Published online May 7, 2021. doi: 10.3748/wjg.v27.i17.2015
Peer-review started: January 18, 2021
First decision: February 9, 2021
Revised: February 22, 2021
Accepted: March 31, 2021
Article in press: March 31, 2021
Published online: May 7, 2021
Processing time: 100 Days and 17.8 Hours
Liver cancer is one of the most common malignant tumors, and ranks as the fourth leading cause of cancer death worldwide. Microvascular invasion (MVI) is considered one of the most important factors for recurrence and poor prognosis of liver cancer. Thus, accurately identifying MVI before surgery is of great importance in making treatment strategies and predicting the prognosis of patients with hepatocellular carcinoma (HCC). Radiomics as an emerging field, aims to utilize artificial intelligence software to develop methods that may contribute to cancer diagnosis, treatment improvement and evaluation, and better prediction.
To investigate the predictive value of computed tomography radiomics for MVI in solitary HCC ≤ 5 cm.
A total of 185 HCC patients, including 122 MVI negative and 63 MVI positive patients, were retrospectively analyzed. All patients were randomly assigned to the training group (n = 124) and validation group (n = 61). A total of 1351 radiomic features were extracted based on three-dimensional images. The diagnostic performance of the radiomics model was verified in the validation group, and the Delong test was applied to compare the radiomics and MVI-related imaging features (two-trait predictor of venous invasion and radioge
A total of ten radiomics features were finally obtained after screening 1531 features. According to the weighting coefficient that corresponded to the features, the radiomics score (RS) calculation formula was obtained, and the RS score of each patient was calculated. The radiomics model exhibited a better correction and identification ability in the training and validation groups [area under the curve: 0.72 (95% confidence interval: 0.58-0.86) and 0.74 (95% confidence interval: 0.66-0.83), respectively]. Its prediction performance was significantly higher than that of the image features (P < 0.05).
Computed tomography radiomics has certain predictive value for MVI in solitary HCC ≤ 5 cm, and the predictive ability is higher than that of image features.
Core Tip: Microvascular invasion (MVI) is considered one of the most important factors for recurrence and poor prognosis of liver cancer. Thus, accurately identifying MVI before surgery is of great importance in making treatment strategies and predicting the prognosis of patients with hepatocellular carcinoma (HCC). This study showed that radiomics as an emerging method at present had a good diagnostic efficiency and exhibited better accuracy in predicting MVI than image features, indicating that radiomics is a more suitable method in predicting MVI in solitary HCC ≤ 5 cm.
- Citation: Liu P, Tan XZ, Zhang T, Gu QB, Mao XH, Li YC, He YQ. Prediction of microvascular invasion in solitary hepatocellular carcinoma ≤ 5 cm based on computed tomography radiomics. World J Gastroenterol 2021; 27(17): 2015-2024
- URL: https://www.wjgnet.com/1007-9327/full/v27/i17/2015.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i17.2015
Liver cancer is one of the most common malignant tumors, and ranks as the fourth leading cause of cancer death worldwide[1]. Furthermore, more than half of liver cancers occur in China, where there is a high incidence of hepatitis B[2]. Moreover, the recurrence rate after hepatectomy is 70%[3], and microvascular invasion (MVI) is considered one of the most important factors for recurrence and poor prognosis of liver cancer[4]. MVI has a relatively high incidence in hepatocellular carcinoma (HCC), which ranges from 12.4% to 57.1%, and may occur even in solitary HCC ≤ 2 cm[5,6]. Thus, accurately identifying MVI before surgery is of great importance in making treatment strategies and predicting the prognosis of patients with HCC[7]. However, MVI can only be confirmed by histopathology via surgical resection at present. Therefore, the accurate prediction of MVI before surgery is desperately needed. Radiomics as an emerging field, which aims to utilize artificial intelligence software to develop methods that may contribute to cancer diagnosis, treatment improvement and evaluation, and better prediction[8]. At present, few studies have focused on the prediction of MVI in the early stage of HCC (which refers to solitary tumor with a size of ≤ 5 cm, without intrahepatic venous invasion[9]). The present study aimed to investigate the predictive value of computed tomography (CT) radiomics for MVI in solitary HCC ≤ 5 cm.
Patients were retrospectively collected from January 1, 2014 to November 15, 2018 (Hunan provincial People's Hospital). The inclusion criteria were: (1) Pathologically diagnosed hepatocellular carcinoma with MVI; (2) Solitary tumor with the maximum diameter of ≤ 5 cm; and (3) Enhanced CT scanning was performed before surgery. The exclusion criteria were: (1) Complicated with other malignant tumors, and multiple primary or recurrent liver cancer; (2) History of preoperative treatment; (3) CT revealed a vascular tumor thrombus or macrovascular invasion; or (4) The tumor boundary was difficult to determine. The flowchart for the screening of patients is presented in Figure 1.
The Philips (Brilliance iCT 256) and Neusoft (NeuViz 64EN) scanners were used, with a tube voltage of 100-200 kV, tube current of 171-313 mAs, scanning layer thickness of 5 mm, layer spacing of 5 mm, and matrix of 1024 × 1024. The contrast medium (iopromide injection, 300 mgI/mL) was injected using a high-pressure syringe through the anterior cubital vein at a rate of 3.5 mL/s and at a dose of 1.2 mL/kg. Dynamic contrast-enhanced imaging data acquisition was performed at fixed time points: For the arterial phase, acquisition occurred at approximately 25-33 s after administration; for the portal vein phase, it was 57-63 s, and for the delayed phase, 117-123 s.
The two-trait predictor of venous invasion (TTPVI) was defined as having two independent imaging characteristics at the same time, and the development of an internal tumor artery without the signs of low density at the tumor margin[10]. Radiogenomic invasion (RVI) comprised of three independent image features: Intratumoral artery, low-density ring, and tumor-liver difference[11]. If there was an intratumoral artery, but there was no low-density ring or tumor-liver difference, the tumor was considered to have RVI. The imaging features (TTPVI and RVI) were evaluated double-blindly by two radiologists (with three years and seven years of experience in abdominal radiology, respectively). If these radiologists had inconsistent evaluation results, a third senior radiologist (with 13 years of experience in abdominal radiology) would make the further confirmation. The detailed description is presented in Figure 2.
Region of interest selection: The CT images of the patients in the arterial phase were exported in DICOM format. Without knowing the pathological results, the radiologist with three years of experience in abdominal radiology used the 3D-Slice software (http://www.slice.org) to delineate the region of interest (ROI) in each layer that contained the tumor, and finally formed the three-dimensional segmented image.
Extraction of radiomic features: After segmenting the images, the plug-in radiologics in the 3D-Slice software was used to analyze the original image data in the ROI, and 1351 candidate texture parameters were extracted, including the histogram features, morphological features, original features, and texture features.
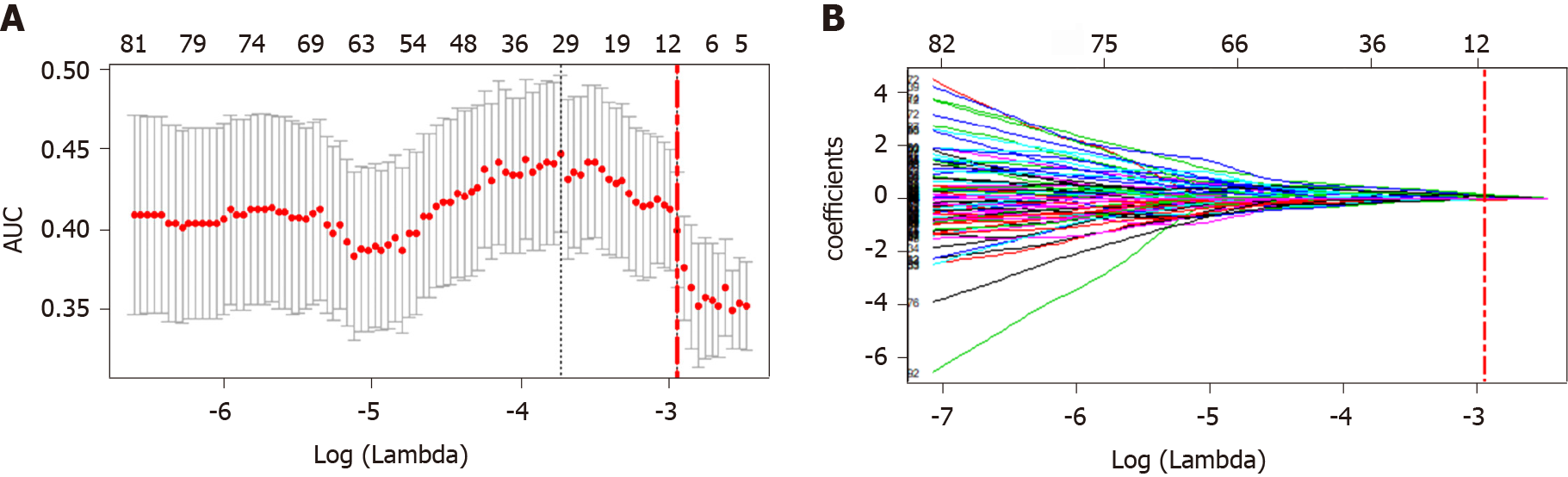
Selection of features correlated to the MVI status and construction of the radiomics tags: First, the extracted intraclass features were evaluated by intraclass correlation coefficient (ICC), and features with ICC < 0.75 were removed. Then, the least absolute shrinkage and selection operator (LASSO) algorithm was used for dimension reduction and feature construction, and the least feature variables were selected by 10-fold cross-validation with the minimal value. The selected features were modeled by Logistic regression, in order to generate the formula of the radiomics score (rad-score, RS) and calculate the RS score of each patient.
The R language (version 3.4.0, https://www.r-project.org) was used for the statistical analyses, and P < 0.05 was considered statistically significant. The chi-square test for two independent samples was used for categorical variables, and Mann-Whitney U-test for two independent samples was used for continuous variables, in order to analyze the difference between the training group and verification group. The receiver operator characteristic curve and the area under the curve (AUC) were used to evaluate the prediction efficiency of the radiomic and image features, and the Delong test was used to determine whether there was a statistical difference between the two methods.
Finally, the study consisted of 185 patients. Tumor size ranged from 10 mm to 50 mm. The clinicopathological and CT features in the training group and verification group are presented in Table 1. There was no significant difference in scores for MVI, other clinical data, imaging features (TTPVI and RVI), or RS between the two groups (P > 0.05), indicating the reasonable arrangement of the training group and verification group.
| Feature | Training group (n = 124) | Verification group (n = 61) | Z value/χ2 value | P value |
| Age (yr), median (quartile) | 54 (47; 63) | 52 (46; 62) | -0.900 | 0.368 |
| Gender/cases | ||||
| Male | 102 | 53 | 0.349 | 0.555 |
| Female | 22 | 8 | ||
| Hepatitis B | 104 | 56 | 1.575 | 0.210 |
| Liver cirrhosis | 84 | 43 | 0.044 | 0.833 |
| AFP (ng/mL) | ||||
| ≤ 20 | 73 | 30 | 1.188 | 0.276 |
| > 20 | 51 | 31 | ||
| MVI | ||||
| Negative | 82 | 40 | 0.000 | 1.000 |
| Positive | 42 | 21 | ||
| Tumor size (mm), median (quartile) | 36 (28; 44) | 34 (27.5; 41) | -0.746 | 0.456 |
| TTPVI | ||||
| Negative | 56 | 25 | 0.145 | 0.703 |
| Positive | 68 | 36 | ||
| RVI | ||||
| Negative | 93 | 40 | 1.362 | 0.243 |
| Positive | 31 | 21 | ||
| Rad-score, median (quartile) | -0.669 (-0.831; -0.546) | -0.640 (-0.780; -0.494) | -0.917 | 0.359 |
After removal by high correlation, 185 of the 1531 features remained. Then, ten features were selected by dimension reduction using the LASSO algorithm, as shown in Figure 3.
According to the weighting coefficient corresponding to the feature (Figure 3), the radiomics formula was obtained and used to calculate the histological score of each lesion in the training group and verification group. The formula is as follows: Rad-score = -0.66692761 - 0.02491645 × originalshapeFlatness + 0.10564798 × log.sigma.
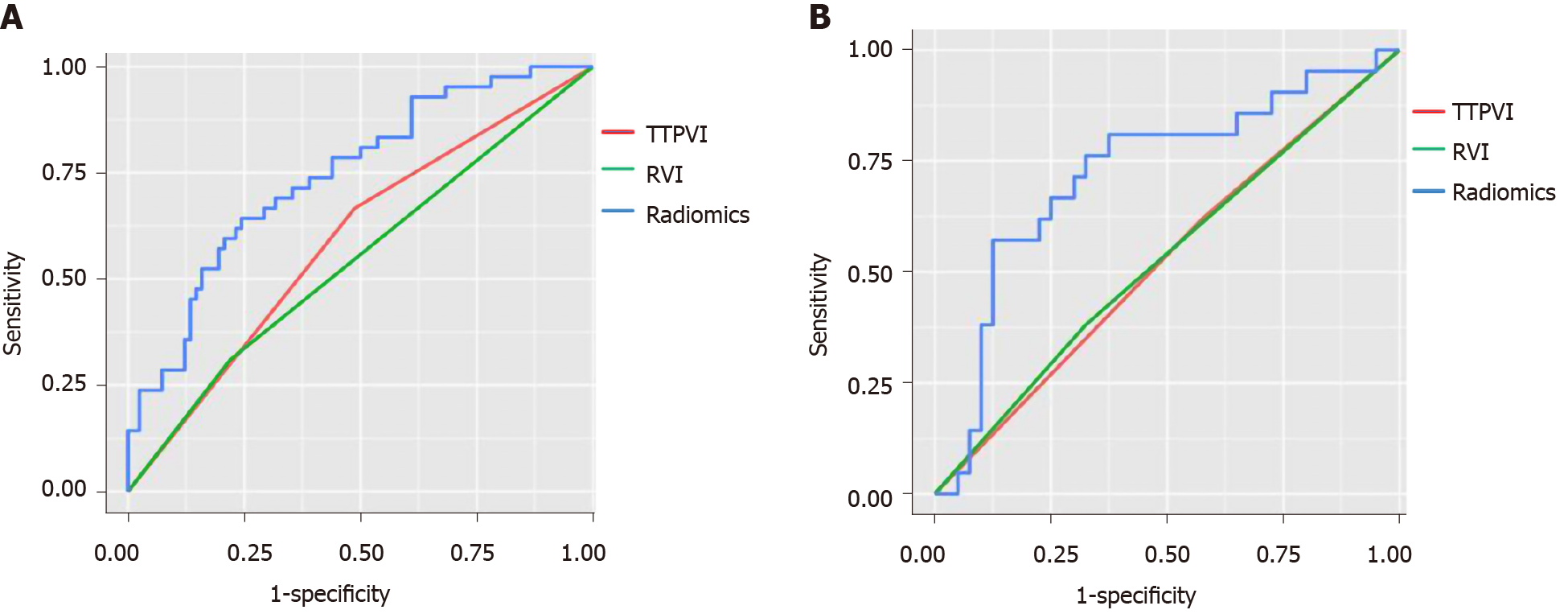
The median RS [quartile interval] of MVI positive patients [training group: -0.574 (-0.695, -0.412); verification group: -0.495 (-0.644, -0.429)] was significantly higher than that of MVI negative patients [training group: -0.710 (-0.899, -0.610); verification group: -0.709 (-0.805, -0.589)] in both the training and verification groups, and the difference was statistically significant (P < 0.05). The radiomics tags exhibited better diagnostic efficacy in both the training and verification groups, as shown in Table 2.
| Training group | Verification group | |||
| AUC (95%CI) | P value | AUC (95%CI) | P value | |
| Rad-score | 0.724 (0.584-0.863) | 0.745 (0.655-0.834) | ||
| TTPVI | 0.590 (0.500-0.679) | 0.522 (0.393-0.651) | ||
| RVI | 0.545 (0.462-0.628) | 0.528 (0.401-0.655) | ||
| Rad vs TTPVI | 0.018 | 0.043 | ||
| Rad vs RVI | 0.002 | 0.048 | ||
As shown in Figure 4 and Table 2, the diagnostic efficacy of the radiomics score was higher than that of the image features in the training group and verification group, and the difference was statistically significant (P < 0.05).
The present study revealed that radiomics, as an emerging method at present, exhibited good diagnostic efficiency and better accuracy in predicting MVI, when compared to image features, indicating that radiomics is a more suitable method for predicting MVI in solitary HCC ≤ 5 cm.
A number of studies have shown that tumor size and imaging features can predict MVI, in which TTPVI and RVI are good predictors with an ideal sensitivity and specificity[11]. However, the diagnostic performance of TTPVI and RVI in the present study was significantly lower than that in previous studies, which might be attributed to the difference in tumor diameter of the study samples. Furthermore, previous studies did not define the tumor size, while the present study merely included patients with a tumor diameter of ≤ 5 cm. In addition, the imaging predictors, such as internal arteries and low-density shadow, were not commonly observed in cases with small tumors, which significantly reduced the positive rate of the MVI-related image features in the present study.
With the recent increase in development of radiomics, numerous studies have indicated that radiomics can reveal the pathological grade, prognosis, MVI, and treatment response of liver cancer. The nomogram of MVI in HCC based on CT radiomics established by Peng et al[12] exhibited good decision-making efficiency (AUC = 0.84), which was slightly higher than the results of the present study, but the model did not involve the tumor diameter. In another study[13], the diagnostic efficacy of predicting MVI in a tumor diameter of ≤ 5 cm based on radiomics (the AUC for the verification and verification group was 0.637 and 0.583, respectively) was slightly lower than that of the present study. Compared to that study[13], the present study adopted more stringent inclusion and exclusion criteria. Moreover, the present study only included solitary liver cancer with a diameter of ≤ 5 cm. Partial hepatectomy is the first choice for patients with solitary liver cancer ≤ 5 cm and good liver function[9]. However, if the lesion is not fully resected, the residual MVI near the surgical margin may be an important cause of recurrence in patients with HCC[14], and some studies have demonstrated that extended resection can reduce the early recurrence rate of patients with liver cancer complicated with MVI[15]. Therefore, the present study has certain reference value for surgery choice in patients with liver cancer. In addition, the present study excluded patients with a visible thrombus or the invasion of large blood vessels, rupture and bleeding of liver cancer, and intangible tumor boundary due to other reasons, because the ROI of these patients was difficult to delineate. Hence, measurement errors were hard to avoid. Furthermore, the present study employed the three-dimensional ROI of tumors to the extract radiomics features, which can better reflect the whole outline of the tumor, and allow for the extraction of more tumor information, when compared to two-dimensional ROI. Some studies have revealed that the feature extraction of the maximum cross-sectional area cannot represent the whole tumor[16]. Hence, the present study has obtained more objective prediction results.
The present study had some limitations. First, the present retrospective and single-center study may have selection bias. Second, the present study only used arterial phase images. Multi-phase images may be utilized to obtain more tumor information and improve the diagnostic efficiency. Therefore, multi-center studies with large samples and multi-phase images would become our future research content.
In conclusion, CT radiomics has certain predictive value for MVI in solitary HCC ≤ 5 cm. Compared to imaging features, the predictive ability of radiomics tags is significantly higher. The radiomics model of MVI would facilitate clinicians in choosing the appropriate treatment.
Liver cancer is one of the most common malignant tumors, and ranks as the fourth leading cause of cancer death worldwide. Microvascular invasion (MVI) is considered one of the most important factors for recurrence and poor prognosis of liver cancer. Radiomics as an emerging field, aims to utilize artificial intelligence software to develop methods that may contribute to cancer diagnosis, treatment improvement, and evaluation and better prediction.
At present, few studies have focused on the prediction of MVI in the early stage of hepatocellular carcinoma (HCC) (which refers to solitary tumor with a size of ≤ 5 cm, without MVI). Our study aimed to investigate the predictive value of computed tomography (CT) radiomics for MVI in solitary HCC ≤ 5 cm.
This study aimed to investigate the predictive value of radiomics for MVI in solitary HCC ≤ 5 cm.
A total of 185 HCC patients, including 122 MVI negative and 63 MVI positive patients, were retrospectively analyzed. All patients were randomly assigned to the training group (n = 124) and validation group (n = 61), at a ratio of 2:1. A total of 1351 radiomic features were extracted based on three-dimensional images. In the training group, the least absolute shrinkage and selection operator feature selection algorithm was used to reduce the dimensions, and the most relevant radiomic features of MVI were selected to calculate the image score (Rad-score, RS) of each patient. The diagnostic performance of the radiomics model was verified in the validation group, and the Delong test was applied to compare the radiomics and MVI-related imaging features (two-trait predictor of venous invasion and radiogenomic invasion).
A total of ten radiomics features were finally obtained after screening 1531 features. According to the weighting coefficient that corresponded to the features, the RS calculation formula was obtained, and the RS score of each patient was calculated. The radiomics model exhibited a better correction and identification ability in the training and validation groups [area under the curve: 0.72 (95% confidence interval: 0.58-0.86) and 0.74 (95% confidence interval: 0.66-0.83), respectively]. Its prediction performance was significantly higher than that of the image features (P < 0.05).
CT radiomics has certain predictive value for MVI in solitary HCC ≤ 5 cm, and the predictive ability is higher than that of image features.
The accurate prediction of MVI before surgery is desperately needed. Radiomics as an emerging field, aims to utilize artificial intelligence software to develop methods that may contribute to cancer diagnosis, treatment improvement and evaluation, and better prediction. At present, few studies have focused on the prediction of MVI in the early stage of HCC (which refers to solitary tumor with a size of ≤ 5 cm, without MVI). The present study aimed to investigate the predictive value of CT radiomics for MVI in solitary HCC ≤ 5 cm.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lim SC, Ni X, Xu PJ S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 454] [Article Influence: 90.8] [Reference Citation Analysis (1)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 3. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1108] [Article Influence: 100.7] [Reference Citation Analysis (1)] |
| 4. | Kim KA, Kim MJ, Jeon HM, Kim KS, Choi JS, Ahn SH, Cha SJ, Chung YE. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging. 2012;35:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 5. | Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 6. | Wang H, Wu MC, Cong WM. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res. 2019;49:344-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 7. | Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford). 2005;7:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 3857] [Article Influence: 296.7] [Reference Citation Analysis (2)] |
| 9. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014; 146: 1691-700. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 10. | Renzulli M, Brocchi S, Cucchetti A, Mazzotti F, Mosconi C, Sportoletti C, Brandi G, Pinna AD, Golfieri R. Can Current Preoperative Imaging Be Used to Detect Microvascular Invasion of Hepatocellular Carcinoma? Radiology. 2016;279:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 293] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 11. | Banerjee S, Wang DS, Kim HJ, Sirlin CB, Chan MG, Korn RL, Rutman AM, Siripongsakun S, Lu D, Imanbayev G, Kuo MD. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology. 2015;62:792-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 12. | Peng J, Zhang J, Zhang Q, Xu Y, Zhou J, Liu L. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 13. | He M, Zhang P, Ma X, He B, Fang C, Jia F. Radiomic Feature-Based Predictive Model for Microvascular Invasion in Patients With Hepatocellular Carcinoma. Front Oncol. 2020;10:574228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Zhao H, Hua Y, Lu Z, Gu S, Zhu L, Ji Y, Qiu Y, Dai T, Jin H. Prognostic value and preoperative predictors of microvascular invasion in solitary hepatocellular carcinoma ≤ 5 cm without macrovascular invasion. Oncotarget. 2017;8:61203-61214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Wang H, Yu H, Qian YW, Cao ZY, Wu MC, Cong WM. Impact of Surgical Margin on the Prognosis of Early Hepatocellular Carcinoma (≤5 cm): A Propensity Score Matching Analysis. Front Med (Lausanne). 2020;7:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Ng F, Kozarski R, Ganeshan B, Goh V. Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol. 2013;82:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |