Published online May 28, 2021. doi: 10.3748/wjg.v27.i20.2474
Peer-review started: January 27, 2021
First decision: February 25, 2021
Revised: March 4, 2021
Accepted: April 7, 2021
Article in press: April 7, 2021
Published online: May 28, 2021
Processing time: 112 Days and 21.8 Hours
Chronic infections by hepatitis B virus (HBV) and hepatitis C virus (HCV) major causes of advanced liver disease and mortality worldwide. Although regarded as benign infections in children, their persistence through adulthood is undoubtedly of concern. Recent advances in HCV treatment have restored the visibility of these conditions and raised expectations for HBV treatment, which is currently far from being curative. Herein we describe direct-acting antivirals available for pediatric HCV (sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, glecaprevir/pibrentasvir) and their real-world use. A critical review of the HBV pediatric classification is provided. Anti-HBV investigational compounds are reviewed in light of the pathophysiology in the pediatric population, including capsid assembly modulators, antigen secretion inhibitors, silencing RNAs, and immune modifiers. Recommendations for screening and management of immunosuppressed children or those with other risk factors or comorbidities are also summarized.
Core Tip: Chronic hepatitis B and C account for substantial morbidity and mortality worldwide. Infection control in children has great clinical and epidemiological implications. This review discusses recent achievements and forthcoming opportunities in the management of pediatric viral hepatitis. Removing barriers to the access to novel direct-acting antivirals, and understanding the relevance of tolerance-breaking new approaches to infection control, are major challenges for pediatric hepatologists.
- Citation: Nicastro E, Norsa L, Di Giorgio A, Indolfi G, D'Antiga L. Breakthroughs and challenges in the management of pediatric viral hepatitis. World J Gastroenterol 2021; 27(20): 2474-2494
- URL: https://www.wjgnet.com/1007-9327/full/v27/i20/2474.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i20.2474
The World Health Organization has estimated that - in 2015 - hepatitis B virus (HBV) and hepatitis C virus (HCV) infections caused 1.34 million deaths worldwide, exceeding the death toll of human immunodeficiency virus (HIV) and malaria and reaching that of tuberculosis[1]. These epidemics now move into the spotlight for the forthcoming innovations in their treatment armamentarium. We are living in the aftermath of the breakthrough of direct-acting antivirals (DAAs) for HCV. Those drugs have radically changed the epidemiology of adult chronic liver disease in developed countries, but they are still poorly accessible in resource-limited settings, and their use in children is just beginning. Improvements in HBV treatment are just around the corner. Notably, most investigational compounds directed against HBV tackle the host immune response towards the virus.
The role of children in this scenario is very important. Treating children chronically infected by HBV and HCV is complementary to prevention of mother-to-child transmission, with the purpose of the epidemiological control. On the clinical side, treatments capable of restoring immune tolerance or exhaustion, and promoting viral clearance, are paramount to prevent advanced liver disease in adulthood. In this review, we identify and discuss the next challenges in treatment of pediatric viral hepatitis. Overcoming the barriers to the availability of treatment for all children is a major objective in HCV infection. Conversely, identifying homogeneous patients phenotypes of chronic infection, selecting timely and appropriate treatment, bearing in mind forthcoming therapeutic opportunities, are keys to meeting the current challenges in the management of pediatric HBV infection.
HCV infection differs in children and adults in the mode of transmission, rate of clearance, progression of fibrosis, and duration of chronic infection when acquired at birth[2]. Vertical transmission of HCV from mother to child is reported to be the leading cause of pediatric infection worldwide. A recent review found that the risk of vertical HCV infection to the children of HCV antibody-positive and HCV-RNA-positive women was 5.8% [95% confidence interval (CI), 4.2-7.8] for children of HIV-negative women and 10.8% (95%CI, 7.6-15.2) for children of HIV-positive women[3].
Symptomatic acute HCV is rare in childhood, but can present with lethargy, fever, and myalgia[4]. Approximately 20%-25% of acute HCV infections can be cleared spontaneously. Spontaneous clearance of the virus following vertical transmission is reported in around 20% of infected patients, usually by 4 years of age[5]. Children who do not clear HCV spontaneously develop chronic infection that, is usually asymptomatic in pediatric patients[6]. During the chronic course, transaminase levels may be normal or intermittently elevated. Serum HCVRNA levels may considerably fluctuate as well, but without immediate prognostic relevance[7,8]. Histological findings are usually unremarkable, but the presence of cirrhosis is reported in 1%-4% of patients[7,9,10]. Overall, the risk of severe hepatic complications in pediatric patients is low[7].
Information on childhood to adult progression of liver is lacking, and the proportion of HCV-infected children who develop serious long-term liver disease is not clear[10]. Hepatic fibrosis is reported in less than 2% of pediatric cases, but the percentage is much higher in patients with long-term follow-up, suggesting that the development of fibrosis correlates with age and the duration of infection[11-14]. In a recent study from the United Kingdom including 1049 patients with a chronic hepatitis C acquired during childhood, one-third developed liver disease, with a median of 33 years after infection. Patients who acquired the infection vertically developed cirrhosis at an earlier age (36 years) compared with patients who acquired HCV in childhood through drug abuse (48 years), blood transfusion (46 years), or with an unknown route of infection (52 years, P < 0.0001). In that cohort, the incidence of hepatocellular carcinoma (HCC) was 5%; 4% required a liver transplant, and death occurred in 3%[11]. Long-term liver complications included cirrhosis, HCC, or requirement for transplantation[11,12,14]. In adults, the natural history of chronic HCV infection is affected by associated medical and social factors including hepatic steatosis, alcohol consumption, malignancy, and viral HIV and HBV coinfection[11,15]. Extrahepatic manifestations of chronic HCV (e.g., positive nonorgan-specific autoantibodies, autoimmune thyroiditis, glomerulonephritis) are rare in children[15,16]. In conclusion, early acquired hepatitis C infection is a clinically and histologically silent condition. Nevertheless, it may become insidious. Although the percentage of children developing liver cirrhosis in adulthood is low, progression beyond the second decade of life is likely. Thus, the main aim of therapeutic interventions in pediatric patients is not the treatment of an ongoing liver disease, but the prevention of progression by early eradication of the infection[17].
In 2011 boceprevir and telaprevir, two first-generation NS3/4A protease inhibitors, were approved for use in combination with pegylated interferon (peg-IFN) and ribavirin for the treatment of adults with chronic HCV infection[18,19]. The response rates to triple therapy with boceprevir or telaprevir was improved when compared with dual therapy with peg-IFN and ribavirin, but was accompanied by significant side effects. Since then, the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA) have approved 13 different DAAs and six fixed-dose combinations for the treatment of adults with chronic HCV infection (sofosbuvir/ledipasvir, ombitasvir/paritaprevir/ritonavir, elbasvir/grazoprevir, glecaprevir/pibrentasvir, sofosbuvir/velpatasvir, and sofosbuvir/ velpatasvir/ voxilaprevir)[20]. DAAs are classified into several categories by their molecular targets. Those agents were designed to inhibit specific viral proteins that have critical roles in HCV replication. They include NS3/4A protease inhibitors (e.g., simeprevir, paritaprevir, grazoprevir, voxilaprevir, and glecaprevir), nucleotide (e.g., sofosbuvir) and non-nucleotide (e.g., dasabuvir) inhibitors of NS5B polymerase, and NS5A inhibitors (e.g., daclatasvir, ledipasvir, ombitasvir, velpatasvir, elbasvir, and pibrentasvir). The development of combinations of DAAs is based on the concept that at least two drugs are needed to achieve the treatment goal of a virological response of > 95%) without selecting resistant mutants. When the backbone of the treatment is a nucleoside NS5B inhibitor like sofosbuvir, only one other drug, an NS3/4A protease inhibitor or a NS5A-inhibitor, is usually required. Conversely, a non-nucleoside NS5B inhibitor like dasabuvir should be used together with both NS3/4A proteases and NS5A inhibitors.
The introduction of DAAs changed the HCV treatment paradigm. These regimens are oral, patient-friendly, have treatment schedules as short as 8 wk, are highly effective, and have few side effects. Given these clear advantages over IFN-based therapies, DAAs have become the preferred treatment for adults with HCV. Remarkably, the latest generation of DAAs (glecaprevir/pibrentasvir, sofosbuvir/ velpatasvir, and sofosbuvir/velpatasvir/voxilaprevir) have pan-genotypic activity, thus simplifying treatment decisions.
The aims of treating HCV with DAAs are multiple. The first is to prevent the complications of HCV-related liver disease, namely cirrhosis, liver failure, and HCC. Secondly, the treatment can improve the quality of life of patients with chronic infection and remove the social stigma of being HCV-positive. Finally, treatment effective for HCV elimination also prevents further transmission of the infection, which targets the goal of global HCV elimination. The excellent efficacy and safety profile of DAAs, have also made antiviral therapy possible for patients with advanced liver disease and for those on the waiting list for liver transplantation (LT), leading to remarkable clinical improvement, avoiding the infection of the graft, and even allowing the delisting of up to one third of the patients[21]. Unfortunately, the initial lack of clinical data specific to DAA treatment in adolescents and children with chronic HCV infection has delayed the introduction of those agents for pediatric use. Several recent studies have demonstrated the safety and efficacy of various DAA-based regimens in pediatric patients[22], supporting their use in that setting (Table 1).
| Drug regimen | Age range in yr | Sample size | Genotype(s) | Number of patients with SVR12, % | Ref. |
| Sofosbuvir/ledipasvir | 12-17 | 100 | 1 | 98/100 (98) | Balistreri et al[25] |
| 6-11 | 92 | 1, 3, 4 | 91/92 (99) | Murray et al[26] | |
| 3-5 | 34 | 1, 4 | 33/34 (97) | Schwarz et al[27] | |
| Sofosbuvir plus ribavirin | 12-17 | 52 | 2, 3 | 51/52 (98) | Wirth et al[33] |
| 6-11 | 41 | 41/41 (100) | Rosenthal et al[34] | ||
| 3-5 | 13 | 12/13 (92) | Rosenthal et al[34] | ||
| Sofosbuvir/velpatasvir | 12-17 | 102 | 1, 2, 3, 4 | 97/102 (95) | Sokal et al[35] |
| 6-11 | 73 | 68/73 (93) | Sokal et al[35] | ||
| 3-5 | 41 | 34/41 (83) | Sokal et al[35] | ||
| Glecaprevir/pibrentasvir | 12-17 | 47 | 1, 2, 3, 4 | 47/47 (100) | Jonas et al[36] |
| 6-11 | 32 | 31/32 (97) | Jonas et al[37] | ||
| 3-5 | 16 | 15/16 (96) | Jonas et al[37] | ||
| Ombitasvir/paritaprevir/ritonavir ± dasabuvir ± ribavirin | 12-17 | 38 | 1, 4 | 38/38 (100) | Leung et al[134] |
| 6-11 | 26 | 1 | 25/26 (95) | Rosenthal et al[135] | |
| Elbasvir/grazoprevir | 12-17 | 22 | 1, 4 | 22/22 (100) | Wirth et al[136] |
| 6-11 | 17 | 17/17 (100) | Wirth et al[136] | ||
| 3-5 | 18 | 18/18 (100) | Wirth et al[136] | ||
| Sofosbuvir/velpatasvir/voxilaprevir | 12-17 | 21 | 1, 2, 3, 4 | 21/21 (100) | Bansal et al[137] |
| Sofosbuvir plus daclatasvir | 15-17 | 13 | 4 | 13/13 (100) | El-Sayed et al[138] |
| 13-17 | 10 | 10/10 (100) | El-Shabrawi et al[139] | ||
| 12-17 | 30 | 29/29 (100) | Yakoot et al[140] | ||
| 8-17 | 40 | 1, 4 | 39/39 (100) | Abdel Ghaffar et al[141] | |
| 7-13 | 14 | 3 | 14/14 (100) | Padhi et al[57] |
IFN-based treatment regimens are no longer recommended as therapeutic options in adolescents and children with HCV, given the high toxicity and modest sustained virological response (SVR) rates[4,23,24]. In 2017, the United States. FDA and the EMA approved the use of sofosbuvir/ledipasvir for adolescents 12 years of age or older with HCV genotype 1, 4, 5, or 6 infection, and sofosbuvir in combination with ribavirin for those with HCV genotype 2 or 3. Since then, four different regimens with age-specific limitations have been approved based on the results of four phase II-III, open-label, multicenter, multicohort studies (Table 2).
| Regimen | Genotype and duration of treatment | Formulations | Dosage |
| Sofosbuvir/ledipasvir | GT 1, 4, 5, 6: 12 wk | Tablet (FDC) 400/90 mg | > 35 kg: 400/90 mg/d |
| GT 1, treatment-experienced, cirrhosis: 24 wk | 17-35 kg: 200/45 mg/d | ||
| < 17 kg, older than 3 yr of age: 150/33.75 mg/d | |||
| Tablet (FDC) 200/45 mg | |||
| Pellets 200/45 mg and 150/33.75 | |||
| Sofosbuvir + ribavirin | GT 2: 12 wk | Sofosbuvir: tablet 400 mg | Sofosbuvir: > 35 kg: 400 mg/d |
| GT 3: 24 wk | 17-35 kg: 200 mg/d | ||
| Tablet 100 mg | < 17 kg, older than 3 yr of age: 200 mg if ≥ 17 kg | ||
| Capsules 50 mg containing granules | |||
| 150 mg/d if < 17 kg. Ribavirin: 15 mg/kg per day in two divided doses | |||
| Sofosbuvir/velpatasvir | All GTs: 12 wkdecompensated cirrhosis: 12 wk + ribavirin | Tablet (FDC) 400/100 mg | > 30 kg: 400/100 mg/d |
| 17-30 kg, older than 6 yr of age: 200/50 mg/d. Ribavirin: 15 mg/kg per day in two divided doses | |||
| Tablet (FDC) 200/50 mg | |||
| Glecaprevir/pibrentasvir | All GTs: 8 wk | Tablet (FDC) 100/40 mg/d | 12-17 yr or > 45 kg: 300/120 mg/d |
| All GTs, cirrhosis: 12 wk | |||
| GT 3 treatment-experienced: 16 wk |
Sofosbuvir/ledipasvir was tested in 100 adolescents 12-17 years of age, 92 children 6-11 years of age, and 34 toddlers 3-5 years of age with chronic HCV infections[25-27]. The efficacy of the combination across the three different age cohorts was high (Table 1). One adolescent discontinued treatment and one did not attend post-treatment follow-up visits after having achieved the end-of-treatment response. One cirrhotic child 6 years of age with HCV genotype 1a infection relapsed by the 4-wk follow-up visit, and one 3-year-old patient discontinued treatment after 5 days because of “abnormal drug taste” and vomiting. The treatment was well tolerated. No serious or grade 3-4 drug-related adverse events were reported, and no patient discontinued treatment because of an adverse event. The efficacy of this combination has been confirmed in real-world studies[28-32].
The combination of sofosbuvir and ribavirin was tested in 52 adolescents, 41 children 6-11 years of age and 13 toddlers 3-5 years of age with chronic HCV infection, showing high efficacy (Table 1)[33,34]. One adolescent with HCV genotype 3 infection who did not achieve SVR 12 wk after the end of treatment (SVR12), was lost to follow-up after achieving the end-of-treatment response and SVR4 (HCV-RNA negative 4 wk after the end of treatment). In the younger cohorts, a 4-year-old child who did not achieve the primary endpoint of SVR12 discontinued treatment after 3 d because of an “abnormal drug taste”. No patients had a virologic nonresponse, breakthrough, or relapse. Treatment was well tolerated. One serious adverse event, accidental ribavirin overdose requiring hospitalization for monitoring was reported in a 3-year-old patient. The child completed treatment and achieved SVR12.
A sofosbuvir/velpatasvir fixed-dose formulation was tested in 102 adolescents and 73 children 6-11 years of age with chronic HCV infection[35]. Two treatment failures were reported, a 17-year-old girl with genotype 1a who became pregnant and discontinued therapy at week 4, and a 10-year-old girl with genotype 1a. Eight patients were lost to follow-up. The treatment was well tolerated. No serious or grade 3-4 drug-related adverse events were reported, and no patient discontinued treatment because of an adverse event. The combination of glecaprevir/pibrentasvir was tested in 47 adolescents[36], and an SVR12 of 100% was reported. No patients had virologic nonresponse, breakthrough infection, or relapse. The treatment was well tolerated. No serious adverse events were reported, and no patient discontinued treatment because of an adverse event.
Sofosbuvir/ledipasvir and sofosbuvir plus ribavirin are approved for children as young as 3 years of age. Sofosbuvir/velpatasvir and glecaprevir/pibrentasvir are approved for those older than 6 years older than 12 years of age, respectively. The results of the trials of those regimens for younger age cohorts (3-5 years of age for sofosbuvir/velpatasvir and 3-11 years of age for glecaprevir/pibrentasvir) are now available[35,37], and expansion of the indication is expected in late 2021. Remarkably, adherence to the treatment regimen seems to be the main factor impacting the effectiveness of DAA regimens. Noncompliance was responsible of most of the treatment failures across the different trials[22].
After the approval of DAAs by international regulatory agencies and their inclusion in clinical guidelines in the early 2010’s, other barriers had to be overcome before they actually became available. In the adult setting, delay in private third-party payer authorization was an obstacle to access to DAA[38,39]. In the pediatric setting, the situation is paradoxical. DAA treatment has been approved by the EMA and the FDA, but the drugs are not available in many countries because of a lack of indications approved by the national agencies[40]. Nevertheless, eradicating HCV infection in early life has been demonstrated to impact favorably on the quality of life and to be cost-effective[41,42]. In fact, reports of real-world experience with the pediatric use of DAAs are increasing, and have shown that the excellent results of the experimental trials can be replicated in adolescents. A study in a cohort of 78 Italian children 12-17 years of age with chronic genotype 1, 3, and 4 HCV infections treated with sofosbuvir/ledipasvir had an SVR12 of 98.7%[28]. Similar results were obtained in three cohorts of Egyptian children. Thirty adolescents with genotype 2 and 144 with genotype 3 infections achieved 99%-100% SVR12s[31,32], and 20 children 6-12 years of age had an SVR12 of 95% after fixed half-dose sofosbuvir/ledipasvir, with only one patient lost to follow-up[43]. Real-world experience has also confirmed that children are ideal candidates for a shortened DAA course, as they typically exhibit little or no fibrosis. In fact, noncirrhotic, treatment-naïve children with genotype 1 and 4 infections and low viremia below 6.8 log10 IU/mL HCV-RNA have been reported to achieve 100% SVR12 after only 8 wk of sofosbuvir/ledipasvir[29,44].
The advent of DAA has radically changed the approach to HCV eradication treatment not only for otherwise healthy children but also for those with comorbidities. The high safety profile of DAA fostered wide experimental use for previously neglected clinical conditions for which IFN-based treatment strategy was proscribed[45]. HCV recurrence after LT is a well-known problem in the adult setting, and can progress rapidly to graft cirrhosis and early loss[46]. HCV eradication with DAA before and after LT brought a tremendous improvement in transplantation outcomes[47-49]. The type of post-LT immunosuppression regimen seems not to impact the DAA treatment response[50], but fluctuation of calcineurin inhibitor trough levels during treatment may account for graft immune-mediated dysfunction[51,52]. Although a similar DAA efficacy might be expected in pediatric LT recipients, no relevant pediatric experience has been published.
So far only seven children receiving DAAs after hemopoietic stem cell transplantation (HSCT) for hematological disease have been described in the literature. One had received HSCT for acute lymphoblastic leukemia (4 years of age, genotype 1a)[53], and another for sickle cell disease (15-year-old, genotype 4)[54], and were treated with a combination of sofosbuvir/simeprevir for 24 wk and 12 wk, respectively. They achieved stable viral clearance with calcineurin inhibitor-based regimens (cyclosporin + mycophenolate mofetil, and tacrolimus + sirolimus, respectively). The other five children were 5-12 years of age, and received haploidentical allogeneic HSCT for genotype 1b refractory lymphoblastic leukemia, and were under cyclosporin maintenance[55]. All of them were treated with 24 wk of sofosbuvir/velpatasvir after a median HSCT follow-up of 15 mo, and achieved SVR by 1 mo. El-Shabrawi et al[56] reported on a 12-wk course of sofosbuvir/daclatasvir in 20 genotype 4-infected children in full remission of a hematological malignancy for more than 18 mo. All patients achieved SVR24 without notable adverse events. Two teams of researchers have reported on DAA-driven HCV eradication in thalassemic patients. In total, 25 children were enrolled, 14 with genotype 3 received 12-wk sofosbuvir/daclatasvir and 11 with genotype 4 received 12-wk sofosbuvir/ledipasvir). All achieved SVR12 without complaining of any serious adverse reactions[57,58].
Due to their efficacy and safety profile, DAA use is expanding to cutting-edge therapeutic scenarios. Hematopoietic stem cell-gene therapy (HSC-GT)—a life-saving option for inborn error diseases—is contraindicated in HCV-infected children because of the infection risk of bone marrow cells used as starting material for the manufacture. A 12-wk course with sofosbuvir/ledipasvir was found to allow autologous HSC-GT for the correction of severe combined immunodeficiency caused by adenosine deaminase deficiency as described in a pioneering study[59]. Pediatric studies have not yet replicated the encouraging results with DAA in adults with HIV/HCV coinfection[60-62], even when vertically transmitted[63], nor for the treatment of HCV in hemodialyzed[64] and kidney transplant patients[65,66].
Similar to HCV, childhood HBV infections occur mostly by mother-to-child transmission. In highly endemic countries, up to one third of the HBV incident transmissions are horizontal, caused by child-to-child, household or intrafamilial contacts[67]. Unlike adults, the prevalence of HBV infections in children reflects the extent of infant and HBV vaccination at birth, which is done with the goal of eradication in the absence of an effective antiviral treatment[68]. According to the last World Health Organization hepatitis report, global hepatitis B surface antigen (HBsAg) prevalence in preschool children dropped from approximately 4.7% between the 1980 and the early 2000s to 1.3% in 2015, following the widespread adoption of universal infant immunization[69]. The age at transmission determines the development of chronic HBV infection, which occurs in approximately 90% of infected newborns and infants compared with only 10% of infections acquired later in childhood or in adults[70,71]. Once chronic infection is established, the subsequent disease progression is the result of the adaptive immune response to HBV, immune-inflammatory liver injury, and the pathogen itself.
Until recently, patients have been classified by immune activity and control criteria, assuming that the clinical phenotype of HBV infection represented a clear-cut immunological state. Due to the lack of immunological data, the latest classification by the European Association for the Study of the Liver (EASL) replaces states with “phases”, which focused more on the infection course in adults, but do not fit the pediatric scenario[72]. In fact, the natural history of chronic HBV infection across all ages has been described by few large, long-term, prospective studies[73-80]. After infection, the vast majority of children enter a state characterized by detectable HBsAg and hepatitis B e antigen (HBeAg), high viremia, and normal or near-normal transaminase levels. This “tolerant” phase can last for decades, and Asian children or those carrying genotypes C or D have a higher likelihood to remain so[81-83]. Eventually, at a median age of 30 years, 3%-6% of the patients achieve HBeAg/hepatitis B e antibody (HBeAb) seroconversion annually, and most of those patients succeed in suppressing viremia[73,75,81,82]. Spontaneous HBsAg/hepatitis B surface antibody (HBsAb) seroconversion, which potentially allows long-term infection control, rarely occurs, with a rate of approximately 0.5%/year[73,75].
The major concern in children with chronic pediatric HBV infection is the risk of cirrhosis and HCC, which occur in 1%-5% and 0.03%-2% of the patients, respectively[73,74]. As this risk increases in parallel with the activity and the duration of chronic hepatitis B (CHB) (e.g., transaminase elevation in the presence of elevated viral load)[84], the ultimate long-term goal is the achievement of sustained viral suppression, either as a result of the immunologic control or with the use of antiviral drugs.
The current classification of chronic HBV infection recognizes different phases and considers their interchangeability[72]. The phases are identified by the presence or absence of HBeAg (i.e. HBeAg positive and HBeAg negative) and by increased transaminases, which distinguishes chronic hepatitis from chronic infection. These categories do not fit the pediatric scenario well, where HBeAg-positive chronic infection tends to persist and HBeAg-negative hepatitis is anecdotal[85].
Classifying patients with HBV infection by the clinical phenotype, which reflects the immune status against the virus, has some advantages. Phenotype classification has been progressively refined to harmonize population data, estimate the need for antiviral treatment, and possibly predict the outcome of the infection[81,82]. It describes three childhood phenotypes: (1) immune-active (HBeAg +/-, elevated transaminases, HBV-DNA > log10 4 IU/mL); (2) immune-tolerant (HBeAg-positive, normal transaminases, HBV-DNA > log10 4 IU/mL); and (3) inactive carriers (HBeAg +/-, normal transaminases, HBV-DNA ≤ log10 4 IU/mL); with the remaining being “indeterminant”[81-83]. So defined, the immune phenotype may help predict the fate towards infection control. Indeed, HBeAg-positive immune-active children are much more likely to achieve spontaneous HBeAg/HBeAb seroconversion (28% and 7.47%/year) compared with immune-tolerant children (11% and 2.29%/year)[81]. In a scenario characterized by the lack of clear treatment indications, and disappointing therapeutic outcomes, the definition of phenotypes has proven to bear prognostic value, and to help define homogeneous groups for patient selection in future trials.
The current pediatric guidelines recommend limiting treatment to children with prolonged (> 6 mo) active hepatitis B and evidence of fibrosis, and to observe those with the immune-tolerant phenotype[86]. The rationale for this approach, is that HBeAg-positive CHB, especially when protracted and beginning early, at < 3 years of age, has the highest risk of progression to cirrhosis, which is between 1% and 5% by the third decade[73,74]. Many centers tend to delay treatment based on the sound conception that CHB is harmless and that transaminase activity heralds spontaneous immune clearance[73]. Moreover, there is evidence that treatment only accelerates HBeAg/HBeAb seroconversion without influencing the proportion of patients who seroconvert over time[75]. On the other hand, treatment could be indicated to break tolerance. The EASL recommends nucleoside or nucleotide analogues (NAs) for long-term viral suppression in immune-tolerant patients 30 years of age or older[72], to reduce the risk of fibrosis and cirrhosis in those with delayed immune clearance [87,88]. In fact, studies highlight that high viremia in persistently HBeAg-positive patients is associated with the highest risk of cirrhosis, HCC, and liver-related mortality and that functional infection control following HBeAg/HBeAb seroconversion lowers those risks[87,89,90]. Thus, the question has arisen whether treatment should also be offered to immune-tolerant children.
The pediatric age has been regarded as a good time window to break viral tolerance. The first encouraging experience of treating immune-tolerant children was published in 2006. HBV-DNA clearance and HBsAg/HBsAb seroconversion were achieved in 5 of 22 children (23%) after 10 mo of sequential combination therapy with lamivudine and IFN-a[91]. That result was confirmed in a case-control study in which 6 of 22 immune-tolerant children achieved HBsAb-dependent functional control[92]. Another study reported a success rate of 33%[93]. Those studies reported rates of HBsAb seroconversion of 20%-25%. Conversely, a clinical trial using a combination of entecavir and peg-IFN-a 2a resulted in only 2 of 60 children achieving the primary endpoint of HBeAg loss and sustained HBV-DNA levels < 1000 IU/mL), with adverse events reported in more than 50% of the children[94].
In favor of treating immune-tolerant children is evidence that their spontaneous seroconversion rate is much lower than that seen in adults[87]. Also, immune-tolerant children treated with a combination of lamivudine and IFN achieved a high rate of loss of HBsAg. Moreover, unlike adults, immune tolerance in young patients is characterized by a high level of HBV DNA integration and clonal hepatocyte expansion with malignant potential despite a relatively preserved anti-HBV T-cell response[95]. Several new compounds in the pipeline that aim at increasing immune responses and overcoming immune exhaustion, with probably play a role in this specific group of children.
Seven different drugs have been approved for children and adolescents with chronic HBV infection by the United States FDA and the EMA (Table 3). IFN- and peg-IFN- are immune modulators that can be administered for a predefined duration with the aim of inducing immune-mediated control of HBV infection and achieving long-lasting suppression of off-treatment viral replication[68]. NA are potent HBV inhibitors that are used as long-term oral treatments to suppress viral replication, or as treatments of finite duration with or without IFN, to obtain a sustained off-treatment virological response. NAs are also classified as genetic barriers to resistance by the threshold of mutations required for clinically meaningful loss of drug susceptibility. Lamivudine, adefovir, and telbivudine have low and tenofovir, and entecavir have high, genetic barriers to resistance[68]. Treatment is indicated for children and adolescents with active viral replication (detectable HBV-DNA levels), prolonged (6-12 mo) active hepatitis (elevated serum transaminase levels) and/or inflammation or fibrosis on liver biopsy. Unless within a clinical trial, treatment is contraindicated when transaminases are normal[72,86,96].
| Drug | Use in children | Dose | Formulation |
| Interferon-α-2b | ≥ 1 yr | 6 million IU/m2 3 times a week | Subcutaneous injections |
| Pegylated interferon-α-2a | ≥ 3 yr | 180 µg/1.73 m2 once a week | Subcutaneous injections |
| Lamivudine | ≥ 3 yr | 3 mg/kg daily (max 100 mg) | Oral solution (5 mg/mL) |
| tablets (100 mg) | |||
| Entecavir | ≥ 2 yr | 10-30 kg: 0.015 mg/kg daily (max 0·5 mg) | Oral solution (0.05 mg/mL) |
| tablets (0.5 mg and 1 mg) | |||
| Adefovir | ≥ 12 yr | 10 mg daily | Tablets (10 mg) |
| Tenofovir disoproxil fumarate | ≥ 12 yr (FDA) | 300 mg daily | Oral powder (40 mg per 1 g) |
| ≥ 2 yr (EMA) | tablets (150, 200, 250 and 300 mg) | ||
| Tenofovir alafenamide | ≥ 12 yr (EMA) | 25 mg daily | Tablets (25 mg) |
IFN--2b, peg-IFN--2a, lamivudine, adefovir, tenofovir disoproxil fumarate, and entecavir were approved for the treatment of children and adolescents with chronic HBV infection following six randomized placebo-controlled trials[97-102]. Tenofovir alafenamide was approved on the basis of studies of its use in HIV infection (Table 4). IFN therapy may be associated with higher rates of HBsAg loss compared with NAs. In all studies, a good overall treatment response defined by reduction of serum HBV-DNA to undetectable concentrations, loss of serum HBeAg, and normalization of transaminases, was associated with and increased baseline histology activity index score, increased baseline transaminase concentrations, and decreased baseline HBV-DNA concentrations.
| Interferon-α-2b | Lamivudine | Adefovir | Tenofovir DF | Entecavir | Pegylated interferon-α-2a | |
| Number treated | 144 | 191 | 173 | 52 | 120 | 101 |
| Duration of treatment in wk | 24 | 52 | 48 | 72 | 48 | 48 |
| Age, median (range) | 5 (1-17) | 9 (2-17) | 11 | 15.5 (12-17) | 12 (2-17) | 11 (3-7) |
| Virological response as HBeAg negative HBV DNA undetectable (% treated vs placebo) | 26 (vs 11) | 23 (vs 13) | 10.6 (vs 0) | 21.2 (vs 0) | 24.2 (vs 3.3) | 19.8 (vs 2) |
| HBsAg negative (% treated vs placebo) | 10 (vs 1) | 2 (vs 0) | 0.8 (vs 0) | 1.9 (vs 0) | 5.8 (vs 0) | 8.9 (0) |
| Ref. | Sokal et al[97] | Jonas et al[98] | Jonas et al[99] | Murray et al[100] | Jonas et al[101] | Wirth et al[102] |
Peg-IFN, entecavir, and tenofovir disoproxil fumarate are considered the drugs of choice for the treatment of CHB in children by the major international societies [72,86,96]. The advantages of IFN and peg-IFN use in children, compared with NAs, are the absence of viral resistance and a predictable, finite duration of treatment. However, the use of IFN and peg-IFN is demanding for children because it requires subcutaneous injection and is associated with a nearly certain occurrence of flu-like symptoms.
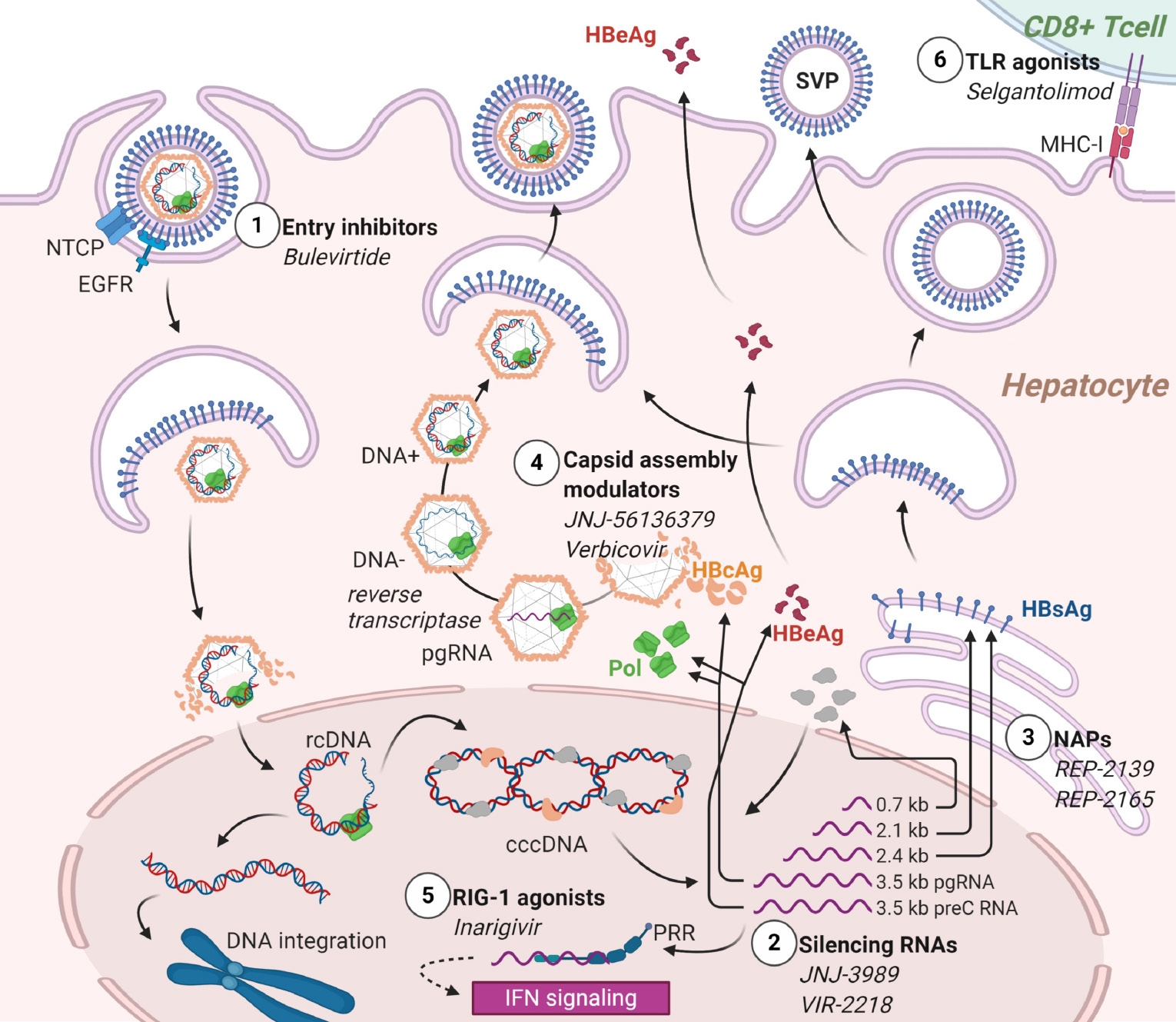
Currently available drugs against HBV have inherent limitations. NAs are safe and well tolerated and usually succeed in suppressing replication. They are not curative, as they act at a late stage of the viral cycle and do not prevent HBV-DNA persistence in episomal or integrated forms[96]. On the other hand, IFNs have limited efficacy and frequent side effects, but they are the most likely to achieve a definite “functional” cure with HBeAg or HBsAg loss, seroconversion, normal transaminases, and undetectable viremia)[103]. Various new classes of compounds are under investigation with the aim of achieving high rates of HBsAg seroconversion[104]. The candidate drugs are relevant to the pediatric field, as almost all act as immune modifiers to achieve tolerance breakthrough. The molecules currently in phase II and later trials are listed and summarized in Table 5.
| Class | Compound | Mechanism | Known side effects | Route | Status | Ref. |
| Entry inhibitors | Bulevirtide | Blocks entry via interaction with NTCP | Injection site reactions, cholestasis | Subcutaneous (iv) | Approved for CHD (HBV/HDV) Phase II for HBeAg-CHB | European Association for the Study of the Liver[117] and Bogomolov et al[118] |
| Capsid assembly modulators | JNJ-56136379 | Prevents encapsidation of pgRNA Prevents formation of cccDNA | Hypertransaminasemia | Oral | Phase II for CHB ± NA | Zoulim et al[105] |
| Capsid assembly modulators | ABI-H0731 (Vebicorvir) | Prevents packaging of pgRNA into capsids | Skin rash, dizziness | Oral | Phase II for CHB ± NA | Yuen et al[106] |
| HBsAg secretion inhibitors | REP-2139 | Inhibits the secretion of HBsAg subviral particles | Fever, chills, hypertransaminasemia, leukopenia | iv | Phase II for CHB + NA Phase II for CHD + peg-IFN-α | Bazinet et al[109,110] |
| HBsAg secretion inhibitors | REP-2165 | Inhibits the secretion of HBsAg subviral particles | Fever, chills, hypertransaminasemia, leukopenia | iv | Phase II for CHD + peg-IFN-α | Bazinet et al[110] |
| RNA interference | JNJ-3989 | Silences the mRNA viral transcripts reducing HBsAg | - | iv | Phase II for CHB | Yuen et al[111] and Gane et al[112] |
| RNA interference | VIR-2218 | Silences the mRNA viral transcripts reducing HBsAg | - | iv | Phase II for CHB | VIR Biotechnology[113] |
| RIG-1 agonist | Inarigivir (SB9200) | Activates PRR RIG-1 and IFN-I response | Headache, dizziness, UTI, ILI, GI symptoms, hypertransaminasemia | Oral | Phase II for CHB + NA | Yuen et al[114] |
| Immune modifier | Selgantolimod (GS-9688) | TLR8 agonist | Nausea, vomiting, chills, headache, UTI | Oral | Phase II for CHB + NA | Gane et al[116] |
Capsid assembly inhibitors are antiviral agents complementary to NAs. JNJ-56136379 is an oral compound with two distinct mechanisms, the inhibition of the encapsidation of the pregenomic RNA (pgRNA) and the formation of covalently closed circular (ccc)DNA. It has been studied in 57 subjects with CHB treated for 28 d. HBV-DNA and HBV-RNA decreased at all tested doses and HBV-DNA was undetectable at the end of the study in one third of the patients. Nonetheless, none achieved HBsAg/HBsAb seroconversion[105]. ABI-H0731 (Vebicorvir) is an oral compound inhibiting encapsidation, binding the core protein, and thus blocking the packaging of pgRNA into nucleocapsids. A phase I study conducted in healthy volunteers and 38 patients with CHB reported good tolerability and a prompt but temporary drop in viremia[106]. Two phase II studies are ongoing, whose interim results show that in already NA-suppressed patients, the addition of Vebicorvir significantly suppressed HBV-RNA levels. In treatment-naïve patients, its association with standard care resulted in a greater decrease in HBV DNA levels[107].
Nucleic acid polymeric secretion inhibitors reduce the release of HBsAg small viral particles, considered crucial in immune system exhaustion, thus favoring HBsAg loss and the seroconversion to HBsAb. The polymer REP-2139, administered intravenously once weekly, has been selected for its tolerability within this class[108]. The sequential use of REP-2139 and peg-IFN-a in chronic HBV/HDV coinfection over a period of 63 wk, resulted in sustained HBsAg loss and seroconversion to HBsAb in six of 12 patients. HBV-DNA and HDV-RNA were negative in seven of the patients at the end of the treatment and in nine after 1 year of follow-up[109]. In HBeAg-negative CHB, REP-2139 or its analogue REP-2165 were used in combination with tenofovir and peg-IFN-a and achieved sustained HBsAg/HBsAb seroconversion in 41% of the patients and functional control (undetectable HBV-DNA and normal transaminases) in 77%, an unprecedented result[110].
RNA interference is another promising strategy that aims at silencing the translation of viral transcripts and subsequently decreasing HBsAg. Preliminary reports of efficacy are available from ongoing phase I/II studies on CHB entailing 3 moly administration of the small interfering (si)RNA JNJ-3989 (ARO-HBV). Regardless of HBeAg status and previous treatment, HBsAg decreased by 97%-100% after one dose and the majority of participants achieved HBsAg loss and dramatically reduced HBV-DNA shortly after protocol completion[111,112]. This drug aims at breaking the immune stall toward the virus, as suggested by an ongoing trial in immune-tolerant adults. Similar interim results have been reported in a trial of the siRNA VIR-2218[113].
Immune modulators are a heterogeneous class of candidate antivirals that target different effectors of innate immunity. Inarigivir is an RIG-1 pattern recognition receptor agonist, whose binding activates the IFN-I response. Final results of the phase II ACHIEVE trial demonstrated dose-dependent HBV-DNA reduction after inarigivir monotherapy, and the endpoint of an HBsAg reduction > 0.5 log10 was achieved in 22% of the patients[114]. Selgantolimod (formerly GS-9688), is a potent, orally administered Toll-like-receptor (TLR8) agonist capable of inducing tumor necrosis factor-, IFN-γ, interleukin (IL)-12, and IL-18 expression[115]. Interim results of a phase II study show that it induced a significant HBsAg reduction in 16%-30% of CHB patients and occasional HBsAg loss 24 wk after treatment onset[116]. Bulevirtide is the only viral-entry inhibitor approved by the EMA in 2020 for HBV/HDV coinfection, while it is on a phase II study for HBeAg-negative CHB (NCT02881008). It binds sodium taurocholate cotransporting polypeptide (NTCP) to prevent HBV from entering hepatocytes. Combined with peg-IFN-a, it was shown to significantly decrease HBV-DNA and HDV-DNA compared with peg-IFN-a alone[117,118]. Figure 1 depicts the different mechanisms of action of the HBV investigational products. Although many steps remain to achieve availability in the pediatric population, these drugs will certainly change the burden of HBV in children as much as in adults. The perspective of feasible and curative treatments aiming to break tolerance will increase the efforts to eradicate HBV early in life.
Data on the risk of hepatitis B reactivation in children who need to start immunosuppressive treatment for concomitant diseases are extremely scarce. For that reason, the recommendations expressed in a recent position paper of the Hepatology committee of the ESPGHAN[119] are mostly derived from adult evidence[72,96,120]. Experts recommend HBV screening, with HBsAg, HBsAb, and hepatitis B core antibody (HBcAb) testing, of all patients at risk of HBV reactivation, including those who are going to start immunosuppressive treatment. The tests should be performed even if HBV vaccination is complete, because, as shown in inflammatory bowel diseases, immunosuppressed children have low serologic protection from childhood vaccines[121]. Patients with negative HBV screening should be vaccinated before starting immunosuppressive treatment. This statement was supported by a recent study of 580 children that demonstrated a high seroconversion rate after the vaccine booster even after immunosuppression initiation[122].
Reactivation risk is classified as mild, moderate, or severe depending on the administered immunosuppressive agents[119]. The risk classes are based exclusively on adult evidence, as no corresponding pediatric study results have been published. Children scheduled for moderate or high-risk drugs should start antiviral prophylaxis, while a preemptive approach is preferred for children starting on low-risk drugs[123,124]. Even in the absence of pediatric evidence, a robust network meta-analysis reported that entecavir or tenofovir should be preferred for HBV reactivation treatment in immunosuppressed patients[125].
Currently, reactivation of HBV infection in pediatric solid organ transplant recipients is anecdotal, as HBcAb-positive donors are almost no longer used, and end-stage liver disease in HBsAg-positive recipients is exceptional. However, current recommendations replicate those for adults. In HBsAg-positive recipients, tenofovir or entecavir treatment should be started as soon as possible before transplant to achieve undetectable HBV-DNA[72,96]. NA treatment should be continued indefinitely, and HBV-specific immunoglobulins can be stopped after 5-7 d, unless there is a history of drug resistance or poor compliance[72,96,126]. The use of grafts from HBcAb positive/HBsAg negative donors might be acceptable in case of organ shortage and in highly endemic countries. In those situations, recipients with HBsAb titers > 200 IU/mL might be protected from infection. However, ultimately the overall risk of developing infection depends on multiple factors and implies that recipients of such organs receive NAs (e.g., entecavir, tenofovir, or tenofovir alafenamide) for at least 1 year after transplant. Discontinuation might then be carefully evaluated on a case-by-case basis in HBsAb-positive children[127].
Another challenge for HBV treatment is represented by HBeAg-negative CHB, which is the most prevalent chronic hepatitis in many countries[128]. In adults, the clinical profile is characterized by wide fluctuations in viral replication and biochemical activity, with temporary spontaneous remissions[129]. The risk of cirrhosis in HBeAg-negative CHB is higher (8%-10%/year) than in HBeAg-positive (2%-5%/year)[130,131]. Infants with fulminant hepatitis caused by mother-to-child transmission of HBV e-minus variants are described[132]. This behavior is thought to result from absence of the tolerogenic effect of HBeAg. Adults are treated with long courses of NAs[133], but evidence of the benefits in children with HBeAg-negative hepatitis is lacking. However, pediatric HBeAg-negative and HBeAg-positive CHB have the same therapeutic approach, which is based on IFN- and indefinite use of NAs. The target should be decrease in HBsAg, HBV-DNA clearance, and normalization of transaminases.
HBV and HCV infections in childhood usually progress to chronic hepatitis through different mechanisms of immune tolerance and exhaustion. For HCV infection, different combinations of DAAs are increasingly available, including pan-genotypic combinations, with very few side effects and extremely high SVRs. For HBV infection, recent cohort studies have clarified that several factors including immune host phenotype, viral genotype, and ethnicity, contribute to spontaneous control. Viral hepatitis should not be a barrier to the use of immunosuppressive medications in the treatment of autoimmune conditions, nor to antineoplastic chemotherapy, provided that timely screening and appropriate pharmacological interventions are performed. Finally, new drugs in development for the treatment of HBV, mostly acting by fostering the breaking of tolerance, could dramatically improve the treatment outcome of CHB in children.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang Y S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | World Health Organization. Viral hepatitis: a hidden killer gains visibility. [cited 2 March 2021]. In: World Health Organization [Internet]. Available from: http://www.who.int/publications/10-year-review/hepatitis/en/. |
| 2. | Robinson JL, Doucette K. The natural history of hepatitis C virus infection acquired during childhood. Liver Int. 2012;32:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 4. | Indolfi G, Easterbrook P, Dusheiko G, El-Sayed MH, Jonas MM, Thorne C, Bulterys M, Siberry G, Walsh N, Chang MH, Meyers T, Giaquinto C, Wirth S, Chan PL, Penazzato M. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | Resti M, Jara P, Hierro L, Azzari C, Giacchino R, Zuin G, Zancan L, Pedditzi S, Bortolotti F. Clinical features and progression of perinatally acquired hepatitis C virus infection. J Med Virol. 2003;70:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 416] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Bortolotti F, Verucchi G, Cammà C, Cabibbo G, Zancan L, Indolfi G, Giacchino R, Marcellini M, Marazzi MG, Barbera C, Maggiore G, Vajro P, Bartolacci S, Balli F, Maccabruni A, Guido M; Italian Observatory for HCV Infection and Hepatitis C in Children. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroenterology. 2008;134:1900-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Jara P, Resti M, Hierro L, Giacchino R, Barbera C, Zancan L, Crivellaro C, Sokal E, Azzari C, Guido M, Bortolotti F. Chronic hepatitis C virus infection in childhood: clinical patterns and evolution in 224 white children. Clin Infect Dis. 2003;36:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Goodman ZD, Makhlouf HR, Liu L, Balistreri W, Gonzalez-Peralta RP, Haber B, Jonas MM, Mohan P, Molleston JP, Murray KF, Narkewicz MR, Rosenthal P, Smith LJ, Robuck PR, Schwarz KB. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds-C Trial. Hepatology. 2008;47:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Indolfi G, Guido M, Azzari C, Resti M. Histopathology of hepatitis C in children, a systematic review: implications for treatment. Expert Rev Anti Infect Ther. 2015;13:1225-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Modin L, Arshad A, Wilkes B, Benselin J, Lloyd C, Irving WL, Kelly DA. Epidemiology and natural history of hepatitis C virus infection among children and young people. J Hepatol. 2019;70:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Jara P, Hierro L. Treatment of hepatitis C in children. Expert Rev Gastroenterol Hepatol. 2010;4:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Guido M, Bortolotti F, Leandro G, Jara P, Hierro L, Larrauri J, Barbera C, Giacchino R, Zancan L, Balli F, Crivellaro C, Cristina E, Pucci A, Rugge M. Fibrosis in chronic hepatitis C acquired in infancy: is it only a matter of time? Am J Gastroenterol. 2003;98:660-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Abdel-Hady M, Bunn SK, Sira J, Brown RM, Brundler MA, Davies P, Kelly DA. Chronic hepatitis C in children--review of natural history at a National Centre. J Viral Hepat. 2011;18:e535-e540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Garazzino S, Calitri C, Versace A, Alfarano A, Scolfaro C, Bertaina C, Vatrano S, Mignone F, Licciardi F, Gabiano C, Tovo PA. Natural history of vertically acquired HCV infection and associated autoimmune phenomena. Eur J Pediatr. 2014;173:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Sugiura T, Yamada T, Kimpara Y, Fujita N, Goto K, Koyama N. Effects of pegylated interferon alpha-2a on hepatitis-C-virus-associated glomerulonephritis. Pediatr Nephrol. 2009;24:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Indolfi G, Thorne C, El Sayed MH, Giaquinto C, Gonzalez-Peralta RP. The Challenge of Treating Children With Hepatitis C Virus Infection. J Pediatr Gastroenterol Nutr. 2017;64:851-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Sarrazin C, Rouzier R, Wagner F, Forestier N, Larrey D, Gupta SK, Hussain M, Shah A, Cutler D, Zhang J, Zeuzem S. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonresponders. Gastroenterology. 2007;132:1270-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 19. | Reesink HW, Zeuzem S, Weegink CJ, Forestier N, van Vliet A, van de Wetering de Rooij J, McNair L, Purdy S, Kauffman R, Alam J, Jansen PL. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase Ib, placebo-controlled, randomized study. Gastroenterology. 2006;131:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 301] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Jakobsen JC, Nielsen EE, Feinberg J, Katakam KK, Fobian K, Hauser G, Poropat G, Djurisic S, Weiss KH, Bjelakovic M, Bjelakovic G, Klingenberg SL, Liu JP, Nikolova D, Koretz RL, Gluud C. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017;9:CD012143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Belli LS, Berenguer M, Cortesi PA, Strazzabosco M, Rockenschaub SR, Martini S, Morelli C, Donato F, Volpes R, Pageaux GP, Coilly A, Fagiuoli S, Amaddeo G, Perricone G, Vinaixa C, Berlakovich G, Facchetti R, Polak W, Muiesan P, Duvoux C; European Liver and Intestine Association (ELITA). Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J Hepatol. 2016;65:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 22. | Indolfi G, Giometto S, Serranti D, Bettiol A, Bigagli E, De Masi S, Lucenteforte E. Systematic review with meta-analysis: the efficacy and safety of direct-acting antivirals in children and adolescents with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2020;52:1125-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Indolfi G, Hierro L, Dezsofi A, Jahnel J, Debray D, Hadzic N, Czubkowski P, Gupte G, Mozer-Glassberg Y, van der Woerd W, Smets F, Verkade HJ, Fischler B. Treatment of Chronic Hepatitis C Virus Infection in Children: A Position Paper by the Hepatology Committee of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Indolfi G, Fischler B, Gonzalez-Peralta RP, Ciocca M, Porta G, Neelam M, El-Guindi M, Kelly D, Ni YH, Sibal A, Leung DH, Chang MH; Hepatitis Expert Team of the Federation of International Societies of Pediatric Gastroenterology; Hepatology, and Nutrition (FISPGHAN). Comparison of Recommendations for Treatment of Chronic Hepatitis C Virus Infection in Children and Adolescents: A Position Paper of the Federation of International Societies of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2020;70:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Balistreri WF, Murray KF, Rosenthal P, Bansal S, Lin CH, Kersey K, Massetto B, Zhu Y, Kanwar B, German P, Svarovskaia E, Brainard DM, Wen J, Gonzalez-Peralta RP, Jonas MM, Schwarz K. The safety and effectiveness of ledipasvir-sofosbuvir in adolescents 12-17 years old with hepatitis C virus genotype 1 infection. Hepatology. 2017;66:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 26. | Murray KF, Balistreri WF, Bansal S, Whitworth S, Evans HM, Gonzalez-Peralta RP, Wen J, Massetto B, Kersey K, Shao J, Garrison KL, Parhy B, Brainard DM, Arnon R, Gillis LA, Jonas MM, Lin CH, Narkewicz MR, Schwarz K, Rosenthal P. Safety and Efficacy of Ledipasvir-Sofosbuvir With or Without Ribavirin for Chronic Hepatitis C in Children Ages 6-11. Hepatology. 2018;68:2158-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Schwarz KB, Rosenthal P, Murray KF, Honegger JR, Hardikar W, Hague R, Mittal N, Massetto B, Brainard DM, Hsueh CH, Shao J, Parhy B, Narkewicz MR, Rao GS, Whitworth S, Bansal S, Balistreri WF. Ledipasvir-Sofosbuvir for 12 Weeks in Children 3 to <6 Years Old With Chronic Hepatitis C. Hepatology. 2020;71:422-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Serranti D, Nebbia G, Cananzi M, Nicastro E, Di Dato F, Nuti F, Garazzino S, Silvestro E, Giacomet V, Forlanini F, Pinon M, Calvo PL, Riva S, Dodi I, Cangelosi AM, Antonucci R, Ricci S, Bartolini E, Mastrangelo G, Trapani S, Lenge M, Gaio P, Vajro P, Iorio R, D'Antiga L, Indolfi G. Efficacy of Sofosbuvir/Ledipasvir in Adolescents With Chronic Hepatitis C Genotypes 1, 3, and 4: A Real-world Study. J Pediatr Gastroenterol Nutr. 2021;72:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Serranti D, Dodi I, Nicastro E, Cangelosi AM, Riva S, Ricci S, Bartolini E, Trapani S, Mastrangelo G, Vajro P, D'Antiga L, Resti M, Indolfi G. Shortened 8-Week Course of Sofosbuvir/Ledipasvir Therapy in Adolescents With Chronic Hepatitis C Infection. J Pediatr Gastroenterol Nutr. 2019;69:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | El-Khayat H, Kamal EM, Yakoot M, Gawad MA, Kamal N, El Shabrawi M, Sameh Y, Haseeb A, Fouad Y, Attia D. Effectiveness of 8-week sofosbuvir/Ledipasvir in the adolescent chronic hepatitis C-infected patients. Eur J Gastroenterol Hepatol. 2019;31:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | El-Khayat HR, Kamal EM, El-Sayed MH, El-Shabrawi M, Ayoub H, RizK A, Maher M, El Sheemy RY, Fouad YM, Attia D. The effectiveness and safety of ledipasvir plus sofosbuvir in adolescents with chronic hepatitis C virus genotype 4 infection: a real-world experience. Aliment Pharmacol Ther. 2018;47:838-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | El-Karaksy H, Mogahed EA, Abdullatif H, Ghobrial C, El-Raziky MS, El-Koofy N, El-Shabrawi M, Ghita H, Baroudy S, Okasha S. Sustained Viral Response in Genotype 4 Chronic Hepatitis C Virus-infected Children and Adolescents Treated With Sofosbuvir/Ledipasvir. J Pediatr Gastroenterol Nutr. 2018;67:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Wirth S, Rosenthal P, Gonzalez-Peralta RP, Jonas MM, Balistreri WF, Lin CH, Hardikar W, Kersey K, Massetto B, Kanwar B, Brainard DM, Shao J, Svarovskaia E, Kirby B, Arnon R, Murray KF, Schwarz KB. Sofosbuvir and ribavirin in adolescents 12-17 years old with hepatitis C virus genotype 2 or 3 infection. Hepatology. 2017;66:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 34. | Rosenthal P, Schwarz KB, Gonzalez-Peralta RP, Lin CH, Kelly DA, Nightingale S, Balistreri WF, Bansal S, Jonas MM, Massetto B, Brainard DM, Hsueh CH, Shao J, Parhy B, Davison S, Feiterna-Sperling C, Gillis LA, Indolfi G, Sokal EM, Murray KF, Wirth S. Sofosbuvir and Ribavirin Therapy for Children Aged 3 to <12 Years With Hepatitis C Virus Genotype 2 or 3 Infection. Hepatology. 2020;71:31-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 35. | Sokal EM, Schwarz KB, Rosenthal, Philip, Verucchi G, Lin C-H, Balistreri W, Wen J, Whitworth S, Leung DH, Bansal S, Wikrom K, Mangia A, Rao GS, Shao J, Hsueh C-H, Parhy B, Gaggar A, Kersey K, Narkewicz MR, Gonzalez-Peralta RP, Murray KF, Romero R, Jonas MM. Safety and efficacy of sofosbuvir/velpatasvir for the treatment of chronic hepatitis c infection in adolescents and children aged 3 to 17 years old through 24 weeks post-treatment. Hepatology. 2020;570A. |
| 36. | Jonas MM, Squires RH, Rhee SM, Lin CW, Bessho K, Feiterna-Sperling C, Hierro L, Kelly D, Ling SC, Strokova T, Del Valle-Segarra A, Lovell S, Liu W, Ng TI, Porcalla A, Gonzalez YS, Burroughs M, Sokal E. Pharmacokinetics, Safety, and Efficacy of Glecaprevir/Pibrentasvir in Adolescents With Chronic Hepatitis C Virus: Part 1 of the DORA Study. Hepatology. 2020;71:456-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 37. | Jonas MM, Lon HK, Rhee SM, Gilmour SM, Gonzalez-Peralta RP, Leung DH, Ling SC, Lobritto S, Narkewicz MR, Sabharwal V, Del Valle-Segarra A, Wen J, Lovell S, Porcalla A, Tripathi R, Sokal EM. Pharmacokinetics of glecaprevir/pibrentasvir in children with chronic hcv infection: interim analysis of part 2 of the Dora study. Hepatology. 2019;934-935. |
| 38. | Do A, Mittal Y, Liapakis A, Cohen E, Chau H, Bertuccio C, Sapir D, Wright J, Eggers C, Drozd K, Ciarleglio M, Deng Y, Lim JK. Drug Authorization for Sofosbuvir/Ledipasvir (Harvoni) for Chronic HCV Infection in a Real-World Cohort: A New Barrier in the HCV Care Cascade. PLoS One. 2015;10:e0135645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Torabi J, Rocca JP, Ajaimy M, Melvin J, Campbell A, Akalin E, Liriano LE, Azzi Y, Pynadath C, Greenstein SM, Le M, Goldstein DY, Fox AS, Carrero J, Weiss JM, Powell T, Racine AD, Reinus JF, Kinkhabwala MM, Graham JA. Commercial insurance delays direct-acting antiviral treatment for hepatitis C kidney transplantation into uninfected recipients. Transpl Infect Dis. 2021;23:e13449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Marascio N, Mazzitelli M, Pavia G, Giancotti A, Barreca GS, Costa C, Pisani V, Greco G, Serapide F, Trecarichi EM, Casalinuovo F, Liberto MC, Matera G, Torti C. Clinical, Virological Characteristics, and Outcomes of Treatment with Sofosbuvir/Ledipasvir in Two Pediatric Patients Infected by HCV Genotype 4. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Younossi ZM, Stepanova M, Balistreri W, Schwarz K, Murray KF, Rosenthal P, Bansal S, Hunt S. Health-related Quality of Life in Adolescent Patients With Hepatitis C Genotype 1 Treated With Sofosbuvir and Ledipasvir. J Pediatr Gastroenterol Nutr. 2018;66:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Ruggeri M, Romano F, Basile M, Coretti S, Rolli FR, Drago C, Cicchetti A. Cost-Effectiveness Analysis of Early Treatment of Chronic HCV with Sofosbuvir/Velpatasvir in Italy. Appl Health Econ Health Policy. 2018;16:711-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | El-Shabrawi MHF, Kamal NM, El-Khayat HR, Kamal EM, AbdElgawad MMAH, Yakoot M. A pilot single arm observational study of sofosbuvir/Ledipasvir (200 + 45 mg) in 6- to 12- year old children. Aliment Pharmacol Ther. 2018;47:1699-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Behairy BE, El-Araby HA, El-Guindi MA, Basiouny HM, Fouad OA, Ayoub BA, Marei AM, Sira MM. Safety and Efficacy of 8 Weeks Ledipasvir/Sofosbuvir for Chronic Hepatitis C Genotype 4 in Children Aged 4-10 Years. J Pediatr. 2020;219:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Suda G, Ogawa K, Morikawa K, Sakamoto N. Treatment of hepatitis C in special populations. J Gastroenterol. 2018;53:591-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Berenguer M, Schuppan D. Progression of liver fibrosis in post-transplant hepatitis C: mechanisms, assessment and treatment. J Hepatol. 2013;58:1028-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Brown RS Jr, O'Leary JG, Reddy KR, Kuo A, Morelli GJ, Burton JR Jr, Stravitz RT, Durand C, Di Bisceglie AM, Kwo P, Frenette CT, Stewart TG, Nelson DR, Fried MW, Terrault NA; Hepatitis C Therapeutic Registry Research Network Study Group. Interferon-free therapy for genotype 1 hepatitis C in liver transplant recipients: Real-world experience from the hepatitis C therapeutic registry and research network. Liver Transpl. 2016;22:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 48. | Agarwal K, Castells L, Müllhaupt B, Rosenberg WMC, McNabb B, Arterburn S, Camus G, McNally J, Stamm LM, Brainard DM, Mani Subramanian G, Mariño Z, Dufour JF, Forns X. Sofosbuvir/velpatasvir for 12 wk in genotype 1-4 HCV-infected liver transplant recipients. J Hepatol. 2018;69:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Rupp C, Hippchen T, Neuberger M, Sauer P, Pfeiffenberger J, Stremmel W, Gotthardt DN, Mehrabi A, Weiss KH. Successful combination of direct antiviral agents in liver-transplanted patients with recurrent hepatitis C virus. World J Gastroenterol. 2018;24:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | El-Hamamsy M, Montasser IF, Mansy AE, Nabet DE, El-Meteini M. Effect of cyclosporine A vs tacrolimus on the response to antiviral therapy after hepatitis C genotype-4 recurrence post-liver transplantation: A prospective cohort trial. J Clin Pharm Ther. 2019;44:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Bixby AL, Fitzgerald L, Leek R, Mellinger J, Sharma P, Tischer S. Impact of direct-acting antivirals for hepatitis C virus therapy on tacrolimus dosing in liver transplant recipients. Transpl Infect Dis. 2019;21:e13078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Chan C, Schiano T, Agudelo E, Paul Haydek J, Hoteit M, Laurito MP, Norvell JP, Terrault N, Verna EC, Yang A, Levitsky J. Immune-mediated graft dysfunction in liver transplant recipients with hepatitis C virus treated with direct-acting antiviral therapy. Am J Transplant. 2018;18:2506-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Thomas P, Santiago T, Dallas MH. Treatment of hepatitis C in a pediatric patient using simeprevir and sofosbuvir immediately after an umbilical cord blood transplantation. Bone Marrow Transplant. 2016;51:735-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Fischler B, Priftakis P, Sundin M. Sofosbuvir and Simeprevir Treatment of a Stem Cell Transplanted Teenager With Chronic Hepatitis C Infection. Pediatr Infect Dis J. 2016;35:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Jaiswal SR, Bhakuni P, Soni M, Gupta M, Thatai A, Chakrabarti S. Safety and efficacy of Sofosbuvir and Velpatasvir in children with active hepatitis C virus infection undergoing haploidentical transplantation. Transpl Infect Dis. 2020;e13490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | El-Shabrawi MH, Sherief LM, Yakoot M, Kamal NM, Almalky MA, AbdElgawad MM, Mahfouz AA, Helmy S, Kamal EM, Attia D, El-Khayat HR. Effects of dual sofosbuvir/daclatasvir therapy on, chronic hepatitis C infected, survivors of childhood malignancy. World J Clin Cases. 2019;7:2247-2255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Padhi S, Maharshi S, Gupta GK, Garg K, Nijhawan S. Efficacy and Safety of Direct Acting Antiviral Therapy for Chronic Hepatitis C in Thalassemic Children. J Pediatr Hematol Oncol. 2018;40:511-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Makhlouf NA, Abdelmalek MO, Ibrahim ME, Abu-Faddan NH, Kheila AE, Mahmoud AA. Ledipasvir/Sofosbuvir in Adolescents With Chronic Hepatitis C Genotype 4 With and Without Hematological Disorders: Virological Efficacy and Impact on Liver Stiffness. J Pediatric Infect Dis Soc. 2021;10:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Tucci F, Calbi V, Barzaghi F, Migliavacca M, Ferrua F, Bernardo ME, Canarutto D, Consiglieri G, Recupero S, Calzatini F, Gabaldo M, Lucano C, Casiraghi M, Darin S, Dionisio F, Marktel S, Cestone E, Finazzi R, Mieli-Vergani G, Boeri E, Appleby J, Abd Elaziz D, Ciceri F, Aiuti A, Cicalese MP. Successful Treatment With Ledipasvir/Sofosbuvir in an Infant With Severe Combined Immunodeficiency Caused by Adenosine Deaminase Deficiency With HCV Allowed Gene Therapy with Strimvelis. Hepatology. 2018;68:2434-2437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, Marks K, Luetkemeyer A, Baden RP, Sax PE, Gane E, Santana-Bagur J, Stamm LM, Yang JC, German P, Dvory-Sobol H, Ni L, Pang PS, McHutchison JG, Stedman CA, Morales-Ramirez JO, Bräu N, Jayaweera D, Colson AE, Tebas P, Wong DK, Dieterich D, Sulkowski M; ION-4 Investigators. Ledipasvir and Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 379] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 61. | Molina JM, Orkin C, Iser DM, Zamora FX, Nelson M, Stephan C, Massetto B, Gaggar A, Ni L, Svarovskaia E, Brainard D, Subramanian GM, McHutchison JG, Puoti M, Rockstroh JK; PHOTON-2 study team. Sofosbuvir plus ribavirin for treatment of hepatitis C virus in patients co-infected with HIV (PHOTON-2): a multicentre, open-label, non-randomised, phase 3 study. Lancet. 2015;385:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 62. | Rockstroh JK, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, Matthews GV, Saag MS, Zamor PJ, Orkin C, Gress J, Klopfer S, Shaughnessy M, Wahl J, Nguyen BY, Barr E, Platt HL, Robertson MN, Sulkowski M. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2:e319-e327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 63. | Carrasco I, Sainz T, Frick MA, Jiménez de Ory S, Fortuny C, Burgos J, Montero M, Gavilán C, Falcón MD, Couceiro JA, Bernardino JI, Bisbal O, Guerrero C, Aldámiz-Echevarría MT, Berenguer J, Navarro ML; the Pediatric National AIDS Research Network of Spain (CoRISpe) integrated in the Translational Research Network in Pediatric Infectious Diseases (RITIP). Response to direct-acting antivirals for hepatitis C treatment in vertically HIV/HCV co-infected patients. J Viral Hepat. 2020;27:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H Jr, Martin P, Pol S, Londoño MC, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen BY, Robertson M, Barr E, Wahl J, Greaves W. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 533] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 65. | Fernández-Ruiz M, Polanco N, García-Santiago A, Muñoz R, Hernández AM, González E, Mercado VR, Fernández I, Aguado JM, Praga M, Andrés A. Impact of anti-HCV direct antiviral agents on graft function and immunosuppressive drug levels in kidney transplant recipients: a call to attention in the mid-term follow-up in a single-center cohort study. Transpl Int. 2018;31:887-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 66. | González-Corvillo C, Beneyto I, Sánchez-Fructuoso A, Perelló M, Alonso A, Mazuecos A, Jiménez C, Zárraga S, Paul J, Lauzurica R, Hernández D, Guirado L, Franco A, Ruiz JC, Llorente S, Crespo M, Rodríguez-Benot A, de Gracia Guindo MDC, Díaz-Corte C, Gentil MÁ. Direct-acting Antivirals for the Treatment of Kidney Transplant Patients With Chronic Hepatitis C Virus Infection in Spain: A Long-term Prospective Observational Study. Transplant Direct. 2019;5:e510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Gupta S, Gupta R, Joshi YK, Singh S. Role of horizontal transmission in hepatitis B virus spread among household contacts in north India. Intervirology. 2008;51:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Indolfi G, Easterbrook P, Dusheiko G, Siberry G, Chang MH, Thorne C, Bulterys M, Chan PL, El-Sayed MH, Giaquinto C, Jonas MM, Meyers T, Walsh N, Wirth S, Penazzato M. Hepatitis B virus infection in children and adolescents. Lancet Gastroenterol Hepatol. 2019;4:466-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 69. | World Health Organization. Global hepatitis report, 2017. [cited 2 March 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/publications-detail-redirect/global-hepatitis-report-2017. |
| 70. | McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, Maynard JE. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 502] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 71. | Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 701] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 72. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 73. | Bortolotti F, Guido M, Bartolacci S, Cadrobbi P, Crivellaro C, Noventa F, Morsica G, Moriondo M, Gatta A. Chronic hepatitis B in children after e antigen seroclearance: final report of a 29-year longitudinal study. Hepatology. 2006;43:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 74. | Wen WH, Chang MH, Hsu HY, Ni YH, Chen HL. The development of hepatocellular carcinoma among prospectively followed children with chronic hepatitis B virus infection. J Pediatr. 2004;144:397-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Marx G, Martin SR, Chicoine JF, Alvarez F. Long-term follow-up of chronic hepatitis B virus infection in children of different ethnic origins. J Infect Dis. 2002;186:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Wu JF, Su YR, Chen CH, Chen HL, Ni YH, Hsu HY, Wang JL, Chang MH. Predictive effect of serial serum alanine aminotransferase levels on spontaneous HBeAg seroconversion in chronic genotype B and C HBV-infected children. J Pediatr Gastroenterol Nutr. 2012;54:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Ni YH, Chang MH, Wang KJ, Hsu HY, Chen HL, Kao JH, Yeh SH, Jeng YM, Tsai KS, Chen DS. Clinical relevance of hepatitis B virus genotype in children with chronic infection and hepatocellular carcinoma. Gastroenterology. 2004;127:1733-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 78. | Roushan MR, Bijani A, Ramzaninejad S, Roushan MH, Amiri MJ, Baiani M. HBeAg seroconversion in children infected during early childhood with hepatitis B virus. J Clin Virol. 2012;55:30-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Tseng YR, Wu JF, Ni YH, Chen HL, Chen CC, Wen WH, Hsu HY, Chang MH. Long-term effect of maternal HBeAg on delayed HBeAg seroconversion in offspring with chronic hepatitis B infection. Liver Int. 2011;31:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 80. | Wu JF, Chiu YC, Chang KC, Chen HL, Ni YH, Hsu HY, Chang MH. Predictors of hepatitis B e antigen-negative hepatitis in chronic hepatitis B virus-infected patients from childhood to adulthood. Hepatology. 2016;63:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Nicastro E, Mangili B, Giacomet V, Benincaso AR, Di Giorgio A, Sansotta N, Callegaro A, D'Antiga L. Longitudinal Immune Phenotype Assessment and Serological Outcome in Foreign-born Children With Chronic Hepatitis B. J Pediatr Gastroenterol Nutr. 2020;71:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Schwarz KB, Lombardero M, Di Bisceglie AM, Murray KF, Rosenthal P, Ling SC, Cloonan YK, Rodriguez-Baez N, Schwarzenberg SJ, Hoofnagle JH, Teckman J; Hepatitis B Research Network (HBRN). Phenotypes of Chronic Hepatitis B in Children From a Large North American Cohort. J Pediatr Gastroenterol Nutr. 2019;69:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | Di Bisceglie AM, Lombardero M, Teckman J, Roberts L, Janssen HL, Belle SH, Hoofnagle JH; Hepatitis B Research Network (HBRN). Determination of hepatitis B phenotype using biochemical and serological markers. J Viral Hepat. 2017;24:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 84. | Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, Lu SN, You SL, Wang LY, Chen CJ; R. E.V.E.A.L.-HBV Study Group. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 85. | Arnone OC, Serranti D, Bartolini E, Mastrangelo G, Stinco M, Trapani S, Ricci S, Resti M, Indolfi G. Chronic hepatitis B in children, report of a single-centre longitudinal study on 152 children. J Viral Hepat. 2020;27:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 86. | Sokal EM, Paganelli M, Wirth S, Socha P, Vajro P, Lacaille F, Kelly D, Mieli-Vergani G; European Society of Pediatric Gastroenterology; Hepatology and Nutrition. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines: consensus of an expert panel on behalf of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Hepatol. 2013;59:814-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 87. | Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 88. | Wong GL, Wong VW, Choi PC, Chan AW, Chim AM, Yiu KK, Chan HY, Chan FK, Sung JJ, Chan HL. Clinical factors associated with liver stiffness in hepatitis B e antigen-positive chronic hepatitis B patients. Clin Gastroenterol Hepatol. 2009;7:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 89. | Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology. 2010;51:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 90. | Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, Lu SN, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL–HBV) Study Group. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 2011; 141: 1240-1248, 1248.e1-1248. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 91. | D'Antiga L, Aw M, Atkins M, Moorat A, Vergani D, Mieli-Vergani G. Combined lamivudine/interferon-alpha treatment in "immunotolerant" children perinatally infected with hepatitis B: a pilot study. J Pediatr. 2006;148:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 92. | Poddar U, Yachha SK, Agarwal J, Krishnani N. Cure for immune-tolerant hepatitis B in children: is it an achievable target with sequential combo therapy with lamivudine and interferon? J Viral Hepat. 2013;20:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Zhu S, Zhang H, Dong Y, Wang L, Xu Z, Liu W, Gan Y, Tang H, Chen D, Wang F, Zhao P. Antiviral therapy in hepatitis B virus-infected children with immune-tolerant characteristics: A pilot open-label randomized study. J Hepatol. 2018;68:1123-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 94. | Rosenthal P, Ling SC, Belle SH, Murray KF, Rodriguez-Baez N, Schwarzenberg SJ, Teckman J, Lin HS, Schwarz KB; Hepatitis B Research Network (HBRN). Combination of Entecavir/Peginterferon Alfa-2a in Children With Hepatitis B e Antigen-Positive Immune Tolerant Chronic Hepatitis B Virus Infection. Hepatology. 2019;69:2326-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 95. | Wong GL. Management of chronic hepatitis B patients in immunetolerant phase: what latest guidelines recommend. Clin Mol Hepatol. 2018;24:108-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 96. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2845] [Article Influence: 406.4] [Reference Citation Analysis (0)] |
| 97. | Sokal EM, Conjeevaram HS, Roberts EA, Alvarez F, Bern EM, Goyens P, Rosenthal P, Lachaux A, Shelton M, Sarles J, Hoofnagle J. Interferon alfa therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology. 1998;114:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 98. | Jonas MM, Mizerski J, Badia IB, Areias JA, Schwarz KB, Little NR, Greensmith MJ, Gardner SD, Bell MS, Sokal EM; International Pediatric Lamivudine Investigator Group. Clinical trial of lamivudine in children with chronic hepatitis B. N Engl J Med. 2002;346:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 196] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 99. | Jonas MM, Kelly D, Pollack H, Mizerski J, Sorbel J, Frederick D, Mondou E, Rousseau F, Sokal E. Safety, efficacy, and pharmacokinetics of adefovir dipivoxil in children and adolescents (age 2 to <18 years) with chronic hepatitis B. Hepatology. 2008;47:1863-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 100. | Murray KF, Szenborn L, Wysocki J, Rossi S, Corsa AC, Dinh P, McHutchison J, Pang PS, Luminos LM, Pawlowska M, Mizerski J. Randomized, placebo-controlled trial of tenofovir disoproxil fumarate in adolescents with chronic hepatitis B. Hepatology. 2012;56:2018-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 101. | Jonas MM, Chang MH, Sokal E, Schwarz KB, Kelly D, Kim KM, Ling SC, Rosenthal P, Oraseanu D, Reynolds L, Thiry A, Ackerman P. Randomized, controlled trial of entecavir vs placebo in children with hepatitis B envelope antigen-positive chronic hepatitis B. Hepatology. 2016;63:377-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 102. | Wirth S, Zhang H, Hardikar W, Schwarz KB, Sokal E, Yang W, Fan H, Morozov V, Mao Q, Deng H, Huang Y, Yang L, Frey N, Nasmyth-Miller C, Pavlovic V, Wat C. Efficacy and Safety of Peginterferon Alfa-2a (40KD) in Children With Chronic Hepatitis B: The PEG-B-ACTIVE Study. Hepatology. 2018;68:1681-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 103. | Smolders EJ, Burger DM, Feld JJ, Kiser JJ. Review article: clinical pharmacology of current and investigational hepatitis B virus therapies. Aliment Pharmacol Ther. 2020;51:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 104. | Hepatitis B Foundation. Drug Watch. [cited 2 March 2021]. In: Hepatitis B Foundation [Internet]. Available from: https://www.hepb.org/treatment-and-management/drug-watch/. |
| 105. | Zoulim F, Lenz O, Vandenbossche JJ, Talloen W, Verbinnen T, Moscalu I, Streinu-Cercel A, Bourgeois S, Buti M, Crespo J, Manuel Pascasio J, Sarrazin C, Vanwolleghem T, Shukla U, Fry J, Yogaratnam JZ. JNJ-56136379, an HBV Capsid Assembly Modulator, Is Well-Tolerated and Has Antiviral Activity in a Phase 1 Study of Patients With Chronic Infection. Gastroenterology 2020; 159: 521-533. e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 106. | Yuen MF, Agarwal K, Gane EJ, Schwabe C, Ahn SH, Kim DJ, Lim YS, Cheng W, Sievert W, Visvanathan K, Ruby E, Liaw S, Yan R, Huang Q, Colonno R, Lopatin U. Safety, pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: a randomised, placebo-controlled phase 1 trial. Lancet Gastroenterol Hepatol. 2020;5:152-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 107. | Ma X, Lalezari J, Nguyen T, Bae H, Schiff ER, Fung S, Yuen M-F, Hassanein T, Hann H-W, Elkhashab M, Dieterich D, Sulkowski PK, Nahass R, Agarwal K, Ramji A, Park J, Ravendhran N, Chan S, Weilert F, Han S-H, Ayoub W, Gane EJ, Jacobson I, Bennett M, Huang Q, Yan R, Huey V, Ruby E, Liaw S, Colonno R, Lopatin U. Interim safety and efficacy results of the ABI-H0731 phase 2a program exploring the combination of ABI-H0731 with Nuc therapy in treatment-naive and treatment-suppressed chronic hepatitis B patients. [cited 2 March 2021]. Available from: https://program.manage.com/ProgramSearch/DownloadAbstractOfPresentation/ilc2019?id=417422. |
| 108. | Al-Mahtab M, Bazinet M, Vaillant A. Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PLoS One. 2016;11:e0156667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 109. | Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, Schmid P, Le Gal F, Gordien E, Krawczyk A, Mijočević H, Karimzadeh H, Roggendorf M, Vaillant A. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus coinfection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 110. | Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Dittmer U, Krawczyk A, Vaillant A. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology. 2020;158:2180-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 111. | Yuen MF, Locarnini S, Huey Lim T, Strasser S, Sievert W, Cheng W, Thompson A, Given B, Schluep T, Hamilton J, Cloherty G, Wong D, Schwabe C, Jackson K, Ferrari C, Lai C-L, Gish R, Gane E. Short term RNA interference therapy in chronic hepatitis B using JNJ-3989 brings majority of patients to HBsAg < 100 IU/mL threshold. [cited 2 March 2021]. Available from: https://program.m-anage.com/ilc2019/en-GB/ProgramSearch/DownloadAbstract/151378. |
| 112. | Gane E, Locarnini S, Huey Lim T, Strasser S, Sievert W, Cheng W, Thompson A, Given B, Schluep T, Hamilton J, Li Z, Cloherty G, Wong D, Schwabe C, Jackson K, Ferrari C, Lai C-L, Gish R, Yuen MF. First results with RNA interference (RNAi) in chronic hepatitis B (CHB) using ARO-HBV. The Liver Meeting; 2018 November; San Francisco, USA. |
| 113. | VIR Biotechnology. VIR-2218 Demonstrates Dose-Dependent and Durable Reductions of Hepatitis B Surface Antigen in Phase 1/2 Trial. [cited 2 March 2021]. In: VIR Biotechnology [Internet]. Available from: https://investors.vir.bio/news-releases/news-release-details/vir-2218-demonstrates-dose-dependent-and-durable-reductions/. |
| 114. | Yuen MF, Chen CY, Liu CJ, Jeng RWJ, Elkhashab M, Coffin C, Kim W, Greenbloom S, Ramji A, Lim YS, Kim YJ, Fung S, Kim DJ, Jang JW, Lee KS, Afdhal N, Radhakrishnan I, Macfarlane C, Jackson K, Locarnini S, Chan H. Ascending dose cohort study of inarigivir - A novel RIG I agonist in chronic HBV patients: Final results of the ACHIEVE trial. J Hepatol. 2019;70:e47-E48. [DOI] [Full Text] |
| 115. | Reyes M, Lutz JD, Lau AH, Gaggar A, Grant EP, Joshi A, Mackman RL, Ling J, Tan SK, Ayithan N, Daffis S, Woo J, Wu P, Lam T, Fletcher SP, Kottilil S, Poonia B, Gane EJ, Mathias A, German P. Safety, pharmacokinetics and pharmacodynamics of selgantolimod, an oral Toll-like receptor 8 agonist: a Phase Ia study in healthy subjects. Antivir Ther. 2020;25:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 116. | Gane E, Zhao Y, Tan SK, Lau AH, Gaggar A, Subramanian M, Wallin J, Brooks AE, Dubar PR, Kottilil S, Tang L. Efficacy and Safety of Oral TLR8 Agonist Selgantolimod in Virally Suppressed Adult Patients With Chronic Hepatitis B: a Phase 2, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study. Hepatology. 2019;. |
| 117. | European Association for The Study of The Liver. Bulevirtide shows promise in the treatment of chronic hepatitis B/D (HBV/HDV) coinfection. [cited 2 March 2021]. In: European Association for The Study of The Liver [Internet]. Available from: https://2019.ilc-congress.eu/press-release/bulevirtide-shows-promise-in-the-treatment-of-chronic-hepatitis-b-d-hbv-hdv-coinfection/. |
| 118. | Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, Lehr T, Lempp FA, Wedemeyer H, Haag M, Schwab M, Haefeli WE, Blank A, Urban S. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol. 2016;65:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 119. | Indolfi G, Abdel-Hady M, Bansal S, Debray D, Smets F, Czubkowski P, van der Woerd W, Samyn M, Jahnel J, Gupte G, Zellos A, Mozer-Glassberg Y, Verkade HJ, Sokal E, Fischler B. Management of Hepatitis B Virus Infection and Prevention of Hepatitis B Virus Reactivation in Children With Acquired Immunodeficiencies or Undergoing Immune Suppressive, Cytotoxic, or Biological Modifier Therapies. J Pediatr Gastroenterol Nutr. 2020;70:527-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 120. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1960] [Article Influence: 217.8] [Reference Citation Analysis (0)] |
| 121. | deBruyn JCC, Soon IS, Fonseca K, Feng S, Purtzki M, Goedhart C, Kuhn S, Vanderkooi OG, Wrobel I. Serologic Status of Routine Childhood Vaccines, Cytomegalovirus, and Epstein-Barr Virus in Children With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 122. | Aljaberi N, Ghulam E, Smitherman EA, Favier L, Dykes DMH, Danziger-Isakov LA, Brady RC, Huggins J. Maintaining Hepatitis B Protection in Immunocompromised Pediatric Rheumatology and Inflammatory Bowel Disease Patients. J Rheumatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 123. | Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215-9; quiz e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 451] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 124. | Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 443] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 125. | Zhang MY, Zhu GQ, Shi KQ, Zheng JN, Cheng Z, Zou ZL, Huang HH, Chen FY, Zheng MH. Systematic review with network meta-analysis: Comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7:30642-30658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 126. | Fung J, Wong T, Chok K, Chan A, Cheung TT, Dai JW, Sin SL, Ma KW, Ng K, Ng KT, Seto WK, Lai CL, Yuen MF, Lo CM. Long-term outcomes of entecavir monotherapy for chronic hepatitis B after liver transplantation: Results up to 8 years. Hepatology. 2017;66:1036-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 127. | Huprikar S, Danziger-Isakov L, Ahn J, Naugler S, Blumberg E, Avery RK, Koval C, Lease ED, Pillai A, Doucette KE, Levitsky J, Morris MI, Lu K, McDermott JK, Mone T, Orlowski JP, Dadhania DM, Abbott K, Horslen S, Laskin BL, Mougdil A, Venkat VL, Korenblat K, Kumar V, Grossi P, Bloom RD, Brown K, Kotton CN, Kumar D. Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant. 2015;15:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 128. | Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment. Semin Liver Dis. 2006;26:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 129. | Brunetto MR, Oliveri F, Colombatto P, Coco B, Ciccorossi P, Bonino F. Treatment of HBeAg-negative chronic hepatitis B with interferon or pegylated interferon. J Hepatol. 2003;39 Suppl 1:S164-S167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 130. | Brunetto MR, Oliveri F, Rocca G, Criscuolo D, Chiaberge E, Capalbo M, David E, Verme G, Bonino F. Natural course and response to interferon of chronic hepatitis B accompanied by antibody to hepatitis B e antigen. Hepatology. 1989;10:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 164] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 131. | Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 132. | Ogunbosi B, Smuts H, Eley B, Korsman S, De Lacy R, Hardie DR. Fulminant hepatitis B virus (HBV) infection in an infant following mother-to-child transmission of an e-minus HBV mutant: Time to relook at HBV prophylaxis in South African infants. S Afr Med J. 2018;108:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 133. | Lampertico P, Viganò M, Di Costanzo GG, Sagnelli E, Fasano M, Di Marco V, Boninsegna S, Farci P, Fargion S, Giuberti T, Iannacone C, Regep L, Massetto B, Facchetti F, Colombo M; PegBeLiver Study Group. Randomised study comparing 48 and 96 wk peginterferon α-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut. 2013;62:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 134. | Leung DH, Wirth S, Yao BB, Viani RM, Gonzalez-Peralta RP, Jonas MM, Lobritto SJ, Narkewicz MR, Sokal E, Fortuny C, Hsu EK, Del Valle-Segarra A, Zha J, Larsen L, Liu L, Shuster DL, Cohen DE, Rosenthal P. Ombitasvir/Paritaprevir/Ritonavir With or Without Dasabuvir and With or Without Ribavirin for Adolescents With HCV Genotype 1 or 4. Hepatol Commun. 2018;2:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 135. | Rosenthal P, Narkewicz MR, Yao BB, Jolley CD, Lobritto SJ, Wen J, Molleston JP, Hsu EK, Jonas MM, Zha J, Liu L, Leung DH. Ombitasvir, Paritaprevir, Ritonavir, and Dasabuvir Mini-Tabs Plus Ribavirin for Children Aged 3-11 Years with Hepatitis C Genotype 1a. Adv Ther. 2020;37:3299-3310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 136. | Wirth S, Gonzalez-Peralta RP, Squires RH, Mutschler F, Lang T, Pawlowska M, Sluzewski W, Majda-Stanislawska E, Fischler B, Balistreri WF, Jonas MM, Blondet N, Rosenthal P, Alkhouri N, Romero R, Grandhi A, Castronuovo P, Caro L, Rosenblum D, Du L, Haber BH. Elbasvir/grazoprevir in children aged 3 to <18 years with chronic hepatitis c virus (hcv) genotype (gt) 1 or 4 infection: final results from an iterative pharmacokinetic (pk) modeling study. Hepatology. 2020;555-556. |
| 137. | Bansal S, Pawlowska M, Nebbia G, Di Dato F, Sluzewski W, Giacomet V, Szenborn L, Shao J, Yue MS, Hsueh C-H, Parhy B, Gaggar A, Kersey K, Mangia A, Kelly DA, Indolfi G. Safety and efficacy of sofosbuvir/velpatasvir/voxilaprevir in adolescents with chronic hepatitis c virus infection. Hepatology. 2020;571. |
| 138. | El-Sayed MH, Hassany M, Asem N. A pilot study for safety and efficacy of 12 wk sofosbuvir plus daclatasvir with or without ribavirin in Egyptian adolescents with chronic hepatitis C virus Infection. J Hepatology. 2017;178. |
| 139. | El-Shabrawi MH, Abdo AM, El-Khayat HR, Yakoot M. Shortened 8 Weeks Course of Dual Sofosbuvir/Daclatasvir Therapy in Adolescent Patients, With Chronic Hepatitis C Infection. J Pediatr Gastroenterol Nutr. 2018;66:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 140. | Yakoot M, El-Shabrawi MH, AbdElgawad MM, Mahfouz AA, Helmy S, Abdo AM, El-Khayat HR. Dual Sofosbuvir/Daclatasvir Therapy in Adolescent Patients With Chronic Hepatitis C Infection. J Pediatr Gastroenterol Nutr. 2018;67:86-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 141. | Abdel Ghaffar TY, El Naghi S, Abdel Gawad M, Helmy S, Abdel Ghaffar A, Yousef M, Moafy M. Safety and efficacy of combined sofosbuvir/daclatasvir treatment of children and adolescents with chronic hepatitis C Genotype 4. J Viral Hepat. 2019;26:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |