Published online Mar 28, 2021. doi: 10.3748/wjg.v27.i12.1132
Peer-review started: November 29, 2020
First decision: December 31, 2020
Revised: January 10, 2021
Accepted: February 24, 2021
Article in press: February 24, 2021
Published online: March 28, 2021
Processing time: 115 Days and 20.9 Hours
Localized gastric amyloidosis (LGA) is a rare disease characterized by abnormal extracellular deposition of amyloid protein restricted to the stomach and it is confirmed by positive results of Congo red staining. Over decades, only a few cases have been reported and studies or research focusing on it are few. Although LGA has a low incidence, patients may suffer a lot from it and require proper diagnosis and management. However, the pathology of LGA remains unknown and no overall review of LGA from its presentations to its prognosis has been published. Patients with LGA are often asymptomatic or manifest atypical symptoms, making it difficult to differentiate from other gastrointestinal diseases. Here, we report the case of a 70-year-old woman with LGA and provide an overview of case reports of LGA available to us. Based on that, we conclude current concepts of clinical manifestations, diagnosis, treatment, and prognosis of LGA, aiming at providing a detailed diagnostic procedure for clinicians and promoting the guidelines of LGA. In addition, a few advanced technologies applied in amyloidosis are also discussed in this review, aiming at providing clinicians with a reference of diagnostic process. With this review, we hope to raise awareness of LGA among the public and clinicians.
Core Tip: Localized gastric amyloidosis (LGA) is rare. Few case reports are available to the public. It is often misdiagnosed as other gastrointestinal diseases due to its atypical manifestation. However, no systemic reviews or guidelines of LGA is published now. Therefore, we present a detailed overview from its clinical manifestations to prognosis for the first time. Based on that, a clinical diagnostic procedure is provided and may benefit clinicians who manage LGA.
- Citation: Lin XY, Pan D, Sang LX, Chang B. Primary localized gastric amyloidosis: A scoping review of the literature from clinical presentations to prognosis . World J Gastroenterol 2021; 27(12): 1132-1148
- URL: https://www.wjgnet.com/1007-9327/full/v27/i12/1132.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i12.1132
Amyloidosis is a group of conformational diseases characterized by the accumulative extracellular deposition of insoluble fibrils in various tissues and organs as a result of protein folding disorders[1]. Large deposits may lead to the loss of the normal structure of tissues, subsequent organ dysfunction, and even death. At present, 36 different amyloid fibrils have been identified that are associated with amyloidosis[1]. Amyloid fibrils determine the properties of the amyloid diseases, and the subtypes of amyloidosis are also named after the corresponding fibrils. For example, light chain (AL) indicates that amyloid fibrils are derived from immunoglobulin light chains, and the resulting disease is referred to as AL amyloidosis[2]. According to the amyloid distribution, amyloidosis is divided into systemic and localized amyloidosis. Systemic amyloidosis is universal, while localized amyloidosis is a rare condition that only comprises 12% of newly identified amyloidosis cases[3].
Amyloidosis is a rare disease, among which AL amyloidosis is the most common type. An epidemiological study in Sweden reported an incidence of nonhereditary amyloidosis of 8.29 per million person-years, among which AL amyloidosis accounted for 3.2 per million person-years[4]. A nationwide study in the United States reported an increasing incidence of AL amyloidosis, from 9.7 per million person-years in 2007 to 14.0 per million person-years in 2015[5]. Although rare, amyloidosis can result in a severe disease burden, as reflected by patients’ poor scores on assessments of the health status compared to the general population in a recent study of 341 patients[6]. Moreover, without proper intervention, it may ultimately develop into a fatal disease. From 2000 to 2008, 0.58 per thousand deaths were due to amyloidosis in England, and its proportion of deaths has doubled, indicating a tendency to increase[7].
Gastrointestinal involvement manifests as systemic amyloidosis (79%), while it is relatively rare in localized cases (21%), according to a retrospective study of 76 patients of biopsy-proven gastrointestinal amyloidosis evaluated in 1998-2011[8]. Localized gastric amyloidosis (LGA) is an extremely unusual condition. Generally, it refers to amyloidosis confined to the stomach without evidence of potential plasma cell dyscrasia or the involvement of other organs, particularly the heart, liver, kidney, or nerve[3,8]. More specifically, the precursor protein of amyloid is produced and deposited in the stomach without detection in a remote site[9]. According to a retrospective study of gastrointestinal biopsies from 542 patients, the most common amyloid subtype in the stomach is AL (λ), followed by transthyretin (ATTR), AL (κ), and serum amyloid A (AA)[10].
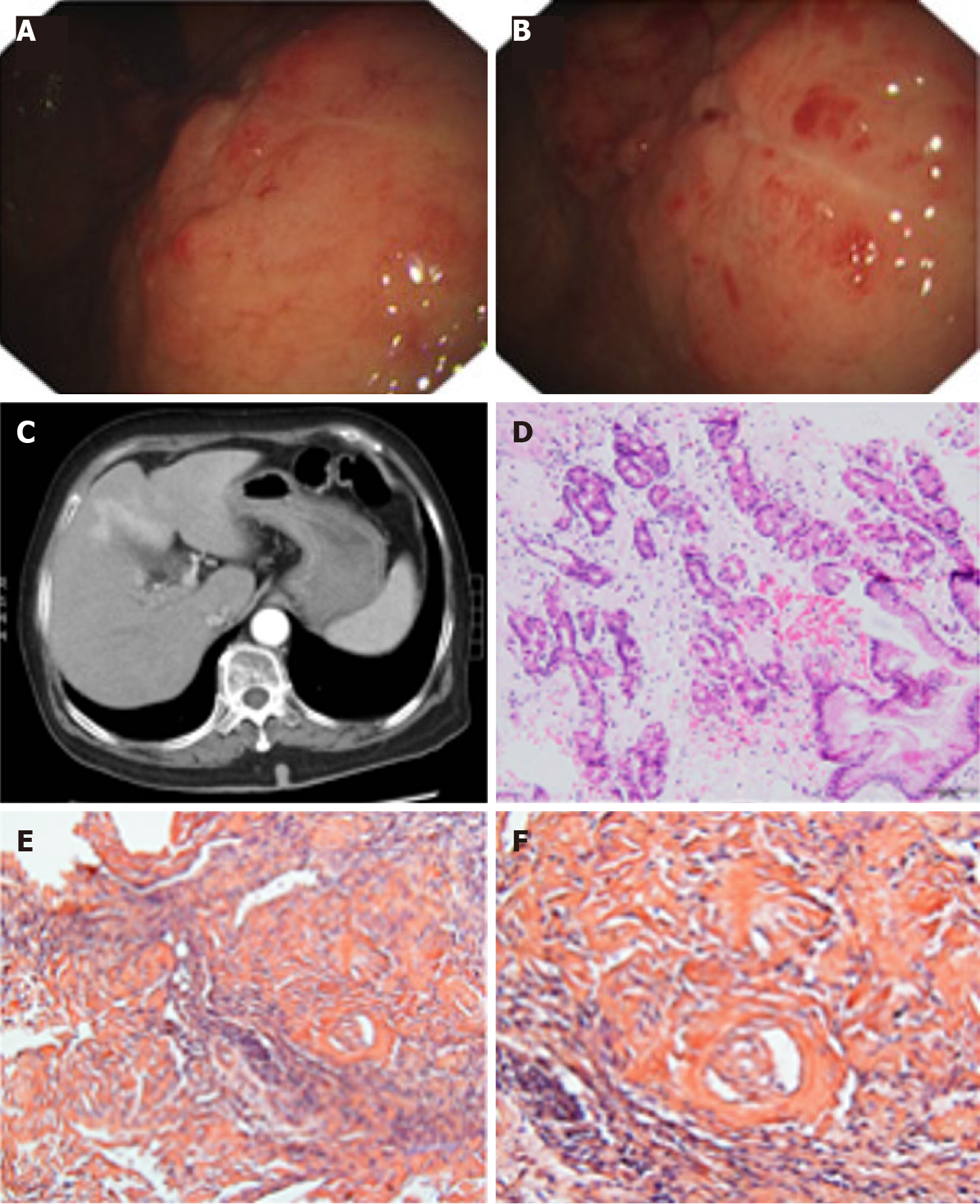
Given the rare reports and unsolved problems associated with LGA, we present a case of LGA (Figures 1 and 2) and collect several recent case reports of LGA available in the literature to present its clinical manifestations, diagnosis and differential diagnosis, and treatment. In addition, we will describe our understanding of its pathogenesis.
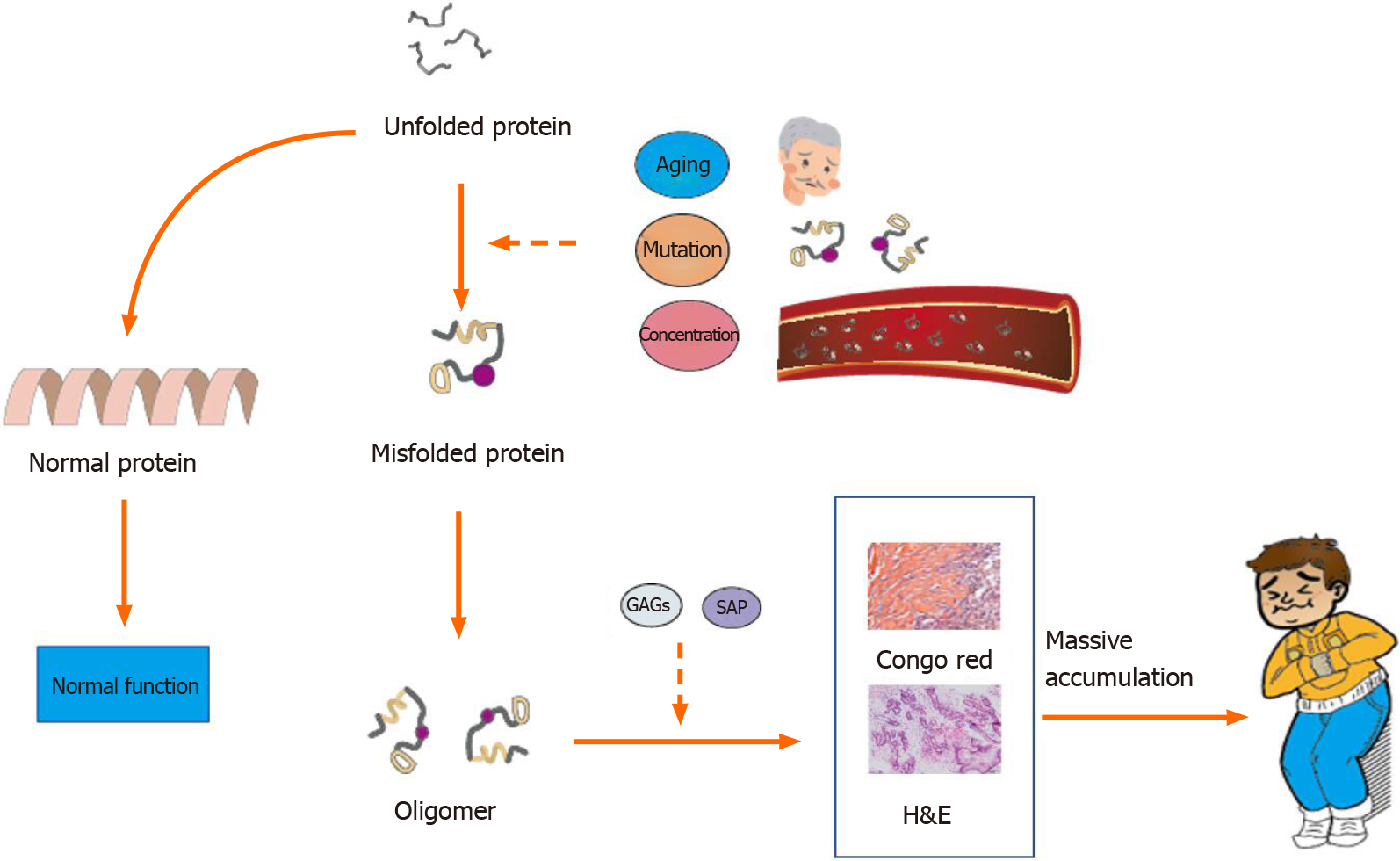
To date, the pathogenesis of amyloidosis is unclear, but current studies and hypotheses provide a few insights. All amyloid fibrils share the same antiparallel cross-β secondary structure, a structure with a high propensity for self-aggregation, as observed under an electron microscope[11,12]. Tightly bound β-sheets form the protofilament, and several protofilaments twist and eventually form amyloid fibrils[13]. Proteins may have a potential intrinsic propensity of misfolding that is influenced by multiple factors, such as aging and stably high concentrations in serum[11]. For example, wild-type TTR forms amyloid fibrils in older individuals, even at normal concentrations, while serum amyloid A and β2M only become amyloidogenic at a persistently high concentration[14]. Different amyloid fibrils may be susceptible to different conditions that trigger misfolding. Mutations may induce the formation of amyloidogenic proteins[15,16]. Mutations in genes that encode amyloid fibrils, such as TTR, trigger familial amyloidosis[17]. Somatic mutations have been identified in AL amyloid fibrils. The N-terminal strand of the light chain variable domain prevents protein aggregation, and its mutation destabilizes the protein and accelerates light chain fibrillogenesis[18,19]. However, the exact relationship between mutations and amyloidosis remains unknown. In addition, further thermodynamic investigations reveal that many environmental factors, such as temperature and pH, are related to the conformational stability of amyloid fibrils and may be critical factors contributing to the conversion of a normal protein into amyloid fibrils[13,20] (Figure 3).
In addition, several protein cofactors, nonfibrillar components, glycosaminoglycans (GAGs), and serum amyloid P (SAP) are present in all amyloid deposits and are believed to function in amyloidogenesis and persistence. GAGs, a main component of the extracellular matrix, are associated with amyloid fibrils in AL amyloidosis, and their size and charge may play an important role in the acceleration and stability of amyloid formation[19]. SAP is a type of plasma glycoprotein that binds to all types of amyloid fibrils in its calcium-bound state and protects them from proteolysis[21] Amyloid fibrils are digested in vitro by proteases and phagocytic cells, while amyloid fibrils likely exhibit relative stability in vivo with the assistance of SAP[22]. According to an in vitro study, SAP may accelerate and stabilize the formation of Aβ42, the amyloid fibrils responsible for Alzheimer’s disease[23]. The severity of amyloid deposition in SAP knockout mice is decreased considerably[24]. Therapies targeting SAP have been tested and confirmed to exert stable effects[25].
The mechanism by which amyloid deposits damage the organs and lead to dysfunction is unclear. The site of deposition may be related to multiple factors, such as the pH, protein concentration, proteolytic processing, and fibril seeds. Different amyloid fibrils exhibit a preference for specific organs; for example, β2-microglobulin prefers joints[11]. In patients with LGA, amyloid is universally present in the walls of small vessels, and most of these amyloid deposits are classified as AL amyloidosis (12/22). In cardiac amyloidosis, amyloid deposits in small vessels lead to symptoms of cardiac ischemia[26]. In gastric amyloidosis, we are able to detect the same distribution patterns, but no connections have been reported to date. Furthermore, the toxicity of amyloidogenic light chain proteins (AL-LC) may be responsible for this condition. An investigation of the potential mechanism revealed that the injection of human AL-LC within a zebrafish model causes cell death. Human AL-LC induces intracellular oxidative stress and alters the cellular redox status, eventually leading to cardiac dysfunction, which is not attributed to the deposition of amyloid protein[27]. As shown in another study, AL-LC mediates cardiomyocyte apoptosis and dysfunction through the activation of p38 mitogen-activated protein kinases[28]. Lysosomal dysfunction has also been reported to provoke the proteotoxicity of AL-LC by contributing to impaired autophagy[29]. Investigations of the precise molecular mechanism of cardiac amyloidosis are ongoing and may explain how the amyloid protein contributes to organ damage. These studies may reveal the mechanism and inspire further studies of gastric amyloidosis.
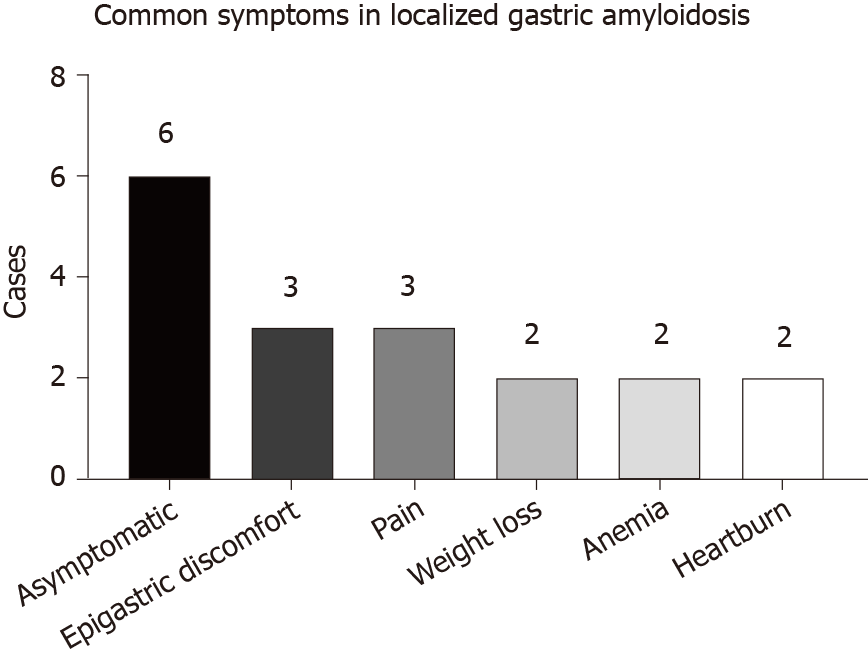
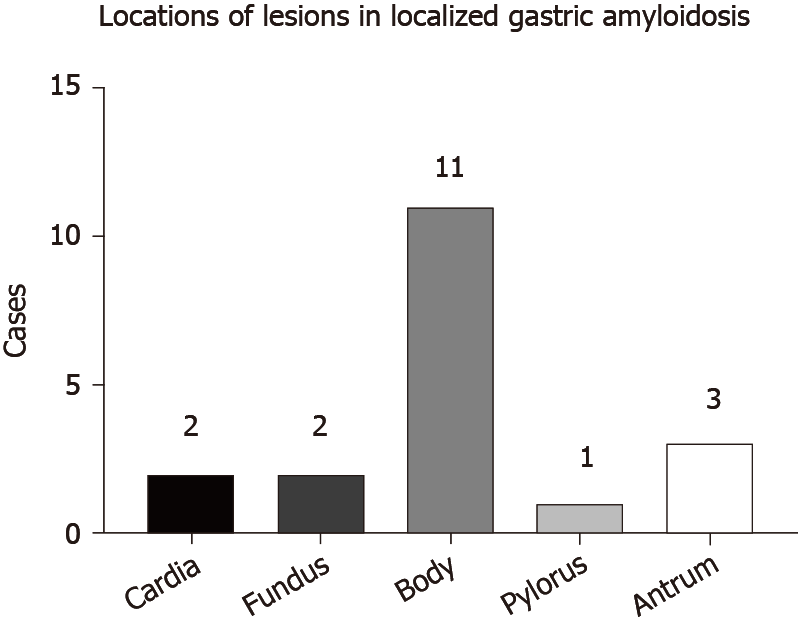
LGA mainly targets middle-aged and elderly people aged from 50 to 80 years. Equal numbers of male and female patients are affected, and no sex differences have been detected. The clinical manifestations of LGA often mimic other common gastric diseases and lack specificity, ranging from an asymptomatic disease to epigastric discomfort, pain, weight loss, anemia, heartburn, nausea, hematemesis, tarry stool, fatigue, and other symptoms (Figure 4). Generally, these manifestations depend on the sites and extent of amyloid involvement[30]. Patients in our reviewed case studies often presented without a chief complaint, and LGA was generally diagnosed based on the results of tests for other gastric diseases. Among the 22 cases that we reviewed, 13 cases of AL LGA and 1 case of AA LGA were identified. Most AL LGA cases manifest asymptomatically[31-35] or with epigastric discomfort[36,37], while AA LGA exclusively manifests as epigastric discomfort[38] (Table 1). To date, no association between symptoms and amyloid types has been confirmed[39]. Further discussion of whether a correlation exists between clinical presentations and amyloid fibrils in patients with LGA is worthwhile.
| Ref. | Age/sex | Symptom | Gross | Size | Location | Endoscopic ultrasound | biopsy | Amyloid type | Exclusion test | Suspected diagnosis | Treatment |
| Ikeda et al[59], 1978 | 68/F | Epigastric pain, nausea | One grayish-white mural elastic soft tumor with an irregular shape and poor margins; thickened and uneven mucosa, partly nodular; swollen mucosal folds | 6 cm × 5 cm | Antrum | / | Congo red (+); amyloid deposits in vessel walls; H&E staining: Amyloid deposits with foreign-body reactions; small nodules of amyloid proteins with a scattered distribution | / | Biopsy of the skin rectum, gingiva and liver; urine Bence-Jones protein levels | Gastric carcinoma | Surgery |
| Dastur et al[40], 1980 | 50/M | Abdominal distension, worse after meals | One mass with central small ulceration and defective mucosa | / | Antrum | / | The mass extended from the mucosa to superficial muscularis and consisted of lymphocytes and germinal centers; normal plasma cells and amyloid proteins; Congo red (+) | / | Urine Bence-Jones protein (-) | / | Surgery |
| Björnsson et al[45], 1987 | 60/F | Hematemesis | A considerable amount of blood, bleeding and irregular, thickened mucosa folds | / | / | / | H&E staining: Amyloid deposits in the lamina propria and muscularis mucosae, infiltration of plasma cells, mucosa atrophy; Congo red staining: Amyloid deposits in the submucosa, muscularis propria and subserosa, mainly around vessels | AL (κ&λ) | Biopsy of rectum, gingiva, cervix and bone marrow; analysis of renal function; urine analysis | / | Surgery |
| Yanai et al[31], 1991 | 52/F | None | One irregular erosion | 2.5 cm | The lower body of the stomach | Thickened mucosa and submucosa | Amyloid deposits in vessels of the mucosa and submucosa | AL (λ) | Examinations of blood, urine and skin; ultrasound and CT of the abdomen; simple X-ray examination of the chest and abdomen; biopsy of the rectum; ECG; ultrasound of the heart; barium enema; tests of antigens, urine Bence-Jones protein, rheumatoid factors and C-reactive protein levels | Gastric cancer | / |
| Wu et al[38], 2003 | 50/F | Epigastric discomfort, dull pain in the upper abdomen | One ulcer with heaped-up rough borders and erosive fragile mucosa | 3 cm × 1 cm | Lesser curvature of the gastric body | Uneven hypoechoic thickened gastric wall with infiltrated submucosa | Amyloid deposits in the mucosa, submucosa and walls visualized using H&E and Congo red staining (-); atrophy of gastric glands and intestinal metaplasia | AA | Biopsy of the bone marrow and other gastrointestinal tissues (esophagus, duodenum, colon and stomach) (-) | Gastric cancer | Subtotal gastrectomy, clearance of perigastric lymph nodes |
| Shibukawa et al[44], 2004 | 51/F | Tarry stool | One irregular ulcer with swollen edges and dirty slough-like advanced cancer; bleeding | / | / | Structural loss of the first three layers of the gastric wall, a small amount of ascites | H&E staining: Amyloid deposits infiltrated the submucosal connective tissues, lamina propria, and muscularis mucosae and were mainly observed around vascular walls in the submucosa; Congo red (+) | AL or primary type | / | Carcinoma | Partial gastric resection |
| Deniz et al[41], 2006 | 67/M | Fatigue, weight loss, poor appetite | One mass | 5 mm × 5 mm × 5 mm | Paracardiac region | / | H&E staining: Amyloid deposits in the mucosa; Congo red (+) | / | Biopsy of other gastrointestinal tissues (-); urine Bence-Jones protein (-) | / | / |
| Rotondano et al[60], 2007 | 55/M | Epigastric pain, heartburn, weight loss | Two white-yellow granular-like circular areas | 3 cm | Distal portion of the gastric body and angle of the stomach | Mucosal and submucosal layers exhibited slight thickening | H&E staining: Lymphocytes and polyclonal plasma cells infiltrated the lamina propria; Congo red (+) | / | Biopsy and endoscopy of the rectum, duodenum and esophagus (-) | / | None |
| Ebato et al[62], 2012 | 77/F | Anemia | One flat, depressed area | 46 mm × 28 mm | Lower gastric body | / | H&E staining: Amyloid deposits in the mucosa and submucosa; DFS staining (+) | AL | / | / | Endoscopic removal |
| Sawada et al[46], 2012 | 72/F | / | Flat elevations, tumors; ulcers resemble advanced cancer, intramural hematomas | / | Scattered distribution in the antrum, proximal and middle stomach | Structural loss, thickened hypoechoic mucosa and submucosa | Congo red (+) | AL (κ&λ) | Biopsy and endoscopy of other gastrointestinal tissues (-) | / | / |
| Rivera et al[42], 2012 | 67/M | Melena, anemia | One round and erosive mass with errhysis | 2.5-3 cm | Cardia | / | Confirmed amyloidosis | / | Biopsy of bone marrow (-) | Gastric adenocarcinoma | Surgery, hematology consultations |
| Kamata et al[36], 2012 | 76/F | Epigastric discomfort | Multiple swollen and reddish folds with a hemorrhagic and erosive mucosa | / | Greater curvature of the gastric body | Thickened submucosal layer | Amyloid deposits in the submucosa and mucosa, Congo red (+) | AL | Biopsy of the rectum and ileum (-); Bence-Jones protein (-); echocardiography (-) | Gastric carcinoma | None |
| Jin et al[61], 2014 | 33/F | Epigastric pain, dyspepsia, heartburn, acid reflux | One area with irregular borders and a hemorrhagic mucosa; another area with normal borders and smooth surfaces | 1.2 cm × 1.2 cm; 10 mm × 20 mm | Lesser curvature of the gastric body; gastric fundus | Hypoechoic thickened stratum mucosum and lamina muscularis protruded in the lesser curvature; nonechoic lesions in the fundus | Amyloid deposits detected from the submucosa to muscularis propria and around small blood vessels using H&E staining; Congo red (+); van Gieson staining (-) | / | / | / | ESD; DMSO |
| Yamaguchi et al[47], 2015 | 49/M | / | One elevated lesion similar to a submucosal tumor | 15 mm | Greater curvature of the lower body | A hypoechoic mass with hyperechoic spots in the submucosa and the muscular layer | Amyloid deposits in the submucosa; Congo red (+) | AL | Biopsy of other tissues in the gastrointestinal tract (-) | / | / |
| Kobara et al[37], 2015 | 80/M | Epigastric discomfort | One granular, elevated lesion | 20 mm | Posterior wall of the prepyloric ring | A hypoechoic mass in the submucosa | Congo red (+) | AL | / | / | / |
| Kagawa et al[32], 2016 | 73/M | None | One pale, depressed area with clear borders | 15 mm | The anterior wall of the lower gastric body | / | H&E staining: Amyloid deposits in the lamina propria and submucosa; Congo red (+) | AL | CT of the chest, abdomen and pelvis (-); urine Bence-Jones protein (-); electrocardiogram, echocardiography | / | None |
| Ahn et al[33], 2018 | 55/F | None | One pale, round, central-depressed area with irregular and heaped-up edges | 20 mm | Lesser curvature of the mid-gastric body | / | Lymphoplasmacytes and Congo red (+) staining in the lamina propria | AL (κ&λ) | Biopsy of colon and duodenum (-); echocardiography (-); CT of chest abdomen and pelvis (-); antineutrophilic antibodies, rheumatoid factors, serum immunoglobulin and components, antinuclear antibodies and urine Bence-Jones protein | cancer | None |
| Ding et al[53], 2018 | 54/M | None | One well-defined lesion with irregularly distorted vessels | / | / | Thinned superficial mucosa, thickened deep mucosa | Congo red (+); H&E: Amyloid deposits in the mucosa | / | / | Early gastric cancer | Surgery |
| Kinugasa et al[34], 2018 | 64/M | None | One submucosal tumor with a hard elastic character | 40 mm | Middle body of the greater curvature | In the second and third layers of the mucosa | Congo red (+) staining in the mucosal propria | AL (λ) | Bone marrow biopsy; biopsy of other gastrointestinal tissues; ultrasound and CT of the liver, kidney and heart | Myoma, malignant lymphoma, gastrointestinal submucosal tumor | / |
| Savant et al[43], 2018 | 64/M | / | One mass | 3.6 cm | / | One hypoechoic mass in the muscularis propria | Congo red (+); H&E staining: Amyloid deposits with a foreign-body giant cell reaction | AL (λ) | CT, urine analysis and serology (-) | Gastrointestinal stromal tumor | / |
| Matsueda et al[35], 2019 | 59/M | None | Multiple pale and depressed lesions | / | Through the stomach | / | Congo red (+) | AL | Biopsy of other gastrointestinal tissues; Bence-Jones protein (-); ultrasound and CT | Healing gastric ulcer | / |
| Present case | 70/F | Hematemesis | Multiple congestive erosions; one area of the mucosa with edema and ulcers exhibited unclear boundaries | 4.0 cm × 4.0 cm | The anterior wall of the gastric body and fundus | / | Congo red (+); H&E staining: Massive amyloid fibrous connective tissues deposited in the interstitium with inflammatory cell infiltration | / | CT of the liver, colon, kidney; HRCT of the lung; ultrasound of the liver, heart, and kidney | / | None |
Endoscopic results have identified variable features in patients. Amyloid deposits mainly invade the gastric body and antrum (Figure 5). Generally, the deposits manifest as single or multiple lesions in the form of a mass[40-43], ulcer[38,44], fold[36,45], elevation[37,46], or submucosal tumor-like feature[34,47]. The borders may be clear or unclear. These findings are consistent with those of previous studies. A remote study of 37 patients with gastrointestinal amyloidosis revealed that erosions were the most common presentation in the stomach, followed by granules, ulcers, and mucosal friability[48]. However, a recent study provided a different order of a normal appearance, followed by erythema, erosions, and nodularity[39]. The samples included both localized and systemic amyloidosis, and thus, the difficulty in distinguishing localized amyloidosis from systemic involvement simply based on endoscopic features is noted given the similarities in appearance. Furthermore, some patients appear normal in endoscopic evaluations, and endoscopy should therefore not be used for an independent diagnosis[39].
After reviewing our data, a reasonable hypothesis is that amyloid subtypes and endoscopic findings are correlated, based on the similar appearance and features noted among the patients with the same amyloid subtype. The endoscopic appearance corresponded to amyloid subtypes in the small intestine, which is the most frequent site of gastrointestinal amyloidosis. For instance, the endoscopic presentation of AL is mostly thickening (75%) and multiple polypoid protrusions (63%), whereas a fine granular appearance is predominant in patients with AA (85%)[49]. However, no association has been confirmed in the gastrointestinal tract according to a study with a small sample size, consistent with the results of the study by Samar M[39,50]. Considering the small sample sizes and limitations of published studies, further studies with large samples are required.
Narrow-band imaging with magnifying endoscopy (NBI-ME), a convenient technique that visualizes the mucosal morphology and microvascular architecture without a dye[51], is also highlighted in the amyloidosis diagnosis. Commonly, it is applied to detect malignancy in the colon and rectum through precise measurements of pit patterns with a promising accuracy (93.4%), sensitivity (100%), and specificity (75%)[52]. To date, the emerging use of NBI-ME has been reported not only in patients with LGA[32,46,47,53] but also in patients with amyloidosis of the rectum, trachea, and pleura[54-56]. Upon enhancement, special pathological patterns are detected in affected lesions, manifesting as abnormal distorted vascular networks on the mucosa with a grayish-green appearance and grooved surface, which may relate to amyloid protein deposits[32,46,56]. The application of NBI-ME provides a better method to reveal the amyloid deposits as a useful tool to assist with the diagnosis.
Endoscopic ultrasound (EUS) is also useful for determining the diagnosis by revealing the loss of the normal structure and thickened hypoechoic gastric walls, which mainly affects the mucosa and submucosa (Table 1). Its use in combination with other methods has certain clinical value, such as EUS-guided fine needle aspiration (EUS-FNA). In a retrospective study of 47 patients with amyloidosis presenting with lymphadenopathy (swollen lymph nodes), the involvement of the gastrointestinal tract was noted in 39% of cases[57]. In addition, EUS-FNA displayed a favorable sensitivity (83%), specificity (94%), and accuracy (86%) for distinguishing malignancy, although the size of the swollen lymph nodes may influence the accuracy[58]. The clearance of peripheral lymph nodes was once suggested as a preferred method to prevent malignancy[38]. However, with the assistance of EUS-FNA, the resection of swollen lymph nodes is not necessary in every case. Further use of EUS is expected.
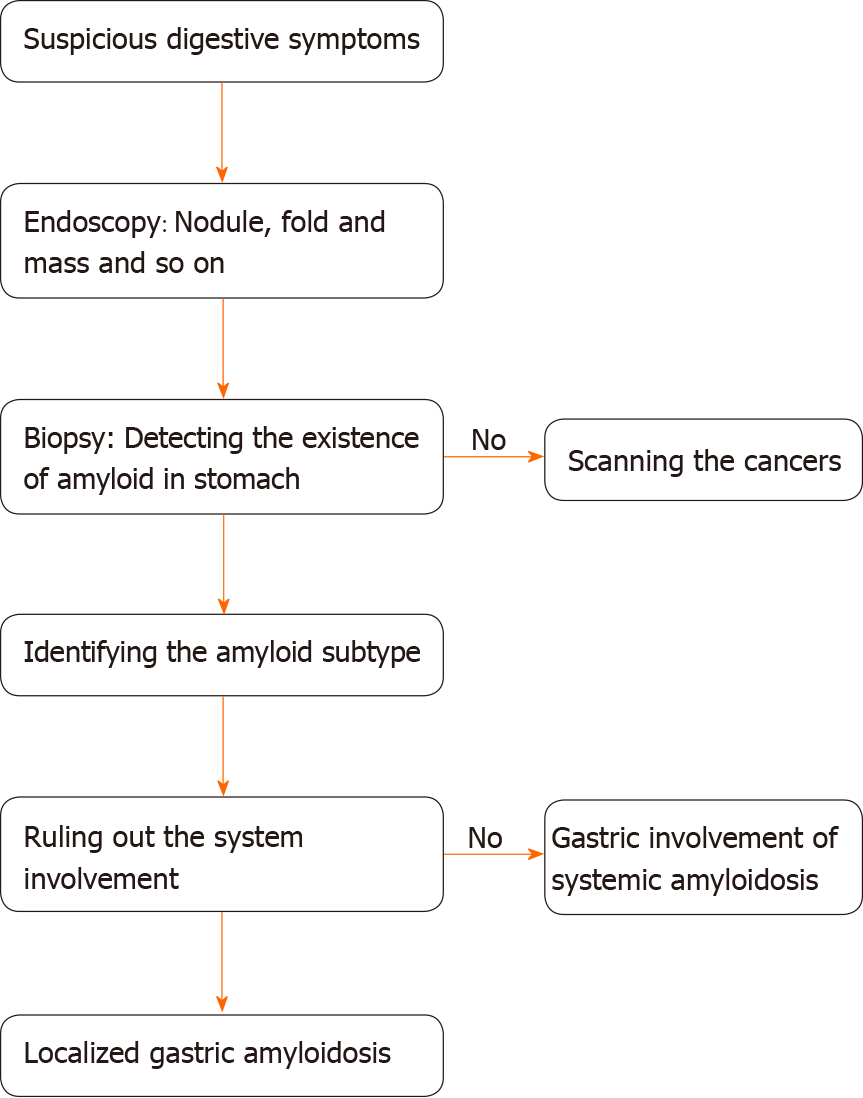
Biopsy is an essential assessment for diagnosing amyloidosis. Direct biopsy verification in patients with symptoms is the main diagnostic criterion for gastrointestinal amyloidosis, according to the guideline of AL amyloidosis[30]. Specific staining methods, such as H&E staining and Congo red staining, are used to visualize the amyloid deposits. Under a light microscope, amyloid deposits typically manifest as amorphous eosinophilic hyaline materials after H&E staining[38]. Amyloids with foreign-body reactions[43,59] and plasma cell infiltration[40,45,60] are also noted. The gold standard for identifying amyloid protein is Congo red staining. When bound to the dye, amyloid deposits are orange under a light microscope, and exhibit green, orange, or yellow birefringence under a polarized microscope[1,12]. Congo red staining is commonly used to diagnose all types of amyloidosis and has been applied to various tissue samples. Most of the case reports collected in the present study use and present the results of both of H&E and Congo red staining to confirm the diagnosis. Biopsy findings also show that amyloids are mainly deposited in the lamina propria, submucosa, and mucosa. Amyloid involvement of blood vessels is also observed[31,38,59,61]. AA LGA is characterized by deposits in the mucosa, submucosa, and vascular walls[38]. AL LGA deposits are mainly located in the mucosal propria, submucosa, and mucosa[32,34,36,47,62]. In a study of 79 cases of gastric amyloidosis, depositions occurred in the muscularis mucosae, but our data do not reveal this characteristic, likely due to the limited number of cases and exclusion of patients with systemic gastric amyloidosis[39]. Interestingly, the amyloid subtypes may account for the difference in deposit locations, which has been verified in several studies. In patients with gastric amyloidosis, AA amyloid deposits are preferentially located in the lamina propria, while AL is mainly deposited in the muscularis mucosae[63].
Although useful, Congo red staining has limitations. For example, when inadequate amyloid labeling occurs or the procedure is performed by inexperienced examiners in poorly controlled conditions, it presents limitations and improved methods have been constantly introduced over the years[14,64]. The application of some hypersensitive techniques may improve the accuracy of amyloid detection. Luminescent dye-conjugated polymer (LCP) spectroscopy detects every deposit that Congo red does using an easy method and effectively reduces false positives[65]. Based on these findings, several probes have been used in experiments. In a study with a small sample of patients with systemic amyloidosis, heptameric formic thiophene acetic acid (h-FTAA) was reported to be more sensitive than Congo red staining when detecting amyloid in abdominal fat[66]. Furthermore, h-FTTA detects small amyloid-like structures that are negative for Congo red staining due to its high quantum field. Thus, this method might potentially achieve the early diagnosis of amyloidosis and allow considerable progress in early treatment. Moreover, h-FTTA possesses a high sensitivity and relatively low specificity, but its combination with Mayer’s hematoxylin staining may compensate for its disadvantage in visual contrast[67]. Although the methods that we mentioned above are still in development, a reasonable expectation is the future development of a hypersensitive approach as a replacement for Congo red staining.
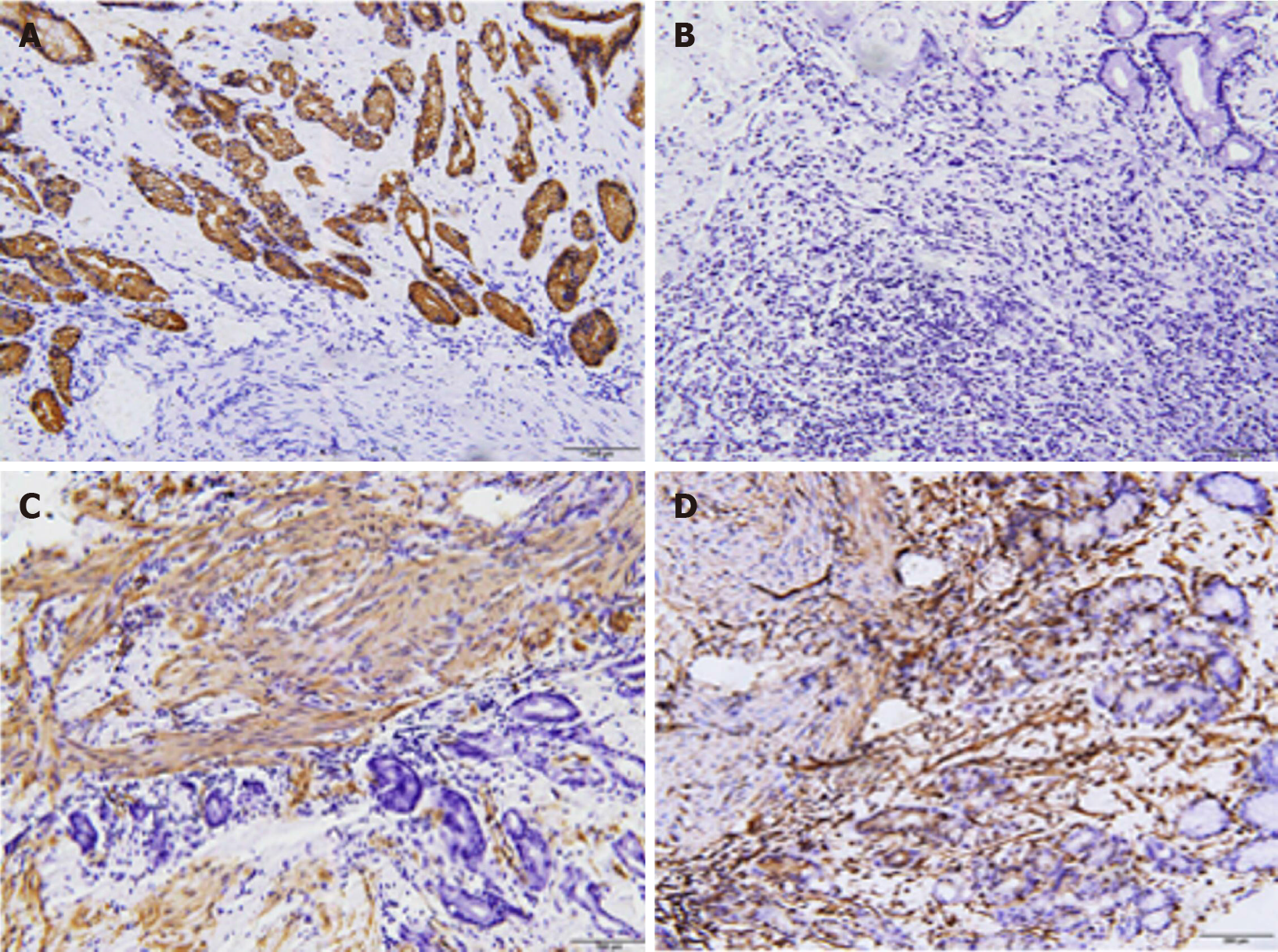
Different forms of amyloid lead to completely different prognoses, and amyloid typing is a routine test performed in patients with amyloidosis. Several assessments have been applied for amyloid typing, including immunohistochemistry (IHC), immunofluorescence (IF) staining, immunoelectron microscopy, and genetic testing[8,16,39]. However, only half (13/22) of the patients receive the test during the diagnosis of LGA, probably due to insufficient tissues and limited techniques[8]. IHC is currently used in the remaining patients and in patients with other forms of amyloidosis, as it is a convenient and rapid method. IHC was demonstrated to be a convincing approach with considerable specificity and sensitivity, according to a systematic study involving 117 patients[68]. However, some of its disadvantages include the presence of unclassifiable and misdiagnosed conditions due to the limited availability, specificity, and sensitivity of antibodies[16,65]. Laser microdissection/mass spectrometry (LMD/MS) requires a small amount of sample and exhibits a high specificity and sensitivity in typing clinical specimens[39,69]. LMD/MS has also been used to analyze samples that do not reach the standards for IF and exhibits a high detection rate of 92% compared to 45% using IHC[70,71]. Moreover, its use in combination with multiple reaction monitoring or liquid chromatography (LMD-LC-MS) results in the detection of amyloid protein that IHC fails to detect and achieves early amyloid detection, even when Congo red results are negative[72,73]. In addition, the decellularization of an amyloid biopsy can facilitate the diagnosis of AL and ATTR amyloidosis, which are rich in plasma proteins. Here, decellularization provides a solution by removing the unnecessary proteins without altering the amyloid deposits and the basic structures of the biopsy[74,75]. Hopefully, these approaches may be used after further testing and reduce costs. Until then, we still call for the urgent increase in the use of IHC to diagnose LGA.
The final diagnosis must be confirmed by the exclusion of the systemic involvement of other organs, which is generally performed using ultrasound of the heart and kidney and biopsy of the bone marrow and regions of the gastrointestinal tract, including the esophagus, duodenum, and colon. Occasionally, urine levels of the Bence-Jones protein and other antigens are tested. Under most conditions, the aforementioned tests are selected based on clinicians’ experience with a screening procedure. Immunohistochemical examinations also provide a lead for the differentiation of systemic and localized amyloidosis, because the deposited protein exhibits specific distribution patterns. For example, AA and ATTR are often detected in the former type, while AL has been detected in both types[2,11].
Some radiopharmaceuticals present potential abilities to distinguish systemic and localized amyloidosis. SAP is one of the nonfibrillar components present in all types of amyloid deposits, and its abundant accumulation makes it an ideal radiotracer to visualize amyloid deposits in images of the body[2,22]. In a study of 189 patients with confirmed amyloidosis, 123I SAP scintigraphy, a noninvasive qualitative method, presented a high sensitivity and specificity and was applied to diagnose most cases of AA and AL amyloidosis[76]. Through the use of whole-body scintigraphy, the injection of 123I SAP obviously reveals the distribution and extent of amyloid deposits in images that a histopathological examination fails to detect, and the organ involvement identified using this technique exceed the results obtained in the clinic, which may be valuable in excluding systemic involvement. The only limitation is its failure in revealing the heart muscle[76]. It is gradually becoming a universal technique used in relevant studies for scanning systemic involvement in the whole body, except for the heart[3,77]. With the development of proper radiotracers and a considerable increase in use, we postulate that nuclear images will significantly contribute to the diagnosis of LGA.
18F-fluorodeocyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is also reported as a potential technique to distinguish systemic and localized amyloidosis. Under most conditions, it is introduced as a method for detecting lung malignancy in patients suspected of having the disease, and it is mainly used to scan amyloidosis in the lung[78,79]. Glaudemans et al[77] reported that 18F-FDG PET/CT is able to visualize the difference between localized and systemic amyloidosis by imaging the inflammatory reaction of multinuclear giant cells, which are unique in localized amyloidosis, manifesting as positive FDG uptake at sites of amyloid deposits in patients with localized amyloidosis but negative uptake in patients with systemic amyloidosis[77]. However, Mekinian et al[80] also reported positive results in patients with systemic amyloidosis, which may be attributed to the limited number of samples or inappropriate designs[80]. To date, 18F-FDG PET/CT is still an immature method to exclude systemic amyloidosis, and patients with gastric amyloidosis were not examined in either study. When used in conjunction with confirmed evidence obtained from other techniques, it may play a supporting role in determining the diagnosis.
Due to its rarity and nonspecific presentations, amyloidosis is typically not the first diagnosis suspected by clinicians, and differential diagnosis becomes an important step. Under most circumstances, patients are scheduled for a precise test screening for potential cancers and are confirmed to have amyloidosis. Most lesions were suspected to be advanced gastric cancers or some gastrointestinal tumors (9/22) since they share common appearances, such as ulcers, elevations, and tumor-like lesions. During gastroscopy or esophagogastroduodenoscopy, some of these lesions are easily suspected to be submucosal tumors[34,46]. The most reliable method for excluding the possibility of tumors is a tissue biopsy that does not detect tumor components and the confirmation of the presence of amyloid based on positive Congo red staining results. Tumor biomarkers are occasionally involved in the examinations based on clinicians’ experience. Although NBI-ME is a new technique, it may also facilitate differentiation, given its ability to exclude malignancies. Based on the clear visualization of the microvasculature and microstructure under NBI-ME, vascular and surface pattern classifications are proposed as a reference, and the typical hallmarks of advanced gastric cancer can be observed, thus providing a reliable evaluation of advanced gastric cancer[81]. A subsequent study reported an obviously increased sensitivity and accuracy of NBI-ME compared with routine approaches in scanning for advanced gastric cancer, and the use of NBI-ME was also beneficial to locate the most suspicious lesion for biopsy[82].
Compared to other case reports, we excluded advanced cancer using a more innovative screening method, namely, immunohistochemical staining, given our restricted conditions. Because gastric carcinoma is suspected in some cases[31,36,59], it may be a wise choice. The combination of several antibodies for immunohistochemical staining, including CKPAN, KL067, Periodic Acid-Schiff, spinal muscular atrophy and vimentin, with biopsy results did not reveal strong support for cancers and excluded the possibility of gastric cancer from various origins.
Generally, the goal of therapy for amyloidosis is to eliminate harmful extracellular amyloid deposits and restore the normal function of affected organs as much as possible. The main treatments include surgery, observation, and radiotherapy[83]. Chemotherapy is recommended for patients with AL who present with myeloma.
Several novel techniques are also introduced here. As we mentioned above, SAP is a type of plasma protein present in all amyloid deposits, and its interaction with amyloid fibrils may prevent the digestion of amyloid, as evidenced by the results of in vitro experiments[21,22]. Because amyloidosis is delayed in SAP knockout mice, SAP clearance is introduced as a potential strategy for treating amyloidosis with the application of relevant antibodies and drugs[24]. Here, (R)-1-{6-[(R)-2-carboxy-pyrrolidin-1-yl]-6-oxo-hexanoyl}pyrrolidine-2-carboxylic acid (CPHPC), a small-molecule drug with a high affinity for SAP, depletes most of the circulating SAP[84,85]. Its use in combination with the anti-SAP antibody dezamizumab results in a considerable reduction in the amount of residual amyloid protein visible in 123I SAP scintigraphy, with no obvious side effects[85,86]. After treatment, patients tend to present an improved or stable state, suggesting its potential ability to ameliorate symptoms and reduce damage in the affected organs[84]. A subsequent study further confirmed its effects on the spleen, kidney, and liver[87]. Currently, studies targeting SAP are mainly conducted in mice and small samples of patients with systemic amyloidosis. Although complete removal of all amyloid deposits has not yet been achieved, its usage in patients with LGA as a rapid method for the early clearance of amyloid deposits is worthy of further exploration.
Currently, the first-line therapy for localized AL gastrointestinal amyloidosis is mainly observation/supportive care and the excision of amyloid deposits, and radiotherapy is rarely used, according to the experience of physicians from the Mayo Clinic[83]. Among 13 patients with LGA presented with clear therapeutic strategies, surgery was the main choice (8/13), and a few patients chose observation (4/13). The administration of dimethyl sulfoxide is also recommended to reduce the digestive symptoms and result in a visible improvement on endoscopy[61].
The prognosis of amyloidosis is generally related to the extent of organ damage. Unlike systemic amyloidosis, localized amyloidosis has an excellent prognosis and minimally affects patient survival[3]. After first-line treatment, most patients improved (53%) or achieved a stable state (31%), and only a few progressed (0.2%), according to a study enrolling 413 patients with localized AL amyloidosis. However, the study also mentioned an undeniable recurrence rate, as two or more recurrences occurred in 5% of the 413 patients, and the first 5 years after diagnosis is a crucial period for recurrence[83]. To date, no recurrence of LGA has been mentioned in the case reports published, and all patients presented a healthy state in follow-up visits, but close follow-up and regular examinations are necessary, particularly within the first 5 years (Figure 6).
Local gastric amyloidosis is such an extremely rare disease that only 22 cases have been reported in the past few decades and the disease is unknown to the public. It is commonly introduced as a human pathological state in which an abnormally misfolded protein accumulates in tissues, causing structure loss, organ dysfunction, and even death. Its pathogenesis remains a mystery, but a few influencing factors that may contribute to the formation of amyloid fibrils are studied and introduced here. Our review may inspire further investigations of the mechanism. Based on the 21 existing cases and our case, we present a detailed description of the main information available on LGA and conclusions regarding its clinical features, diagnostic tools, and treatment, with the goal of establishing future guidelines. Its clinical manifestations are complex and similar to those of other gastric diseases, such as advanced cancer, resulting in minimal awareness among clinicians. The diagnostic tools include biopsy, imaging, and amyloid typing. The final diagnosis mainly depends on the biopsy results, and Congo red staining remains the gold standard. Treatments for LGA mainly include supportive care and surgery. After treatment, most patients receive a good prognosis. Currently, due to its rare incidence, LGA lacks public awareness, and studies that explore its pathogenesis and its clinical features are often unspecific. For clinicians, LGA is a challenge to diagnose using regular tests. Building on that information, we describe the main clinical features and take the lead in proposing a process for diagnosing LGA from the clinicians’ perspective, with the aims of promoting awareness of LGA and potentially contributing to the development of LGA guidelines.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Filipec Kanizaj T, Slomiany BL S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Sipe JD, Benson MD, Buxbaum JN, Ikeda SI, Merlini G, Saraiva MJ, Westermark P. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid. 2016;23:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 409] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 2. | Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, Sipe JD, Westermark P. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2018;25:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 392] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 3. | Mahmood S, Bridoux F, Venner CP, Sachchithanantham S, Gilbertson JA, Rowczenio D, Wagner T, Sayed R, Patel K, Fontana M, Whelan CJ, Lachmann HJ, Hawkins PN, Gillmore JD, Wechalekar AD. Natural history and outcomes in localised immunoglobulin light-chain amyloidosis: a long-term observational study. Lancet Haematol. 2015;2:e241-e250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Hemminki K, Li X, Försti A, Sundquist J, Sundquist K. Incidence and survival in non-hereditary amyloidosis in Sweden. BMC Public Health. 2012;12:974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2:1046-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 6. | Bayliss M, McCausland KL, Guthrie SD, White MK. The burden of amyloid light chain amyloidosis on health-related quality of life. Orphanet J Rare Dis. 2017;12:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Pinney JH, Smith CJ, Taube JB, Lachmann HJ, Venner CP, Gibbs SD, Dungu J, Banypersad SM, Wechalekar AD, Whelan CJ, Hawkins PN, Gillmore JD. Systemic amyloidosis in England: an epidemiological study. Br J Haematol. 2013;161:525-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | Cowan AJ, Skinner M, Seldin DC, Berk JL, Lichtenstein DR, O'Hara CJ, Doros G, Sanchorawala V. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, Masters CL, Merlini G, Saraiva MJ, Sipe JD. A primer of amyloid nomenclature. Amyloid. 2007;14:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Freudenthaler S, Hegenbart U, Schönland S, Behrens HM, Krüger S, Röcken C. Amyloid in biopsies of the gastrointestinal tract-a retrospective observational study on 542 patients. Virchows Arch. 2016;468:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1350] [Cited by in RCA: 1342] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 12. | Pande M, Srivastava R. Molecular and clinical insights into protein misfolding and associated amyloidosis. Eur J Med Chem. 2019;184:111753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Eisenberg DS, Sawaya MR. Structural Studies of Amyloid Proteins at the Molecular Level. Annu Rev Biochem. 2017;86:69-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 388] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 14. | Pepys MB. Amyloidosis. Annu Rev Med. 2006;57:223-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 468] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 15. | Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011;29:1924-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 359] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 16. | Leung N, Nasr SH, Sethi S. How I treat amyloidosis: the importance of accurate diagnosis and amyloid typing. Blood. 2012;120:3206-3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Westermark GT, Fändrich M, Westermark P. AA amyloidosis: pathogenesis and targeted therapy. Annu Rev Pathol. 2015;10:321-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 18. | del Pozo-Yauner L, Wall JS, González Andrade M, Sánchez-López R, Rodríguez-Ambriz SL, Pérez Carreón JI, Ochoa-Leyva A, Fernández-Velasco DA. The N-terminal strand modulates immunoglobulin light chain fibrillogenesis. Biochem Biophys Res Commun. 2014;443:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Blancas-Mejia LM, Misra P, Dick CJ, Cooper SA, Redhage KR, Bergman MR, Jordan TL, Maar K, Ramirez-Alvarado M. Immunoglobulin light chain amyloid aggregation. Chem Commun (Camb). 2018;54:10664-10674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Shammas SL, Knowles TP, Baldwin AJ, Macphee CE, Welland ME, Dobson CM, Devlin GL. Perturbation of the stability of amyloid fibrils through alteration of electrostatic interactions. Biophys J. 2011;100:2783-2791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Tennent GA, Lovat LB, Pepys MB. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proc Natl Acad Sci USA. 1995;92:4299-4303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 295] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Pepys MB. The Pentraxins 1975-2018: Serendipity, Diagnostics and Drugs. Front Immunol. 2018;9:2382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Mold M, Shrive AK, Exley C. Serum amyloid P component accelerates the formation and enhances the stability of amyloid fibrils in a physiologically significant under-saturated solution of amyloid-β42. J Alzheimers Dis. 2012;29:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Botto M, Hawkins PN, Bickerstaff MC, Herbert J, Bygrave AE, McBride A, Hutchinson WL, Tennent GA, Walport MJ, Pepys MB. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3:855-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 183] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Hirschfield GM. Amyloidosis: a clinico-pathophysiological synopsis. Semin Cell Dev Biol. 2004;15:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Tsai SB, Seldin DC, Wu H, O'Hara C, Ruberg FL, Sanchorawala V. Myocardial infarction with "clean coronaries" caused by amyloid light-chain AL amyloidosis: a case report and literature review. Amyloid. 2011;18:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, Apstein CS, Liao R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 312] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 28. | Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, Connors LH, Sawyer DB, Semigran MJ, Macgillivray TE, Seldin DC, Falk R, Liao R. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci USA. 2010;107:4188-4193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 29. | Guan J, Mishra S, Qiu Y, Shi J, Trudeau K, Las G, Liesa M, Shirihai OS, Connors LH, Seldin DC, Falk RH, MacRae CA, Liao R. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol Med. 2014;6:1493-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Gillmore JD, Wechalekar A, Bird J, Cavenagh J, Hawkins S, Kazmi M, Lachmann HJ, Hawkins PN, Pratt G; BCSH Committee. Guidelines on the diagnosis and investigation of AL amyloidosis. Br J Haematol. 2015;168:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Yanai H, Fuji T, Tada M, Aibe T, Karita M, Fujimura H, Okita K, Uchino F. Localized amyloidosis of the stomach. Gastrointest Endosc. 1991;37:408-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Kagawa M, Fujino Y, Muguruma N, Murayama N, Okamoto K, Kitamura S, Kimura T, Kishi K, Miyamoto H, Uehara H, Takayama T. Localized amyloidosis of the stomach mimicking a superficial gastric cancer. Clin J Gastroenterol. 2016;9:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Ahn YH, Rhee YY, Choi SC, Seo GS. Localized Gastric Amyloidosis with Kappa and Lambda Light Chain Co-Expression. Clin Endosc. 2018;51:285-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Kinugasa H, Tanaka T, Okada H. Primary Localized Gastric Amyloidosis Mimicking a Submucosal Tumor-Like Gastrointestinal Tumor. Clin Gastroenterol Hepatol. 2020;18:e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Matsueda K, Kawano S, Okada H. Primary localized amyloidosis of the stomach mimicking healing gastric ulcer. Gastrointest Endosc. 2020;91:947-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Kamata T, Suzuki H, Yoshinaga S, Nonaka S, Fukagawa T, Katai H, Taniguchi H, Kushima R, Oda I. Localized gastric amyloidosis differentiated histologically from scirrhous gastric cancer using endoscopic mucosal resection: a case report. J Med Case Rep. 2012;6:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Kobara H, Oryu M, Mori H, Fujihara S, Nishiyama N, Goda Y, Chiyo T, Matsunaga T, Ayaki M, Yachida T, Nakatsu T, Masaki T. Tissue sampling using a submucosal tunnelling technique for indefinite gastric amyloidosis. Can J Gastroenterol Hepatol. 2015;29:70-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Wu D, Lou JY, Chen J, Fei L, Liu GJ, Shi XY, Lin HT. A case report of localized gastric amyloidosis. World J Gastroenterol. 2003;9:2632-2634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Said SM, Grogg KL, Smyrk TC. Gastric amyloidosis: clinicopathological correlations in 79 cases from a single institution. Hum Pathol. 2015;46:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Dastur KJ, Ward JF. Amyloidoma of the stomach. Gastrointest Radiol. 1980;5:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Deniz K, Sari I, Torun E, Patiroğlu TE. Localized gastric amyloidosis: a case report. Turk J Gastroenterol. 2006;17:116-119. [PubMed] |
| 42. | Rivera R, Kaul V, DeCross A, Whitney-Miller C. Primary gastric amyloidosis presenting as an isolated gastric mass. Gastrointest Endosc. 2012;76:186-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Savant D, Adler M, Kahn L, Cocker R. Amyloidoma of Stomach: A Case Report. Acta Cytol. 2018;62:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Shibukawa G, Irisawa A, Takagi T, Hikichi T, Yamamoto G, Wakatsuki T, Takahashi Y, Ogura G, Hojyo H, Obara K, Sato Y. Endosonographic features of solitary gastric amyloidosis. J Ultrasound Med. 2004;23:719-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 45. | Björnsson S, Jóhannsson JH, Sigurjónsson F. Localized primary amyloidosis of the stomach presenting with gastric hemorrhage. Acta Med Scand. 1987;221:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Sawada T, Adachi Y, Akino K, Arimura Y, Ishida T, Ishii Y, Endo T. Endoscopic features of primary amyloidosis of the stomach. Endoscopy. 2012;44 Suppl 2 UCTN:E275-E276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Yamaguchi T, Inoue T, Nishida T, Kato M, Hayashi Y, Tsujii Y, Maekawa A, Kawai S, Fujinaga T, Araki M, Nagai K, Yoshii S, Hiyama S, Shinzaki S, Iijima H, Tsujii M, Takehara T. Localized gastric amyloidosis mimicking a submucosal tumor-like gastric cancer. Gastrointest Endosc. 2015;82:175-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Tada S, Iida M, Iwashita A, Matsui T, Fuchigami T, Yamamoto T, Yao T, Fujishima M. Endoscopic and biopsy findings of the upper digestive tract in patients with amyloidosis. Gastrointest Endosc. 1990;36:10-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Tada S, Iida M, Yao T, Kawakubo K, Okada M, Fujishima M. Endoscopic features in amyloidosis of the small intestine: clinical and morphologic differences between chemical types of amyloid protein. Gastrointest Endosc. 1994;40:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Alcalde-Vargas A, Leo-Carnerero E, Rojas-Mercedes N, Trigo-Salado C, Herrera-Justiniano JM, Márquez-Galán JL. Correlation between location of amyloid deposits and endoscopic and clinical manifestations in symptomatic gastrointestinal amyloidosis. Rev Esp Enferm Dig. 2015;107:49-51. [PubMed] |
| 51. | Hirata M, Tanaka S, Oka S, Kaneko I, Yoshida S, Yoshihara M, Chayama K. Magnifying endoscopy with narrow band imaging for diagnosis of colorectal tumors. Gastrointest Endosc. 2007;65:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, Yoshida S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 371] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 53. | Ding Y, Li Y, Sun L. A Rare Case of Asymptomatic Primary Gastric Localized Amyloidosis. Clin Gastroenterol Hepatol. 2019;17:A41-A42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Serrano-Fernández ML, Alvarez-Maldonado P, Aristi-Urista G, Valero-Gómez A, Cicero-Sabido R, Redondo CN. Narrow-band imaging bronchoscopy in tracheobronchial amyloidosis. J Bronchology Interv Pulmonol. 2014;21:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Kanno Y, Furuya N, Okamoto M, Noguchi A, Inoue T, Mineshita M. Narrow-band imaging thoracoscopy in pleural amyloidosis. Respirol Case Rep. 2018;6:e00305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 56. | Hui YT, Lam TW, Yee Lam PW, Yan Wu WH, Lam WM. Narrow-band imaging system with magnifying endoscopy for rectal amyloidosis. Gastrointest Endosc. 2008;68:400-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Fu J, Seldin DC, Berk JL, Sun F, O'Hara C, Cui H, Sanchorawala V. Lymphadenopathy as a manifestation of amyloidosis: a case series. Amyloid. 2014;21:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Fujii Y, Kanno Y, Koshita S, Ogawa T, Kusunose H, Masu K, Sakai T, Yonamine K, Kawakami Y, Murabayashi T, Kozakai F, Noda Y, Okada H, Ito K. Predictive Factors for Inaccurate Diagnosis of Swollen Lymph Nodes in Endoscopic Ultrasound-Guided Fine Needle Aspiration. Clin Endosc. 2019;52:152-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Ikeda K, Murayama H. A case of amyloid tumor of the stomach. Endoscopy. 1978;10:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Rotondano G, Salerno R, Cipolletta F, Bianco MA, De Gregorio A, Miele R, Prisco A, Garofano ML, Cipolletta L. Localized amyloidosis of the stomach: a case report. World J Gastroenterol. 2007;13:1877-1878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (11)] |
| 61. | Jin SZ, Qu B, Han MZ, Cheng YQ, Liang GY, Chu YJ, Zhu F, Liu BR. Endoscopic submucosal dissection combined with orally administered dimethyl sulfoxide for primary gastric localized amyloidosis. Clin Res Hepatol Gastroenterol. 2014;38:e79-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Ebato T, Yamamoto T, Abe K, Ishii T, Kuyama Y. Endoscopic removal of localized gastric amyloidosis. Endoscopy. 2012;44 Suppl 2 UCTN:E351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Yamada M, Hatakeyama S, Tsukagoshi H. Gastrointestinal amyloid deposition in AL (primary or myeloma-associated) and AA (secondary) amyloidosis: diagnostic value of gastric biopsy. Hum Pathol. 1985;16:1206-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 64. | Westermark GT, Johnson KH, Westermark P. Staining methods for identification of amyloid in tissue. Methods Enzymol. 1999;309:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 65. | Nilsson KP, Ikenberg K, Aslund A, Fransson S, Konradsson P, Röcken C, Moch H, Aguzzi A. Structural typing of systemic amyloidoses by luminescent-conjugated polymer spectroscopy. Am J Pathol. 2010;176:563-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Sjölander D, Bijzet J, Hazenberg BP, Nilsson KP, Hammarström P. Sensitive and rapid assessment of amyloid by oligothiophene fluorescence in subcutaneous fat tissue. Amyloid. 2015;22:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Sjölander D, Röcken C, Westermark P, Westermark GT, Nilsson KP, Hammarström P. Establishing the fluorescent amyloid ligand h-FTAA for studying human tissues with systemic and localized amyloid. Amyloid. 2016;23:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Schönland SO, Hegenbart U, Bochtler T, Mangatter A, Hansberg M, Ho AD, Lohse P, Röcken C. Immunohistochemistry in the classification of systemic forms of amyloidosis: a systematic investigation of 117 patients. Blood. 2012;119:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 69. | Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957-4959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 621] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 70. | Mollee P, Boros S, Loo D, Ruelcke JE, Lakis VA, Cao KL, Renaut P, Hill MM. Implementation and evaluation of amyloidosis subtyping by laser-capture microdissection and tandem mass spectrometry. Clin Proteomics. 2016;13:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 71. | Sethi S, Vrana JA, Theis JD, Leung N, Sethi A, Nasr SH, Fervenza FC, Cornell LD, Fidler ME, Dogan A. Laser microdissection and mass spectrometry-based proteomics aids the diagnosis and typing of renal amyloidosis. Kidney Int. 2012;82:226-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 72. | Park J, Lee GY, Choi JO, Jeon ES, Kim K, Kim JS, Lee SY. Development and Validation of Mass Spectrometry-Based Targeted Analysis for Amyloid Proteins. Proteomics Clin Appl. 2018;12:e1700106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Sethi S, Fervenza FC, Miller D, Norby S, Leung N. Recurrence of amyloidosis in a kidney transplant. Am J Kidney Dis. 2010;56:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Mangione PP, Mazza G, Gilbertson JA, Rendell NB, Canetti D, Giorgetti S, Frenguelli L, Curti M, Rezk T, Raimondi S, Pepys MB, Hawkins PN, Gillmore JD, Taylor GW, Pinzani M, Bellotti V. Increasing the accuracy of proteomic typing by decellularisation of amyloid tissue biopsies. J Proteomics. 2017;165:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Mazza G, Simons JP, Al-Shawi R, Ellmerich S, Urbani L, Giorgetti S, Taylor GW, Gilbertson JA, Hall AR, Al-Akkad W, Dhar D, Hawkins PN, De Coppi P, Pinzani M, Bellotti V, Mangione PP. Amyloid persistence in decellularized liver: biochemical and histopathological characterization. Amyloid. 2016;23:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Hazenberg BP, van Rijswijk MH, Piers DA, Lub-de Hooge MN, Vellenga E, Haagsma EB, Hawkins PN, Jager PL. Diagnostic performance of 123I-labeled serum amyloid P component scintigraphy in patients with amyloidosis. Am J Med 2006; 119: 355.e15-355. e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 77. | Glaudemans AW, Slart RH, Noordzij W, Dierckx RA, Hazenberg BP. Utility of 18F-FDG PET(/CT) in patients with systemic and localized amyloidosis. Eur J Nucl Med Mol Imaging. 2013;40:1095-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 78. | Seo JH, Lee SW, Ahn BC, Lee J. Pulmonary amyloidosis mimicking multiple metastatic lesions on F-18 FDG PET/CT. Lung Cancer. 2010;67:376-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Baqir M, Lowe V, Yi ES, Ryu JH. 18F-FDG PET scanning in pulmonary amyloidosis. J Nucl Med. 2014;55:565-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Mekinian A, Jaccard A, Soussan M, Launay D, Berthier S, Federici L, Lefèvre G, Valeyre D, Dhote R, Fain O; Centre de Référence des Amyloses immunoglobulinémiques et autres maladies liées aux dépots des immunoglobulines monoclonales. 18F-FDG PET/CT in patients with amyloid light-chain amyloidosis: case-series and literature review. Amyloid. 2012;19:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 82. | Yu H, Yang AM, Lu XH, Zhou WX, Yao F, Fei GJ, Guo T, Yao LQ, He LP, Wang BM. Magnifying narrow-band imaging endoscopy is superior in diagnosis of early gastric cancer. World J Gastroenterol. 2015;21:9156-9162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 83. | Kourelis TV, Kyle RA, Dingli D, Buadi FK, Kumar SK, Gertz MA, Lacy MQ, Kapoor P, Go RS, Gonsalves WI, Warsame R, Lust JA, Hayman SR, Rajkumar SV, Zeldenrust SR, Russell SJ, Lin Y, Leung N, Dispenzieri A. Presentation and Outcomes of Localized Immunoglobulin Light Chain Amyloidosis: The Mayo Clinic Experience. Mayo Clin Proc. 2017;92:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 84. | Gillmore JD, Tennent GA, Hutchinson WL, Gallimore JR, Lachmann HJ, Goodman HJ, Offer M, Millar DJ, Petrie A, Hawkins PN, Pepys MB. Sustained pharmacological depletion of serum amyloid P component in patients with systemic amyloidosis. Br J Haematol. 2010;148:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 85. | Bodin K, Ellmerich S, Kahan MC, Tennent GA, Loesch A, Gilbertson JA, Hutchinson WL, Mangione PP, Gallimore JR, Millar DJ, Minogue S, Dhillon AP, Taylor GW, Bradwell AR, Petrie A, Gillmore JD, Bellotti V, Botto M, Hawkins PN, Pepys MB. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature. 2010;468:93-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 86. | Richards DB, Cookson LM, Berges AC, Barton SV, Lane T, Ritter JM, Fontana M, Moon JC, Pinzani M, Gillmore JD, Hawkins PN, Pepys MB. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med. 2015;373:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 87. | Richards DB, Cookson LM, Barton SV, Liefaard L, Lane T, Hutt DF, Ritter JM, Fontana M, Moon JC, Gillmore JD, Wechalekar A, Hawkins PN, Pepys MB. Repeat doses of antibody to serum amyloid P component clear amyloid deposits in patients with systemic amyloidosis. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |