Published online Feb 14, 2020. doi: 10.3748/wjg.v26.i6.645
Peer-review started: November 25, 2019
First decision: December 23, 2019
Revised: January 8, 2020
Accepted: January 15, 2020
Article in press: January 15, 2020
Published online: February 14, 2020
Processing time: 83 Days and 20.9 Hours
Reports on bacterial infection (BI) in decompensated cirrhosis (DC) is mainly from alcoholic cirrhosis. The role of BI as a trigger or complication of acute-on-chronic liver failure (ACLF) in patients with hepatitis B virus decompensated cirrhosis (HBV-DC) remains to be investigated.
To investigate the impact of BI on the outcomes of the patients with HBV-DC admitted into the hospital with or without ACLF.
This retrospective study included patients with HBV-DC admitted to two tertiary centers in China. In-hospital overall survival, 90-d transplant-free survival, 5-year post-discharge survival, and cumulative incidence of ACLF were evaluated. Risk factors for death were analyzed considering liver transplantation as a competing event.
A total of 1281 hospitalized HBV-DC patients were included; 284 had ACLF at admission. The overall prevalence of BI was 28.1%. The patients with BI had a significantly lower in-hospital survival and transplant-free 90-d survival than those without, in both the patients admitted with and without ACLF. The presence of BI significantly increased the risk of developing ACLF [sub-distribution hazard ratio (sHR) = 2.52, 95%CI: 1.75-3.61, P < 0.001] in the patients without ACLF. In the patients discharged alive, those who had an episode of BI had a significantly lower 5-year transplant-free survival. BI was an independent risk factor for death in the patients admitted without ACLF (sHR = 3.28, 95%CI: 1.93-5.57), while in ACLF admissions, the presence of pneumonia, but not other type of BI, independently increased the risk of death (sHR = 1.87, 95%CI: 1.24-2.82).
BI triggers ACLF in patients with HBV-DC and significantly impairs short-term survival. HBV-DC patients should be monitored carefully for the development of BI, especially pneumonia, to avoid an adverse outcome.
Core tip: In our cohort of 1281 patients with hepatitis B virus-related decompensated cirrhosis (HBV-DC), bacterial infection (BI) significantly reduced both short-term and long-term survival independent of the presence of acute-on-chronic liver failure (ACLF) and the severity of the underlying liver disease. BI precipitated ACLF in patients admitted without this syndrome and in those with ACLF, BI was significantly associated with a reduced rate of liver transplantation. Thus, our data suggest that patients with HBV-DC should be monitored carefully for the development of BI to avoid an adverse outcome.
- Citation: Cao ZJ, Liu YH, Zhu CW, Yin S, Wang WJ, Tang WL, Zhao GD, Xu YM, Chen L, Zhou TH, Cai MH, Wang H, Cai W, Bao SS, Li H, Xie Q. Bacterial infection triggers and complicates acute-on-chronic liver failure in patients with hepatitis B virus-decompensated cirrhosis: A retrospective cohort study. World J Gastroenterol 2020; 26(6): 645-656
- URL: https://www.wjgnet.com/1007-9327/full/v26/i6/645.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i6.645
Chronic hepatitis B virus (HBV) infection remains a global health burden, affecting 257 million people and leading to 900 thousand deaths worldwide in 2015[1]. Most deaths from HBV are related to complications of cirrhosis[1]. Bacterial infection (BI) is a turning point in the natural course of cirrhosis[2]. BI is responsible for 30% of cases of acute-on-chronic liver failure (ACLF) syndrome in patients admitted for acute decompensation of cirrhosis mainly related to alcoholic liver disease[3]. It is also reported that BI is a common precipitating event of HBV-ACLF[4,5], suggesting BI as a trigger of extra-hepatic organ failure in the context of HBV-related decompensated cirrhosis (HBV-DC) where hepatic organ failure is usually triggered by flare-up of HBV in the absence of appropriate anti-viral management. Taken together, the literature emphasizes the importance of BI prevention and control in patients hospitalized with cirrhosis.
Currently, BI is investigated as a whole in the disease progression in patients with HBV-DC, however, the data regarding source of acquisition, site of infection, and their association with clinical outcome remains unknown. Further investigation of the role BI as a complication of HBV-ACLF is also needed. Such investigations would be particularly important in improving the current management of HBV-DC and ACLF, especially in Asian countries where health care resources are relatively limited and access to liver transplantation is much more restricted. The aim of the current study was to investigate the impact of BI on the outcomes of patients with HBV-DC admitted into the hospital with or without ACLF.
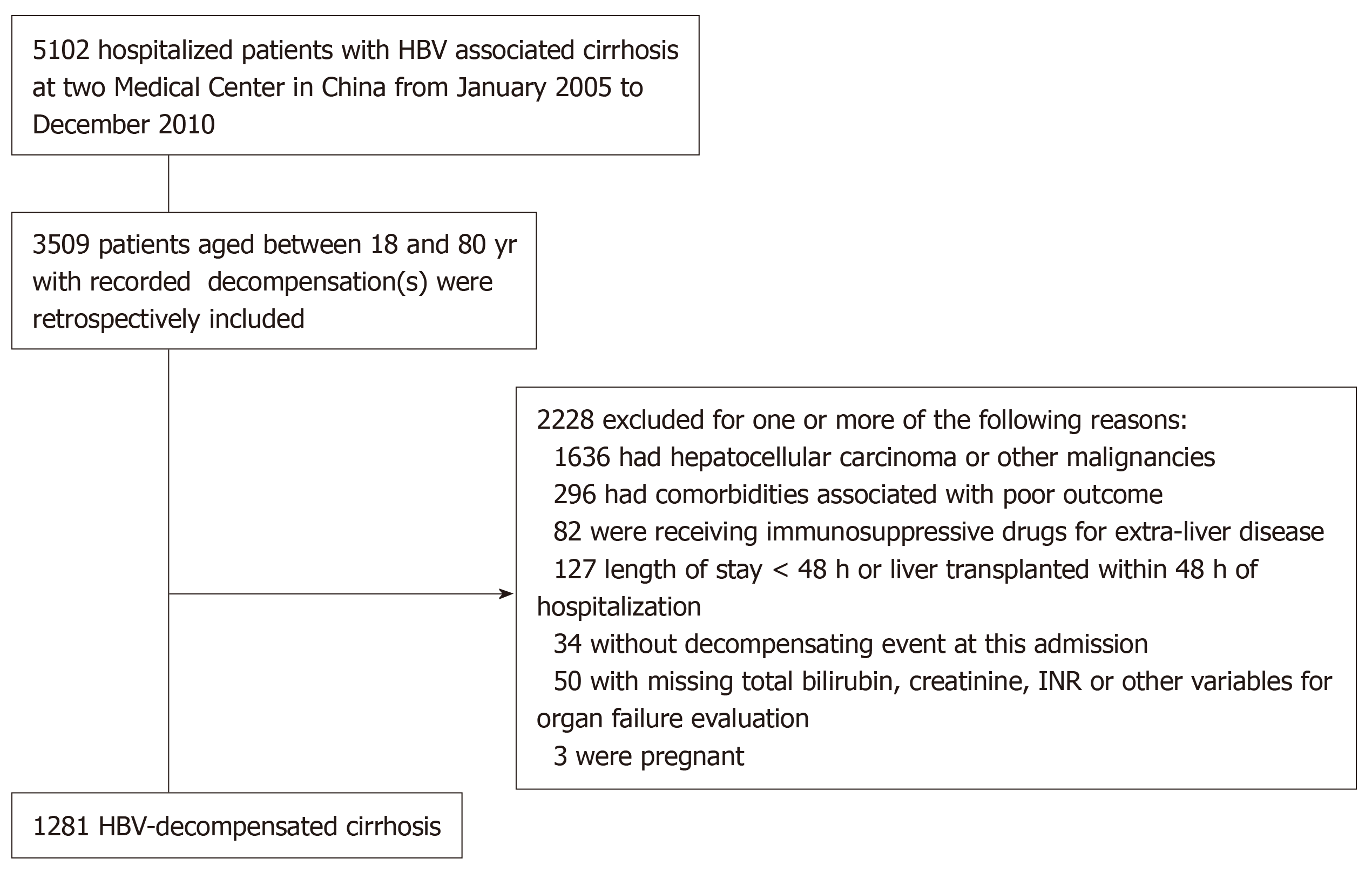
This study is part of a large retrospective cohort study from January 2005 to December 2010, which assessed the 5-year outcomes of patients with chronic HBV infection admitted into the hospital. For the establishment of this cohort, patient’s charts were identified from the hospital information system, which were reviewed by two investigators from each center for cross-checking. The inclusion criteria were: (1) Age between 18 and 80 years; (2) Chronic HBV infection (HBsAg positivity for ≥ 6 mo); (3) Cirrhosis diagnosed according to biochemistry, radiology, endoscopic appearance, and/or histopathology; and (4) Admitted with decompensating events: Ascites, hepatic encephalopathy (HE), gastrointestinal variceal bleeding, and/or jaundice. The exclusion criteria were: (1) Active solid organ malignancy; (2) Immunosuppressive medications or co-infection with HIV; (3) Severe extra-hepatic comorbidities that might impair short-term survival (e.g., chronic kidney disease on dialysis, chronic obstructive pulmonary disease with respiratory failure, or coronary heart disease grade III/IV using New York Heart Association classification); (4) Died, discharged, or received liver transplantation within 48 h of admission; (5) Missing key data for evaluating disease severity and ACLF diagnosis; and (6) Pregnancy.
A detailed flowchart for the patient selection is presented in Figure 1. Each patient was included once (using the first encountered decompensation episode to either center).
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved with a waiver of written informed consent by the Institutional Ethics Review Committee at Ruijin Hospital and Renji Hospital. Oral informed consent was obtained from all of the patients and/or their relatives for the usage of the clinical data.
The study was aimed at investigating the prevalence, characteristics, and outcome of BI in patients with HBV-DC. BI was considered both on admission and during hospitalization. The diagnosis of BI was made by two independent investigators after reviewing all patients’ information. Any discrepancy between the two investigators was referred to a senior investigator for adjudication. Vital status and liver transplantation information in patients discharged alive were collected via the hospital information system or telephone contact. Previous antibiotics including quinolones or rifaximin for the prophylaxis of spontaneous bacterial peritonitis (SBP) or HE were not available in the patient’s records during the study period.
BI was diagnosed according to the conventional criteria[6]: (1) Pneumonia: New radiological pulmonary infiltration with the presence of dyspnea, cough, purulent sputum, pleuritic chest pain, or signs of consolidation; positive findings on auscultation (rales or crepitation) or at least one sign of infection: Core body temperature > 38 °C or < 36 °C, or leukocyte count > 10000/mm3 or < 4000/mm3 in the absence of antibiotics; (2) SBP: Ascitic fluid polymorphonuclear cells > 250/mm3; (3) Urinary tract infection (UTI): Urine leucocytes > 15/high-power field with either positive urine Gram staining or culture; (4) Spontaneous bacteremia: Positive blood cultures without a source of infection; (5) Softtissue/skin infection: Fever with cellulitis; (6) Bacterial enterocolitis: Diarrhea with an increase in fecal leucocyte count ≥ 15/high-power field or a positive stool culture for Salmonella, Shigella, Yersinia, Campylobacter, or pathogenic Escherichia coli; (7) Secondary bacterial peritonitis: Ascitic fluid polymorphonuclear cells > 250/mm3 in the presence of an intraabdominal source of peritonitis and multiple organisms cultured from ascitic fluid; (8) Intra-abdominal infections: Diverticulitis, appendicitis, and cholangitis; (9) Clostridium difficile infection: Diarrhea with a positive Clostridium difficile assay; (10) catheterrelated infection: Positive blood and catheter cultures; and (11) Unproven infection: Presence of fever and leukocytosis requiring antibiotic therapy without any identifiable source.
All BI episodes were categorized into community-acquired, if diagnosis was made within 48 h of admission without hospitalizations in the previous 6 mo; healthcare-associated, if diagnosis was made within 48 h of admission with at least 2 d of hospitalization in the previous 6 mo; or nosocomial infection, if diagnosis was made beyond 48 h of admission[6].
Diagnostic criteria for organ dysfunction and failure were based on the CLIF-COF criteria[3,7]. ACLF was diagnosed according to the EASL-CLIF consortium definition[3], which has been validated also in HBV-related cirrhosis[4,5].
The primary endpoint was in-hospital overall survival. The secondary endpoint included: (1) 90-d transplant-free survival; (2) Liver transplant rate; (3) Five-year transplant-free survival in patients discharged alive; and (4) Cumulative incidence of ACLF during 28-d follow-up in patients admitted without ACLF.
Continuous data are described according to their distribution: mean ± SD for normal distributions and median with interquartile range for skewed distributions. Categorical data are presented as counts and percentages. Comparisons between two groups were performed using Student’s t-test, Mann-Whitney U test, or χ2 or Fisher’s exact test, as appropriate.
The in-hospital overall survival rate was compared using χ2 test. The cumulative incidence of ACLF during hospitalization was calculated and compared between patients with and without BI. In this analysis, liver transplantation or death before the onset of ACLF was considered a competing event. Patients who were free of ACLF throughout hospitalization were censored on the date of discharge. Comparisons of cumulative incidence curves were performed using Gray’s test[8]. Transplant-free survival probability was estimated and compared between patients with and without BI in a similar way using competing risk method in which liver transplantation was considered as a competing event and patients who were lost to follow-up were censored on the date of last medication recorded in the hospital information system.
The proportional sub-distribution hazards regression model proposed by Fine and Gray[8] was used to identify risk factors for mortality in a competing risk framework where liver transplantation was considered as a competing event of death. The standard Cox model was not applied in the current study, because Cox model does not cover the competing effect of liver transplantation on death and therefore results in upwards biased estimates[9]. Sub-distribution hazard ratio (sHR) is reported for each factor. Factors that were significant in the univariate analysis were introduced into the multivariate model with a backward elimination strategy. At each step of the backward procedure, the variable that produced the largest value of the Bayesian information criterion (BIC)[10] were removed. The procedure of elimination of effects was considered finished when the removal of any further variable increased the BIC. The final model was the one characterized by the lowest value of BIC.
All statistical analyses were performed using R 3.4.3 (http://www.r-project.org/). A two-tailed P value < 0.05 was considered statistically significant.
Among these 1281 patients, 1012 were male with a median age of 51 (interquartile range, 42-57) years (Table 1). Thirty-seven percent of the patients were HBeAg positive. The anti-viral treatment rate before admission was low (24.2%), contributing to the development of decompensation in these cirrhotic patients. The most common type of decompensation was ascites (85.8%) followed by jaundice (41.7%), gastrointestinal variceal bleeding (26.2%), and encephalopathy (14.8%). Twenty-two percent of patients fulfilled ACLF diagnosis upon admission as per the EASL-CLIF-C criteria. Laboratory tests and severity scores are summarized in Table 1.
| Characteristic | All patients (n = 1281) | Without BI (n = 921) | With BI (n = 360) | P value |
| Median age (IQR) -yr | 51 (42-57) | 52 (43-57) | 50 (42-57) | 0.16 |
| Male sex (%) | 1012 (79) | 726 (78.8) | 286 (79.4) | 0.88 |
| Concomitant etiology of cirrhosis (%) | ||||
| Alcohol | 142 (11.1) | 102 (11.1) | 40 (11.1) | 1.00 |
| Others | 48 (3.7) | 36 (3.9) | 12 (3.3) | 0.74 |
| HBeAg positive HBV (%)1 | 421/1122 (37.5) | 298/794 (37.5) | 123/328 (37.5) | 1.00 |
| Experience of anti-HBV treatment (%)1 | 284/1176 (24.2) | 183/851 (21.5) | 101/325 (31.1) | < 0.001 |
| Decompensations (%)2 | ||||
| Ascites | 1099 (85.8) | 773 (83.9) | 326 (90.6) | < 0.01 |
| Jaundice | 534 (41.7) | 297 (32.2) | 237 (65.8) | < 0.001 |
| Gastrointestinal variceal bleeding | 336 (26.2) | 280 (30.4) | 56 (15.6) | < 0.001 |
| Hepatic encephalopathy | 190 (14.8) | 82 (8.9) | 108 (30) | < 0.001 |
| ACLF at admission (%) | 284 (22.2) | 130 (14.1) | 154 (42.8) | < 0.001 |
| Median value for laboratory tests (IQR) | ||||
| Log10 HBV DNA (copies/mL)3 | 4.7 (3.1-6.1) | 4.8 (3-6.2) | 4.6 (3.1-5.7) | 0.33 |
| Hemoglobin (g/L) | 103 (83-121) | 102 (82-119) | 107 (87-127) | < 0.001 |
| White cell count: × 109 cells/L | 4.7 (2.9-7.5) | 4.2 (2.7-6.1) | 6.9 (4.3-10.9) | < 0.001 |
| Platelet count: × 109 cells/L | 63 (40-102) | 60 (40-96) | 70 (42-117.5) | < 0.01 |
| International normalized ratio | 1.6 (1.3-2) | 1.4 (1.3-1.9) | 1.8 (1.5-2.6) | < 0.001 |
| Serum sodium (mmol/L) | 136 (131.2-139) | 136 (132.3-139.3) | 134 (129-137) | < 0.001 |
| Serum creatinine (mg/dL) | 0.8 (0.7-1) | 0.8 (0.7-1) | 0.8 (0.6-1.1) | 0.20 |
| Aspartate aminotransferase (IU/L) | 67 (42-124) | 60 (40-107) | 97 (56.5-192) | < 0.001 |
| Alanine aminotransferase (IU/L) | 47 (30-97) | 42 (29-80) | 67 (37-185.8) | < 0.001 |
| Total bilirubin (mg/dL) | 3.4 (1.5-15.5) | 2.6 (1.4-8) | 10.9 (3-27.4) | < 0.001 |
| Serum albumin (g/dL) | 2.8 (2.5-3.2) | 2.9 (2.5-3.3) | 2.8 (2.4-3.1) | < 0.001 |
| Median value for severity scores (IQR) | ||||
| Child-Pugh | 11 (9-12) | 10 (8-12) | 12 (10-13) | < 0.001 |
| MELD | 16.8 (11.8-25.5) | 14.8 (11.1-22.3) | 24.4 (17.1-30.2) | < 0.001 |
Three hundred and sixty (28.1%) patients were either admitted with or developed BI (s) during their hospitalization. Among these 360 patients, 99 had documented bacterial isolation (27.5%), and 56 bacterial isolates were Gram negative (56/99, 56.6%). Demographic and clinical characteristics, laboratory tests, and severity scores of patients on admission were compared between the patients with and without BI (Table 1). As expected, the patients with BI were systemically different from patients without BI, mainly demonstrating more complications of cirrhosis, higher severity of liver disease, and more ACLF. Part of the results were previously reported in a study investigating the role of HBV flare in patients with cirrhosis and BI[11].
One hundred and eighty patients died while hospitalizing and the overall in-hospital survival rate was 85.95%. The most common cause for death was multiple organ failure without shock (56.7%), followed by septic shock (13.3%), hypovolemic shock (10.6%), and other reasons (8.3%).
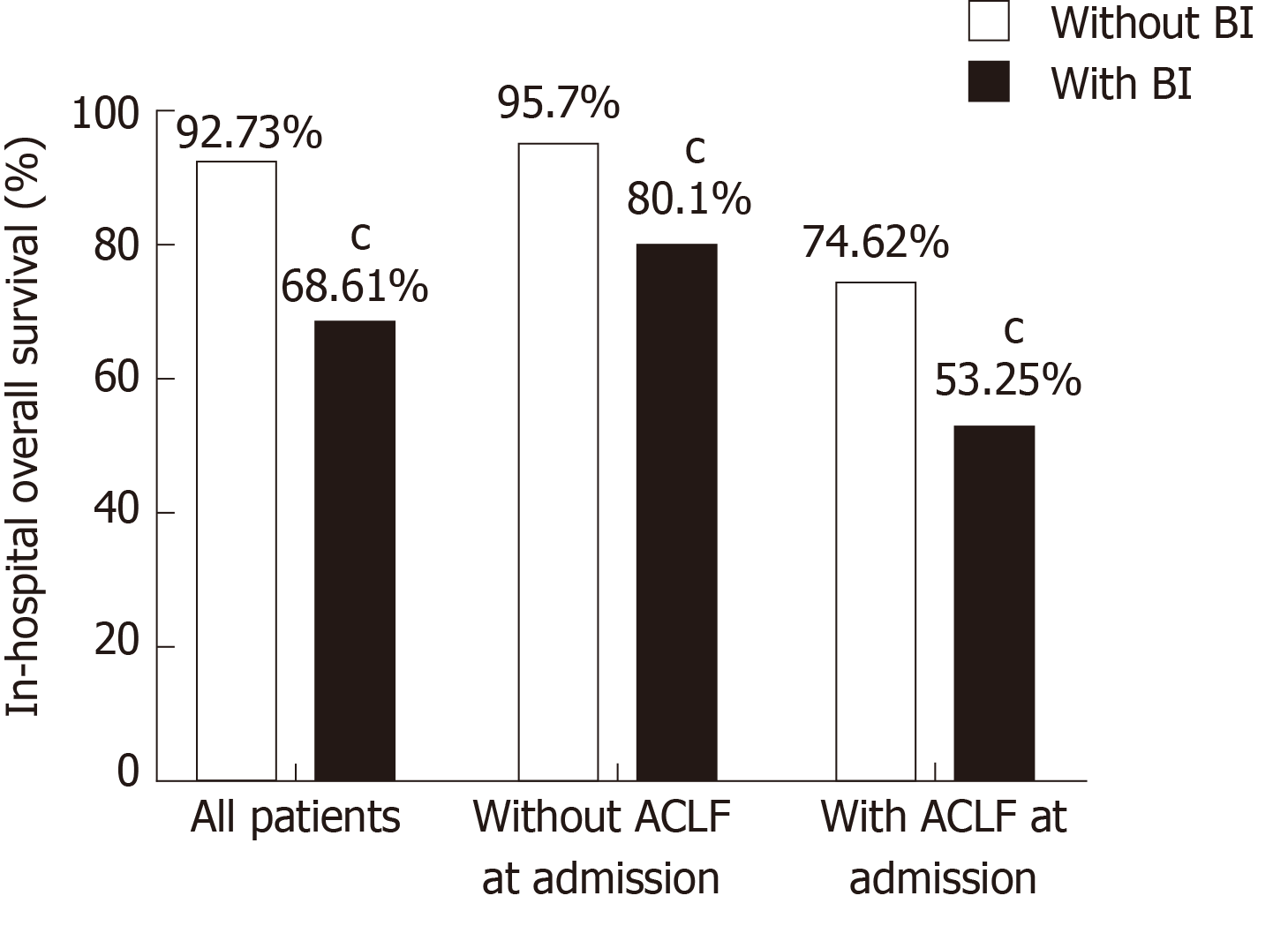
Patients with BI had a significantly lower survival rate than those without (68.61% vs 92.73%, P < 0.001, Figure 2). The negative impact of BI on survival was independent of disease stage as suggested by the subgroup analysis, showing that both patients with and without ACLF had a significantly lower survival when BI occurred (Figure 2). It was also independent of the severity of liver disease as was shown by the stratification analysis by the MELD score (Supplementary Figure 1). Patients with BI mostly died from multiple organ failure (50.4%) and septic shock (38.1%), whereas those without BI mostly died from multiple organ failure (67.2%), hypovolemic shock (14.9%), and other reasons (16.4%).
Among all the 360 patients with BI, 76 (21%) or 136 (37.8%) patients had community-acquired or healthcare-associated BI, respectively. The remaining 162 (45%) patients acquired BI during hospitalization, i.e., nosocomial infections. All these three types of BI were significantly more prevalent in non-survivors than in survivors (Table 2). Pneumonia was the most common infection followed by SBP, UTI, spontaneous bacteremia, skin or soft tissue infection, and others (Table 2). Thirty-four (9.4%) patients had more than one site of infection and 60 (16.7%) patients had BI with unknown site. Non-survivors had significantly more pneumonia, SBP, and multisite and unknown site of infection, but not UTI, spontaneous bacteremia, skin or soft tissue infection, or others (Table 2).
| Characteristic | All patient (n = 1281) | Survivors (n = 1101) | Non-survivors (n = 180) | P value1 |
| Bacterial infection | 360 (28.1) | 247 (22.4) | 113 (62.8) | < 0.001 |
| Source of acquisition | ||||
| Community-acquired | 76 (5.9) | 58 (5.3) | 18 (10) | 0.02 |
| Healthcare-associated | 136 (10.6) | 89 (8.1) | 47 (26.1) | < 0.001 |
| Nosocomial infection | 162 (12.6) | 107 (9.7) | 55 (30.6) | < 0.001 |
| Single site | ||||
| Pneumonia | 126 (9.8) | 75 (6.8) | 51 (28.3) | < 0.001 |
| Spontaneous bacterial peritonitis | 69 (5.4) | 47 (4.3) | 22 (12.2) | < 0.001 |
| Urinary tract infection | 29 (2.3) | 26 (2.4) | 3 (1.7) | 0.79 |
| Spontaneous bacteremia | 16 (1.3) | 14 (1.3) | 2 (1.1) | 1.00 |
| Skin or soft tissue infection | 9 (0.7) | 7 (0.6) | 2 (1.1) | 0.37 |
| Others2 | 17 (1.3) | 15 (1.4) | 2 (1.1) | 1.00 |
| Multi sites | 34 (2.7) | 20 (1.8) | 14 (7.8) | < 0.001 |
| Unknown site3 | 60 (4.7) | 43 (3.9) | 17 (9.4) | < 0.01 |
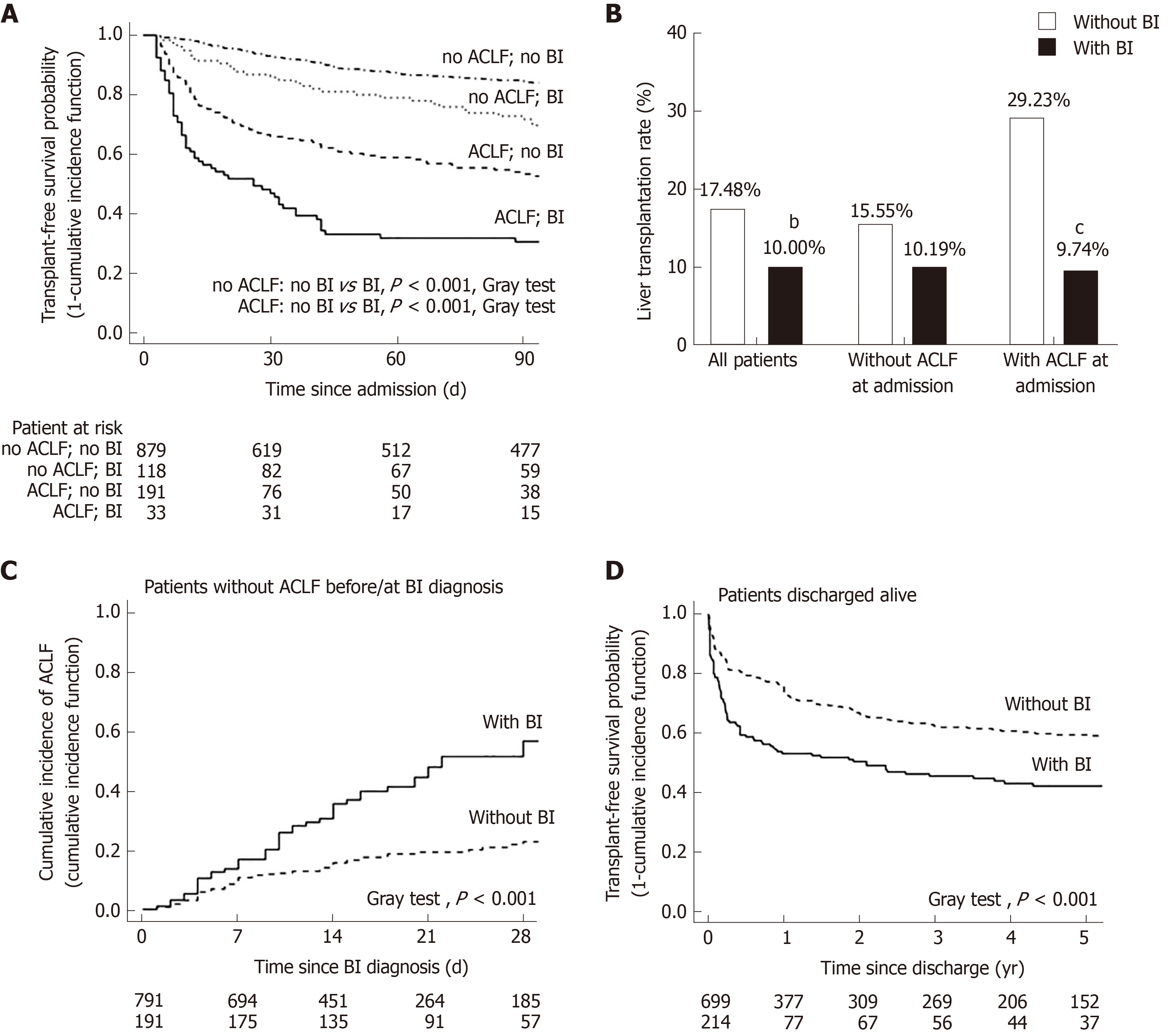
The negative impact of BI on survival was validated in the competing risk analysis taking liver transplantation as a competing event for death. The 90-d transplant-free survival was significantly lower in patients with BI than in those without, regardless of the presence of ACLF on admission (Figure 3A). Moreover, the patients with BI had a significantly lower rate of liver transplantation than those without (BI vs no BI: 10.00% vs 17.84%, P < 0.01), especially in the patients admitted with ACLF (BI vs no BI: 9.74% vs 29.33%, P < 0.001) (Figure 3B). For the patients without ACLF before or at the diagnosis of BI, the cumulative incidence of ACLF during follow-up was significantly increased after the onset of BI (Figure 3C). A total of 913 patients were discharged alive without receiving liver transplantation. Among them, 214 patients had at least one episode of BI during their hospitalizations. These patients had a significantly lower 5-year transplant-free survival compared to the patients who were free of BI during hospitalization (Figure 3D).
Univariate analysis of risk factors for in-hospital mortality was performed separately in patients admitted with and without ACLF (Table 3). BI (sHR: 3.28), serum bilirubin (sHR: 1.03), serum albumin (sHR: 0.46), and serum sodium (sHR: 0.93) at admission were identified as independent predictors of in-hospital mortality in patients without ACLF (Table 4). When the analysis was performed in patients admitted with ACLF, pneumonia (sHR: 1.87), HE (sHR: 1.73), serum bilirubin (sHR: 1.03), and serum albumin (sHR: 0.49) at admission were identified as independent predictors of death (Table 4).
| Parameters | Without ACLF at admission | ACLF at admission | ||
| sHR (95%CI) | P value | sHR (95%CI) | P value | |
| Age (yr) | 1.01 (0.99-1.03) | 0.22 | 1.02 (1.00-1.03) | 0.03 |
| Male sex (%) | 1.37 (0.74-2.54) | 0.32 | 0.97 (0.59-1.60) | 0.91 |
| Ascites (%) | 2.88 (1.05-7.93) | 0.04 | 1.11 (0.57-2.17) | 0.76 |
| V Gastrointestinal variceal bleeding (%) | 0.65 (0.37-1.14) | 0.13 | 0.64 (0.22-1.81) | 0.40 |
| Hepatic encephalopathy (%) | 0.96 (0.36-2.57) | 0.93 | 1.90 (1.28-2.83) | < 0.01 |
| Jaundice (%) | 5.10 (3.10-8.39) | < 0.001 | 2.45 (1.20-5.03) | < 0.001 |
| Bacterial infection (%) | 4.94 (3.08-7.94) | < 0.001 | 2.34 (1.52-3.59) | < 0.001 |
| Pneumonia (%) | 4.79 (2.86-8.03) | < 0.001 | 2.06 (1.39-3.05) | < 0.001 |
| Spontaneous bacterial peritonitis (%) | 4.05 (2.19-7.48) | < 0.001 | 1.49 (0.88-2.53) | 0.13 |
| Urinary tract infection (%) | 0.94 (0.23-3.81) | 0.93 | 1.34 (0.67-2.68) | 0.41 |
| HBeAg positive HBV (%) | 0.92 (0.56-1.54) | 0.76 | 1.08 (0.78-1.63) | 0.70 |
| Log10 HBV DNA (copies/mL) | 1.04 (0.90-1.21) | 0.57 | 1.10 (0.96-1.25) | 0.18 |
| White cell count (× 109 cells/L) | 1.11 (1.07-1.16) | < 0.001 | 1.04 (1.01-1.06) | 0.02 |
| Total bilirubin (mg/dL) | 1.05 (1.03-1.06) | < 0.001 | 1.02 (1.01-1.03) | < 0.01 |
| Serum creatinine (mg/dL) | 1.87 (0.59-5.93) | 0.29 | 1.05 (0.96-1.15) | 0.25 |
| International normalized ratio | 1.97 (1.56-2.49) | < 0.001 | 1.09 (0.93-1.29) | 0.31 |
| Serum albumin (g/dL) | 0.41 (0.27-0.64) | < 0.001 | 0.63 (0.45-0.88) | < 0.01 |
| Serum sodium (mmol/L) | 0.91 (0.88-0.93) | < 0.001 | 0.98 (0.95-1.01) | 0.14 |
| Parameters | Estimate | Standard error | sHR (95%CI) | P value |
| Without ACLF at admission | ||||
| Bacterial infection | 1.1872 | 0.2708 | 3.28 (1.93-5.57) | < 0.001 |
| Total bilirubin at admission | 0.0292 | 0.0104 | 1.03 (1.01-1.05) | < 0.01 |
| Albumin at admission | -0.7695 | 0.2357 | 0.46 (0.29-0.74) | < 0.01 |
| Sodium at admission | -0.0738 | 0.0178 | 0.93 (0.9-0.96) | < 0.001 |
| ACLF at admission | ||||
| Pneumonia | 0.6250 | 0.2106 | 1.87 (1.24-2.82) | < 0.01 |
| Hepatic encephalopathy | 0.5453 | 0.2068 | 1.73 (1.15-2.59) | < 0.01 |
| Total bilirubin at admission | 0.0277 | 0.0081 | 1.03 (1.01-1.04) | < 0.001 |
| Albumin at admission | -0.7110 | 0.1945 | 0.49 (0.34-0.72) | < 0.001 |
The current results were obtained in a large cohort of HBV-DC patients by analysis of both short-term and long-term outcome. Data were collected systematically according to a rigorous protocol with cross-checking. BI on admission and during hospitalization was diagnosed according to the standard criteria to cover a broad spectrum[6]. The data from our study could provide better knowledge of BI in patients with HBV-DC that might have a positive impact on clinical practice.
This cohort is unique in that it consisted of 1281 patients with HBV-DC. The development of DC in these HBV patients was largely due to the lack of anti-viral treatment, as supported by our data that only 24% of the patients had an experience of anti-HBV treatment. It further highlighted the barriers for care engagement in these patients, which were rather complicated, including absence of clinical signs and symptoms, fear of stigmatization, preference to traditional herbal medicine, and inadequate HBV education from the health-care system[12]. In this cohort, BI was diagnosed in 360 patients with an overall prevalence of 28%, which is as common as in the alcoholic cirrhosis[13,14]. Our data demonstrated that patients with HBV-related cirrhosis were also at a high risk of developing BI, suggesting that the susceptibility of these patients to BI were mainly due to the increased bacterial translocation[15] and the immuno-compromised state of cirrhotic patients which reduces their ability to fight against infection[16]. It is also interesting to note that there was no significant difference regarding the frequency and etiologies of bacterial infection, prevalence of ACLF as well as survival rate among patients with isolated chronic HBV-related liver disease vs those with concomitant alcoholic and HBV-related liver disease (Supplementary Tables 1 and 2), suggesting a little impact of the etiology of cirrhosis on the development of bacterial infection and associated outcome.
The presence of BI worsens the prognosis of cirrhosis as frequently reported in alcoholic cirrhosis[17-19]. However, data is limited in viral cirrhosis until recently, and the adverse effect of BI on survival was highlighted in compensated viral cirrhosis as reported in the Civir cohort study from France, a multicenter longitudinal study on compensated viral cirrhosis (79% HCV, 19% HBV, and 2% co-infection with HBV/HCV)[20]. In this French study, the 5-year cumulative occurrence of BI was 13.6%, which is very close to that of the composite of all other complications (13.7%). These BIs significantly increase the probability of hepatic decompensation and thus increase mortality[20]. Our study, by the analysis of BI in HBV-DC, extended knowledge in the following five aspects: First, the presence of BI significantly reduced in-hospital overall survival and 90-d transplant-free survival and this negative impact was independent of the presence of ACLF; second, BI was associated with a significant reduction of liver transplantation rate, especially in patients admitted with ACLF; third, the negative impact of BI on survival extended into the post-discharge long-term period; fourth, BI precipitated ACLF in patients admitted without ACLF, thus leading to a poorer short-term outcome. Fifth, Patients admitted with HBV-ACLF in our cohort was at a higher risk of pneumonia which further independently increased the risk of death.
These results provided robust clinical evidence that the presence of BI should be considered as a major complication of cirrhosis which significantly impaired clinical outcome both in HBV-DC patients with and without ACLF[21,22]. In this study, we analyzed the impact of the source of acquisition and site of infection on the clinical outcome but the type of bacteria according to the virulence or the susceptibility to the antibiotics are the two topics not addressed in the current study. HCV infection is beyond the scope of the current study, which will be investigated in future. It is also not clear whether the prior antibiotics including quinolones or rifaximin for the prophylaxis of SBP or HE play a role in the development of BI and affecting the survival. Future studies are warranted.
In line with the survival analyses in our current study, BI was identified as an independent risk factor for death in the multivariate analysis in HBV-DC patients admitted without ACLF. Indeed in the patients without ACLF, the presence of BI precipitates organ failures including hepatic, kidney, brain, circulation, and respiratory systems[19] and these organ failures collectively contribute to the development of ACLF, as was also confirmed in our current study. It is interesting to note that pneumonia rather than other types of BI was identified as an independent risk factor for death in patients admitted with ACLF. Similar findings were also recently reported in the CANONIC study[13] and another single-center study in Germany[21]. These results emphasized the role of pneumonia in worsening prognosis. Preventative strategies for pneumonia, such as smoking cessation, prompt treatment of upper airway infection, and oral hygiene care[23], are some of the measures that could reduce the likelihood of pneumonia and hence decrease ACLF and its associated mortality. Moreover, in the hospital, a well-functional environment and equipment as well as effective program for infection prevention and control and water, sanitation, and hygiene should be enhanced because it minimizes the spread of the organism, particularly those resistant to multi-antibiotics, and reduces hospital acquired infection by at least 30%[24].
The main limitation of the present study is its retrospective design. We, therefore, chose in-hospital survival as the primary endpoint to avoid the missing information on clinical outcome and the negative impact of BI on survival was validated with various secondary endpoints to support the main findings. Due to the lack of systemic assessment of respirovirus, we were not able to exclude the possibility that some pneumonia we defined in this study are viral related. Some of the patients were classified as BI based on clinical judgement without microbiology evidence. Although we strictly adhered to the well-established diagnostic criteria[6], this could still be a source of potential investigator bias. Leukopenia due to the cirrhosis associated hypersplenism would also introduce false positive sign of infection[25] that was not accounted for in the diagnostic criteria we used in the current study. Another limitation is the lack of information about the resistance profile in our report which has been recently shown to be related to clinical outcomes in patients with cirrhosis[26]. Future study should be performed in a multi-center prospective way to establish the resistance profile of bacteria in the HBV-DC population.
In conclusion, BI is prevalent and a major risk factor for survival in our large HBV-DC cohort. It is imperative to minimize/prevent the risk of BI, as this has a negative impact on patient survival, extending well into the post-discharge period. Once BI is suspected, proper antibiotic treatment should be initiated early to prevent adverse outcomes.
Most deaths from hepatitis B virus (HBV) infection are related to complications of cirrhosis, among which bacterial infection (BI) frequently develops in decompensated cirrhosis (DC) as reported in Western countries where alcoholic cirrhosis is frequent.
Investigation on BI in patients with HBV-DC would be particularly important in improving the current management of HBV-DC and acute-on-chronic liver failure (ACLF), especially in Asian countries where health care resources are relatively limited and access to liver transplantation is much more restricted.
To investigate the impact of BI on the outcomes of patients with HBV-DC admitted into the hospital with or without ACLF.
This retrospective study included the patients with HBV-DC admitted to two tertiary centers in China. In-hospital overall survival, 90-d transplant-free survival, 5-year post-discharge survival, and cumulative incidence of ACLF were evaluated. Risk factors for death were analyzed considering liver transplantation as a competing event.
A total of 1281 hospitalized HBV-DC patients were included; 284 had ACLF at admission. The overall prevalence of BI was 28.1%. The patients with BI had a significantly lower in-hospital survival and transplant-free 90-d survival than those without, in both the patients admitted with and without ACLF. The presence of BI significantly increased the risk of developing ACLF [sub-distribution hazard ratio (sHR) = 2.52, 95%CI: 1.75-3.61, P < 0.001)] in the patients without ACLF. In the patients discharged alive, those who had an episode of BI had a significantly lower 5-year transplant-free survival. BI was an independent risk factor for death in the patients admitted without ACLF (sHR = 3.28, 95%CI: 1.93-5.57), while in ACLF admissions, the presence of pneumonia, but not other type of BI, independently increased the risk of death (sHR = 1.87, 95%CI: 1.24-2.82).
BI triggers ACLF in patients with HBV-DC and significantly impairs short-term survival.
It is imperative to minimize/prevent the risk of BI, as this has a negative impact on patient survival, extending well into the post-discharge period. Once BI is suspected, proper antibiotic treatment should be initiated early to prevent adverse outcomes.
The authors acknowledge all the clinical and research staff from the Department of Infectious Diseases of the Ruijin Hospital and the Renji Hospital. We appreciate Professor Florence Wong of the University of Toronto, Canada for the constructive comments in the preparation of this manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Elbahrawy A, Esmat S, Fedeli U, Karagiannakis D, Sudhamshu K S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Nayagam S, Thursz M, Sicuri E, Conteh L, Wiktor S, Low-Beer D, Hallett TB. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 2. | Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 3. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1-1437.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2160] [Article Influence: 180.0] [Reference Citation Analysis (5)] |
| 4. | Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, Zhang S, Xu Z, Wu Y, Yan H, Chen Z. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 5. | Li H, Chen LY, Zhang NN, Li ST, Zeng B, Pavesi M, Amorós À, Mookerjee RP, Xia Q, Xue F, Ma X, Hua J, Sheng L, Qiu DK, Xie Q, Foster GR, Dusheiko G, Moreau R, Gines P, Arroyo V, Jalan R. Characteristics, Diagnosis and Prognosis of Acute-on-Chronic Liver Failure in Cirrhosis Associated to Hepatitis B. Sci Rep. 2016;6:25487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS, O'Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, Brown G, Noble NA, Thacker LR, Kamath PS; NACSELD. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 7. | Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, Hopf C, Alessandria C, Rodriguez E, Solis-Muñoz P, Laleman W, Trebicka J, Zeuzem S, Gustot T, Mookerjee R, Elkrief L, Soriano G, Cordoba J, Morando F, Gerbes A, Agarwal B, Samuel D, Bernardi M, Arroyo V; CANONIC study investigators of the EASL-CLIF Consortium. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 730] [Article Influence: 66.4] [Reference Citation Analysis (1)] |
| 8. | Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496-509. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8085] [Cited by in RCA: 9858] [Article Influence: 379.2] [Reference Citation Analysis (0)] |
| 9. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 362] [Article Influence: 51.7] [Reference Citation Analysis (1)] |
| 10. | Volinsky CT, Raftery AE. Bayesian information criterion for censored survival models. Biometrics. 2000;56:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Cao Z, Liu Y, Wang S, Lu X, Yin S, Jiang S, Chen L, Cai M, Zeng B, Yao Y, Tang W, Zhao G, Xiang X, Wang H, Cai W, Zhu C, Li H, Xie Q. The impact of HBV flare on the outcome of HBV-related decompensated cirrhosis patients with bacterial infection. Liver Int. 2019;39:1943-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Adjei CA, Stutterheim SE, Naab F, Ruiter RAC. Barriers to chronic Hepatitis B treatment and care in Ghana: A qualitative study with people with Hepatitis B and healthcare providers. PLoS One. 2019;14:e0225830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, Welzel T, Pavesi M, Hernández-Tejero M, Ginès P, Arroyo V; European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 393] [Article Influence: 56.1] [Reference Citation Analysis (2)] |
| 14. | Gustot T, Fernandez J, Szabo G, Albillos A, Louvet A, Jalan R, Moreau R, Moreno C. Sepsis in alcohol-related liver disease. J Hepatol. 2017;67:1031-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Ponziani FR, Zocco MA, Cerrito L, Gasbarrini A, Pompili M. Bacterial translocation in patients with liver cirrhosis: physiology, clinical consequences, and practical implications. Expert Rev Gastroenterol Hepatol. 2018;12:641-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 844] [Article Influence: 76.7] [Reference Citation Analysis (1)] |
| 17. | Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1-1256.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 18. | Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, Albillos A, Lammert F, Wilmer A, Mookerjee R, Vila J, Garcia-Martinez R, Wendon J, Such J, Cordoba J, Sanyal A, Garcia-Tsao G, Arroyo V, Burroughs A, Ginès P. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 641] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 19. | Piano S, Brocca A, Mareso S, Angeli P. Infections complicating cirrhosis. Liver Int. 2018;38 Suppl 1:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Nahon P, Lescat M, Layese R, Bourcier V, Talmat N, Allam S, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Goria O, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Benhamou Y, Pilette C, Silvain C, Christidis C, Capron D, Bernard-Chabert B, Hillaire S, Di Martino V, Trinchet JC, Moreau R, Roudot-Thoraval F; ANRS CO12 CirVir and Microcir Groups. Bacterial infection in compensated viral cirrhosis impairs 5-year survival (ANRS CO12 CirVir prospective cohort). Gut. 2017;66:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Mücke MM, Rumyantseva T, Mücke VT, Schwarzkopf K, Joshi S, Kempf VAJ, Welsch C, Zeuzem S, Lange CM. Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 2018;38:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Dionigi E, Garcovich M, Borzio M, Leandro G, Majumdar A, Tsami A, Arvaniti V, Roccarina D, Pinzani M, Burroughs AK, O'Beirne J, Tsochatzis EA. Bacterial Infections Change Natural History of Cirrhosis Irrespective of Liver Disease Severity. Am J Gastroenterol. 2017;112:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | Hua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2016;10:CD008367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Weber N, Martinsen AL, Sani A, Assigbley EKE, Azzouz C, Hayter A, Ayite K, Baba AAB, Davi KM, Gelting R. Strengthening Healthcare Facilities Through Water, Sanitation, and Hygiene (WASH) Improvements: A Pilot Evaluation of "WASH FIT" in Togo. Health Secur. 2018;16:S54-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M; International Ascites Club. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 26. | Piano S, Singh V, Caraceni P, Maiwall R, Alessandria C, Fernandez J, Soares EC, Kim DJ, Kim SE, Marino M, Vorobioff J, Barea RCR, Merli M, Elkrief L, Vargas V, Krag A, Singh SP, Lesmana LA, Toledo C, Marciano S, Verhelst X, Wong F, Intagliata N, Rabinowich L, Colombato L, Kim SG, Gerbes A, Durand F, Roblero JP, Bhamidimarri KR, Boyer TD, Maevskaya M, Fassio E, Kim HS, Hwang JS, Gines P, Gadano A, Sarin SK, Angeli P; International Club of Ascites Global Study Group. Epidemiology and Effects of Bacterial Infections in Patients with Cirrhosis Worldwide. Gastroenterology. 2019;156:1368-1380.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 325] [Article Influence: 54.2] [Reference Citation Analysis (0)] |