Published online Dec 7, 2020. doi: 10.3748/wjg.v26.i45.7258
Peer-review started: September 26, 2020
First decision: November 8, 2020
Revised: November 9, 2020
Accepted: November 21, 2020
Article in press: November 21, 2020
Published online: December 7, 2020
Processing time: 68 Days and 11.4 Hours
Endoscopic resection of non-invasive lesions is now the standard of care for lesions in the GI tract. However, resection techniques require extensive training, are not available in all endoscopy centers and are prone to complications. Endoscopic mucosal ablation (EMA) is a combination of resection and ablation techniques and it may offer an alternative in the management of such lesions.
In this case series we report the successful treatment of three flat colonic polyps using the EMA technique. Two lesions were treatment naïve and 1 was a recurrence after an endoscopic mucosal resection. The sizes ranged from 2 to 4 cm. All three polyps were ablated successfully with no immediate or delayed complications. The recurrence rate at 1 year of follow up was 0%.
Based on this initial experience, we conclude that EMA is a safe and effective technique for the treatment of non-invasive colonic polyps when endoscopic resection techniques are not available.
Core Tip: Endoscopic resection via endoscopic mucosal resection or endoscopic submucosal dissection is currently the standard of care for non-invasive colonic polyps. However, these resection techniques require extensive training, are not available in all endoscopy centers, and are prone to adverse events such as perforation and bleeding. Endoscopic mucosal ablation appears to be a safe and effective alternative in the treatment of colonic polyps without invasive features.
- Citation: Mendoza Ladd A, Espinoza J, Garcia C. Endoscopic mucosal ablation - an alternative treatment for colonic polyps: Three case reports. World J Gastroenterol 2020; 26(45): 7258-7262
- URL: https://www.wjgnet.com/1007-9327/full/v26/i45/7258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i45.7258
Endoscopic mucosal resection (EMR) and/or endoscopic submucosal dissection (ESD) is now the standard of care for non-dysplastic, dysplastic and early malignant lesions of the gastrointestinal tract. The sine qua non of these techniques is the adequate formation of a submucosal cushion prior to resection, in order to avoid damage of the muscularis propria and hence, perforation. However, these resection techniques require rigorous training, are prone to complications and are not always available at all centers. Recently the submucosal cushion principle has been adopted in the management of dysplastic Barrett’s esophagus (BE) prior to ablation with argon plasma in a technique called Hybrid APC[1]. We explored this concept in the management of 3 flat colonic polyps.
All patients presented in this case series had colon polyps requiring treatment.
All three patients originally presented to their gastroenterology providers for routine colon cancer screening or surveillance and were referred to our endoscopy center.
Case 1: Hypertension (HTN), DM2.
Case 2: DM2, HTN, hypercholesterolemia and treated tuberculosis.
Case 3: Hypercholesterolemia and osteoarthritis.
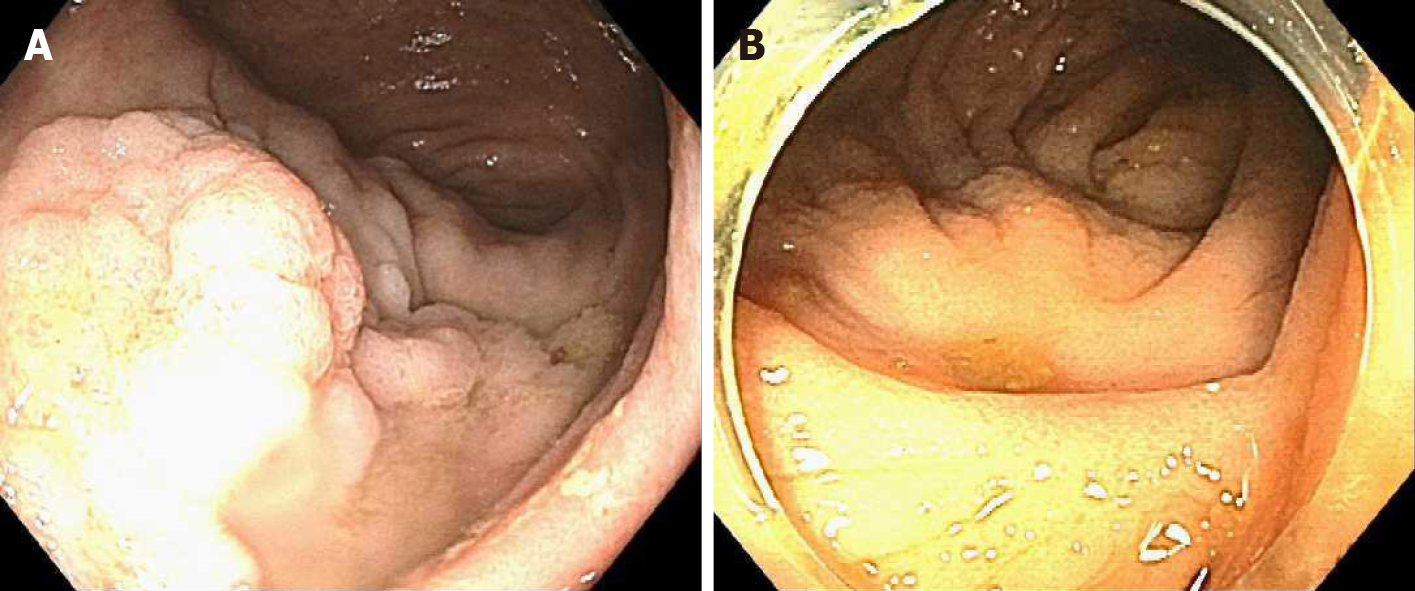
Case 1: An 85 y/o M with previous medical history (PMH) described above was referred for evaluation of a cecal polyp. Colonoscopy revealed a 4 cm 0-IIa +Is lesion in the cecum that involved the IC valve (Figure 1A). Due to morphology of the lesion and the risk of incomplete resection via EMR (we did not perform ESD at our center at the time), removal was not attempted. The lesion was biopsied and pathological analysis established it was a tubular adenoma with no dysplasia. The patient was offered referral to an outside facility for ESD. However due to his lack of medical insurance, referral was not a possibility. Therefore, endoscopic mucosal ablation (EMA) was offered. After detailed explanation of the potential risks and benefits, he agreed to the procedure. The colonoscopy was repeated 2 mo later. The lesion was injected with a mix of O’rise gelTM (Boston Scientific, Natick, MA, United States) + epinephrine at 1:10000 to create the submucosal cushion. The lesion raised easily. Once raised, ablation of the lesion was performed using Argon Plasma Coagulation in forced coagulation mode at 1 L/min, 60 W, effect 2 (Video). The patient did well and was discharged the same day of the procedure. Colonoscopy 1 year later showed a scar with no evidence of polyp recurrence (Figure 1B). Biopsies of the scar confirmed eradication of the lesion.
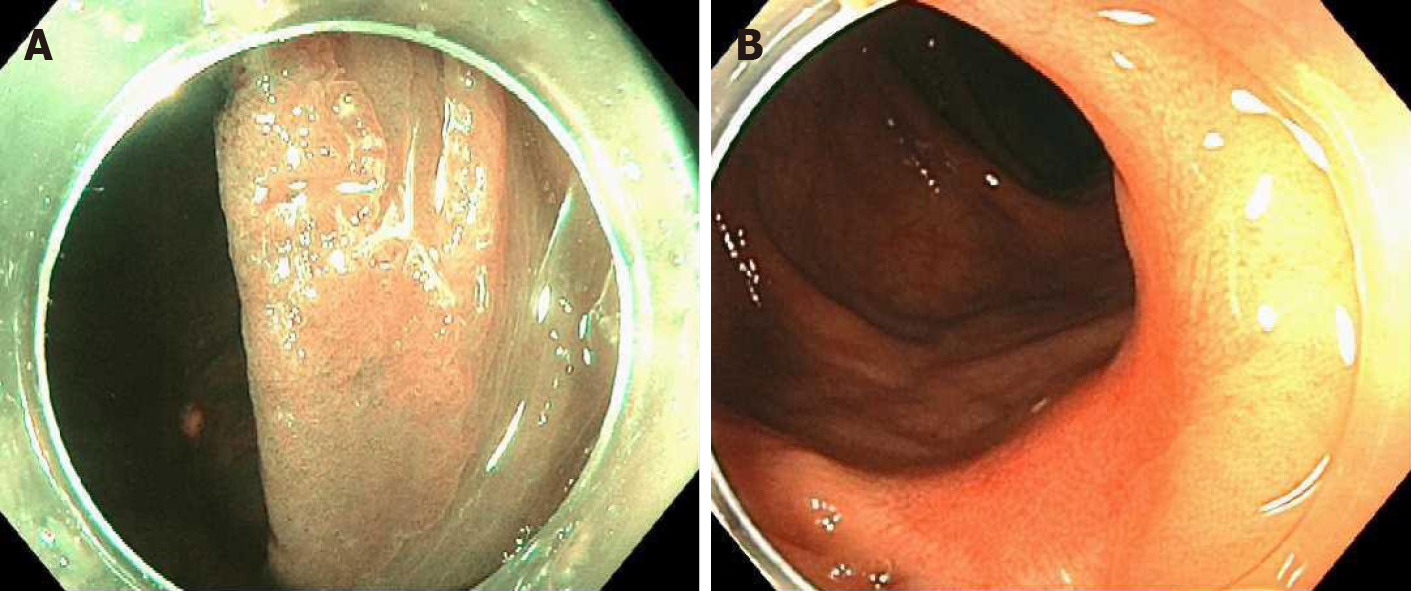
Case 2: This was a 69 y/o M with PMH described above who was referred for removal of polyp recurrence after a previous EMR. Colonoscopy showed a 0-IIa lesion of approximately 3 cm at the previous EMR scar in the hepatic flexure (Figure 2A). The lesion was biopsied prior to ablation. After the biopsy, EMA was performed in a similar manner as in case 1 (video). The biopsy of the lesion revealed a tubular adenoma with no dysplasia. Colonoscopy at 11 mo showed a healthy scar with no signs of polyp recurrence (Figure 2B). Biopsy of the scar confirmed eradication.
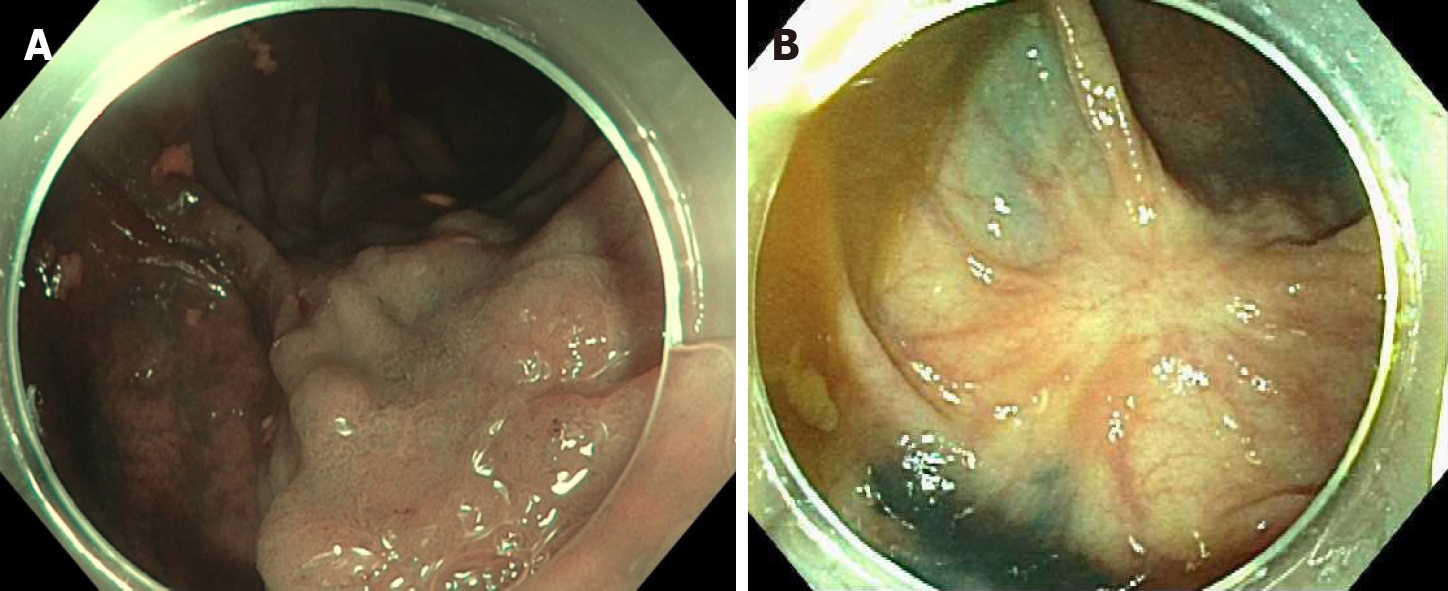
Case 3: A 79 y/o F with a PMH described above was referred for evaluation of a flat polyp. Colonoscopy revealed a 2 cm 0-IIa lesion in the hepatic flexure (Figure 3A). The lesion was biopsied prior to ablation. Ablation ensued as described above (video). Biopsy of the lesion revealed tubular adenoma features with no dysplasia. Colonoscopy 13 mo later revealed a healthy scar with no polyp recurrence (Figure 3B). Biopsy of the scar confirmed eradication.
All patients were diagnosed with TA with no dysplasia.
All patients were treated with the EMA technique.
All patients had follow-up colonoscopy at 1 year and biopsy of the ablation site revealed no evidence of recurrence.
EMA is a combination of established resection and ablation techniques already utilized in different sections of the GI tract. The EMA technique has been previously applied successfully in gastric lesions. Kothari et al[2] successfully treated a flat dysplastic lesion at the GJ anastomosis of a patient with a previous Roux-en-Y gastric bypass[2]. Estifan et al[3] also obtained adequate results using EMA to treat lesion recurrence after an ESD for intestinal metaplasia in the incisura[3]. The only available report of the application of this technique in the colon was published by Tsiamoulos et al[4]. In contrast to ours, this case series included only recurrent lesions after prior endoscopic resection. They reported no complications and a recurrence rate of 82% at one year.
When utilizing EMA, a natural concern is the risk of complications such as perforation. However, studies in porcine models have demonstrated that ablation with argon plasma does not cause injury to the musclularis propria when a submucosal cushion is created[5,6]. This finding led to the novel technique of Hybrid APC. In this technique, a submucosal cushion is created and the affected mucosa is ablated. This technique has been applied in the treatment of BE and has shown promising results[1].
The advantages of EMA include its simplicity, safety and its availability in most community endoscopy centers. The main disadvantage is that it does not produce a surgical specimen for pathological analysis. Although previous biopsy of the lesion can give preliminary information about the histology, it may not reflect that of the entire lesion. Therefore, endoscopists should only use EMA in colonic polyps when endoscopic resection via EMR or ESD is not available. Furthermore, this technique should not be utilized when the preparation of the colon is poor due to the risk of colonic explosion[7,8].
To our knowledge, this is the first report of the use of EMA in treatment naïve colonic lesions. We encountered no complications and our recurrence rate at 1 year was 0% with biopsies of the scars confirming eradication in all cases. Our results suggest that EMA is safe and effective in the treatment of colonic polyps when endoscopic resection is not possible or available.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy, No. 136083; and American College of Gastroenterology, No. 35849.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fu TL, Inal V, Protopapas A S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Manner H, May A, Kouti I, Pech O, Vieth M, Ell C. Efficacy and safety of Hybrid-APC for the ablation of Barrett's esophagus. Surg Endosc. 2016;30:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Kothari TH, Kothari S, Kaul V. Hybrid argon plasma coagulation: a new modality for treatment of a diffuse foregut anastomotic dysplastic lesion. VideoGIE. 2019;4:209-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Estifan E, Cavanagh Y, Grossman MA. Hybrid Argon Plasma Coagulation for Treatment of Gastric Intestinal Metaplasia. Cureus. 2020;12:e7427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Tsiamoulos ZP, Bourikas LA, Saunders BP. Endoscopic mucosal ablation: a new argon plasma coagulation/injection technique to assist complete resection of recurrent, fibrotic colon polyps (with video). Gastrointest Endosc. 2012;75:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 5. | Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ichinose M, Omata M. Submucosal injection of normal saline may prevent tissue damage from argon plasma coagulation: an experimental study using resected porcine esophagus, stomach, and colon. Surg Laparosc Endosc Percutan Tech. 2006;16:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Goulet CJ, Disario JA, Emerson L, Hilden K, Holubkov R, Fang JC. In vivo evaluation of argon plasma coagulation in a porcine model. Gastrointest Endosc. 2007;65:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Manner H, Plum N, Pech O, Ell C, Enderle MD. Colon explosion during argon plasma coagulation. Gastrointest Endosc. 2008;67:1123-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Pichon N, Maisonnette F, Cessot F, Sodji M, Sautereau D. Colonic perforations after gas explosion induced by argon plasma coagulation. Endoscopy. 2004;36:573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |