making it an attractive target for attenuating transcription. HBx promotes ubiquitin-mediated degradation of structural maintenance of chromosomes 5/6 (Smc5/6), a restriction factor of cccDNA transcription[57]. Nitazoxide, a thiazolid-derived antiprotozoal agent, blocks the interaction of HBx and host protein DNA damage-binding protein 1, which is essential for ubiquitination of Smc5/6, and reduces viral antigen production in the HBV minicircle system[58]. Administration of nitazoxide in humans has produced favorable results, with serum HBV DNA undetectable in 8 of 9 patients after 4 to 20 wk of treatment[59]. A 12-mo, placebo-controlled, Phase II trial of nitazoxanide in virologically suppressed HBeAg-negative patients is ongoing to evaluate the efficacy of nitazoxide and the pharmacokinetic relationships with standard NUC treatment (NCT03905655). In the same context, pevonedistat inhibits activation of neural precursor cell expressed, developmentally downregulated 8 (NEDD-8), a ubiquitin-like protein critical for the ubiquitin-proteosome pathway, and minimizes cccDNA transcription by restoring Smc5/6 levels[60]. In addition, HBx promotes overexpression of the histone deacetylase sirtuin-2, which in turn promotes HBV replication and has been linked to hepatocarcinogenesis. AGK2 is a selective inhibitor of sirtuin-2, and was found to reduce HBV antigen production in both in vivo and in vitro studies[61]. Finally, significant antiviral activity was observed in a transgenic mouse model using CRV431, a cyclophilin inhibitor that blocks the formation of the HBx-cyclophilin A complex in a dose-dependent manner[62]. Genetic engineering has made it possible to knock down HBx gene expression using CRISPR/Cas9 technology. Specifically, a custom CRISPR/Cas9 system targeting a specific sequence of the HBx gene has been developed and tested in HCC cells containing an integrated HBV genome. Apart from decreasing HBx and HBsAg production, HBx/CRISPR reduced the epithelial-mesenchymal transition capacity and stem-cell properties of HCC cells, revealing a perspective for controlling HBV-associated tumor progression[63].
HBV hijacks host translational machinery to produce proteins that ensure survival and replication. It is becoming clear that agents that accelerate the degradation of viral transcripts or silence their expression could be a useful means of controlling the viral antigen load. Reduced viral replication and the emerging stimulation of the host immune response could therefore pave the way for a functional cure. RNA therapeutics have gained popularity on the battlefield against HBV, with two basic approaches being used in clinical trials, double-stranded small interfering RNAs (siRNAs) and antisense oligonucleotides (ASOs)[64]. siRNA interacts with the RNA-induced silencing complex (RISC) and the endonuclease argonaut 2 (AGO2). When siRNA docks with RISC, its two strands are separated and the coding strand is destroyed. The antisense strand binds to the corresponding sequence of the target RNA, which is cleaved by AGO2, and the residues are degraded by exonucleases[64]. All HBV transcripts, including subgenomic and pgRNA, have an identical 3' terminus that is a suitable target for simultaneous silencing of all viral transcripts. Several siRNAs have been developed and tested in animal models, and some of them have found their way from the laboratory bench to clinical trials. The first anti-HBV RNAi-based therapeutic to be administered in humans was ARC-520, which contains two different siRNAs conjugated to cholesterol that promotes uptake into hepatocytes and a polymer-based excipient (ARC -EX1) that enhances endosomal release into the cytoplasm. A significant reduction in HBsAg was observed (mean reduction of 0.38 log10 IU/mL in HBeAg-negative and 0.54 log10 IU/mL and in HBeAg-positive patients) that persisted for at least 80 d after the last dose[65]. Nevertheless, in HBeAg-negative and NUC-experienced individuals, a substantial portion of HBsAg is thought to be derived from integrated HBV DNA[10]. Deletion of HBV DNA regions upon integration resulted in truncated mRNAs lacking the sequence recognized by siRNAs, which reduced the efficiency of RNAi in the aforementioned patients[65].
JNJ-3989 (formerly ARO-HBV), which consists of two siRNAs that target hepatocytes and the entire HBV transcriptome was developed in an attempt to circumvent that problem and target both cccDNA and integrated transcripts[66]. Regardless of HBe serostatus, three monthly doses of JNJ-3989 on days 1, 27, and 57, combined with daily NUC treatment, resulted in profound HBsAg reduction along with significant reductions of HBV DNA, HBV RNA, and hepatitis B core antigen (HBcAg). Remarkably, 22/39 (56%) of patients achieved a sustained HBsAg response (defined as ≥ 1.0 log10 IU/mL reduction) even 9 mo after the last JNJ-3989 injection[66]. VIR-2218 (formerly ALN-HBV) is another a liver-targeted pangenotypic siRNA. In a Phase II clinical trial, 24 CHB patients undergoing NUC treatment were administered two monthly doses of VIR-2218 on days 1 and 29. Preliminary results found a pivotal HBsAg decline that persisted in a subset of patients for 28 wk after treatment (a mean decline of 1.0 log10 IU/mL) and suggest that different patterns of response may underestimate the ultimate therapeutic effect of VIR-2218[67]. The lipid nanoparticle platform-based delivery technology ARB-1467 consists of three siRNAs directed against all HBV transcripts. Single and multiple doses of ARB-1467 induced significant HBsAg decline in a Phase II study, independent of HBeAg status[68]. An alternative delivery technology utilizes the RNAi therapeutic AB-729 conjugated to a trimer of N-acetylgalactosamine (GalNAc) ligand that promotes uptake into hepatocytes via the asialoglycoprotein receptor (ASGR). Preliminary results of a phase I study show that a single dose of AB-729 resulted in a profound HBsAg decline in CHB patients on stable NUC therapy that persisted for 12 wk after treatment[69]. RG6346 also uses GalNAc-RNAi technology and has demonstrated a reduction in markers of viral activity and stimulation of the immune-mediated host response in a Phase Ib/IIa study, as evidenced by self-resolving ALT relapses. No serious adverse events were noted and 11 of 12 (92%) NUC-suppressed CHB patients receiving four monthly doses of RG6346 achieved an HBsAg drop ≥ 1.0 log10 IU/mL[70].
ASOs are small single-stranded DNA or RNA oligomers that silence the target mRNA by various mechanisms including inhibition of 5' cap formation, steric disruption of translation, or activation of RNase H[64]. GSK3389404 (Ionis-HBV-LRx) is an ASO-GalNAc conjugate that targets all four ORFs of HBV and exerts its function via its active metabolite GSK3228836 (Ionis-HBVRx), which binds to the complementary target mRNA. The ASO/RNA duplex is degraded in the nucleus via an RNAase H1-dependent pathway[71]. The results of a recent double-blind, placebo-controlled Phase II trial in which CHB patients were treated with multiple doses of GSK3228836 over a 4-wk period showed a significant reduction in HBsAg in patients treated with NUC (mean decrease of 2.5 log10 IU/mL) and in NUC-naïve patients (mean decrease of 1.6 log10 IU/mL)[72]. The simultaneous suppression of HBcAg and HBV RNA suggests a remarkable downregulation of cccDNA transcriptional activity[73]. ALG-020572 and ALG-020576 are GalNAc-conjugated ASOs that have superior antiviral efficacy compared with GSK3228836 in mouse models. Promisingly, the combination of the two agents in a 1:1 ratio shows potent antiviral activity with pangenotypic coverage even in viral genome integration, suggesting a synergism with a variety of anti-HBV therapeutics[74]. Similarly, RO7062931 is a single-stranded oligonucleotide based on locked nucleic acid (LNA) technology. It was tested in a phase I clinical trial in which 59 CHB patients on established NUC therapy were randomized to receive either placebo or 2-5 injections of the compound. A dose-dependent reduction in HBsAg was observed and was independent of HBe serostatus, but no sustained response to treatment was observed[75]. GapmeR is another antisense LNA that benefits from hyperconserved regions at the 5' end of the HBx gene and efficiently suppresses HBV expression in vitro[76]. Reducing off-target toxicity, improving hepatocyte availability, and increasing affinity for a wide range of quasispecies are essential for the new generation of RNAi therapeutics. However, the inability to directly eradicate cccDNA and evolutionary pressures leading to increased HBV mutation rates diminish their efficacy and may limit their application as only a component of upcoming combination therapy.
Small molecule drug candidates that silence gene expression have performed well in preclinical studies. RG7834 is the first orally bioavailable, highly selective dihydroquinolizinone-based small molecule to produce significant HBV DNA suppression and HBsAg regression in animal models[77]. The proposed mechanism of action involves accelerated intranuclear viral mRNA degradation, which requires the posttranscriptional regulatory element of HBV rather than inhibition of transcrip
The Cp is a relatively small protein comprising 183 residues and is the basic element of the nucleocapsid. The N-terminal region is responsible for the assembly function and associated homodimer structure of Cp. Multimerization of 120 core dimers generates the icosahedral structure of the mature nucleocapsid. However, Cp is not only a static carrier of the HBV genome but also has a multifunctional role. The arginine-rich C-terminal domain is required for pgRNA capsidation, coordinates reverse transcription initiation, and regulates intracellular transport pathways via nuclear localization signals. In addition, Cp makes epigenetic modifications to cccDNA and interacts with HBsAg to promote virion morphogenesis[79]. Imprinting of the nucleocapsid at various points in the viral life cycle implies that allosteric modulation of Cp may be an effective therapeutic option. Nuclear protein allosteric modulators (CpAMs) interfere with the assembly process by binding to a hydrophobic pocket within the dimer-dimer interface, thereby triggering changes in the quaternary structure[80]. Based on their pharmacological properties, we recognize two classes of CpAMs. Type I CpAMs, typically heteroaryldihydropyrimidine analogs (HAP), alter the kinetics of Cp oligomerization and promote the formation of large aberrant, nonencapsulated polymers that are prone to degradation or intracellular aggregation. Phenylpropenamides and sulfamoylbenzamides (SBAs) are the major II type CpAMs and cause acceleration of the assembly process, resulting in nascent capsids that lack the pgRNA polymerase complex-important for HBV replication[80]. In addition to limiting rcDNA new synthesis, certain CpAMs inhibit de novo cccDNA formation by disrupting nucleocapsid uncoating during the initial steps of the viral life cycle[81].
Numerous pharmaceuticals targeting Cp have been tested in clinical trials. RO7049389 is an orally administered compound that belongs to class I of CpAMs and has shown a satisfactory safety and tolerability profile. When administered to patients with CHB over a 28-d period, RO7049389 resulted in a significant reduction in HBV DNA (median decrease of 2.66-3.2 log10 IU/mL). Remarkably, HBV DNA decreased below the limit of detection in 13/16 HBeAg-negative patients[82]. Currently, RO7049389 is being studied in a Phase II trial as part of a combination therapy that also includes an immunomodulator and an NUC (NCT04225715). GLS4 and KL060332, which exhibit potent antiviral activity and low cytotoxic effects in experimental models, are among the HAP derivatives that promote defective capsid assembly and have successfully entered clinical development[83,84].
The first SBA derivative NVR 3-778 was administered in a placebo-controlled, multicenter Phase I study to 73 HBeAg-negative patients over a treatment period of 4 wk. Significant reductions in HBV DNA and RNA were observed at daily doses > 1000 mg, but HBsAg levels were unaffected. The mean HBV DNA change was more pronounced in patients receiving NVR 3-778 plus Peg-IFN (−1.97 log10 IU/mL) than in patients receiving NVR 3-778 monotherapy (−1.43 log10 IU/mL) or Peg-IFN monotherapy (−1.06 log10 IU/mL)[85]. After meeting safety standards, JNJ-6379, another potent SBA derivative that manipulates nuclear protein to form empty capsids and affects the kinetics of nucleocapsid degradation, was enrolled in a Phase II clinical trial[86]. Preliminary data from 88 unpretreated and 84 virologically suppressed patients randomized to receive 75 mg or 250 mg of JNJ-6379 plus NUC or placebo plus NUC support more pronounced HBV DNA and RNA suppression in untreated patients receiving dual therapy. Importantly, a median HBsAg reduction of 0.4 log10 IU/mL was observed in untreated HBeAg-positive patients receiving 250 mg JNJ-6379 plus NUC[87]. JNJ-6379 is under investigation in a Phase II trial evaluating the safety and efficacy of a triple combination of anti-HBV therapies along with a NUC and the RNAi-based JNJ-3989 (NCT03982186). AB-423, AB-506 and EDP-514 are II CpAMs that have entered clinical trials (Table 1). AB-506 was discontinued due to 2 cases of acute hepatitis during a 28-d phase Ia study in healthy volunteers (ACTRN-12618000 987268).
| Compound | Class and action | Phase of development | Ref. or trial number | |
| Entry Inhibitors | HBIG | Polyclonal antibodies neutralizing HBsAg | Available | Herrscher et al[29] |
| GC1102 | Monoclonal antibody neutralizing HBsAg | Phase II | NCT03801798 | |
| HzKR359-1, HzKR127-3.2 | Anti-preS1 monoclonal antibodies | Preclinical | Wi et al[30] | |
| Heparin, Suramin | Inhibition of HBV-HSPGs interaction | Preclinical | Herrscher et al[29] and Petcu et al[33] | |
| SALPs | Inhibition of HBV-HSPGs interaction | Preclinical | Krepstakies et al[32] | |
| PAC and analogs | Anti-preS1 oligomeric flavonoid analogs | Preclinical | Tsukuda et al[35] | |
| Conjugated bile acids (TCA, UDCA, TUDCA) | NTCP inhibitors | Preclinical | Yan et al[36] | |
| Ezetimibe, Irbesartan | NTCP inhibitors | Available | Lucifora et al[37] and Ko et al[38] | |
| Cyclosporine A analogs (SCY450, SCY995) | NTCP inhibitors | Preclinical | Watashi et al[39] and Shimura et al[40] | |
| Bulevirtide(Myrcludex B) | NTCP inhibitor | Phase III | Wedemeyer et al[41] | |
| TargetingcccDNA | Interferons, TNF-α, Lymphotoxin-β receptor agonists | cccDNA degradation | Available | Bockmann et al[44] and Xia et al[45] |
| ccc-R08 | cccDNA destabilizer | Preclinical | Wang et al[46] | |
| Zinc finger nucleases | Gene editing technology | Preclinical | Cradick et al[47] | |
| TALENs | Gene editing technology | Preclinical | Chen et al[49] | |
| CRISPR‐associated (Cas) nucleases | Gene editing technology | Preclinical | Ramanan et al[48], Seeger and Sohn[50], and Martinez et al[51] | |
| C646 | CBP and p300 inhibitorEpigenetic silencing | Preclinical | Cougot et al[54] | |
| GS-5801 | Lysine demethylase 5 inhibitorEpigenetic silencing | Phase I | Gilmore et al[55] | |
| EYP001 | FXR agonist | Phase II | Erken et al[56] | |
| TargetingHBx | Nitazoxide | HBx-DDB1 interaction inhibitor | Phase II | Sekiba et al[58], Rossignol and Bréchot[59] |
| Pevonedistat | NEDD8-activating enzyme inhibitor | Preclinical | Sekiba et al[60] | |
| AGK2 | SIRT-2 inhibitor | Preclinical | Yu et al[61] | |
| CRV431 | Cyclophilin inhibitor | Phase I | NCT03596697 | |
| RNA interference | JNJ-3989 | siRNA | Phase II | Gane et al[66] |
| VIR-2218 (or ALN-HBV) | siRNA | Phase II | Gane et al[67] | |
| ARB-1467 | siRNA | Phase II | Streinu-Cerce et al[68] | |
| ARB-1740 | siRNA | Phase I | Thi et al[191] | |
| AB-729 | siRNA | Phase I | Yuen et al[69] | |
| RG6346 (or DCR-HBVS) | siRNA | Phase II | Yuen et al[70] | |
| GSK3228836, GSK33389404 | ASOs | Phase II | Han et al[71] and Yuen et al[72] | |
| ALG-020572, ALG-020576 | ASOs | Preclinical | Hong et al[74] | |
| RO7062931 (or RG6004) | Antisense LNA | Phase I | Yuen et al[75] | |
| Gapmers | Antisense LNA | Preclinical | Cortese et al[76] | |
| RG7834 | RNA destabilizer | Preclinical | Mueller et al[77] and Zhou et al[78] | |
| Core protein allosteric modulators (CpAMs) | RO7049389 (or RG7907) | Class I CpAM (HAP derivative) | Phase II | Gane et al[82] |
| GLS4 (or morphothiadine) | Class I CpAM (HAP derivative) | Phase II | Zhao et al[83] | |
| KL060332 | Class I CpAM (HAP derivative) | Phase I | Tai et al[84] | |
| NVR3-778 | Class II CpAM (SBA derivative) | Phase I | Yuen et al[85] | |
| JNJ-6379 | Class II CpAM (SBA derivative) | Phase II | Berke et al[86] and Janssen et al[87] | |
| AB-423 | Class II CpAM (SBA derivative) | Phase I | Mani et al[192] | |
| EDP-514 | Class II CpAM | Phase I | NCT04470388 | |
| ABI-H0731 | CpAM | Phase II | Fung et al[88] and Yuen et al[89] | |
| ABI-H2158 | CpAM | Phase II | Agarwal et al[90] | |
| ABI-H3733 | CpAM | Phase I | NCT04271592 | |
| QL-007 | CpAM | Phase I | NCT03770624 | |
| ZM-H1505R | CpAM (Pyrazole derivative) | Phase I | NCT04220801 | |
| ALG-001075, ALG-000184 | Class II CpAMs | Preclinical | Zhang et al[193] | |
| GLP-26 | Class II CpAM (SBA derivative) | Preclinical | Amblard et al[194] | |
| AT-61, AT-130 | Class II CpAMs (PPA derivatives) | Preclinical | Delaney et al[195] | |
| Phthalazinone derivatives | CpAMs | Preclinical | Chen et al[196] | |
| HBsAg inhibitors | REP 2139, REP 2156 | NAPsSVP release inhibitors | Phase II | Bazinet et al[95] |
| ALG-010133 | STOPS | Phase I | Nie et al[94] and Gohil et al[96] |
Data from two phase II studies of the first-generation core inhibitor ABI-H0731 strongly support further development of that agent. The first is an ongoing, double-blind, placebo-controlled study in virologically suppressed patients randomized to receive either ABI-H0731 (300 mg daily) plus NUC or placebo plus NUC (NCT03576066). After 24 wk of treatment, a higher proportion of patients treated with combination therapy achieved undetectable HBV DNA, as measured by high-sensitivity PCR, compared with those receiving placebo plus NUC [15/16 (94%) vs 7/10 (70%)][88]. The second study was an open-label extension study (NCT03780543) that demonstrated sustained reductions in HBV DNA, RNA, and viral antigens in patients treated with ABI-H0731 plus NUC for more than 1 year[89]. ABI-H2158 is a second-generation core inhibitor. Recently, data were published from a phase Ib study in which HBeAg-positive patients were blindly randomized to receive 100, 300, or 500 mg ABI-H2158 or placebo once daily for 14 d. The changes in HBV DNA from baseline to day 15 were −2.3 log10 IU/mL, −2.5 log10 IU/mL, −2.7 log10 IU/mL, and −0.1 log10 IU/mL, respectively[90]. QL-007, ZM-H1505R, ALG-001075, ALG-000184, GLP-26, AT-61, AT-130, and phthalazinone derivatives are other CpAMs in the pipeline for an HBV cure (Table 1).
In the sera of infected patients, in addition to infectious Dane particles, we find an excess (even 10000-fold) of empty SVPs in the form of spheres and filaments composed of envelope proteins. SVPs transport most of the circulating HBsAg, interfere with signaling pathways of innate and adaptive immunity, and thus contribute to viral persistence[91]. Nucleic acid polymers (NAPs) act through phosphorothioated oligonucleotides to disrupt apolipoprotein interactions involved in SVP assembly and secretion[92]. Analysis of the relative changes in HBsAg isoforms during NAP administration in CHB patients suggests a stronger effect on S-HBsAg. As S-HBsAg is the major component of SVPs, it can be inferred that NAPs act by selectively inhibiting SVP release[93]. S-antigen transport inhibitory oligonucleotide polymers (STOPS) share structural similarities with NAPs, implying a comparable mechanism of action. Moreover, STOPs are characterized by distinct chemical properties that provide enhanced efficacy in different HBV cell lines, including those with a high degree of HBV integration[94]. Therefore, elimination of HBsAg by inhibition of SVP secretion and consequent abrogation of immunosuppressive effect reflects a significant therapeutic opportunity.
REP-401 was an open-label phase II study to validate the safety and efficacy of the NAPs REP 2139-Mg and REP 2156-Mg in HBeAg-negative patients with CHB. After 24 wk of NUC treatment, 40 subjects were randomized to two groups; the first group received experimental treatment (NUC + Peg-IFN + REP 2139-Mg or REP 2165-Mg) for 48 wk. The second group received control therapy (NUC + Peg-IFN) for 24 wk followed by experimental treatment for 48 wk. The addition of NAPs to Peg-IFN + NUC therapy resulted in remarkable HBsAg clearance and seroconversion without serious adverse effects. Importantly, at the 48-wk post-treatment follow-up, 14 of 40 patients achieved a functional cure with durable HBsAg seroconversion. Efficacy and safety parameters between REP 2139-Mg and REP 2165-Mg were equivalent[95]. As for STOPS, ALG-010133 is the leading candidate that has progressed to clinical testing after demonstrating minimal genotoxic effects and favorable pharmacokinetic properties in mice and monkeys (NCT04485663)[96].
Control of HBV infection requires an alert innate immune system and coordinated specific humoral and cellular anti-HBV responses. Resolution of acute HBV infection in adults reflects an optimal host response involving early control of viral replication possibly by IFN-mediated non-cytolytic mechanisms. The human immune system is equipped with a series of pattern-recognition receptors (PRRs) that recognize HBV and trigger signal transduction pathways leading to the production of proinflammatory cytokines and IFN by effector cells. In parallel, functional antigen-presenting cells (APCs) activate the two arms of adaptive immunity, polyclonal and multispecific CD4+ and CD8+ T-cell responses, and properly regulated B cells that differentiate into plasma cells that secrete neutralizing antibodies. Their relative contribution to viral clearance remains to be elucidated. In a chimpanzee model, effective CD4+ T-cell priming and cytotoxic T-cell activity were shown to be essential for limiting HBV infection[97,98]. On the other hand, the importance of humoral defense was highlighted in patients with previously cleared HBV infection who received rituximab for treatment of malignancies. In those patients, anti-HBs seropositivity was highly protective against viral reactivation[99]. It remains controversial whether HBV is a "stealth" virus that evades detection or acts as a suppressor of the innate defense system. Interestingly, coinfection with other hepatotropic viruses stimulates the host response and suppresses HBV replication, suggesting that agents that activate innate immunity offer a therapeutic prospect[100]. In established infection, HBV polarizes monocytes/macrophages toward an anti-inflammatory phenotype by stimulating the synthesis of TGF-β and ΙL-10 and recruiting myeloid derived suppressive cells (MDSCs) with inhibitory functions. In vitro, MDSCs secrete arginase that downregulates IFN-γ expression by HBV-specific T cells and promotes regulatory T-cell activity. Under those circumstances, HBV educates natural killer (NK) cell differentiation to regulatory NK cells and promotes the creation of deficient and tolerogenic dendritic cells (DCs). Moreover, CD4+ and CD8+ T cells respond inadequately with decreased cytokine secretion and weak cytotoxicity, whereas expression of programmed cell death protein 1 (PD-1) and other negative signaling pathways indicate T-cell exhaustion in CHB resulting from long-term exposure to a high antigen load[12]. A better understanding of HBV viral and host immune responses has led to attempts to develop therapeutic factors that target a dysfunctional immune system. A variety of immunomodulatory strategies are currently being investigated, including PRR agonists, agents targeting immune checkpoint proteins, T-cell engineering, and therapeutic vaccination to promote efficient and long-term anti-HBV activity (Table 2).
| Compound | Class and action | Phase of development | Ref. or trial number | |
| TLR agonists | GS-9620 (or Vesatolimod) | TLR-7 agonist | Phase II | Janssen et al[112] and Boni et al[113] |
| RO7020531 (RG7854) | TLR-7 agonist | Phase II | Luk et al[116] | |
| JNJ-64794964 | TLR-7 agonist | Phase I | Wu et al[117] | |
| APR002 | TLR-7 agonist | Preclinical | Korolowizc et al[115] | |
| T7-EA | TLR-7 agonist | Preclinical | Hu et al[118] | |
| GS-9688 (or Selgantolimod) | TLR-8 agonist | Phase II | Mackman et al[121] and Janssen et al[122] | |
| ImmunoTAC™ | ASGR1-TLR8 agonist conjugate | Preclinical | Baum et al[123] | |
| RIG-I, NOD agonists | SB9200 (or Inarigivir) | RIG-I/NOD-2 agonist | Phase II | Yuen et al[129] |
| STING agonists | DMXAA (or Vadimezan or ASA404) | STING agonist | Preclinical | Guo et al[132] |
| T-cell engineering | TCR-engineered T cells | TCR-specific for MHC-I restricted HBV epitopes | Phase I | NCT03899415 NCT02719782 NCT02686372 |
| S-CAR engineered T cells | CAR targeting envelope proteins | Preclinical | Festag et al[145] | |
| IMC-I109V | ImmTAV targeting HLA-A restricted HBV epitopes | Phase II | EudraCT2019-004212-64 | |
| ImmTAV-HLA-E | ImmTAV targeting HBV epitopes presented by HLA-E | Preclinical | Leonard et al[151] | |
| Immune checkpoint inhibitors | Nivolumab | Monoclonal antibodyPD-1 inhibitor | Phase Ib | Gane et al[155] |
| ASC22 (Envafolimab) | Monoclonal antibodyPD-L1 inhibitor | Phase II | NCT04465890 | |
| HSV-1 glycoprotein D (gD) | BTLA-HVEM pathway inhibitor | Preclinical | Hasanpourghadi et al[158] | |
| APG-1387 | IAP antagonist | Phase II | Zhang et al[159] | |
| Vaccines | INO-1800 | DNA vaccine | Phase I | NCT02431312 |
| HB110 | DNA vaccine | Phase I | Yoon et al[164] | |
| JNJ-64300535 | DNA vaccine | Phase I | NCT03463369 | |
| AIC649 | Viral vector vaccine (iPPVO) | Phase I | Addy et al[167] | |
| TG1050 | Viral vector vaccine (Ad5) | Phase Ib | Zoulim et al[168] | |
| VTP-300 | Viral vector vaccine (ChAdOx1) | Phase I | Vaccitech[169] | |
| HepTcell | T cell vaccine | Phase II | Lim et al[171] and Altimmune[172] | |
| GS-4774 | Yeast-based, T-cell vaccine | Phase II | Boni et al[173] | |
| Chimigen® HBV (C-HBV) | Immune complex vaccine | Preclinical | Ma et al[177] | |
| YIC (HBsAg-HBIG) | Vaccine based on yeast-derived immune complexes | Phase II | Zhou et al[178] and Xu et al[179] | |
| ABX-203 (or Nasvac) | HBsAg and HBcAg | Phase III | Al Mahtab et al[183] and Yoshida et al[184] | |
| VBI-2601 (or BRII-179) | eVLP-based vaccine | Phase II | ACTRN-12619001210167 | |
| NP-preS1 | Nanoparticle-based vaccine | Preclinical | Wang et al[190] |
Toll-like receptors (TLRs) are a distinct class of PRRs that recognize pathogen-associated molecular patterns and damage-associated molecular patterns. Activation of TLRs initiates a cascade of signaling pathways leading to the production of proinflammatory cytokines, chemokines, type I IFNs, and antimicrobial peptides[101,102]. In CHB, abnormal (mainly downregulated) expression and impaired function of TLRs have been reported in both in vivo and in vitro studies [103-106]. Moreover, in vivo and in vitro studies have shown that TLR-mediated innate immune responses can lead to inhibition of HBV replication[107-110]. Therefore, TLRs represent a promising target for stimulating the immune system and rebalancing immune responses during chronic infection. GS-9620, also known as vesatolimod, is an orally bioavailable TLR-7 agonist that has shown promising results in preclinical studies by increasing IFN production, activating immune cells, and reducing viral DNA in serum and liver[109,111]. Although phase II studies with vesatolimod have shown a dose-dependent induction of IFN-stimulated gene expression, no significant decrease in HBsAg has been observed[112,113]. Attempts to optimize hepatoselectivity led to development of APR002, a novel TLR-7 agonist that has a better serum-to-liver ratio than GS-9620 because its uptake is mediated by the organic-anion-transporting polypeptide 1B1/3 transporters, which are highly expressed in the sinusoidal membrane of hepatocytes[114]. A weekly oral dose of APR002 in combination with entecavir over a 12-wk period contributed to sustained immune-mediated suppression of CHB and cccDNA elimination by inducing antibody production and stimulating IFN gene expression[115]. RO7020531 is another TLR-7 agonist that has shown a favorable safety profile in clinical trials. A phase II trial evaluating the safety and efficacy of triple anti-HBV therapy with RO7020531 along with NUC and the CpAM RO7049389 (NCT04225715) is ongoing[116]. Single and multiple doses of JNJ-64794964 (JNJ-4964), which is a TLR-7 agonist, were administered to healthy adult volunteers to clarify its pharmacokinetic/pharmacodynamic properties. The bioavailability and safety parameters of a weekly dosing regimen and JNJ-4964-mediated immune stimulation as demonstrated by IFN-α, IFN-γ-inducible protein 10, IFN-stimulated gene 15 (ISG15), and neopterin changes, support further development of this agent[117]. Interestingly, a novel TLR-7 agonist (T7-EA) has been developed as a component of a therapeutic vaccine that is under investigation. T7-EA has demonstrated the ability to reverse immune tolerance to the HBV by inducing HBsAg-specific antibody production and Th1-type immune responses in healthy and HBV-infected mice[118].
In contrast to TLR-7 agonists, TLR-8 activation induces mainly proinflammatory cytokines and chemokines [IL-12, and TNF-α, macrophage inflammatory protein (MIP)- 1α] rather than IFN production because of the different receptor expression pattern between different populations of immune cells[119]. GS-9688 or selgantolimod is a selective TLR-8 agonist that has shown potent antiviral activity in preclinical studies, reducing HBV RNA, HBV DNA, HBsAg, and HBeAg[120,121]. A phase II multicenter, double-blind study was conducted to evaluate the safety and efficacy of GS-9688 in 67 (39 HBeAg-positive) viremic CHB patients randomized (2:2:1) to receive 3 mg or 1.5 mg of GS-9688 or placebo once weekly for 24 wk in combination with daily NUC therapy. Oral administration of GS-9688 up to 3 mg once weekly for 24 wk was safe, well tolerated and elicited dose-dependent changes in cytokine/cytokine receptor levels (IL-12p40, IL-1Rα and IFN-γ) and shifts in immune populations. Although the primary endpoint of an HBsAg reduction ≥ 1 log10 IU/mL was not met, participants who showed a reduction ≥ 0.5 log10 IU/mL (n = 3) were exclusively in the GS-9688 treatment group. Stronger HBsAg responses were associated with higher baseline ALT and IFN-γ levels[122]. A novel ImmunoTAC™ therapeutic is now available, consisting of a TLR-8 agonist bound to a monoclonal antibody directed against liver-specific ASGR1, and shown to result in hepatoselective immune activation. In cell cultures, the ASGR1-TLR8 conjugate strongly stimulates myeloid cells, which subsequently upregulate IFN-γ expression and activate B cells. In HBV-infected mice, this compound exhibited minimal hepatotoxicity and induced significant HBsAg seroconversion by enhancing anti-HBs and anti-HBc responses of IFNγ+ T cells and triggering anti-HBs B-cell responses[123].
Retinoic acid-inducible gene I (RIG-I)-like receptors and nucleotide-binding oligomerization domain (NOD)-like receptors are members of the PRR receptor family. When stimulated by viral cytosolic RNA, RIG-I-like receptors (RLRs) undergo conformational changes that trigger intracellular signaling pathways and transcription factors [nuclear factor-κB, interferon regulatory factor (IRF) 3, IRF7] that activate ISGs to produce IFN-α and other cytokines[124]. Similarly, NOD-like receptors (NLRs) are important coordinators of innate immunity involved in inflammasome activation, cell death regulation, antigen presentation, and differentiation of adaptive immunity[125]. Evidence suggests that HBV has evolved multiple mechanisms to evade RLR- and NLR-mediated responses[126,127]. In addition to direct inhibition of the HBV replication complex, the orally administered dinucleotide inarigivir (SB9200) acts preferentially via stimulation of RIG-I and NOD-2 receptors and has demonstrated a suitable pharmacokinetic profile and robust antiviral efficacy in a marmoset HBV model[128]. Final results from the phase II ACHIEVE study, in which 80 patients were randomized 4:1 to receive inarigivir (25 mg, 50 mg, 100 mg, and 200 mg) or placebo for 12 wk followed by 12 wk of tenofovir administration in both arms, reported a dose-dependent decline in HBV DNA independent of HBeAg serostatus. The efficacy and safety of inarigivir were highlighted at a daily dose of up to 200 mg. Further studies investigating higher doses or additional inarigivir treatment in NUC-suppressed patients are ongoing (NCT03434353)[129].
The stimulator of IFN genes (STING) downstream signaling pathway is an integral component of innate immunity and recognizes cyclic dinucleotides that are products of aberrant cytosolic DNA. STING recruits and activates the transcription factors IRF-3 and STAT-6 and induces the expression of type I IFNs and STAT-6-dependent chemokines[130]. Preclinical studies have focused on manipulating the STING antiviral drive to overcome tolerance in CHB. The STING-ligand cyclic guanosine monophosphate adenosine monophosphate (cGAMP) was used as an adjuvant for an HBsAg-derived vaccine and tested in HBV-infected mice. Indeed, cGAMP enhanced the effect of the vaccine on humoral and cellular immunity. Specifically, the STING cascade upregulated cytokines that triggered the synthesis of specific anti-HBV antigens, activated APCs in lymphoid tissues, and elicited strong CD4+ and CD8+ T-cell responses[131]. The STING agonist DMXAA (also known as vadimezan or ASA404) has shown positive results in curbing HBV infection in vivo. DMXAA induced macrophages to produce antiviral cytokines with a unique profile, resulting in a significant reduction of HBV DNA and cytoplasmic HBV capsids in infected mice[132].
T cells are key players in the coordination of the adaptive immune response. CD4+ T cells contribute to the activation of B cells, regulate their differentiation into antibody-producing plasma cells, promote the formation of memory CD8+ T cells, and trigger the proliferation of CD8+ T cells directed against pathogen-infected cells. CD4+ T cells secrete a variety of cytokines and generate specific environmental stimuli responsible for the activation of APCs and the development of specific types of T effector cells[133]. CD4+ regulatory T cells (Tregs) downregulate immune responses and provide tolerance to self-antigens by maintaining immune homeostasis[134]. Although a vigorous immune response against the HBV virus is induced during acute infection, T-cell exhaustion or anergy is observed in chronically infected patients[135]. Exhausted CD4+ and CD8+ T cells are no longer effectively stimulated by their respective ligands, and are characterized by upregulated co-inhibitory receptors (PD-1, cytotoxic T-lymphocyte-associated protein 4, T-cell immunoglobulin and mucin domain, CD244)[136,137], death receptors and pro-apoptotic molecules (tumor necrosis factor-related apoptosis-inducing ligand-2, bcl-2-interacting mediator of cell death)[138,139]. As a result, clonal expansion of HBV-specific T cells is impeded, pro-apoptotic signals are triggered, and antiviral cytokines are downregulated, leading to a weak anti-HBV response[135,140]. To reprogram immune function, a new concept is emerging using autologous transfer of genetically restored HBV-specific T cells. Indeed, adoptive immunity transfer has been demonstrated in CHB patients who achieved HBsAg clearance after receiving allogeneic bone marrow transplantation from donors with cleared HBV infection, indicating that efficient donor HBV-specific T cells are able to overcome immunological exhaustion or anergy[141].
T-cell engineering is a revolutionary strategy to repair the function of HBV-specific T cells. In this method, T cells are engineered with a chimeric antigen receptor (CAR) that targets HBV envelope proteins[142] or with a natural T-cell receptor (TCR) specific for HBV epitopes restricted to major histocompatibility complex (MHC) class I[143]. To generate a genetically engineered T-cell population, T cells are isolated from peripheral blood and activated in vitro to receive a transgene consisting of a CAR or a TCR using viral transduction techniques. Subsequently, CAR or TCR-manipulated T cells are cultured for clonal expansion and transfused back to the patient after a quality assessment process[144]. In vivo experiments have been performed to investigate the antiviral effect of CAR or TCR-manipulated T cells in HBV-infected animals. Festag et al[145] developed an HBV mouse model that received human CAR receptor targeting protein S (S-CAR) containing a human B-cell-derived single-chain antibody fragment, a human immunoglobulin G (IgG) spacer, and CD28- and CD3-signaling immunogenic domains. Specifically, murine CD8+ T cells were manipulated to express S-CAR and transferred into HBV transgenic mice. S-CAR T cells exhibited robust antiviral activity and achieved significant decreases in serum HBsAg, HBeAg, and HBV DNA and increases in anti-HBs without therapy-associated side effects. Since human-derived domains of S-CAR could induce rejection of transplanted cells in immunocompetent organisms, further technical manipulations were performed to ensure sustained expansion and persistence of S-CAR T cells[145].
Irradiation of immunocompetent HBV mice and specific S-CAR tolerance treatment [involving transfer of nonfunctional, signal-deficient S-Decoy (Δ) CAR T cells] were essential for maintaining the long-lasting antiviral efficacy of S-CAR T cells. Similarly, a preclinical study using T cells engineered to carry HBV-specific TCRs addressed to HBV core or envelope proteins yielded positive results. CD4+ and CD8+ T cells derived from either healthy donors or CHB patients, after transplantation of the TCR into HBV-infected mice, showed potent antiviral effects and achieved significant reductions in HBsAg, HBeAg, HBV DNA (4 log10 mL/IU) and pgRNA. Importantly, the engineered T cells induced clearance of HBV-infected hepatocytes without significant toxicity to healthy liver tissue. Coadministration with bulevirtide provided durable control of HBV infection[146]. Although preclinical models suggest T-cell engineering as a promising strategy for efficient elimination of HBV virus, further research is needed to clarify the immunological implications and safety parameters of genetically engineered T cells. Adverse events associated with the binding of transplanted T cells to other cognate or "off-target" antigens have been reported in oncology patients[147,148]. Achieving sustained activity of transplanted T cells and defining the optimal mechanism of T-cell delivery, cell volume, and frequency of infusions required are among the challenges that need to be addressed[149]. Research is ongoing and three clinical trials (NCT03899415, NCT02719782, NCT02686372) investigating the safety and efficacy of TCR-engineered T cells in patients with HBV-related HCC are currently recruiting participants.
Redirection of endogenous, non-HBV-specific T cells to eliminate HBV-infected cells can be mediated by immunomobilizing monoclonal TCRs against virus (ImmTAV) molecules. ImmTAV molecules are bispecific molecules that combine a TCR with increased affinity that targets HBV-specific human leukocyte antigen (HLA)-limited epitopes and a recombinant anti-CD3 antibody that triggers T-cell activation. ImmTAV molecules designed for HLA-A*02:01-restricted HBV antigen epitopes generated T cells that specifically lyse in vitro HCC cells containing integrated HBV DNA and HBV-infected cells[150]. A phase II study is currently evaluating the safety, antiviral activity, and pharmacokinetics of the ImmTAV molecule IMC-I109V in HLA-A*02:01-positive patients with CHB (EudraCT 2019-004212-64). However, the highly polymorphic nature of HLA class I complexes compromises the efficacy of ImmTAV therapeutics. Leonard et al[151] used HLA-E, which is universally expressed and highly conserved, to overcome HLA-related limitations and developed an ImmTAV platform that recognizes HBsAg and HBV Cps presented by HLA-E alleles (HLA-E*01:01 and 01:03). ImmTAV targeting HLA-E has demonstrated potent antiviral efficacy in vitro without significant cytotoxic effects on non-infected cells, joining the long line of immunotherapeutic agents pursuing HBV eradication[151].
T-cell exhaustion has been associated with upregulation of inhibitory receptors on the cell surface, resulting in a weak cellular response[152]. Therefore, the design of immune checkpoint inhibitors that target these receptors and suppress their activity may be a useful means of restoring T-cell function in HBV infection.
Nivolumab is an immune checkpoint inhibitor that has been clinically tested in certain cancers and has shown a favorable safety profile[153,154]. It is a genetically engineered human IgG4 monoclonal antibody that binds to PD-1. By inhibiting the PD-1 interaction with its respective ligands (PD-L1 or PD-L2), nivolumab blocks the signal transduction pathway, resulting in functionally active T cells[154]. A phase Ib study (ACTRN12615001133527) evaluated the efficacy of this immunomodulator alone or in combination with the immunostimulatory vaccine GS-4774 (discussed later in the text) in virally suppressed CHB patients. Patients received a single intravenous dose of nivolumab, either 0.1 mg/kg (n = 2) or 0.3 mg/kg (n = 12). In addition, 10 patients received 40 yeast units (YU) of GS-4774 subcutaneously at baseline and 40 YU of GS-4774 plus 0.3 mg/kg nivolumab at week 4. Mean changes in HBsAg levels were estimated 12 wk after nivolumab administration, while safety and immunological outcomes were assessed at week 24 (end of follow-up). Sustained and strong binding of nivolumab to the PD-1 receptor was observed in all patients and a significant decrease in HBsAg levels (0.5 log10) was observed in patients in the higher dose group. Nivolumab also induced HBsAg-specific T-cell responses without serious adverse events until the end of follow-up, results that support further clinical testing of this agent[155].
In addition, ACS22 (Envafolimab) is a single-domain antibody generated by a fusion of the PD-L1 domain with the Fc fragment of the human IgG1 antibody. This chimeric molecule binds with high affinity to PD-L1 and inhibits the PD-1/PD-L1 pathway, thereby improving T-cell function[156]. A phase II trial is ongoing to evaluate the safety, tolerability and efficacy of ASC22 in CHB patients (NCT04465890).
Herpes simplex virus-1 glycoprotein D (gD) is one of the checkpoint inhibitors of the B and T lymphocyte attenuator/herpes virus entry mediator pathway that revives T-cell response and antigen recognition by CD8+ T cells[157]. A viral vector-based vaccine expressing HBc and polymerase antigens in genetic combination with gD was tested in a mouse model. Vaccination resulted in a reduction of HBV genome copies by approximately 2 log10 IU/mL for a period of more than 8 wk. The use of gD expanded the antigen repertoire recognized by CD8+ T cells and optimized their response. If these results are confirmed in clinical trials, a shift toward subdominant epitopes may be necessary to circumvent irreversibly depleted T cells targeting immunodominant HBV epitopes[158].
Apoptosis is an additional biological checkpoint process that promotes the elimination of pathogen-infected cells and contributes to viral control. Inhibitors of apoptosis proteins (IAPs) have been associated with impaired clearance of HBV virus by preventing IFN-mediated death of virus-infected cells and are currently being investigated as potential targets of anti-HBV therapeutics. APG-1387 is an IAP antagonist that shows robust anti-HBV activity in vivo. Intravenous or intraperitoneal injection of 20 mg/kg APG-1387 once weekly for 4-20 wk in HBV mice induced clearance of serum HBsAg, HBeAg, HBV DNA, and elimination of liver HBcAg and achieved sustained HBV clearance after treatment. These effects on HBV markers mediated by APG-1387 were attributed to activation of intrahepatic HBV-specific CD4+ and CD8+ T-cell responses and stimulation of immune-related genes[159]. A phase II open-label dose-escalation study to evaluate the safety, tolerability, and pharmacodynamics of APG-1387 in CHB patients is ongoing (NCT04568265).
Vaccination against HBV virus is a safe, effective, and globally accepted prevention strategy that is part of routine childhood immunization programs[160]. To date, attempts to use vaccination as part of anti-HBV treatment against established infection have not met the expectations raised by experimental models[161]. The basic mechanism of action of the vaccine involves the injection of an inactive compound of the target pathogen that is recognized by APCs, which in turn activate T and B cells. This activation results in the production of specific antibodies, lysis of pathogen-infected cells, and clonal expansion of T and B cells including memory subpopulations that contribute to long-term immunity against the virus[162]. Several categories of HBV vaccines are being tested, including DNA vaccines, viral vector-based vaccines, T-cell epitope peptides, and immune complex (IC) vaccines[163].
DNA vaccines: INO-1800 is a DNA-based vaccine containing plasmids encoding surface and core antigens. A phase I dose-escalation clinical trial (NCT02431312) was conducted to evaluate the safety and immunogenicity of INO-1800 either alone or in combination with INO-9112 (DNA plasmid expressing human IL-12) as an adjuvant in 90 virally suppressed CHB patients. This study is complete and final results are awaited. HB-110 is an investigational vaccine equipped with a broader antigenic arsenal consisting of three DNA plasmids (pGX10-S/L, pGX10-C/P and pGX10-hIL-12m) expressing HBV envelope proteins (S, L), Cp/polymerase and human IL-12, respectively. Although they elicit potent T-cell and antibody-mediated responses in animal models, the effect of HB-110 was weak in 27 Korean patients receiving NUC[164]. A phase I study is ongoing to evaluate the safety and efficacy of an additional DNA vaccine JNJ-64300535 in virally suppressed CHB patients receiving NUC therapy (NCT03463369). Intracellular delivery of the aforementioned DNA vaccines, instead of viral vectors, is based on electroporation, a technology that disrupts cell permeability by applying a potential difference across the membrane. Optimization of electroporation platforms is critical to transfect larger cell populations and improve vaccine immunogenicity[165].
Viral vaccines: AIC649 is an immunostimulatory vaccine consisting of inactivated parapoxvirus particles. In animal models, AIC649 induced significant upregulation of Th1 cytokines, activation of APCs, and HBV-specific T-cell responses, resulting in a remarkable decrease in HBV DNA comparable to that induced by tenofovir or entecavir[166]. Single intravenous doses of AIC649 were tested in CHB patients in a randomized, placebo-controlled trial. The study included 32 patients divided into four ascending dose groups (6 AIC649:2 placebo in each group) and followed for 3 mo. A single dose of this agent was reported to stimulate innate immunity, as evidenced by increased plasma levels of IL-1β, IL-6, IL-8, IFN-γ, and reduced levels of IL-10. In addition, a significant expansion of the CD4+ T-cell memory population was observed, whereas HBsAg levels did not change significantly[167].
The vector-based vaccine TG1050 uses a non-replicative serotype 5 adenovirus expressing a large fusion protein containing the polymerase and domains of envelope and core antigens. Forty-eight virally suppressed CHB patients were randomized in a 1:1:1 ratio to three dose levels (109, 1010, 1011 viral particles) of TG1050 in a phase I study; in the first phase, 12 participants were assigned to a single-dose cohort (9 vaccine:3 placebo) and the remaining participants were assigned to a multiple (3) dose cohort (27 vaccine:9 placebo). TG1050 demonstrated a favorable safety profile and induced IFN-γ-producing T cells that reacted to HBV antigens. Although HBcAg was undetectable in 6 patients, the decrease in HBsAg was not significant[168].
VTP-300 is based on a viral vector platform and consists of replication-deficient chimpanzee adenovirus oxford 1, which encodes three full-length HBV antigens and is designed to elicit specific CD4+ and CD8+ T-cell responses[169]. A phase I study to evaluate the safety and efficacy of VTP-300 in viral suppressed CHB patients is ongoing (NCT04297917).
T-cell vaccines: The initiation of T-cell-mediated eradication of a virus-infected cell depends on the interaction of the TCR with the target antigen presented by the MHC. T-cell vaccines consist of HBV epitope peptides that target specific TCR receptors and aim to elicit T-cell responses against HBV antigens[170].
The HepTcell vaccine is a synthetic immunomodulator consisting of nine peptides designed to induce CD4+ and CD8+ T-cell responses against multiple HBV epitopes. A formulation of these nine peptides in combination with a TLR-9 agonist adjuvant was prepared to enhance HBV-specific immune responses. Lim et al[171] evaluated the safety and efficacy of HepTcell against HBV infection in a placebo-controlled phase Ib study. A total of 60 HBeAg-negative CHB patients on established NUC treatment were randomized to three dose-escalating cohorts of HepTcell with or without IC31. They received 3 injections with a 28-d injection interval and were followed up for 6 mo[171]. The results support further development of this immunomodulator in a phase II trial[172], as HepTcell administration was safe and induced virus-specific cellular immune responses, especially in combination with TLR9 adjuvant[171].
GS-4774 is a therapeutic vaccine containing HBx, core and envelope proteins together with recombinant Saccharomyces cerevisiae as a natural adjuvant to elicit HBV-specific T-cell responses. In a multicenter, randomized phase II trial, 169 HBsAg-positive, treatment-naïve patients were recruited. Participants were randomized 1:2:2:2 into groups receiving oral TDF (300 mg) alone or in combination with 2, 10, or 40 GS-4774 YU. Vaccination was administered subcutaneously in six monthly doses, while oral administration of NUCs continued until the end of follow-up (week 48). The addition of GS-4774 to NUC treatment was well tolerated, induced a reduction of Tregs, and stimulated IFN-γ, TNF-α, and IL-2-producing CD8+ T cells directed against specific HBV epitopes. However, HBsAg levels remained stable and CD4+ T-cell responses were anemic[173]. Therefore, restoration of T-cell immune responses by vaccination is an achievable strategy, but clinical benefits may occur in the context of combination treatment.
IC vaccines: Research aimed at HBV eradication has used IC-based therapies to increase host immune activation. ICs, which consist of immunoglobulins bound to their cognate antigens, are able to exert significant immunoregulatory effects. Specifically, ICs can activate APCs via binding of their Fc fragment to Fc receptors (FcγR) expressed on these cells[174]. Activation of FcγR-mediated signaling in DCs, as well as the effect of ICs on cross-presentation, leads to upregulation of antigen delivery to MHC-II molecules, resulting in increased antibody production and clonal expansion of immune memory cells[175]. In addition, ICs trigger molecular signaling pathways associated with phagocyte action (ITAM/Syk) and other signal transduction pathways that regulate cellular processes, tumor suppression, and inflammation[176].
Chimigen® HBV is a therapeutic vaccine based on a fusion molecule consisting of HBV antigens (PreS1 and PreS2 peptide fragments and Cp) and a murine Fc fragment targeting FcγRII (CD32) and mannose receptor (CD206). The Fc-receptor interaction leads to internalization of the chimeric molecule by immature DCs and promotes MHC-I and -II surface expression. In preclinical studies, vaccination induced clonal expansion of HBV-specific T cells, upregulated IFN-γ, TNF-α, perforin, and granzyme B expression by CD4+/CD8+ T cells, and induced apoptosis of Tregs[177].
Yeast-derived immune complexes (YIC) of HBsAg in combination with high-titer immunoglobulin (HBsAg-HBIG) were developed to facilitate antigen uptake by APCs and initiate strong adaptive immune responses. The immunomodulatory effects of ICs were illustrated in a pilot study in which 44 HBsAg- and HBeAg-positive CHB patients were randomly assigned to receive intramuscular injections of YIC, alum, or normal saline as adjuvants to adenofovir. Patients treated with YIC experienced a relative increase in Th1 and Th2 CD4+ T cells and an increase in Tc1 and Tc17 CD8+ T cells, whereas the number of Tregs decreased. Regarding the cytokine profile, IL-2 and IFN-γ were significantly upregulated, whereas the inhibitory cytokines IL-10 and TGF-β, and transcription factor Foxp3 were reduced[178]. Although the primary endpoint was not met, administration of YIC showed strong antiviral efficacy with respect to HBeAg seroconversion in a phase IIb study[179].
Not to be neglected are the possible implications of ICs in immunopathological reactions. It has been described that an inability of phagocytes to clear ICs leads to deposition, which can trigger inflammation and tissue damage by activating complement receptors on innate immune cells. Furthermore, paradoxical enhancement of bacterial infections can occur when non-neutralizing IgG-antibody complexes activate FcγR signaling in infected monocytes/macrophages (intrinsic antibody-dependent enhancement)[180,181]. The investigation of ICs in HBV vaccinology has just begun and further studies are needed to evaluate IC-associated effects and assess their potential as part of combination therapies[182].
Breakthroughs in biomedical engineering have contributed to improved drug delivery systems involving either routes of administration or delivery vehicles. The ABX-203 vaccine (Nasvac), which contains HBsAg and HBcAg antigens, was developed for nasal administration. Nasvac has been enrolled in a phase III open-label, randomized clinical trial (NCT01374308) comparing the therapeutic efficacy of Nasvac to Peg-IFN in therapy-naïve CHB patients. A total of 160 patients were assigned 1:1 to either Nasvac or Peg-IFN. Nasvac was administered via nasal spray (1 mL) (baseline to week 12) or via nasal spray and subcutaneously (1 mL) (from week 12 to week 24). Patients in the Peg-IFN group received 180 μg Peg-IFN once weekly for 48 wk. A 24-wk follow-up period was conducted. Nasvac showed a better safety profile in terms of adverse effects and induced a higher HBV DNA drop compared to Peg-IFN. In parallel, transient ALT flares were more frequent in the Nasvac group than in the Peg-IFN group (85% vs 30%), suggesting that vaccination induces a higher rate of positive immune activation. In addition, Nasvac administration prevented progression of liver cirrhosis, as measured by liver stiffness, and induced HBeAg seroconversion more frequently[183]. The efficacy of Nasvac was also evaluated in a clinical trial of 71 CHB patients (including 29 participants on a stable NUC regimen) who received ten doses of the drug once every two weeks. Nasal administration of Nasvac was associated with sustained reductions in HBsAg and increases in anti-HBs in both groups, and notably, 6 of 45 patients monitored 18 mo after treatment achieved functional recovery[184].
Optimization of vaccine delivery by using innovative viral vectors (chimapanzee adenovirus, ChAd or modified vaccinia Ankara) encoding HBV antigens was investigated in normal and HBV transgenic mice. Stimulation of HBV-specific T-cell responses in the liver and increased production of HBc- and HBs-specific IgG were observed without significant hepatotoxicity, providing new insights for clinical investigations[185,186].
VBI-2601 (or BRII-179) is a protein-based vaccine candidate that has entered a phase II trial (ACTRN12619001210167) using enveloped virus-like particle (eVLP) technology. Briefly, eVLPs are noninfectious nanostructures that mimic bona fide virions and carry viral antigenic epitopes to elicit immune responses[187]. In the case of VBI-2601, the immunogenic preS1 domain was selected to trigger immune-mediated clearance of HBV-infected cells. Nanoparticles are a novel strategy for vaccine delivery, as ferritin-based nanoparticles have been found to elicit B-cell responses and antibody production in Epstein-Barr virus or influenza virus infections[188,189]. A nanoparticle vaccine was developed that delivers the pre-S1 domain of HBsAg to specific DCs and lymphoid sinus-associated macrophages that activate follicular helper T cells and B cells, respectively. Encouragingly, administration of ferritin nanoparticle pre-S1 vaccine in HBV-infected mice resulted in significant decreases or even undetectable HBV DNA and HBsAg. Moreover, vaccination resulted in impressive rates of HBsAg seroconversion and cccDNA reduction[190]. Clearly, among the new therapeutics targeting functional cure, nanoparticle vaccines represent promising candidates worthy of future evaluation.
So far, the introduction of NUCs has been the hallmark of therapeutic interventions against HBV infection, achieving suppression of viral replication and thus significantly improving the prognosis of patients. Nevertheless, the impact of existing treatment regimens on cccDNA stability is negligible, implying that the intranuclear cccDNA pool is constantly renewed, leading to chronification of HBV infection. Although HBsAg clearance has long been an endpoint of clinical trials, the performance of NUCs and IFN-based therapies has been more or less subpar. Considering that HBsAg can originate from integrated viral DNA, it is clear that despite highly efficient drugs that achieve functional cure, hepatocytes harboring reservoirs of HBV DNA may be responsible for persistent subclinical antigen expression after resolution of infection or residual risk for HCC. In parallel, with current therapeutics, there is little or no reversal of defective innate immune responses and depletion of adaptive immunity effectors; ergo, HBV establishes and replicates by exploiting the tolerogenic microenvironment of the liver.
Innovative advances in biotechnology, as well as a better understanding of the complex life cycle of HBV and its intricate effects on the host immune system, have reinvigorated the field of HBV therapeutics. A number of drug candidates with antiviral activity through various mechanisms have entered phase II/III clinical trials, demonstrating comparable or greater reductions in HBV DNA and/or RNA compared to NUCs. The breakthrough entry inhibitor bulevirtide has demonstrated synergistic activity with Peg-IFN, resulting in significant viral suppression and HBsAg reduction in HBV/HDV-coinfected patients. Consistent reduction of HBV DNA was achieved with CpAMs, and remarkably, coadministration with NUCs resulted in even more profound inhibition of viral replication. RNAi-based therapeutics with the lead agents JNJ-3989 and VIR-2218, as well as NAPs, provided more exciting results and achieved sustained HBsAg loss off-treatment in a substantial proportion of patients. In contrast, gene editing technology, although providing direct cccDNA eradication, has not yet been tested in clinical trials. Therefore, a sterilizing cure that results in elimination of cccDNA and integrated DNA still seems a long way off, as it would require genome-targeting tools.
In the field of immunotherapies, TLR agonists and other PRR agonists that stimulate innate immunity have induced a substantial decrease in HBV DNA with weak effects on HBsAg serostatus, whereas immune checkpoint inhibitors and especially the PD-1 blocker nivolumab induced stronger HBsAg responses in clinical trials. T-cell depletion due to activation-induced cell death or T-cell exhaustion or anergy, caused by high levels of viral antigens circulating in soluble form in patient sera, and the concomitant inability to elicit potent cytotoxic responses, are possibly the reasons why a conventional therapeutic HBV vaccine is elusive. Moreover, chronic inflammation leads to a tolerogenic intrahepatic environment and suppresses T-cell effector functions, which compromises vaccine efficacy. Nonetheless, efforts to improve immunogenicity, the use of new vectors, and the introduction of innovative delivery platforms have optimized HBV vaccine efficacy. Several vaccines have elicited HBV-specific immune responses in clinical trials, and results from a phase III trial of Nasvac offer the possibility of achieving a functional cure through immunotherapy. However, enhancement of host immunity is a risk factor for induction of uncontrollable immune activation and severe liver injury. Due to the variable hepatic inflammatory status and the different pattern of intrinsic immune responses, different degrees of hepatotoxicity may occur when the same treatment strategy is applied. Therefore, the implemen
Obviously, a functional cure is more likely in the context of a combination therapy that simultaneously targets HBV through multifaceted mechanisms. In parallel, an immunomodulator could be used to restore innate immunity, while vaccination or T-cell engineering could reprogram and enhance HBV multispecific cellular and humoral responses. However, the extent to which the combination of different therapeutic agents has an additive or synergistic effect on HBV suppression remains to be elucidated. Therefore, it is important to design clinical trials that examine not only the efficacy of concomitantly administered drugs, but also the resulting toxicity or potential drug-drug interactions. Due to altered bioavailability and underlying comorbidities, cirrhotic patients are more susceptible to hepatotoxicity and may be harmed by hepatic flares, so this population should receive special consideration.
Interestingly, phase II trials are already underway investigating concurrent administration of three DAAs (RNAi (JNJ-3989) plus CpAM (JNJ-6379) plus NUC) (NCT04129554) or two DAAs together with an immunomodulator (NUC plus CpAM (RO7049389) plus TLR7 agonist (RO7020531)) (NCT04225715). IFNs, either in their native form or modified to increase their half-life, remain a major weapon in the armamentarium against HBV providing antiviral efficacy not only through direct mechanism (cccDNA degradation or epigenetic silencing) but also by exerting robust immunoregulatory functions. IFNs due to their distinct mechanism of action retain a position as part of “add-on” or “switch” to IFN treatment strategies in selected patients on long-term NUCs to enhance HBsAg clearance[5]. Accordingly, numerous clinical trials investigating experimental combination therapies include Peg-IFN. PENGUIN (NCT04667104) and Piranga (NCT04225715) are ongoing phase II trials assessing the efficacy of quadruple (RNAi (JNJ-3989) plus CpAM (JNJ-6379) plus NUC plus Peg-IFΝ) and triple (siRNA (RO7445482) plus Peg-IFN plus NUC) combination regimens, respectively, in virologically suppressed participants with CHB. Apart from add-on strategies, the use of sequential treatment regimens could repair step by step the dysregulated immunity and eventually enable HBV eradication. A randomized, multicenter, phase II study is intended to evaluate if sequential ASO (GSK3228836) and Peg-IFN treatment increases the rate of HBV surface antigen (HBsAg) loss in participants on stable NUC therapy (NCT04676724). Undoubtedly, further studies are needed to divide patients into groups that would benefit most from each combination therapy.
We are in the midst of a storm of evolving pharmaceutical agents that tip the balance toward a functional cure for HBV infection. First, a number of DAA drugs have provided encouraging results targeting multiple points in the HBV life cycle; inhibition of viral entry into the host cell, suppression of gene expression, blocking HBsAg release, or even direct elimination of cccDNA are among the features of antiviral drugs in the pipeline. Second, immune-based therapies have entered clinical trials, and sustained restoration of innate immunity and enhancement of HBV-specific cellular and humoral responses without induction of uncontrolled flares seems more realistic than ever. There is a growing consensus that a future treatment could consist of a NUC-based backbone, at least one novel DAA, and one or more immunomodulators. Further studies are needed to clarify the parameters of overlapping toxicity and to establish the most efficient combination strategy.
Manuscript source: Invited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A, A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Tamori A, Tanaka Y, Xu XY S-Editor: Liu M L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1215] [Article Influence: 173.6] [Reference Citation Analysis (2)] |
| 2. | World Health Organization. Hepatitis B. WHO Fact sheets. 27 Jul 2020. [cited 10 January 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. |
| 3. | European Association for the Study of the Liver. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3801] [Article Influence: 475.1] [Reference Citation Analysis (1)] |
| 4. | Suk-Fong Lok A. Hepatitis B Treatment: What We Know Now and What Remains to Be Researched. Hepatol Commun. 2019;3:8-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Viganò M, Grossi G, Loglio A, Lampertico P. Treatment of hepatitis B: Is there still a role for interferon? Liver Int. 2018;38 Suppl 1:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Cornberg M, Lok AS, Terrault NA, Zoulim F; 2019 EASL-AASLD HBV Treatment Endpoints Conference Faculty. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference‡. J Hepatol. 2020;72:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 7. | Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18:827-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 405] [Article Influence: 67.5] [Reference Citation Analysis (1)] |
| 8. | Burdette D, Cathcart A, Shauf A, Win R, Zaboli S, Hedskog C, Svarovskaia SE, Barry V, Inzunza D, Flaherty JF, Gaggar A, Fletcher S, Chan H, Mo H, Lazerwith S, Feierbach B, Delaney B. Evidence for the presence of infectious virus in the serum from chronic hepatitis B patients suppressed on nucleos (t) ide therapy with detectable but not quantifiable HBV DNA. J Hepatol. 2019;70:e95. [DOI] [Full Text] |
| 9. | Boyd A, Lacombe K, Lavocat F, Maylin S, Miailhes P, Lascoux-Combe C, Delaugerre C, Girard PM, Zoulim F. Decay of ccc-DNA marks persistence of intrahepatic viral DNA synthesis under tenofovir in HIV-HBV co-infected patients. J Hepatol. 2016;65:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 694] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 11. | Wooddell CI, Yuen MF, Chan HL, Gish RG, Locarnini SA, Chavez D, Ferrari C, Given BD, Hamilton J, Kanner SB, Lai CL, Lau JYN, Schluep T, Xu Z, Lanford RE, Lewis DL. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 371] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 12. | Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J Gastroenterol. 2019;25:3527-3537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 13. | Schulze A, Gripon P, Urban S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology. 2007;46:1759-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 14. | Verrier ER, Colpitts CC, Bach C, Heydmann L, Weiss A, Renaud M, Durand SC, Habersetzer F, Durantel D, Abou-Jaoudé G, López Ledesma MM, Felmlee DJ, Soumillon M, Croonenborghs T, Pochet N, Nassal M, Schuster C, Brino L, Sureau C, Zeisel MB, Baumert TF. A targeted functional RNA interference screen uncovers glypican 5 as an entry factor for hepatitis B and D viruses. Hepatology. 2016;63:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 15. | Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1280] [Cited by in RCA: 1600] [Article Influence: 123.1] [Reference Citation Analysis (1)] |
| 16. | Blanchet M, Sureau C. Infectivity determinants of the hepatitis B virus pre-S domain are confined to the N-terminal 75 amino acid residues. J Virol. 2007;81:5841-5849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Huang HC, Chen CC, Chang WC, Tao MH, Huang C. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J Virol. 2012;86:9443-9453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Schmitz A, Schwarz A, Foss M, Zhou L, Rabe B, Hoellenriegel J, Stoeber M, Panté N, Kann M. Nucleoporin 153 arrests the nuclear import of hepatitis B virus capsids in the nuclear basket. PLoS Pathog. 2010;6:e1000741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Rabe B, Delaleau M, Bischof A, Foss M, Sominskaya I, Pumpens P, Cazenave C, Castroviejo M, Kann M. Nuclear entry of hepatitis B virus capsids involves disintegration to protein dimers followed by nuclear reassociation to capsids. PLoS Pathog. 2009;5:e1000563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Xia Y, Carpentier A, Cheng X, Block PD, Zhao Y, Zhang Z, Protzer U, Liang TJ. Human stem cell-derived hepatocytes as a model for hepatitis B virus infection, spreading and virus-host interactions. J Hepatol. 2017;66:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Koumbi L, Karayiannis P. The Epigenetic Control of Hepatitis B Virus Modulates the Outcome of Infection. Front Microbiol. 2015;6:1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Karayiannis P. Hepatitis B virus: virology, molecular biology, life cycle and intrahepatic spread. Hepatol Int. 2017;11:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Kim S, Wang H, Ryu WS. Incorporation of eukaryotic translation initiation factor eIF4E into viral nucleocapsids via interaction with hepatitis B virus polymerase. J Virol. 2010;84:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Nassal M. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J Virol. 1992;66:4107-4116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 373] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Lentz TB, Loeb DD. Roles of the envelope proteins in the amplification of covalently closed circular DNA and completion of synthesis of the plus-strand DNA in hepatitis B virus. J Virol. 2011;85:11916-11927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Gerelsaikhan T, Tavis JE, Bruss V. Hepatitis B virus nucleocapsid envelopment does not occur without genomic DNA synthesis. J Virol. 1996;70:4269-4274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Watanabe T, Sorensen EM, Naito A, Schott M, Kim S, Ahlquist P. Involvement of host cellular multivesicular body functions in hepatitis B virus budding. Proc Natl Acad Sci USA. 2007;104:10205-10210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | Allweiss L, Volz T, Giersch K, Kah J, Raffa G, Petersen J, Lohse AW, Beninati C, Pollicino T, Urban S, Lütgehetmann M, Dandri M. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut. 2018;67:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 29. | Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020;9:1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 30. | Wi J, Jeong MS, Hong HJ. Construction and Characterization of an Anti-Hepatitis B Virus preS1 Humanized Antibody that Binds to the Essential Receptor Binding Site. J Microbiol Biotechnol. 2017;27:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Kim SH, Shin YW, Hong KW, Chang KH, Ryoo KH, Paik SH, Kim JM, Brotman B, Pfahler W, Prince AM. Neutralization of hepatitis B virus (HBV) by human monoclonal antibody against HBV surface antigen (HBsAg) in chimpanzees. Antiviral Res. 2008;79:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Krepstakies M, Lucifora J, Nagel CH, Zeisel MB, Holstermann B, Hohenberg H, Kowalski I, Gutsmann T, Baumert TF, Brandenburg K, Hauber J, Protzer U. A new class of synthetic peptide inhibitors blocks attachment and entry of human pathogenic viruses. J Infect Dis. 2012;205:1654-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Petcu DJ, Aldrich CE, Coates L, Taylor JM, Mason WS. Suramin inhibits in vitro infection by duck hepatitis B virus, Rous sarcoma virus, and hepatitis delta virus. Virology. 1988;167:385-392. [PubMed] |
| 34. | Xia Y, Cheng X, Blossey CK, Wisskirchen K, Esser K, Protzer U. Secreted Interferon-Inducible Factors Restrict Hepatitis B and C Virus Entry In Vitro. J Immunol Res. 2017;2017:4828936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Tsukuda S, Watashi K, Hojima T, Isogawa M, Iwamoto M, Omagari K, Suzuki R, Aizaki H, Kojima S, Sugiyama M, Saito A, Tanaka Y, Mizokami M, Sureau C, Wakita T. A new class of hepatitis B and D virus entry inhibitors, proanthocyanidin and its analogs, that directly act on the viral large surface proteins. Hepatology. 2017;65:1104-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Yan H, Peng B, Liu Y, Xu G, He W, Ren B, Jing Z, Sui J, Li W. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J Virol. 2014;88:3273-3284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 37. | Lucifora J, Esser K, Protzer U. Ezetimibe blocks hepatitis B virus infection after virus uptake into hepatocytes. Antiviral Res. 2013;97:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 38. | Ko C, Park WJ, Park S, Kim S, Windisch MP, Ryu WS. The FDA-approved drug irbesartan inhibits HBV-infection in HepG2 cells stably expressing sodium taurocholate co-transporting polypeptide. Antivir Ther. 2015;20:835-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 39. | Watashi K, Sluder A, Daito T, Matsunaga S, Ryo A, Nagamori S, Iwamoto M, Nakajima S, Tsukuda S, Borroto-Esoda K, Sugiyama M, Tanaka Y, Kanai Y, Kusuhara H, Mizokami M, Wakita T. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP). Hepatology. 2014;59:1726-1737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 40. | Shimura S, Watashi K, Fukano K, Peel M, Sluder A, Kawai F, Iwamoto M, Tsukuda S, Takeuchi JS, Miyake T, Sugiyama M, Ogasawara Y, Park SY, Tanaka Y, Kusuhara H, Mizokami M, Sureau C, Wakita T. Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity. J Hepatol. 2017;66:685-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | Wedemeyer H, Schöneweis K, Bogomolov PO, Chulanov V, Stepanova T, Viacheslav M, Allweiss L, Dandri M, Ciesek S, Dittmer U, Haefeli W-E, Alexandrov A, Urban S. 48 wk of high dose (10 mg) bulevirtide as monotherapy or with peginterferon alfa-2a in patients with chronic HBV/HDV coinfection. J Hepatol. 2020;73 Suppl 1:S52-S53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 42. | Lucifora J, Protzer U. Attacking hepatitis B virus cccDNA--The holy grail to hepatitis B cure. J Hepatol. 2016;64:S41-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 43. | Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, Remouchamps C, Chou WM, Thasler WE, Hüser N, Durantel D, Liang TJ, Münk C, Heim MH, Browning JL, Dejardin E, Dandri M, Schindler M, Heikenwalder M, Protzer U. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 741] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 44. | Bockmann JH, Stadler D, Xia Y, Ko C, Wettengel JM, Schulze Zur Wiesch J, Dandri M, Protzer U. Comparative Analysis of the Antiviral Effects Mediated by Type I and III Interferons in Hepatitis B Virus-Infected Hepatocytes. J Infect Dis. 2019;220:567-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, Michler T, Wisskirchen K, Cheng X, Zhang K, Chou WM, Wettengel JM, Malo A, Bohne F, Hoffmann D, Eyer F, Thimme R, Falk CS, Thasler WE, Heikenwalder M, Protzer U. Interferon-γ and Tumor Necrosis Factor-α Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology. 2016;150:194-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 46. | Wang L, Zhu Q, Zeng J, Yan Z, Feng A, Young J, Gao L. PS-074-A first-in-class orally available HBV cccDNA destabilizer ccc_R08 achieved sustainable HBsAg and HBV DNA suppression in the HBV circle mouse model through elimination of cccDNA-like molecules in the mouse liver. J Hepatol. 2019;70 Suppl 1:e48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Cradick TJ, Keck K, Bradshaw S, Jamieson AC, McCaffrey AP. Zinc-finger nucleases as a novel therapeutic strategy for targeting hepatitis B virus DNAs. Mol Ther. 2010;18:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Ramanan V, Shlomai A, Cox DB, Schwartz RE, Michailidis E, Bhatta A, Scott DA, Zhang F, Rice CM, Bhatia SN. CRISPR/Cas9 cleavage of viral DNA efficiently suppresses hepatitis B virus. Sci Rep. 2015;5:10833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 49. | Chen J, Zhang W, Lin J, Wang F, Wu M, Chen C, Zheng Y, Peng X, Li J, Yuan Z. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Ther. 2014;22:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 50. | Seeger C, Sohn JA. Complete Spectrum of CRISPR/Cas9-induced Mutations on HBV cccDNA. Mol Ther. 2016;24:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 51. | Martinez MG, Inchauspe A, Delberghe E, Chapus F, Neveu G, Alam A, Carter K, Testoni B, Zoulim F. Targeting hepatitis B virus with CRISPR/Cas9 approach. J Hepatol. 2020;73 Suppl 1:S841-S842. [DOI] [Full Text] |
| 52. | Murai K, Kodama T, Hikita H, Shimoda A, Fukuaka M, Fukutomi K, Tahata Y, Makino Y, Yamada R, Sakamori R, Tatsumi T, Takehara T. Novel Anti-HBV Therapies Using CRISPR/Cas9 Targeting HBV Genome Strongly Suppress HBV. Hepatology. 2020;72 Suppl 1:61A-62A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest. 2012;122:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 54. | Cougot D, Wu Y, Cairo S, Caramel J, Renard CA, Lévy L, Buendia MA, Neuveut C. The hepatitis B virus X protein functionally interacts with CREB-binding protein/p300 in the regulation of CREB-mediated transcription. J Biol Chem. 2007;282:4277-4287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Gilmore S, Tam D, Dick R, Appleby T, Birkus G, Willkom M, Delaney WE, Notte GT, Feierbach B. Antiviral activity of GS-5801, a liver-targeted prodrug of a lysine demethylase 5 inhibitor, in a hepatitis B virus primary human hepatocyte infection model. J Hepatol. 2017;66 Suppl 1:S690-S691. [DOI] [Full Text] |
| 56. | Erken R, Stelma F, Roy E, Sampson D, Radreau P, André P, Vonderscher J, Meldrum E, Sousa CM, Jacob E, Schreuder T, Pierrillas P, Laveille C, Scalfaro P, Reesink H. First clinical evaluation in chronic hepatitis B patients of the synthetic farnesoid X receptor agonist EYP001. J Hepatol. 2018;68 Suppl 1:S488-S489. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 57. | Decorsière A, Mueller H, van Breugel PC, Abdul F, Gerossier L, Beran RK, Livingston CM, Niu C, Fletcher SP, Hantz O, Strubin M. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 414] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 58. | Sekiba K, Otsuka M, Ohno M, Yamagami M, Kishikawa T, Suzuki T, Ishibashi R, Seimiya T, Tanaka E, Koike K. Inhibition of HBV Transcription From cccDNA With Nitazoxanide by Targeting the HBx-DDB1 Interaction. Cell Mol Gastroenterol Hepatol. 2019;7:297-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 59. | Rossignol JF, Bréchot C. A Pilot Clinical Trial of Nitazoxanide in the Treatment of Chronic Hepatitis B. Hepatol Commun. 2019;3:744-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Sekiba K, Otsuka M, Ohno M, Yamagami M, Kishikawa T, Seimiya T, Suzuki T, Tanaka E, Ishibashi R, Funato K, Koike K. Pevonedistat, a Neuronal Precursor Cell-Expressed Developmentally Down-Regulated Protein 8-Activating Enzyme Inhibitor, Is a Potent Inhibitor of Hepatitis B Virus. Hepatology. 2019;69:1903-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Yu HB, Jiang H, Cheng ST, Hu ZW, Ren JH, Chen J. AGK2, A SIRT2 Inhibitor, Inhibits Hepatitis B Virus Replication In Vitro And In Vivo. Int J Med Sci. 2018;15:1356-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Gallay P, Ure D, Bobardt M, Chatterji U, Ou J, Trepanier D, Foster R. The cyclophilin inhibitor CRV431 inhibits liver HBV DNA and HBsAg in transgenic mice. PLoS One. 2019;14:e0217433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 63. | Rawal PR, Tripathi DM, Sarin SK, Nain V, Kaur S. Targeting HBx by CRISPR/Cas9 system effectively reduced the EMT and stemness characteristics of HCC cells in vitro. Hepatology. 2020;72 Suppl 1:61A. |
| 64. | Chery J. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc J. 2016;4:35-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 65. | Yuen MF, Schiefke I, Yoon JH, Ahn SH, Heo J, Kim JH, Lik Yuen Chan H, Yoon KT, Klinker H, Manns M, Petersen J, Schluep T, Hamilton J, Given BD, Ferrari C, Lai CL, Locarnini SA, Gish RG. RNA Interference Therapy With ARC-520 Results in Prolonged Hepatitis B Surface Antigen Response in Patients With Chronic Hepatitis B Infection. Hepatology. 2020;72:19-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 66. | Gane E, Locarnini S, Lim TH, Strasser S, Sievert W, Cheng W, Thompson A, Given B, Schluep T, Hamilton J, Biermer M, Kalmeijer R, Beumont-Mauviel M, Lenz O, Cloherty G, Ka-Ho Wong D, Schwabe C, Jackson K, Ferrari C, Lai CL, Gish RG, Yuen MF. Short-term treatment with RNA interference therapy, JNJ-3989, results in sustained hepatitis B surface antigen supression in patients with chronic hepatitis B receiving nucleos(t)ide analogue treatment. J Hepatol. 2020;73 Suppl 1:S20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Gane E, Lim YS, Tangkijvanich P, O’Beirne J, Lim TH, Bakardjiev A, Ding X, Connolly L, Huang S, Kim J, Pang P, Yuen MF. Preliminary safety and antiviral activity of VIR-2218, an X-targeting HBV RNAi therapeutic, in chronic hepatitis B patients. J Hepatol. 2020;73 Suppl 1:S50-S51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 68. | Streinu-Cercel A, Gane E, Cheng W, Sievert W, Roberts S, Ahn SH, Kim YJ, Agarwal K, Niforos D, Symonds B, Mendez P. A phase 2a study evaluating the multi-dose activity of ARB-1467 in HBeAg positive and negative virally suppressed subjects with hepatitis B. J Hepatol. 2017;66 Suppl 1:S688-S689. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Yuen MF, Berliba E, Kim YJ, Holmes JA, Lim Y-S, Strasser SI, Schwabe C, Jucov A, Lee ACH, Thi EP, Harasym T, Pamulapati GR, Wattamwar P, Kunta J, Sofia M, Sevinsky H, Gray K, Eley T, Picchio G, Sims KD, Gane E. Safety and Pharmacodynamics of the GalNAc-siRNA AB-729 in subjects with Chronic Hepatitis B infection. Hepatology. 2020;72 Suppl 1:62A-63A. |
| 70. | Yuen MF, Lim TH, Kim W, Tongkijvonich P, Yoon JH, Sievert W, Sukeepoisornjoroen W, Thompson A, Schwabe C, Brown B, Achnecl H, Gane E. HBV RNAi inhibitor RG6346 in Phase 1b-2a trial was safe, well-tolerated, and resulted in substantial and durable reductions in serum HBsAg levels. Late breaking abstract to the AASLD’s The Liver Meeting® Digital Experience™ 2020; American Association for the Study of Liver Diseases, 2020: 14. |
| 71. | Han K, Cremer J, Elston R, Oliver S, Baptiste-Brown S, Chen S, Gardiner D, Davies M, Saunders J, Hamatake R, Losos J, Leivers M, Hood S, van der Berg F, Paff M, Ritter JM, Theodore D. A Randomized, Double-Blind, Placebo-Controlled, First-Time-in-Human Study to Assess the Safety, Tolerability, and Pharmacokinetics of Single and Multiple Ascending Doses of GSK3389404 in Healthy Subjects. Clin Pharmacol Drug Dev. 2019;8:790-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 72. | Yuen MF, Heo J, Jang JW, Yoon JH, Kweon YO, Park SJ, Bennett CF, Kwoh J. AS067-Hepatitis B virus (HBV) surface antigen (HBsAg) inhibition with isis 505358 in chronic hepatitis B (CHB) patients on stable nucleos (t)ide analogue (NA) regimen and in NA -naive CHB patients: phase 2a, randomized, double-blind, placebo-controlled study. J Hepatol. 2020;73 Suppl 1:S49-S50. [DOI] [Full Text] |
| 73. | You S, Yates P, Elston R, Tao Y, Paff M, Theodore D. Short-term therapy with GSK3228836 in chronic hepatitis B (CHB) patients results in reductions in HBcrAg and HBV RNA: Phase 2a, randomized, double-blind, placebo-controlled study. Late breaking abstract to the AASLD’s The Liver Meeting® Digital Experience™ 2020; American Association for the Study of Liver Diseases, 2020: 52. |
| 74. | Hong J, Tan H, Lin T-I, Kang H, Nie Y, Bhattacharya A, Rajendra P, Blatt LM, Symons JA, Beigelman LN. Combination of Antisense Oligonucleotides (ASOS) ALG-020572 and ALG-020576 against Hepatitis B Virus (HBV) improves activity and can be combined with other anti-HBV agents. Hepatology. 2020;72 Suppl 1:63A. |
| 75. | Yuen MF, Gane E, Kim DJ, Chan H, Surujbally B, Pavlonic V, Triyatni M, Grippo J, Kim HJ, Leerapun A, Lim TH, Lim YS, Tanwandee T, Kim W, Cheng W, HuTH, Wat C. RO7062931 antisense oligonucleotide phase 1 study demonstrates target engagement in patients with chronic hepatitis B on established nucleos(t)ide therapy. J Hepatol. 2020;73 Suppl 1:S51. [DOI] [Full Text] |
| 76. | Cortese MF, Garcia-Garcia S, Casillas R, Lopez-Martinez R, Ruiz BP, Sopena S, Tabernero D, Ferrer-Costa RM, Barciela MR, Buti Ferret MA, Rodriguez‐Frías F. Gene Silencing by GAPMERS: A New Therapeutic Tool in HBV Infection. Hepatology. 2020;72 Suppl 1:504A-505A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | Mueller H, Wildum S, Luangsay S, Walther J, Lopez A, Tropberger P, Ottaviani G, Lu W, Parrott NJ, Zhang JD, Schmucki R, Racek T, Hoflack JC, Kueng E, Point F, Zhou X, Steiner G, Lütgehetmann M, Rapp G, Volz T, Dandri M, Yang S, Young JAT, Javanbakht H. A novel orally available small molecule that inhibits hepatitis B virus expression. J Hepatol. 2018;68:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 78. | Zhou T, Block T, Liu F, Kondratowicz AS, Sun L, Rawat S, Branson J, Guo F, Steuer HM, Liang H, Bailey L, Moore C, Wang X, Cuconatti A, Gao M, Lee ACH, Harasym T, Chiu T, Gotchev D, Dorsey B, Rijnbrand R, Sofia MJ. HBsAg mRNA degradation induced by a dihydroquinolizinone compound depends on the HBV posttranscriptional regulatory element. Antiviral Res. 2018;149:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 79. | Zlotnick A, Venkatakrishnan B, Tan Z, Lewellyn E, Turner W, Francis S. Core protein: A pleiotropic keystone in the HBV lifecycle. Antiviral Res. 2015;121:82-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 80. | Zhang X, Cheng J, Ma J, Hu Z, Wu S, Hwang N, Kulp J, Du Y, Guo JT, Chang J. Discovery of Novel Hepatitis B Virus Nucleocapsid Assembly Inhibitors. ACS Infect Dis. 2019;5:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Guo F, Zhao Q, Sheraz M, Cheng J, Qi Y, Su Q, Cuconati A, Wei L, Du Y, Li W, Chang J, Guo JT. HBV core protein allosteric modulators differentially alter cccDNA biosynthesis from de novo infection and intracellular amplification pathways. PLoS Pathog. 2017;13:e1006658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 82. | Gane E, Yuen MF, Bo Q, Schwabe C, Tanwandee T, Das S, Jin Y, Gao L, Zhou X, Wang Y, Feng S, Meinel D, Zhu M. FRI-219-RO7049389, a core protein allosteric modulator, demonstrates robust decline in HBV DNA and HBV RNA in chronic HBV infected patients. J Hepatol. 2019;70 Suppl 1:e491. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Zhao N, Jia B, Zhao H, Xu J, Sheng X, Luo L, Huang Z, Wang X, Ren Q, Zhang Y, Zhao X, Cui Y. A First-in-Human Trial of GLS4, a Novel Inhibitor of Hepatitis B Virus Capsid Assembly, following Single- and Multiple-Ascending-Oral-Dose Studies with or without Ritonavir in Healthy Adult Volunteers. Antimicrob Agents Chemother. 2019;64:e01686-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 84. | Tai Z, Tian Q, Zhao X, Xie J, Lu Y, Tan Y, Zhao W, Ma X, Yuan X, Song H, Xue T, Wang J. Discovery of KL060332, a potential best-in-class capsid inhibitor. Hepatology. 2020;72 Suppl 1:504A. |
| 85. | Yuen MF, Gane EJ, Kim DJ, Weilert F, Yuen Chan HL, Lalezari J, Hwang SG, Nguyen T, Flores O, Hartman G, Liaw S, Lenz O, Kakuda TN, Talloen W, Schwabe C, Klumpp K, Brown N. Antiviral Activity, Safety, and Pharmacokinetics of Capsid Assembly Modulator NVR 3-778 in Patients with Chronic HBV Infection. Gastroenterology 2019; 156: 1392-1403. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 86. | Berke JM, Dehertogh P, Vergauwen K, Mostmans W, Vandyck K, Raboisson P, Pauwels F. Antiviral Properties and Mechanism of Action Studies of the Hepatitis B Virus Capsid Assembly Modulator JNJ-56136379. Antimicrob Agents Chemother. 2020;64:e02439-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 87. | Janssen H, Hou J, Asselah T, Chan H, Zoulim F, Tanaka Y, Janczewska E, Nahass R, Bourgeois S, Buti M, Lampertico P, Lenz O, Verbinnen T, Vandenbossche JJ, Talloen W, Biermer M, Kalmeijer R, Beumont-Mauviel M, Shukla U. LBP12-Efficacy and safety results of the phase 2 JNJ-56136379 JADE study in patients with chronic hepatitis B: Interim week 24 data. J Hepatol. 2020;73 Suppl 1:S129-S130. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 88. | Fung S, Sulkowski M, Lalezari J, Schiff ER, Dieterich D, Hassanein T, Kwo P, Elkhashab M, Nahass R, Ayoub W, Han SH, Bonacini M, Alves K, Zayed Hany, Huang Q, Colonno R, Knox S, Ramji A, Bennett M, Gane E, Ravendhran N, Park J, Jacobson I, Bae H, Chan S, Hann HW, Ma X, Nguyen T, Yuen MF. Antiviral activity and safety of the hepatitis B core inhibitor ABIH0731 administered with a nucleos(t)ide reverse transcriptase inhibitor in patients with HBeAg-negative chronic hepatitis B infection. J Hepatol. 2020;73 Suppl 1:S51-S52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Yuen MF, Agarwal K, Ma X, Nguyen T, Schiff ER, Hann HW, Dieterich D, Nahass R, Park J, Chan S, Han HS, Gane E, Bennett M, Alves K, Zayed H, Huang Q, Colonno R, Knox S, Stamm L, Bonacini M, Jacobson I, Ayoub W, Weilert F, Ravendhran N, Ramji A, Kwo P, Elkhashab M, Hassanein T, Bae H, Lalezari J, Fung S, Sulkowski M. Antiviral activity and safety of the hepatitis B core inhibitor ABIH0731 administered with a nucleos(t)ide reverse transcriptase inhibitor in patients with HBeAg-positive chronic hepatitis B infection in a long-term extension study. J Hepatol. 2020;73 Suppl 1:S140. [DOI] [Full Text] |
| 90. | Agarwal K, Niu J, Ding Y, Gane E, Nguyen T, Alves K, Evanchick M, Zayed H, Huang Q, Knox S, Stamm L, Colonno R, Hassanein T, Kim DJ, Lim YS, Yuen MF. Antiviral activity, pharmacokinetics and safety of the secondgeneration hepatitis B core inhibitor ABI-H2158 in Phase 1b study of patients with HBeAg-positive chronic hepatitis B infection. J Hepatol. 2020;73 Suppl 1:S125. [DOI] [Full Text] |
| 91. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1700] [Cited by in RCA: 1712] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 92. | Real CI, Werner M, Paul A, Gerken G, Schlaak JF, Vaillant A, Broering R. Nucleic acid-based polymers effective against hepatitis B Virus infection in patients don't harbor immunostimulatory properties in primary isolated liver cells. Sci Rep. 2017;7:43838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Bazinet M, Anderson M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Gersch J, Holzmayer V, Kuhns M, Cloherty G, Vaillant A. Analysis of HbsAg isoforms during and after NAP-based combination therapy in the REP 301, REP 301-LTF and REP 401 studies. Hepatology. 2020;72 Suppl 1:500A-501A. |
| 94. | Nie Y, Tan H, Kao C, Ren S, Pandey R, Chanda S, Blatt L, Beigelman LN, Symons JA, Hong J. S-Antigen Traffic-Inhibiting Oligonucleotide Polymers (STOPs) Effectively Inhibit Hepatitis B Surface Antigen (HbsAg) Secretion in Both Hepatitis B Virus (HBV) Cell Lines and HBV Infected Cells. Hepatology. 2020;72 Suppl 1:502A-503A. |
| 95. | Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Dittmer U, Krawczyk A, Vaillant A. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients with Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology. 2020;158:2180-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 96. | Gohil V, Misner D, Chanda S, Zhang Q, Liu J, Hong J, Pandey R, Rajwanshi VK, Williams C, Yogaratnam J, Fry J, Smith DB, Symons JA, Blatt LM, Beigelman LN, Lin TI. The S-Antigen Transport-Inhibiting Oligonucleotide Polymer (STOPS™) ALG‐010133 Demonstrates a Favorable Preclinical Profile for the Treatment of Chronic Hepatitis B. Hepatology. 2020;72 Suppl 1:508A. |
| 97. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 764] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 98. | Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, Chisari FV. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol. 2009;83:9652-9662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 99. | Paul S, Dickstein A, Saxena A, Terrin N, Viveiros K, Balk EM, Wong JB. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: A meta-analysis. Hepatology. 2017;66:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 100. | Liaw YF, Chen YC, Sheen IS, Chien RN, Yeh CT, Chu CM. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology. 2004;126:1024-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 101. | Zhang Y, Liang C. Innate recognition of microbial-derived signals in immunity and inflammation. Sci China Life Sci. 2016;59:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 102. | Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488-R493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 372] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 103. | Williams JB, Hüppner A, Mulrooney-Cousins PM, Michalak TI. Differential Expression of Woodchuck Toll-Like Receptors 1-10 in Distinct Forms of Infection and Stages of Hepatitis in Experimental Hepatitis B Virus Infection. Front Microbiol. 2018;9:3007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y, Zhang Q, Wang J, Zhang Z, Shen F, Yuan Z. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 105. | Riordan SM, Skinner N, Kurtovic J, Locarnini S, Visvanathan K. Reduced expression of toll-like receptor 2 on peripheral monocytes in patients with chronic hepatitis B. Clin Vaccine Immunol. 2006;13:972-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 106. | Vincent IE, Zannetti C, Lucifora J, Norder H, Protzer U, Hainaut P, Zoulim F, Tommasino M, Trépo C, Hasan U, Chemin I. Hepatitis B virus impairs TLR9 expression and function in plasmacytoid dendritic cells. PLoS One. 2011;6:e26315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 107. | Wu J, Lu M, Meng Z, Trippler M, Broering R, Szczeponek A, Krux F, Dittmer U, Roggendorf M, Gerken G, Schlaak JF. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology. 2007;46:1769-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 108. | Zhang X, Ma Z, Liu H, Liu J, Meng Z, Broering R, Yang D, Schlaak JF, Roggendorf M, Lu M. Role of Toll-like receptor 2 in the immune response against hepadnaviral infection. J Hepatol. 2012;57:522-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, Halcomb RL, Tumas DB. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013; 144: 1508-1517, 1517.e1-1517. 10;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 110. | Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269-7272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 360] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 111. | Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, Bellezza CA, Cote PJ, Zheng J, Halcomb R, Fosdick A, Fletcher SP, Daffis S, Li L, Yue P, Wolfgang GH, Tennant BC. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol. 2015;62:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 112. | Janssen HLA, Brunetto MR, Kim YJ, Ferrari C, Massetto B, Nguyen AH, Joshi A, Woo J, Lau AH, Gaggar A, Subramanian GM, Yoshida EM, Ahn SH, Tsai NCS, Fung S, Gane EJ. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol. 2018;68:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 113. | Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, Lampertico P, Grossi G, Facchetti F, Brunetto MR, Coco B, Cavallone D, Mangia A, Santoro R, Piazzolla V, Lau A, Gaggar A, Subramanian GM, Ferrari C. TLR7 Agonist Increases Responses of Hepatitis B Virus-Specific T Cells and Natural Killer Cells in Patients With Chronic Hepatitis B Treated With Nucleos(T)Ide Analogues. Gastroenterology 2018; 154: 1764-1777. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 114. | Bertoletti A, Le Bert N. Fine-Tuning TLR-7-Based Therapy for Functional HBV Cure. Hepatol Commun. 2019;3:1289-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 115. | Korolowizc KE, Li B, Huang X, Yon C, Rodrigo E, Corpuz M, Plouffe DM, Kallakury BV, Suresh M, Wu TY, Miller AT, Menne S. Liver-Targeted Toll-Like Receptor 7 Agonist Combined With Entecavir Promotes a Functional Cure in the Woodchuck Model of Hepatitis B Virus. Hepatol Commun. 2019;3:1296-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Luk A, Jiang Q, Glavini K, Triyatni M, Zhao N, Racek T, Zhu Y, Grippo JF. A Single and Multiple Ascending Dose Study of Toll-Like Receptor 7 Agonist (RO7020531) in Chinese Healthy Volunteers. Clin Transl Sci. 2020;13:985-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 117. | Wu L, Niu J, Gane EJ, Slaets L, Creus AD, Ding Y, Schwabe C, Goeyvaerts N, Xu Z, Huo D, Remoortere PV, Schwertschlag U, Vandenbossche JJ. Population pharmacokinetic/pharmacodynamic (PK/PD) models of JNJ-64794964, a toll-like receptor (TLR)-7 agonist in healthy adult subjects. Hepatology. 2020;72 Suppl 1:506A. |
| 118. | Hu Y, Tang L, Zhu Z, Meng H, Chen T, Zhao S, Jin Z, Wang Z, Jin G. A novel TLR7 agonist as adjuvant to stimulate high quality HBsAg-specific immune responses in an HBV mouse model. J Transl Med. 2020;18:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 546] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 120. | Daffis S, Balsitis S, Chamberlain J, Zheng J, Santos R, Rowe W, Ramakrishnan D, Pattabiraman D, Spurlock S, Chu R, Kang D, Mish M, Ramirez R, Li L, Li B, Ma S, Hung M, Voitenleitner C, Yon C, Suresh M, Menne S, Cote P, Delaney WE 4th, Mackman R, Fletcher SP. Toll-Like Receptor 8 Agonist GS-9688 Induces Sustained Efficacy in the Woodchuck Model of Chronic Hepatitis B. Hepatology. 2021;73:53-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 121. | Mackman RL, Mish M, Chin G, Perry JK, Appleby T, Aktoudianakis V, Metobo S, Pyun P, Niu C, Daffis S, Yu H, Zheng J, Villasenor AG, Zablocki J, Chamberlain J, Jin H, Lee G, Suekawa-Pirrone K, Santos R, Delaney WE 4th, Fletcher SP. Discovery of GS-9688 (Selgantolimod) as a Potent and Selective Oral Toll-Like Receptor 8 Agonist for the Treatment of Chronic Hepatitis B. J Med Chem. 2020;63:10188-10203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 122. | Janssen HL, Lim YS, Kim HJ, Tseng CH, Coffin CS, Elkhashab M, Anh SH, Nguyen AH, Chen D, Wallin JJ, Tan SK, Yang JC, Gaggar A, Brainard DM, Fung S, Kim YJ, Kao JH, Chuang WL. Safety and efficacy of 24 wk treatment with oral TLR8 agonist, Selgantolimod, in viremic adult patients with chronic Hepatitis B: a phase 2 study. Hepatology. 2020;72 Suppl 1:506A-507A. |
| 123. | Baum P, Odegard V. ASGR1-TLR8, an ASGR1-directed TLR8 Immunotac(TM) therapeutic, is a potent myeloid cell agonist with liver-localized activity for the treatment of chronic HBV. Hepatology. 2020;72 Suppl 1:503A. |
| 124. | Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 2020;20:537-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1164] [Cited by in RCA: 1093] [Article Influence: 218.6] [Reference Citation Analysis (0)] |
| 125. | Motta V, Soares F, Sun T, Philpott DJ. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev. 2015;95:149-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 126. | Hou Z, Zhang J, Han Q, Su C, Qu J, Xu D, Zhang C, Tian Z. Hepatitis B virus inhibits intrinsic RIG-I and RIG-G immune signaling via inducing miR146a. Sci Rep. 2016;6:26150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 127. | Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 128. | Korolowicz KE, Iyer RP, Czerwinski S, Suresh M, Yang J, Padmanabhan S, Sheri A, Pandey RK, Skell J, Marquis JK, Kallakury BV, Tucker RD, Menne S. Antiviral Efficacy and Host Innate Immunity Associated with SB 9200 Treatment in the Woodchuck Model of Chronic Hepatitis B. PLoS One. 2016;11:e0161313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 129. | Yuen R, Chen C-Y, Liu C-J, Jeng RW-J, Elkhashab M, Coffin C, Kim W, Greenbloom S, Ramji A, Lim YS, Kim YJ, Fung S, Kim DJ, Jang J, Lee KS, Afdhal N, Lyer R, Macfarlane C, Jackson aka, Locarnini S, Chan H. Ascending dose cohort study of inarigivir-A novel RIG I agonist in chronic HBV patients: Final results of the ACHIEVE trial. J Hepatol. 2019;70 Suppl 1:e47-e48. [DOI] [Full Text] |
| 130. | Tao J, Zhou X, Jiang Z. cGAS-cGAMP-STING: The three musketeers of cytosolic DNA sensing and signaling. IUBMB Life. 2016;68:858-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 131. | Ito H, Kanbe A, Hara A, Ishikawa T. Induction of humoral and cellular immune response to HBV vaccine can be up-regulated by STING ligand. Virology. 2019;531:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 132. | Guo F, Han Y, Zhao X, Wang J, Liu F, Xu C, Wei L, Jiang JD, Block TM, Guo JT, Chang J. STING agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrob Agents Chemother. 2015;59:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 133. | Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 423] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 134. | Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3906] [Article Influence: 229.8] [Reference Citation Analysis (0)] |
| 135. | Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 284] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 136. | Dong Y, Li X, Zhang L, Zhu Q, Chen C, Bao J, Chen Y. CD4+ T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B. BMC Immunol. 2019;20:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 137. | McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu Rev Immunol. 2019;37:457-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 1281] [Article Influence: 213.5] [Reference Citation Analysis (0)] |
| 138. | Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A, Maini MK. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest. 2008;118:1835-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 139. | Peppa D, Gill US, Reynolds G, Easom NJ, Pallett LJ, Schurich A, Micco L, Nebbia G, Singh HD, Adams DH, Kennedy PT, Maini MK. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med. 2013;210:99-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 140. | Thaventhiran T, Sethu S, Yeang H, Al-Huseini L, Hamdam J, Sathish J. T cell co-inhibitory receptors-functions and signalling mechanisms. J Clin Cell Immunol. 2012;S12:004. [DOI] [Full Text] |
| 141. | Lau GK, Lok AS, Liang RH, Lai CL, Chiu EK, Lau YL, Lam SK. Clearance of hepatitis B surface antigen after bone marrow transplantation: role of adoptive immunity transfer. Hepatology. 1997;25:1497-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 142. | Bohne F, Chmielewski M, Ebert G, Wiegmann K, Kürschner T, Schulze A, Urban S, Krönke M, Abken H, Protzer U. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology. 2008;134:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 143. | Gehring AJ, Xue SA, Ho ZZ, Teoh D, Ruedl C, Chia A, Koh S, Lim SG, Maini MK, Stauss H, Bertoletti A. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol. 2011;55:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 144. | Tan AT, Schreiber S. Adoptive T-cell therapy for HBV-associated HCC and HBV infection. Antiviral Res. 2020;176:104748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 145. | Festag MM, Festag J, Fräßle SP, Asen T, Sacherl J, Schreiber S, Mück-Häusl MA, Busch DH, Wisskirchen K, Protzer U. Evaluation of a Fully Human, Hepatitis B Virus-Specific Chimeric Antigen Receptor in an Immunocompetent Mouse Model. Mol Ther. 2019;27:947-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 146. | Wisskirchen K, Kah J, Malo A, Asen T, Volz T, Allweiss L, Wettengel JM, Lütgehetmann M, Urban S, Bauer T, Dandri M, Protzer U. T cell receptor grafting allows virological control of Hepatitis B virus infection. J Clin Invest. 2019;129:2932-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 147. | Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, Phan GQ, Hughes MS, Kammula US, Miller AD, Hessman CJ, Stewart AA, Restifo NP, Quezado MM, Alimchandani M, Rosenberg AZ, Nath A, Wang T, Bielekova B, Wuest SC, Akula N, McMahon FJ, Wilde S, Mosetter B, Schendel DJ, Laurencot CM, Rosenberg SA. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 876] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 148. | Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee CC, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1091] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 149. | Bertoletti A, Tan AT. Challenges of CAR- and TCR-T cell-based therapy for chronic infections. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 150. | Fergusson JR, Wallace Z, Connolly MM, Woon AP, Suckling RJ, Hine DW, Barber C, Bunjobpol W, Choi BS, Crespillo S, Dembek M, Dieckmann N, Donoso J, Godinho LF, Grant T, Howe D, McCully ML, Perot C, Sarkar A, Seifert FU, Singh PK, Stegmann KA, Turner B, Verma A, Walker A, Leonard S, Maini MK, Wiederhold K, Dorrell L, Simmons R, Knox A. Immune-Mobilizing Monoclonal T Cell Receptors Mediate Specific and Rapid Elimination of Hepatitis B-Infected Cells. Hepatology. 2020;72:1528-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 151. | Leonard S, Paterson R, Godinho L, Howe D, Monteiro M, Hague RM, Atkin K, Sarkar A, Suckling R, Bunjobpol W, Kay D, Grant T, Perot C, Knox A. Novel HLA-E specific ImmTAVR molecules for the treatment of Hepatitis B. Hepatology. 2020;72 Suppl 1:59A-60A. |
| 152. | Fuertes Marraco SA, Neubert NJ, Verdeil G, Speiser DE. Inhibitory Receptors Beyond T Cell Exhaustion. Front Immunol. 2015;6:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 153. | Johnson DB, Peng C, Sosman JA. Nivolumab in melanoma: latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 154. | Sundar R, Cho BC, Brahmer JR, Soo RA. Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 155. | Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, Schwabe C, Dunbar PR. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol. 2019;71:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 156. | Zhang F, Wei H, Wang X, Bai Y, Wang P, Wu J, Jiang X, Wang Y, Cai H, Xu T, Zhou A. Structural basis of a novel PD-L1 nanobody for immune checkpoint blockade. Cell Discov. 2017;3:17004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 157. | Yu X, Zheng Y, Mao R, Su Z, Zhang J. BTLA/HVEM Signaling: Milestones in Research and Role in Chronic Hepatitis B Virus Infection. Front Immunol. 2019;10:617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 158. | Hasanpourghadi M, Luber A, Magowan C, Zhou X, Ertl H. Glycoprotein D, a checkpoint inhibitor of early T cell activation, broadens HBV T cell antigen recognition and produces sustained antiviral declines in preclinical studiesa. Hepatology. 2020;72 Suppl 1:505A. |
| 159. | Zhang XY, Huang KY, Zhu W, Zhang Ge, Chen JH, Huang X, Liu HY, Zhai YF, Yang DJ, Hou JL. Targeting inhibitor of apoptosis proteins (IAPs) enhances intrahepatic antiviral immunity to clear hepatitis B virus infection in vivo. J Hepatol. 2020;73 Suppl 1:S5-S6. [DOI] [Full Text] |
| 160. | Stasi C, Silvestri C, Voller F. Hepatitis B vaccination and immunotherapies: an update. Clin Exp Vaccine Res. 2020;9:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 161. | Vandepapelière P, Lau GK, Leroux-Roels G, Horsmans Y, Gane E, Tawandee T, Merican MI, Win KM, Trepo C, Cooksley G, Wettendorff M, Ferrari C; Therapeutic HBV Vaccine Group of Investigators. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine. 2007;25:8585-8597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 162. | Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 851] [Cited by in RCA: 726] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 163. | Kosinska AD, Bauer T, Protzer U. Therapeutic vaccination for chronic hepatitis B. Curr Opin Virol. 2017;23:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 164. | Yoon SK, Seo YB, Im SJ, Bae SH, Song MJ, You CR, Jang JW, Yang SH, Suh YS, Song JS, Kim BM, Kim CY, Jeong SH, Sung YC. Safety and immunogenicity of therapeutic DNA vaccine with antiviral drug in chronic HBV patients and its immunogenicity in mice. Liver Int. 2015;35:805-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 165. | Shi J, Ma Y, Zhu J, Chen Y, Sun Y, Yao Y, Yang Z, Xie J. A Review on Electroporation-Based Intracellular Delivery. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 166. | Paulsen D, Weber O, Ruebsamen-Schaeff H, Tennant BC, Menne S. AIC649 Induces a Bi-Phasic Treatment Response in the Woodchuck Model of Chronic Hepatitis B. PLoS One. 2015;10:e0144383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 167. | Addy I, Jambrecina A, Berg T, Boemmel F, Kropeit D, Vank C, Bigge A, Nedoschinsky K, Stobernack HP, Rangaraju M, Kummer S, Matschl U, Gehrimg A, Eberhard J, Altfeld M, Wiesch JSZ, Zimmermann H, Paulsen D. First in Human, single ascending dose clinical trial of AIC649 in patients with chronic hepatitis. J Hepatol. 2019;70 Suppl 1:e478-e479. [DOI] [Full Text] |
| 168. | Zoulim F, Fournier C, Habersetzer F, Sprinzl M, Pol S, Coffin CS, Leroy V, Ma M, Wedemeyer H, Lohse AW, Thimme R, Lugardon K, Martin P, Bastien B, Sansas B, Adda N, Halluard C, Bendjama K, Brandely M, Inchauspé G. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: a phase 1b placebo-controlled trial. Hum Vaccin Immunother. 2020;16:388-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 169. | Vaccitech Ltd. First participant dosed in Phase 1 clinical study with VTP-300 immunotherapeutic to treat chronically infected Hepatitis B patients. 2020 June 23. [cited 20 September 2020]. In: B3C newswire [Internet]. Available from: https://www.vaccitech.co.uk/first-participant-dosed-in-phase-1-clinical-study-with-vtp-300-immunotherapeutic-to-treat-chronically-infected-hepatitis-b-patients/. |
| 170. | Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11:S25-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 171. | Lim YS, Mutimer D, Heo J, Tak WY, Rosenberg W, Jang BK, Kim YJ, Forton D, Tasker S, Georges B. A phase 1b evaluation of HepTcell HBV-specific immunotherapy in nuc-controlled, eAg negative chronic HBV infection. J Hepatol. 2019;70 Suppl 1:e50-e51. [DOI] [Full Text] |
| 172. | Altimmune Inc. Altimmune Announces IND Clearance for a Phase 2 Trial of HepTcell™ Immunotherapeutic for the Treatment of Chronic Hepatitis B. 2020 June 22. [cited 25 September 2020]. In: GlobeNewswire [Internet]. Available from: https://www.globenewswire.com/news-release/2020/06/22/2051121/0/en/Altimmune-Announces-IND-Clearance-for-a-Phase-2-Trial-of-HepTcell-Immunotherapeutic-for-the-Treatment-of-Chronic-Hepatitis-B.html. |
| 173. | Boni C, Janssen HLA, Rossi M, Yoon SK, Vecchi A, Barili V, Yoshida EM, Trinh H, Rodell TC, Laccabue D, Alfieri A, Brillo F, Fisicaro P, Acerbi G, Pedrazzi G, Andreone P, Cursaro C, Margotti M, Santoro R, Piazzolla V, Brunetto MR, Coco B, Cavallone D, Zhao Y, Joshi A, Woo J, Lau AH, Gaggar A, Subramanian GM, Massetto B, Fung S, Ahn SH, Ma X, Mangia A, Ferrari C. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients With Chronic Hepatitis. Gastroenterology 2019; 157: 227-241. e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 174. | Swisher JF, Feldman GM. The many faces of FcγRI: implications for therapeutic antibody function. Immunol Rev. 2015;268:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 175. | Wen YM, Mu L, Shi Y. Immunoregulatory functions of immune complexes in vaccine and therapy. EMBO Mol Med. 2016;8:1120-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 176. | Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1112] [Cited by in RCA: 1040] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 177. | Ma A, Motyka B, Gutfreund K, Shi YE, George R. A dendritic cell receptor-targeted chimeric immunotherapeutic protein (C-HBV) for the treatment of chronic hepatitis B. Hum Vaccin Immunother. 2020;16:756-778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 178. | Zhou C, Li C, Gong GZ, Wang S, Zhang JM, Xu DZ, Guo LM, Ren H, Xu M, Xie Q, Pan C, Xu J, Hu Z, Geng S, Zhou X, Wang X, Mi H, Zhao G, Yu W, Wen YM, Huang L, Wang XY, Wang B. Analysis of immunological mechanisms exerted by HBsAg-HBIG therapeutic vaccine combined with Adefovir in chronic hepatitis B patients. Hum Vaccin Immunother. 2017;13:1989-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 179. | Xu DZ, Zhao K, Guo LM, Li LJ, Xie Q, Ren H, Zhang JM, Xu M, Wang HF, Huang WX, Bai XF, Niu JQ, Liu P, Chen XY, Shen XL, Yuan ZH, Wang XY, Wen YM. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS One. 2008;3:e2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 180. | Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J Immunol. 2013;190:4315-4323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 181. | Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect Dis. 2010;10:712-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 315] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 182. | Wang XY, Wang B, Wen YM. From therapeutic antibodies to immune complex vaccines. NPJ Vaccines. 2019;4:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 183. | Al Mahtab M, Akbar SMF, Aguilar JC, Guillen G, Penton E, Tuero A, Yoshida O, Hiasa Y, Onji M. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment controlled phase III clinical trial). PLoS One. 2018;13:e0201236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 184. | Yoshida O, Imai Y, Shiraishi K, Tokumoto Y, Sanada T, Kohara-Tsukiyama K, Miyazaki T, Kamishita T, AI Mahtab M, Aguilar JC, Guillen Ge, Fazle Akbar SM, Kohara M, Hiasa Y. HBsAg reduction by nasal administration of a therapeutic vaccine containing HBsAg and HBcAg (Nasvac) in patients with chronic HBV infection: the result of 18 mo follow up. Hepatology. 2020;72 Suppl 1:80. |
| 185. | Cargill T, Narayan S Chinnakannan SK, Lee LN, Hutchings C, Klenerman P, Barnes E. Optimising delivery of therapeutic hepatitis B vaccines to induce resident memory T cells in the liver. J Hepatol. 2020;73 Suppl 1:S886. [DOI] [Full Text] |
| 186. | Bayat B, Lorin C, Vassilev V, Warter L. A new therapeutic candidate vaccine can overcome hepatitis B virus-induced immune tolerance in a mouse model of chronic infection. J Hepatol. 2020;73 Suppl 1:S571. [DOI] [Full Text] |
| 187. | Dai S, Wang H, Deng F. Advances and challenges in enveloped virus-like particle (VLP)-based vaccines. J Immunol Sci. 2018;2:36-41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 188. | Bu W, Joyce MG, Nguyen H, Banh DV, Aguilar F, Tariq Z, Yap ML, Tsujimura Y, Gillespie RA, Tsybovsky Y, Andrews SF, Narpala SR, McDermott AB, Rossmann MG, Yasutomi Y, Nabel GJ, Kanekiyo M, Cohen JI. Immunization with Components of the Viral Fusion Apparatus Elicits Antibodies That Neutralize Epstein-Barr Virus in B Cells and Epithelial Cells. Immunity 2019; 50: 1305-1316. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 189. | Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, Wheatley AK, Fisher BE, Ambrozak DR, Creanga A, Leung K, Yang ES, Boyoglu-Barnum S, Georgiev IS, Tsybovsky Y, Prabhakaran MS, Andersen H, Kong WP, Baxa U, Zephir KL, Ledgerwood JE, Koup RA, Kwong PD, Harris AK, McDermott AB, Mascola JR, Graham BS. Author Correction: Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol. 2019;20:765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 190. | Wang W, Zhou X, Bian Y, Wang S, Chai Q, Guo Z, Wang Z, Zhu P, Peng H, Yan X, Li W, Fu YX, Zhu M. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat Nanotechnol. 2020;15:406-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 191. | Thi EP, Dhillon AP, Ardzinski A, Bidirici-Ertekin L, Cobarrubias KD, Cuconati A, Kondratowicz AS, Kwak K, Li AHL, Miller A, Pasetka C, Pei L, Phelps JR, Snead NM, Wang X, Ye X, Sofia MJ, Lee ACH. ARB-1740, a RNA Interference Therapeutic for Chronic Hepatitis B Infection. ACS Infect Dis. 2019;5:725-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 192. | Mani N, Cole AG, Phelps JR, Ardzinski A, Cobarrubias KD, Cuconati A, Dorsey BD, Evangelista E, Fan K, Guo F, Guo H, Guo JT, Harasym TO, Kadhim S, Kultgen SG, Lee ACH, Li AHL, Long Q, Majeski SA, Mao R, McClintock KD, Reid SP, Rijnbrand R, Snead NM, Micolochick Steuer HM, Stever K, Tang S, Wang X, Zhao Q, Sofia MJ. Preclinical Profile of AB-423, an Inhibitor of Hepatitis B Virus Pregenomic RNA Encapsidation. Antimicrob Agents Chemother. 2018;62:e00082-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 193. | Zhang Q, Jekle A, Serebryany V, Welch M, Liu J, Vendeville S, Debing Y, Buh Kum D, Ren S, Liu C, Deval J, Misner D, Chanda S, Mukherjee S, Raboisson P, Symons JA, Blatt LM, Biegelman LN, Smith DB. Best in class preclinical characteristics of ALG-000184, a prodrug of the capsid assembly modulator ALG-001075 for the treatment of chronic hepatitis B. Hepatology. 2020;72 Suppl 1:503A. |
| 194. | Amblard F, Boucle S, Bassit L, Cox B, Sari O, Tao S, Chen Z, Ozturk T, Verma K, Russell O, Rat V, Rocquigny HD, Fiquet O, Boussand M, Santo JD, Strick-Marchand H, Schinazi RF. Novel Hepatitis B Virus Capsid Assembly Modulator Induces Potent Antiviral Responses In Vitro and in Humanized Mice. Antimicrob Agents Chemother. 2020;64:01701-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 195. | Delaney WE 4th, Edwards R, Colledge D, Shaw T, Furman P, Painter G, Locarnini S. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrob Agents Chemother. 2002;46:3057-3060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 196. | Chen W, Liu F, Zhao Q, Ma X, Lu D, Li H, Zeng Y, Tong X, Zeng L, Liu J, Yang L, Zuo J, Hu Y. Discovery of Phthalazinone Derivatives as Novel Hepatitis B Virus Capsid Inhibitors. J Med Chem. 2020;63:8134-8145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |