Published online Feb 28, 2020. doi: 10.3748/wjg.v26.i8.865
Peer-review started: September 24, 2019
First decision: November 27, 2019
Revised: December 23, 2019
Accepted: January 19, 2020
Article in press: January 19, 2020
Published online: February 28, 2020
Processing time: 156 Days and 18.9 Hours
Bariatric procedures are considered superior to medical therapies in managing type 2 diabetes mellitus (T2DM). Laparoscopic Roux-en-Y gastric bypass (LRYGB) and laparoscopic sleeve gastrectomy (LSG) are the most commonly used procedures for weight loss and comorbidity resolution worldwide. However, it is not yet known whether the degree of T2DM is influenced by the choice of bariatric procedure.
To quantitatively compare T2DM resolution over 1-5 years follow-up by LRYGB and LSG in morbidly obese patients.
We searched the selected databases for full-text English language clinical studies that compared the effectiveness of LRYGB and LSG for T2DM resolution. Review manager 5.3 was used for data analysis, and the overall effect summary was represented in a forest plot.
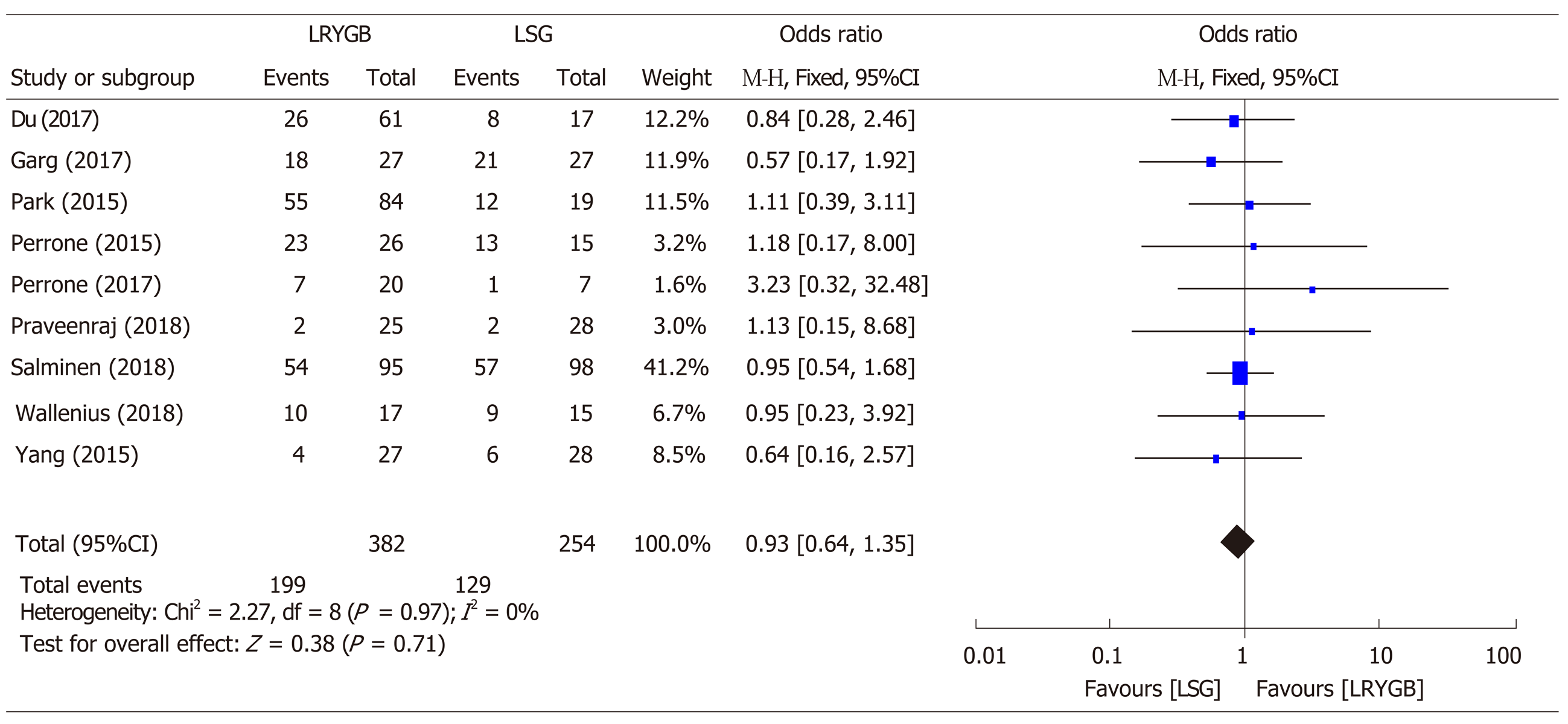
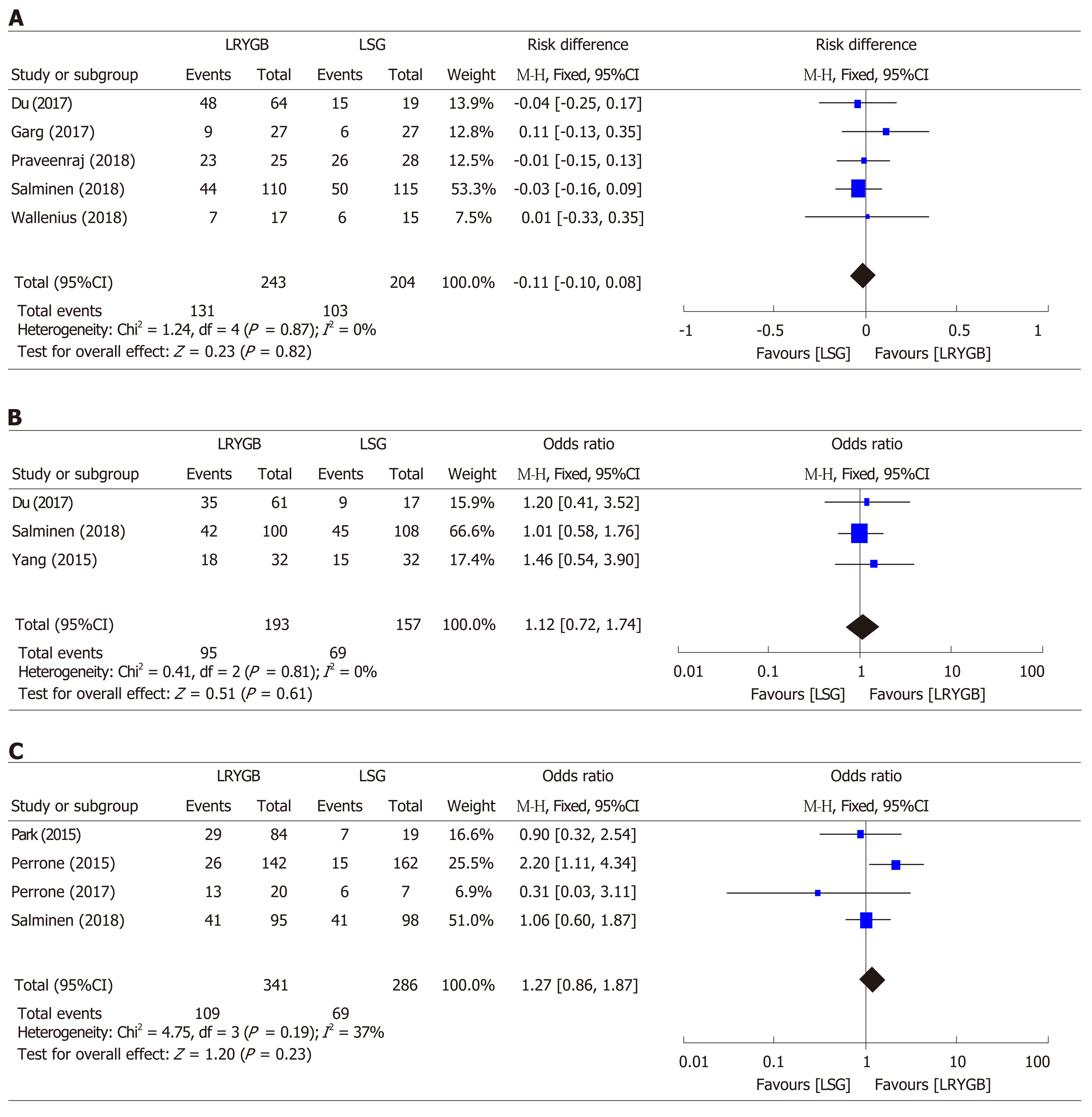
From 1,650 titles retrieved by an initial search, we selected nine studies for this research. We found insignificant differences for T2DM resolution by LRYGB and LSG, with an odds ratio of 0.93 (95%CI: 0.64-1.35, Z statistics = 0.38, P = 0.71). Additionally, subset analyses for T2DM resolution showed insignificant differences after 24 mo (χ2 = 1.24, df = 4, P = 0.87, overall Z effect = 0.23), 36 mo (χ2 = 0.41, df = 2, P = 0.81, overall Z effect = 0.51), and 60 mo (χ2 = 4.75, df = 3, P = 0.19, overall Z effect = 1.20) by LRYGB and LSG. This study reports a T2DM remission rate of 82.3% by LRYGB and 80.7% by LSG.
This study reports similar T2DM resolution rates by both LRYGB and LSG during 1-5 years of follow-up. However, long-term follow-up of 10 years is needed to further substantiate these findings.
Core tip: Based on our research findings, both laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy can be used for type 2 diabetes mellitus resolution in morbidly obese patients.
- Citation: Guraya SY, Strate T. Surgical outcome of laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass for resolution of type 2 diabetes mellitus: A systematic review and meta-analysis. World J Gastroenterol 2020; 26(8): 865-876
- URL: https://www.wjgnet.com/1007-9327/full/v26/i8/865.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i8.865
Bariatric surgery is effective in treating morbid obesity, and in the resolution of its associated co-morbidities including metabolic syndrome, hyperlipidemia, type 2 diabetes mellitus (T2DM), sleep apnea, osteoarthritis, and psychological disorders[1]. Out of a host of surgical remedies for morbid obesity, laparoscopic Roux-en-Y gastric bypass (LRYGB) and laparoscopic sleeve gastrectomy (LSG) remain the most commonly performed bariatric surgery procedures worldwide[2]. Both LRYGB and LSG have been shown to be safe, feasible and effective in accomplishing excess weight loss and resolution of co-morbidities[3,4]. Literature has shown a rapid escalation in the prevalence of T2DM and its associate complications, particularly cardiovascular[5], gallstones[6], peripheral arterial system and foot ulcers[7,8], stroke[9], and colorectal cancer[10]. In this perspective, bariatric procedures such as LRYGB and LSG have been successfully used for the resolution of T2DM and its comorbidities[11].
The mechanisms of T2DM resolution by LRYGB and LSG are largely unknown. It has been postulated that changes in gastrointestinal hormone secretions following LRYGB account for the resolution of T2DM, as the duodenum and upper jejunum are bypassed for direct delivery of nutrients to the midgut[12]. In contrast, weight loss induced by LSG is mediated by a primarily restrictive mechanism that leads to the simultaneous resolution of T2DM.
Literature has reported a T2DM resolution rate of 81.2% by LRYGB and 80.9% by LSG[13]. However, the selection of weight loss procedure is influenced by a wealth of factors. Praveenrai et al[14] proposed that the choice of bariatric surgery procedure is primarily driven by therapy goals (weight loss or glycemic control), associated gastro-esophageal reflux or nutritional deficiencies, patient preferences and expertise of the surgeon. Generally, LRYGB is recommended for patients with long-standing, refractory T2DM with low serum C peptides, and LSG for other patients with morbid obesity. Interestingly, the majority of patients prefer LSG over LRYGB for the weight loss and resolution of associated metabolic disorders. In a double blind randomized clinical trial by Lee et al[15], the authors investigated T2DM resolution by LRYGB and LSG (fasting glucose 126 mg/dL and HbA1c 6.5% without anti-glycemic treatment)[15]. The study concluded that the patients in the LRYGB group had greater weight loss, a lower waist circumference, and had rapid T2DM regression and lipid control compared to LSG. On the other hand, Vidal et al[16] showed that LSG was as effective as LRYGB in securing the resolution of T2DM and metabolic syndrome at a 12-mo follow-up after surgery. Similarly, in a systematic review of randomized clinical trials by Osland et al[17], the authors reported significant remission of T2DM by both LRYGB as well as by LSG across all stages of follow-up.
There seems to be no standard consensus about the superiority of LRYGB or LSG in achieving the resolution of T2DM. The available data are scarce, and very few studies have rigorously compared the outcome of LRYGB and LSG for attaining remission of T2DM in obese patients. This systematic review and meta-analysis provides quantitative comparison of the effectiveness of LRYGB and LSG for the resolution of T2DM in morbidly obese patients.
In January 2019, this systematic review and meta-analysis was performed using the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[18]. The Medical Subject Headings (MeSH) terms used for systematic review included: Morbid obesity, type 2 diabetes mellitus, laparoscopic sleeve gastrectomy, laparoscopic Roux-en-Y gastric bypass, and fasting blood glucose. The databases of PubMed, Ovid, Wiley online library, Cochrane library, CINAHL, ISI Web of knowledge, ScienceDirect, and EBSCO were searched for full-text English original clinical studies published from Jan 2013 to Jan 2018. The original studies that compared the effectiveness of LSG and LRYG for the resolution of T2DM in morbidly obese patients during 1-5 years of follow-up were included in this search. The remission of T2DM was considered when HbA1c < 6.0% without anti-diabetic therapy was achieved by bariatric procedures[19]. As defined by review articles, editorials, expert opinions, commentaries, and short communications were excluded. The original studies with incomplete data as mean ± SD (for continuous outcome), number, percentage (for dichotomous outcome) or an average follow-up of less than one year were excluded. In addition, research articles that attempted to determine surgical outcomes of LSG and LRYGB in patients with a body BMI < 27 kg/m2 or < 18- or > 65-years-old were excluded. The indicators of glycemic control were HbA1C and fasting blood glucose levels. Finally, research showing combined data of revision or conversion bariatric procedures was not included in this study.
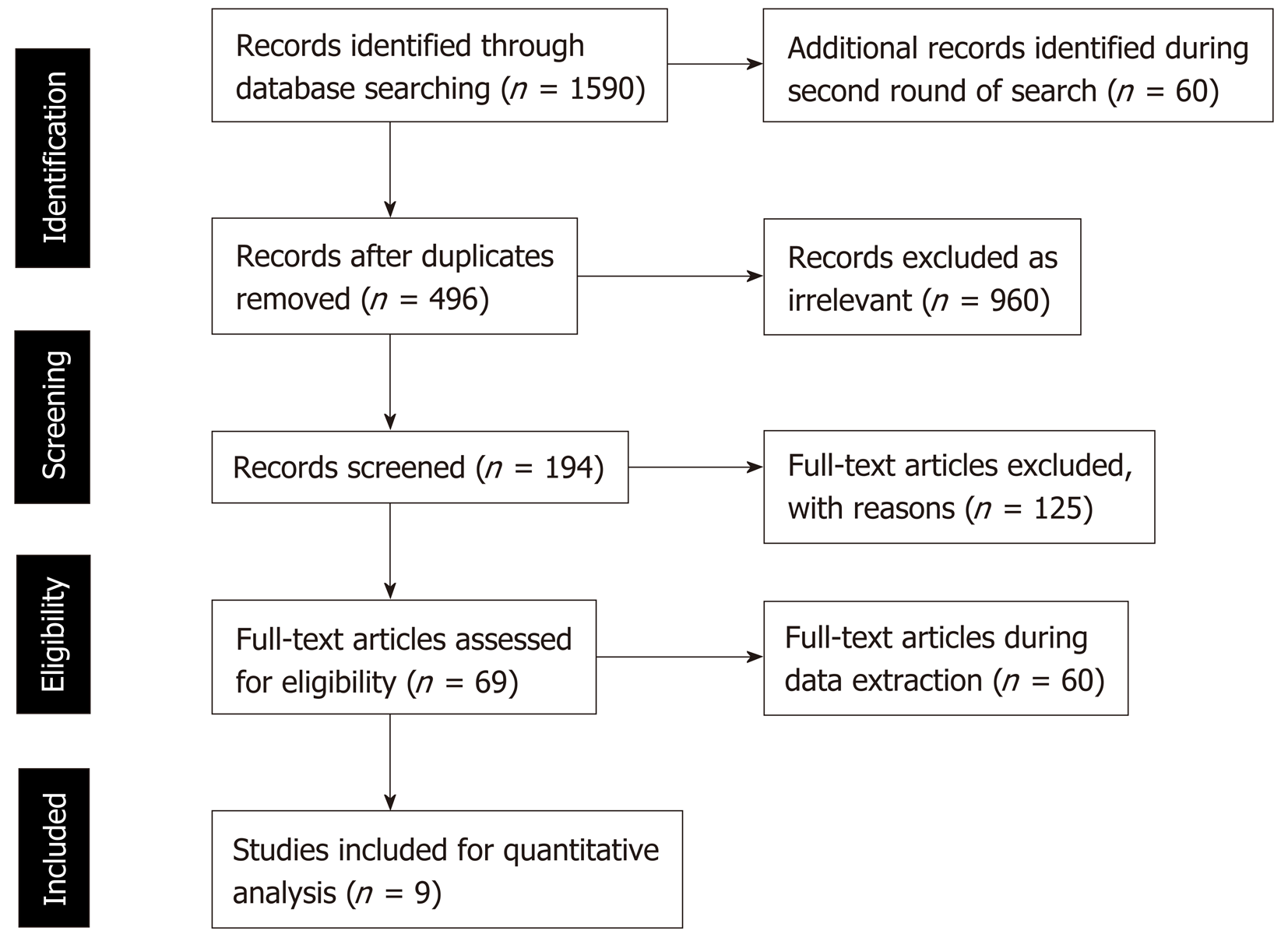
During two rounds of searches in the selected databases, 1650 studies were retrieved. Review of the searched titles showed 496 duplicate and 960 irrelevant titles that were excluded from further literature review. At the next stage of analysis of the 194 titles, another 125 articles were excluded, as the contents and study outcomes did not match our systematic review primary outcomes. As many as 69 articles were found to be relevant, as they compared the effectiveness of LSG and LRYGB for T2DM resolution. However, during full text review of these 69 articles, 60 studies were further excluded, as they contained incomplete data and inconsistent findings. Finally, a total of nine relevant studies were selected in this meta-analysis (Figure 1).
Two independent researchers (Salman Yousuf Guraya and Tim Strate) searched, analyzed, reviewed the retrieved titles and full-text articles for suitability and study representativeness. Using the Cochrane Collaboration tool, we found some element of selection bias that was reflected by the blinding of participants and personnel preferences (performance bias)[10,20]. The risk of bias and conflict of interest were eliminated by discussions and by reaching a general consensus.
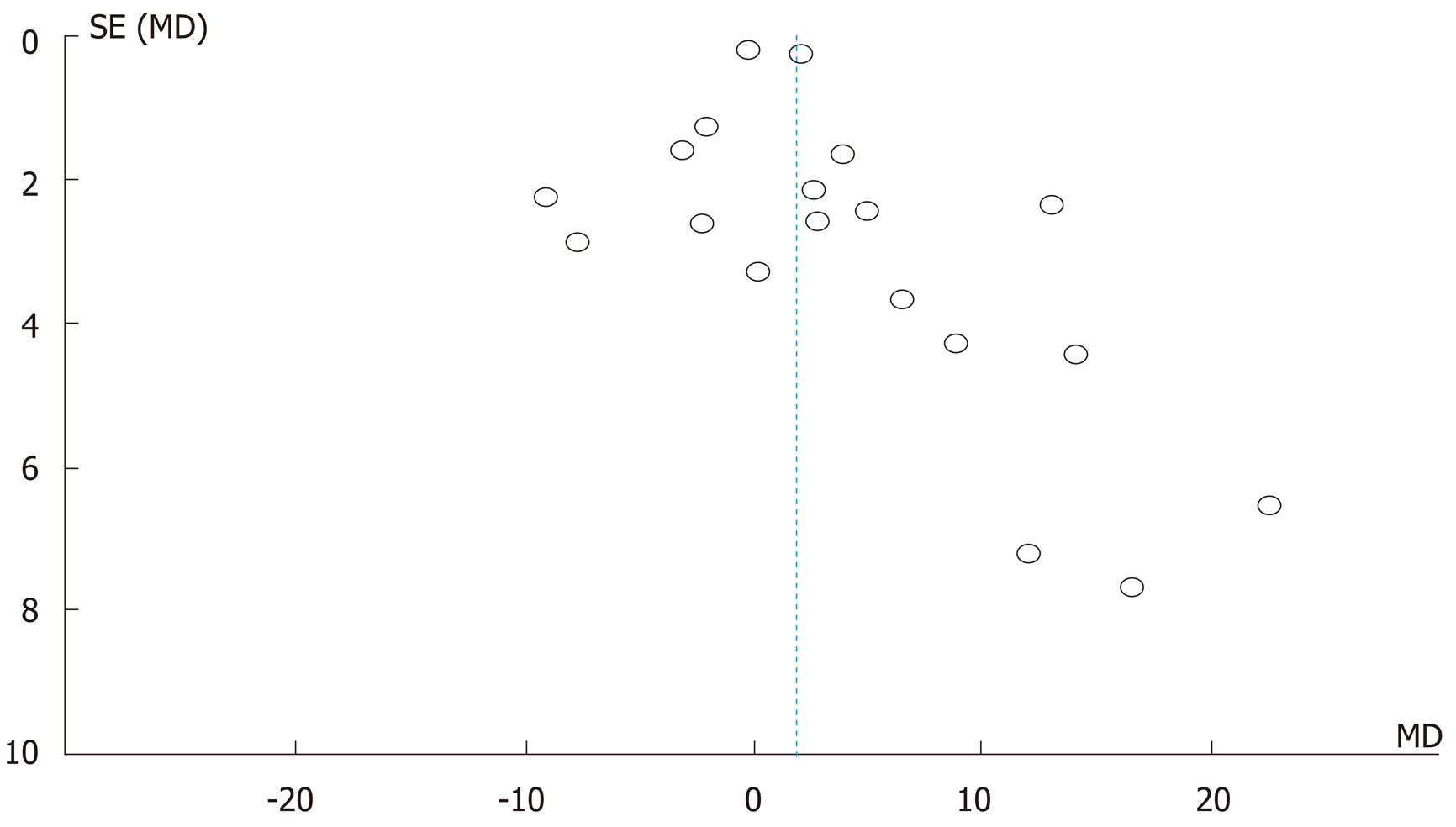
Review Manger 5.3 software, developed by Cochrane Library, was used for the quantitative analysis of data from the selected studies in this meta-analysis[21]. The findings of the meta-analysis were graphed on a forest plot, which quantitatively demonstrates the consistency and reliability of results. In a forest plot, the effect size of each study is computed as an outcome measure, and pooled effect summary is calculated to determine heterogeneity across sleeted studies. Statistically, Q tests reflect heterogeneity in the selected studies using the null hypothesis that all studies are identical. In this meta-analysis, the I squared (I2) statistical analysis was used to validate heterogeneity in percentage terms[20]. In the case of low heterogeneity (P > 0.10, I2 < 50%), a fixed effects model is recommended. On the other hand, a random effects model is employed in the case of higher heterogeneity (P < 0.10 or I2 > 50%). The Tau squared (Tau2) test is applied to estimate variance in the calculated data using the random effects model. We estimated publication bias by visual inspection of the funnel plot, and the level of significance in this study was considered as 5% (P < 0.05).
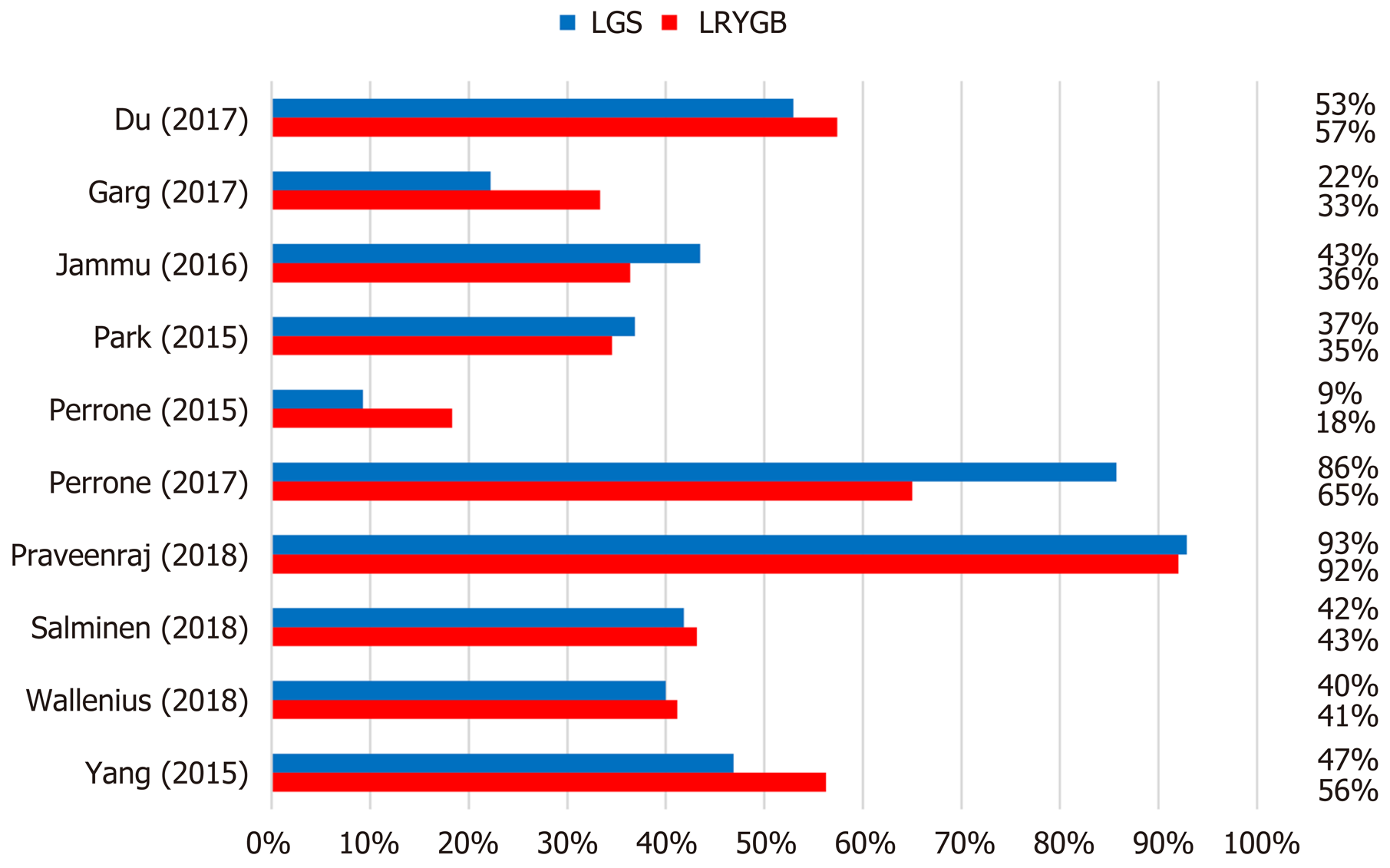
A close comparative analysis of the resolution of T2DM by LSG and LRYGB is illustrated in Figure 2. We found that the Cochrane Q (χ2 = 154.43) test was significant at 5% (P < 0.05). This rejected the null hypothesis that all studies were identical. Due to substantial heterogeneity (I2 = 88%), a random effect model was deemed necessary. In our meta-analysis, the forest plot generated by Review Manager 5.3 for the comparison of resolution of T2DM by LSG and LRYGB showed insignificant differences for T2DM resolution by LSG and LRYGB, with an odds ratio of 0.93 (95%CI: 0.64-1.35, Z statistics = 0.38, P = 0.71), as shown in Figure 3. Figure 4 includes a forest plot that compares the resolution of T2DM by LSG and LRYGB after 24, 36 and 60 mo. Insignificant differences are reported between the two weight loss surgical procedures. The scientific evidence of symmetry and homogeneity of the selected articles for effectiveness of LSG and LRYGB in resolution of T2DM is demonstrated in the funnel plot in Figure 5. The remission rate of T2DM by LRYGB and LSG is estimated to be 82.3% and 80.7%, respectively. Characteristics of the selected studies, including the complete citation of articles, study design, number of patients at the time of surgery, and key outcomes by both LSG and LRYGB are shown in Table 1.
| Ref. | Country | Study type | Sample size | Key findings | |
| LSG | LRYGB | ||||
| Du et al[1], 2017 | China | Randomized clinical trial | 19 | 74 | Overall remission rate achieved with LRYGB and LSG was 75.9% at 1 yr and 56.4% at 3 yr |
| Safety profile, T2DM resolution and other morbid obesity-related comorbidities by both procedures are comparable | |||||
| Garg et al[42], 2017 | India | Retrospective clinical | 40 | 40 | The median duration of T2DM was higher in LRYGB than LSG (2.2 vs 1.8), respectively |
| Both LRYGB and LSG showed significant but similar improvement in T2DM remission | |||||
| Park and Kim[43], 2015 | South Korea | Retrospective Cohort Study | 104 | 236 | The study found comparable results with insignificant differences between LRYGB and LSG |
| Perrone et al[27], 2016 | Italy | Prospective clinical trials | 162 | 142 | LSG is more effective in obese men than in women for excess weight loss. However, there is no difference in terms of the remission of comorbidities |
| LRYGB showed similar findings in both genders for excess weight loss and comorbidity resolution, including T2DM | |||||
| Perrone et al[44], 2017 | Italy | Prospective clinical trial | 162 | 142 | LRYGB showed better T2DM resolution rate in the short-term |
| Neither LRYGB nor LSG showed a significant difference in T2DM remission in the long-term | |||||
| Praveenraj et al[14], 2016 | India | Retrospective clinical trial | 54 | 32 | LRYGB showed better surgical outcomes than LSG in patients > 50 yr |
| LSG had shorter operative times and shorter hospital stays | |||||
| Peterli et al[45], 2018 | Finland | The SLEEVEPASS multicenter, randomized clinical trial | 120 | 120 | Complete or partial remission of T2DM was reported in 37% after LSG and in 45% LRYGB. No significant difference in terms of T2DM resolution is reported between LRYGB and LSG |
| Wallenius et al[29], 2018 | Sweden | Prospective clinical trial | 15 | 18 | There was a similar decrease in post-op fasting blood glucose in both the LRYGB and LSG groups |
| LRYGB and LSG showed similar outcomes in glycemic control, with insignificant differences in short- and mid-term follow-up | |||||
| Yang et al[22], 2015 | China | Randomized clinical study | 22 | 23 | LSG and LRYGB had comparable effects on T2DM remission in the Chinese study cohort with a BMI of 28-35 kg/m2 |
Our systematic review and meta-analysis showed insignificant differences in T2DM resolution by LSG and LRYGB. Additionally, a deeper analysis of subgroups also demonstrated insignificant differences after 24 mo (χ2 = 1.24, df = 4, P = 0.87, overall Z effect = 0.23), 36 mo (χ2 = 0.41, df = 2, P = 0.81, overall Z effect = 0.51), and 60 mo (χ2 = 4.75, df = 3, P = 0.19, overall Z effect = 1.20) in T2DM resolution by LSG and LRYGB. In the absence of a clear consensus about the superiority of LRYGB over LSG for T2DM resolution, the findings of our study provide landmark evidence for the management of morbid obesity and its comorbidities.
In the clinical trial by Yang et al[22], the researchers followed up with patients after LRYGB and LSG for 3 years, and have reported similar T2DM remission rates. Similar results have been reported elsewhere[23,24]. In the same study, complete T2DM resolution rates, as defined by HbA1c < 6.0% without anti-diabetic therapy, were 78.6% in the LSG group and 85.2% in the LRYGB group. These findings are in agreement with the previously published prospective clinical studies on patients with a BMI > 35 kg/m2[25,26]. However, in the randomized controlled trial by Lee et al[15], the investigators argued that LRYGB was superior in achieving better blood glucose control than LSG at 1 and 5 years after surgery on patients with a BMI of 25-35 kg/m2. Nonetheless, our systematic review and meta-analysis could not find superiority of LRYGB or LSG in T2DM throughout 1-5 years of follow-up.
In the study by Perrone et al[27], the authors compared long-term results on weight loss and comorbidity resolution for LRYGB and LSG on a cohort of 304 consecutive patients. Though LSG was more effective in the percentage of excess weight loss at 180 d and at 1 year of follow-up, however, LRYGB and LSG showed similar results at 5 years of follow-up; 72.34 vs 70.26, respectively. Generally, LRYGB was shown to be more effective in T2DM remission than LSG. These findings reflect the lack of a gold standard bariatric procedure that can help achieve excess weight loss and comorbidity resolution[28]. The study by Wallenius et al[29] compared early weight-independent and later weight-dependent influence by LRYGB and LSG on glycemic control. Initially, there was a similar drop in fasting blood glucose levels in both the LRYGB and LSG groups; 8.1 ± 0.6 mmol/L vs 8.2 ± 0.4 mmol/L, 2 d; 7.8 ± 0.5 mmol/L vs 7.4 ± 0.3 mmol/L, 21 d; 6.6 ± 0.4 mmol/L vs 6.6 ± 0.3 mmol/L, respectively. This study reported similar effects on glycemic controls by both surgical procedures. On the other hand, the study by Gray et al[30] reported a greater improvement in T2DM, hypertension, hyperlipidemia, and gastroesophageal reflux disease by LRYGB over LSG during a median follow-up of 39 mo. From a different perspective, some investigators have recommended LRYGB as a gold standard for effective weight loss and resolution of comorbidities, and to keep LSG as an attractive substitute[31,32]. Unfortunately, as of today, the literature is divided on this argument, and we need more concrete evidence to validate these observations.
By and large, the mechanisms of T2DM remission by LRYGB and LSG are unclear, although several hypotheses exist. The literature has shown a greater inclination toward LSG as an anti-diabetic surgical remedy, which induces early glucose homeostasis similar to that of LRYGB[33]. In an interesting study by Nannipier et al[34], the authors investigated the mechanism for T2DM remission by GI hormones, and found similar results from LRYGB and LSG. The study concluded that glucagon-like peptide (GLP-1) and polypeptide YY (PYY) were the key predators of glucose homeostasis in the post-operative follow-up. Though the exact mechanisms underlying better glucose homeostasis following LSG is uncertain, a fall in ghrelin levels and unexpected changes in serum levels of distal intestinal hormones (GLP-1, GLP-2 and PYY) are considered to play some role. Furthermore, insulin resistance is decreased and incretin hormones levels are substantially elevated. On the other hand, since LRYGB bypasses the proximal intestine, a host of neurohormonal changes ensue, particularly low insulin resistance, as well as changes in ghrelin, GLP-1, GLP-2 and PYY level[35]. On a similar note, Peterli et al[36] found that 1 year after operation, post-prandial serum cholecystokinin levels increased less in the LRYGB than in the LSG group. The authors have argued that bypassing the foregut is not the sole underlying reason for improved glucose homeostasis.
LSG has several advantages over LRYGB: Easier to perform, preserves pylorus and antrum with less chance of dumping syndrome, no risk of internal herniation, and decreased risk of nutritional deficiencies[37,38]. In terms of its shorter learning curve, LSG is gaining popularity over LRYGB among bariatric surgeons. The results of our meta-analysis would further strengthen the value of LSG in achieving weight loss and remission of comorbidities, particularly T2DM due to its comparable profile with LRYGB. Additionally, improving surgeons skills[39] and adhering to professional codes tend to lead to better surgical outcomes[40].
In conclusion, this study reports similar T2DM resolution rates by both LRYGB and LSG during 1 to 5 years of follow-up. However, long-term follow-up of 10 years is needed to further endorse these findings.
There is a staggering rise in the incidence of obesity worldwide. A sedentary lifestyle, unhealthy food, and multiple comorbidities such as Type 2 diabetes mellitus (T2DM), hypertension and hyperlipidemia are major risk factors for obesity. In order to curtail the epidemic of obesity, a host of treatment strategies are offered, including lifestyle change, dietary consultations, medications, as well as surgical therapies. Of these, surgical remedies carry great promise in achieving effective weight loss and the resolution of comorbidities. Generally, bariatric procedures are considered superior to medical therapies in treating obesity-related T2DM. Though Laparoscopic Roux-en-Y gastric bypass (LRYGB) and Laparoscopic sleeve gastric bypass (LSG) are the most popular bariatric surgical procedures worldwide, there is no consensus about the superiority of one procedure over the other in terms of the resolution of obesity-related T2DM.
This study determines the effectiveness of LSG and LRYGB for treating obesity-related T2DM. Short-, mid- and long-term follow-up results after bariatric surgery were analyzed. The literature is divided about the estimated outcomes by various bariatric surgical procedures in achieving excess percentage weight loss and T2DM. This study quantitatively compares the resolution of T2DM by LSG and LRYGB.
We conducted the current study to quantitatively compare the impact of LSG and LRYGB in T2DM resolution over 1 to 5 years post-surgery follow-up.
We conducted a literature search by using selected keywords in pre-defined databases for full-text English language clinical studies. This study compared short-, mid- and long-term outcomes of T2DM resolution by LRYGB and LSG. The data from all selected studies were analyzed by Review Manager® 5.3. Forest plots were generated for overall effect summaries. The homogeneity of the selected studies was determined by funnel plots and, finally, the findings were interpreted and compared with published reports.
A total of 1650 titles were retrieved from the selected databases. Using PRISMA guidelines, both investigators shortlisted and then finally selected nine studies for further analysis. We report a T2DM remission rate of 82.3% by LRYGB and 80.7% by LSG. This study shows insignificant differences for T2DM resolution by LRYGB and LSG, with an odds ratio of 0.93 (95%CI: 0.64-1.35, Z statistics = 0.38, P = 0.71). Deeper analysis of subsets for T2DM resolution for short-, mid- and long-term follow-up showed similar results at 24 mo (χ2 = 1.24, df = 4, P = 0.87, overall Z effect = 0.23), 36 mo (χ2 = 0.41, df = 2, P = 0.81, overall Z effect = 0.51), and 60 mo (χ2 = 4.75, df = 3, P = 0.19, overall Z effect = 1.20).
This study provides comparative quantitative evidence regarding the role of LSG and LRYGB in treating obesity-related T2DM. Technically, compared to LRYGB, LSG is much easier to perform, and takes significantly shorter operative time. Being a relatively easier bariatric surgical procedure, LSG may be favored in achieving T2DM resolution. However, before we can reach a consensus, the results of long-term follow-up over 10 years should be quantitatively analyzed. By and large, this study implies a comparable achievement in T2DM resolution by both procedures up to 5 years follow-up.
LSG and LRYGB, although quite different bariatric surgical procedures, achieve similar T2DM resolution up to 5 years post-surgery. Future research should investigate different neurohormonal mechanisms that lead to a common goal of T2DM resolution by both surgical procedures.
We acknowledge the support in literature review and meta-analysis provided by Mr B Bilal, Associate Professor Hubei Centre for Accounting Development Research School of Accountancy Hubei University of Economics, Wuhan, China.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and metabolic disorder
Country of origin: United Arab Emirates
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Das U, Emile S, Saligram S S-Editor: Zhou JJ L-Editor: Filipodia E-Editor: Zhang YL
| 1. | Hariri K, Guevara D, Dong M, Kini SU, Herron DM, Fernandez-Ranvier G. Is bariatric surgery effective for co-morbidity resolution in the super-obese patients? Surg Obes Relat Dis. 2018;14:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Singhal S, Agarwal D, Kanojiya R, Arora D, Avesthi A, Kothari A. Effect of laparoscopic sleeve gastrectomy on lipid profile of obese patients in complete nine month follow up. International Surgery Journal. 2016;3:42-46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Dicker D, Yahalom R, Comaneshter DS, Vinker S. Long-Term Outcomes of Three Types of Bariatric Surgery on Obesity and Type 2 Diabetes Control and Remission. Obes Surg. 2016;26:1814-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Du X, Fu XH, Peng BQ, Luo R, Hu JK, Cheng Z. Resolution of metabolic syndrome and related metabolic disorders after bariatric surgery: comparison of sleeve gastrectomy and gastric bypass. Surg Obes Relat Dis. 2018;14:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3405] [Article Influence: 486.4] [Reference Citation Analysis (0)] |
| 6. | Khairy GA, Guraya SY, Murshid KR. Cholesterolosis. Incidence, correlation with serum cholesterol level and the role of laparoscopic cholecystectomy. Saudi Med J. 2004;25:1226-1228. [PubMed] |
| 7. | Almaramhy H, Mahabbat NA, Fallatah KY, Al-Ahmadi BA, Al-Alawi HH, Guraya SY. The correlation of fasting blood glucose levels with the severity of diabetic foot ulcers and the outcome of treatment strategies. Biomedical Research. 2018;29:1961-1967. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Guraya SY, London N. The prevalence and management strategies for peripheral artery disease associated with diabetes mellitus in the Arab world. Journal of Taibah University Medical Sciences. 2016;11:310-316. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Balakumar P, Maung-U K, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 385] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 10. | Guraya SY. Association of type 2 diabetes mellitus and the risk of colorectal cancer: A meta-analysis and systematic review. World J Gastroenterol. 2015;21:6026-6031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (2)] |
| 11. | Rubino F. Bariatric surgery: effects on glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2006;9:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 552] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 13. | Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F, Basso N. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosc. 2010;24:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Praveenraj P, Gomes RM, Kumar S, Perumal S, Senthilnathan P, Parthasarathi R, Rajapandian S, Palanivelu C. Comparison of weight loss outcomes 1 year after sleeve gastrectomy and Roux-en-Y gastric bypass in patients aged above 50 years. J Minim Access Surg. 2016;12:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, Tsai MH, Chuang LM. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 332] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 16. | Vidal J, Ibarzabal A, Romero F, Delgado S, Momblán D, Flores L, Lacy A. Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. Obes Surg. 2008;18:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Osland E, Yunus RM, Khan S, Memon B, Memon MA. Diabetes improvement and resolution following laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic Roux-en-Y gastric bypass (LRYGB) procedures: a systematic review of randomized controlled trials. Surg Endosc. 2017;31:1952-1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Guraya SY, Norman RI, Khoshhal KI, Guraya SS, Forgione A. Publish or Perish mantra in the medical field: A systematic review of the reasons, consequences and remedies. Pak J Med Sci. 2016;32:1562-1567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Shen SC, Wang W, Tam KW, Chen HA, Lin YK, Wang SY, Huang MT, Su YH. Validating Risk Prediction Models of Diabetes Remission After Sleeve Gastrectomy. Obes Surg. 2019;29:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Guraya SY, Barr H. The effectiveness of interprofessional education in healthcare: A systematic review and meta-analysis. Kaohsiung J Med Sci. 2018;34:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 21. | Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. John Wiley &. Sons;. 2011;. [DOI] [Full Text] |
| 22. | Yang J, Wang C, Cao G, Yang W, Yu S, Zhai H, Pan Y. Long-term effects of laparoscopic sleeve gastrectomy versus roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28-35 kg/m2. BMC Surg. 2015;15:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Li K, Gao F, Xue H, Jiang Q, Wang Y, Shen Q, Tian Y, Yang Y. Comparative study on laparoscopic sleeve gastrectomy and laparoscopic gastric bypass for treatment of morbid obesity patients. Hepatogastroenterology. 2014;61:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Zerrweck C, Sepúlveda EM, Maydón HG, Campos F, Spaventa AG, Pratti V, Fernández I. Laparoscopic gastric bypass vs. sleeve gastrectomy in the super obese patient: early outcomes of an observational study. Obes Surg. 2014;24:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 25. | Keidar A, Hershkop KJ, Marko L, Schweiger C, Hecht L, Bartov N, Kedar A, Weiss R. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia. 2013;56:1914-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Pham S, Gancel A, Scotte M, Houivet E, Huet E, Lefebvre H, Kuhn JM, Prevost G. Comparison of the effectiveness of four bariatric surgery procedures in obese patients with type 2 diabetes: a retrospective study. J Obes. 2014;2014:638203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Perrone F, Bianciardi E, Benavoli D, Tognoni V, Niolu C, Siracusano A, Gaspari AL, Gentileschi P. Gender Influence on Long-Term Weight Loss and Comorbidities After Laparoscopic Sleeve Gastrectomy and Roux-en-Y Gastric Bypass: a Prospective Study With a 5-Year Follow-up. Obes Surg. 2016;26:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Guraya SY, Strate T. Effectiveness of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity in achieving weight loss outcomes. Int J Surg. 2019;70:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Wallenius V, Dirinck E, Fändriks L, Maleckas A, le Roux CW, Thorell A. Glycemic Control after Sleeve Gastrectomy and Roux-En-Y Gastric Bypass in Obese Subjects with Type 2 Diabetes Mellitus. Obes Surg. 2018;28:1461-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Gray KD, Moore MD, Bellorin O, Abelson JS, Dakin G, Zarnegar R, Pomp A, Afaneh C. Increased Metabolic Benefit for Obese, Elderly Patients Undergoing Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Obes Surg. 2018;28:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Dogan K, Gadiot RP, Aarts EO, Betzel B, van Laarhoven CJ, Biter LU, Mannaerts GH, Aufenacker TJ, Janssen IM, Berends FJ. Effectiveness and Safety of Sleeve Gastrectomy, Gastric Bypass, and Adjustable Gastric Banding in Morbidly Obese Patients: a Multicenter, Retrospective, Matched Cohort Study. Obes Surg. 2015;25:1110-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Pekkarinen T, Mustonen H, Sane T, Jaser N, Juuti A, Leivonen M. Long-Term Effect of Gastric Bypass and Sleeve Gastrectomy on Severe Obesity: Do Preoperative Weight Loss and Binge Eating Behavior Predict the Outcome of Bariatric Surgery? Obes Surg. 2016;26:2161-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301:R15-R27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. 2013;98:4391-4399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 35. | Samat A, Malin SK, Huang H, Schauer PR, Kirwan JP, Kashyap SR. Ghrelin suppression is associated with weight loss and insulin action following gastric bypass surgery at 12 mo in obese adults with type 2 diabetes. Diabetes Obes Metab. 2013;15:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22:740-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 37. | Navarrete A, Corcelles R, Del Gobbo GD, Perez S, Vidal J, Lacy A. Sleeve gastrectomy in the elderly: A case-control study with long-term follow-up of 3 years. Surg Obes Relat Dis. 2017;13:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Khairy G, Guraya SY, Murshid KR. Incidence, correlation with serum cholesterol I and the role of laparoscopic cholecystectomy. Saudi Med J. 2005;26:1058. |
| 39. | Forgione A, Kislov V, Guraya SY, Kasakevich E, Pugliese R. Safe introduction of laparoscopic colorectal surgery even in remote areas of the world: the value of a comprehensive telementoring training program. J Laparoendosc Adv Surg Tech A. 2015;25:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Guraya SY, Norman RI, Roff S. Exploring the climates of undergraduate professionalism in a Saudi and a UK medical school. Med Teach. 2016;38:630-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Du X, Zhou HX, Zhang SQ, Tian HM, Zhou ZG, Cheng Z. A comparative study of the metabolic effects of LSG and LRYGB in Chinese diabetes patients with BMI < 35 kg/m2. Surg Obes Relat Dis. 2017;13:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Garg H, Priyadarshini P, Aggarwal S, Agarwal S, Chaudhary R. Comparative study of outcomes following laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in morbidly obese patients: A case control study. World J Gastrointest Endosc. 2017;9:162-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Park JY, Kim YJ. Laparoscopic gastric bypass vs sleeve gastrectomy in obese Korean patients. World J Gastroenterol. 2015;21:12612-12619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Perrone F, Bianciardi E, Ippoliti S, Nardella J, Fabi F, Gentileschi P. Long-term effects of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass for the treatment of morbid obesity: a monocentric prospective study with minimum follow-up of 5 years. Updates Surg. 2017;69:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, Schultes B, Beglinger C, Drewe J, Schiesser M, Nett P, Bueter M. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. JAMA. 2018;319:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 881] [Article Influence: 125.9] [Reference Citation Analysis (0)] |