Published online May 28, 2021. doi: 10.3748/wjg.v27.i20.2643
Peer-review started: February 6, 2021
First decision: February 27, 2021
Revised: March 13, 2021
Accepted: April 23, 2021
Article in press: April 23, 2021
Published online: May 28, 2021
Processing time: 102 Days and 9.6 Hours
Studies on the association of oral contraceptive (OC) use and pancreatic cancer showed inconsistent findings.
To evaluate the relationship between OC use and pancreatic cancer risk.
A literature search for observational studies (case-control and cohort studies) was conducted up to December 2020. A meta-analysis was performed by calculating pooled relative risks (RRs) and 95% confidence intervals (CIs). Heterogeneity was assessed using Cochran’s chi-square test and I2 statistic. Subgroup analyses were performed by study design, source of controls in case-control studies, number of cases of pancreatic cancers, study quality according to Newcastle-Ottawa Scale score, geographical region and menopausal status. All analyses were performed using Review Manager 5.3 (RevMan 5.3).
A total of 21 studies (10 case-control studies and 11 cohort studies) were finally included in the present meta-analysis, comprising 7700 cases of pancreatic cancer in total. A significant association was observed between the ever use of OC and pancreatic cancer risk in the overall analysis (RR = 0.85; 95%CI = 0.73-0.98; P = 0.03). Duration of OC use (< 1 year, < 5 years, 5-10 years, > 10 years) was not significantly associated with the risk of pancreatic cancer. Subgroup analyses revealed a statistically significant subgroup difference for the geographic region in which the study was conducted (Europe vs Americas vs Asia; P = 0.07). Subgroup analyses showed a statistically significant decrease in pancreatic cancer risk and OC use in high-quality studies, studies conducted in Europe, and in postmenopausal women.
Despite the suggested protective effects of OC use in this meta-analysis, further epidemiological studies are warranted to fully elucidate the association between the use of OC and pancreatic cancer risk.
Core Tip: Although the understanding of the etiology of pancreatic cancer has improved dramatically over the past decades, the link between pancreatic cancer risk and oral contraceptive (OC) use is still insufficiently known. This meta-analysis showed a significant association between OC use and pancreatic cancer risk (overall relative risk = 0.85; 95% confidence interval = 0.73-0.98). A better understanding of the risks of pancreatic cancer occurrence in women who use OC may be relevant for pancreatic cancer prevention strategy.
- Citation: Ilic M, Milicic B, Ilic I. Association between oral contraceptive use and pancreatic cancer risk: A systematic review and meta-analysis . World J Gastroenterol 2021; 27(20): 2643-2656
- URL: https://www.wjgnet.com/1007-9327/full/v27/i20/2643.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i20.2643
Pancreatic cancer is the seventh most common cause of death among malignant tumors in females, with about 220000 deaths worldwide in 2018[1,2]. Pancreatic cancer is one of the deadliest types of cancer, with an estimated overall 5-year survival rate less than 10%[1]. Pancreatic cancer mortality is characterized by a dramatic increase after the age of 30 years, reaching the highest burden in women at about 80-years-old[1,3].
An understanding of the etiology of pancreatic cancer has improved dramatically over the past decades and certain risk factors have been established including tobacco use, obesity, diabetes mellitus, chronic pancreatitis, positive family history and inherited genetic syndromes, high alcohol consumption, dietary factors, physical inactivity, workplace exposure to some chemicals, infections[4-7]. Although some risk factors have been identified, the causes of pancreatic cancer are still insufficiently known.
Regarding the link between pancreatic cancer risk and oral contraceptive (OC) use, epidemiological studies have shown conflicting results: Some findings have shown positive associations with risk of pancreatic cancer[8,9], whereas some studies have indicated inverse associations[10,11]. One previous meta-analysis of observational studies did not support the hypothesis that OC use is associated with pancreatic cancer risk (the pooled relative risk [RR] = 1.09, 95% confidence interval [CI] 0.96–1.23)[12].
A better understanding of risks of pancreatic cancer occurrence in women who use OC may be relevant for pancreatic cancer prevention strategy. The purpose of this study was to evaluate the relationship between the use of OC and pancreatic cancer risk by performing a meta-analysis of case-control and cohort studies.
This meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[13].
This study is a part of a research project approved by the Ethics Committee of the Faculty of Medical Sciences, University of Kragujevac (No. 01-14321).
We searched PubMed from inception through December 2020 using combinations of keywords: (“oral contraceptives” or “birth control pills”) and (“pancreatic cancer” or “pancreatic tumor” or “pancreatic neoplasm”) and (“risk” or “risk factors” or “risk assessment”). Additionally, references of retrieved studies and reviews were hand-searched to identify additional relevant studies (up to 31 December 2020). No language restrictions were applied in the search.
Two authors (II and MI) independently screened the titles and abstracts of studies retrieved by literature search. Subsequently, the full texts of articles that were identified as relevant were assessed. Any disagreements between the reviewers were resolved through consensus. Studies which reported on the association between the use of OC (the exposure of interest) and risk of pancreatic cancer (the outcome of interest) and that were designed as case-control studies and cohort studies were included. In cases of multiple publications reporting results from the same population, the most recent report and the one with the most data was used. Case reports, case-series, reviews, letters, animal studies and studies with incomplete data were excluded. Studies that did not report separate data for OC use, but instead reported “any hormone therapy” were excluded.
Data extraction and quality appraisal were performed independently by two authors (II and MI). Details regarding the study’s author and year of publication, study design, sample size, methods of exposure assessment, methods of outcome assessment, and main findings regarding the investigated outcome were extracted. Methodological quality of studies was assessed using the Newcastle-Ottawa Scale (NOS) for quality assessment of case-control studies and cohort-studies[14]. This tool rates three categories: Selection, comparability and exposure (in case-control studies) or outcome (in cohort studies) in observational studies using a star-system. We considered studies with 8 and 9 stars as high quality, 6 and 7 stars as medium quality, and ≤ 5 stars as low quality.
A meta-analysis of the comparison of nonusers vs users of OC was performed. Odds ratios (ORs), RRs, and hazard ratios (HRs) were extracted from included studies and transformed into RRs. It can be considered that OR and HR approximate RR because the absolute risk of pancreatic cancer is low[15]. For studies that did not report risk estimates for the comparison of ever vs never use of OC, we calculated ORs and RRs based on the available published data. We pooled risk estimates for pancreatic cancer and calculated overall RRs with 95%CIs. Risk effects were combined using the random-effects model[16]. The P < 0.05 was considered significant.
Heterogeneity was quantified using Cochran’s chi-square test and I2 statistic, with I2 values of 30%-60%, 50%-90%, and 75%-100% indicating moderate, substantial and considerable heterogeneity, respectively[17]. To explore heterogeneity, we performed subgroup analyses by study design (case-control and cohort), source of controls in case-control studies (population and hospital), number of cases of pancreatic cancers (< 200 and ≥ 200), and assessed study quality (NOS score ≤ 7 and > 7), geographical region (Europe, Americas, Asia), and menopausal status (premenopausal and postmenopausal). Statistically significant subgroup differences were considered for P < 0.1[18].
The pooled RRs with corresponding 95%CIs were presented graphically with forest plots. Publication bias was assessed with a funnel plot. All statistical analyses were performed using Review Manager 5 software (RevMan version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration)[19].
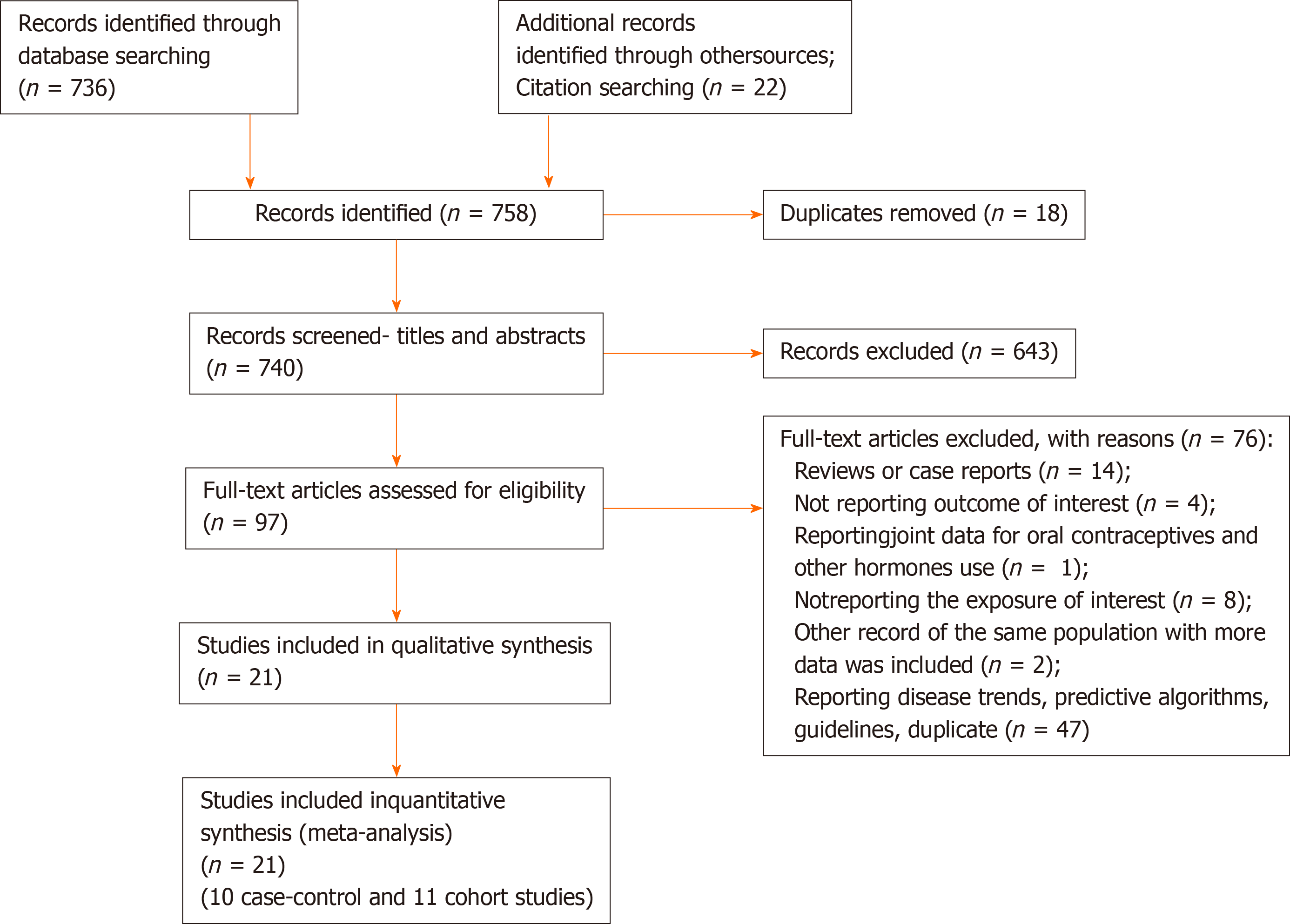
The results of the literature search are presented in Figure 1. We identified 21 studies that investigated the association between the use of OC and risk of pancreatic cancer and that fulfilled the inclusion criteria[8-11,20-36]. There were 10 case-control studies and 11 cohort studies included in the meta-analysis. Characteristics of the included studies are presented in Table 1. In total, the included studies comprised 7700 cases of pancreatic cancer. Among the case-control studies, six had population-based controls and four had hospital based-controls. The use of OC was assessed via interviews with trained interviewers in 11 studies by means of self-reported questionnaires in 9 studies, and one study used data from the National Register of Medicinal Products. Outcome assessment was verified through cancer and hospital registries, and in most of the studies, there was pathohistological verification of the diagnosis of pancreatic cancer. Seven studies were conducted in the European region, ten in the Americas (the United States and Canada), three in Asia, and one study was multicentric and conducted in all three regions. According to NOS scores, 12 studies were of high quality (3 case-control and 9 cohort), 8 of moderate quality (6 case-control and 2 cohort), and 1 case-control study was of low quality.
| Study design | Ref. | Country | Sample size1 | Exposure assessment | Outcome assessment | Risk estimate (95%CI)2 | Adjustments | NOS score |
| Case-control | ||||||||
| Bueno de Mesquita et al[20], 1992 | Netherlands | 82 cases and 252 controls | Interviewer administered questionnaire | Clinical diagnosis (histological verification, clinicians, laboratory records, registries) | OR = 0.83 (0.31-2.23) | Age, response status and life-time smoking of cigarettes | 7 | |
| Ji et al[21], 1996 | China | 184 cases and 680 controls | Interviewer administered questionnaire | Rapid reporting system within cancer registry | OR = 1.78 (0.91-3.47) | Age, income, education, smoking, BMI, green tea drinking, respondent status, age at first birth, intake of dietary vitamin C | 7 | |
| Kreiger et al[10], 2001 | Canada | 52 cases and 233 controls | Mailed questionnaire | Cancer registry | OR = 0.36 (0.13-0.96); P = 0.031 | Age, smoking status, BMI, tofu, dietary fat, coffee consumption, age at menarche, age at menopause, parity, estrogen replacement therapy age at first full term pregnancy | 8 | |
| Duell et al[22], 2005 | United States | 241 cases and 818 controls | Interviewer administered questionnaire | Physician, SEER and histologic confirmation | OR = 0.95 (0.65-1.4) | Age, education, smoking | 8 | |
| Duell et al[23], 2009 | Australia, Canada, The Netherlands, Poland | 367 cases and 821 controls | Interviewer administered questionnaire | Clinicians, hospital records, pathology records, cancer registries | OR = 0.74 (0.43-1.26) | Smoking, schooling, age, center, type of interview | 8 | |
| Zhang et al[24], 2010 | United States | 284 cases and 1096 controls | Interviewer administered questionnaire | Pathology report confirmation | 3 | - | 6 | |
| Lucenteforte et al[8], 2011 | Italy | 285 cases and 713 controls | Interviewer administered questionnaire | Histologically confirmed | OR = 1.04 (0.55-1.98) | Study/center, age, education, area of residence, year of interview, history of diabetes, tobacco smoking | 7 | |
| Azeem et al[11], 2015 | Czech Republic | 129 cases and 97 controls | Interviewer administered questionnaire | Hospital diagnosis | OR = 0.21 (0.07-0.69) | Not specified (other monitored factors) | 5 | |
| Masoudi et al[9], 2017 | Iran | 153 cases and 202 controls | Interviewer administered questionnaire | Pathological reports | OR = 1.07 (0.62-1.84) | Smoking status, BMI, diabetes | 6 | |
| Archibugi et al[25], 2020 | Italy | 253 cases and 506 controls | Interviewer administered questionnaire | Histologically proven diagnosis | OR = 0.52 (0.31-0.89) | Age, BMI, first degree family history of pancreatic cancer, history of diabetes > 1 yr, history of chronic pancreatitis, heavy alcohol intake, smoking habit | 7 | |
| Cohort | ||||||||
| Skinner et al[26], 2003 | United States | 243 cases and 115474 non-cases | Mailed questionnaire | Self-reported confirmed via medical records, pathology reports, death certificates, physicians, next of kin interview | RR = 1.21 (0.91-1.61) | Age, time period, cigarette smoking, diabetes, BMI, height, parity | 8 | |
| Teras et al[27], 2005 | United States | 1959 cases among 387981 participants | Self-administered questionnaire | National Death Index and death certificates | 3 | - | 8 | |
| Navarro Silvera et al[28], 2005 | Canada | 187 cases and 89645 noncases | Self-administered questionnaire | Canadian Cancer Database and the National mortality database | HR = 1.10 (0.81-1.50) | Age, cigarette smoking intensity, cigarette smoking duration, BMI, height, study center, randomization group, parity | 8 | |
| Prizment et al[29], 2007 | United States | 228 cases and 37231 noncases | Self-administered questionnaire | National Death Index and State Health Registry of Iowa | HR = 0.90 (0.62-1.30) | Age | 7 | |
| Dorjgochoo et al[30], 2009 | China | 78 cases among 66661 participants | In-person interview and a self-reported questionnaire | Shanghai Cancer registry and Shanghai Vital Statistics Registry | HR = 0.83 (0.45-1.55) | Education, age at menarche, number of live births, cumulative breast feeding months, BMI, exercised regularly in past 5 yr, smoking, menopausal status, first-degree family history of cancer, other contraceptive methods | 9 | |
| Duell et al[31], 2013 | Denmark, France, Germany, Greece, Italy, Netherlands, Norway, Spain, Sweden, United Kingdom | 304 cases among 328610 participants | Self-administered questionnaire | Population cancer registries, health insurance records, hospital-based and pathology registries | 3 | - | 8 | |
| Lee et al[32], 2013 | United States | 323 cases among 118164 participants | Self-administered questionnaire | California Cancer Registry | 3 | - | 8 | |
| Kabat et al[33], 2017 | United States | 1003 cases and 157295 non-cases | Self-administered questionnaire | Self-reports verified by physician adjudicators through records of hospitalizations, surgeries, pathology reports and procedures | HR = 0.92 (0.80-1.06) | Age, smoking status, pack-years of smoking, BMI, educational level, ethnicity, allocation to study component, diabetes | 7 | |
| Andersson et al[34],2018 | Sweden | 110 cases and 16921 noncases | On-site questionnaires and examinations | Swedish Cancer register confirmed by pathology records, autopsy | HR = 0.68 (0.44-1.06) | Age, smoking, alcohol consumption, BMI | 8 | |
| Butt et al[35], 2018 | Denmark | 235 cases among 1.9 million women | National Register of Medicinal Product Statistics | Danish Cancer Register and Danish National Patient Register | RR = 0.90 (0.68-1.19) | Age, year, education, PCOS, endometriosis, parity | 9 | |
| Michels et al[36], 2018 | United States | 1000 cases and 195536 noncases | Mailed questionnaire | Cancer registries | HR = 1.11 (0.97-1.28) | Age, race, BMI, smoking status, alcohol use, number of cigarettes smoked per day | 8 |
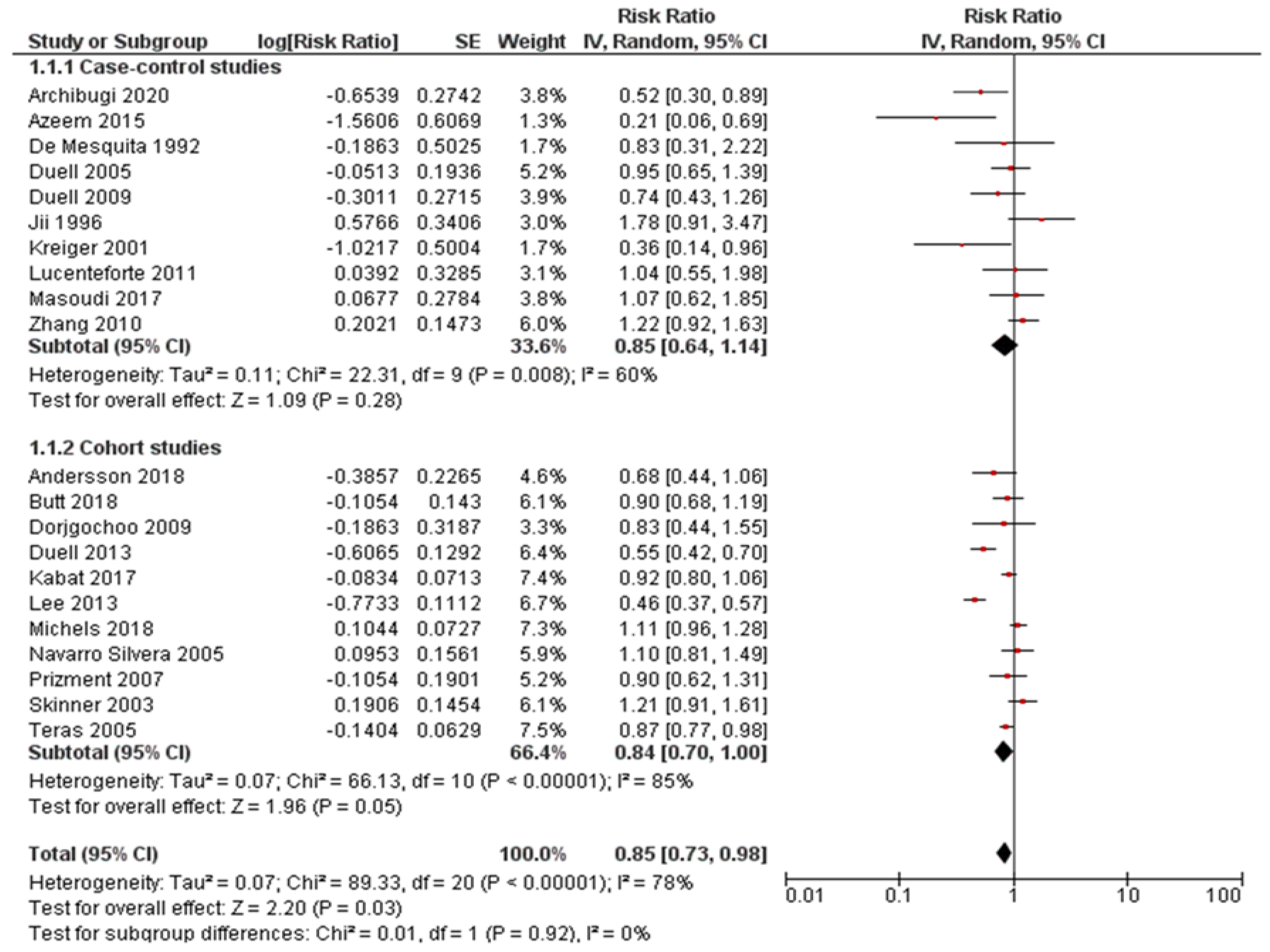
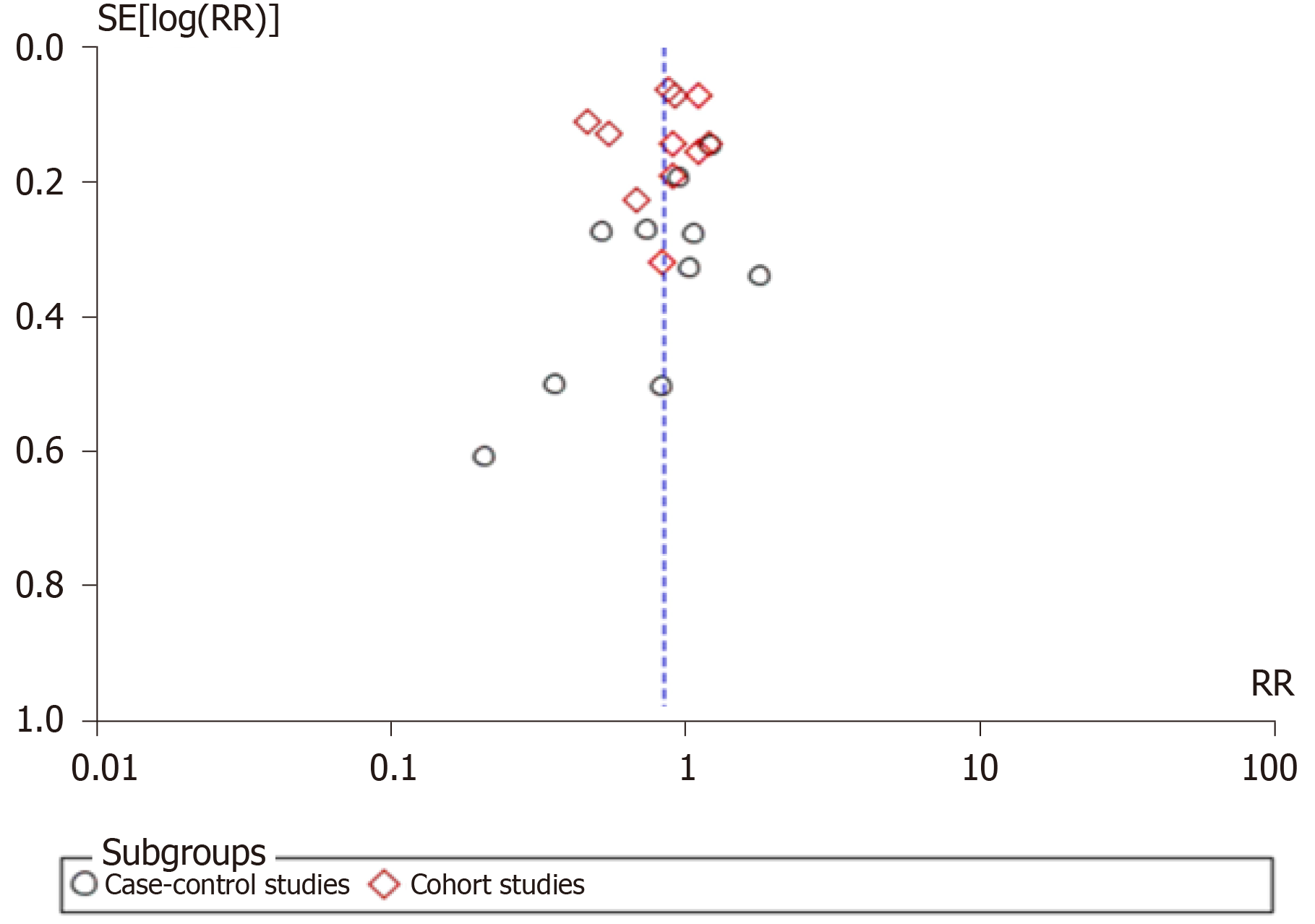
Pooled risk estimates of all included studies showed that the ever use of OC was statistically significantly associated with a decreased risk of pancreatic cancer (RR = 0.85, 95%CI: 0.73-0.98; P = 0.03) (Figure 2). There was substantial heterogeneity for this estimate (I2 = 78%; P < 0.00001). Pooled risk estimates from case-control studies were not statistically significant (RR = 0.85, 95%CI: 0.64-1.14), while the association between ever use of OC and decreased risk of pancreatic cancer was borderline significant in cohort studies (RR = 0.84, 95%CI: 0.70-1.00). Visual inspection of funnel plot (Figure 3) did not indicate any apparent presence of publication bias.
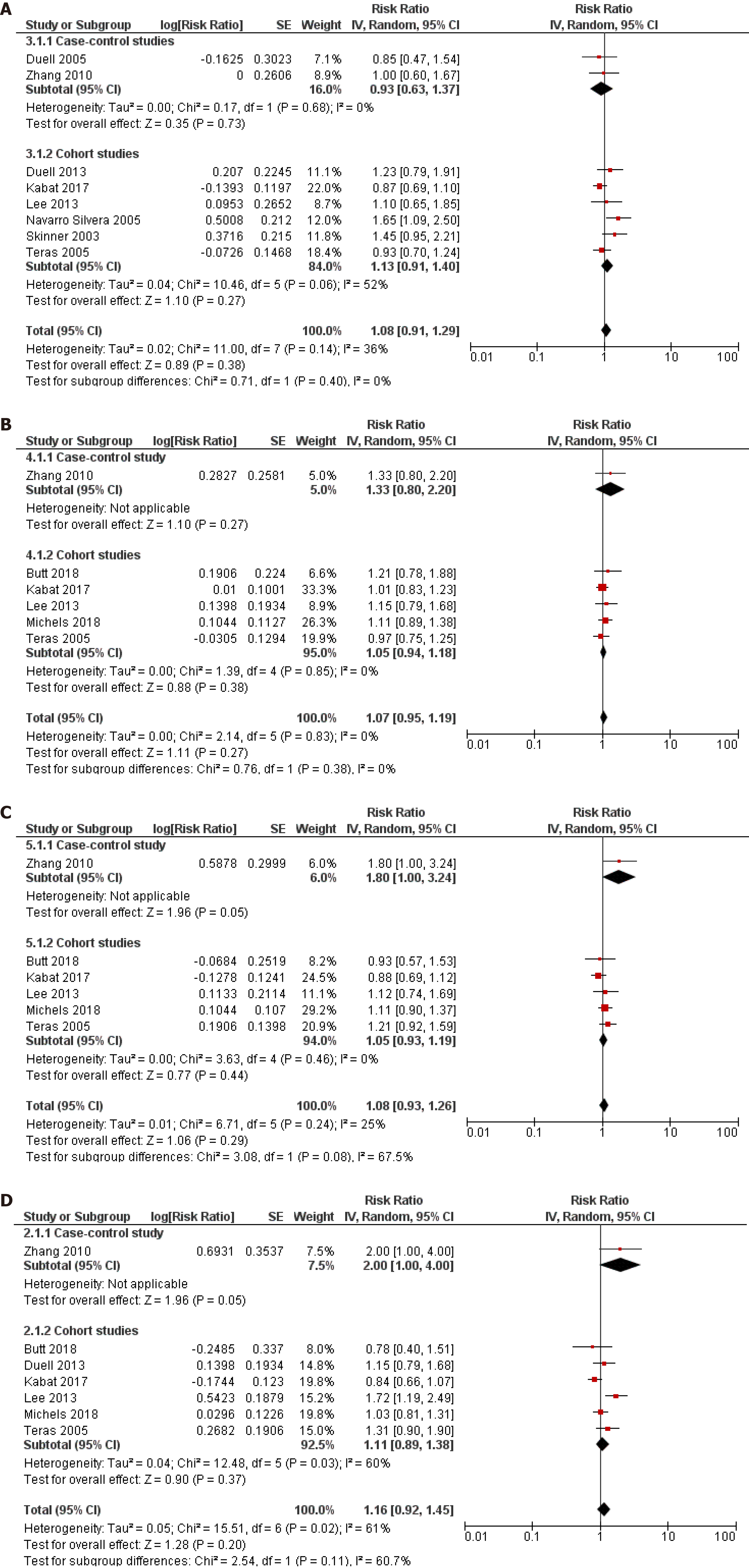
Ever use vs never use of OC was chosen as the primary assessment of exposure because some studies have reported that it was not possible to assess the years of oral contraceptive use in their sample[10] or simply did not inquire participants about the duration of use[8-11,23,29,34]. However, we pooled the results from the subset of studies that investigated the duration of use of OC and risk of pancreatic cancer and that reported comparable cut-off periods. Eight studies (2 case-control and 6 cohort) assessed the < 1 year duration of OC use and risk of pancreatic cancer and the pooled risk estimate was not statistically significant (RR = 1.08, 95%CI: 0.91-1.29; P = 0.38) (Figure 4A). Similarly, across one case-control and six cohort studies, the results were not significant for durations of use: < 5 years (RR = 1.07, 95%CI: 0.95-1.19; P = 0.27) (Figure 4B), 5-10 years (RR = 1.08, 95%CI: 0938-1.26; P = 0.29) (Figure 4C) and > 10 years (RR = 1.16, 95%CI: 0.92-1.45; P = 0.20) (Figure 4D).
Results of the subgroup analyses by selected characteristics are presented in Table 2. Statistically significant results for subgroup differences were noted only for geographic region (Europe vs Americas vs Asia) where studies were conducted (P = 0.07), while there were no significant subgroup differences noted for study design (case-control vs cohort studies), source of controls in case-control studies (population vs hospital), number of pancreatic cancer cases (< 200 vs ≥ 200), study quality (NOS ≤ 7 vs NOS > 7), or menopausal status (premenopausal vs postmenopausal). Finally, subgroup analyses revealed a statistically significant association between the use of OC and decreased risk of pancreatic cancer in studies of high quality (RR = 0.80, 95%CI: 0.66-0.98; P = 0.03), studies conducted in Europe (OR = 0.67, 95%CI: 0.51-0.88; P = 0.004) and in postmenopausal women (r = 0.88, 95%CI: 0.79-0.98; P = 0.02).
| Characteristic | Subgroup | Number of studies | RR (95%CI) | I2 within each subgroup | Test for subgroups differences |
| Study design | Case-control | 10 | 0.85 (0.64-1.14) | 60% (P = 0.008) | P = 0.92 |
| Cohort | 11 | 0.84 (0.70-1.00) | 85% (P < 0.00001) | ||
| Source of controls in case-control studies | Population | 6 | 0.75 (0.47-1.21) | 63% (P = 0.02) | P = 0.47 |
| Hospital | 4 | 0.94 (0.64-1.39) | 61% (P = 0.06) | ||
| Number of pancreatic cancer cases | < 200 | 8 | 0.80 (0.56-1.14) | 66% (P = 0.004) | P = 0.77 |
| ≥ 200 | 13 | 0.85 (0.71-1.00) | 83% (P < 0.00001) | ||
| Assessed study quality | NOS ≤ 7 | 9 | 0.93 (0.75-1.17) | 56% (P = 0.02) | P = 0.33 |
| NOS > 7 | 12 | 0.80 (0.66-0.98) | 84% (P < 0.00001) | ||
| Geographic region1 | Europe | 7 | 0.67 (0.51-0.88) | 55% (P = 0.04) | P = 0.07 |
| Americas | 10 | 0.91 (0.74-1.11) | 86% (P < 0.00001) | ||
| Asia | 3 | 1.14 (0.76-1.73) | 27% (P = 0.25) | ||
| Menopausal status | Premenopausal | 1 | 0.90 (0.68-1.19) | N/A | 0.90 |
| Postmenopausal | 4 | 0.88 (0.79-0.98) | 17% (P = 0.31) |
In this systematic review and meta-analysis, we identified 10 case-control and 11 cohort studies that investigated the association between the use of OC and pancreatic cancer. Our pooled analysis of these 21 studies, which comprised 7700 cases of pancreatic cancer, showed that the ever use of OC was statistically significantly associated with a decreased risk of pancreatic cancer. However, the association was not significant when the duration of OC use less than 1 year, less than 5 years, 5-10 years, and longer than 10 years was assessed in relation to pancreatic cancer risk. A significantly reduced risk of pancreatic cancer in women using OC was noted in higher quality studies, studies conducted in Europe, and in postmenopausal women.
Differences in pancreatic cancer incidence rates between sexes, namely a higher incidence of pancreatic cancer in men than in women[7], have led to investigations into possible reasons behind these differences. Apart from the possible influence of environmental factors, it was hypothesized that female sex hormones could be responsible for a lower incidence of pancreatic cancer in women. In vitro and in vivo studies have shown that the pancreas contains estrogen and androgen receptors and that estrogen inhibits and testosterone promotes the occurrence of some pancreatic cancers[37].
Numerous observational studies have investigated the role of the use of OC and risk of pancreatic cancer; however, the results were not consistent. While some authors have reported an inverse relationship between the use of OC and risk of pancreatic cancer[10,11,25,31,32], other studies have not confirmed these findings. However, none of the published studies found a significant positive relationship between the ever use of OC and pancreatic cancer. With regard to the duration of OC use, our pooled analyses did not identify a significant association with pancreatic cancer risk. Still, one cohort study revealed a significant increase in pancreatic cancer risk in women using OC < 1 year (HR = 1.65, 95%CI: 1.08-2.50)[28], but the number of cases of pancreatic cancer in the group of women who were taking OC less than 1 year was small. Also, one hospital-based case-control study (NOS score assessed as 6) found a borderline positive association for the duration of use of OC of 5-10 years and > 10 years and risk of pancreatic cancer[24], and, despite the small numbers of cases in these groups, the P for trend was significant (< 0.01). In contrast, Kreiger et al[10] found a significant decrease in pancreatic cancer risk in women using OC longer than 6 mo. These discrepancies in results across the studies might be explained by differences in study design, study population, assessment of exposure assessment, definitions of exposure, and different cut-offs for duration of use of OC. Additionally, most of the studies have reported risk estimates adjusted for known and potential pancreatic cancer risk factors (age, diabetes, cigarette smoking, obesity), but with fewer studies also providing estimates adjusted for history of pancreatitis, positive family history of pancreatic cancer and high level of alcohol consumption[25,30,34,36] and one study reporting estimates adjusted only for age[29]. While some of the included studies have adjusted for factors which refer to diet, this most often involved body mass index (BMI)[9,10,21,25,26,28,30,33,34,36], with only two studies investigating nutrition variables such as green tea drinking and intake of dietary vitamin C[21], and coffee and tofu consumption and dietary fat intake[10]. Obesity is a risk factor for pancreatic cancer; however, the mechanisms are not fully known and may involve sex hormones[21]. High BMI might reflect high intake of dietary fat, although the findings regarding its association with pancreatic cancer risk are inconsistent[21,34]. Notably, adipose tissue produces estrogens and might have a protective role[34]. Therefore, dietary factors could confound the association between the risk for pancreatic cancer and the use of OC[10]. Similarly, studies investigating nutrition and pancreatic cancer risk should adjust for reproductive factors such as the use of OC.
Subgroup analyses identified a significantly lower risk of pancreatic cancer in women in European region who used OC (RR = 0.67, 95%CI: 0.51-0.88). Worldwide, highest incidence of pancreatic cancer in women was noted in Northern America, Western and Northern Europe[7]. Prevalence of OC use was the highest in Northern America, followed by Europe and Asia at 75%, 69% and 68%, respectively[38]. The differences in pancreatic cancer risk associated with the use of OC between different geographic regions could be explained by differences in exposure to environmental risk factors, genetic or cultural differences. Notably, most of the studies pooled in this meta-analysis have included relevant known or potential risk factors as covariates in the adjustments of risk estimates. It is also possible that regional differences in diagnosis and outcome assessment and reporting could contribute to the observed significant difference in subgroup analyses by geographic region. Possible explanations for the observed inverse relationship between the use of OC and pancreatic cancer in postmenopausal women could be related to the age of menopause, duration of menopause, duration of use of OC, and formulation of used OC.
Our literature search revealed one previously published meta-analysis that assessed the association between pancreatic cancer risk and female hormonal and menstrual factors[12], and one pooled analysis from the international pancreatic cancer case-control consortium[39]. In contrast to our results, a previous meta-analysis found no significant associations between the risk of pancreatic cancer and OC use–pooled RR from six case-control studies and eight cohort studies was 1.09 (95%CI: 0.96-1.23)[12]. Subsequently, the authors noted that their subgroup analyses by study design showed a marginally significant increased risk of pancreatic cancer associated with OC use in cohort studies (RR = 1.09, 95%CI: 1.00-1.29). Our subgroup analyses by study design identified the opposite, namely, a borderline insignificant result for inverse association (RR = 0.84, 95%CI: 0.70-1.00). However, the authors identified publication bias for studies on exposure to OC, which could mask the true association. Our meta-analysis included an additional 4 case-control studies and 3 cohort studies, totaling 7700 cases of pancreatic cancer vs 5084 in the previous meta-analysis, and our analysis did not identify publication bias. Also, our study did not have language limitations in the literature search in contrast to previously conducted meta-analyses. A previous meta-analysis did not assess the quality of included studies, unlike our study that also explored subgroup differences in relation to study quality and found a statistically significant reduction in pancreatic cancer risk associated with the use of OC (RR = 0.67, 95%CI: 0.51-0.88) when pooling results from studies of high quality. The previous pooled analysis that included only case-control studies did not find a significant association between the ever use of OC and pancreatic cancer (OR = 0.83, 95%CI: 0.69-1.01).
Finally, studies included in this meta-analysis have been conducted in different time periods and the influence of different formulations of OC cannot be excluded. However, only one of the included studies inquired about the type of OC used by women[35], and did not find a significant association between pancreatic cancer and use of different types of hormonal contraceptives (RR = 0.92, 95%CI: 0.62-1.36 for oral combined 20-40 ug ethinyl estradiol, and RR = 1.16, 95%CI: 0.71-1.89 for progestin only). Further on, two studies made assumptions regarding the dose of OC by using the calendar year of use as a proxy for whether women were taking high-dose or low dose formulations[32,34]. Andersson et al[34] did not find a significant association between the risk of pancreatic cancer and use of OC in 1960-1970, after 1970, 1960-1980 or after 1980. However, Lee et al[32] found that a duration dose was present for high-dose use of OC (women who stopped using OC before 1974, P for trend 0.027), and in particular the risk for pancreatic cancer was increased in women using high-dose OC ≥ 10 years (RR = 2.08, 95%CI: 1.05-4.12). Due to the inconsistent results obtained by case-control and cohort studies, it is important to address this issue when planning future observational studies in order to provide further insight into the association between the use of OC and risk of pancreatic cancer.
To the best of our knowledge, this represents the most comprehensive meta-analysis of observational studies which have investigated the association between the use of OC and risk of pancreatic cancer to date. Our analysis included 21 published studies with 7700 from various geographic regions which could add to the generalizability of the presented results. Also, the quality of included studies was relatively high and assessment of outcome involved pathohistological confirmation in majority of the cases.
However, our study had several limitations. First, this was a meta-analysis of observational studies and inherit limitations of study design of included studies cannot be excluded. Second, assessment of exposure was mostly based on self-report or obtained through interview, with only one study investigating the use of OC through a national medicinal registry. Further on, even though we applied a random-effects model in our meta-analyses, a high level of heterogeneity was found in some comparisons in our analyses, which we tried to explore by performing subgroup analyses. We did not assess the association between pancreatic cancer and age at initiation of OC use, years since last use and intensity of use because only two studies reported this data and used different cut-offs. Our analyses pooled risk estimates adjusted for most potential cofactors as available in original studies, however it is not possible to exclude the influence of some confounding factors. Namely, the use of OC could be related with a higher socio-economic status which can in turn be related to a healthier diet[25]. Presence of bias due to the lack of confounding control cannot be excluded in included studies which have not considered dietary factors as possible confounding factors when investigating the association between the use of OC and risk for pancreatic cancer. Further on, for a few studies for which we needed to derive the necessary risk estimates there were no available adjusted risk estimates. Finally, an analysis of individual-patient data would have provided more precise results regarding the association between the use of OC and risk of pancreatic cancer.
Ever use of OC was associated with a decreased risk of pancreatic cancer in the present meta-analysis. However, more well-designed and detailed epidemiological studies are necessary in order to fully elucidate the association between the use of OC and pancreatic cancer.
Pancreatic cancer is the seventh most common cause of death among malignant tumors in women. It represents one of the deadliest types of cancer with overall 5-year survival rate < 10%.
Although the understanding of the etiology of pancreatic cancer has improved over the past decades and certain risk factors have been established, the causes of pancreatic cancer are still insufficiently known. Results of epidemiological studies show conflicting results regarding the association of the use of oral contraceptives (OC) and risk for pancreatic cancer.
The aim of this study was to evaluate the relationship between the use of OC and risk for pancreatic cancer.
A comprehensive literature search was performed based on defined inclusion and exclusion criteria. Quality of included observational studies was assessed and data was extracted. A meta-analysis of ever-use vs never-use of OC and risk for pancreatic cancer was performed using Review Manager 5.3. In addition, the association between the duration of use of OC and pancreatic cancer risk was also assessed, and a subgroup analysis was performed.
A total of 7700 cases of pancreatic cancer from 21 studies (10 case-control and 11 cohort) were included in this meta-analysis. A significant association was observed between the ever-use of OC and pancreatic cancer risk (relative risk = 0.85; 95% confidence interval: 0.73-0.98), while the duration of use (< 1 year, < 5 years, 5-10 years, > 10 years) did not show a significant association. Subgroup analysis revealed a statistically significant decrease in pancreatic cancer and use of OC in high quality studies, studies conducted in Europe and in postmenopausal women.
This meta-analysis suggests a protective effect of the use of OC and pancreatic cancer occurrence, however more epidemiological studies are necessary to fully elucidate this association.
Further epidemiological studies are warranted to fully assess the association between the use of OC and risk for pancreatic cancer. These future studies investigating the risk for pancreatic cancer should be well-designed and include detailed questions regarding the use of OC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sergi C S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | International Agency for Research on Cancer. Cancer Incidence in Five Continents. Vol. XI (electronic version). Lyon: International Agency for Research on Cancer. [cited 20 January 2021]. Available from: http://ci5.iarc.fr. |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55828] [Article Influence: 7975.4] [Reference Citation Analysis (132)] |
| 3. | Ilić M, Vlajinac H, Marinković J, Kocev N. Pancreatic cancer mortality in Serbia from 1991-2010 - a joinpoint analysis. Croat Med J. 2013;54:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 5. | Zanini S, Renzi S, Limongi AR, Bellavite P, Giovinazzo F, Bermano G. A review of lifestyle and environment risk factors for pancreatic cancer. Eur J Cancer. 2021;145:53-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Lin QJ, Yang F, Jin C, Fu DL. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21:7988-8003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 7. | Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694-9705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1003] [Cited by in RCA: 957] [Article Influence: 106.3] [Reference Citation Analysis (24)] |
| 8. | Lucenteforte E, Zucchetto A, Bosetti C, Talamini R, Negri E, Serraino D, Franceschi S, Lipworth L, La Vecchia C. Reproductive and hormonal factors and pancreatic cancer risk in women. Pancreas. 2011;40:460-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Masoudi S, Momayez Sanat Z, Mahmud Saleh A, Nozari N, Ghamarzad N, Pourshams A. Menstrual and Reproductive Factors and Risk of Pancreatic Cancer in Women. Middle East J Dig Dis. 2017;9:146-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Kreiger N, Lacroix J, Sloan M. Hormonal factors and pancreatic cancer in women. Ann Epidemiol. 2001;11:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Azeem K, Horáková D, Tomášková H, Ševčíková J, Vlčková J, Pastucha D, Procházka V, Shonová O, Martínek A, Janout V, Žídková V, Kollárová H. [A multifactor epidemiological analysis of risk factors for pancreatic cancer in women]. Epidemiol Mikrobiol Imunol. 2015;64:34-40. [PubMed] |
| 12. | Tang B, Lv J, Li Y, Yuan S, Wang Z, He S. Relationship between female hormonal and menstrual factors and pancreatic cancer: a meta-analysis of observational studies. Medicine (Baltimore). 2015;94:e177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47183] [Article Influence: 2948.9] [Reference Citation Analysis (0)] |
| 14. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute, 2014. [cited 20 January 2021]. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 15. | Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1192] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 16. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30421] [Article Influence: 780.0] [Reference Citation Analysis (0)] |
| 17. | Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. [cited 20 January 2021]. Available from: https://www.handbook.cochrane.org. |
| 18. | Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health. 2019;7:192-198. [RCA] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 19. | RevMan. 2014 [Computer program] Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014. |
| 20. | Bueno de Mesquita HB, Maisonneuve P, Moerman CJ, Walker AM. Anthropometric and reproductive variables and exocrine carcinoma of the pancreas: a population-based case-control study in The Netherlands. Int J Cancer. 1992;52:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Ji BT, Hatch MC, Chow WH, McLaughlin JK, Dai Q, Howe GR, Gao YT, Fraumeni JF Jr. Anthropometric and reproductive factors and the risk of pancreatic cancer: a case-control study in Shanghai, China. Int J Cancer. 1996;66:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Duell EJ, Holly EA. Reproductive and menstrual risk factors for pancreatic cancer: a population-based study of San Francisco Bay Area women. Am J Epidemiol. 2005;161:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Duell EJ, Maisonneuve P, Baghurst PA, Bueno-de-Mesquita HB, Ghadirian P, Miller AB, Zatonski W, Vrieling A, Boffetta P, Boyle P. Menstrual and reproductive factors and pancreatic cancer in the SEARCH program of the IARC. Cancer Causes Control. 2009;20:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. A case-control study of reproductive factors, female hormone use, and risk of pancreatic cancer. Cancer Causes Control. 2010;21:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Archibugi L, Graglia B, Valente R, Stigliano S, Roberto M, Capalbo C, Marchetti P, Nigri G, Capurso G. Gynecological and reproductive factors and the risk of pancreatic cancer: A case-control study. Pancreatology. 2020;20:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Skinner HG, Michaud DS, Colditz GA, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev. 2003;12:433-438. [PubMed] |
| 27. | Teras LR, Patel AV, Rodriguez C, Thun MJ, Calle EE. Parity, other reproductive factors, and risk of pancreatic cancer mortality in a large cohort of U.S. women (United States). Cancer Causes Control. 2005;16:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Navarro Silvera SA, Miller AB, Rohan TE. Hormonal and reproductive factors and pancreatic cancer risk: a prospective cohort study. Pancreas. 2005;30:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Prizment AE, Anderson KE, Hong CP, Folsom AR. Pancreatic cancer incidence in relation to female reproductive factors: Iowa Women's Health Study. JOP. 2007;8:16-27. [PubMed] |
| 30. | Dorjgochoo T, Shu XO, Li HL, Qian HZ, Yang G, Cai H, Gao YT, Zheng W. Use of oral contraceptives, intrauterine devices and tubal sterilization and cancer risk in a large prospective study, from 1996 to 2006. Int J Cancer. 2009;124:2442-2449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Duell EJ, Travier N, Lujan-Barroso L, Dossus L, Boutron-Ruault MC, Clavel-Chapelon F, Tumino R, Masala G, Krogh V, Panico S, Ricceri F, Redondo ML, Dorronsoro M, Molina-Montes E, Huerta JM, Barricarte A, Khaw KT, Wareham NJ, Allen NE, Travis R, Siersema PD, Peeters PH, Trichopoulou A, Fragogeorgi E, Oikonomou E, Boeing H, Schuetze M, Canzian F, Lukanova A, Tjønneland A, Roswall N, Overvad K, Weiderpass E, Gram IT, Lund E, Lindkvist B, Johansen D, Ye W, Sund M, Fedirko V, Jenab M, Michaud DS, Riboli E, Bueno-de-Mesquita HB. Menstrual and reproductive factors in women, genetic variation in CYP17A1, and pancreatic cancer risk in the European prospective investigation into cancer and nutrition (EPIC) cohort. Int J Cancer. 2013;132:2164-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Lee E, Horn-Ross PL, Rull RP, Neuhausen SL, Anton-Culver H, Ursin G, Henderson KD, Bernstein L. Reproductive factors, exogenous hormones, and pancreatic cancer risk in the CTS. Am J Epidemiol. 2013;178:1403-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Kabat GC, Kamensky V, Rohan TE. Reproductive factors, exogenous hormone use, and risk of pancreatic cancer in postmenopausal women. Cancer Epidemiol. 2017;49:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Andersson G, Borgquist S, Jirström K. Hormonal factors and pancreatic cancer risk in women: The Malmö Diet and Cancer Study. Int J Cancer. 2018;143:52-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Butt SA, Lidegaardi Ø, Skovlund C, Hannaford PC, Iversen L, Fielding S, Mørch LS. Hormonal contraceptive use and risk of pancreatic cancer-A cohort study among premenopausal women. PLoS One. 2018;13:e0206358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Michels KA, Brinton LA, Pfeiffer RM, Trabert B. Oral Contraceptive Use and Risks of Cancer in the NIH-AARP Diet and Health Study. Am J Epidemiol. 2018;187:1630-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Wahi MM, Shah N, Schrock CE, Rosemurgy AS 2nd, Goldin SB. Reproductive factors and risk of pancreatic cancer in women: a review of the literature. Ann Epidemiol. 2009;19:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | United Nations. United Nations, Department of Economic and Social Affairs, Population Division (2015). Trends in Contraceptive Use Worldwide 2015 (ST/ESA/SER.A/349). [cited 20 January 2021]. Available from: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd_report_2015_trends_contraceptive_use.pdf. |
| 39. | Lujan-Barroso L, Zhang W, Olson SH, Gao YT, Yu H, Baghurst PA, Bracci PM, Bueno-de-Mesquita HB, Foretová L, Gallinger S, Holcatova I, Janout V, Ji BT, Kurtz RC, La Vecchia C, Lagiou P, Li D, Miller AB, Serraino D, Zatonski W, Risch HA, Duell EJ. Menstrual and Reproductive Factors, Hormone Use, and Risk of Pancreatic Cancer: Analysis From the International Pancreatic Cancer Case-Control Consortium (PanC4). Pancreas. 2016;45:1401-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |