Published online Apr 28, 2021. doi: 10.3748/wjg.v27.i16.1751
Peer-review started: January 23, 2021
First decision: February 10, 2021
Revised: February 18, 2021
Accepted: March 25, 2021
Article in press: March 25, 2021
Published online: April 28, 2021
Processing time: 87 Days and 10.9 Hours
Gastroesophageal reflux disease (GERD) is one of the most commonly encountered digestive diseases in the world, with the prevalence continuing to increase. Many patients are successfully treated with lifestyle modifications and proton pump inhibitor therapy, but a subset of patients require more aggressive intervention for control of their symptoms. Surgical treatment with fundoplication is a viable option for patients with GERD, as it attempts to improve the integrity of the lower esophageal sphincter (LES). While surgery can be as effective as medical treatment, it can also be associated with side effects such as dysphagia, bloating, and abdominal pain. Therefore, a thorough pre-operative assessment is crucial to select appropriate surgical candidates. Newer technologies are becoming increasingly available to help clinicians identify patients with true LES dysfunction, such as pH-impedance studies and high-resolution manometry (HRM). Pre-operative evaluation should be aimed at confirming the diagnosis of GERD, ruling out any major motility disorders, and selecting appropriate surgical candidates. HRM and pH testing are key tests to consider for patients with GERD like symptoms, and the addition of provocative measures such as straight leg raises and multiple rapid swallows to HRM protocol can assess the presence of underlying hiatal hernias and to test a patient’s peristaltic reserve prior to surgery.
Core Tip: The goal of this review is to discuss recent technological advancements that have utility for patients with gastroesophageal reflux disease (GERD) as a pre-operative assessment for anti-reflux surgery. Surgical treatment of GERD is centered around improving the integrity of the lower esophageal sphincter, therefore it is crucial to rule out other esophageal pathologies that may present with GERD-like symptoms. Advances in pH-impedance studies allow for assessment of patients with weak acid reflux of non-erosive reflux disease. High resolution manometry with the addition of provocative measures can uncover underlying esophageal motility disorders with GERD-like symptoms.
- Citation: Yodice M, Mignucci A, Shah V, Ashley C, Tadros M. Preoperative physiological esophageal assessment for anti-reflux surgery: A guide for surgeons on high-resolution manometry and pH testing. World J Gastroenterol 2021; 27(16): 1751-1769
- URL: https://www.wjgnet.com/1007-9327/full/v27/i16/1751.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i16.1751
Gastroesophageal reflux disease (GERD), defined as an abnormal reflux of gastric contents with associated symptoms, is one of the most common digestive diseases in the world, and currently affects up to 30% of individuals in North America[1,2]. While the disease is commonly encountered in the outpatient setting, the true number of individuals with GERD could be even higher, as some patients may self-treat with over-the-counter medications[3]. Studies have also found the number of patients with GERD continues to rise, which may be due to the growing obesity epidemic[1]. Additionally, GERD is one of the costliest digestive diseases in the United States, with up to half of the cost attributed to the long term use of proton pump inhibitor (PPI) therapy[4].
The American College of Gastroenterology (ACG) guidelines state that a presumptive diagnosis of GERD can be made in patients with classical symptoms such as heartburn or regurgitation, with further diagnostic evaluation such as an upper endoscopy recommended for patients with alarm symptoms[5]. The ACG recommends management of these patients begin with lifestyle modifications, including weight loss and dietary changes, along with an 8-week course of PPI therapy[5]. While this approach is effective for most patients, a subset of patients may require further management including surgical options such as fundoplication. Surgical treatment of GERD is as effective as medical management in appropriate candidates and is currently recommended for reasons including complications from PPIs, medication non-compliance, and large hiatal hernias[3,5]. Additionally, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recommends surgical therapy for patients with GERD who: have failed medical management, request surgery due to quality of life issues related to long term medication use, have complications such as Barrett’s esophagus, or have extra-esophageal symptoms related to their GERD[6].
While surgical treatment of GERD may be as effective as medical therapy, complications are also possible. The most commonly reported side effects of surgery include dysphagia, belching, and increased abdominal bloating, though it is also possible to develop more severe complications requiring repeat surgery[7]. A total 360° Nissen Fundoplication is the most common surgical treatment for GERD, but variations also exist involving differing degrees of wrapping. Studies have found partial fundoplication to be similarly effective for reducing reflux symptoms, and may also have less post-operative dysphagia and bloating[8]. This is supported by a level 1 recommendation from the SAGES that partial fundoplication is associated with less dysphagia and similar patient satisfaction[6]. Additionally, newer techniques such as the Linx procedure [which uses a circlet of magnets to augment the lower esophageal sphincter (LES)] have shown similar short-term outcomes for the treatment of GERD when compared to fundoplica
While surgical treatment of GERD can successfully relieve symptoms in up to 90% of patients[10], a thorough pre-operative assessment is necessary to identify appropriate surgical candidates. The underlying mechanism of anti-reflux surgery is to improve the integrity of the LES. Therefore, in order to achieve the best post-operative outcomes, it is crucial to confirm a patient’s symptoms are due to GERD and not another underlying pathology. Otherwise, a patient would likely continue experiencing symptoms post-operatively along with potentially developing complications such as dysphagia.
Newer technologies are becoming increasingly available for the assessment of patients with reflux symptoms and can help confirm the diagnosis of GERD. Improvements in pH studies with the inclusion of impedance measurements may help identify patients with non-erosive reflux disease (NERD). Additionally, advancements in high resolution manometry (HRM) help further classify esophageal pathologies and identify patients as having disorders with GERD like symptoms (Table 1). In this review, we aim to provide a guide for the pre-operative assessment and evaluation of patients with reflux. We will focus on newer technologies that can be used to identify appropriate candidates for anti-reflux surgery by confirming the diagnosis of GERD and ruling out other esophageal diseases.
| Diagnosis | Definition | Clinical symptoms | Pathophysiology | Diagnostic evaluation |
| Structural disorders | ||||
| GERD | Symptoms and complications secondary to the reflux of gastic contents above the lower esophageal sphincter[5] | Regurgitation, reflux, dysphagia, retrosternal non-cardiac chest pain, globus sensation, extra esophageal symptoms | Abnormal transient LES relaxation, LES dysfunction secondary to anatomic abnormality such as hiatal hernia | Upper endoscopy, high resolution manometry, ambulatory pH testing, ambulatory impedance testing |
| Weak acid reflux | Symptoms secondary to reflux of gastric contents above the LES with pH ranging from 4-7[32] | Reflux, regurgitation, non-cardiac chest pain | Persistent reflux with pH from 4-7 due to transient LES relaxation | pH studies - on maximum PPI therapy |
| Eosinophilic esophagitis | Presence of symptoms of esophageal dysfunction such as reflux or dysphagia, eosinophilic inflammation on esophageal biopsy with ≥ 15 eosinophils per high power field, and exclusion of other disorders with similar presentations[81] | Dysphagia, reflux, non-cardiac chest pain | Eosinophil mediated inflammatory response in the esophagus secondary to allergenic antigens | Upper endoscopy with biopsy |
| Motility disorders | ||||
| Achalasia | Elevated IRP > 15 mmHg and absence of normal peristalsis[44] | Dysphagia, regurgitation, non-cardiac chest pain | Failure of LES relaxation and absence of normal peristalsis | High resolution manometry, upper endoscopy, barium studies |
| Absent peristalsis | Systemic symptoms with aperistalsis with failed peristalsis on 100% of swallows[49] | Reflux, dysphagia, non-cardiac chest pain | Lower esophageal collagen deposition leading to LES dysfunction | High resolution manometry, autoimmune antibody workup |
| Distal esophageal spasm | Normal IRP and ≥ 20% premature contractions with DCI > 450 mmHg[44] | Dysphagia, regurgitation, reflux, non-cardiac chest pain | Impaired inhibition and coordination of esophageal muscle contraction | High resolution manometry, Barium swallow “corkscrew esophagus” |
| Hypercontractile esophagus | Minimum of 2 swallows with DCI > 8000 mmHg[44] | Retrosternal non-cardiac chest pain, dysphagia, regurgitation | Increased contraction of esophageal smooth muscle | Upper endoscopy, barium studies, high resolution manometry |
| Esophagogastric junction outflow obstruction | Elevated median IRP > 15 mmHg with evidence of peristalsis on swallows[44] | Dysphagia, reflux, regurgitation | Impairment of esophagogastric junction relaxation with normal or weakened esophageal peristalsis | High resolution manometry, needs to be confirmed with further studies such as barium swallow or endoflip, must rule out artifact that can be seen with a hiatal hernia |
| Opioid induced esophageal dysfunction | Presence of symptoms of esophageal dysfunction with manometric evidence of esophageal dysmotility in the presence of chronic opioid use[55] | Regurgitation, dysphagia, reflux | Opioid induced blocking of esophageal inhibitory signals leading to increased spastic contraction and decreased LES relaxation | Clinical history, high resolution manometry |
| Gastroparesis | Presence of symptoms such as nausea, vomiting, and early satiety with mechanical obstruction ruled out and evidence of delayed gastric emptying on testing[82] | Nausea, reflux, regurgitation, early satiety, abdominal pain and bloating | Multiple etiologies caused slowed peristalsis and delayed gastric emptying | Gastric emptying study |
| Functional disorders | ||||
| Functional heartburn | Presence of burning retrosternal discomfort, no symptoms relief on optimal therapy, absence of GERD or EOE as cause of symptoms, and absence of major motility disorder[83] | Reflux, regurgitation, globus sensation | Potentially secondary to increased esophageal sensitivity | Upper endoscopy, high resolution manometry, pH-impedance studies |
| Reflux hypersensitivity | Presence of retrosternal chest pain, normal endoscopy and absence of EOE, absence of major motility disorder, and symptom association with reflux events with normal acid exposure on pH-impedance tests[83] | Reflux | Hypersensitization of esophageal nerve endings leading to pain secondary to physiologic esophageal stimuli | Upper endoscopy, high resolution manometry, pH-impedance studies |
| Rumination | Must include both persistent regurgitation of recently ingested food with subsequent spitting or re-mastication, and regurgitation that is not preceded by retching[83] | Regurgitation (frequently after meals), reflux | Behavioral contraction of abdominal muscles leading to increased intragastric pressure and reflux | Clinical history, high resolution manometry, pH-impedance studies |
| Supragastric belching | Presence of frequent repetitive belching, no established clinical correlate for gastric belching, and evidence of supragastric origin on impedance testing[83] | Frequent belching, reflux, regurgitation, globus sensation | Behavioral swallowing of air without LES relaxation | Clinical history, high resolution manometry, pH-impedance studies |
The ACG guidelines state that a presumptive diagnosis of GERD can be made in patients with classical symptoms such as heartburn or regurgitation, with further diagnostic evaluation such as an upper endoscopy recommended for patients with alarm symptoms[5]. The ACG recommends management of these patients begin with lifestyle modifications, including weight loss and dietary changes, along with an 8-wk course of PPI therapy[5]. While this approach is effective for most patients, a subset of patients may require further management including surgical options such as fundoplication. Surgical treatment of GERD is as effective as medical management in appropriate candidates and is currently recommended for reasons including complications from PPIs, medication non-compliance, and large hiatal hernias[3,5]. Additionally, the SAGES recommends surgical therapy for patients with GERD who: have failed medical management, request surgery due to quality of life issues related to long term medication use, have complications such as Barrett’s esophagus, or have extra-esophageal symptoms related to their GERD[6].
While surgical treatment of GERD may be as effective as medical therapy, complications are also possible. The most commonly reported side effects of surgery include dysphagia, belching, and increased abdominal bloating, though it is also possible to develop more severe complications requiring repeat surgery[7]. A total 360° Nissen fundoplication is the most common surgical treatment for GERD, but variations also exist involving differing degrees of wrapping. Studies have found partial fundoplication to be similarly effective for reducing reflux symptoms, and may also have less post-operative dysphagia and bloating[8]. This is supported by a level 1 recommendation from the SAGES that partial fundoplication is associated with less dysphagia and similar patient satisfaction[6]. Additionally, newer techniques such as the Linx procedure (which uses a circlet of magnets to augment the LES) have shown similar short-term outcomes for the treatment of GERD when compared to fundoplication. More research is needed on the long-term outcomes[9].
While surgical treatment of GERD can successfully relieve symptoms in up to 90% of patients[10], a thorough pre-operative assessment is necessary to identify appropriate surgical candidates. The underlying mechanism of anti-reflux surgery is to improve the integrity of the LES. Therefore, in order to achieve the best post-operative outcomes, it is crucial to confirm a patient’s symptoms are due to GERD and not another underlying pathology. Otherwise, a patient would likely continue experiencing symptoms post-operatively along with potentially developing complications such as dysphagia.
Newer technologies are becoming increasingly available for the assessment of patients with reflux symptoms and can help confirm the diagnosis of GERD. Improvements in pH studies with the inclusion of impedance measurements may help identify patients with NERD. Additionally, advancements in HRM help further classify esophageal pathologies and identify patients as having disorders with GERD like symptoms (Table 1). In this review, we aim to provide a guide for the pre-operative assessment and evaluation of patients with reflux. We will focus on newer technologies that can be used to identify appropriate candidates for anti-reflux surgery by confirming the diagnosis of GERD and ruling out other esophageal diseases.
The most important step in evaluating a patient’s candidacy for anti-reflux surgery is to confirm the diagnosis of GERD. This starts with careful history taking to assess the signs and symptoms a patient is suffering from. While the presence of typical GERD symptoms is enough for a presumptive diagnosis[5], these symptoms can also be present in a wide range of diseases and do not confirm the diagnosis of GERD. One recent systematic review found that classic symptoms such as heartburn and regurgitation had a varying degree of sensitivity, ranging from 30%-76%, for the presence of underlying GERD[11]. Additionally, the researchers found the studied symptoms were associated with a wide range of specificities (62%-96%)[11]. It is also important to identify if patients are suffering from extra-esophageal symptoms of GERD, as they are less likely to find symptom relief with surgery. One randomized control study compared patients with asthma on medical vs surgical therapy for reflux. While all patients experienced clinical improvement of their symptoms, researchers found no significant difference between the two modalities[12]. Overall, while a patient may display classic symptoms of GERD, further assessment must be completed to determine their appropriateness for surgery.
Another important aspect of the patient’s history is to assess their response to PPI therapy, which may be a good predictor of outcomes after anti-reflux surgery[13]. One recent study involving 370 patients undergoing laparoscopic Nissan fundoplication found patients who responded well to PPI therapy had a significantly greater reduction in reflux symptoms after surgery[14]. Previous studies have also indicated that patients with symptoms refractory to PPI therapy may have a higher likelihood of poor surgical outcomes[15]. However, while PPI effectiveness may help predict surgical outcomes, it should not be used to confirm or rule out a diagnosis. One meta-analysis found that a successful response to PPI therapy was only 78% sensitive and 54% specific for an underlying diagnosis of GERD[16].
When assessing a patient for anti-reflux surgery, it is also important to understand the role of obesity in surgical outcomes. It is known that GERD is more common in obese patients, as greater central adipose tissue may lead to increased intraabdominal pressure stressing the LES[17]. While some studies indicated the morbidly obese are at a higher risk for surgical failure, more recent studies found similar outcomes of fundoplication in obese patients[18,19]. An alternative surgical option for obese patients includes Roux-en-Y gastric bypass surgery. The SAGES currently recommends bypass surgery for morbidly obese patients with GERD, as it can help decrease GERD symptoms in addition to the weight loss benefits for the patient[6]. However, evidence is still unclear regarding the optimal surgical treatment for patients with class I obesity, and while anti-reflux surgery is a safe option, these patients should be evaluated on a case-by-case basis. Additionally, it is still important to fully assess the reflux symptoms of patients undergoing weight loss surgery. While obese patients are more likely to have GERD, they may also have an underlying esophageal motility disorders, and the use of HRM can help distinguish between these conditions[20].
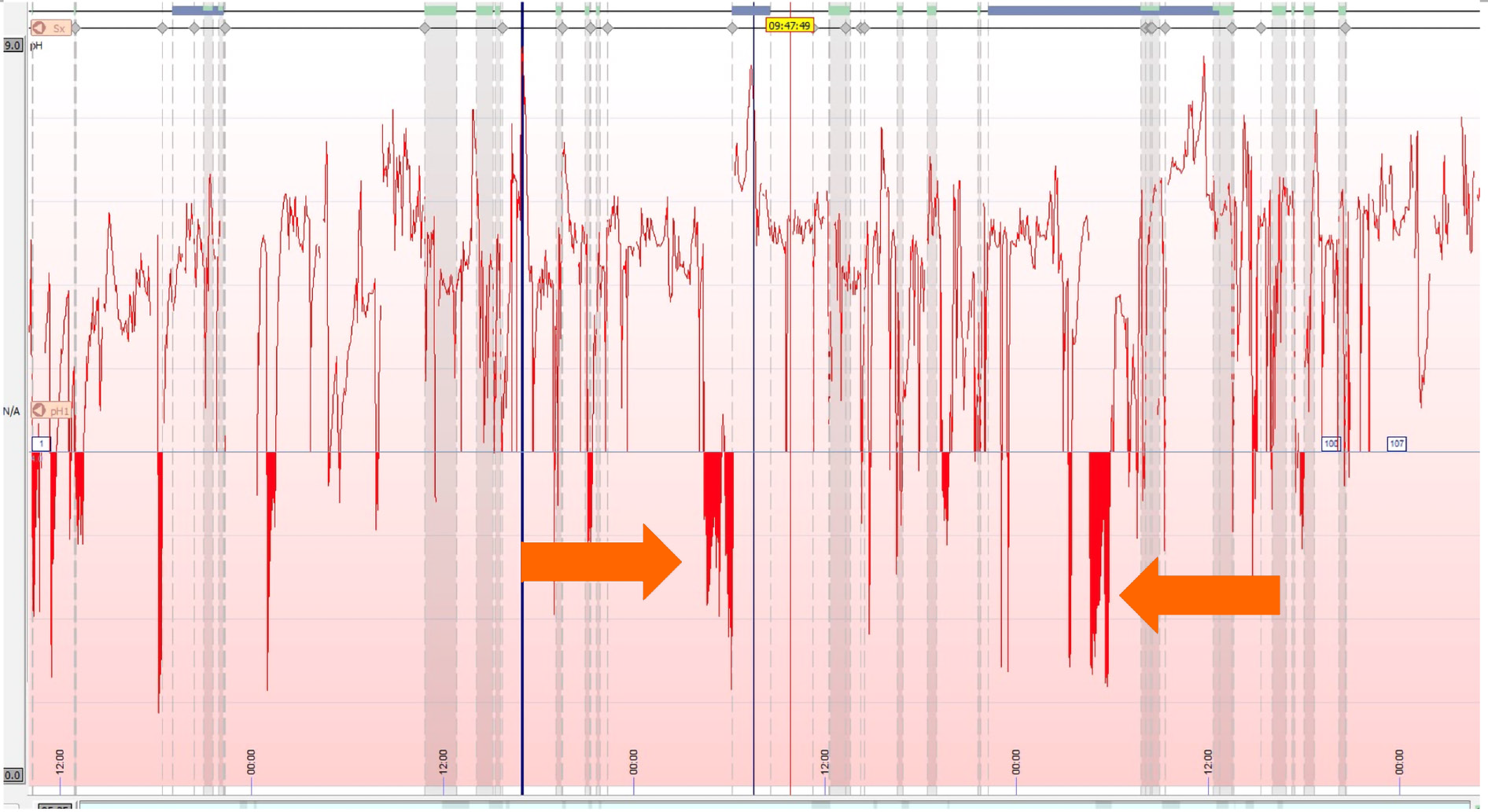
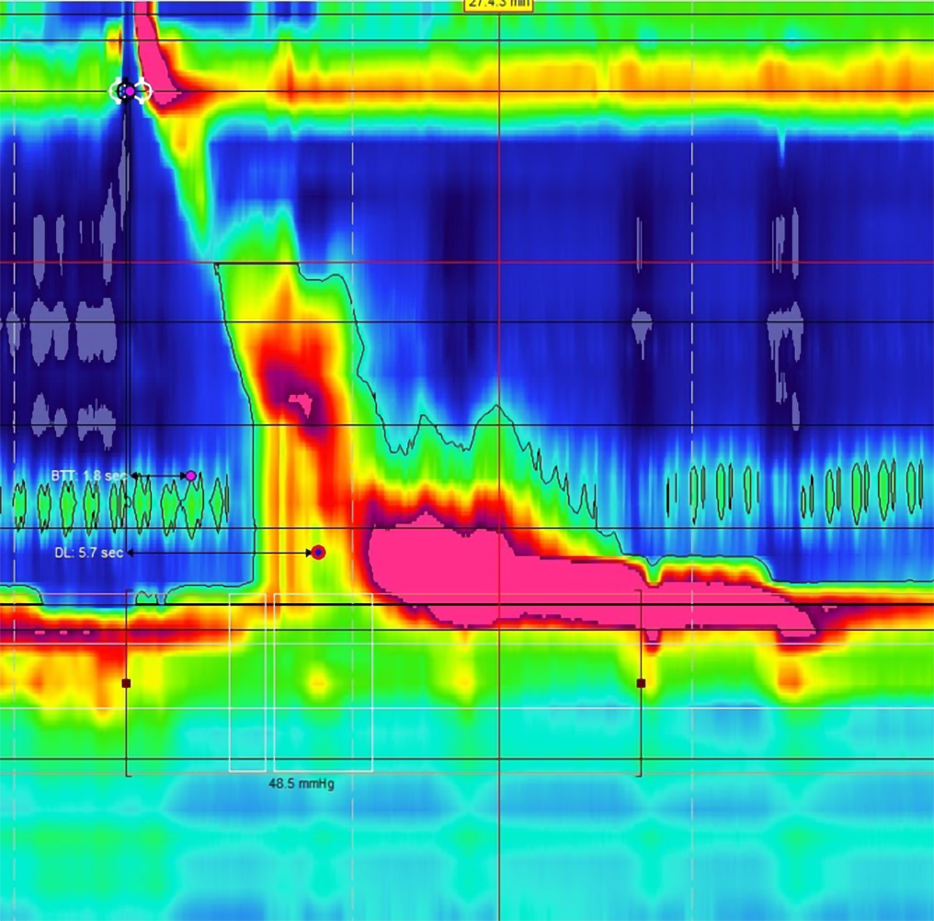
After thorough history taking to identify patients with GERD like symptoms, a upper endoscopy can help make a diagnosis by directly visualizing esophageal lesions such as esophagitis. However, a subset of patients may still have gastric reflux without evidence of any erosions[5]. In these patients with NERD, further testing is required to accurately diagnose reflux and confirm if a patient might be a good candidate for surgical correction. Esophageal pH testing can be a useful modality to identify acidic reflux above the LES[21,22]. Standard ambulatory pH monitoring involves placement of a trans-nasal probe 5 cm above the LES[23]. The probe then measures acid exposure time (AET) by recording any drops in pH below 4 over a 24-h period (Figure 1). The DeMeester score was a system developed in the 1970’s to categorize a patient’s reflux based on parameters including the number and timing of reflux events as well as supine vs upright positioning during these periods[24]. More recently, the Lyon consensus recommends using only AET when determining GERD, and defined pathologic reflux as having greater than 6% AET[22]. Although there is debate regarding which method is more reliable, both have similar strengths and weaknesses, and there is no current evidence that one method is superior to the other[25]. In general, the ACG recommends pH monitoring for all patients with NERD as a pre-operative assessment, and more recent studies have also found utility in measuring AET for patients with LA grade A or B esophagitis prior to surgery[5,26]. Additionally, pH testing has utility for confirming reflux in patients with GERD or NERD who are unresponsive to PPI therapy[21].
One drawback of ambulatory pH monitoring is the discomfort patients may experience from the trans-nasal catheter, with some studies showing patients tend to decrease typical daily activities that might promote reflux after the probe is placed[27]. Wireless capsule pH monitoring is also available, and while it must be placed endoscopically, it is often better tolerated than the catheter probe and may provide better measurements of physiologic reflux (Figure 2)[28]. In addition to improved patient tolerance, the capsule can record AET for 48-96 h depending on battery life, improving the sensitivity of the test[29]. However, both catheter and capsule pH monitoring are limited by the fact that they only measure acidic reflux. Studies have shown up to 30% of patients with pathologic reflux may have normal pH measurements on ambulatory monitoring[30]. This indicates up to 1/3 of patients with LES dysfunction who may benefit from surgery are missed with this form of testing (Table 2).
| Overview | Benefits | Limitations | |
| Twenty-four hours ambulatory catheter | Trans-nasal catheter placed 5 cm above the LES. Measures time of pH < 4 | Can be placed in office | Catheter may cause discomfort; Patients may deviate from daily routine; Patients should refrain from taking PPI therapy during testing; False positives secondary eating/drinking acidic food |
| Wireless capsule | Small probe that is placed endoscopically in esophagus 5-6 cm above LES. Measures time of pH < 4 | Little patient discomfort; Battery life of 48-96 h allows for better measurement of physiologic acid exposure | Must be placed endoscopically; Patients should refrain from taking PPI therapy during testing; False positives secondary eating/drinking acidic food |
| MII-pH catheter | Trans-nasal catheter placed 5 cm above LES. Contains pH probe along with electrodes to measure reflux episodes | Can be done on or off PPI; Measures pH and reflux independently; Patients can continue taking PPIs; Can identify patients with weak acid reflux | Catheter may cause discomfort; Patients must have prior manometry testing; False positive possible in patients with rumination, achalasia, and scleroderma |
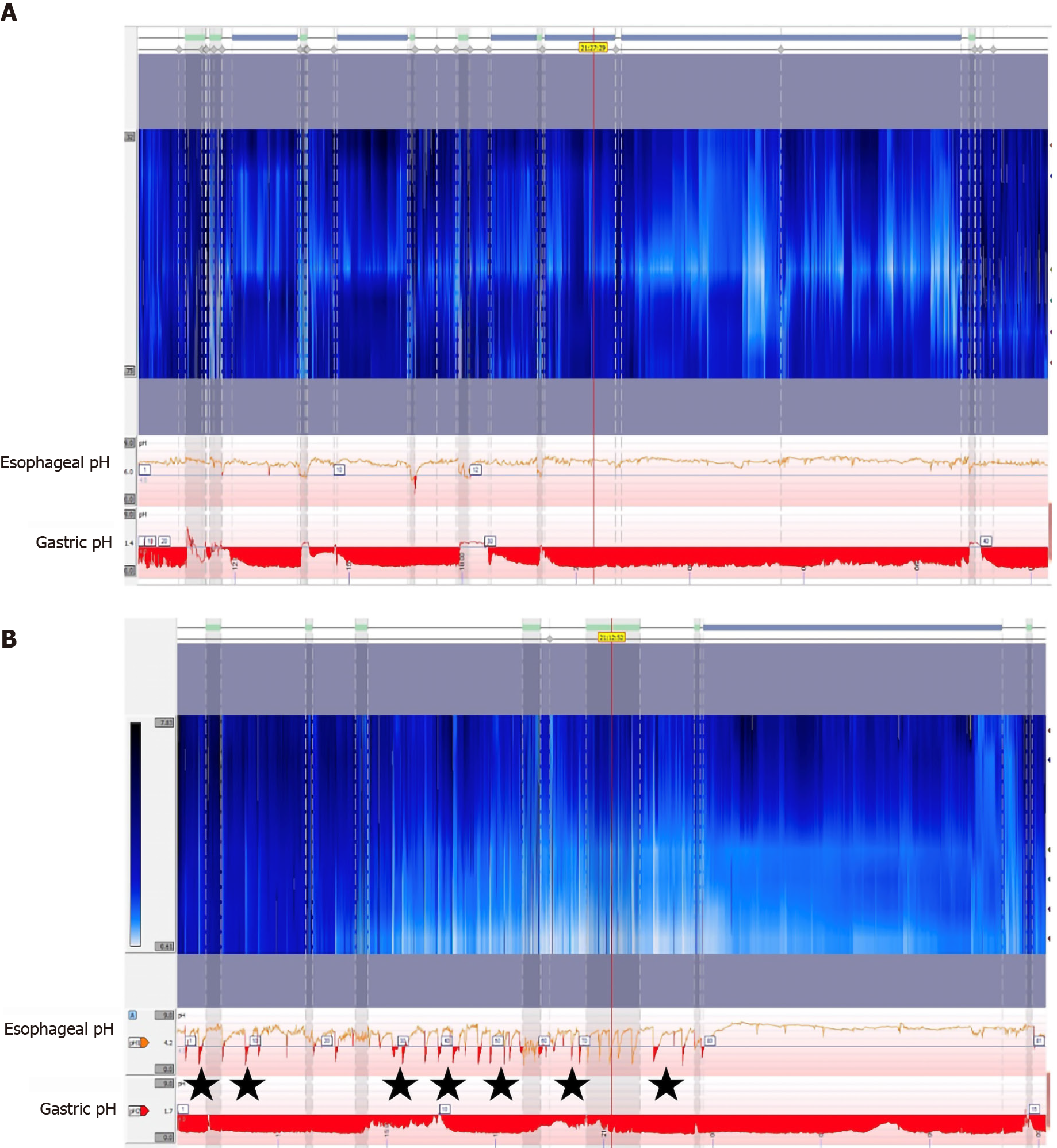
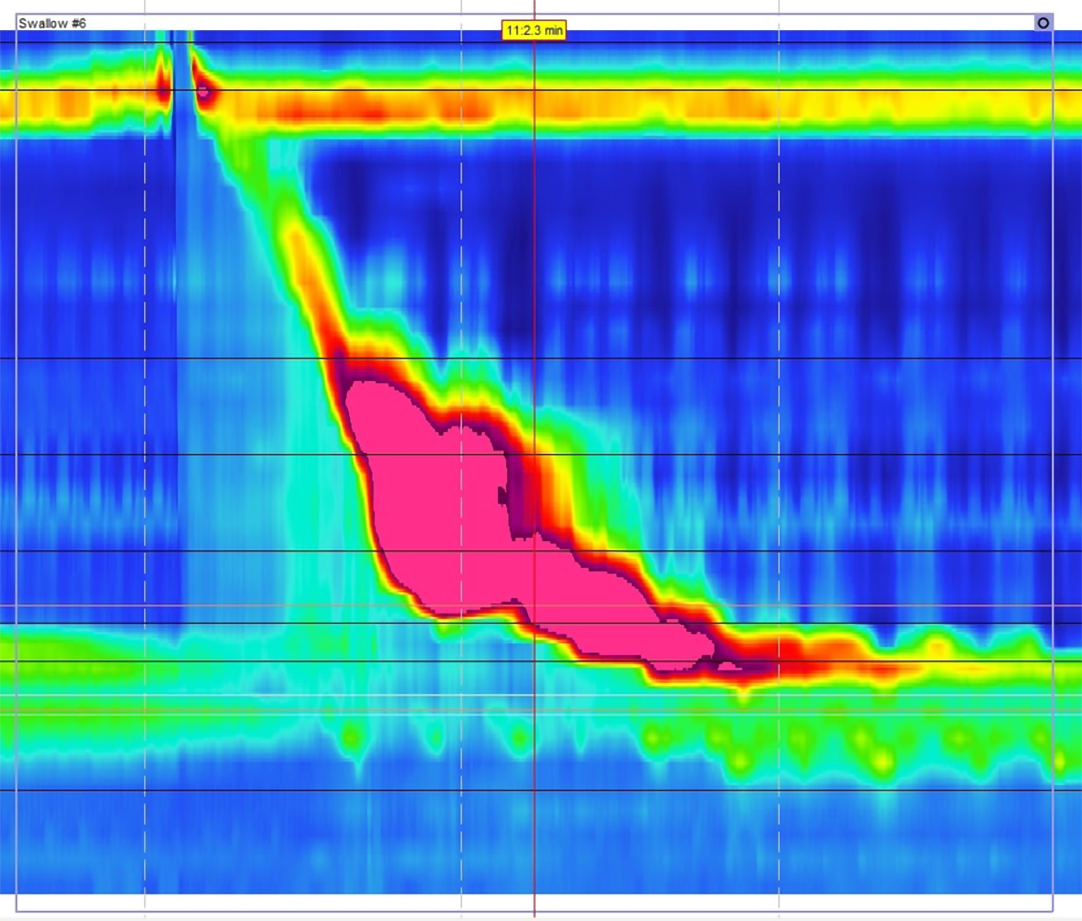
Multichannel intraluminal impendence pH monitoring (MII-pH) is a newer technology that measures changes in electrical conductivity between multiple points on the probe[23]. This data is combined with the pH probe to measure reflux of any material above the LES, independent of pH (Figure 3)[30]. The Lyon consensus defines physiologic reflux as 40 or less reflux events, with pathologic defined as greater than 80 episodes of reflux[22]. This definition allows for patients to continue PPI therapy during testing, as pH does not factor into the diagnosis[31]. MII-pH testing also has utility in identifying patients with weak acid reflux (pH 4-7). Up to 30% of patients with GERD may continue to have symptoms refractory to acid suppression therapy[32]. While these symptoms could potentially be secondary to any of the diagnoses seen in Table 1, MII-pH testing can identify patients with weak reflux by independently measuring reflux and pH (Figure 3). Treatment of patients with weak acid reflux is controversial, especially because previous studies have shown failure of PPIs is an indicator for poor outcomes after anti-reflux surgery[15]. However, if clinicians can confirm refractory GERD is secondary to weak acid reflux and not alternative diagnoses such as functional heartburn, they can identify patients who would still be good surgical candidates[33].
Limitations of MII-pH testing include using a trans-nasal catheter and required manometry testing for all patients prior to placement[29]. Additionally, visual analysis of the data is time consuming, but computerized electronic review software can be imprecise. One study found that computer interpretation of results identified only 74% of reflux events confirmed by visual analysis[34]. The authors suggested visual review of the data may be necessary to confirm test accuracy[34]. There also remains a potential for patients to have increased AET and reflux events secondary to other esophageal pathologies not related to LES dysfunction or GERD. This is important to keep in mind during pre-operative assessment for anti-reflux surgery, as patients without underlying LES dysfunction would not be good candidates for surgical treatment. Patients with rumination syndrome can have false positives on pH testing due to frequent regurgitation, not transient LES relaxation[35]. Increased AET can also be secondary to a hypomotility disorders, such as in patients with achalasia or scleroderma. This is not limited to only pH testing, as impedance studies may also have inconclusive or misleading results in patients with achalasia, scleroderma, or rumination syndrome[29]. Due to these possibilities, the ACG recommends that all patients undergo manometry as part of their pre-operative assessment to rule out any motility disorders that may cause reflux[5].
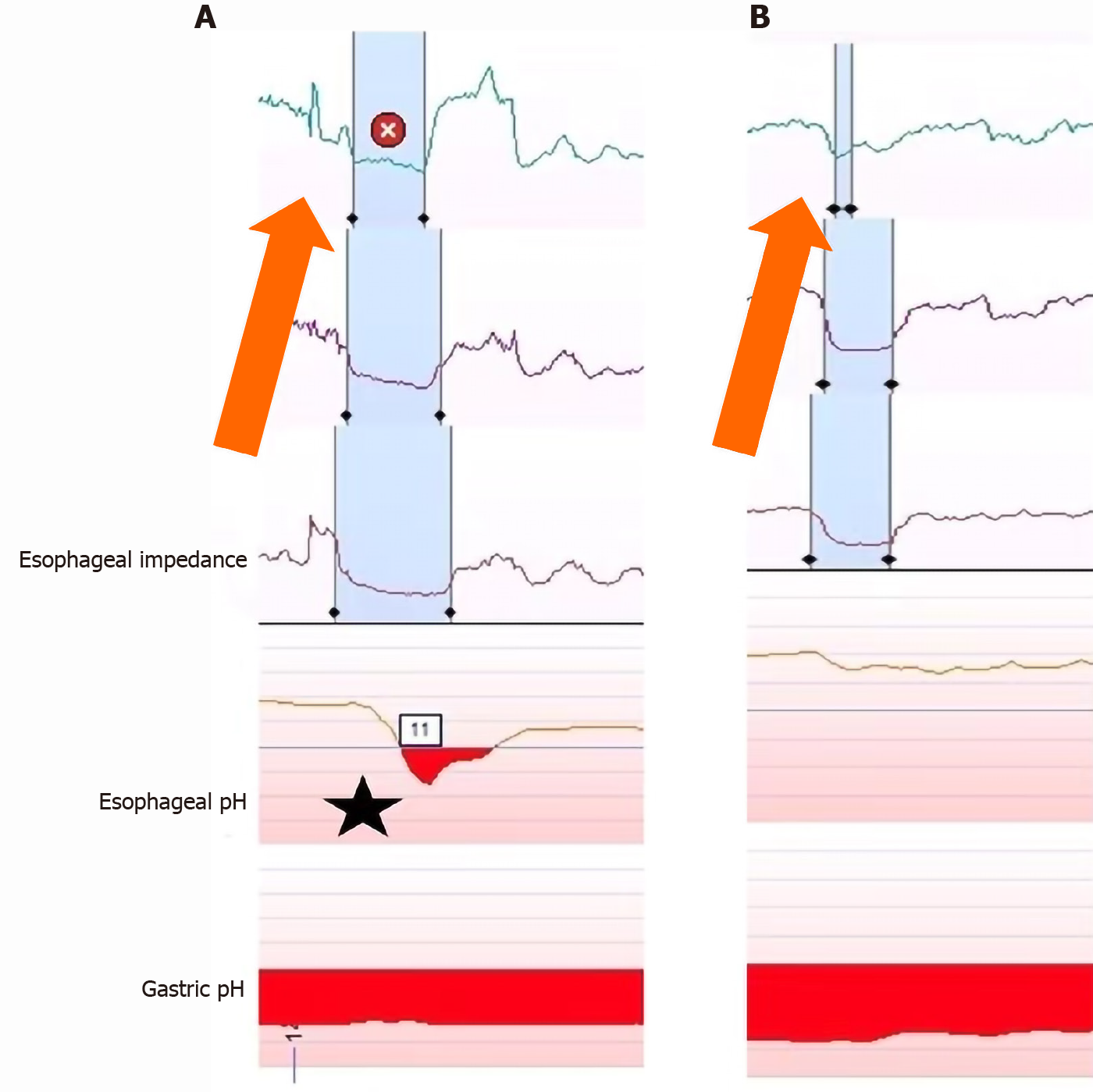
Another important aspect of utilizing pH or pH-impedance testing is the measurement of any associated symptoms during testing (Figure 4). Since there is still the potential for false positives when looking at AET or number of reflux episodes during testing, it is also important for a patient to log any symptoms they experience during testing[36]. This also has utility with extra-esophageal symptoms and can help in identifying if symptoms are causing reflux or are secondary to reflux, such as coughing[36], The Symptoms Association Probability (SAP) is one example of a methods to assess the association of symptoms with reflux events. This involves using a 2 × 2 table assessing the presence of symptoms and reflux events in 2-min blocks over a 24 h period[37]. Analysis then allows the determination of whether the symptoms were more likely secondary to reflux events or caused by chance. While there are other methods to assess symptom association, a SAP can provide a probability of symptom association and is thought to have the best clinical utility[36].
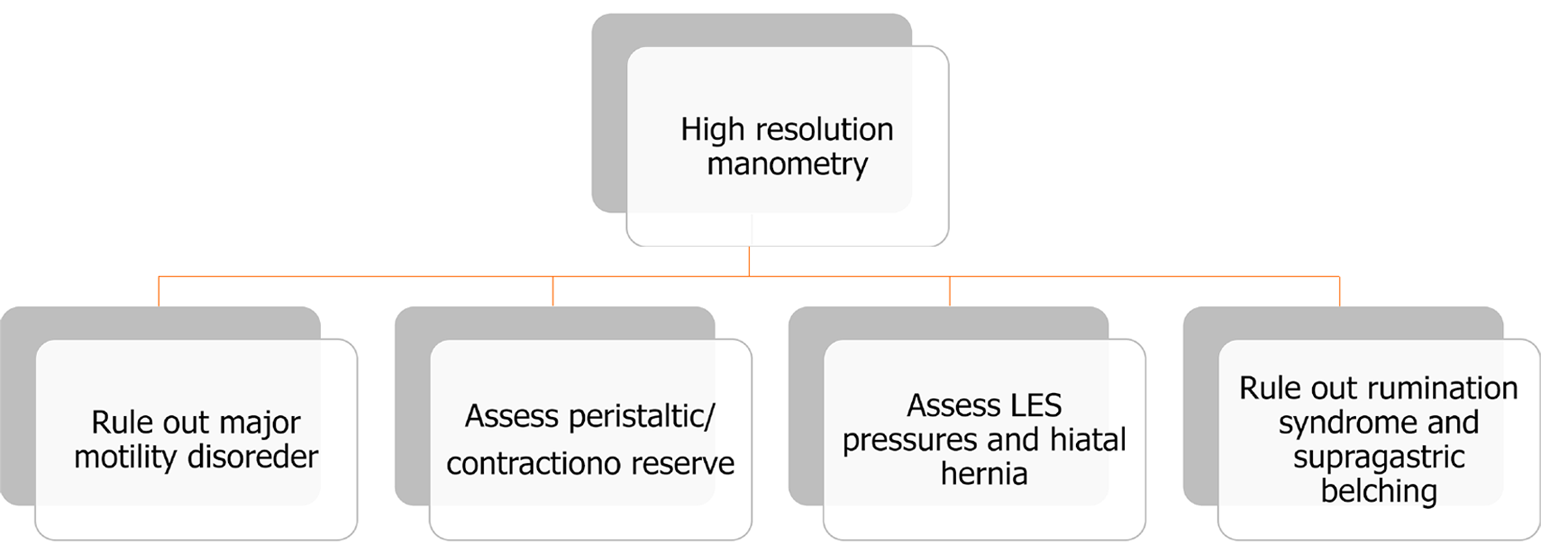
Another important aspect of the pre-operative assessment is to rule out major esophageal motility disorders with HRM (Figure 5). Patients with GERD often have findings of decreased LES pressures on HRM, indicating an impaired ability for the LES to act as an anti-reflux barrier and prevent gastric contents from entering the esophagus[38]. The smooth muscle in the LES is also augmented by pressure generated from the crural diaphragm, and because HRM utilizes an increased number of sensors, these pressures can be differentiated when evaluating the LES strength[39]. The esophagogastric junction contractile integral can also be quantified on HRM and may identify patients with severe reflux, but further research is needed to create a standardized metric for evaluating patients[22]. Additionally, LES physiology can be altered in the presence of a hiatal hernia, and HRM can be used to assess and measure the size of a patient’s hiatal hernia[40]. While esophageal manometry is not a confirmatory test for diagnosing GERD, HRM is useful in identifying any underlying motility issues (Figure 6)[41,42]. While there is limited data to support mandatory pre-operative manometry testing, the ACG still recommends manometry to specifically rule out achalasia and absent peristalsis seen in conditions such as scleroderma[5]. In the 2019 ICARUS guidelines, 94% of experts also strongly agreed with pre-op HRM testing, but this could only be supported with grade D evidence[42]. Additionally, HRM can be used to assess baseline esophageal physiology to predict the risk of post-operative dysphagia.
The main indication for HRM should be used to rule out major motility disorders, as these may be the underlying cause of a patient’s reflux symptoms, and may also be a contraindication for fundoplication[6].
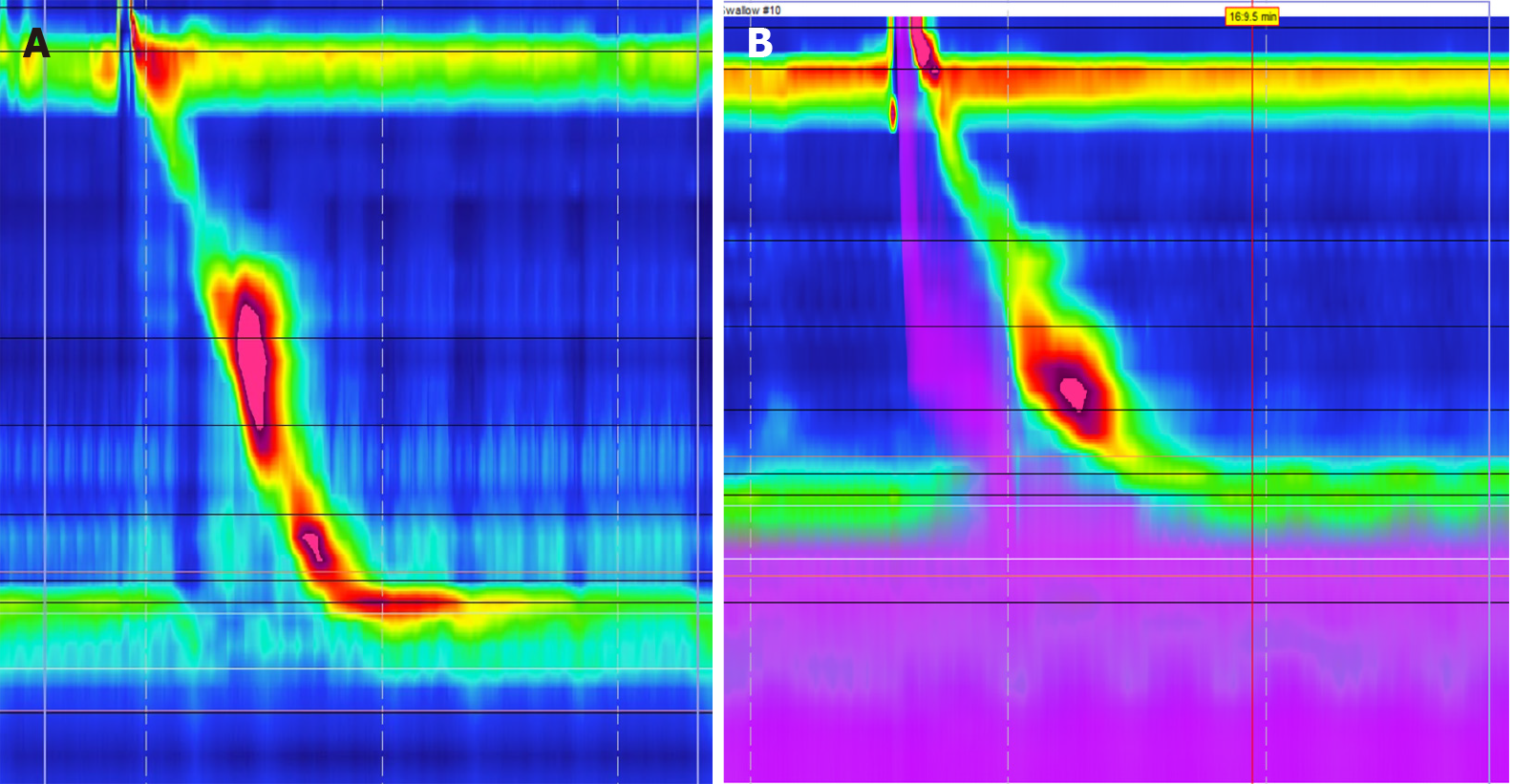
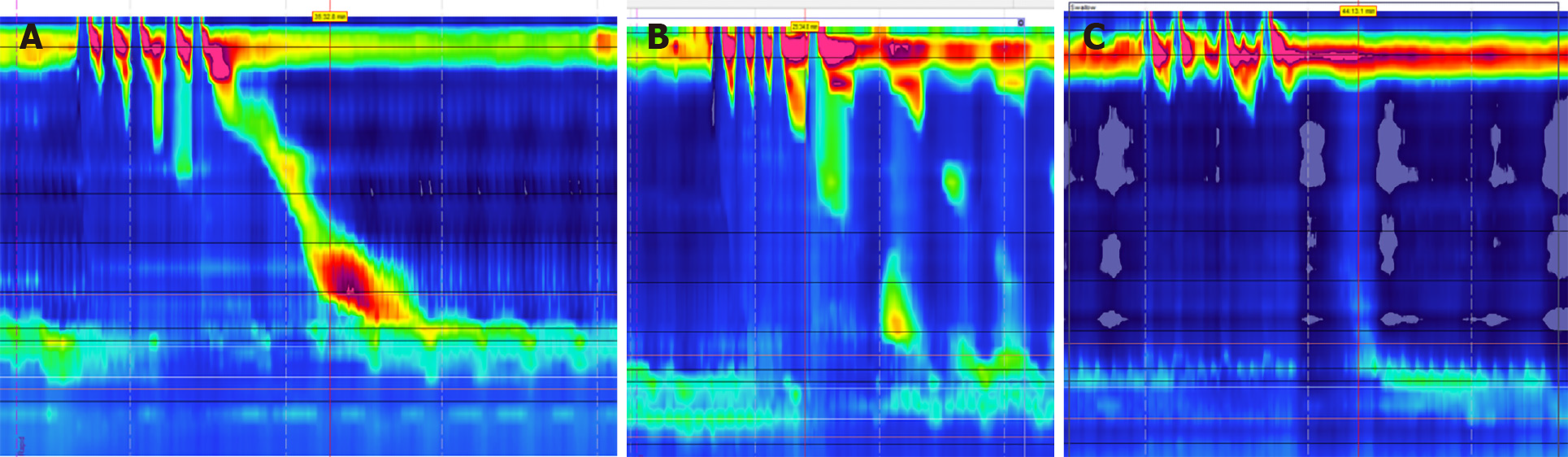
Achalasia: Abnormal esophageal peristalsis can manifest as GERD-like symptoms in patients with achalasia. The integrated relaxation pressure (IRP) is an important measurement during manometry that assesses esophageal pressures and transit across the esophagogastric junction[43]. According to the Chicago Classification (CC), type I achalasia is diagnosed in patients found to have an IRP > 15 mmHg and 100% failed peristalsis on HRM, with type II having the same characteristics as well as panesophageal pressurization in ≥ 20% of swallows[44]. Type III achalasia is defined as an elevated IRP > 15 mmHg, the absences of any normal peristalsis, and spastic contractions greater > 450 mmHg on ≥ 20% of swallows (Figure 7)[44]. As previously discussed, the goal of fundoplication is to improve the integrity of the LES, but patients with achalasia suffer from impaired LES relaxation along with esophageal aperistalsis. While these patients may also have underlying GERD, surgical treatment revolves around addressing the LES with myotomy[42,45]. However, after myotomy weakens the LES, GERD can become a common side effect. While these patients would benefit from fundoplication, there is debate regarding the risk of post-operative dysphagia[46]. One prospective randomized study compared outcomes of myotomy alone vs myotomy with fundoplication for 43 patients with achalasia. Researchers found that patients receiving myotomy with fundoplication had significantly less GERD and AET, with no significant difference in dysphagia between the two groups[47]. The group also followed patients long-term and concluded there was no significant differences in post-op dysphagia in patients who received myotomy with fundoplication[48]. Current guidelines from both the ACG and the SAGES recommend patients with achalasia undergo both myotomy and fundoplication for the best outcomes[45,46].
Absent peristalsis: Conflicting data also exists regarding fundoplication for patients with absent peristalsis. Evidence of aperistalsis, defined as failed peristalsis with 100% of swallows, on HRM can indicate patients with scleroderma-like esophagus (Figure 8)[49]. Older studies have confirmed anti-reflux surgery can lead to improved symptoms, but more recent studies indicated these patients might have a higher risk for post-operative dysphagia[42,50]. Additionally, studies have shown that alternative surgeries such as a Roux-en-Y bypass may lead to better outcomes. A 2018 study followed patients with systemic sclerosis undergoing surgical treatment for their GERD and compared fundoplication vs Roux-en-Y bypass. While the sample size was small, researchers found patients had better GERD symptom relief after Roux-en-Y compared to fundoplication[51].
Esophagogastric junction outflow obstruction: Esophagogastric junction outflow obstruction (EGJOO) is another esophageal motility disorder that can present with GERD like symptoms. In this disorder, patients have normal or weakened esophageal peristalsis on HRM with an elevated IRP[44]. While the current CC provides a guideline on making a diagnosis with HRM, it is important to remember that other factors can lead to an increased IRP. In some patients with hiatal hernias, the manometry catheter may be incorrectly positioned due to the underlying anatomical abnormality, which can contribute to increased IRP measurements[52]. These patients require confirmatory testing, and some centers have adopted the use of upright HRM measurements with an IRP > 12 mmHg as a cutoff[53]. Additionally, other esophageal abnormalities such as Schatzki’s ring can lead to altered HRM results[54]. Data is currently limited regarding the outcomes of fundoplication in patients with EGJOO, but an accurate diagnosis is still important as it identifies esophageal dysmotility as the cause of a patient’s GERD like symptoms.
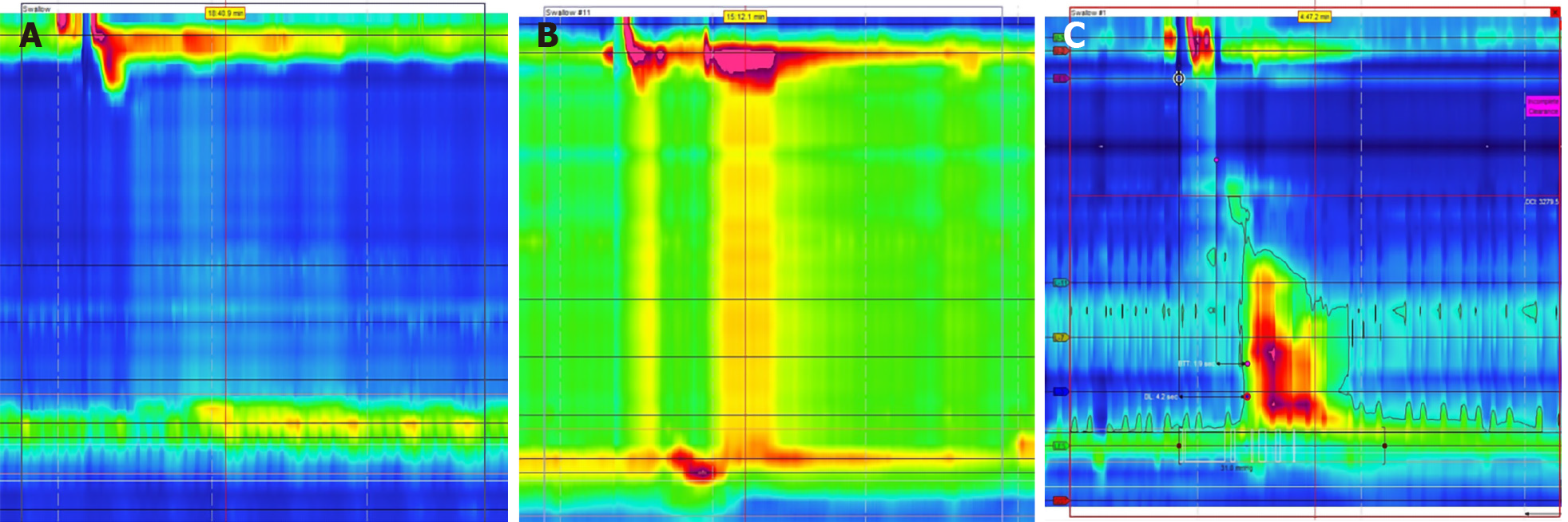
Opioid induced esophageal dysfunction is increasingly being recognized as a potential cause of esophageal dysmotility. Just as chronic opioid use can lead to bowel hypomotility, it is theorized that opioids may block esophageal inhibitory signals leading to increased contractions and decreased LES sphincter relaxation[55]. This can in turn lead to findings of EGJOO, esophageal spasm, and hypercontractile esophagus on HRM (Figure 9)[56]. It is important to recognize the effect of chronic opioid use on esophageal motility, as cessation of opioid drugs may lead to resolution of GERD like symptoms.
Hypercontractile disorders: In addition to uncovering evidence of esophageal hypomotility, HRM can also identify hypercontractile patterns such as jackhammer esophagus and esophageal spasm (Figure 10). While the pathophysiology behind these diseases is still under investigation, both involve impaired inhibition and coordination of esophageal peristalsis and can present with dysphagia, non-cardiac chest pain, and reflux[57,58]. Jackhammer esophagus can be diagnosed on HRM if at least 20% of swallows have a distal contractile integral (DCI) of greater than 8000 mmHg[44]. According to the 2019 ICARUS guidelines, 64% of experts agreed that patients with jackhammer esophagus are still good candidates for anti-reflux surgery, but this assertion is only supported by grade D evidence[42]. Data is limited regarding outcomes of patients with jackhammer esophagus after anti-reflux surgery, but one retrospective study found no difference in outcomes when compared to patients with physiologic esophageal motility[59]. Distal esophageal spasm is diagnosed in patients with a normal IRP but ≥ 20% of premature contractions with a DCI of > 450 mmHg[44]. 64% of experts agreed that these patients were not good surgical candidates, but this again was only supported with grade D evidence[42]. Instead, the authors suggested specific therapeutic measures for esophageal spasm such as botulinum injections and myotomy instead of anti-reflux surgery[42].
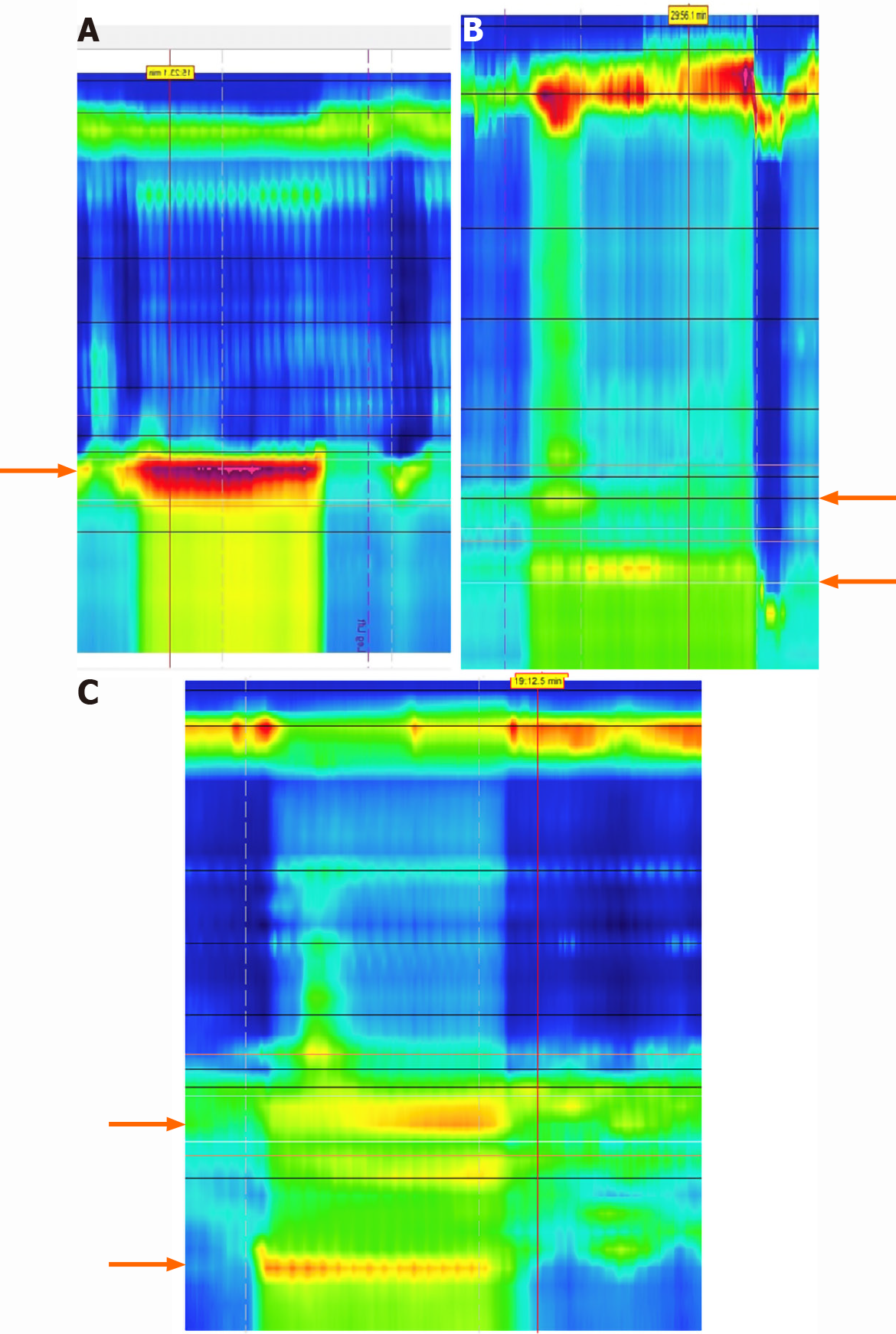
Provocative studies such as multiple rapid swallow (MRS) or rapid drink challenge (RDC) tests can also be added to HRM to further evaluate esophageal physiology. MRS testing involves swallowing small amounts of water over a quick period of time which stresses the coordination of esophageal contraction and LES relaxation[60]. Recent studies have found the addition of MRS during HRM can help uncover pathologies that might have been missed on manometry alone such as distal esophageal spasm and achalasia variants[61]. MRS also allows for visualization of esophageal pressurization patterns which can help identify hypercontractility and EGJOO[62,63]. RDC is also a simple test that can be added to HRM to stress the esophagus and uncover motility disorders. RDC is a similar test to MRS, but involves drinking a larger total amount of water. A recent study in 2017 compared HRM and RDC results between healthy individuals and a cohort of patients with dysphagia and reflux. They found that the addition of RDC improved the sensitivity and specificity of HRM for identifying esophageal motility disorders to 85% and 95% respectively[64].
Another provocative test that can be added to HRM is apple viscous swallows (AVS). AVS involves patients swallowing apple sauce during manometry, and the increased viscosity may help better simulate physiologic swallowing of food. A study from 2011 Looked at patients with dysphagia and compared results of HRM with AVS and standard water swallows. The researchers found that 4% of patients had abnormal results with water swallows, but this increased to 30% with AVS[65]. Overall, the additions of provocative maneuvers such as MRS, RDC, and AVS to HRM are quick and simple and can help identify esophageal motility disorders in patients with GERD like symptoms.
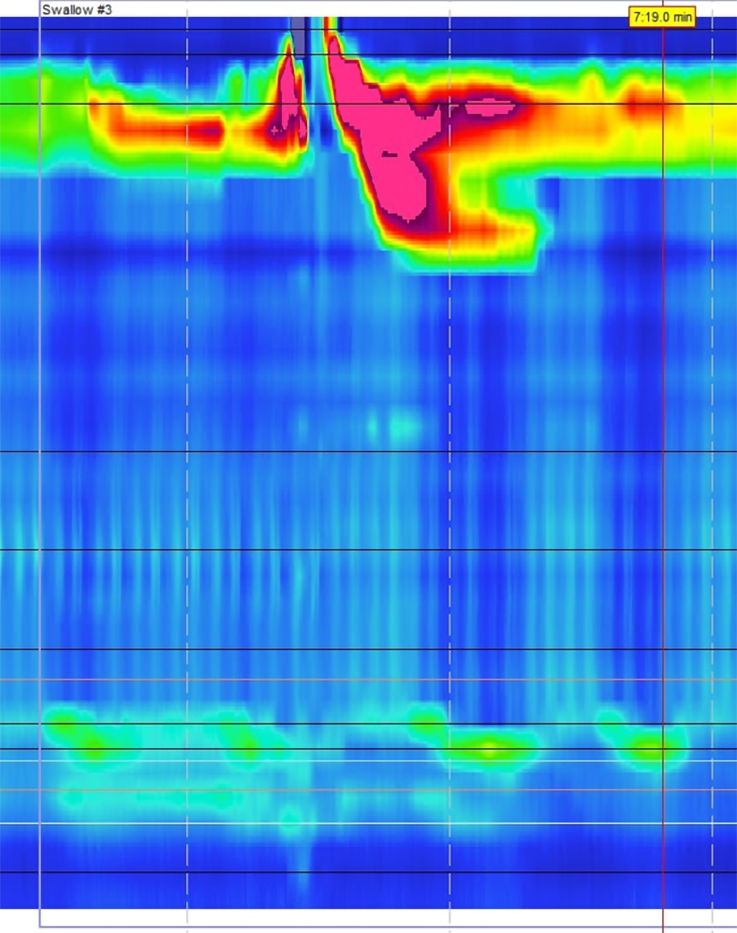
Provocative testing also has important utility as a pre-operative assessment by predicting the risk of post-operative dysphagia. The DCI is another measurement that can be used to assess the strength of esophageal contractions during HRM[63]. According to the CC, a DCI ratio can be calculated from standard manometry and MRS testing to assess peristaltic or contraction reserve (Figure 11)[44]. This can help indicate the ability of the esophageal body to augment contractions and may be predictive of multiple pathologies in patients with impaired esophageal motility[22,66]. Patients with weak contractions on HRM with MRS may have low peristaltic reserve and a DCI ratio cutoff of > 0.85 was found to be 67% sensitive and 64% specific for identifying late post-operative dysphagia in patients with GERD[67]. Additionally, a lack of manometric response can identify patients with esophageal involvement of their systemic sclerosis, as peristaltic reserve is typically absent in this population[68].
It is known from previous studies that impaired LES pressures are associated with increased esophageal acid exposure[69]. A 2012 retrospective study included over 2000 patients who underwent manometry and pH testing and found that patients with incompetent LES pressures had significantly higher DeMeester scores[70]. A hiatal hernia is one example of why the LES can be compromised in patients and lead to lower closing pressures[71]. The straight leg raise is another simple provocative maneuver that can be completed during HRM to assess patients with a hiatal hernia (Figure 12). Previous studies have indicated the size of a hiatal hernia is the best predictor for the severity of GERD[72]. Straight leg raises during HRM can increase intraabdominal pressure and stress the LES. A recent study measured trans-esophagogastric junction gradient pressures during straight leg raises on HRM and found a significant decrease in peak pressure gradient in patients with a hiatal hernia of 3 cm or greater[73]. Straight leg raises are a simple provocative maneuver to add during HRM and can identify patients that could benefit from surgical treatment for both their GERD and hiatal hernia with good outcomes[74] (Table 3).
| Measurement | Utility |
| Integrated relaxation pressure | Measures esophageal pressures during transit and passage through esophagogastric junction. Can be used to diagnose achalasia and other hypomotility disorders |
| Distal contractile integral | Measures strength of esophageal contractions. Can diagnose hypercontractile disorders such as jackhammer esophagus |
| Distal latency | Measurement of esophageal transit and contraction time. Can indicate impaired or spastic peristalsis |
| DCI ratio | Ratio of DCI on normal swallows and MRS testing. Used to assess peristaltic reserve. This can be used to predict risk of post-operative dysphagia |
Patients with GERD may also commonly complain of increased belching[75]. Supragastric belching is another disorder with many GERD-like symptoms, and is common in patients with GERD, NERD, reflux hypersensitivity, and functional dyspepsia[76,77]. Distinguishing between the two disorders is important, as both are due to different underlying pathologies requiring specific treatment approaches. Supragastric belching is seen as a learned behavioral disorder where air is pulled into the esophagus, but there is no transient relaxation of the LES as seen in physiologic belching[78]. Even though these patients present with symptoms of reflux, the treatment is directed at behavioral therapy to decrease bringing air into the esophagus. These patients would not be good candidates for anti-reflux surgery. Recent improvements in high resolution impedance manometry (HRIM) allow for clinicians to measure both esophageal pressure and flow during swallows[79]. While supragastric belching can induce GERD, HRIM can identify episodes of supragastric belching with no LES relaxation and confirm only esophageal air is released instead of gastric air[80].
Rumination syndrome is another functional disorder involving patients suffering with GERD-like symptoms of frequent reflux and regurgitation. Like supragastric belching, HRM can be useful in identifying the underlying mechanism of the dysfunction. A recent study found that different subtypes of rumination can be identified on HRM, with some having pathophysiologies similar to supragastric belching, and others showing evidence of gastric contents refluxing above the LES[35]. While the first line treatment of rumination syndrome involves cognitive behavioral therapy, HRM can help identify patients with gastric rumination as the cause of their reflux symptoms, and these patients may benefit from further treatments such as anti-reflux surgery[35].
Fundoplication is a safe and effective treatment for individuals with GERD, but a thorough pre-operative assessment is critical to achieving good surgical outcomes. Newer technologies continue to become more widely available and can help clinicians in selecting appropriate surgical candidates. By accurately diagnosing GERD, assessing peristaltic reserve, and ruling out major motility disorders and diseases with GERD-like symptoms, clinicians can confidently identify patients with true LES dysfunction who would benefit from surgical intervention.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Y, Sawada A, Uygun I, Wang D S-Editor: Zhang H L-Editor: A P-Editor: Ma YJ
| 1. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1263] [Article Influence: 114.8] [Reference Citation Analysis (2)] |
| 2. | Dent J, Becher A, Sung J, Zou D, Agréus L, Bazzoli F. Systematic review: patterns of reflux-induced symptoms and esophageal endoscopic findings in large-scale surveys. Clin Gastroenterol Hepatol 2012; 10: 863-873. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Patti MG. An Evidence-Based Approach to the Treatment of Gastroesophageal Reflux Disease. JAMA Surg. 2016;151:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Gawron AJ, French DD, Pandolfino JE, Howden CW. Economic evaluations of gastroesophageal reflux disease medical management. Pharmacoeconomics. 2014;32:745-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308-28; quiz 329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1119] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 6. | Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD; SAGES Guidelines Committee. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:2647-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 7. | Frazzoni M, Piccoli M, Conigliaro R, Frazzoni L, Melotti G. Laparoscopic fundoplication for gastroesophageal reflux disease. World J Gastroenterol. 2014;20:14272-14279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (4)] |
| 8. | Broeders JA, Roks DJ, Ahmed Ali U, Watson DI, Baigrie RJ, Cao Z, Hartmann J, Maddern GJ. Laparoscopic anterior 180-degree versus nissen fundoplication for gastroesophageal reflux disease: systematic review and meta-analysis of randomized clinical trials. Ann Surg. 2013;257:850-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 9. | Skubleny D, Switzer NJ, Dang J, Gill RS, Shi X, de Gara C, Birch DW, Wong C, Hutter MM, Karmali S. LINX® magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: a systematic review and meta-analysis. Surg Endosc. 2017;31:3078-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Davis CS, Baldea A, Johns JR, Joehl RJ, Fisichella PM. The evolution and long-term results of laparoscopic antireflux surgery for the treatment of gastroesophageal reflux disease. JSLS. 2010;14:332-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Moayyedi P, Talley NJ, Fennerty MB, Vakil N. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 12. | Larrain A, Carrasco E, Galleguillos F, Sepulveda R, Pope CE 2nd. Medical and surgical treatment of nonallergic asthma associated with gastroesophageal reflux. Chest. 1991;99:1330-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 155] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Oelschlager BK, Quiroga E, Parra JD, Cahill M, Polissar N, Pellegrini CA. Long-term outcomes after laparoscopic antireflux surgery. Am J Gastroenterol. 2008;103:280-7; quiz 288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Hamdy E, El Nakeeb A, Hamed H, El Hemaly M, ElHak NG. Outcome of laparoscopic Nissen fundoplication for gastroesophageal reflux disease in non-responders to proton pump inhibitors. J Gastrointest Surg. 2014;18:1557-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Campos GM, Peters JH, DeMeester TR, Oberg S, Crookes PF, Tan S, DeMeester SR, Hagen JA, Bremner CG. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg. 1999;3:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 233] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Numans ME, Lau J, de Wit NJ, Bonis PA. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med. 2004;140:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Herbella FA, Sweet MP, Tedesco P, Nipomnick I, Patti MG. Gastroesophageal reflux disease and obesity. Pathophysiology and implications for treatment. J Gastrointest Surg. 2007;11:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Luketina RR, Koch OO, Köhler G, Antoniou SA, Emmanuel K, Pointner R. Obesity does not affect the outcome of laparoscopic antireflux surgery. Surg Endosc. 2015;29:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Morgenthal CB, Lin E, Shane MD, Hunter JG, Smith CD. Who will fail laparoscopic Nissen fundoplication? Surg Endosc. 2007;21:1978-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Kuna M, Tran V, Tadros M. The Role of High-Resolution Manometry in Management of Patients with Sleeve Gastrectomy. Obes Surg. 2021;31:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Savarino E, Bredenoord AJ, Fox M, Pandolfino JE, Roman S, Gyawali CP; International Working Group for Disorders of Gastrointestinal Motility and Function. Advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol. 2018;15:323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM, Vaezi M, Sifrim D, Fox MR, Vela MF, Tutuian R, Tack J, Bredenoord AJ, Pandolfino J, Roman S. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 672] [Cited by in RCA: 944] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 23. | Vardar R, Keskin M. Indications of 24-h esophageal pH monitoring, capsule pH monitoring, combined pH monitoring with multichannel impedance, esophageal manometry, radiology and scintigraphy in gastroesophageal reflux disease? Turk J Gastroenterol. 2017;28:S16-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Johnson LF, DeMeester TR. Development of the 24-hour intraesophageal pH monitoring composite scoring system. J Clin Gastroenterol. 1986;8 Suppl 1:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 242] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Neto RML, Herbella FAM, Schlottmann F, Patti MG. Does DeMeester score still define GERD? Dis Esophagus. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Schwameis K, Lin B, Roman J, Olengue K, Siegal S, DeMeester SR. Is pH Testing Necessary Before Antireflux Surgery in Patients with Endoscopic Erosive Esophagitis? J Gastrointest Surg. 2018;22:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Fass R, Hell R, Sampliner RE, Pulliam G, Graver E, Hartz V, Johnson C, Jaffe P. Effect of ambulatory 24-hour esophageal pH monitoring on reflux-provoking activities. Dig Dis Sci. 1999;44:2263-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Richter JE, Pandolfino JE, Vela MF, Kahrilas PJ, Lacy BE, Ganz R, Dengler W, Oelschlager BK, Peters J, DeVault KR, Fass R, Gyawali CP, Conklin J, DeMeester T; Esophageal Diagnostic Working Group. Utilization of wireless pH monitoring technologies: a summary of the proceedings from the esophageal diagnostic working group. Dis Esophagus. 2013;26:755-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Chae S, Richter JE. Wireless 24, 48, and 96 Hour or Impedance or Oropharyngeal Prolonged pH Monitoring: Which Test, When, and Why for GERD? Curr Gastroenterol Rep. 2018;20:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Frazzoni M, de Bortoli N, Frazzoni L, Tolone S, Savarino V, Savarino E. Impedance-pH Monitoring for Diagnosis of Reflux Disease: New Perspectives. Dig Dis Sci. 2017;62:1881-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Zerbib F, Roman S, Ropert A, des Varannes SB, Pouderoux P, Chaput U, Mion F, Vérin E, Galmiche JP, Sifrim D. Esophageal pH-impedance monitoring and symptom analysis in GERD: a study in patients off and on therapy. Am J Gastroenterol. 2006;101:1956-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 319] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 32. | Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61:1340-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 33. | Scarpellini E, Ang D, Pauwels A, De Santis A, Vanuytsel T, Tack J. Management of refractory typical GERD symptoms. Nat Rev Gastroenterol Hepatol. 2016;13:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Koop AH, Francis DL, DeVault KR. Visual and Automated Computer Analysis Differ Substantially in Detection of Acidic Reflux in Multichannel Intraluminal Impedance-pH Monitoring. Clin Gastroenterol Hepatol. 2018;16:979-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Tucker E, Knowles K, Wright J, Fox MR. Rumination variations: aetiology and classification of abnormal behavioural responses to digestive symptoms based on high-resolution manometry studies. Aliment Pharmacol Ther. 2013;37:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Kamal AN, Clarke JO, Oors JM, Smout AJ, Bredenoord AJ. The Role of Symptom Association Analysis in Gastroesophageal Reflux Testing. Am J Gastroenterol. 2020;115:1950-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Weusten BL, Roelofs JM, Akkermans LM, Van Berge-Henegouwen GP, Smout AJ. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology. 1994;107:1741-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 407] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 38. | Pandolfino JE, Roman S. High-resolution manometry: an atlas of esophageal motility disorders and findings of GERD using esophageal pressure topography. Thorac Surg Clin. 2011;21:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Pandolfino JE, Kim H, Ghosh SK, Clarke JO, Zhang Q, Kahrilas PJ. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 40. | Tolone S, Savarino E, Zaninotto G, Gyawali CP, Frazzoni M, de Bortoli N, Frazzoni L, Del Genio G, Bodini G, Furnari M, Savarino V, Docimo L. High-resolution manometry is superior to endoscopy and radiology in assessing and grading sliding hiatal hernia: A comparison with surgical in vivo evaluation. United European Gastroenterol J. 2018;6:981-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | Mittal R, Vaezi MF. Esophageal Motility Disorders and Gastroesophageal Reflux Disease. N Engl J Med. 2020;383:1961-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Pauwels A, Boecxstaens V, Andrews CN, Attwood SE, Berrisford R, Bisschops R, Boeckxstaens GE, Bor S, Bredenoord AJ, Cicala M, Corsetti M, Fornari F, Gyawali CP, Hatlebakk J, Johnson SB, Lerut T, Lundell L, Mattioli S, Miwa H, Nafteux P, Omari T, Pandolfino J, Penagini R, Rice TW, Roelandt P, Rommel N, Savarino V, Sifrim D, Suzuki H, Tutuian R, Vanuytsel T, Vela MF, Watson DI, Zerbib F, Tack J. How to select patients for antireflux surgery? Gut. 2019;68:1928-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 43. | Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878-G885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE; International High Resolution Manometry Working Group. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1373] [Cited by in RCA: 1450] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 45. | Stefanidis D, Richardson W, Farrell TM, Kohn GP, Augenstein V, Fanelli RD; Society of American Gastrointestinal and Endoscopic Surgeons. SAGES guidelines for the surgical treatment of esophageal achalasia. Surg Endosc. 2012;26:296-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 46. | Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RT. ACG Clinical Guidelines: Diagnosis and Management of Achalasia. Am J Gastroenterol. 2020;115:1393-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 47. | Richards WO, Torquati A, Holzman MD, Khaitan L, Byrne D, Lutfi R, Sharp KW. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: a prospective randomized double-blind clinical trial. Ann Surg. 2004;240:405-12; discussion 412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 362] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 48. | Kummerow Broman K, Phillips SE, Faqih A, Kaiser J, Pierce RA, Poulose BK, Richards WO, Sharp KW, Holzman MD. Heller myotomy versus Heller myotomy with Dor fundoplication for achalasia: long-term symptomatic follow-up of a prospective randomized controlled trial. Surg Endosc. 2018;32:1668-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Bakhos CT, Petrov RV, Parkman HP, Malik Z, Abbas AE. Role and safety of fundoplication in esophageal disease and dysmotility syndromes. J Thorac Dis. 2019;11:S1610-S1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Poirier NC, Taillefer R, Topart P, Duranceau A. Antireflux operations in patients with scleroderma. Ann Thorac Surg. 1994;58:66-72; discussion 72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Yan J, Strong AT, Sharma G, Gabbard S, Thota P, Rodriguez J, Kroh M. Surgical management of gastroesophageal reflux disease in patients with systemic sclerosis. Surg Endosc. 2018;32:3855-3860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Roman S, Kahrilas PJ, Kia L, Luger D, Soper N, Pandolfino JE. Effects of large hiatal hernias on esophageal peristalsis. Arch Surg. 2012;147:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Triggs JR, Carlson DA, Beveridge C, Jain A, Tye MY, Kahrilas PJ, Pandolfino JE. Upright Integrated Relaxation Pressure Facilitates Characterization of Esophagogastric Junction Outflow Obstruction. Clin Gastroenterol Hepatol 2019; 17: 2218-2226. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 54. | Müller M, Gockel I, Hedwig P, Eckardt AJ, Kuhr K, König J, Eckardt VF. Is the Schatzki ring a unique esophageal entity? World J Gastroenterol. 2011;17:2838-2843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 55. | Snyder DL, Vela MF. Opioid-induced esophageal dysfunction. Curr Opin Gastroenterol. 2020;36:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Snyder DL, Crowell MD, Horsley-Silva J, Ravi K, Lacy BE, Vela MF. Opioid-Induced Esophageal Dysfunction: Differential Effects of Type and Dose. Am J Gastroenterol. 2019;114:1464-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 57. | Jung HY, Puckett JL, Bhalla V, Rojas-Feria M, Bhargava V, Liu J, Mittal RK. Asynchrony between the circular and the longitudinal muscle contraction in patients with nutcracker esophagus. Gastroenterology. 2005;128:1179-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Rohof WOA, Bredenoord AJ. Chicago Classification of Esophageal Motility Disorders: Lessons Learned. Curr Gastroenterol Rep. 2017;19:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 59. | Dell'Acqua-Cassão B, Mardiros-Herbella FA, Farah JF, Bonadiman A, Silva LC, Patti MG. Outcomes of laparoscopic Nissen fundoplication in patients with manometric patterns of esophageal motility disorders. Am Surg. 2013;79:361-365. [PubMed] |
| 60. | Leopold A, Yu D, Bhuta R, Kataria R, Lu X, Jehangir A, Harrison M, Friedenberg F, Malik Z, Schey R, Parkman HP. Multiple Rapid Swallows (MRS) Complements Single-Swallow (SS) Analysis for High-Resolution Esophageal Manometry (HREM). Dig Dis Sci. 2019;64:2206-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Tadros M, Tran V, Shah V, Yodice M. Patterns of esophageal dysmotility elicited by multiple rapid swallows. Esophagus. 2021;18:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Marin I, Serra J. Patterns of esophageal pressure responses to a rapid drink challenge test in patients with esophageal motility disorders. Neurogastroenterol Motil. 2016;28:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 63. | Tadros M, Yodice M. The Challenges of Esaphagogastric Junction Outflow Obstruction, Is It Really a Diagnosis? Dysphagia. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Ang D, Hollenstein M, Misselwitz B, Knowles K, Wright J, Tucker E, Sweis R, Fox M. Rapid Drink Challenge in high-resolution manometry: an adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 65. | Basseri B, Pimentel M, Shaye OA, Low K, Soffer EE, Conklin JL. Apple sauce improves detection of esophageal motor dysfunction during high-resolution manometry evaluation of dysphagia. Dig Dis Sci. 2011;56:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Shaker A, Stoikes N, Drapekin J, Kushnir V, Brunt LM, Gyawali CP. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol. 2013;108:1706-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 67. | Stoikes N, Drapekin J, Kushnir V, Shaker A, Brunt LM, Gyawali CP. The value of multiple rapid swallows during preoperative esophageal manometry before laparoscopic antireflux surgery. Surg Endosc. 2012;26:3401-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 68. | Carlson DA, Crowell MD, Kimmel JN, Patel A, Gyawali CP, Hinchcliff M, Griffing WL, Pandolfino JE, Vela MF. Loss of Peristaltic Reserve, Determined by Multiple Rapid Swallows, Is the Most Frequent Esophageal Motility Abnormality in Patients With Systemic Sclerosis. Clin Gastroenterol Hepatol. 2016;14:1502-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 69. | Zaninotto G, DeMeester TR, Schwizer W, Johansson KE, Cheng SC. The lower esophageal sphincter in health and disease. Am J Surg. 1988;155:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 236] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Tsuboi K, Hoshino M, Sundaram A, Yano F, Mittal SK. Role of the lower esophageal sphincter on esophageal acid exposure - a review of over 2000 patients. Trop Gastroenterol. 2012;33:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 71. | Tolone S, Gualtieri G, Savarino E, Frazzoni M, de Bortoli N, Furnari M, Casalino G, Parisi S, Savarino V, Docimo L. Pre-operative clinical and instrumental factors as antireflux surgery outcome predictors. World J Gastrointest Surg. 2016;8:719-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Jones MP, Sloan SS, Rabine JC, Ebert CC, Huang CF, Kahrilas PJ. Hiatal hernia size is the dominant determinant of esophagitis presence and severity in gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Rogers B, Hasak S, Hansalia V, Gyawali CP. Trans-esophagogastric junction pressure gradients during straight leg raise maneuver on high-resolution manometry associate with large hiatus hernias. Neurogastroenterol Motil. 2020;32:e13836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Lei Y, Li JY, Jiang J, Wang J, Zhang QY, Wang TY, Krasna MJ. Outcome of floppy Nissen fundoplication with intraoperative manometry to treat sliding hiatal hernia. Dis Esophagus. 2008;21:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Lin M, Triadafilopoulos G. Belching: dyspepsia or gastroesophageal reflux disease? Am J Gastroenterol. 2003;98:2139-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Conchillo JM, Selimah M, Bredenoord AJ, Samsom M, Smout AJ. Air swallowing, belching, acid and non-acid reflux in patients with functional dyspepsia. Aliment Pharmacol Ther. 2007;25:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Sawada A, Guzman M, Nikaki K, Sonmez S, Yazaki E, Aziz Q, Woodland P, Rogers B, Gyawali CP, Sifrim D. Identification of Different Phenotypes of Esophageal Reflux Hypersensitivity and Implications for Treatment. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 78. | Bredenoord AJ. Excessive belching and aerophagia: two different disorders. Dis Esophagus. 2010;23:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Holloway RH. Combined impedance-manometry for the evaluation of esophageal disorders. Curr Opin Gastroenterol. 2014;30:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Kessing BF, Bredenoord AJ, Smout AJ. The pathophysiology, diagnosis and treatment of excessive belching symptoms. Am J Gastroenterol. 2014;109:1196-203); (Quiz) 1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, Spechler SJ, Attwood SE, Straumann A, Aceves SS, Alexander JA, Atkins D, Arva NC, Blanchard C, Bonis PA, Book WM, Capocelli KE, Chehade M, Cheng E, Collins MH, Davis CM, Dias JA, Di Lorenzo C, Dohil R, Dupont C, Falk GW, Ferreira CT, Fox A, Gonsalves NP, Gupta SK, Katzka DA, Kinoshita Y, Menard-Katcher C, Kodroff E, Metz DC, Miehlke S, Muir AB, Mukkada VA, Murch S, Nurko S, Ohtsuka Y, Orel R, Papadopoulou A, Peterson KA, Philpott H, Putnam PE, Richter JE, Rosen R, Rothenberg ME, Schoepfer A, Scott MM, Shah N, Sheikh J, Souza RF, Strobel MJ, Talley NJ, Vaezi MF, Vandenplas Y, Vieira MC, Walker MM, Wechsler JB, Wershil BK, Wen T, Yang GY, Hirano I, Bredenoord AJ. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018; 155: 1022-1033. e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 815] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 82. | Camilleri M, Chedid V, Ford AC, Haruma K, Horowitz M, Jones KL, Low PA, Park SY, Parkman HP, Stanghellini V. Gastroparesis. Nat Rev Dis Primers. 2018;4:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 83. | Rome IV Criteria. Rome Foundation. 2016. [cited 20 January 2021]. Available from: https://theromefoundation.org/rome-iv/rome-iv-criteria/. |