Published online Dec 14, 2020. doi: 10.3748/wjg.v26.i46.7299
Peer-review started: August 17, 2020
First decision: October 18, 2020
Revised: October 27, 2020
Accepted: November 9, 2020
Article in press: November 9, 2020
Published online: December 14, 2020
Processing time: 118 Days and 15.6 Hours
Nonalcoholic fatty liver disease (NAFLD) has become one of the most common chronic liver diseases in the world. In our early clinical data and questionnaire analysis of NAFLD, it was found that the body mass index of some patients did not meet the diagnostic criteria for overweight or obesity. The consumption of high-temperature-processed foods such as fried food, hot pot and barbecue is closely related to the occurrence of nonobese NAFLD. Reducing the intake of this kind of food can reduce disease severity and improve prognosis.
To explore the untargeted metabolomics characteristics of nonobese nonalcoholic fatty liver disease in Sprague-Dawley rats induced by high-temperature-processed feed.
Fifty-four male Sprague-Dawley rats were divided into three groups: The control group received a standard diet; the nonfried soybeans (NDFS) group received 60% NDFS and 40% basic feed and the dry-fried soybeans (DFS) group received 60% DFS and 40% basic feed. Six rats were sacrificed at week 4, 8, and 12 in each group. The food intake, body weight, Lee’s index, liver index, serological index and hepatic histopathology were assessed. Untargeted metabolomics characteristics were used to analyze the changes in liver metabolites of rats at week 12. Correlations between metabolites and pathology scores between the DFS and control groups and between the DFS and NDFS groups were analyzed. We selected some of the metabolites, both within the pathway and outside of the pathway, to explain preliminarily the difference in liver pathology in the three groups of rats.
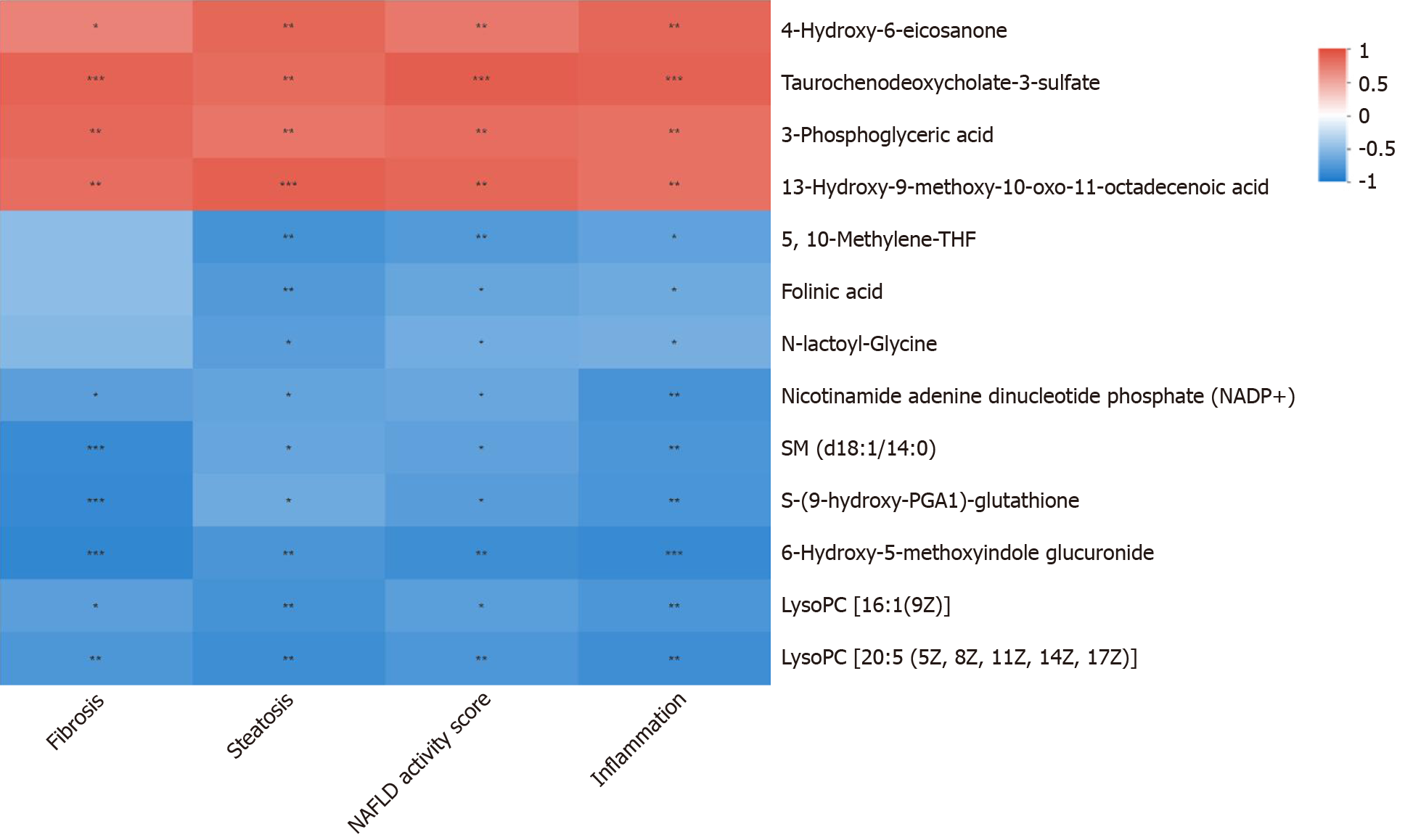
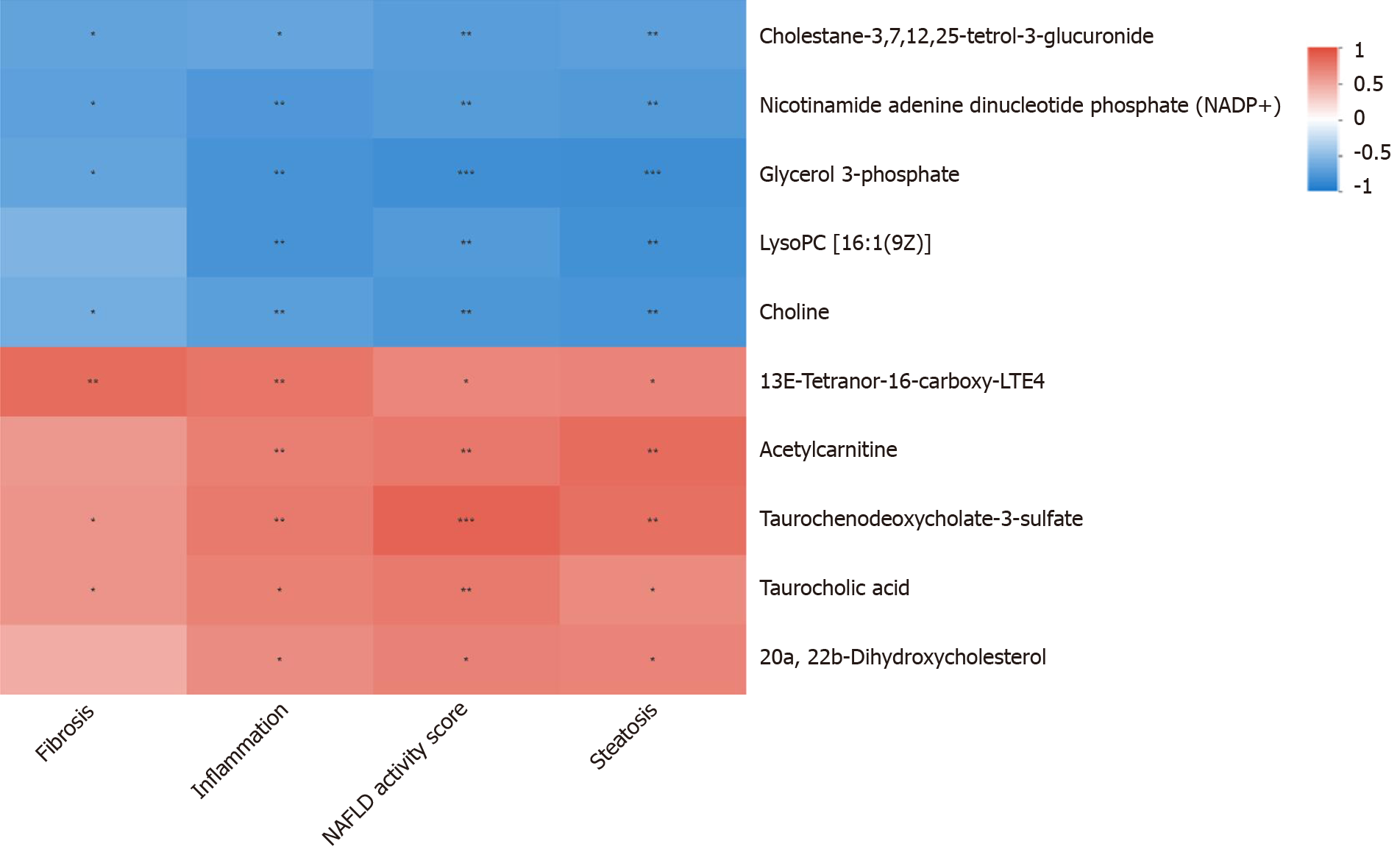
There were no statistically significant differences in the food intake, body weight, Lee's index or serological index between the DFS group and the control group (P > 0.05). At week 8 and week 12, the steatosis scores in the DFS group were significantly higher than those in the other two groups (P < 0.05). At week 12, the liver index of the DFS group was the lowest (NDFS group vs DFS group, P < 0.05). The fibrosis score in the DFS group was significantly higher than those in the other two groups (P < 0.05). The correlation analysis of the liver pathology score and differential metabolites in the DFS and NDFS groups showed that there were 10 strongly correlated substances: Five positively correlated substances and five negatively correlated substances. The positively correlated substances included taurochenodeoxycholate-3-sulfate, acetylcarnitine, 20a,22b-dihydroxycholesterol, 13E-tetranor-16-carboxy-LTE4 and taurocholic acid. The negatively correlated substances included choline, cholesterane-3,7,12,25-tetrol-3-glucuronide, nicotinamide adenine dinucleotide phosphate, lysoPC [16:1 (9Z)] and glycerol 3-phosphate. The correlation analysis of the liver pathology score and differential metabolites in the DFS and control groups showed that there were 13 strongly correlated substances: Four positively correlated substances and 9 negatively correlated substances. The positively correlated substances included 4-hydroxy-6-eicosanone, 3-phosphoglyceric acid, 13-hydroxy-9-methoxy-10-oxo-11-octadecenoic acid and taurochenodeoxycholate-3-sulfate. The negatively correlated substances included lysoPC [16:1(9Z)], S-(9-hydroxy-PGA1)-glutathione, lysoPC [20:5 (5Z, 8Z, 11Z, 14Z, 17Z)], SM (d18:1/14:0), nicotinamide adenine dinucleotide phosphate, 5,10-methylene-THF, folinic acid, N-lactoyl-glycine and 6-hydroxy-5-methoxyindole glucuronide.
We successfully induced liver damage in rats by using a specially prepared high-temperature-processed feed and explored the untargeted metabolomics characteristics.
Core Tip: For some patients with normal body mass index and normal serum indexes, liver damage may already exist. In our previous analysis of clinical data and a high-risk factor questionnaire for patients with nonalcoholic fatty liver disease (NAFLD), we found that the body mass index of some patients did not meet the diagnostic criteria for overweight or obesity. The consumption of high-temperature-processed foods such as fried food and hot pot is closely associated with the occurrence of NAFLD. Dietary intervention to reduce the consumption of such foods can alleviate NAFLD and improve prognosis. For patients with mild liver fat changes and early fibrosis, some clinical equipment and means of diagnosis are prone to misdiagnosis. Untargeted metabolomics can preliminarily explain the difference in liver pathology in the three groups of rats.
- Citation: Xue LJ, Han JQ, Zhou YC, Peng HY, Yin TF, Li KM, Yao SK. Untargeted metabolomics characteristics of nonobese nonalcoholic fatty liver disease induced by high-temperature-processed feed in Sprague-Dawley rats. World J Gastroenterol 2020; 26(46): 7299-7311
- URL: https://www.wjgnet.com/1007-9327/full/v26/i46/7299.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i46.7299
Nonalcoholic fatty liver disease (NAFLD) is a clinicopathological syndrome characterized by fat accumulation in the liver that is not caused by certain liver-damaging factors such as alcohol abuse. The spectrum includes simple steatosis, nonalcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma. NAFLD has become one of the most common chronic liver diseases in the world, with a global prevalence rate of 25.24% among adults[1]. The prevalence rate of NAFLD in China in 2018 was 29.6%[2]. NAFLD has surpassed viral hepatitis in becoming the largest liver disease in China, representing a growing challenge in terms of prevention and treatment. To date, the pathogenesis of NAFLD has not been fully elucidated, and the classic theory is the "two-hit hypothesis"[3]. A high-fat diet, obesity and insulin resistance act as the “first hit”. A "second hit", such as oxidative stress, leads to inflammation, necrosis and fibrosis progression[4,5]. Recently, some scholars have proposed the theory of the "multiple-hit hypothesis", suggesting that insulin resistance, lipid metabolism disorder, nutritional factors, intestinal flora and genetic factors jointly contribute to the occurrence of NAFLD. However, the specific mechanism has not been fully explained.
The existing animal models of NAFLD are represented by a high-fat diet, which can simulate the pathological features and metabolic syndrome manifestations of human NASH. However, liver inflammation and fibrosis are relatively mild, and a high-fat diet does not conform to the dietary pattern of patients with NAFLD. In recent years, high-temperature-processed foods such as fried food, grilled food and hot pot have been increasingly deeply enjoyed by people. In our early clinical data and questionnaire analysis of NAFLD, it was found that the body mass index (BMI) of some patients did not meet the diagnostic criteria for overweight or obesity. The intake of high-temperature-processed food is closely related to the occurrence of NAFLD. Reducing the intake of this kind of food can reduce disease severity and improve prognosis. The prevalence of nonobese NAFLD (3%-30%) varies widely globally[6]. This may be related to differences in study populations, lifestyles, dietary habits and diagnostic patterns. Nonobese patients with NAFLD can be defined as BMI < 28 kg/m2 and BMI < 30 kg/m2 in Asian and Western countries, respectively[7]. Among them, the change in dietary patterns is one of the most basic and important factors. The etiology of nonobese NAFLD has not been fully elucidated, and clinically, nonobese patients with NAFLD are often overlooked. For patients with normal BMI and no abnormal serological examination, liver damage may have occurred, which should be given sufficient attention by clinicians. The purpose of our study was to study the effects of high-temperature-processed feed on rats and to explore the metabolomics pathogenesis of nonobese NAFLD. Considering the complexity of fried food, we added dry-fried soybeans to the normal diet of rats in a certain proportion to simulate high-temperature-processed feed.
Fifty-four healthy male Sprague-Dawley rats, 6-8 wk, specific pathogen free, were provided by Beijing Huafukang Biotechnology Co., Ltd., and adaptive feeding was performed for 2 wk. The animal protocol was designed to minimize pain or discomfort to the animals. Animals were maintained at 23 ± 3 °C with a 12:12-h light/dark cycle and fed with a commercial standard diet and tap water ad libitum. Then, they were randomly divided into three groups with 18 rats in each group. The control group was fed normal feed. The nonfried soybeans (NDFS) group was fed 40% basal feed + 60% nonfried soybeans. The dry-fried soybeans (DFS) group was fed 40% basal feed + 60% high-temperature dry-fried soybeans. Every 4, 8 and 12 weeks, six rats were sacrificed in each group. The rat serum and liver were retained. Our experiment was repeated twice. The animal experimental protocol was approved by the experimental animal welfare ethics committee of China-Japan Friendship Hospital (Approval No: 190201).
Body length, body weight and Lee's index were measured. Blood was collected from the abdominal aorta of the rats. Serum was collected after centrifugation for 10 min at 3000 rpm, and serum alanine transaminase, aspartate aminotransferase, cholesterol and triglyceride (TG) levels were detected. Six lobes of the rat liver were removed separately, weighed and fixed in 10% formalin and embedded in paraffin for hematoxylin-eosin staining (H and E staining), Masson staining and immunohistochemical staining. Liver index = liver weight (g) ÷ body weight (g) × 100%. Lee's index = [body weight (g)1/3 × 1000]/body length (cm).
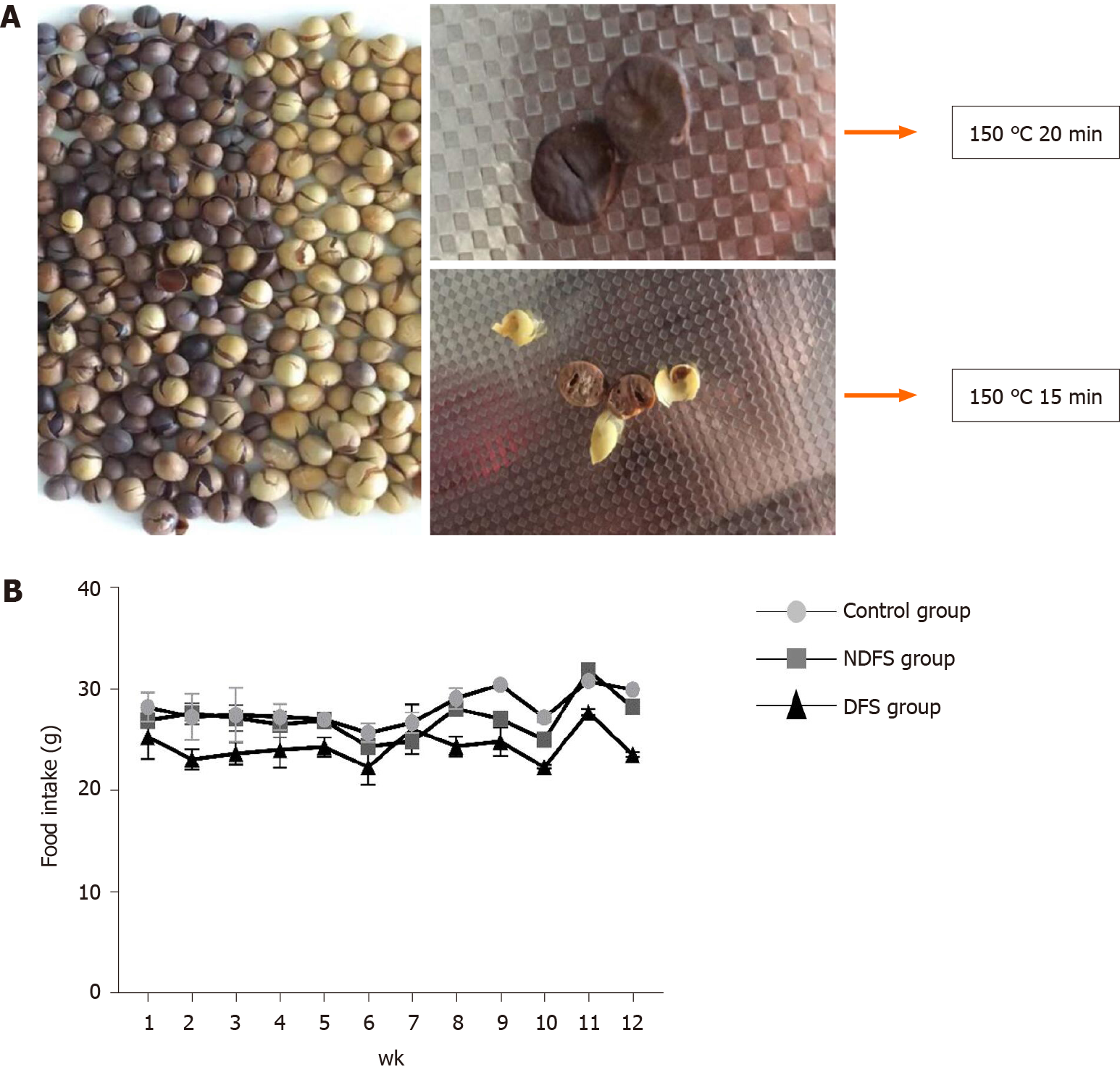
Zhonghuang 13 non-genetically modified organism soybeans were purchased from the Chinese Academy of Agricultural Science. Dry-fried soybean feed was prepared by Beijing Spaifu Biotechnology Co., Ltd. Soybeans were dried in a 150 °C temperature-controlled machine for 15 min, cooled to 70 °C and crushed. Then, 60% of the above soybeans was mixed with 40% of the commercial standard rat feed evenly. The detection of feed ingredients was accomplished by the Pony Testing International Group (www.ponytest.com).
The paraffin-embedded liver tissue was cut into 4 µm-thick sections for standard H and E staining. Ten light microscopic fields (× 200) were viewed in each section and scored for the severity of hepatic steatosis, inflammation and fibrosis. Pathological diagnostic criteria refer to NAFLD activity points (NAS points) and fibrosis score stage 0-4[8,9]. Masson's trichrome staining was performed using standard histological techniques. At 400 × magnification, Image-Pro Plus 6.0 software was used to randomly select five frames for semiquantitative analysis, and the ratio of collagen to liver tissue was recorded. The prepared paraffin-embedded sections were dewaxed, dehydrated and incubated in 3% hydrogen peroxide. After incubating with 3% bovine serum albumin in phosphate buffer for 30 min, liver sections were incubated with primary antibodies against alpha-smooth muscle actin (α-SMA, 1:200) and transforming growth factor beta (TGF-β, 1:200) overnight at 4 °C. Then, the tissues were incubated with a 1:2000 dilution of hypothalamic regulatory peptides-conjugated secondary antibody for 50 min at room temperature. The nuclei were counterstained with hematoxylin for 3 min. The sections were viewed by a fluorescence microscope (Nikon, Tokyo, Japan). At 400 × magnification, the area of the α-SMA and TGF-β staining in five random fields was measured using Image-Pro Plus software 6.0. The mean optical density of the staining area was used to measure the expression of TGF-β and α-SMA. All sections were inspected independently by two blinded observers, and the mean values of the readings were used for final analysis.
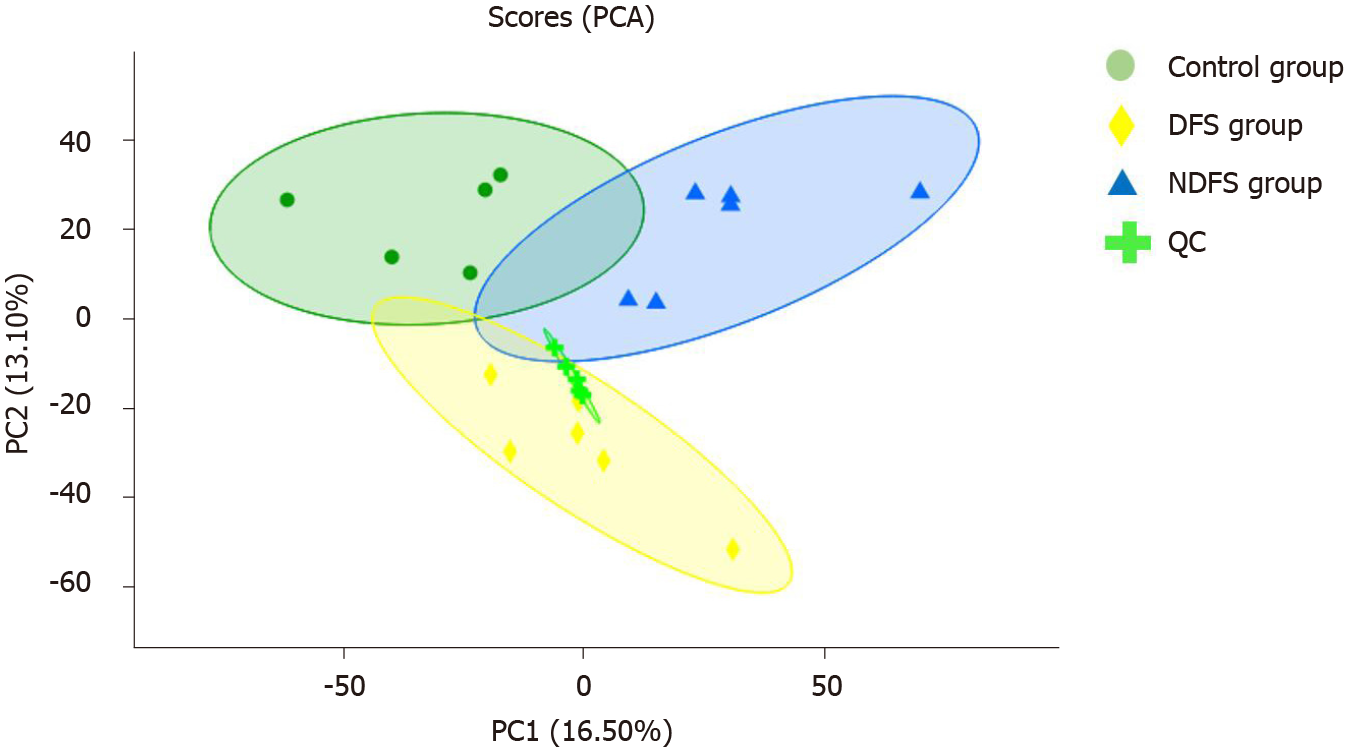
The relative abundances of metabolites, differential metabolites, and related metabolic pathways were tested and analyzed. In this project, 18 rat liver samples at 12th week were studied metabolically using an liquid chromatography-mass spectrometry analysis platform. The samples were first pretreated to remove impurities and extract metabolites, and then the metabolite information was obtained by computer detection and collection under liquid chromatography-mass spectrometry positive and negative modes. Metabolite annotation and data preprocessing were performed using progenesis quality index (Waters Corporation, Milford, CT, United States) to obtain the metabolite list and data matrix. Combined with the T test and variable importance in projection, differential metabolites were screened out, and advanced analyses, such as pathway analysis, correlation analysis and cluster analysis, were further used to mine the biological information of differential metabolism. The correlation analyses of the liver pathology score and differential metabolites in the DFS and NDFS groups and DFS and control groups were assessed. The results of correlation analysis in this study were determined by P < 0.05 and absolute value of correlation coefficient greater than or equal to 0.5. Different colors in the correlation analysis graph represent the size of the correlation coefficient. Red represents positive correlation, and blue represents negative correlation. The darker the color, the greater the absolute value of the correlation coefficient. The asterisk represents the P value, one asterisk, two asterisks and three asterisks respectively represent the P < 0.05, P < 0.01 and P < 0.001.
GraphPad Prism 8.0 software (La Jolla, CA, United States) was used for statistical analysis. The measurement data are expressed as the mean ± standard deviation (x ± s). The comparison of multiple sample means was performed by one-way analysis of variance if the values conformed to the normal distribution and homogeneity of variance. If the values did not conform to a normal distribution and homogeneity of variance, multiple sample nonparametric tests were used. P < 0.05 indicated that the difference was statistically significant.
Unsupervised principal component analysis (PCA) was used to observe the overall distribution among samples and the degree of dispersion among groups. Orthogonal partial least squares discrimination analysis was used to distinguish the differences in the total metabolic profile between groups and to find the differentially produced metabolites. In orthogonal partial least squares discrimination analysis, variables with variable importance in projection values greater than one were considered difference variables. To prevent the model from overfitting, 200 permutation tests were used to investigate the fitting effect of the model.
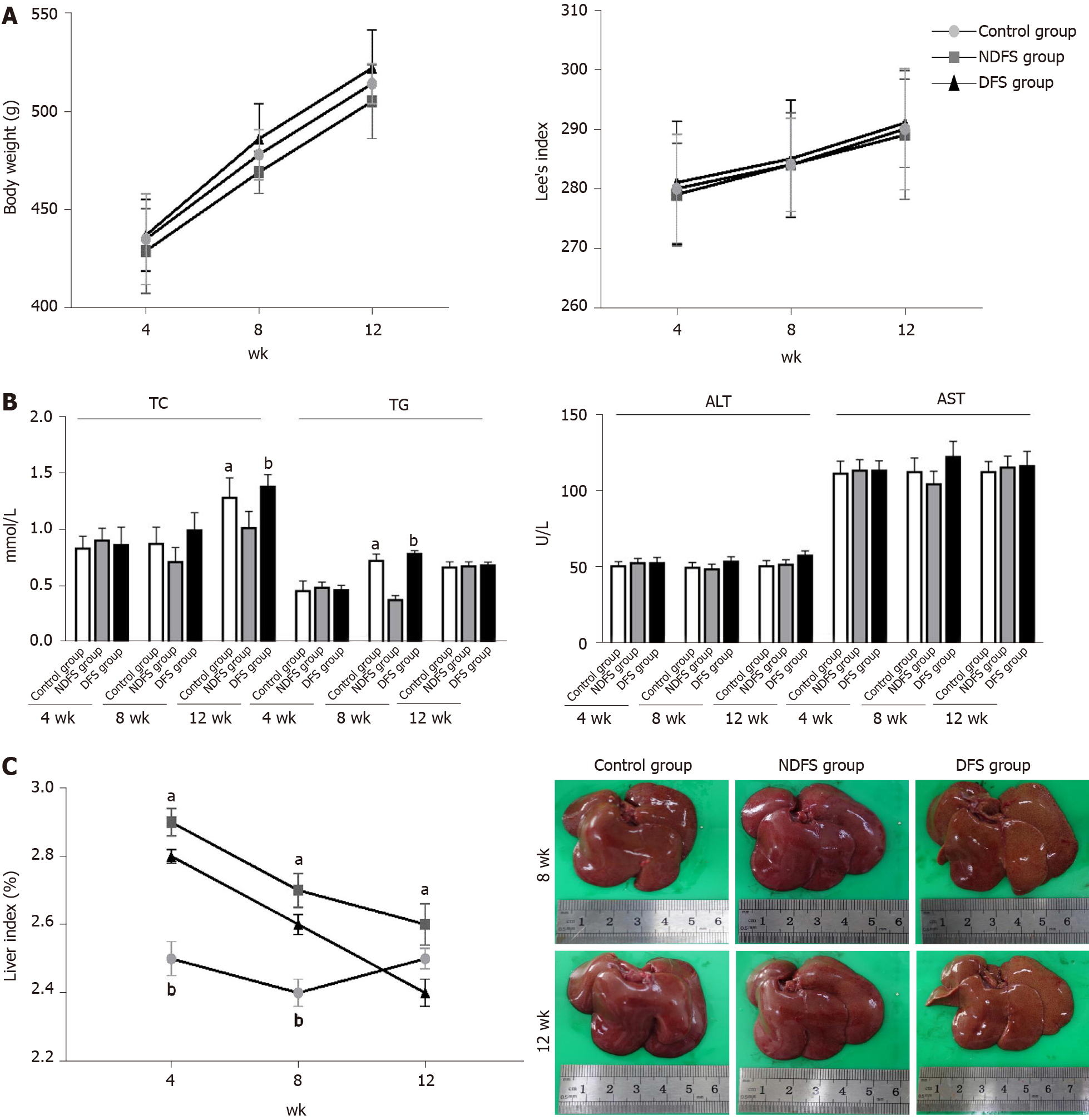
The feed components of the three groups are shown in Table 1. The preparation of dry-fried soybeans feed and food intake of rats are shown in Figure 1. In the process of feed preparation, the nutrient composition ratio of feeds in the three groups all met the feeding standards for rats. There was no significant difference in the total feeding energy among the three groups (P > 0.05). At weeks 4, 8 and 12, there were no statistically significant differences in body weight or Lee's index in the DFS group compared with the control group (P > 0.05). Among the three groups, the liver index of rats in the NDFS group was the largest. The liver index of rats in the NDFS group and DFS group decreased gradually, and the decrease was more obvious in the DFS group. At week 12, the liver index of the DFS group was the lowest, and the difference was statistically significant compared with that of the NDFS group (NDFS group vs DFS group, P < 0.05). Compared with the control group, serum cholesterol, triglyceride, aspartate aminotransferase and alanine aminotransferase levels of the DFS group were not significantly increased (P > 0.05), as shown in Figure 2.
| Control group | NDFS group | DFS group | Reference method | |
| Water, % | 8.1 | 9.0 | 7.1 | GB/T 6435-2014 8.1 |
| Fat, % | 4.6 | 14.2 | 11.0 | GB/T 6433-2006 |
| Protein, % | 21.0 | 29.6 | 31.2 | GB/T 6432-2018 |
| Carbohydrates, g/100 g | 59.9 | 41.8 | 45.2 | GB/Z 21922-2008 |
| Energy, KJ/100 g | 1546 | 1739 | 1706 | GB/Z 21922-2008 |
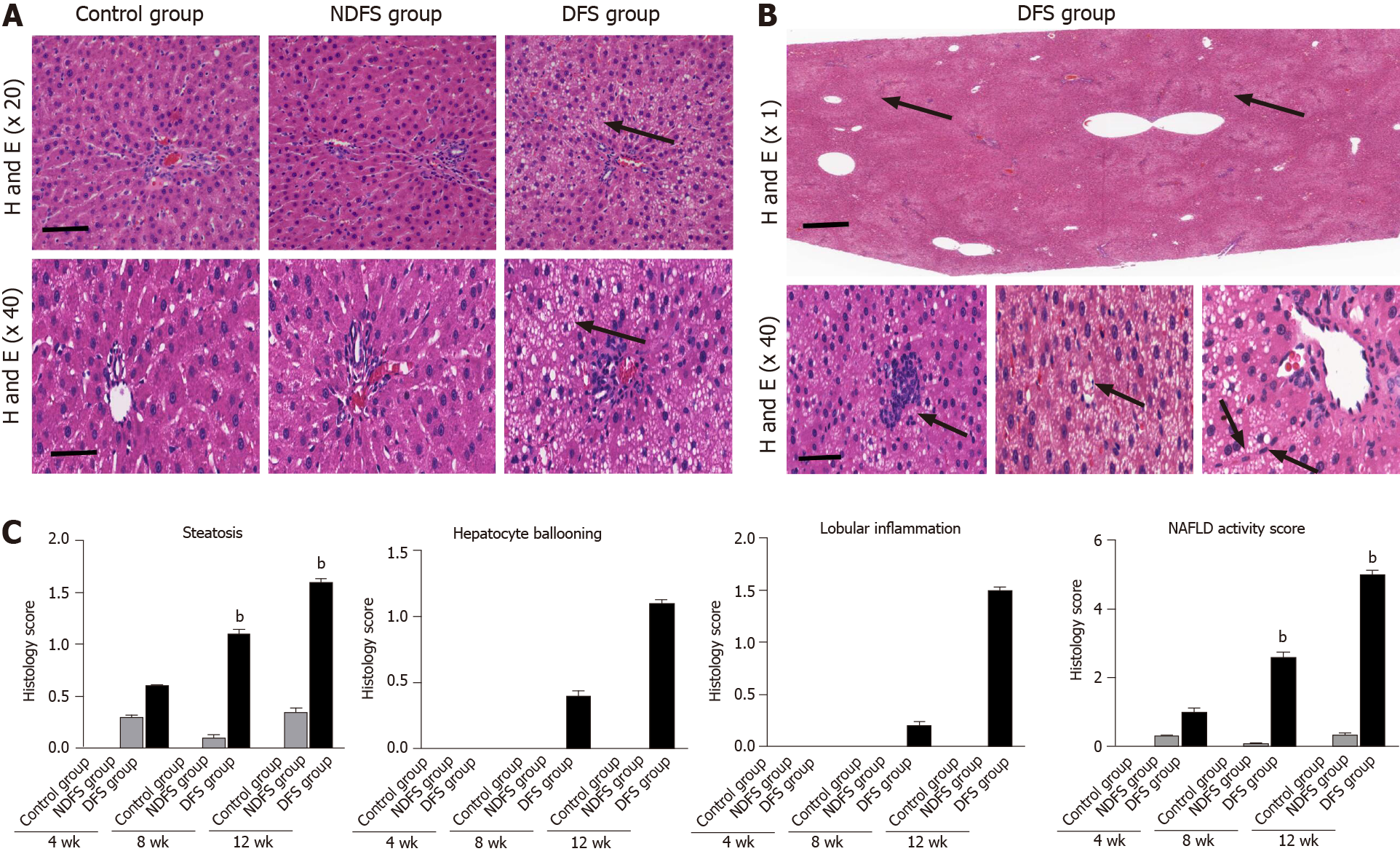
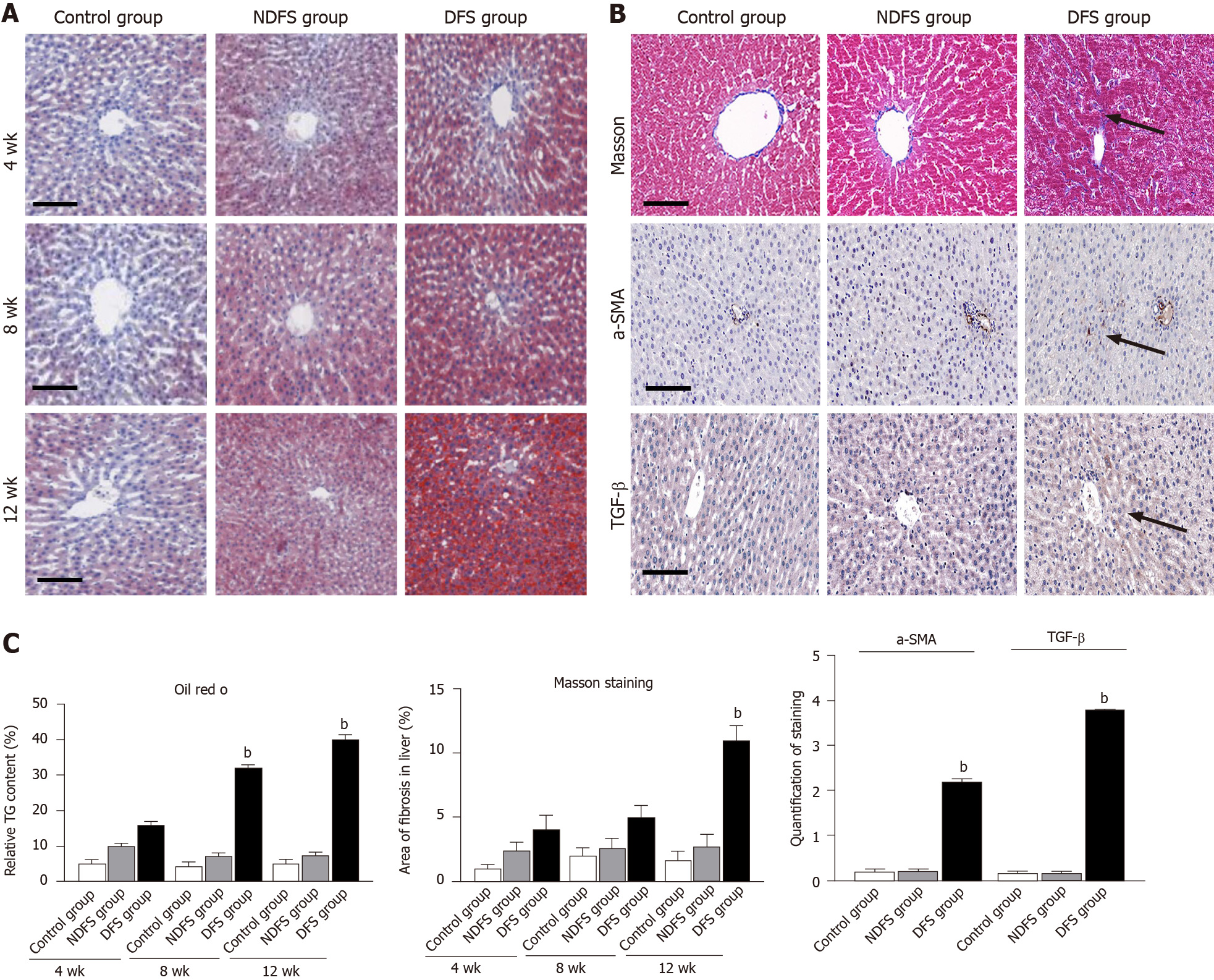
In each rat, H and E staining showed no significant difference among the six lobes of the liver, so we selected the right lobe of the liver as representative to observe its pathological change. The steatosis was further confirmed by Oil red O staining. In terms of steatosis score, at week 8 and week 12, there were statistically significant differences between the DFS group and the other two groups (P < 0.05). In the DFS group, vesicular lipidosis was predominant in the liver, mainly occurring around the portal area. At week 8 and week 12, one-third and two-thirds of liver fat changes were observed in four and six rats, respectively. In terms of fibrosis, compared with the other two groups, the degree of liver fibrosis in the DFS group was increased at week 12, and the difference was statistically significant (P < 0.05). Immunohistochemistry showed increased expression of α-SMA and TGF-β in the DFS group compared with the other two groups (P < 0.05) at week 12. Pathological features and scores are shown in Figures 3 and 4.
PCA analysis showed that the separation trend was obvious among the three groups. According to PCA analysis, we excluded abnormal deviation value control 2. The correlation analysis of liver pathology score and differential metabolites in the DFS and NDFS groups showed that there were 10 strongly correlated substances, specifically five positively correlated substances and five negatively correlated substances. The positively correlated substances included taurochenodeoxycholate-3-sulfate, acetylcarnitine, 20a,22b-dihydroxycholesterol, 13E-tetranor-16-carboxy-LTE4 and taurocholic acid. The negatively correlated substances included choline, cholesterane-3,7,12,25-tetrol-3-glucuronide, nicotinamide adenine dinucleotide phosphate (NADP+), lysoPC [16:1 (9Z)] and glycerol 3-phosphate. The correlation analysis of the liver pathology score and differential metabolites in the DFS and control groups showed that there were 13 strongly correlated substances, specifically four positively correlated substances and nine negatively correlated substances. The positively correlated substances included 4-hydroxy-6-eicosanone, 3-phosphoglyceric acid, 13-hydroxy-9-methoxy-10-oxo-11-octadecenoic acid and taurochenodeoxycholate-3-sulfate. The negatively correlated substances included lysoPC [16:1 (9Z)], S-(9-hydroxy-PGA1)-glutathione, lysoPC [20: 5 (5Z,8Z,11Z,14Z,17Z)], SM (d18: 1/14: 0), nicotinamide adenine dinucleotide phosphate (NADP+), 5,10-methylene-THF, folinic acid, N-lactoyl-glycine and 6-hydroxy-5-methoxyindole glucuronide. Compared with the NDFS group and control group, the DFS group mainly had effects on the metabolism of glycerophospholipid, folic acid, fatty acids and bile acids in normal rats, indicating that the dry-fried soybean diet may cause liver damage by changing the structure and permeability of the liver cell membrane in normal rats or by the accumulation of lipid peroxides and triggering of inflammatory reactions, as shown in Figures 5-7.
To verify the liver damage caused by high-temperature-processed feed, our research involved feeding rats a mixed diet (60% dry-fried soybeans + 40% basal diet) for 8-12 wk to induce the occurrence of NAFLD without obesity, and the pathological manifestations are different from those of other animal models. It was previously found that feeding 1.5 mL of repeatedly heated oil at 160-190 °C to rats every day for 6 consecutive weeks can damage the metabolism of glycerin and change the structure of the intestinal histology and microbiota compared with rats that have not been fed heated oil[10]. However, liver damage was not elucidated in that article. Soybean is one of the most abundant plant sources of dietary protein. It contains isoflavones that have a cholesterol-lowering effect. However, the potential effects of soy foods on human health remain controversial, and the effects of heat-treated soy on humans remain unclear. Raw soy contains anti-trypsin and other substances, which can harm the growth of livestock and poultry. At week 4, we found that the livers of the rats in the DFS group showed steatosis, but the steatosis did not increase with the extension of time. In the early stage, we explored the temperature required for dry-fried soybeans. When the temperature was below 150 °C, we found that the inside of the soybean was not ripe, and when the temperature was above 150 °C, the surface of the soybean became charred. Therefore, we used a temperature of 150 °C. The surface of the soybean was scorched when processed at 150 °C for 20 min. Therefore, we used 150 °C for 15 min as the condition for dry-fried soybeans. In preliminary experiments, when dry-fried soybeans were added as 40% of the feed, we did not find liver damage in rats. Then, when we increased the proportion of dry-fried soybeans to 60%, the liver of the rats was damaged. Therefore, we believe that only when high-temperature-processed feed reaches a certain temperature and a certain amount can it cause damage to rats.
In this experiment, DFS group rats showed no signs of obesity. We know that some patients with NAFLD have no symptoms of obesity, and we believe that inflammation and oxidative stress play an important role in the early development of fatty liver in our study. In this work, metabolomics has identified a number of metabolites that are closely related to inflammation and oxidative stress, such as dihydroxycholesterol, 13E-tetranor-16-carboxy-LTE4, eicosanoic acid and 13-hydroxy-9-methoxy-10-oxo-11-octadecenoic acid. In the DFS group, the latter three substances were positively correlated with the steatosis score, inflammation score, fibrosis score and NAFLD pathological score, while dihydroxycholesterol was positively correlated with the steatosis score, inflammation score and NAFLD pathological score. Dihydroxycholesterol is the cholesterol that is oxidized in food. Fat is oxidized when food is heated. To avoid excessive intake of hydroxycholesterol, foods such as fried chicken, grilled burgers or steaks should be avoided. Thirteen-E-Tetranor-16-carboxy-LTE4 is a lipid oxidation product of leukotriene E4. It is synthesized by the conversion of polyunsaturated fatty acids by lipoxygenase. Leukotriene E4 is a potent inflammatory mediator[11,12]. Correlation analysis showed that 13E-tetranor-16-carboxy-LTE4 was the substance most positively correlated with fibrosis in the DFS group compared with the NDFS group. Eicosanoids are a family of lipids derived from 20-carbon polyunsaturated fatty acids. These compounds contain 20 carbon atoms and are therefore known as "eicosanoids". Their precursor is mainly arachidonic acid, which is involved in the contraction or relaxation of smooth muscle, platelet aggregation and the inflammatory response. In biochemistry, arachidonic acids are "signaling molecules" formed by the oxidation of fatty acids linked by 20 carbons. They are subcategories of lipins (oxylipins) formed by enzymatic or nonenzymatic oxidation of polyunsaturated fatty acids. Soybean fatty acids mainly contain oleic acid, linoleic acid, linolenic acid, palmitic acid and stearic acid, among which the linoleic acid content is the highest. Linoleic acid is unstable and easily oxidized. Thirteen-hydroxy-9-methoxy-10-oxo-11-octadecenoic acid is an oxidation product derivative of linoleic acid, and its production increases when oxidative stress increases. Therefore, the addition of dry-fried soybean feed may cause liver damage by enhancing oxidative stress and triggering inflammation by the accumulation of lipid peroxidation products.
Lysophospholipids (LPCs) and NADP+ decreased in the DFS group compared with the control group and the NDFS group. LPCs are phospholipids with various physiological functions that are mainly metabolized in the liver. LPCs are closely related to metabolic diseases such as diabetes, atherosclerosis and fatty liver. Impaired metabolism of LPCs may be a signal of liver inflammation. It has been reported that taurocholic acid increased and LPCs decreased in patients with NASH[13]. This is consistent with the results in our study. The depletion of NADP+ is an important marker of aging and many age-related diseases, such as neuromuscular, cardiometabolic, liver and kidney diseases. In the liver, reduced NADP+ content is associated with the development of alcoholic and NAFLD, while retention of NADP+ content in the liver reduces the associated damage. Bile acid is the transformation product of cholesterol in hepatocytes, which is secreted by hepatocytes and discharged into the intestine through the biliary system. In hepatobiliary diseases, the excretion of total bile acid is inhibited, resulting in an increase in peripheral blood total bile acid concentration. Abnormally elevated bile acids can lead to the accumulation of toxic bile acids in the liver, resulting in mitochondrial dysfunction and excessive production of reactive oxygen species and reactive nitrogen species. In this study, taurochenodeoxycholate-3-sulfate and taurocholic acid were increased in the DFS group, indicating abnormal liver function of rats.
Meanwhile, the antioxidant S-(9-hydroxy-PGA1)-glutathione was decreased in the DFS group compared with the control group. Glutathione is an important antioxidant in the body, and glutamine is involved in its synthesis. Glutamine is an essential nutrient for the metabolism of intestinal mucosal cells and plays an important role in maintaining the integrity of the intestinal mucosal epithelial structure. Especially under severe stress, such as trauma, infection and fatigue, glutamine is rapidly depleted in intestinal mucosal epithelial cells. By maintaining and increasing the storage of glutathione in tissue cells, the antioxidant capacity of the body is improved. Glycine is a constituent amino acid of the endogenous antioxidant reduced glutathione. Reductions in L-glutamine, glutathione and glycine were found in the DFS group. Folic acid is a B vitamin widely distributed in the body that plays an important role in the metabolism of one-carbon units. Tetrahydrofolic acid is a coenzyme of one-carbon group transferase, which has the function of transferring one carbon group and is a necessary coenzyme in many biosynthesis reactions. A carbon group is one of the raw materials for the synthesis of purine nucleotides and thymine nucleotides in organisms, so folic acid plays an important role in the formation of nucleic acids and affects the synthesis of proteins and the growth of cells. Tetrahydrofolic acid carries these carbon units and forms 5-CH3-THF, which binds with plasma proteins and is mainly transported to the liver for storage. In this study, the levels of folic acid and 5,10-methylene-THF were decreased in the DFS group compared with the control group. Therefore, metabolic disorders of folic acid and tetrahydrofolic acid may be involved in the occurrence and development of fatty liver.
Choline is a component of the cell membrane, lipoproteins and phospholipid proteins and plays an important role in cell membrane integrity and lipid metabolism. In choline-deficient rats, a high-fat diet resulted in fat accumulation in the liver due to reduced phosphatidylcholine synthesis. Choline decreased in the DFS group compared with the NDFS group. The DFS group mainly showed effects on the metabolism of glycerophospholipids in normal rats, which may lead to liver damage by changing the structure and permeability of the liver cell membrane. Acetylcarnitine increased in the DFS group compared with the NDFS group. Acetylcarnitine carries fatty acids from the cytoplasm to the mitochondria, where they are involved in the production and release of energy during the oxidation of fatty acids. Elevated acetylcarnitine levels are associated with oxidative disorders in the lipid metabolism of macrophages, and the transformation of macrophage lipid overload into macrophage foam cells may play a key role in the formation of fatty liver[14].
In conclusion, our research also has some limitations. We did not include other high-temperature-processed foods, such as fried and grilled foods. We did not investigate the effects of high-temperature-processed foods on other organs in rats. However, our study may be of great significance for the in-depth understanding of the mechanism in nonobese nonalcoholic fatty liver disease. The main pathological changes in the rat liver in the DFS group were vesicular lipidosis and early fibrosis. Clinically, many people have normal serological indicators but early liver damage. Especially for patients with mild liver fat changes and early fibrosis, some clinical equipment and means of testing are prone to misdiagnosis.
Nonalcoholic fatty liver disease (NAFLD) has surpassed viral hepatitis to become the most common liver disease in China and is facing increasing challenges in prevention and treatment. The etiology of nonobese NAFLD has not been fully elucidated and is often neglected clinically. For patients with normal body mass index and no abnormal serological indicators, liver damage may have occurred, which should be given full attention by clinicians. It is of great significance to develop new animal models to study the pathogenesis of this disease.
In modern society, more and more young people suffer from fatty liver disease with normal body mass index. They prefer high temperature processed food such as barbecue, hot pot and fried food, which may be associated with the development of nonobese NAFLD.
The purpose of our study was to investigate the effects of high-temperature-processed feed on rats and to explore the metabolomics pathogenesis of nonobese NAFLD.
Fifty-four male Sprague-Dawley rats were divided into three groups: The control group received a standard diet; the nonfried soybeans (NDFS) group received 60% nonfried soybeans and 40% basal feed and the dry-fried soybeans (DFS) group received 60% dry-fried soybeans and 40% basal feed. Six rats were sacrificed at weeks 4, 8 and 12 in each group. The food intake, body weight, Lee’s index, liver index, serological index and hepatic histopathology were assessed. Untargeted metabolomics characteristics were used to analyze the changes in liver metabolites of rats at week 12. Correlations between selected metabolites and pathology scores between the DFS and control groups and between the DFS and NDFS groups were analyzed. The results of correlation analysis in this study were determined by P < 0.05 and absolute value of correlation coefficient greater than or equal to 0.5.
There was no statistically significant difference in the food intake, body weight, Lee's index or serological index between the DFS group and the control group (P > 0.05). At week 8 and week 12, the steatosis score in the DFS group were significantly higher than those in the other two groups (P < 0.05). At week 12, the fibrosis score in the DFS group was significantly higher than those in the other two groups (P < 0.05). The correlation analysis of the liver pathology score and differential metabolites in the DFS and NDFS groups showed that there were 10 strongly correlated substances: Five positively correlated substances and five negatively correlated substances. The correlation analysis of the liver pathology score and differential metabolites in the DFS and control groups showed that there were 13 strongly correlated substances: Four positively correlated substances and nine negatively correlated substances.
We successfully induced liver damage in rats by using a specially prepared high-temperature-processed feed and preliminarily explored the untargeted metabolomics characteristics. Our study provides new insights into the diagnosis, treatment and health education of patients with NAFLD.
In the future, we will further study the molecular mechanism of fatty liver induced by high-temperature-processed feed in rats and explore the effects of other high-temperature-processed feed on rat liver.
The authors would like to acknowledge Ji-Kuan Sun and Feng Zhang for skillful technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bramhall SR, Can G S-Editor: Zhang L L-Editor: Filipodia P-Editor: Liu JH
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7528] [Article Influence: 836.4] [Reference Citation Analysis (0)] |
| 2. | Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71:1851-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 449] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 3. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3125] [Article Influence: 115.7] [Reference Citation Analysis (36)] |
| 4. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2112] [Article Influence: 234.7] [Reference Citation Analysis (1)] |
| 5. | Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014;15:8591-8638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 6. | Kim D, Kim WR. Nonobese Fatty Liver Disease. Clin Gastroenterol Hepatol. 2017;15:474-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (1)] |
| 7. | Aby E, Saab S. Nonobese nonalcoholic fatty liver disease. Clin Liver Dis (Hoboken). 2017;10:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8229] [Article Influence: 411.5] [Reference Citation Analysis (5)] |
| 9. | Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 647] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 10. | Zhou Z, Wang Y, Jiang Y, Diao Y, Strappe P, Prenzler P, Ayton J, Blanchard C. Deep-fried oil consumption in rats impairs glycerolipid metabolism, gut histology and microbiota structure. Lipids Health Dis. 2016;15:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Assies J, Mocking RJ, Lok A, Ruhé HG, Pouwer F, Schene AH. Effects of oxidative stress on fatty acid- and one-carbon-metabolism in psychiatric and cardiovascular disease comorbidity. Acta Psychiatr Scand. 2014;130:163-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Hennebelle M, Otoki Y, Yang J, Hammock BD, Levitt AJ, Taha AY, Swardfager W. Altered soluble epoxide hydrolase-derived oxylipins in patients with seasonal major depression: An exploratory study. Psychiatry Res. 2017;252:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H, Watkins SM, Sanyal AJ. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 520] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 14. | Blair HC, Sepulveda J, Papachristou DJ. Nature and nurture in atherosclerosis: The roles of acylcarnitine and cell membrane-fatty acid intermediates. Vascul Pharmacol. 2016;78:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |