Published online Mar 7, 2020. doi: 10.3748/wjg.v26.i9.933
Peer-review started: December 5, 2019
First decision: December 30, 2019
Revised: January 8, 2020
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: March 7, 2020
Processing time: 92 Days and 0.4 Hours
As the most common biliary malignancy, gallbladder cancer (GC) is an elderly-biased disease. Although extensive studies have elucidated the molecular mechanism of microRNA 182 (miR-182) and reversion-inducing-cysteine-rich protein with kazal motifs (RECK) in various cancers, the specific role of exosomal miR-182 and RECK in GC remains poorly understood.
To explore the relationship between exosomal miR-182/RECK and metastasis of GC.
Paired GC and adjacent normal tissues were collected from 78 patients. Quantitative polymerase chain reaction was employed to detect miR-182 and exosomal miR-182 expression, and Western blotting was conducted to determine RECK expression. In addition, the effects of exosomal miR-182/RECK on the biological function of human GC cells were observed. Moreover, the double luciferase reporter gene assay was applied to validate the targeting relationship between miR-182 and RECK.
Compared with normal gallbladder epithelial cells, miR-182 was highly expressed in GC cells, while RECK had low expression. Exosomal miR-182 could be absorbed and transferred by cells. Exosomal miR-182 inhibited RECK expression and promoted the migration and invasion of GC cells.
Exosomal miR-182 can significantly promote the migration and invasion of GC cells by inhibiting RECK; thus miR-182 can be used as a therapeutic target for GC.
Core tip: Gallbladder cancer (GC) is the most common biliary malignancy that mostly affects the elderly. At present, however, the specific role of exosomal miR-182 and RECK in GC remains unknown. The results of this study indicated that exosomal miR-182 could markedly promote the migration and invasion of GC cells by inhibiting RECK. Therefore, miR-182 could serve as a therapeutic target for GC.
- Citation: Zheng H, Wang JJ, Zhao LJ, Yang XR, Yu YL. Exosomal miR-182 regulates the effect of RECK on gallbladder cancer. World J Gastroenterol 2020; 26(9): 933-946
- URL: https://www.wjgnet.com/1007-9327/full/v26/i9/933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i9.933
Gallbladder cancer (GC) is the most common biliary malignancy, ranking sixth among gastrointestinal cancers, with a high recurrence rate and poor prognosis[1,2]. Diabetes, gallstone size, age, sugary drinks, and genetic mutations are all risk factors affecting GC. At present, the effect of surgical treatment for GC is largely dependent on tumor metastasis[3], worsened by the fact that GC cells are highly invasive[1]. So gene target therapy has gradually become a research hotspot in GC. Many studies[4,5] have suggested that regulation of target gene expression by microRNA (miRNA) has potential therapeutic prospects.
MiR-182, a miRNA approximately 110 base pairs in length, is located on chromosome 7. It is well established that the abnormal expression of miR-182 is associated with cancer progression. For example, Kulkarni et al[6] found that miR-182 inhibited mitosis of renal cancer cells by downregulating metastasis associated lung adenocarcinoma transcript 1 expression. According to Livingstone et al[7], the serum expression of miR-182 in liver cancer model rats was significantly increased when aflatoxin was applied to construct liver cancer model rats, and the authors believed that the high expression of serum miR-182 was closely related to liver cancer. In addition, Perilli et al[8] found that the high expression of miR-182 in colorectal cancer cells could increase the activity of G0/G1 phase cells and downregulate the apoptosis pathway. MiR-182 can inhibit the Notch pathway through the NF-kB-miR-182-HES1 axis, ultimately promoting malignant invasion of medullary thyroid cancer cells[9]. While in GC, miR-182 promotes tumor metastasis by downregulating cell adhesion molecule 1 expression[10].
Reversion-inducing-cysteine-rich protein with kazal motifs (RECK) protein was expressed in 26 tissues including gallbladder, endometrium, and lung. As an important regulator of extracellular matrix remodeling, it can negatively regulate matrix metalloproteinase 9 (MMP9) and MMP2 to inhibit malignant behaviors such as migration and invasion of cancer cells[11-13]. Apart from that, RECK can promote apoptosis by altering p53, p53, B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), and other p53 pathway proteins[14]. Moreover, the influence of RECK on tumor progression was regulated by miRNA. For example, Chen et al[15] found that miR-15b promoted the proliferation and invasion of prostate cancer cells by targeting inhibition of RECK. Besides, Wang et al[16] demonstrated that RECK was negatively regulated by miR-21, which could upregulate the invasion and migration of colon cancer cells. In colorectal cancer, it has been reported that the effects of miR-92a[17], miR-96[18] and miR-375[19] on the biological function of cancer cells are all related to the regulation of RECK expression.
Although the molecular mechanism of miR-182 and RECK has been clarified in a variety of cancers, the co-role of exosomal miR-182 combined with RECK in GC remains unclear. Therefore here, by regulating their expression in GC, we explored the role of exosomal miR-182 and RECK in GC.
Paired cancer tissues and adjacent normal tissues were obtained from 78 diagnosed GC patients, including 36 males and 42 females. Inclusion criteria were patients diagnosed with GC. In contrast, the exclusion criteria were as follows: Patients with psychiatric disorders, previous treatment history of surgery, chemotherapy, radiotherapy or antibiotic, combined with other tumors or those who did not cooperate with the treatment. The collected tissues were sectioned and stored in at -80 °C for testing. Venous blood samples from GC patients and healthy subjects were collected on an empty stomach and stored in Eppendorf tube without anticoagulant. Then it was centrifuged at room temperature for 15 min at 3 × 103 rpm to obtain the supernatant, which was then placed in a RNase-free Eppendorf tube for a 5-min centrifugation at a rotational speed of 1.2 × 104 rpm. After centrifugation, the supernatant was collected and stored at -80 °C until subsequent measurement. All participants were fully informed of the study, and the Medical Ethics Committee of the Affiliated Hospital of Zunyi Medical University approved the study.
GC cell lines (GBC-SD, EHGB1, NOZ) and human gallbladder epithelial cells (HGBEC) were purchased from the cell bank of American Type Culture Collection (Gaithersburg, MD, United States), and the cells were cultured in an animal cell incubator at 37 °C and 5% CO2. The medium used for the GC cell lines was 1640 medium (HyClone Laboratories, South Logan, UT, United States) supplemented with 10% fetal bovine serum (FBS; Gibco Laboratories, Gaithersburg, MD, United States) and 1% penicillin/streptomycin (100X; Beijing Solarbio Science & Technology Co. Ltd., Beijing, China), while that used for the HGBEC cell line was Dulbecco’s Modified Eagle Medium (DMEM) (Hyclone) supplemented with 10% FBS (Gibco Company) and 1% penicillin/streptomycin (100X; Solarbio). Follow-up experiments were carried out until the cells reached 80%-90% confluency.
The culture medium was renewed with FBS-free medium 1 d before transfection, and cells were seeded into 6-well plates at 1 × 105/well. MiR-182 mimics, RECK small interfering RNA (siRNA), and negative control (NC) si vectors were purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China) Then the Lipofectamine 2000 Transfection Kit (Invitrogen, Carlsbad, CA, United States) was employed to transfect the abovementioned vectors into the cell lines according to the manufacturer’s instructions. The culture medium was renewed at 37 °C and 5% CO2 8 h after transfection.
Cell (NOZ cell) suspension was prepared by enzymatic hydrolysis before centrifuging at 1 × 105 g for 8 h. Then the cells were cultured in RPMI 1640 medium containing 10% FBS at 37 °C and 5% CO2. When the cells were cultured to 80%-90% confluency, the supernatant of the medium was collected, the exosomes were extracted, and the latter was centrifuged at 1 × 105 g for 1.5 h to collect the lower exosome sediments. Next, phosphate-buffered saline (PBS) was added to the exosome precipitate for repeated pipetting. Finally, the expression of cluster of differentiation 63 (CD63) and CD81 was detected by Western blot analysis.
The total RNA from the tissue samples or cells was extracted by Trizol, while that of the exosomes was detected using the Total Exosome RNA & Protein Isolation Kit (No. 4475545; Invitrogen). Then the concentration and purity of total RNA at 260-280 nm was detected by an ultraviolet spectrophotometer, and RNA with OD260/OD280 > 1.8 was selected for follow-up quantitative PCR (qPCR) detection. The FastKing One-Step Reverse Transcription-Fluorescence Quantification Kit [Catalog No. FP314; Tiangen Biotech (Beijing) Co. Ltd., Beijing, China) and ABI PRISM 7000 (Applied Biosystems, Foster City, CA, United States] were applied for qPCR quantification. MiR-182 primer was designed and synthesized by Sangon Biotech (Shanghai) Co., Ltd. The reaction system was performed in strict accordance with the kit instructions (50 μL): Upstream primer: 1.25 μL, downstream primer: 1.25 μL, probe: 1.0 μL, RNA template: 10 pg/μg, 50 × ROX Reference Dye ROX: 5 μL, and RNase-FreeddH_2 was added to reach an overall reaction volume of 50 μL. Reaction process: reverse transcription at 50 °C for 30 min, cycled once; pre-denaturation at 95 °C for 3 min, cycled once; denaturation at 95 °C for 15 s, and annealing at 60 °C for 30 s, cycled 40 times. The results were analyzed by the ABI PRISM 7000 instrument with U6 and GAPDH as the internal reference genes; the primer sequences are shown in Table 1.
| Upstream primer (5'→3') | Downstream primer (5'→3') | |
| miR-182 | TGCGGTTTGGCAATGGTAGAAC | CCAGTGCAGGGTCCGAGGT |
| RECK | TCTGCAGGGGAAGTTGGTTG | CAGTTACAGGGCAGACCTGT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| GAPDH | AGAAGGCTGGGGCTCATTTG | AGGGGCCATCCACAGTCTTC |
An volume of 1 mL cell protein extract (cell lysate: protease inhibitor: phosphatase inhibitor = 98:1:1, v/v/v) was added to the cells. Then the solution was pipetted several times and centrifuged at 1.2 × 104 r/min for 15 min to collect the supernatant. The proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis before transfer to the nitrocellulose membrane, followed by blocking at room temperature for 1 h in 5% skim milk/PBS solution. Next, the membrane was incubated overnight at 4 °C with primary antibodies against RECK, caspase-3, caspase-9, E-cadherin, Bax, Bcl-2, N-cadherin, β-catenin and β-actin (all from Shanghai Abcam Co., Shanghai, China). Then the nitrocellulose membrane was washed three times with PBS, followed by a 1 h incubation at room temperature with goat anti-rabbit secondary antibody (horseradish peroxidase cross-linked; Shanghai Abcam Co.). Finally, the NC membrane was rinsed with PBS and proteins were visualized by enhanced chemiluminescence. With β-actin as the internal reference protein, the relative expression level of the protein to be measured was equal to the gray value of the band to be measured/the gray value of the β-actin band.
The adherent cells were digested with trypsin and the supernatant was obtained by 1103 x g centrifugation for 1 min, and then added to the cell medium to re-suspend the cells. Then the cells were seeded in the upper migration chamber (containing 200 μL 10% FBS + 1% DMEM) at 2 × 104 cells/well, and the DMEM containing 10% FBS (total volume of 500 μL) was added to the lower chamber. After 24 h of cell culture, the upper chamber fluid was removed and the parenchyma cells were wiped off. Then the Transwell cells were fixed in 4% methanol for 20 min, and the Transwell chamber was washed with PBS after crystal violet staining for 15 min. Photographs of cell migration were taken under a 200-fold microscope, and three fields of view were randomly selected to calculate the number of cells, with the average value as the number of permeabilized cells. The experiment was repeated three times. The invasion experiment shared the same steps with those of migration experiment, except that the former was treated with 8% matrix glue and the number of cells per well was increased to 5 × 104.
The transfected cells were hydrolyzed with trypsin and centrifuged to remove the enzyme solution, followed by the addition of fresh medium and pipetting to prepare the cell suspension. Then four 96-well plates were inoculated with cells of 5 × 103/100 μL per well, with 3 wells per set. One plate was taken out every 24 h, and 5 mg/mL MTT solution was added at 10 μL/well for additional culture for 1 h. Then the culture medium was removed and the OD value at 570 nm was measured by a microplate reader. The experiment was repeated three times to plot the cell viability-time curve.
The adherent cells were digested and prepared as a cell suspension. The CountessTM automatic counter (Invitrogen) was applied to dilute the cells to 1 × 106. Then cells were fixed in a 70% ethanol ice-cold solution at 4 °C for 30 min. Thereafter, the ethanol solution was removed, and the cells were incubated in Annexin V-FITC/7-AAD solution. Finally, apoptosis was analyzed using the FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, United States).
The pmirGLO-HMGB1-wt and pmirGLO-HMGB1-mut vectors were constructed and co-transfected into EH-GB1 and GBS-SD cells with miR-182 mimics and NC mimics, respectively. After 48 h of transfection, the luciferase activity was detected by double luciferase reporter gene assay (Promega, Madison, WI, United States) in strict accordance with the instructions.
Statistical analysis of the collected data of indicators mentioned above was performed using SPSS20.0 (Asia Analytics Formerly SPSS, China) and GraphPad Prism 6.0. Each experiment was repeated three times. The data are represented by the mean ± standard deviation, and the count data are expressed as n. Intergroup comparison was conducted by an independent samples t-test, multi-group comparison was analyzed by one-way analysis of variance, and post-hoc pairwise comparison was performed by an least significant difference test. Pearson’s analysis was applied to the correlation between miR-182 and RECK. The relationship between miR-182 and clinical features was determined by the two-sample Student's t-test. All data were double-tailed. With 95% as its confidence interval, a statistically significant difference was assumed at P < 0.05.
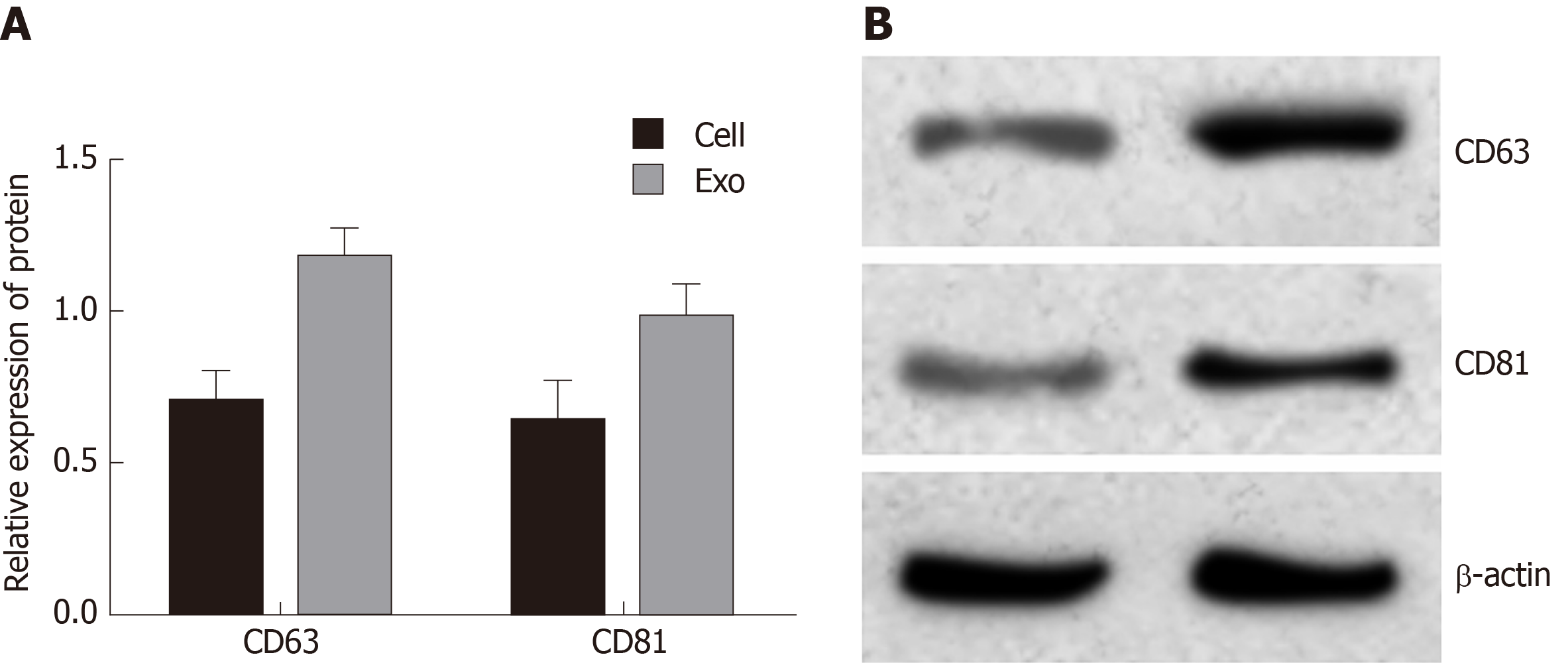
Exosomes were extracted and Western blot analysis was used to detect exosome protein markers CD63 and CD81 (Figure 1). The results showed that CD63 and CD81 were highly expressed in exosomes compared with cell samples, indicating that the sample was indeed exosomes.
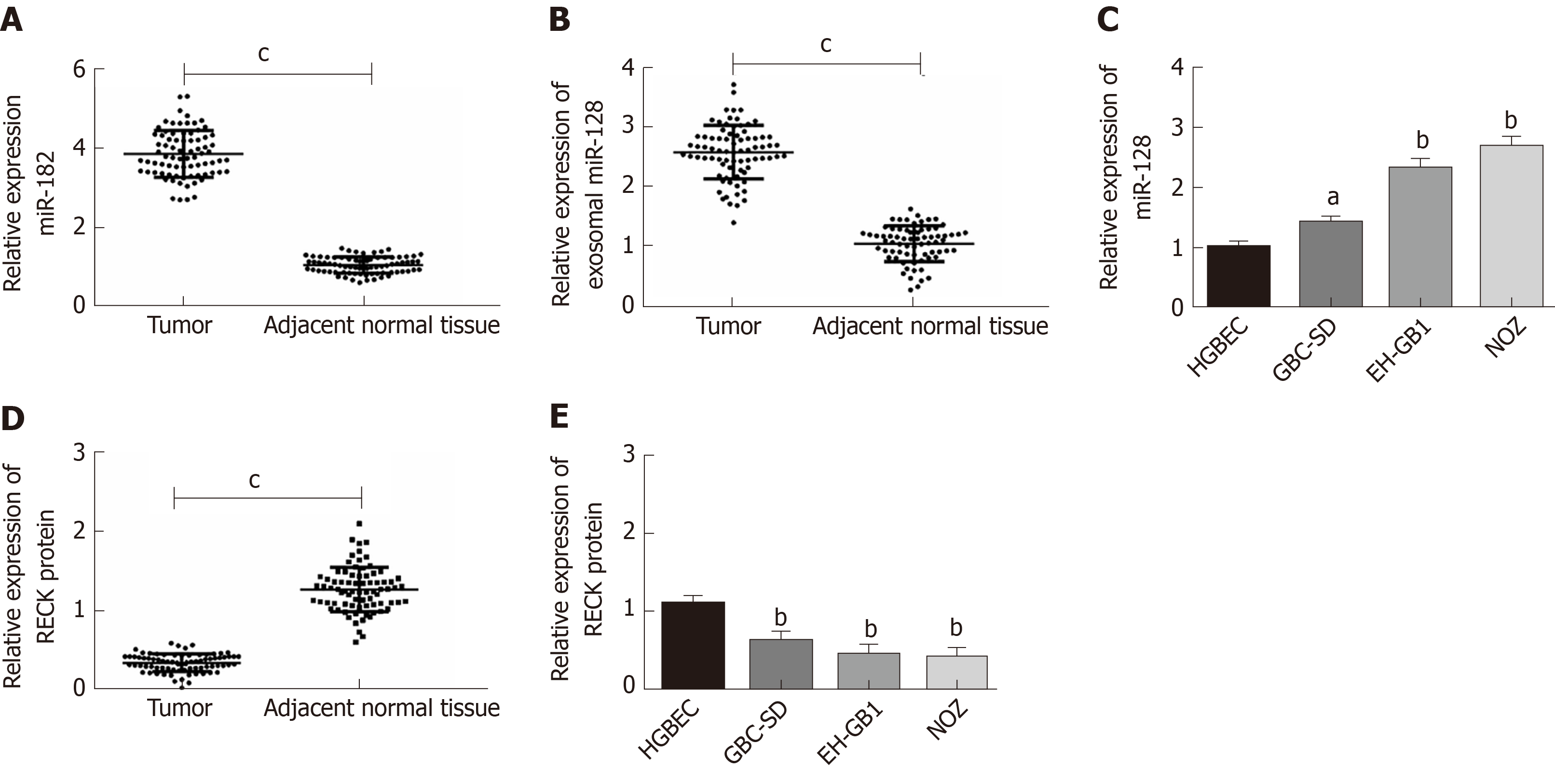
This study included 78 cases of paired GC and adjacent normal tissues. qPCR was employed to quantify the expression levels of miR-182 and exosomal miR-182 in tissues and cells, and the expression of RECK protein in tissues and cells was determined by Western blot analysis(Figure 2). The expression of miR-182 was markedly increased in cancer tissues (Figure 2A), whereas that of RECK was notably decreased in cancer tissues compared with normal adjacent tissues (Figure 2D). Compared with normal human blood samples, serum miR-182 level was significantly elevated in patients with GC (Figure 2B). However, compared with HGBEC, miR-182 expression was markedly increased (Figure 2C), whereas RECK was significantly decreased in GC cells (Figure 2E). The abovementioned results indicated that miR-182 was highly expressed in GC, whereas there was low expression of RECK. It is worth mentioning that the expression levels of miR-182 and RECK were the highest and lowest, respectively in NOZ cell lines.
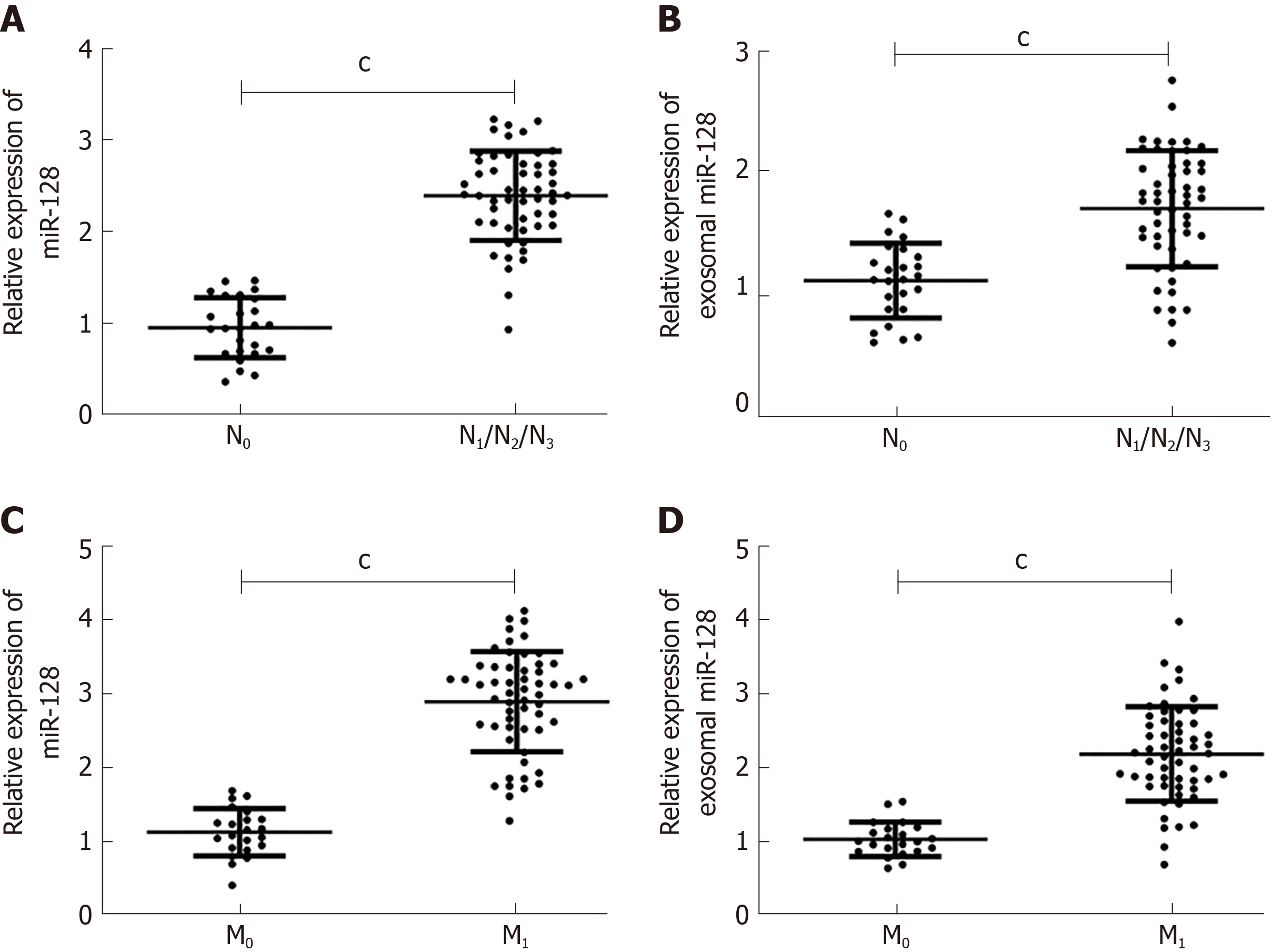
Here, we compared the expression of miR-182 and exosomal miR-182 in different tumor-node-metastasis (TNM) phases and analyzed the correlation between miR-182 and clinical features (Figure 3, Table 2). As shown in Figure 3, miR-182 and exosomal miR-182 were highly expressed in the N1/N2/N3 and M1 phases. Table 2 illustrates that miR-182 was associated with the N and M phases and RECK expression.
| Categories | n | miR-182 | χ2 | P value | |
| Low expression | High expression | ||||
| Gender | 0.125 | 0.819 | |||
| Male | 36 | 17 | 25 | ||
| Female | 42 | 16 | 20 | ||
| Age in yr | 0.273 | 0.646 | |||
| ≤ 60 | 45 | 19 | 26 | ||
| > 60 | 33 | 12 | 21 | ||
| Smoking history | 0.075 | 0.823 | |||
| Yes | 41 | 22 | 19 | ||
| No | 37 | 21 | 16 | ||
| Drinking history | 0.396 | 0.645 | |||
| Yes | 46 | 22 | 24 | ||
| No | 32 | 15 | 17 | ||
| N staging | 5.128 | 0.035 | |||
| N0 | 25 | 12 | 13 | ||
| N1/N2/N3 | 53 | 12 | 41 | ||
| M staging | 5.349 | 0.027 | |||
| M0 | 22 | 10 | 12 | ||
| M1 | 56 | 11 | 45 | ||
| RECK expression | |||||
| Low | 31 | 15 | 16 | 5.246 | 0.028 |
| High | 47 | 11 | 36 | ||
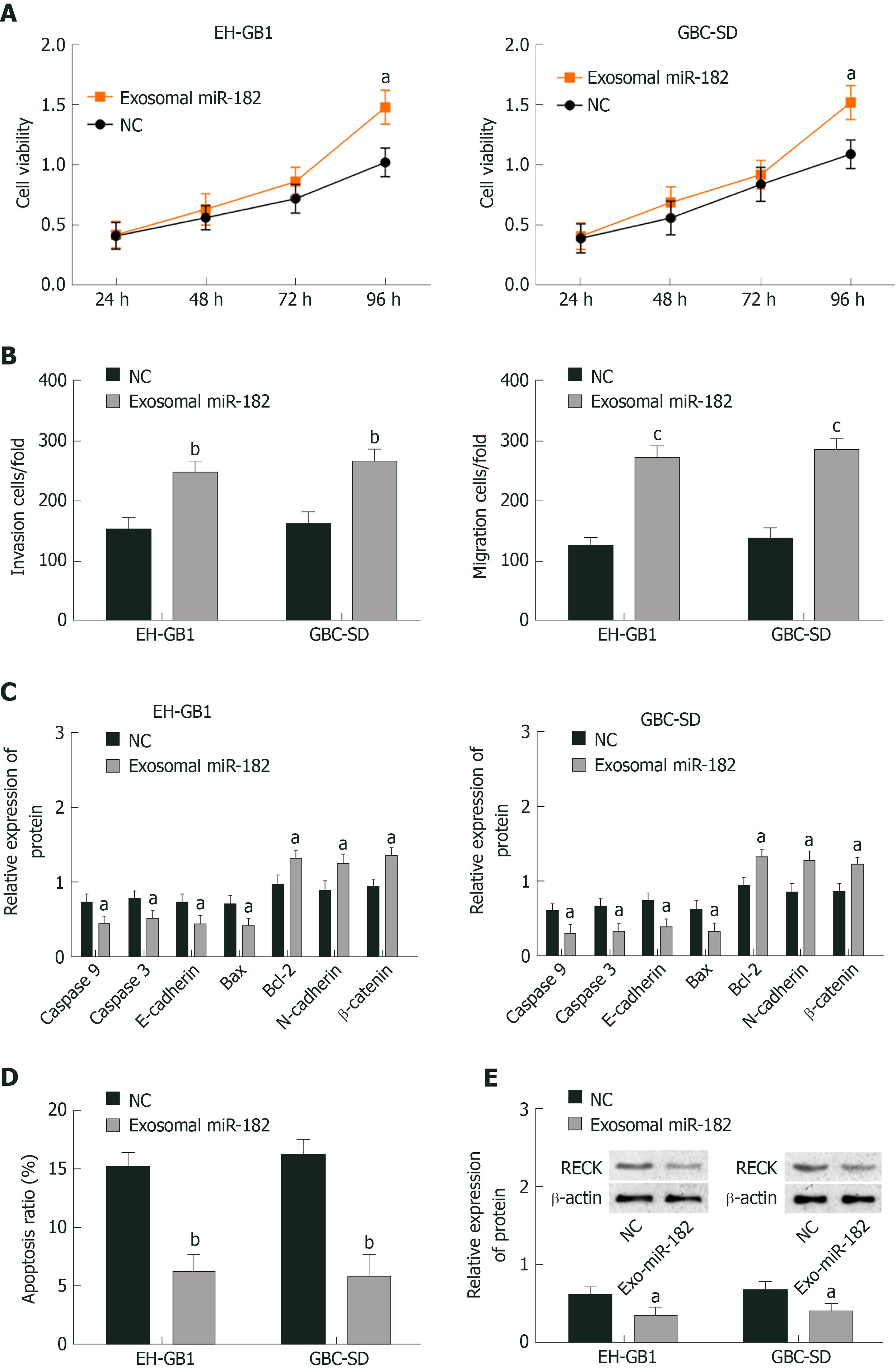
As mentioned earlier, miR-182 and RECK presented the highest and lowest expression, respectively in NOZ cell lines; thus exosomal miR-182 in NOZ cells was extracted and co-cultured with EH-GB1 and GBS-SD cells to observe the effect of miR-182 on cell biological function. Cell viability was detected by MTT, cell invasion and migration were measured by the Transwell assay, protein expression was detected by Western blotting, and apoptosis was determined by flow cytometry. Compared with the NC group, the cell viability (Figure 4A and B), cell invasion (Figure 4C), and migration (Figure 4D) of the exosomal miR-182 group were enhanced, whereas apoptosis (Figure 4E, Supplementary Figure 1A and C) was attenuated. In addition, compared with the NC group, the expression levels of RECK, caspase-3, caspase-9, E-cadherin, and Bax were downregulated in the exosomal miR-182 group, whereas the expression levels of Bcl-2, N-cadherin and β-catenin were upregulated (Figure 4E). These results revealed that exosomal miR-182 promoted cell proliferation, migration and invasion, and inhibited apoptosis and RECK expression.
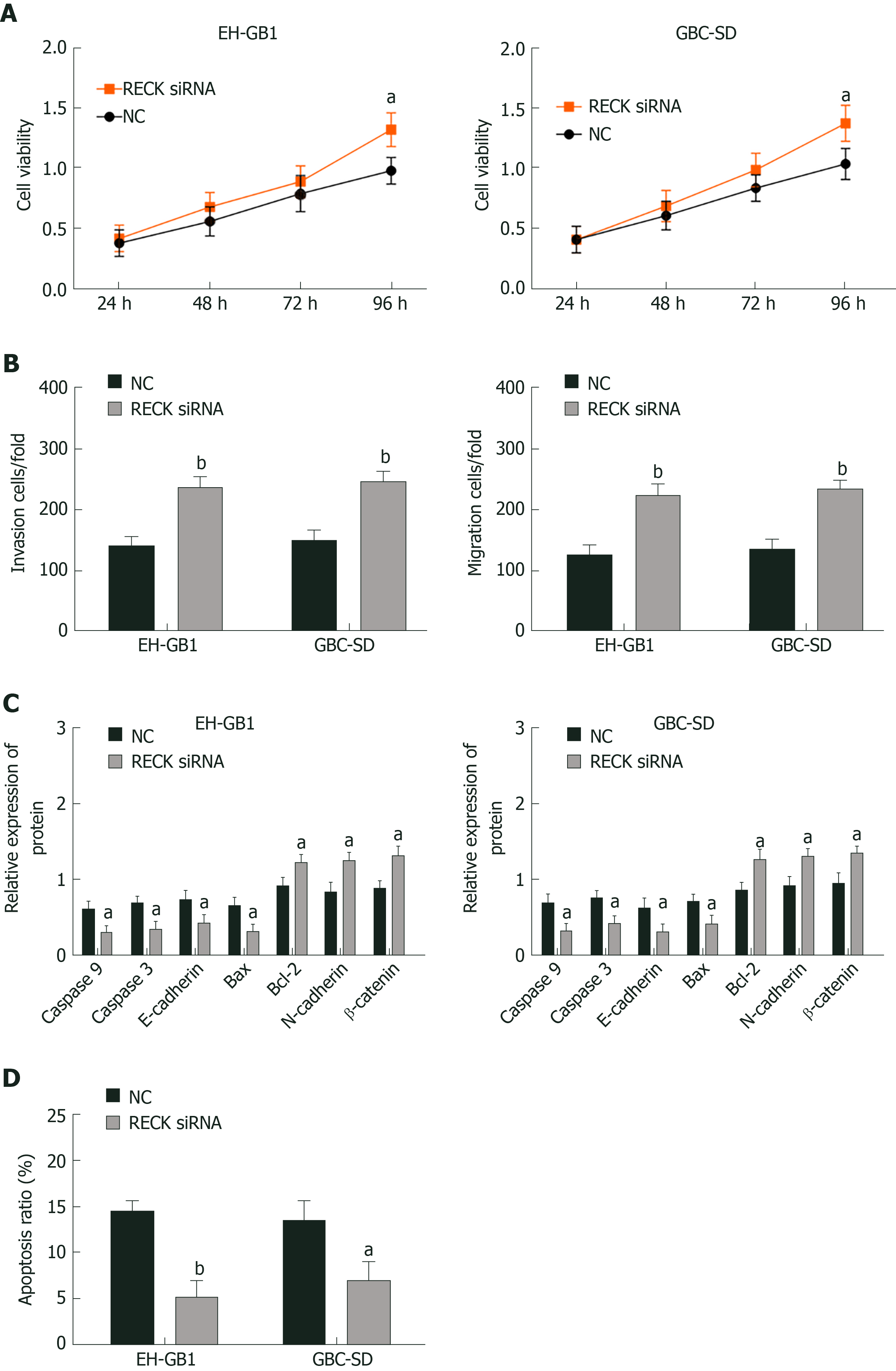
The expression of RECK was regulated by exosomal miR-182. In order to understand whether the change of RECK expression was related to the occurrence of GC, we inhibited RECK and observed its effect on the biological function of GC cells (Figure 5). Compared with the NC group, the RECK siRNA group had increased cell viability (Figure 5A and B), enhanced cell invasion (Figure 5C) and migration (Figure 5D), and decreased apoptosis (Supplementary Figure 1B and D). In addition, the expression levels of caspase-3, caspase-9, E-cadherin, and Bax in the RECK siRNA group were downregulated, while the expression levels of Bcl-2, N-cadherin, and β-catenin were upregulated in contrast with the NC group. These results indicated that RECK promoted apoptosis and inhibited cell proliferation, migration, and invasion.
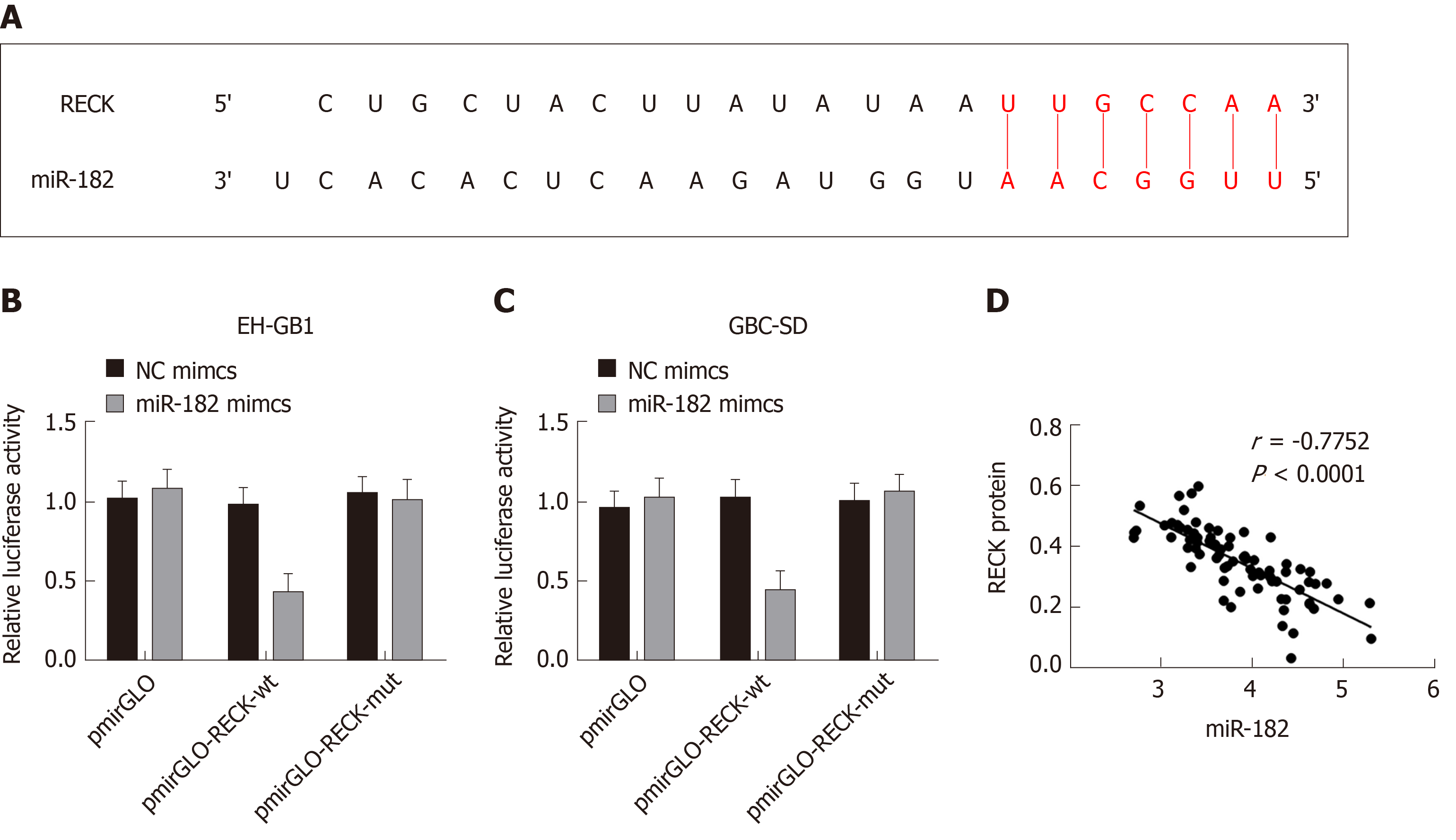
The TargetScan database predicted that miR-182 had binding sites on RECK mRNA (Figure 6A). The double luciferase reporter gene assay confirmed that miR-182 had a targeting relationship with RECK mRNA (Figure 6B and C). In addition, a negative correlation between miR-182 and RECK protein was validated by Pearson’s analysis. The above results indicated that miR-182 could target to inhibit RECK.
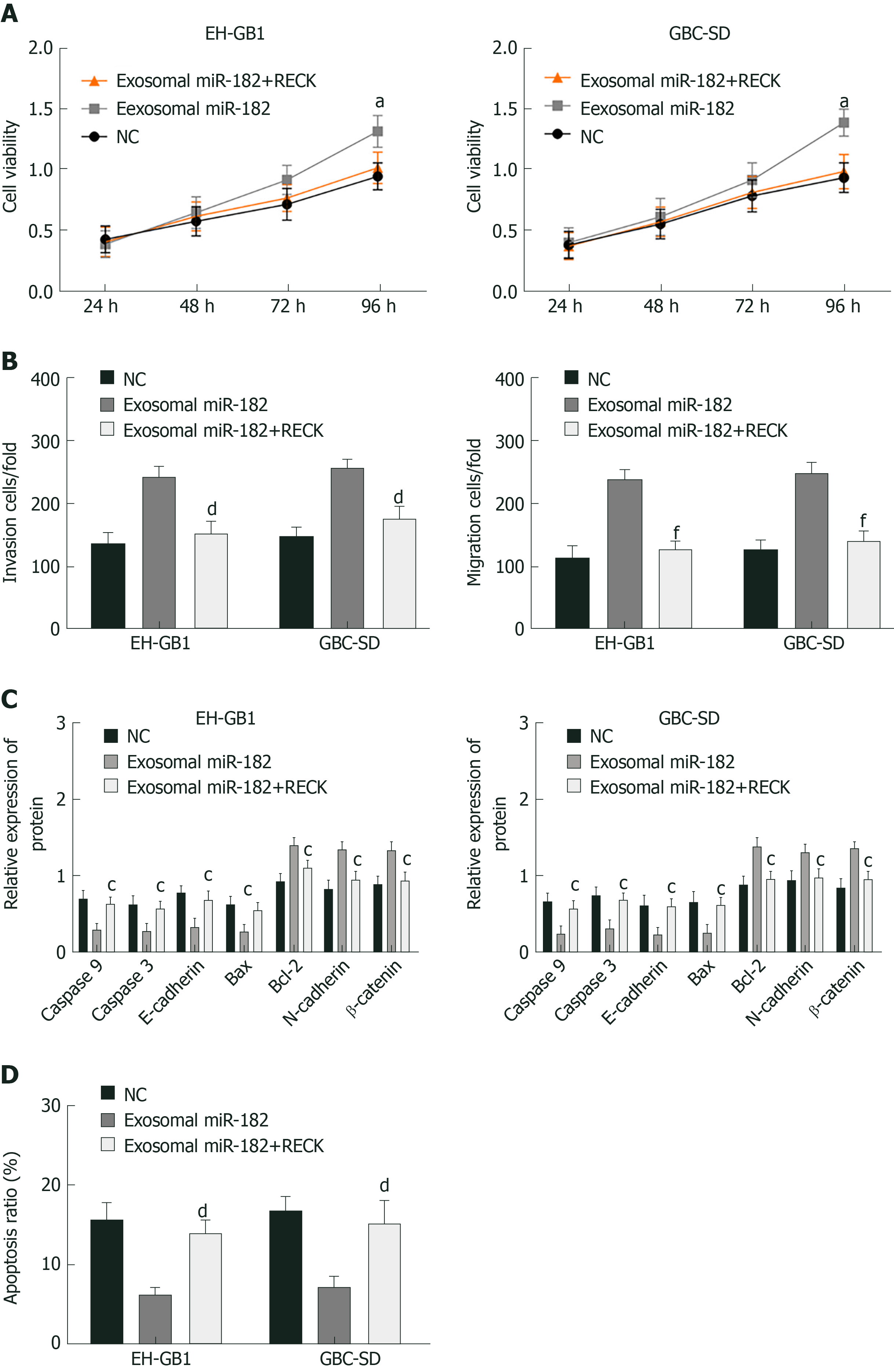
Exosomal miR-182 and exosomal miR-182+RECK overexpression vectors were added to GC cells to perform the MTT assay, Western blot analysis, and Transwell assay. The results showed that compared with the exosomal miR-182 group, the cell viability, migration, and invasion of the exosomal miR-182+RECK group decreased, and apoptosis increased. Meanwhile, the exosomal miR-182+RECK group showed upregulated caspase-3, caspase-9, E-cadherin and Bax and downregulated Bcl-2, N-cadherin, and β-catenin expression (Figure 7). The above results suggested that RECK overexpression could counteract the enhancement of cell proliferation, invasion, and migration caused by exosomal miR-182.
As essential carriers for cell-cell information communication, exosomes are extracellular vesicles with a diameter of 40-150 nm in nature, which contain a variety of proteins, DNA, and RNA. CD63, CD81, and CD9 on the surface of vesicles are vesicles markers[20]. Exosomes can stimulate extracellular matrix remodeling or information transmission when they are released out of the cell, which is of great significance for human health and disease[21]. Due to the presence of exosomes, miRNAs can be actively secreted outside the cell without degradation of RNA enzymes, and play a role by being absorbed by other cells through endocytosis[22].
In this study, miR-182 and exosomal miR-182 were found to be highly expressed in GC, and had the highest expression levels in NOZ cells. Therefore, exosomal miR-182 was isolated from NOZ cells and co-cultured with EH-GB1 and GBS-SD cells. The results showed that exosomal miR-182 could promote the proliferation, migration, and invasion of EH-GB1 and GBC-SD cells and inhibit cell apoptosis. Meanwhile, the expression of RECK, caspase-9, caspase-9, E-cadherin, and Bax was found to be downregulated, whereas the expression of Bcl-2, N-cadherin and β-catenin was upregulated. In the study of Qiu et al[10], miR-182 could act as a carcinogen to promote the metastasis of GC. The results of this study further supplemented the possibility that miR-182 could regulate other cell biological functions through exosomes.
In addition, we found that RECK was downregulated when exosomal miR-182 was co-cultured with cancer cells. TargetScan further predicted that there were binding sites between miR-182 and RECK mRNA, and the negative correlation between the two. Therefore, we speculated that the effect of miR-182 on cell biological function might be achieved by regulating RECK expression. In this study, the effect of RECK on GC cells was observed by interfering with RECK expression in cancer cells by siRNA, and the targeting relationship between miR-182 and RECK was validated by the double luciferase reporter gene assay. The results demonstrated that the absence of RECK promoted cell proliferation, migration and invasion and inhibited cell apoptosis; meanwhile downregulating the levels of caspase-3, caspase-9, E-cadherin, Bax, and Bcl-2, while upregulating N-cadherin and β-catenin. In addition, it was found that luciferase activity of cells was the lowest when 182 mimics were co-transfected with pmirGLO-RECK-wt. RECK is a protein related to cell migration and invasion. By binding to RECK mRNA, exosomal miR-182 inhibited the expression of RECK at the transcriptional level, resulting in increased expression of N-cadherin, β-catenin, MMP9 and MMP2 in cells, and enhanced cell migration and invasion. Although studies[23-25] have reported that miR-182/RECK affects the development of breast, bladder and prostate cancer, the role exosomal miR-182/RECK plays in GC has not been studied. Here, we proved that, by inhibiting RECK, exosomal miR-182 could downregulate the expression levels of caspase-9, caspase-9, E-cadherin, and Bax, and upregulate Bcl-2, N-cadherin and β-catenin, ultimately promoting the proliferation, migration, and invasion of GC cells.
The present study demonstrated that by regulating RECK, exosomal miR-182 could promote cell proliferation, migration and invasion and inhibit apoptosis, ultimately leading to tumor formation or metastasis, which aggravated the deterioration of GC. Although the relationship between miR-182/RECK and tumor metastasis was preliminarily studied, there is still room for improvement. For example, the signaling pathways implicated in miR-182/RECK should be explored in future experimental design to supplement the oncogenic network of exosomal miR-182. In addition, the clinical value of exosomal miR-182 in GC remains to be discussed.
To summarize, this study investigated the effect of miR-182/RECK axis on the biological function of GC cells by studying the expression mechanism of exosomal miR-182 and RECK in GC. MiR-182 is highly expressed in GC cells, thereby acting on other cells through endocytosis to inhibit intracellular RECK expression, and ultimately promoting cell proliferation, migration, and invasion. In addition, exosomal miR-182 is positively correlated with tumor metastasis of GC. Therefore, miR-182/RECK axis has potential application value in targeted therapy of GC.
As the most common biliary malignancy, GC is an elderly-biased disease. Although extensive studies have elucidated the molecular mechanism of miR-182 and RECK in various cancers, the specific roles of exosomal miR-182 and RECK in GC remain poorly understood.
The expression of miR-182 and exosomal miR-182 was increased in gallbladder cancer, while RECK decreased. Targetscan7.2 predicted RECK could bind with miR-182 via 3’UTR. RECK was negatively correlated with miR-182.
This study was set out to explore the relationship between exosomal miR-182/RECK and metastasis of GC.
Paired GC and adjacent normal tissues were collected from 78 patients. qPCR was employed to detect miR-182 and exosomal miR-182 expression, and Western blot was adopted to determine RECK expression. In addition, the effect of exosomal miR-182/RECK on the biological function of human GC cells were observed. Moreover, double luciferase reporter gene assay was applied to validate the targeting relationship between miR-182 and RECK.
Compared with normal gallbladder epithelial cells, miR-182 was highly expressed in GC cells, while RECK was lowly expressed. Exosomal miR-182 could be absorbed and transferred by cells. Exosomal miR-182 inhibited RECK expression and promoted migration and invasion of GC cells.
Exosomal miR-182 can significantly promote the migration and invasion of GC cells by inhibiting RECK, and thus miR-182 can be used as a therapeutic target for GC.
The signaling pathways implicated in miR-182/RECK should be explored in future experimental design to supplement the oncogenic network of exosomal miR-182. The clinical value of exosomal miR-182 in GC remains to be discussed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ho HK, Li C, Magnani M S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Schmidt MA, Marcano-Bonilla L, Roberts LR. Gallbladder cancer: epidemiology and genetic risk associations. Chin Clin Oncol. 2019;8:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 2. | Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 488] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 3. | de Savornin Lohman EAJ, de Bitter TJJ, van Laarhoven CJHM, Hermans JJ, de Haas RJ, de Reuver PR. The diagnostic accuracy of CT and MRI for the detection of lymph node metastases in gallbladder cancer: A systematic review and meta-analysis. Eur J Radiol. 2019;110:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Niu J, Li Z, Li F. Overexpressed microRNA-136 works as a cancer suppressor in gallbladder cancer through suppression of JNK signaling pathway via inhibition of MAP2K4. Am J Physiol Gastrointest Liver Physiol. 2019;317:G670-G681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Jin YP, Hu YP, Wu XS, Wu YS, Ye YY, Li HF, Liu YC, Jiang L, Liu FT, Zhang YJ, Hao YJ, Liu XY, Liu YB. miR-143-3p targeting of ITGA6 suppresses tumour growth and angiogenesis by downregulating PLGF expression via the PI3K/AKT pathway in gallbladder carcinoma. Cell Death Dis. 2018;9:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Kulkarni P, Dasgupta P, Bhat NS, Shahryari V, Shiina M, Hashimoto Y, Majid S, Deng G, Saini S, Tabatabai ZL, Yamamura S, Tanaka Y, Dahiya R. Elevated miR-182-5p Associates with Renal Cancer Cell Mitotic Arrest through Diminished MALAT-1 Expression. Mol Cancer Res. 2018;16:1750-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Livingstone MC, Johnson NM, Roebuck BD, Kensler TW, Groopman JD. Serum miR-182 is a predictive biomarker for dichotomization of risk of hepatocellular carcinoma in rats. Mol Carcinog. 2019;58:2017-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Perilli L, Tessarollo S, Albertoni L, Curtarello M, Pastò A, Brunetti E, Fassan M, Rugge M, Indraccolo S, Amadori A, Bortoluzzi S, Zanovello P. Silencing of miR-182 is associated with modulation of tumorigenesis through apoptosis induction in an experimental model of colorectal cancer. BMC Cancer. 2019;19:821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Spitschak A, Meier C, Kowtharapu B, Engelmann D, Pützer BM. MiR-182 promotes cancer invasion by linking RET oncogene activated NF-κB to loss of the HES1/Notch1 regulatory circuit. Mol Cancer. 2017;16:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Qiu Y, Luo X, Kan T, Zhang Y, Yu W, Wei Y, Shen N, Yi B, Jiang X. TGF-β upregulates miR-182 expression to promote gallbladder cancer metastasis by targeting CADM1. Mol Biosyst. 2014;10:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Liu LT, Chang HC, Chiang LC, Hung WC. Histone deacetylase inhibitor up-regulates RECK to inhibit MMP-2 activation and cancer cell invasion. Cancer Res. 2003;63:3069-3072. [PubMed] |
| 12. | Noda M, Oh J, Takahashi R, Kondo S, Kitayama H, Takahashi C. RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev. 2003;22:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Takahashi C, Sheng Z, Horan TP, Kitayama H, Maki M, Hitomi K, Kitaura Y, Takai S, Sasahara RM, Horimoto A, Ikawa Y, Ratzkin BJ, Arakawa T, Noda M. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci USA. 1998;95:13221-13226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 365] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Liu Y, Li L, Liu Y, Geng P, Li G, Yang Y, Song H. RECK inhibits cervical cancer cell migration and invasion by promoting p53 signaling pathway. J Cell Biochem. 2018;119:3058-3066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Chen R, Sheng L, Zhang HJ, Ji M, Qian WQ. miR-15b-5p facilitates the tumorigenicity by targeting RECK and predicts tumour recurrence in prostate cancer. J Cell Mol Med. 2018;22:1855-1863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Wang J, Lin Y, Jiang T, Gao C, Wang D, Wang X, Wei Y, Liu T, Zhu L, Wang P, Qi F. Up-regulation of TIMP-3 and RECK decrease the invasion and metastasis ability of colon cancer. Arab J Gastroenterol. 2019;20:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Qin J, Luo M. MicroRNA-221 promotes colorectal cancer cell invasion and metastasis by targeting RECK. FEBS Lett. 2014;588:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Iseki Y, Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Hirakawa K, Ohira M. MicroRNA-96 Promotes Tumor Invasion in Colorectal Cancer via RECK. Anticancer Res. 2018;38:2031-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Wei LJ, Bai DM, Wang ZY, Liu BC. MicroRNA-375 accelerates the invasion and migration of colorectal cancer through targeting RECK. Eur Rev Med Pharmacol Sci. 2019;23:4738-4745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 291] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 21. | Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 1818] [Article Influence: 363.6] [Reference Citation Analysis (0)] |
| 22. | Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Würdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328-6333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1304] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 23. | Chiang CH, Hou MF, Hung WC. Up-regulation of miR-182 by β-catenin in breast cancer increases tumorigenicity and invasiveness by targeting the matrix metalloproteinase inhibitor RECK. Biochim Biophys Acta. 2013;1830:3067-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Hirata H, Ueno K, Shahryari V, Tanaka Y, Tabatabai ZL, Hinoda Y, Dahiya R. Oncogenic miRNA-182-5p targets Smad4 and RECK in human bladder cancer. PLoS One. 2012;7:e51056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Hirata H, Ueno K, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, Hinoda Y, Dahiya R. MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PLoS One. 2013;8:e55502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |