Published online Feb 28, 2020. doi: 10.3748/wjg.v26.i8.818
Peer-review started: January 13, 2020
First decision: January 16, 2020
Revised: January 20, 2020
Accepted: February 21, 2020
Article in press: February 21, 2020
Published online: February 28, 2020
Processing time: 45 Days and 19 Hours
The benefit of neoadjuvant chemotherapy for patients with signet-ring cell carcinoma of the stomach is controversial.
To evaluate the perioperative and long-term outcomes of neoadjuvant chemotherapy for locally advanced gastric signet-ring cell carcinoma.
This retrospective study identified patients with locally advanced signet-ring cell carcinomas of the stomach (cT3/4 and cN any) diagnosed from January 2012 to December 2017 by using the clinical Tumor-Node-Metastasis (cTNM) staging system. We performed 1:1 propensity score matching (PSM) to reduce bias in patient selection. The histologic and prognostic effects of neoadjuvant chemotherapy were assessed. The overall survival rates were used as the outcome measure to compare the efficacy of neoadjuvant chemotherapy vs surgery-first treatment in the selected patients.
Of the 144 patients eligible for this study, 36 received neoadjuvant chemotherapy, and 108 received initial surgery after diagnosis. After adjustment by PSM, 36 pairs of patients were generated, and baseline characteristics, including age, sex, American Society of Anesthesiologists score, tumor location, and cTNM stage, were similar between the two groups. The R0 resection rates were 88.9% and 86.1% in the surgery-first and neoadjuvant chemotherapy groups after PSM, respectively (P = 1.000). The median follow-up period was 46.4 mo. The 5-year overall survival rates of the neoadjuvant chemotherapy group and surgery-first group were 50.0% and 65.0% (P = 0.235), respectively, before PSM and 50% and 64.7% (P = 0.192), respectively, after PSM. Multivariate analyses conducted before and after PSM showed that NAC was not a prognostic factor.
Neoadjuvant chemotherapy provides no survival benefit in patients with locally advanced gastric signet-ring cell carcinoma. For resectable gastric signet-ring cell carcinoma, upfront surgery should be the primary therapy.
Core tip: Gastric cancer is the fifth most frequently diagnosed cancer and the second leading cause of cancer-related death globally. Despite a decrease in the overall incidence of gastric cancer in recent decades, the incidence of the subgroup of patients with signet-ring cell carcinoma is growing. This study provides evidence that neoadjuvant chemotherapy does not provide any survival advantage in gastric signet-ring cell carcinoma. For resectable gastric signet-ring cell carcinoma, surgery should be the primary therapy.
- Citation: Li Y, Ma FH, Xue LY, Tian YT. Neoadjuvant chemotherapy vs upfront surgery for gastric signet ring cell carcinoma: A retrospective, propensity score-matched study. World J Gastroenterol 2020; 26(8): 818-827
- URL: https://www.wjgnet.com/1007-9327/full/v26/i8/818.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i8.818
Gastric cancer is the fifth most frequently diagnosed cancer and the third leading cause of cancer-related deaths worldwide[1]. Despite a decrease in the overall incidence of gastric cancer in recent decades, the incidence of the patient subgroup with signet-ring cell carcinoma (SRCC) is growing[2,3]. According to the World Health Organization classification, SRCC is a histological type where more than 50% of the tumor contains extracellular mucinous pools. Studies have suggested that the biological behavior of SRCC is essentially different from that of other cancers with other cell types[4,5]. SRCC is associated with a worse prognosis in advanced stages[6-8]. Therefore, a multimodal treatment strategy, including neoadjuvant chemotherapy (NAC), needs to be developed and established to improve patient outcomes. Theoretically, the administration of chemotherapy before surgical resection can address micrometastatic lesions and down-stage the disease.
Some studies have investigated the benefit of NAC in gastric SRCC. However, the results are inconsistent. Earlier research showed that NAC was associated with better outcomes, though the response to NAC was relatively weak in gastric SRCC[9]. Conversely, other studies suggested that NAC provided no survival benefit in patients with gastric SRCC[10,11]. Moreover, some studies found that NAC is an independent predictor of poor survival because of its toxicity[12]. Therefore, we conducted a single-center, large-scale retrospective study to determine if there are benefits of NAC for treating gastric SRCC.
A total of 144 patients [staged using the clinical Tumor, Node, and Metastasis (cTNM) staging system] with cT3/4 stage SRCC (cN any) were identified from a database containing all gastric cancer diagnosed at the National Cancer Center of China between January 2010 and December 2017. Of the 144 SRCC patients, 36 had undergone NAC (NAC group), and 108 were surgery-first (surgery-first group). The decision for NAC had been discussed in a multidisciplinary treatment board and was made at the treating physician’s discretion.
Pre-therapeutic staging was performed for all patients using intravenous contrast-enhanced computed tomography (CT), which was repeated after the end of NAC. This study was approved by the institutional review board, which waived the requirement for informed consent due to the retrospective nature of this research.
The median number of NAC courses was four (2.25-4.75), and eleven (30.6%), nine (25.0%), seven (19.4%), four (11.1%), and five (13.9%) for the treated patients who received the SOX, DOS, Xelox, Folfox6, and DOX regimens, respectively. Toxicity and adverse events of NAC were evaluated according to the World Health Organization (WHO) standard criteria. Response to chemotherapy was evaluated by gastroscopy or radiographic examination of the stomach or a CT scan. Post-treatment evaluation of the target lesions was divided into four categories: Complete remission, partial remission, stable disease, and progressive disease according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1).
Patients in the NAC group underwent radical gastrectomy after the completion of NAC (3-4 wk). All patients underwent gastrectomy with standard D2 lym-phadenectomy. Specific surgical gastrectomy procedures, including subtotal and total gastrectomy, were selected depending on the location of the primary tumor. The resection margins were intraoperatively examined in frozen sections. Reconstruction of the gastrointestinal passage was performed according to the type of gastrectomy. Intra- and post-operative complications and corresponding outcomes were documented.
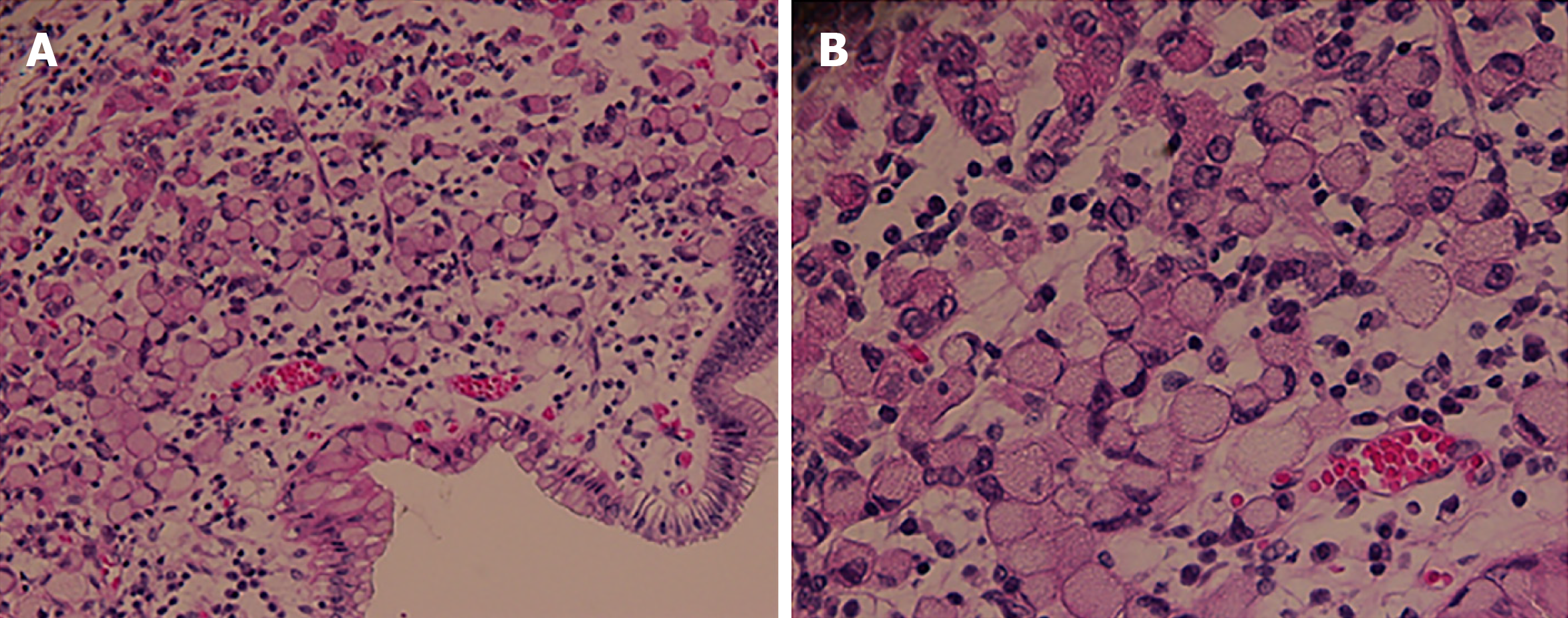
The WHO definition of SRCC was based on a resected specimen or the initial biopsy in cases where there was a complete histopathological response. The WHO defined SRCC by the content of signet ring cell-type cells greater than 50% (Figure 1). Histological staging of all tumors was based on the 8th edition of the International Union Against Cancer TNM classification system. The tumor size and presence of lymphovascular or neural invasion were evaluated. A tumor regression grade from 1 to 5 was used as described by Mandard et al[13]. R0 resection was defined as radical resection with tumor-free margins, while R1 and R2 resections were defined as resections with microscopically positive and macroscopically positive resection margins, respectively. Patients who were found to have metastatic disease at the time of surgery but underwent resection were graded with R2 resection.
Overall survival (OS) was calculated from the start of any treatment occurring since diagnosis to the point of death (for any reason), or the most recent follow-up. Data on surviving patients without relapses were censored at the last follow-up.
Categorical variables were compared using the χ2 test or Fisher’s exact test, while continuous variables were compared using Student’s t-test or the Mann-Whitney U test. We analyzed and compared the NAC and surgery-first groups before and after 1:1 matching on the basis of estimated propensity scores for each patient. The propensity scores were calculated using a logistic regression model to balance the following covariates: Age, sex, comorbidities, tumor location, American Society of Anesthesiology score, and cN stage. Kaplan-Meier survival analysis was conducted for determination of OS and generation of survival curves. Differences in survival curves were analyzed using the log rank test. A Cox proportional hazard model was used to adjust for confounding factors affecting OS. A P value < 0.05 was considered statistically significant. All data analyses were performed using SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp).
Table 1 shows the clinicopathological characteristics of patients in the NAC and surgery-first groups. Age and tumor location significantly differed between the two groups. On the basis of 1:1 propensity score matching (PSM), 72 patients (36 in the NAC group and 36 in the surgery-first group) were selected for analysis. After PSM, age, sex, body mass index, American Society of Anesthesiologists score, tumor location, cT stage, cN stage, cTNM stage, and comorbidities showed no significant differences between the two groups.
| Variable | All patients | P value | Matched patients | P value | ||
| NAC and surgery (n = 36) | Surgery first (n = 108) | NAC and surgery (n = 36) | Surgery first (n = 36) | |||
| Age, yr (mean ± SD) | 52 ± 11 | 57 ± 12 | < 0.05 | 51.6 ± 11.4 | 49.9 ± 12.2 | 0.531 |
| Sex | 0.307 | 1 | ||||
| Male | 21 (58.3) | 75 (69.4) | 21 (58.3) | 21 (58.3) | ||
| Female | 15 (41.7) | 33 (30.6) | 15 (41.7) | 15 (41.7) | ||
| BMI, kg/m2 (mean ± SD) | 24.1 ± 50 | 23.7 ± 3.4 | 0.527 | 24.1 ± 5.0 | 23.6 ± 3.4 | 0.649 |
| ASA | 0.731 | 1 | ||||
| I-II | 34 (94.4) | 99 (91.7) | 34 (94.4) | 33 (91.7) | ||
| III-IV | 2 (5.6) | 9 (8.3) | 2 (5.6) | 3 (8.3) | ||
| Comorbidity | 0.152 | 1 | ||||
| Absent | 28 (77.8) | 68 (63.0) | 28 (77.8) | 27 (75.0) | ||
| Present | 8 (22.2) | 40 (37.0) | 8 (22.2) | 9 (25.0) | ||
| Tumor location | 0.069 | 0.896 | ||||
| Upper | 4 (11.1) | 32 (29.6) | 4 (11.1) | 5 (13.9) | ||
| Middle | 13 (36.1) | 36 (33.3) | 13 (36.1) | 14 (38.9) | ||
| Lower | 19 (52.8) | 40 (37.0) | 19 (52.8) | 17 (47.2) | ||
| Clinical T stage | 1 | 0.462 | ||||
| 3 | 15 (41.7) | 46 (42.6) | 15 (41.7) | 11 (30.6) | ||
| 4 | 21 (58.3) | 62 (57.4) | 21 (58.3) | 25 (69.4) | ||
| Clinical N stage | < 0.05 | 0.514 | ||||
| 0 | 4 (11.1) | 35 (32.4) | 4 (11.1) | 7 (19.4) | ||
| 1-3 | 32 (88.9) | 73 (67.6) | 32 (88.9) | 29 (80.6) | ||
| Clinical TNM stage | 0.181 | 0.829 | ||||
| IIB | 4 (11.1) | 27 (25.0) | 4 (11.1) | 6 (16.7) | ||
| III | 29 (80.6) | 70 (64.8) | 29 (80.6) | 28 (77.8) | ||
| IVA | 3 (8.3) | 11 (10.2) | 3 (8.3) | 2 (5.6) | ||
In this study, 18 (50.0%) patients exhibited partial remission while 18 (50.0%) exhibited stable disease; no patient exhibited progressive disease according to contrast-enhanced CT before and after NAC. After surgery, pathologic tumor regression grades of 1 (n = 3), 2 (n = 4), 3 (n = 4), 4 (n = 3), and 5 (n = 22) were observed (Table 2). Eight (22.2%) of the thirty-six treated patients experienced at least grade 3-4 toxicity during NAC treatment (Table 3). The most common grade 3-4 toxicities were leukopenia/neutropenia (16.7%), nausea and vomiting (8.3%), and thrombocytopenia (2.8%).
| Neoadjuvant therapy | n = 36 |
| Clinical response | |
| PR | 18 (50) |
| SD | 18 (50) |
| Mandard grade | |
| 1 | 3 (8.3) |
| 2 | 4 (11.1) |
| 3 | 4 (11.1) |
| 4 | 3 (8.3) |
| 5 | 22 (61.1) |
| Adverse event | Total | Grade 3/4 |
| Leukopenia/neutropenia | 9 (25.0) | 6 (16.7) |
| Nausea/vomiting | 6 (16.7) | 3 (8.3) |
| Thrombocytopenia | 2 (5.6) | 1 (2.8) |
| Diarrhea | 2 (5.6) | 0 |
| Hand-foot skin reaction | 1 (2.8) | 0 |
| Allergy | 1 (2.8) | 0 |
| Alopecia | 1 (2.8) | 0 |
| Liver dysfunction | 3 (8.3) | 0 |
The incidences of postoperative complications in the NAC and surgery-first groups were 8.3% and 13% (P = 0.563), respectively. After PSM, these incidences were 8.3% and 16.7% (P = 0.478), respectively. R0 resection was performed for 88.9% patients in the NAC group and 88.9% for patients in the surgery-first group (P = 1.000). After PSM, the rate of R0 resection was comparable between the two groups. Type of surgery, operation time, blood loss, and blood transfusion were similar between the two groups before and after PSM. The ratio of patients who received postoperative adjuvant chemotherapy was similar between the groups before and after PSM (Table 4).
| Variable | All patients | P value | Matched patients | P value | ||
| NAC and surgery (n = 36) | Surgery first (n = 108) | NAC and surgery (n = 36) | Surgery first (n = 36) | |||
| Type of surgery | 0.109 | 0.430 | ||||
| Total gastrectomy | 24 (66.7) | 87 (80.6) | 24 (66.7) | 28 (77.8) | ||
| Subtotal gastrectomy | 12 (33.3) | 21 (19.4) | 12 (33.3) | 8 (22.2) | ||
| Combined resection | 1.000 | 1.000 | ||||
| Yes | 1 (2.8) | 4 (3.7) | 1 (2.8) | 2 (5.6) | ||
| No | 35 (97.2) | 104 (96.3) | 35 (97.2) | 34 (94.4) | ||
| Resection | 1.000 | 1.000 | ||||
| R0 | 32 (88.9) | 96 (88.9) | 32 (88.9) | 31 (86.1) | ||
| R1/R2 | 4 (11.1) | 12 (11.1) | 4 (11.1) | 5 (13.9) | ||
| Operation time (min) | 196 ± 54 | 197 ± 56 | 0.961 | 196 ± 54 | 190 ± 49 | 0.600 |
| Blood loss (mL) | 144 ± 139 | 127 ± 140 | 0.527 | 144 ± 139 | 129 ± 122 | 0.628 |
| Blood transfusion | 0.495 | 1.000 | ||||
| Yes | 32 (88.9) | 100 (92.6) | 4 (11.1) | 4 (11.1) | ||
| No | 4 (11.1) | 8 (7.4) | 32 (88.9) | 32 (88.9) | ||
| Morbidity | 0.563 | 0.478 | ||||
| Yes | 3 (8.3) | 14 (13.0) | 3 (8.3) | 6 (16.7) | ||
| No | 33 (91.7) | 94 (87.0) | 33 (91.7) | 30 (83.3) | ||
| Postoperative hospital stay (d) | 13.3 ± 4.9 | 13.9 ± 13.2 | 0.821 | 13.3 ± 4.9 | 14.2 ± 14.0 | 0.743 |
| Borrmann type | 0.082 | 0.450 | ||||
| I | 3 (8.3) | 10 (9.3) | 3 (8.3) | 1 (2.8) | ||
| II | 10 (27.8) | 11 (10.2) | 10 (27.8) | 6 (16.7) | ||
| III | 17 (47.2) | 69 (63.9) | 17 (47.2) | 22 (61.1) | ||
| IV | 6 (16.7) | 18 (16.7) | 6 (16.7) | 7 (19.4) | ||
| Tumor size (cm) | 5.9 ± 3.3 | 5.9 ± 2.9 | 0.995 | 5.9 ± 3.2 | 6.0 ± 3.5 | 0.927 |
| Neural invasion | 0.700 | 0.474 | ||||
| Yes | 18 (50.0) | 74 (68.5) | 17 (47.2) | 13 (36.1) | ||
| No | 18 (50.0) | 34 (31.5) | 19 (52.8) | 23 (63.9) | ||
| Lymphovascular invasion | 0.848 | 0.341 | ||||
| Yes | 17 (47.2) | 55 (50.9) | 18 (50.0) | 23 (63.9) | ||
| No | 19 (52.8) | 53 (49.1) | 18 (50.0) | 13 (36.1) | ||
| Pathological tumor classification | 0.001 | 0.039 | ||||
| (y)pT0-1 | 5 (13.9) | 0 (0) | 5 (13.9) | 0 (0) | ||
| (y)pT2 | 0 (0) | 1 (0.9) | 0 (0) | 1 (2.8) | ||
| (y)pT3 | 9 (25.0) | 40 (37.0) | 9 (25.0) | 15 (41.7) | ||
| (y)pT4a/4b | 22 (61.1) | 67 (62.0) | 22 (61.1) | 20 (55.6) | ||
| Pathologic nodal classification | 0.09 | 0.671 | ||||
| (y)pN0 | 12 (34.3) | 15 (13.9) | 12 (34.3) | 8 (22.2) | ||
| (y)pN1 | 3 (8.6) | 12 (11.1) | 3 (8.6) | 3 (8.3) | ||
| (y)pN2 | 7 (20.0) | 28 (25.9) | 7 (20.0) | 7 (19.4) | ||
| (y)pN3 | 13 (37.1) | 53 (49.1) | 13 (37.1) | 18 (50.0) | ||
| Adjuvant chemotherapy | 1.000 | 0.396 | ||||
| Yes | 26 (72.2) | 77 (71.3) | 26 (72.2) | 30 (83.3) | ||
| No | 10 (27.8) | 31 (28.7) | 10 (27.8) | 6 (16.7) | ||
Following PSM, (y)pT categories after NAC were significantly less advanced than those after initial gastrectomy in the overall cohort; this finding was not observed for the (y)pN stage (Table 4).
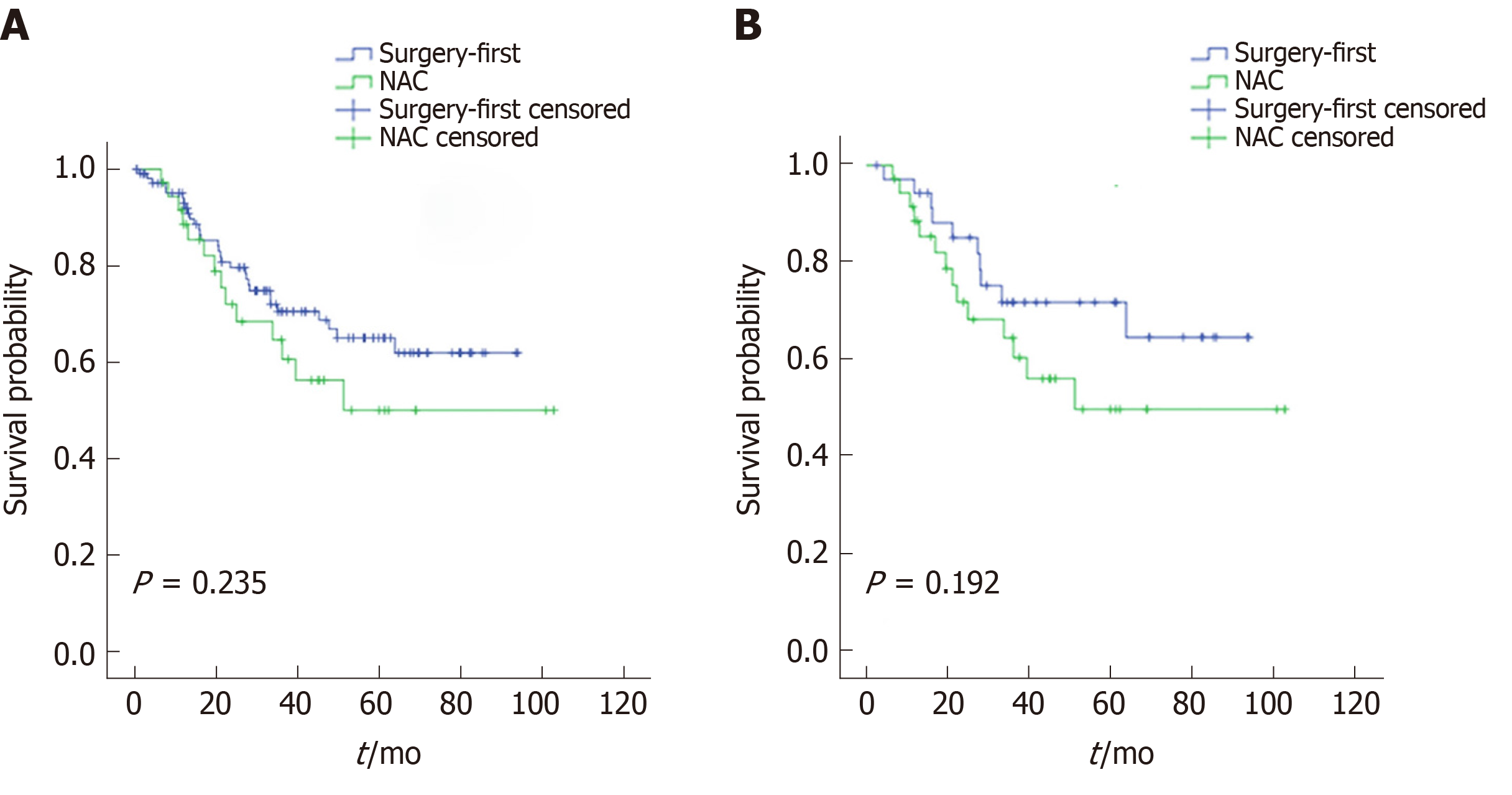
The median follow-up was 46.4 mo and comparable between the NAC and surgery-first groups. The 5-year OS rates in the NAC and surgery-first groups were 50.0% and 65.0% (P = 0.235), respectively, before PSM and 50% and 64.7% (P = 0.192), respectively, after PSM (Figure 2). Univariate and multivariate analyses conducted before PSM revealed that lymphovascular invasion [hazard ratio (HR) = 2.210; 95%CI: 1.168-4.184; P = 0.015) and tumor size (≥ 5 cm; HR = 2.154; 95%CI: 1.129-4.109; P = 0.020) were associated with OS. Multivariate analysis after PSM revealed that tumor size (≥ 5 cm) was a significant predictor of OS (HR = 3.804; 95%CI: 1.471-10.027; P = 0.006). However, NAC was not an independent prognostic factor both before and after PSM (Table 5).
| Variable | All patients (n = 144) | Matched patients (n = 72) | ||
| Hazard ratio | P value | Hazard ratio | P value | |
| Age: < 60 yr vs ≥ 60 yr | 1.248 (0.669-2.330) | 0.486 | 0.778 (0.308-1.965) | 0.595 |
| Sex: Male vs female | 0.991 (0.525-1.869) | 0.977 | 0.974 (0.432-2.194) | 0.949 |
| Comorbidity: Present vs absent | 1.228 (0.664-2.271) | 0.513 | 1.830 (0.782-4.281) | 0.163 |
| NAC: Yes vs No | 1.466 (0.777-2.768) | 0.235 | 1.709 (0.756-3.816) | 0.192 |
| Extent of gastrectomy: Subtotal vs total | 1.394 (0.966-1.883) | 0.079 | 1.282 (0.479-3.435) | 0.621 |
| Tumor location: Lower vs upper | 1.128 (0.507-2.514) | 0.767 | 0.676 (0.218-2.522) | 0.676 |
| Tumor location: Middle vs upper | 1.750 (0.758-3.832) | 0.197 | 0.758 (0.228-2.522) | 0.651 |
| Tumor size: > 5 cm vs ≤ 5 cm | 2.429 (1.282-4.603) | 0.006 | 4.765 (1.886-12.038) | 0.001 |
| cT stage: T4a/4b vs T3 | 0.834 (0.461-1.570) | 0.548 | 1.347 (0.575-3.153) | 0.492 |
| cN stage: N1-3 vs N0 | 1.748 (0.839-3.641) | 0.136 | 0.971 (0.332-2.844) | 0.957 |
| Lymphovascular invasion: Yes vs No | 2.291 (1.233-4.256) | 0.009 | 2.600 (1.141-5.928) | 0.023 |
| Neural invasion: Yes vs No | 1.390 (0.736-2.652) | 0.310 | 2.304 (0.953-5.567) | 0.064 |
| Adjuvant chemotherapy: Yes vs No | 0.673 (0.357-1.270) | 0.222 | 0.654 (0.271-1.581) | 0.346 |
The incidence of gastric SRCC is increasing, especially among young patients. It is still unclear whether NAC contributes to improved survival of patients with gastric SRCC. To our knowledge, there have been only three reports on this subject, and the results of those studies are inconsistent[9-11]. Our study revealed that the 5-year cumulative survival rates were comparable between the NAC and surgery-first groups before and after PSM, with the findings suggesting that upfront surgery may be a reasonable treatment option for patients with locally advanced gastric SRCC.
The FRench Eso-GAstric Tumors working group published the results of a retrospective national French survey study, including 924 locally advanced gastric SRCC patients from 1997 to 2010. In this cohort, 18.5% of the patients received NAC. The study found that NAC provided no survival benefit in patients with gastric SRCC and similar rates of R0 tumor resection between the NAC and surgery-first groups. Moreover, no significant differences were observed in (y)pT category, (y)pN category, mean number of dissected lymph nodes, mean number of invaded lymph nodes, and pathological Tumor-Node-Metastasis (pTNM) stage[10]. In other words, NAC did not result in any tumor downsizing. However, Heger et al[9] reported that NAC was associated with significantly increased rates of complete resection (76.0% in the NAC group vs 60.7% in the surgery-first group; P = 0.010), improved (y)pT category, improved (y)pN category, and significantly less explorations or palliative resections, and was an independent predictor of improved survival for patients with gastric SRCC[9]. In our study, we detected significantly less advanced (y)pT for patients after NAC despite comparable pretreatment stages between the two groups after PSM. However, NAC did not improve the R0 resection rate or result in lymph node downstaging. Furthermore, the 5-year cumulative survival rates were comparable between the NAC and surgery-first groups both before and after PSM. Ronellenfitsch et al[14] conducted a meta-analysis to identify predictors of postoperative survival following NAC for gastric cancer and found the (y)pT stage lost significance as an independent predictor of postoperative survival, while only (y)pN stage and resection status after NAC remained independent prognostic predictors. In our study, we observed downstaging of the T stage, but not the N stage, which may explain why there was no survival benefit from NAC for gastric SRCC.
Many studies have demonstrated that SRCC had different infiltrative and metastatic mechanisms, and SRCC is relatively insensitive to chemotherapy[15]. Roncati et al[16] also demonstrated that SRCC has a great capacity of diffusion through the gastric wall and extended neo-lymph angiogenesis and is not accompanied by an effective immunological response. However, there is no real understanding of the chemoresistance mechanisms of SRCC. It is reported that SRCC lacks free ribosomes but is rich in lysosomes and mucus, which impedes anticancer drugs[17]. Therefore, there is a pressing need to explore SRCC biology to understand the chemoresistance mechanisms involved. Although there is no standardized NAC, regimens based on oxaliplatin, docetaxel, or 5-fluorouracil and their modifications are the most common options for gastric cancer. In our study, SOX, Xelox, DOX, and DOS regimens were used. Additionally, it is reported that toxicity is associated with higher rates of non-R0 resection, increased postoperative mortality, and reduced long-term survival[12]. In our study, toxicity remained acceptable, and NAC did not increase postoperative mortality.
The classification of gastric cancer is complicated due to the coexistence of different histological features in the same tumor[18]. Lauren’s classification and the WHO system are the most widely used. The WHO defined true SRCC by the content of signet ring cell-type cells greater than 50%. The concordance of the pre-therapeutic biopsy and the final histopathological results in SRCC after resection should be considered. The high agreement is reported with an accuracy of 92.5% in a retrospective analysis with particular emphasis on SRCC[19], whereas the experienced group from Cologne reported an agreement in 74% of biopsies and resected specimens in untreated patients[18]. As initial diagnostic biopsies prove to be reliable in making an SRCC diagnosis, they may also be relied upon to define the therapeutic strategy.
Nevertheless, there are several limitations to the current study. First, as a retrospective analysis, this study is subject to possible selection bias, even though we used PSM to reduce bias, which was intended to mimic randomized controlled trials. Second, regimens and the indications for NAC were not standardized; therefore, the effects of different NAC regimens were not analyzed. Third, the median follow-up period was as short as 46.4 mo. Thus, there is a possibility that, in some cases, death could still have occurred and missed during the short follow-up period.
This study suggests that NAC does not provide a survival advantage for gastric SRCC, and upfront surgery should be the primary therapy for resectable gastric SRCC. Future studies that better stratify the SRCC components are warranted.
Some studies have investigated the benefit of neoadjuvant chemotherapy (NAC) in gastric signet-ring cell carcinoma (SRCC). However, the results are inconsistent. Earlier research showed that NAC was associated with better outcomes, though the response to NAC was relatively weak in gastric SRCC. The benefit of NAC for patients with SRCC of the stomach is controversial.
Earlier research showed that NAC was associated with better outcomes, though the response to NAC was relatively weak in gastric SRCC. Conversely, other studies suggested that NAC provided no survival benefit in patients with gastric SRCC. Moreover, some studies found that NAC is an independent predictor of poor survival because of its toxicity. We conducted a single-center, large-scale retrospective study to determine if there are benefits of NAC for treating gastric SRCC.
This study aimed to evaluate the perioperative and long-term outcomes of NAC for locally advanced gastric SRCC.
This study identified patients with locally advanced SRCCs of the stomach diagnosed by using the clinical Tumor-Node-Metastasis staging system. The histologic and prognostic effects of NAC were assessed. The overall survival rates were used as the outcome measure to compare the efficacy of NAC vs surgery-first treatment in the selected patients.
The R0 resection rates were 88.9% and 86.1% in the surgery-first and NAC groups after propensity score matching (PSM), respectively. The median follow-up period was 46.4 mo. The 5-year overall survival rates of the NAC group and surgery-first group were 50.0% and 65.0%, before PSM and 50% and 64.7% after PSM.
NAC provides no survival benefit in patients with locally advanced gastric SRCC. And, for resectable gastric SRCC, upfront surgery should be the primary therapy.
Future studies that better stratify the SRCC components are warranted.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dudek M, Levick C, Trivedi P S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55842] [Article Influence: 7977.4] [Reference Citation Analysis (132)] |
| 2. | Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, Brennan MF, Coit DG, Strong VE. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Taghavi S, Jayarajan SN, Davey A, Willis AI. Prognostic significance of signet ring gastric cancer. J Clin Oncol. 2012;30:3493-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, Noh SH. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Kao YC, Fang WL, Wang RF, Li AF, Yang MH, Wu CW, Shyr YM, Huang KH. Clinicopathological differences in signet ring cell adenocarcinoma between early and advanced gastric cancer. Gastric Cancer. 2019;22:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428-11438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (3)] |
| 7. | Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Jiang CG, Wang ZN, Sun Z, Liu FN, Yu M, Xu HM. Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: results from a Chinese mono-institutional study. J Surg Oncol. 2011;103:700-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Heger U, Sisic L, Nienhüser H, Blank S, Hinz U, Haag GM, Ott K, Ulrich A, Büchler MW, Schmidt T. Neoadjuvant Therapy Improves Outcomes in Locally Advanced Signet-Ring-Cell Containing Esophagogastric Adenocarcinomas. Ann Surg Oncol. 2018;25:2418-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C, FREGAT working group - FRENCH. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg. 2011;254:684-93; discussion 693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 11. | Kim S, Fiteni F, Paget-Bailly S, Ghiringhelli F, Lakkis Z, Jary M, Fein F, Bonnetain F, Mariette C, Borg C. The impact of taxane-based preoperative chemotherapy in gastroesophageal signet ring cell adenocarcinomas. J Hematol Oncol. 2015;8:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Robb WB, Messager M, Gronnier C, Tessier W, Hec F, Piessen G, Mariette C; FREGAT (French EsoGastric Tumor) working group - FRENCH (Fédération de Recherche en Chirurgie). High-Grade Toxicity to Neoadjuvant Treatment for Upper Gastrointestinal Carcinomas: What is the Impact on Perioperative and Oncologic Outcomes? Ann Surg Oncol. 2015;22:3632-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 14. | Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Nowak K, Kieser M, Slanger TE, Burmeister B, Kelsen D, Niedzwiecki D, Schuhmacher C, Urba S, van de Velde C, Walsh TN, Ychou M, Jensen K. Predictors of overall and recurrence-free survival after neoadjuvant chemotherapy for gastroesophageal adenocarcinoma: Pooled analysis of individual patient data (IPD) from randomized controlled trials (RCTs). Eur J Surg Oncol. 2017;43:1550-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Chen L, Shi Y, Yuan J, Wu Q, Han Y, Qin R, Jia B, Wei B, Wei L, Dai G, Jiao S. Evaluation of docetaxel- and oxaliplatin-based adjuvant chemotherapy in postgastrectomy gastric cancer patients reveals obvious survival benefits in docetaxel-treated mixed signet ring cell carcinoma patients. Med Oncol. 2014;31:159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Roncati L, Manenti A, Barbolini G, Maiorana A. Deep inside of gastric signet-ring cell carcinoma. Neoplasma. 2018;65:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Jonckheere N, Van Seuningen I. The membrane-bound mucins: how large O-glycoproteins play key roles in epithelial cancers and hold promise as biological tools for gene-based and immunotherapies. Crit Rev Oncog. 2008;14:177-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Flucke U, Mönig SP, Baldus SE, Zirbes TK, Bollschweiler E, Thiele J, Dienes HP, Hölscher AH. Differences between biopsy- or specimen-related Laurén and World Health Organization classification in gastric cancer. World J Surg. 2002;26:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Piessen G, Amielh D, Messager M, Vinatier E, Leteurtre E, Triboulet JP, Mariette C. Is pretreatment endoscopic biopsy a good predictor of signet ring cell histology in gastric carcinoma? World J Surg. 2012;36:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |