Published online Apr 28, 2021. doi: 10.3748/wjg.v27.i16.1770
Peer-review started: November 20, 2020
First decision: December 17, 2020
Revised: January 29, 2021
Accepted: March 24, 2021
Article in press: March 24, 2021
Published online: April 28, 2021
Processing time: 152 Days and 2.4 Hours
Sulongga-4 (SL-4) is a herbal formula used in traditional Mongolian medical clinics for the treatment of peptic ulcers and gastroenteritis, even though its pharmacological mechanism has not been well characterized.
To evaluate the protective effect and identify the mechanisms of action of SL-4 on gastroduodenal ulcer induced by pyloric ligation (PL) in rats.
PL was performed to induce gastric and duodenal ulcers in rats, which were then treated with oral SL-4 (1.3, 2.6, or 3.9 g/kg per day) for 15 d. PL-induced gastroduodenal ulceration. Therapeutic effects were characterized by pathological and histological evaluations and inflammatory indicators were analyzed by enzyme-linked immunosorbent assay. Microarray analyses were conducted to identify gene expression profiles of gastroduodenal tissue in PL rats with or without SL-4 treatment. The candidate target genes were selected and verified by quantitative reverse transcription polymerase chain reaction (qRT-PCR).
SL-4 decreased histopathological features in the PL-induced ulcerated rats. SL-4 significantly (P < 0.05) decreased expression of tumor necrosis factor-α, interleukin (IL)-1β, IL-6, endotoxin, platelet-activating factor, and increased prostaglandin E2 and epidermal growth factor in ulcer tissue. Microarray analysis was used to identify a panel of candidate target genes for SL-4 acting on PL-induced ulceration. Genes included some complement and coagulation cascade and retinol metabolism pathways that are closely associated with inflammatory responses and gastric mucosal protective mechanisms. qRT-PCR showed that altered expression of the selected genes, such as CYP2b2, UGT2b1, A2m, and MASP1 was consistent with the microarray results.
SL-4 exerts protective effects against PL-induced gastroduodenal ulcers via reducing inflammatory cytokines and elevating expression of gastric acid inhibitory factors. Downregulation of CYP2b2 and UGT2b1 genes in retinol metabolism and upregulation of A2m and MASP1 genes in the complement and coagulation cascades pathways are possibly involved in SL-4-mediated protection against gastroduodenal ulcer.
Core Tip: Sulongga-4 (SL-4) is a classic herbal formula used in Mongolian medical clinics for the treatment of peptic ulcers and gastroenteritis. This study investigated the protective effects and molecular mechanisms of SL-4 in pyloric ligation (PL)-induced gastroduodenal ulcers in rats via microarray analysis. Our results suggest that SL-4 was strongly protective against PL-induced gastroduodenal ulcers via reducing inflammatory cytokines and elevating the expression of gastric acid inhibitory factors. Downregulation of CYP2b2 and UGT2b1, and upregulation of A2m and MASP1 may be involved in the gastroduodenal ulcer protection mechanism of SL-4.
- Citation: Tong S, Wang H, A LS, Bai TN, Gong JH, Jin WJ, Dai LL, Ba GN, Cho SB, Fu MH. Protective effect and mechanisms of action of Mongolian medicine Sulongga-4 on pyloric ligation-induced gastroduodenal ulcer in rats. World J Gastroenterol 2021; 27(16): 1770-1784
- URL: https://www.wjgnet.com/1007-9327/full/v27/i16/1770.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i16.1770
Peptic ulcer disease is the most prevalent chronic disease of the digestive system, with complications resulting in high morbidity and mortality[1]. Many factors can lead to the formation of peptic ulcers, and one of the basic mechanisms is impairment of gastric mucosal barrier function by acidic gastric secretion[2]. Inflammation or injury of the digestive system cause visceral hypersensitivity that can drive the development of chronic pain and patient discomfort[3], while inflammation is a key driver of gastric ulcer formation[4]. For protection against inflammation, cells respond with attenuated production of proinflammatory cytokines such as interleukin (IL)-12, tumor necrosis factor (TNF)-α, IL1β, IL-6, IL-8, and interferon (IFN)[5,6]. These cytokines drive the aggregation and trigger activation of T cells in areas of inflammation. Mediators of inflammation protect against peptic ulcer formation. Although peptic ulcer disease can be treated with drugs such as proton pump inhibitors, H2 receptor antagonists and prostaglandin analogs, most of these drugs have adverse reactions and limitations when used for the long term[7]. Safer and more effective antiulcer agents are therefore needed.
The use of plant extracts as natural medications is well documented, with multifunctional effects reported such as immunostimulatory, anti-inflammatory, antiviral, anticancer, and radioprotective effects[8]. Plant or herbal extracts are also recognized as a source of therapeutics for gastric ulcers[9]. Traditional Mongolian medicine (TMM) has been used to treat peptic ulcers and is proven to be effective in clinical treatment. The use of TMM is widespread in North China, Mongolia, and the Buryat Republic. The use of TMM is spreading to more Asian countries because of its profound curative effects on some common diseases in the northern region. The Mongolian medicine Sulongga-4 (SL-4) is composed of four medicinal herbs: Forsythia suspensa, Persicaria bistorta, Caulis clematidis armandii, and Ophiopogon japonicus. According to ethnopharmacological records, SL-4 has shown good efficacy against Hododenbaoru, a term in TMM referring to peptic ulcer[10]. Modern clinical applications and preliminary studies have shown that SL-4 is generally used for abdominal pain, gastroenteritis, peptic ulcer, and diarrhea. For example, SL-4 has been found to have clinical therapeutic effects against infantile diarrhea and to improve intestinal health[11-13]. Wang et al[14,15] reported that SL-4 was protective against acute liver damage induced by pyloric ligation (PL) and CCl4. To further evaluate the efficacy of SL-4, we investigated whether it had a protective effect on PL-induced gastroduodenal ulcer in a rat model. Additionally, combined microarray analysis and data mining were conducted to uncover the underlying molecular mechanisms of the gastroprotective effects of SL-4.
Male Sprague-Dawley rats (200 ± 20 g, 6 wk of age, SPF grade, batch no. SCXK 2015-0001) were purchased from Liaoning Chang-Sheng Biotechnology Co. The experimental procedures were approved by the Committee on the Ethics of Animal Experiments of Inner Mongolia University for Nationalities (approval no. NM-LL-2016-12-15-01), in accordance with the requirements and general guidelines of the Chinese Experimental Animals Administration Legislation. All surgery was conducted under sodium pentobarbital anesthesia, and every attempt was made to alleviate suffering.
Sixty male rats were housed in environmentally controlled conditions (22 ± 2 °C, relative humidity of 50% ± 5%) with a 12-h light/dark cycle and had free access to food and water for 7 d acclimatization. SL-4 was provided by the Drug Manufacturing section of the Affiliated Hospital of Inner Mongolia University for Nationalities (batch No.: 20190312). Rats were randomly divided into five groups (n = 12) that were treated by a sham operation, PL, and 1.3, 2.6, or 3.9 g/kg SL-4. Rats in the sham operation and PL groups were given 0.5% carboxymethylcellulose sodium (CMC-Na, Sigma). Rats in the three SL-4 groups were given 1.3, 2.6, or 3.9 g/kg SL-4 in 0.5% CMC-Na suspension by gastric lavage once daily. The rats in all five groups were pretreated for 15 consecutive days.
One hour after the last drug administration, all rats were fasted for 24 h with free access to water prior to ulceration. Each rat was anesthetized by intraperitoneal injection of pentobarbital for induction of PL-induced gastric ulcer as previously described[16]. The abdominal cavity was opened and the pylorus was ligated, avoiding injury of the adjacent blood vessels. The stomach was gently replaced and the belly was sutured. After being deprived of food and water for 18 h, the animals were killed and the stomach and duodenum were dissected for subsequent analysis.
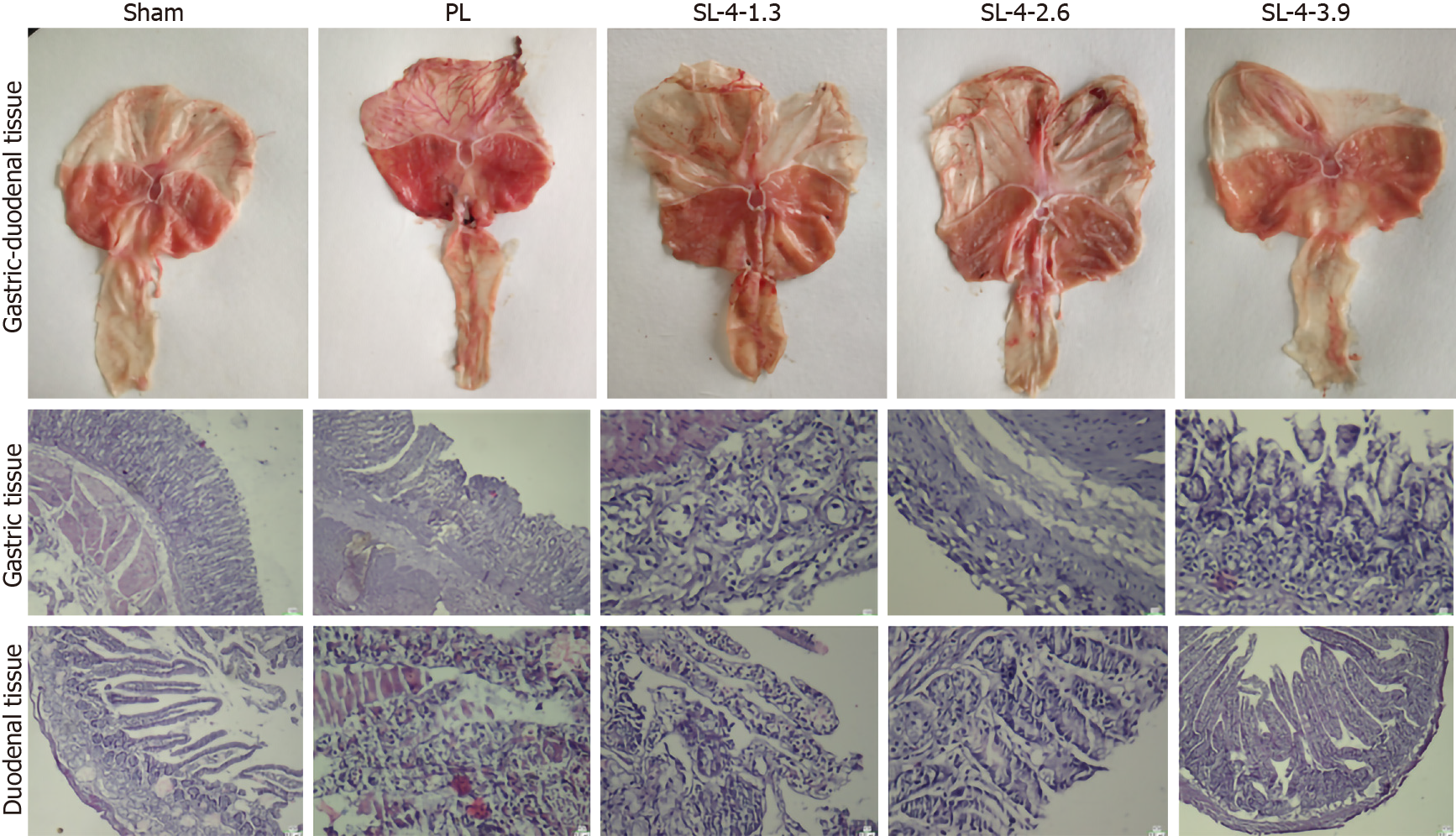
Rats were anesthetized intraperitoneally with sodium pentobarbital and killed. Gastric and duodenal sections were harvested for pathological examination. For histological examination, gastroduodenal ulcer tissue was fixed with 4% paraformaldehyde for 48 h; decalcified in 10% aqueous EDTA solution (Sigma-Aldrich, St. Louis, MO, United States) at room temperature and constant agitation, processed, and embedded in paraffin. The tissues were cut into 4 µm serial sections and dewaxed twice in xylene at 37 °C for 15 min; rehydrated through decreasing concentrations of ethanol, and washed in distilled water at room temperature for 5 min. The tissues were stained with hematoxylin and eosin (H&E, Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
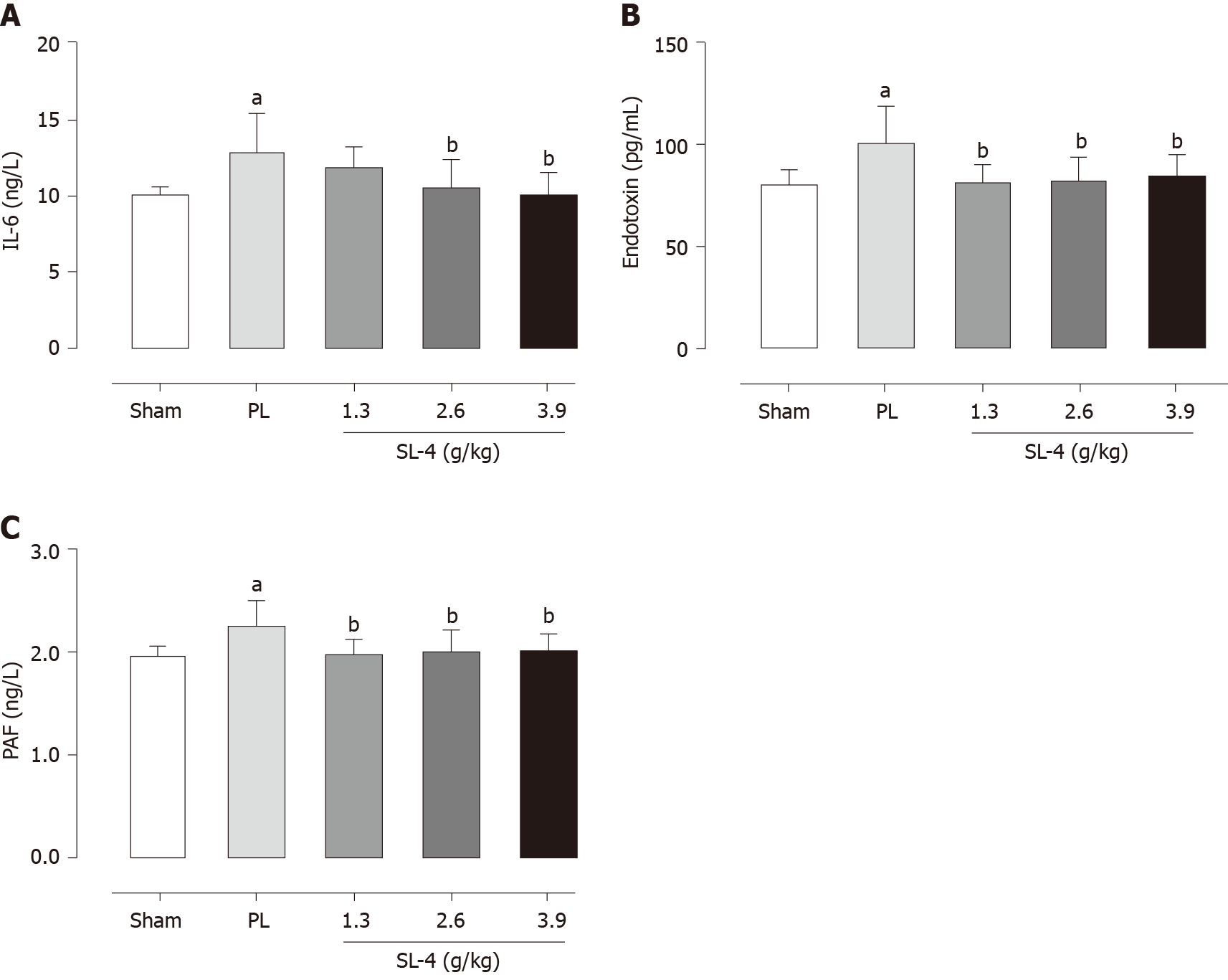
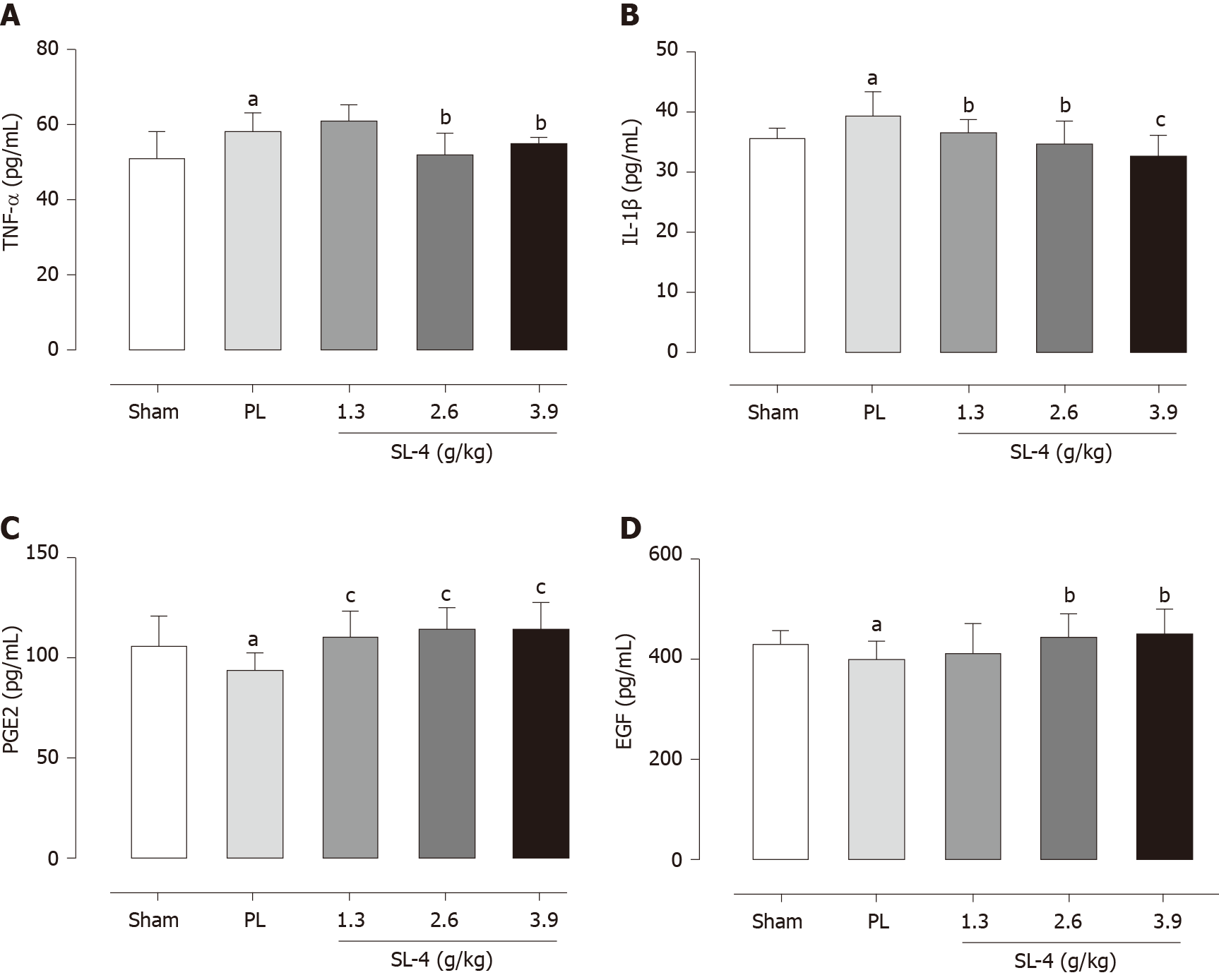
Gastroduodenal tissues were homogenized in Tris buffer on ice and centrifuged at 12 000 g at 4 °C for 10 min. The supernatants were used to determine the activities of endotoxin (ET), IL-6, and platelet-activating factor (PAF) in the duodenal tissue and TNF-α, prostaglandin E2 (PGE2), epidermal growth factor (EGF) and IL-1β in the gastric tissue. Enzyme activity was determined with commercial assay kits (Nanjing Jiancheng Bioengineering Institute).
Total RNA was extracted for microarray and qRT-PCR assay from gastric and duodenal tissue with TRIzol reagent (Invitrogen, Gaithersburg, MD, United States) and purified with mirVana miRNA Isolation Kits (Ambion, Austin, TX, United States). RNA was quantified with a Qubit fluorometer (Thermo Fisher Scientific, United States), and RNA integrity was assessed with a Bioanalyzer 2100 (Agilent, CA, United States). Only RNA extracts with an RNA integrity of > 6 were used in subsequent analyses.
Microarray hybridization, scanning, and analysis were performed by the Capital Biotechnology Corporation (Beijing, China). Using 100 ng total RNA, double-stranded cDNA containing the T7 RNA polymerase promoter sequence was prepared with CbcScript reverse transcriptase using a cDNA synthesis system (Capitalbio, Beijing, China) with the T7 Oligo(dT). cDNA labeled with a fluorescent dye (Cy5 or Cy3-dCTP) was produced by Eberwine’s linear RNA amplification method and subsequent enzymatic reactions. The procedure was improved by using CapitalBio cRNA Amplification and Labeling Kits to produce higher yields of labeled cDNA. The microarray slides were read with an Agilent G2565CA Microarray Scanner to obtain the microarray hybridization images. The array data were analyzed for data summation, normalization, and quality control with GeneSpring V13 (Agilent).
Chip signal intensities ≥ 400 were included for comparison. A two-class unpaired algorithm in the CapitalBio expression microarray analyzer software (CBC analyzer) was applied to identify differentially expressed genes in the sham operation and PL groups, and the PL and SL-4 groups in both gastric and duodenal tissues. Differentially expressed genes with fold-change ≥ 2.0 or ≤ 2 and P values ≤ 0.05 were evaluated. KEGG pathways were analyzed with a CaptitalBio Molecule Annotation System integrated with the KEGG database as previously described.
To validate the microarray results, qRT-PCR was used to validate the expression of the selected genes in the SL-4 (3.9 g/kg), PL and sham groups. The RNA samples used for qRT-PCR were the same samples from individual rats that made up the pools. One microgram of total RNA and an oligo(dT) primer were used for cDNA synthesis following the manufacturer’s protocol for SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, United States). Primers for the four genes chosen for verification were designed based on sequences published in GeneBank (Table 1). The validated genes were CYP2b2, UGT2b1, MASP1, and A2m. qRT-PCR was performed with an ABI PRISM 7900 sequence detection system (PE Applied Biosystems, Foster City, CA, United States) under the following conditions: 50 °C for 10 min, 40 cycles of 95 °C for 1 min, and 60 °C for 1 min. The program was ended with a melting curve from 72 °C to 95 °C and cooling to 40 °C. The GAPDH gene was used as a housekeeping control. Relative gene expression (ΔΔct) was calculated and graphed as a fold-change.
| Gene | Accession No. | Forward primer | Reverse primer |
| MASP1 | NM_022257.1 | CAACTACATCGGCGGCTACTACTG | GCTGGTGATTGTGCCTGTCCTC |
| A2m | NM_012488.2 | TCATCCAAGTCTGGTTCTTCTC | CCAGAACCATATACTGCGGT |
| CYP2b2 | NM_001198676.1 | GGAAGAACGGATTCAGGAGGAAGC | CTGTGATGCACTGGAAGAGGAAGG |
| UGT2b1 | NM_173295.1 | ATGTCATTCTCGCAGATGCTGTGG | ATAGGAAGGAGGCAGTGGAAGTCC |
Pharmacological data were analyzed using SPSS 17.0 and reported as means ± SD. Intergroup comparisons was conducted with t-tests, and P < 0.05 was taken to indicate a significant difference. Regarding the microarray, a CBC analyzer (expression profile analysis procedures based on R Bioconductor and PERL) was used for the analysis. To select the DEGs, the threshold values were ≥ 2 and ≤ 2 fold-change and a t-test P value of 0.05. The data were Log2 transformed and median centered by genes using the adjust data function of CLUSTER 3.0 software and further analyzed by hierarchical clustering with average linkage.
In the PL group, rats showed severe injury and inflammation of the gastroduodenal epithelium and edema of the submucosa. SL-4 improved these alterations dose-dependently, and showed less mucosal damage and milder inflammation than the PL group (Figure 1). H&E staining confirmed the pathological alterations of gastroduodenal tissues. The SL-4-treated groups showed a recovery trend toward normal histology and only superficial lesions. More precisely, the mucosal structure and the epithelium were intact, with normal glandular size, morphology, and distribution in the SL-4 3.9 g/kg group compared with PL untreated rats, which was essentially the same as the control sham group.
The levels of enzymes present in tissue homogenates were determined by enzyme-linked immunosorbent assays. SL-4 pretreatment significantly ameliorated elevation of the inflammatory factors TNF-α and IL-1β in rat gastric tissue and increased the expression of prostaglandin E2 (PGE2) and EGF hormones that inhibit gastric acid secretion compared with the PL group (Figure 2). In comparison, SL-4 treatment attenuated the expression of IL-6, ET and PAF in ulcerated duodenal tissue compared with the PL group in (Figure 3).
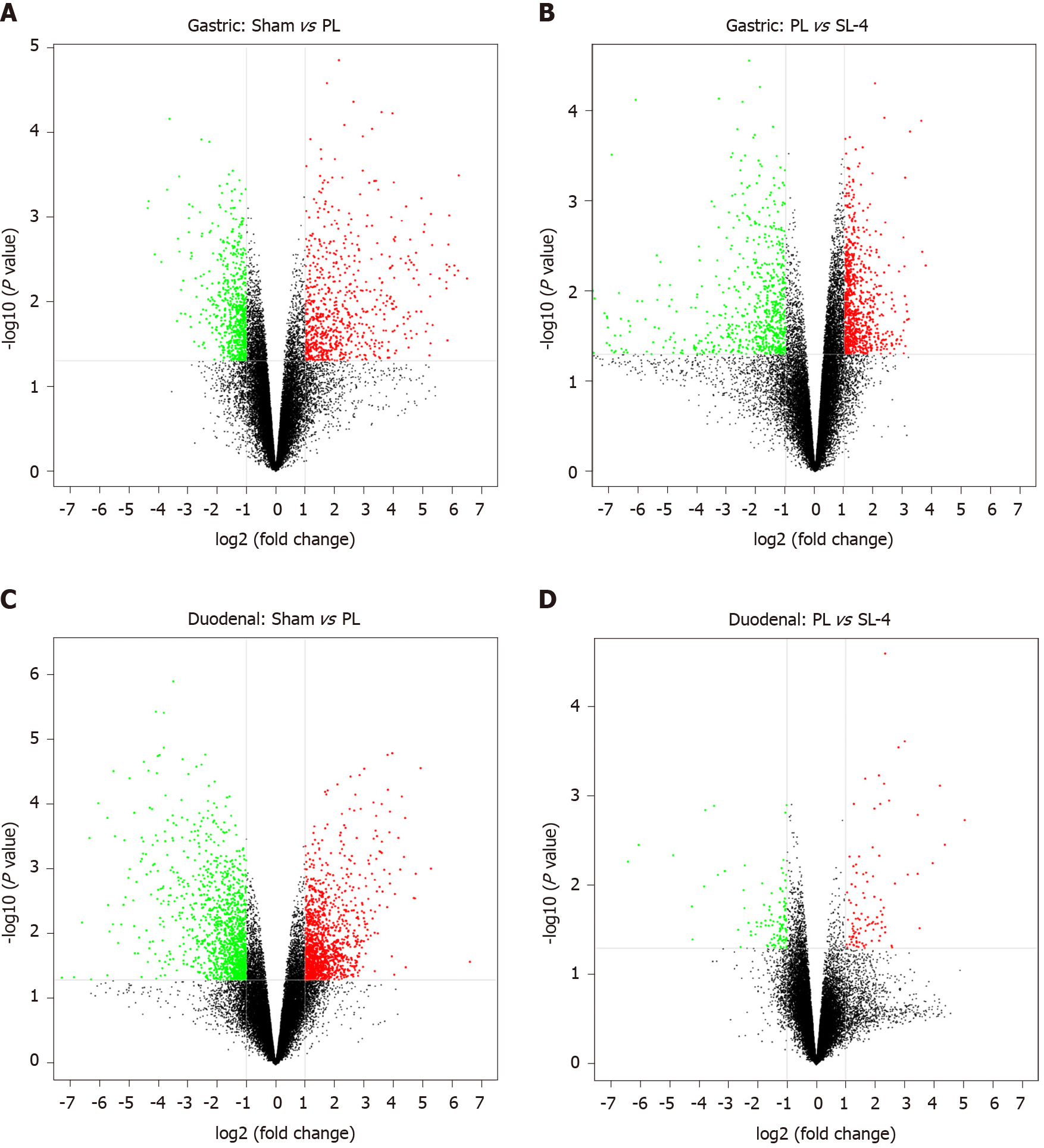
DEGs were identified in the ulcerated gastroduodenal tissues by an mRNA fold-change between two experimental groups of ≥ 2 and a P value ≤ 0.05. Volcano plot analysis showed that 1415 genes (731 upregulated and 784 downregulated) and 1460 genes (792 upregulated and 668 downregulated) were differentially expressed in gastric tissue from sham and PL, and PL and SL-4 groups (Figure 4A and B), and 2610 (1344 upregulated and 1266 downregulated) and 209 (100 upregulated and 109 downregulated) DEGs were identified in sham vs PL and PL vs SL-4 in the duodenal tissue (Figure 4C and D).
To define the biological pathways associated with PL and SL-4 treatment in rat gastroduodenal ulceration, we analyzed the microarray data using R software, which was based on the KEGG database. The top ten DEG-enriched pathways in the sham vs PL and PL vs SL-4 groups in gastric and duodenal tissue are summarized in Tables 2 and 3. In gastric tissue, four pathways were engaged in the crosstalk of sham vs PL and PL vs SL-4, including Staphylococcus aureus infection, retinol metabolism, cytokine-cytokine receptor interaction, and the hematopoietic cell lineage pathway. In duodenal tissue, among the top 10 KEGG pathways in the sham vs PL and PL vs SL-4, two were the same in both groups, which were the complement and coagulation cascade pathways and S. aureus infection pathway.
| Treatment | KEGG pathway | P value |
| Sham vs PL | Staphylococcus aureus infection | 1.83 × 10-5 |
| Cytokine-cytokine receptor interaction | 2.93 × 10-4 | |
| Renin-angiotensin system | 7.29 × 10-4 | |
| Inflammatory bowel disease | 8.59 × 10-4 | |
| Rheumatoid arthritis | 1.52 × 10-3 | |
| Retinol metabolism | 1.57 × 10-3 | |
| Amoebiasis | 1.59 × 10-3 | |
| Mineral absorption | 1.75 × 10-3 | |
| Hematopoietic cell lineage | 2.35 × 10-3 | |
| Ascorbate and aldarate metabolism | 2.51 × 10-3 | |
| PL vs SL-4 | Complement and coagulation cascades | 2.59 × 10-11 |
| Staphylococcus aureus infection | 5.95 × 10-8 | |
| Cytokine-cytokine receptor interaction | 2.06 × 10-7 | |
| Steroid hormone biosynthesis | 8.49 × 10-6 | |
| Hematopoietic cell lineage | 3.10 × 10-5 | |
| Malaria | 6.08 × 10-5 | |
| Chemical carcinogenesis | 1.22 × 10-4 | |
| Metabolism of xenobiotics by cytochrome P450 | 1.28 × 10-4 | |
| Pertussis | 1.99 × 10-4 | |
| Retinol metabolism | 2.25 × 10-4 |
| Treatment | KEGG pathway | P value |
| Shame vs PL | Complement and coagulation cascades | 3.93 × 10-9 |
| Staphylococcus aureus infection | 4.53 × 10-9 | |
| Cytokine-cytokine receptor interaction | 6.48 × 10-7 | |
| Hematopoietic cell lineage | 9.84 × 10-6 | |
| Rheumatoid arthritis | 2.78 × 10-5 | |
| Osteoclast differentiation | 4.40 × 10-5 | |
| PI3K-Akt signalling pathway | 2.13 × 10-4 | |
| Tuberculosis | 2.89 × 10-4 | |
| ECM-receptor interaction | 3.02 × 10-4 | |
| Amoebiasis | 3.37 × 10-4 | |
| PL vs SL-4 | Maturity onset diabetes of the young | 2.10 × 10-7 |
| Complement and coagulation cascades | 7.39 × 10-6 | |
| Staphylococcus aureus infection | 7.99 × 10-5 | |
| Regulation of autophagy | 2.26 × 10-3 | |
| Type II diabetes mellitus | 8.47 × 10-3 | |
| Longevity regulating pathway—multiple species | 1.51 × 10-2 | |
| Phototransduction | 2.05 × 10-2 | |
| Mucin type O-Glycan biosynthesis | 2.35 × 10-2 | |
| Porphyrin and chlorophyll metabolism | 3.51 × 10-2 | |
| Longevity regulating pathway | 4.01 × 10-2 |
According to the rat phenotype, retinol metabolism in the gastric tissue and complement and coagulation cascade pathways in the duodenal tissue were both closely associated with inflammatory responses and gastric mucosal protection mechanisms. Twelve genes were found to have > 2-fold expression and P values ≤ 0.05 in the two pathways. Gene expression pattern variations had the same trends in the PL and SL-4 groups. More precisely, when gene expression associated with retinol metabolism was upregulated in the PL group, SL-4 downregulated the same genes relative to the PL group. In contrast, the pathways for certain genes in the complement and coagulation cascade were downregulated in the PL group and upregulated in the SL-4 group (Table 4).
| Tissue pathway | Gene symbol | Gene name | Sham vs PL | PL vs SL-4 | GeneBank accession No. |
| Gastric (retinol metabolism) | UGT2b1 | UDP glucuronosyl transferase 2 family, polypeptide B1" | +13.71 | -30.56 | NM_173295 |
| SDR16c5 | Short chain dehydrogenase/reductase family 16C, member 5 | +7.16 | +3.12 | NM_001106634 | |
| RDH7 | Retinol dehydrogenase 7 | +5.89 | -45.38 | NM_133543 | |
| CYP2b2 | Cytochrome P450, family 2, subfamily b, polypeptide 2 | +5.57 | -12.51 | NM_001198676 | |
| CYP4a1 | Cytochrome P450, family 4, subfamily a, polypeptide 1 | +3.93 | -83.27 | NM_175837 | |
| CYP1a1 | Cytochrome P450, family 1, subfamily a, polypeptide 1 | -3.47 | +1.86 | NM_012540 | |
| Duodenal (complement and coagulation cascades) | A2m | Alpha-2-macroglobulin | -20.37 | +33.00 | NM_012488 |
| FGG | Fibrinogen gamma chain | -41.01 | +15.50 | NM_012559 | |
| FGA | Fibrinogen alpha chain | -23.51 | +6.49 | NM_001008724 | |
| MASP1 | Mannan-binding lectin serine peptidase 1 | -11.09 | +3.19 | AY149996 | |
| VSIG4 | V-set and immunoglobulin domain containing 4 | -6.37 | +3.26 | NM_001025004 | |
| CFB | Complement factor B | +3.08 | -5.43 | NM_212466 |
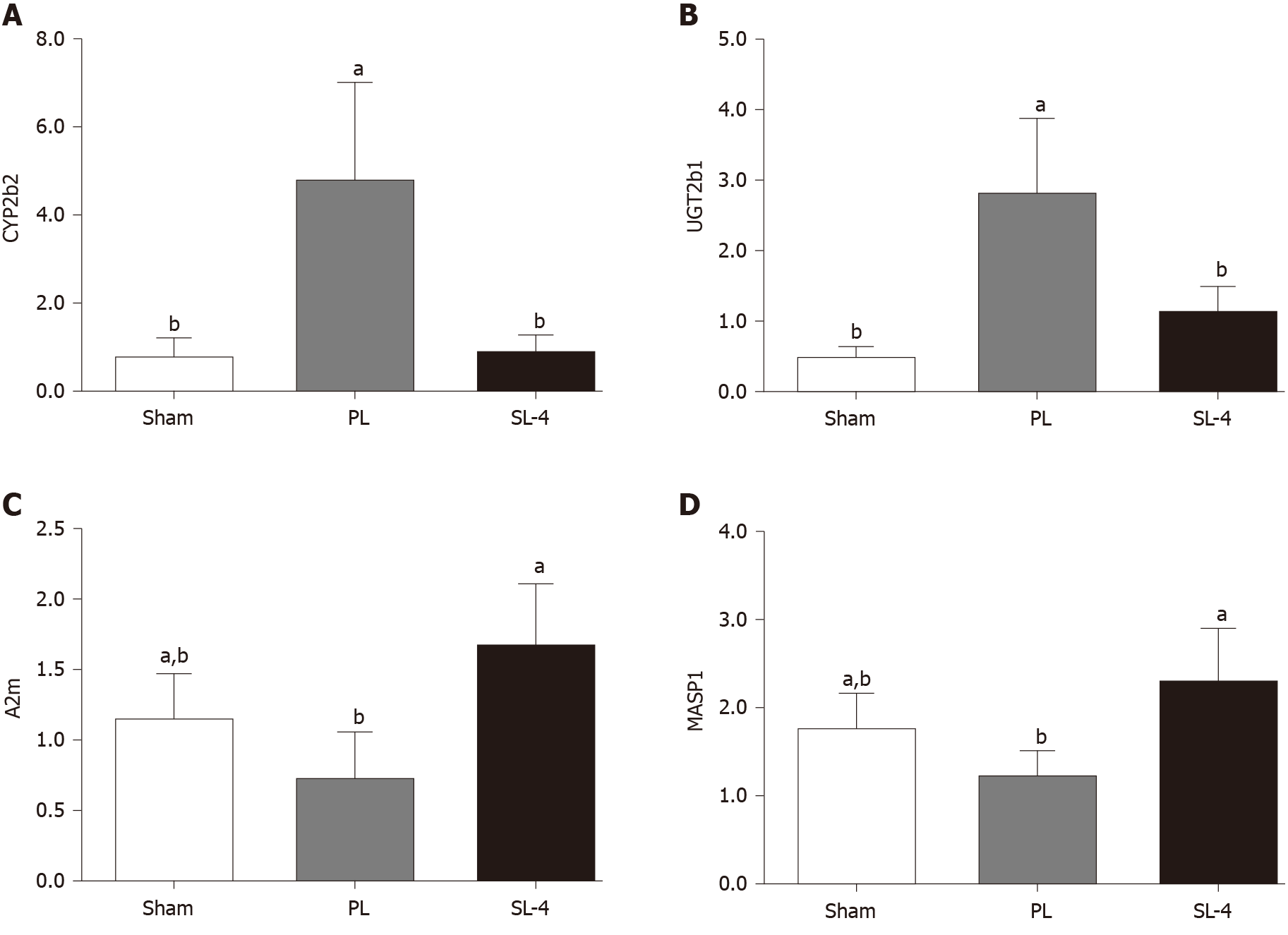
The SL-4-mediated increase or decrease in CYP2b2 and UGT2b1 in the retinol metabolism pathway, and A2m and MASP1 in the complement and coagulation cascades pathway were verified by qRT-PCR. Changes in the expression patterns of the four selected genes were consistent with the microarray results (Figure 5). Expression of CYP2b2 and UGT2b1 mRNA were increased in the PL group and decreased by SL-4 treatment. In contrast, expression of A2m and MASP1 were decreased in the PL group and increased in the SL-4 group.
In Mongolian folk medicine, SL-4 is traditionally used to treat peptic ulcers and gastroenteritis. Previous studies have shown that SL-4 has therapeutic effects against infantile diarrhea and improves intestinal health[13-15]. However, the anti-ulcerogenic effect of SL-4 and its pharmacological mechanism have not been well investigated. Thus, we aimed to study whether SL-4 had a protective effect on PL-induced gastroduodenal ulcers in a rat model and examined its molecular mechanism by microarray analysis.
PL is a well-established methodology to develop gastric ulceration in rats[16]. PL induces accumulation of gastric acid and pepsin, resulting in breakdown of the gastric mucosal barrier and causes inflammatory injury, which are key drivers of gastric ulcer formation[17]. To protect against inflammation, the cells respond with attenuated production of proinflammatory cytokines such as IL-12, TNF-α, IL-1, IL-6, and IFN[18]. Our findings show that SL-4 significantly improved the protective effects against gastroduodenal mucosal hemorrhage and inflammation, as confirmed by histopathology. Additionally, inflammatory factors such as IL-6, TNF-α and IL-1β were significantly reduced by SL-4 in contrast to the PL group, which indicates that the antiulcer effects of SL-4 can be attributed to anti-inflammatory activity. PAF, a potent phospholipid mediator of leukocyte activation, is a potent mediator of endogenous ulcer formation[19]. ET is a hormone secreted by the endothelial cells of blood vessels that can also promote ulceration[18]. Our data showed that SL-4 suppressed expression of PAF and ET in the gastroduodenal tissues in PL model mice in a dose-dependent manner, suggesting an inhibitory role for SL-4 in the activation of endothelial cells during ulcer maintenance. Additionally, SL-4 increased expression of PGE2 and EGF in gastroduodenal tissue, which indicates that it may effectively inhibit the secretion of gastric acid, enhance the gastric mucosal defensive function, promote the repair of gastric mucosal injury, and reduce recurrence rate of gastric ulcer[20]. A previous study reported that PGE2 was responsible for the production and maintenance of the cellular integrity of the gastric mucosa, and that depletion of PGE2 could cause gastric ulcers[21]. Histological evaluation showed that SL-4 reduced mean ulcer size and minimized ulcer formation, congestion, inflammation, hemorrhage, and necrosis of the gastric mucosa. Together, these findings demonstrated the antiulcer effect of SL-4 in PL rats.
Forsythia suspensa is a major herbal component of SL-4. It has been reported that its active ingredients forsythiaside and forsythin exert potent anti-inflammatory and antibacterial activities, which may have possible use as antiulcer agents[22]. A number of medicinal plants have been reported useful for the treatment of gastrointestinal disorders. Wu et al[23] reported that a Chinese medicinal herb, Pogostemon cablin, and its main active compound β-patchoulene profoundly inhibited ulcer formation by reducing the inflammatory response and improving angiogenesis. Kumar et al[24] studied the antiulcer activity of an Indian medicine Cedrus deodara and found that its volatile oil had a potent gastroprotective effect on ulceration and inflammation in PL- and ethanol-treated rats. Al-Wajeeh et al[25] reported that a Malaysian medicine Cibotium barometz hair protected against ethanol-induced ulceration through regulation of antioxidant enzymes and gastric secretory actions. Consistent with those findings, our data confirmed that the gastroprotective effects of SL-4 components primarily work through modulation of inflammatory and gastric secretory mechanisms.
Microarray techniques are crucial for understanding and interpretating the activity of traditional medicines, particularly in revealing their mechanisms of action and drug targets[26]. To investigate the underlying molecular mechanisms of SL-4, we determined the gene expression profiles of gastric and duodenal tissues obtained from rats with PL-induced ulcers with or without SL-4 treatment. Retinol metabolism was one of the most significantly modified pathways in gastric tissue after SL-4 treatment. Retinoic acid (RA) intake and metabolism change dramatically during the acute-phase response (APR) to inflammation[27]. The APR is a metabolic system responding to infection, trauma, or tissue injury, and the PL method used in this study may have caused a strong APR in the intestinal tract. Inflammation drives decreased retinol usage by a reduction of retinol-binding protein synthesis[28]. RA increases the synthesis of inflammatory cytokines including transforming growth factor (TGF)-β, IL-10, IL-4, IL-5, and IL-6, and enhances white blood cell production[29]. Microarray analysis found that SL-4 decreased the expression of key transcriptomes associated with retinol metabolism. RA concentration is controlled by glucuronosyltransferases, including UGT2b1, and P450 cytochromes including CYP26A1, CYP26B1, and CYP26C1. These subfamilies catalyze RA oxidation to forms including 5,8-epoxy RA, 4-oxo RA, 4-hydroxy RA, and 18-hydroxy RA[30,31]. Thus, decreased enzyme activation blocks RA accumulation in the other areas and maintains a suitable physiological RA concentration to achieve the anti-inflammatory function of the gastrointestinal tract. Therefore, SL-4 can enhance the role of RA in maintaining homeostasis at the intestinal barrier and equilibrating immunity and tolerance. Together with vitamin A supplementation, SL-4 treatment may improve the protective effect against gastric ulcers in a clinical setting. The current results also suggest that SL-4 accelerates the anti-inflammatory effects of retinol metabolism. This further indicates the therapeutic effect of SL-4 on inflammatory bowel disease and other clinical inflammatory symp
Additionally, KEGG analysis found significant differences in the complement and coagulation cascade pathways that could have been caused by an inflammatory response. Damage of the intestinal tract activates the coagulation cascade. Fibrin formation provides a physical barrier that can be a potential shield from inflammation[32]. Our microarray results indicated that SL-4 elevated the transcriptome expression of A2m, which acts as a carrier protein to bind numerous growth factors and cytokines, such as platelet-derived growth factor, basic fibroblast growth factor, TGF-β and IL-1β, thus inhibiting inflammation[33]. MASP1 is a serine protease that functions as a component of the lectin pathway of complement systems, which plays a pivotal role in the defense against infection[34]. Elevation of MASP1 expression by SL-4 may be responsible for improving the immune activity and defense against gastric infections. The above results suggest that SL-4 may have several active substances that promote anti-inflammatory responses that prevent peptic ulcer formation via retinol metabolism and the complement and coagulation systems.
S. aureus infection was found in both gastric and duodenal tissues, which indicated that PL activated the immune system against S. aureus infection. Although our KEGG analysis did not find a direct protective effect, antimicrobial activity for SL-4 has been reported[12]. The intestinal immune system plays a vital role in the prevention of and defense against inflammation caused by harmful pathogens. In our study, PL induced an increase in IL-1β secretion in gastric tissues. Although the IL-1β-mediated immune response to S. aureus has not been confirmed, IL-1β is a key cytokine in orchestrating host defenses against the organism[35]. Therefore, the effect of SL-4 on bacterial infection of the intestinal tract should be investigated in a future study.
Our data demonstrate that SL-4 protected against PL-induced gastroduodenal ulceration by reducing inflammatory cytokines and increasing the expression of gastric acid inhibitory factors. We propose that regulation of CYP2b2 and UGT2b1 gene expression in the retinol metabolism pathway and A2m and MASP1 genes in the complement and coagulation cascade pathways are may be involved in mechanism by which SL-4 protects against gastroduodenal ulcers. Most notable are the effects of SL-4 on the transcriptome involved in retinol metabolism, which has not been described previously. The Mongolian folk medicine SL-4 is a promising gastroprotective agent with potential use for treating gastric ulcers in clinical practice.
Sulongga-4 (SL-4) is a classic herbal formula used in traditional Mongolian medical clinics for the treatment of peptic ulcers and gastroenteritis, even though its pharmacological mechanism has not been well characterized.
The study objective was to identify the protective effect and mechanism of SL-4 against peptic ulcer disease.
To evaluate the protective effect and identify the mechanisms of action of SL-4 on gastroduodenal ulcer induced by pyloric ligation (PL) in rats.
PL was performed to induce gastric and duodenal ulcers in rats that were then treated with oral SL-4 (1.3, 2.6, or 3.9 g/kg per day) for 15 d. PL-induced gastroduodenal ulceration. Therapeutic effects were evaluated by pathological and histological evaluation. Inflammatory indicators were analyzed by enzyme-linked immunosorbent assay. Microarray analyses were conducted to determine the gene expression profiles of gastroduodenal tissue in PL rats with or without SL-4 treatment. The candidate target genes were selected and verified by quantitative reverse transcription polymerase chain reaction (qRT-PCR).
SL-4 improved the histopathology of the PL-induced ulcers. SL-4 significantly (P < 0.05) decreased the expression of tumor necrosis factor-α, interleukin (IL)-1β, IL-6, endotoxin, PAF, and increased prostaglandin E2 and EGF in ulcer tissue. Microarray analysis was used to identify a list of candidate target genes for SL-4 acting on PL-induced ulceration. The genes included some that modulate complement and coagulation cascade pathways, and retinol metabolism pathways that are closely associated with inflammatory responses and gastric mucosal protective mechanisms. qRT-PCR showed that altered expression of the selected genes, such as CYP2b2, UGT2b1, A2m, and MASP1, was consistent with the microarray results.
SL-4 exerted protective effects against PL-induced gastroduodenal ulcers via reducing inflammatory cytokines and elevating the expression of gastric acid inhibitory factors. Downregulation of the CYP2b2 and UGT2b1 genes in retinol metabolism and upregulation of the A2m and MASP1 genes in the complement and coagulation cascade pathways may have been involved in the protection against gastroduodenal ulcers.
SL-4, a Mongolian folk medicine, is a promising gastroprotective agent with potential clinical use as a treatment of gastric ulcers.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carri JH, Nakajima N, Shelat VG S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 561] [Article Influence: 70.1] [Reference Citation Analysis (37)] |
| 2. | Chung KT, Shelat VG. Perforated peptic ulcer - an update. World J Gastrointest Surg. 2017;9:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 183] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (5)] |
| 3. | Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N, Whorwell P; Clinical Services Committee of The British Society of Gastroenterology. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770-1798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 609] [Cited by in RCA: 538] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 4. | Aziz RS, Siddiqua A, Shahzad M, Shabbir A, Naseem N. Oxyresveratrol ameliorates ethanol-induced gastric ulcer via downregulation of IL-6, TNF-α, NF-ĸB, and COX-2 Levels, and upregulation of TFF-2 Levels. Biomed Pharmacother. 2019;110:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 1015] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 6. | Scully P, McKernan DP, Keohane J, Groeger D, Shanahan F, Dinan TG, Quigley EM. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol. 2010;105:2235-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Beg S, Swain S, Hasan H, Barkat MA, Hussain MS. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacogn Rev. 2011;5:120-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Kuna L, Jakab J, Smolic R, Raguz-Lucic N, Vcev A, Smolic M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 9. | Bi WP, Man HB, Man MQ. Efficacy and safety of herbal medicines in treating gastric ulcer: a review. World J Gastroenterol. 2014;20:17020-17028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 10. | Ba DRH, Li J. Encyclopedia of Mongolian studies: medical science. 1st ed. Huhhot: IM People's Publishing House, 2012: 253-254. |
| 11. | Ge RL. Traditional Mongolian medicine treatment of infantile diarrhea. Menggu Chuantong Yiyao Zazhi. 1995;1:24-25. |
| 12. | Gang B, Tu Y. A comparative study on the bacterial inhibitory effect of Sulongga-4, Saorilao-4 decoction and Yongwa-4 decoction. Zhongguo Minzu Yiyao Zazhi. 2005;11:21-22. |
| 13. | Qi WM, Wu L, Bai YH. Effect of Sulongga-4 decoction on the intestinal villus epithelial cell movement and goblet cells in diarrhea young rats. Zhongguo Minzu Yiyao Zazhi. 2015;21:61-63. |
| 14. | Wang H, Tong S, Xiao M, Wang BGL, Wang TY. A study on the protective effect of traditional Mongolian medicine Lianqiao-4 decoction on pyloric ligation-induced liver damage. Zhongguo Shiyan Fangjixue Zazhi. 2013;20:238-241. |
| 15. | Wang H, Bai MR, Bao ML, Wang TY, Ba GN. A study on screening for effective sites for the liver-protecting and enzyme-lowering effects of traditional Mongolian medicine Lianqiao-4 decoction in CCl4-induced acute liver damage. Zhongguo Mianyixue Zazhi. 2014;20:45-46. |
| 16. | Zhang SL, Li H, He X, Zhang RQ, Sun YH, Zhang CF, Wang CZ, Yuan CS. Alkaloids from Mahonia bealei posses anti-H⁺/K⁺-ATPase and anti-gastrin effects on pyloric ligation-induced gastric ulcer in rats. Phytomedicine. 2014;21:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Wang XY, Yin JY, Zhao MM, Liu SY, Nie SP, Xie MY. Gastroprotective activity of polysaccharide from Hericium erinaceus against ethanol-induced gastric mucosal lesion and pylorus ligation-induced gastric ulcer, and its antioxidant activities. Carbohydr Polym. 2018;186:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 18. | Liu D, Cao S, Zhou Y, Xiong Y. Recent advances in endotoxin tolerance. J Cell Biochem. 2019;120:56-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Jeong YI, Jung ID, Lee CM, Chang JH, Chun SH, Noh KT, Jeong SK, Shin YK, Lee WS, Kang MS, Lee SY, Lee JD, Park YM. The novel role of platelet-activating factor in protecting mice against lipopolysaccharide-induced endotoxic shock. PLoS One. 2009;4:e6503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Fang YF, Xu WL, Wang L, Lian QW, Qiu LF, Zhou H, Chen SJ. Effect of Hydrotalcite on Indometacin-Induced Gastric Injury in Rats. Biomed Res Int. 2019;2019:4605748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Ercan G, Ilbar Tartar R, Solmaz A, Gulcicek OB, Karagulle OO, Meric S, Cayoren H, Kusaslan R, Kemik A, Gokceoglu Kayali D, Cetinel S, Celik A. Potent therapeutic effects of ruscogenin on gastric ulcer established by acetic acid. Asian J Surg. 2020;43:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Qu H, Zhang Y, Wang Y, Li B, Sun W. Antioxidant and antibacterial activity of two compounds (forsythiaside and forsythin) isolated from Forsythia suspensa. J Pharm Pharmacol. 2008;60:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Wu JZ, Liu YH, Liang JL, Huang QH, Dou YX, Nie J, Zhuo JY, Wu X, Chen JN, Su ZR, Wu QD. Protective role of β-patchoulene from Pogostemon cablin against indomethacin-induced gastric ulcer in rats: Involvement of anti-inflammation and angiogenesis. Phytomedicine. 2018;39:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Kumar A, Singh V, Chaudhary AK. Gastric antisecretory and antiulcer activities of Cedrus deodara (Roxb.) Loud. in Wistar rats. J Ethnopharmacol. 2011;134:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Al-Wajeeh NS, Hajerezaie M, Noor SM, Halabi MF, Al-Henhena N, Azizan AH, Kamran S, Hassandarvish P, Shwter AN, Karimian H, Ali HM, Abdulla MA. The gastro protective effects of Cibotium barometz hair on ethanol-induced gastric ulcer in Sprague-Dawley rats. BMC Vet Res. 2017;13:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Kiyama R. DNA Microarray-Based Screening and Characterization of Traditional Chinese Medicine. Microarrays (Basel). 2017;6:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Couch Y, Akbar N, Roodselaar J, Evans MC, Gardiner C, Sargent I, Romero IA, Bristow A, Buchan AM, Haughey N, Anthony DC. Circulating endothelial cell-derived extracellular vesicles mediate the acute phase response and sickness behaviour associated with CNS inflammation. Sci Rep. 2017;7:9574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Gieng SH, Raila J, Rosales FJ. Accumulation of retinol in the liver after prolonged hyporetinolemia in the vitamin A-sufficient rat. J Lipid Res. 2005;46:641-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Akanda MR, Kim IS, Ahn D, Tae HJ, Nam HH, Choo BK, Kim K, Park BY. Anti-Inflammatory and Gastroprotective Roles of Rabdosia inflexa through Downregulation of Pro-Inflammatory Cytokines and MAPK/NF-κB Signaling Pathways. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, Rossant J, Hamada H. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 2001;15:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 351] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 31. | Takeuchi H, Yokota A, Ohoka Y, Iwata M. Cyp26b1 regulates retinoic acid-dependent signals in T cells and its expression is inhibited by transforming growth factor-β. PLoS One. 2011;6:e16089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J Thromb Haemost. 2007;5 Suppl 1:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Mocchegiani E, Malavolta M. Zinc dyshomeostasis, ageing and neurodegeneration: implications of A2M and inflammatory gene polymorphisms. J Alzheimers Dis. 2007;12:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Beltrame MH, Boldt AB, Catarino SJ, Mendes HC, Boschmann SE, Goeldner I, Messias-Reason I. MBL-associated serine proteases (MASPs) and infectious diseases. Mol Immunol. 2015;67:85-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Gutierrez Jauregui R, Fleige H, Bubke A, Rohde M, Weiss S, Förster R. IL-1β Promotes Staphylococcus aureus Biofilms on Implants in vivo. Front Immunol. 2019;10:1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |