INTRODUCTION
The rise of coronavirus disease 2019 (COVID-19) at the end of 2019 became a real and significant challenge to humanity, especially as the outbreak escalated to a global pandemic in March 2020. The challenge was related not only to the rapid transmission of COVID-19 but also to the dilemma and conflict in the clinical presentations, rate of mutation, laboratory diagnosis, and management of the disease. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a virus that is related to a large group of enveloped, positive-stranded RNA viruses called coronaviruses because of the crown-like spikes on their surface. There are four subgroups (genera) of coronaviruses, named alpha, beta, gamma, and delta; the alpha and beta subgroups can cause disease in humans, causing respiratory infections, gastrointestinal infections, and hepatic and central nervous system diseases[1]. The most recent outbreaks of coronavirus belong to beta coronaviruses and include the SARS-CoV outbreak, the Middle East respiratory syndrome coronavirus outbreak, and the recent SARS-CoV-2 pandemic. In humans, coronaviruses primarily affect the upper respiratory tract, causing different manifestations of respiratory infections. However, gastrointestinal manifestations could be the initial presenting manifestation in many patients, especially in children. These manifestations could also be the sole manifestations in a small percentage of patients. This article will review the different gastrointestinal disorders in association with SARS-CoV-2 infection (COVID-19) and discuss their epidemiological and clinical implications[2].
EPIDEMIOLOGY OF COVID-19 AND GASTROINTESTINAL DISORDERS IN CHILDREN
Although it predominantly affects adults of higher age groups, SARS-CoV-2 can infect children at any age, even during the neonatal period; intrauterine or perinatal transmission of the virus is uncertain, and vertical transmission is not yet proven[3]. Children typically form 1%-8% of all laboratory-confirmed cases of COVID-19: 7% in neonates, 29% in infants less than 1 year old, 10% in the 2nd year of life, 11% between 2 and 5 years old, 16% between 5 and 10 years old, and 34% between 10 and 18 years of age[4]. However, the percentage of the disease in children may differ from one country to another and from one ethnic group to another according to the underlying health, social, and economic status of individuals[5]. As the number of children infected is currently increasing, studies have shown that most infected children have mild disease. However, approximately 4.4% of infected children have severe disease, with a mortality rate of 0.2%, with no deaths reported below the age of 9 years. Infants less than 1 year old and children who have other underlying comorbidities are more prone to severe COVID-19. There was no sex difference in the infected children, unlike that observed in adults with a male to female ratio of 1.1:1. The source of infection was usually an infected family member as a part of a family cluster outbreak[6].
The low prevalence of COVID-19 in children is multifactorial. Children have few outdoor activities, especially with school shutdowns, lower travelling rates than adults, and less exposure to smoking and air pollution; hence, children are less likely to be exposed to infection. Their immune status plays an important role in modifying their risk of infection with SARS-CoV-2. Children have a more active innate immune response and a healthier respiratory system than adults, especially children less than 10 years of age. They also respond with a normal or high lymphocytic count in response to infection with SARS-CoV-2, in contrast to adults, who respond with a decreased lymphocytic count. Coinfection with other viruses may limit the replication of SARS-CoV-2 via direct virus-to-virus interactions and competitive inhibition and may be a possible mechanism like what is observed with the common cold and influenza. However, the main factor for the low incidence of COVID-19 in children is related to the age-dependent immaturity of angiotensin-converting enzyme 2 (ACE2) receptors in children and their different distribution and function compared to adults. These receptors serve as a binding site for SARS-CoV-2 and a portal of entry to the inside of the cell by the binding of the viral spike proteins to ACE2 receptors. The degree of cell susceptibility to viral infection is correlated with the number of ACE2 receptors on those cells. However, ACE2 has dual effects on cell infection by the virus. In addition to ACE2 receptors acting as a binding site for the virus, they have a very important pulmonary protective role, as they protect against severe lung injury caused by the virus. Therefore, this dual role of ACE2 pushes us to perform more research to confirm its implication in COVID-19 pathogenesis[7-10]. The low number of children infected with SARS-CoV-2 does not necessarily mean a high resistance to the infection. Another possible theoretical reason is related to Bacillus Calmette-Guérin (BCG) vaccination in children. BCG may provide some protection against COVID-19 as it modulates cell-mediated immunity, including innate cells such as macrophages, monocytes, and epithelia, and many children are vaccinated with BCG[11]. However, the protective effects of BCG against COVID-19 are still unknown. As more than 95% of children infected with SARS-CoV-2 are asymptomatic and less likely to be tested, epidemiological surveillance may be inadequate, and children may still contribute to viral transmission.
Although fever and respiratory manifestations are the most common features of COVID-19, approximately 17.6% of patients present with gastrointestinal symptoms that usually appear 1-2 d before respiratory symptoms. Verified cases of COVID-19 with the sole gastrointestinal manifestation have been described in both adults and children. Gastrointestinal manifestations were more common in patients with severe disease (17.1%) than in patients with non-severe disease (11.8%). Approximately 48.1% of the patients had stool that was positive for viral RNA, even in stool samples collected after the respiratory samples turned negative for the virus. The exact rate of gastrointestinal symptoms is a matter of debate, with various incidences among the different studies. Anorexia and poor appetite were the most common gastrointestinal symptoms (a range between 347% and 67% and a mean of 47%), followed by diarrhoea (range between 2.1% and 32.5% and a mean of 11.6%), abdominal pain and discomfort (range between 1% and 11.9% and a mean of 5.2%), and nausea and vomiting (range between 1% and 11.7%, and a mean of 5.1%), while the incidence of liver damage ranged from 15% to 53%[12-18]. Liver damage is doubled in the presence of gastrointestinal manifestations compared to in the absence of gastrointestinal manifestations. Gastrointestinal manifestations usually deteriorate with disease progression, and severe gastrointestinal bleeding has been reported in some cases[19].
Gastrointestinal manifestations of COVID-19 in children are not rare, with a prevalence between 0 and 88%, and a wide variety of presentations, including diarrhoea, vomiting, and abdominal pain, that can develop before, with or after the development of respiratory symptoms. Atypical manifestations such as acute appendicitis or liver injury could also appear, especially in the presence of multisystem inflammatory disease[20]. An American study including 44 children showed that gastrointestinal manifestations were present in 84.1% of children admitted to the hospital and were most often associated with fever and rash[21]. In a meta-analysis including 280 children from 9 studies, the pooled prevalence of gastrointestinal manifestations was 22.8%. Diarrhoea was the most common presentation (12.4%), followed by vomiting (10.3%) and abdominal pain (5.4%)[22]. In a prospective study including 992 healthy children of healthcare workers in the United Kingdom, approximately 7% tested positive for SARS-CoV-2 antibodies, half of them were asymptomatic, and 19% had gastrointestinal symptoms, including diarrhoea, vomiting, and abdominal cramps[23]. Another study in Wuhan, China showed that gastrointestinal manifestations were observed in 15.2% of children infected with SARS-CoV-2 (diarrhoea 8.8% and vomiting 6.4%). In contrast to adults, gastrointestinal manifestations in children were associated with less severe disease and less need for oxygen therapy[24].
Pathogenesis of gastrointestinal infection in COVID-19
The exact mechanism of gastrointestinal symptoms caused by SARS-CoV-2 infection is still unclear. The detection of SARS-CoV-2 RNA in the stool suggests faecal-oral transmission. The presence of gastrointestinal manifestations before respiratory symptoms in a group of patients, along with the presence of viral shedding from the gastrointestinal tract in cases with a more aggressive clinical course, may indicate the role of the gastrointestinal tract in modifying the course of COVID-19 and help in predicting prognosis[25]. Several studies suggest that the virus actively infects the cells of the gastrointestinal tract, replicating itself in the epithelium of the small and large intestine and producing an excessive immunological reaction in the host[26]. ACE2 is highly expressed in lung alveolar type II cells and the upper oesophagus, in absorptive enterocytes from theileum and colon, and in hepatocytes and cholangiocytes. The ACE2 receptor is an important receptor on the cell membrane of host cells. The interaction between the S protein of SARS-CoV-2 and ACE2 promotes the invasion of host cells by SARS-CoV-2. Abundant expression of ACE2 throughout the endothelial surface of the gastrointestinal tract, especially in enterocytes, may act as a secondary entry site for SARS-CoV-2 infection. The magnitude of ACE2 expression in the gut may ameliorate or worsen gut dysbiosis and gastrointestinal leakage[27]. Dysbiosis and the consequent leaky gut further impair the gut-lung axis and are related to the onset of pulmonary hypertension as well as the hyperactivation of the ACE/Ang II/AT1R (angiotensin II type 1 receptor) axis from ACE2 Loss. At the same time, infection with SARS-CoV-2 causes infiltration with many plasma cells and lymphocytes and potentially induces interstitial oedema and the degeneration of the gut-blood barrier, leading to the spread of viruses, bacteria, endotoxins, and microbial metabolites into the systemic circulation, affecting the host’s response to SARS-CoV-2 infection and inducing multisystem dysfunction and septic shock[28]. Persistent viral faecal shedding alters the gut into a base for sustained viral replication and consequently may explain the associated poor prognosis. On the other side of the gut-lung axis, SARS-CoV-2-infection-induced respiratory lesions impair the respiratory tract microbiota, which adversely compromises the digestive tract through immune dysregulation[29].
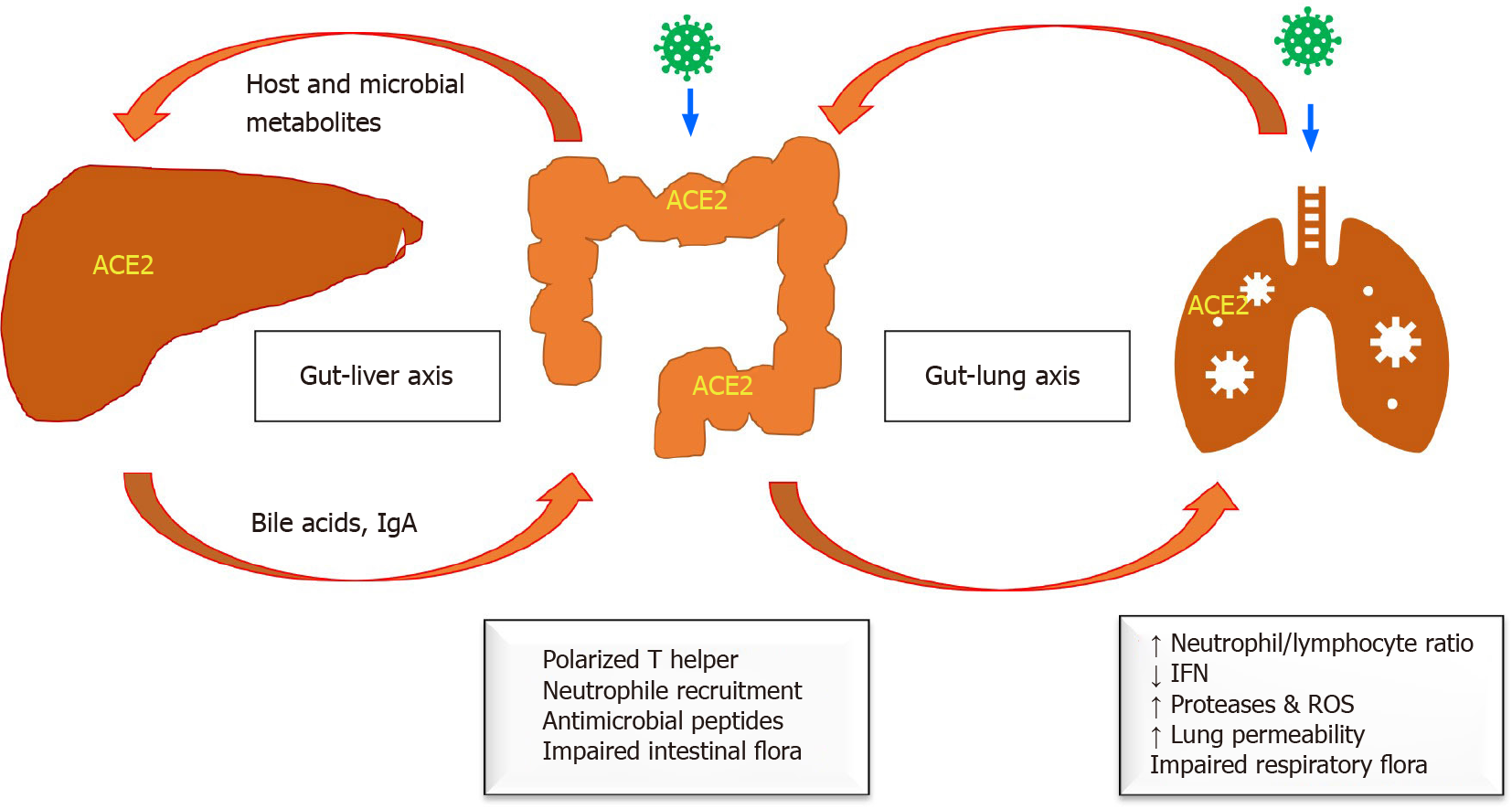
Failure to detect viral RNA in the stool in some patients infected with SARS-CoV-2 that have gastrointestinal manifestations suggests the presence of other mechanisms of indirect gastrointestinal injury in COVID-19 patients. Disturbed cellular immunity has a significant impact on gastrointestinal health in patients with COVID-19. Lung-derived C-C chemokine receptor type 9-CD4- T cells were significantly increased in patients infected with SARS-CoV-2 compared to healthy individuals. These cells are important for mucosal immunity and have a significant role in the development of chronic enteritis by intestinal immune damage and the disruption of the intestinal flora[30]. The resulting dysbiosis will further promote the polarization of Th17 cells in the small intestine, and the production of excessive interleukin (IL)-17A leads to the recruitment of neutrophils, causing more intestinal immune damage, and inducing diarrhoea and other gastrointestinal symptoms[31]. The induced intestinal inflammation impairs the intestinal mucosal barrier, allowing easy access of the bacteria and its toxin to circulate in the blood and affect other organs, including the liver (gut-liver axis). Intestinal inflammation-induced host and microbial metabolites can reach the liver through the portal vein, impairing liver function[32]. Consequently, the inflamed liver releases bile acids and other bioactive materials into the biliary and systemic circulation, reaching the intestines and causing more intestinal lesions (bidirectional effect). The liver could also be affected by the adverse effects of various drugs used to treat COVID-19 (Figure 1).
Figure 1 Pathogenesis of gastrointestinal infection in coronavirus disease 2019.
ACE2: Angiotensin-converting enzyme 2; IFN: Interferon; IgA: Immunoglobulin A; ROS: Reactive oxygen species.
Diagnosis
Gastrointestinal manifestations are present in more than half (57%) of the patients infected with commonly circulating coronaviruses, especially in children. These manifestations are considered a warning in the presence of other medical comorbidities[33]. Children infected with SARS-CoV-2 usually have milder clinical manifestations of the disease than adults, and occasionally, they act as asymptomatic carriers. Gastrointestinal symptoms are common in children with COVID-19; approximately 25% of them exhibit at least one gastrointestinal symptom. These symptoms may occur in the absence of respiratory symptoms. The most common gastrointestinal symptoms in children are diarrhoea, followed by vomiting and abdominal pain. The manifestations usually come in combination. Gastrointestinal bleeding was reported in adults, but no data were reported in children. Abdominal pain is sometimes severe enough to be mistaken with acute abdomen such as acute appendicitis or systemic sepsis[20].
Infants less than one year of age may present with food intolerance or feeding difficulties. These atypical presentations, especially as initial symptoms, may be misleading and lead to delayed diagnosis of COVID-19. Diagnosis is usually based on the detection of viral nucleic acids in respiratory tract specimens, while faecal nucleic acid detection is often neglected. Therefore, children with gastrointestinal symptoms as the predominant manifestation of COVID-19 are often misdiagnosed[34]. Gastrointestinal manifestations are a prominent presenting feature of multisystem inflammatory syndrome in children (MIS-C). When presenting with MIS-C, COVID-19 could be confused with gastrointestinal infections or even inflammatory bowel disease. Therefore, any child with a history of recent SARS-CoV-2 exposure or infection and prominent gastrointestinal symptoms should be considered to have MIS-C, especially in the presence of other clinical comorbidities and extremely high inflammatory markers[21].
Diagnostic tools
The initial diagnostic tools to monitor infected children are the same as those used for adults, i.e., tracing the history of contact with any infected individuals. Real-time reverse transcription-polymerase chain reaction (RT-PCR) testing of nose and throat swabs for the detection of SARS-CoV-2 nucleic acid is the gold-standard confirmatory test for COVID-19. The virus can be detected from the stool until 12 d after disease onset. Stool can still give positive results despite negative respiratory tests. Patients who present with gastrointestinal symptoms may have a longer duration between symptom onset and viral clearance and may have increased faecal virus positivity compared with those who present with respiratory symptoms[35]. Children have a higher nucleic acid positivity rate of faeces and prolonged faecal viral shedding compared to adults[34]. SARS-CoV-2 RNA can be found in stool specimens and anal/rectal swabs, often with more positivity than oral samples, in the late phase of the disease. Next-generation sequencing is utilized to identify SARS-CoV-2 strains and mutations for epidemiological and research purposes. Rapid antigen detection tests depend on the detection of the viral antigen in the nose and throat swab in cases with high viral load and in pre-symptomatic and early symptomatic cases up to five days from symptom onset. These tests offer multiple benefits in comparison to RT-PCR tests, including shorter turnaround times, easier techniques, and reduced costs, especially in situations with limited RT-PCR testing capacity. However, there is a high chance of cross-reactivity with other coronavirus families. Additionally, rapid antigen detection tests have lower sensitivity and specificity than RT-PCR. Therefore, positive results should be confirmed with RT-PCR. On the other hand, antibody detection tests are usually negative during the first 7–10 d of infection, with a high risk of missing cases of SARS-CoV-2 infection[36,37].
There are relatively lower rates of lymphopenia and higher inflammatory markers in children than in adults, and thrombocytopenia may occur. Monitoring the lymphocyte count and C-reactive protein level as signs of severe infection while using procalcitonin levels to detect potential bacterial coinfection is encouraged. Patients with severe infection may have high plasma levels of IL-2, IL-7, IL-10, granulocyte colony-stimulating factor, interferon-gamma-inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-alpha and tumour necrosis factor alpha. Levels of bilirubin and hepatic enzymes are excellent markers for the severity of infections. Elevated lactate dehydrogenase levels, abnormal coagulation, elevated D-dimers, and progressively decreased lymphocytic count may also be observed in severe cases[6,38,39].
Positive faecal occult blood testing indicates upper gastrointestinal bleeding. Endoscopy may show mucosal damage to the oesophageal, gastric, duodenal, and colonic mucosa. Biopsy could show numerous infiltrating plasma cells and lymphocytes, as well as interstitial oedema in the lamina propria of the stomach, duodenum, and rectum. Viral-induced gastrointestinal inflammation causes elevated serum IL-6 levels and elevated levels of faecal calprotectin with significantly higher concentrations in patients with COVID-19 suffering from diarrhoea[26]. The ACE2 protein, used by the virus as a receptor for entry, could be stained mainly in the cytoplasm of gastrointestinal epithelial cells. Immunofluorescence testing may show abundant expression of ACE2 in the glandular cells of the gastric, duodenal, and rectal epithelia[40]. Lung imaging examination has been considered a complimentary confirmation method. Chest X-ray findings in children appear to be nonspecific. However, children with mild disease should not routinely have computed tomography chest imaging in view of high radiation exposure[13].
IMPLICATIONS OF GASTROINTESTINAL INFECTION IN COVID-19
Faecal-oral transmission
Oral gastrointestinal infection with SARS-CoV-2 alters the gastrointestinal tract into a reservoir for viral replication, leading to virus spread to other organs, as well as viral shedding in the stool. Faecal viral shedding could continue for 1 wk after clearance from the respiratory tract. This was observed to be more prevalent in children than in adults, with possible long-term faecal-oral transmission, increasing the potential risk for stools to become a source of the contamination of airdrops and several environmental surfaces and may play an important role in viral spread. Therefore, negative nasopharyngeal or oral samples for the virus may not be sufficient to confirm “non-infectivity”, as the virus might still be intermittently shed in body fluids and excreta, including stool. Adding one negative stool sample may increase the negativity yield, especially in patients with gastrointestinal symptoms. However, intermittent shedding should also be considered. The risk of faecal-oral transmission of SARS-CoV-2 is suspected to increase, especially with studies reporting that viral particles in environmental settings may remain viable in aerosols for up to 3 h and for 72 h on solid surfaces. When combined with poor hygiene, including poor handwashing techniques, children have a high risk of transmitting the disease to their contacts. Children should be taught the importance of proper hygiene in an easily understandable language. They should also avoid contact with elderly people if they test positive for COVID-19[41-43].
Impact on the gut microbiome
The gut microbiome is the collection of microorganisms (bacteria, yeast, fungi, archaea, protozoa, and viruses) found in the gastrointestinal tract, mostly in the large bowel, and it performs many beneficial functions, including immune modulation, with pro-/anti-inflammatory effects. The relation between infection with SARS-CoV-2 and the microbiota is bidirectional. Infection with SARS-CoV-2 is well known to cause the dysbiosis of both the respiratory and gastrointestinal microbiota, which negatively impacts gastrointestinal health and contributes to the development of gastrointestinal disease. The dysbiosis of the gut microbiota creates a suitable environment for SARS-CoV-2 replication and subsequent effects. The inflammatory proteins, cytokines and other mediators that are released because of gut dysbiosis will be augmented with coronavirus infection, which may induce a “cytokine storm” causing more damage than the virus alone, which can ultimately result in multiorgan injury[44]. Zuo et al[45] examined the effect of infection with SARS-CoV-2 on the gut microbiome. They found that the virus induces dysbiosis, an effect that begins even before the start of antiviral therapy. They observed the enrichment of opportunistic pathogens and the depletion of beneficial commensals, an effect that could persist even after the clearance of SARS-CoV-2 from the body, suggesting a more long-lasting detrimental effect on the gut microbiome. They observed a decline in beneficial microbes such as Lactobacillus, Bifidobacterium and Faecalibacterium prausnitzii, which are inversely correlated with the severity of the disease. They also observed the enrichment of Clostridium hathewayi, Clostridium ramosum, and Coprobacillus, which are positively correlated with disease severity[45]. Zuo et al[46] also found heterogeneous configurations of the faecal mycobiome, with the enrichment of fungal pathogens from the genera Candida and Aspergillus, during the hospitalization of patients with COVID-19 compared with controls. This effect persisted even after nasopharyngeal clearance of SARS-CoV-2[46]. Other factors could also play a role in inducing dysbiosis in patients with COVID-19, including the presence of other comorbidities that negatively affect the gut microbiota, such as diabetes mellitus, hypertension, and old age; the use of antibiotics, antivirals, antifungals, and steroids; and other intensive care units milia that could negatively affect and alter the gut microbiome[47].
Impact on patient nutrition
COVID-19 affects the nutrition of patients in many aspects. The poor appetite found in a significant number of patients with COVID-19 makes it difficult to achieve the desired nutrition goals with an oral diet alone, causing undernutrition and sarcopenia. Diarrhoea and vomiting, which are common presentations in children with COVID-19, play an important role in undernutrition development. Anosmia may impair taste perception and interfere with nutritional supplementation or food intake. At the same time, the disruption of normal intestinal mucosal integrity by SARS-CoV-2 could compromise the digestion and absorption of nutrients and may even prevent trophic enteral nutrition[48]. Mechanical ventilation that may be needed in the management of children with severe respiratory disease causes excessive swallowing of air and further gastric distention, which predisposes them to gastroesophageal reflux. Pneumonia and respiratory distress may also cause delayed gastric emptying and intestinal hypomotility, leading to constipation, which is an important factor able to interfere with proper nutritional therapy[49].
Impact on patient management
Gut dysbiosis is an important cause of/and results from gastrointestinal inflammation in patients with COVID-19. Gut microbiota diversity and the presence of beneficial microorganisms in the gut may play an important role in determining the course of the disease. The restoration of gut microbiota diversity could help ameliorate the severity of the disease. Since the gut microbiota is malleable and can be modulated by dietary modification, the addition of specialized pre-/probiotics such as fructooligosaccharides, galactooligosaccharides and various Lactobacillus strains to the diet could help to improve gut dysbiosis, especially for patients presenting with diarrhoea, thereby improving the overall immune response in these patients[50]. Fermented food with probiotics can produce bioactive peptides able to inhibit ACE enzymes by blocking the active sites. Furthermore, fragments of dead probiotic cells can also serve as ACE inhibitors. Consequently, probiotics could have the potential to block the ACE receptor, which acts as an entryway for SARS-CoV-2 to attack gastrointestinal cells[51]. At the same time, prebiotics, probiotics, or symbiotics could be supplied as a prophylactic measure to high-risk groups, such as front-line caregivers and healthy contacts with a suspected case of COVID-19[50]. However, microbiota modulation as a method of treatment of patients with COVID-19 is based on indirect evidence and needs further study.
Dietary management plays an important role in patients with COVID-19. Oral intake is the preferred method of nutrition if the patient condition allows. Oral intake is of paramount importance, as a lack of nutrient contact with intestinal mucosa could lead to the atrophy of lymphoid tissue and functional decline of the immune system, as well as the intensification of bacterial translocation. The patients are advised to take a high-calorie and high-protein diet (1-2.5 g/kg/d) to maintain adequate metabolic functions and proper body weight. If the nutrition targets are not met by the oral diet, oral nutrition supplements are recommended and should be started within 1-2 d of hospitalization. Supplementation with digestive enzymes can be given for patients with poor general conditions and impaired digestion. Breast milk is a true immune nutrient, and breastfeeding should be encouraged[52]. If oral intake fails or is not tolerated, enteral nutrition is still the preferred method of nutrition therapy, as it stimulates the gut. Gastric feeding is preferred to optimize digestive activity, and the post-pyloric route (preferably jejunal) is recommended in the presence of a high risk of reflux aspiration, intolerance, or the failure of nasogastric tube feeding. Prokinetic agents may be used to enhance motility prior to this intervention. As enteral feeding (especially when using post-pyloric tubes) carries a high risk of aerosol generation, healthcare professionals should use proper personal protective equipment during insertion of the tubes. Enteral nutrition ensures adequate anthropometric progression and decreases the length of hospital stay, costs, mortality rate, and septic complications compared with parenteral nutrition. Overfeeding should be avoided in patients with COVID-19 and severe respiratory disease, as this can increase the risk of hypercapnia. The prone position during enteral feeding is permitted and may lead to a better prognosis. Parenteral nutrition is indicated when nutrition targets cannot be met by enteral nutrition or gastrointestinal intolerance is present despite different measures to address intolerance[53-55].
Occasionally, children with severe gastrointestinal symptoms may need endoscopy. This procedure could stimulate excessive saliva and other digestive secretions that increase the risk of choking, vomiting, or diarrhoea with a greater risk of transmission of infection during digestive endoscopy, biopsy, and treatment via an airborne route, including the aspiration of oral and faecal material via endoscopes. Therefore, these procedures should be performed in a specialized endoscopic operation room, if possible, with negative pressure with standard level-three protection and strict disinfection procedures[56,57].
Impact on misdiagnosis and prognosis
Children with COVID-19 have been reported to present as asymptomatic or with only mild symptoms of the respiratory or gastrointestinal system. Infants are more likely to present with atypical clinical symptoms than older children. A study reported five infants diagnosed with COVID-19 who presented with isolated fever and reduced oral intake without associated respiratory symptoms[58,59]. The characteristics of gastrointestinal symptoms in COVID-19 are more insidious than the characteristics of respiratory symptoms, making them easy to overlook. However, some patients might have only gastrointestinal symptoms during the whole course of the disease, and some continue to shed the virus in faeces, despite respiratory samples testing negative[60]. An awareness of these insidious or atypical presentations will allow appropriate investigation and help to decrease the risk of misdiagnosis and consequently decrease the risk of the spread of the disease in the community.
Compared to patients without digestive symptoms, those presenting with digestive symptoms have a longer time from onset to admission and a worse prognosis[61]. A study by Chen et al[62] showed that the presence of gastrointestinal symptoms was associated with a high risk of adult respiratory distress syndrome, non-invasive mechanical ventilation, and tracheal intubation, but not mortality, in patients with COVID-19[62]. Another study by Zhou et al[63] showed that patients with gastrointestinal symptoms have a similar rate of complications, treatment, and clinical prognosis as patients without gastrointestinal symptom in medical and nonmedical staff[63]. However, these studies were performed in the adult population and cannot be extrapolated in children.