Published online Dec 7, 2020. doi: 10.3748/wjg.v26.i45.7242
Peer-review started: June 1, 2020
First decision: June 12, 2020
Revised: July 10, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 7, 2020
Processing time: 185 Days and 11.9 Hours
Anemia is considered a public health issue and is often caused by iron deficiency. Iron-deficiency anemia (IDA) often originates from blood loss from lesions in the gastrointestinal tract in men and postmenopausal women, and its prevalence among patients with gastrointestinal bleeding has been estimated to be 61%. However, few guidelines regarding the appropriate investigation of patients with IDA due to gastrointestinal bleeding have been published.
To review current evidence and guidelines concerning IDA management in gastrointestinal bleeding patients to develop recommendations for its diagnosis and therapy.
Five gastroenterology experts formed the Digestive Bleeding and Anemia Workgroup and conducted a systematic literature search in PubMed and professional association websites. MEDLINE (via PubMed) searches combined medical subject headings (MeSH) terms and the keywords “gastrointestinal bleeding” with “iron-deficiency anemia” and “diagnosis” or “treatment” or “management” or “prognosis” or “prevalence” or “safety” or “iron” or “transfusion” or “quality of life”, or other terms to identify relevant articles reporting the management of IDA in patients over the age of 18 years with gastrointestinal bleeding; retrieved studies were published in English between January 2003 and April 2019. Worldwide professional association websites were searched for clinical practice guidelines. Reference lists from guidelines were reviewed to identify additional relevant articles. The recommendations were developed by consensus during two meetings and were supported by the published literature identified during the systematic search.
From 494 Literature citations found during the initial literature search, 17 original articles, one meta-analysis, and 13 clinical practice guidelines were analyzed. Based on the published evidence and clinical experience, the workgroup developed the following ten recommendations for the management of IDA in patients with gastrointestinal bleeding: (1) Evaluation of hemoglobin and iron status; (2) Laboratory testing; (3) Target treatment population identification; (4) Indications for erythrocyte transfusion; (5) Treatment targets for erythrocyte transfusion; (6) Indications for intravenous iron; (7) Dosages; (8) Monitoring; (9) Indications for intravenous ferric carboxymaltose treatment; and (10) Treatment targets and monitoring of patients. The workgroup also proposed a summary algorithm for the diagnosis and treatment of IDA in patients with acute or chronic gastrointestinal bleeding, which should be implemented during the hospital stay and follow-up visits after patient discharge.
These recommendations may serve as a starting point for clinicians to better diagnose and treat IDA in patients with gastrointestinal bleeding, which ultimately may improve health outcomes in these patients.
Core Tip: Iron-deficiency anemia (IDA) is a public health issue often caused by gastrointestinal bleeding. Few clinical practice guidelines regarding the appropriate investigation of IDA due to gastrointestinal bleeding were published. Therefore, five gastroenterology experts conducted a systematic search in PubMed and medical association websites to analyze the current evidence and guidelines on IDA management in patients with gastrointestinal bleeding. From 494 search results, 13 clinical practice guidelines, 17 original articles, and one meta-analysis were analyzed. Ten recommendations were developed for screening, treatment indications, appropriate therapies, and treatment goals, being a starting point for diagnosing and treating IDA in gastrointestinal bleeding patients.
- Citation: Cotter J, Baldaia C, Ferreira M, Macedo G, Pedroto I. Diagnosis and treatment of iron-deficiency anemia in gastrointestinal bleeding: A systematic review. World J Gastroenterol 2020; 26(45): 7242-7257
- URL: https://www.wjgnet.com/1007-9327/full/v26/i45/7242.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i45.7242
Anemia is a public health issue affecting approximately 25% of the world’s population[1]. Several anemia etiologies exist, and of these, iron deficiency is the most widespread and is estimated to cause up to 50% of all anemia cases[1]. Iron-deficiency anemia (IDA) often originates from blood loss from lesions in the gastrointestinal tract in men and postmenopausal women[2,3]. The prevalence of IDA among patients with gastrointestinal bleeding has been estimated to be 61%[4]. However, studies have shown that IDA is often underdiagnosed, underrecognized, and undertreated in hospitalized patients with gastrointestinal bleeding[5-7]. Moreover, evidence suggests that therapeutic approaches for iron-deficiency correction have been poorly implemented[5-7], and clinical practice guidelines are not being followed[6].
Treatment options for correcting IDA in patients with gastrointestinal bleeding include the administration of oral or intravenous iron therapy and transfusion. Oral iron is often considered a first-line treatment because it is safe, inexpensive, and convenient[8]. However, many patients with gastrointestinal bleeding have a poor response to oral iron therapeutics because of gastrointestinal side effects, malabsorption, or requirements of higher supplemental iron doses to correct iron deficiency that consequently aggravate side effects[9]. In these situations, intravenous iron formulations may be a more effective and better tolerated therapeutic alternative than oral formulations[10-13]. Proper treatment of IDA alleviates symptoms of iron deficiency, such as fatigue[14], and improves quality of life[14-16].
Clinical practice recommendations and guidelines on the management of IDA in gastrointestinal bleeding patients are still scarce[12,13,17], and there is no standardization on the management of these patients[13], in which different strategies have been used in daily clinical practice. Therefore, it is urgent to develop evidence-based recommendations to better diagnose and treat IDA in patients with gastrointestinal bleeding. The main purpose of this systematic review, developed by the Digestive Bleeding and Anemia Workgroup, is to provide recommendations for simple and uniform diagnostic and therapeutic approaches for IDA in patients with gastrointestinal bleeding.
The Digestive Bleeding and Anemia Workgroup was formed in 2016 by five key opinion leaders in gastroenterology in Portugal. The Workgroup members have significant experience in the management of gastroenterology departments and clinical practice in gastroenterology emergencies. Two meetings were held in Coimbra (Portugal) in March 2017, with the sole purpose of reaching a consensus among the five experts regarding the diagnosis and treatment of IDA in patients with gastrointestinal bleeding. A consensus was reached through discussions during the meetings and was further supported by a systematic literature search.
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement to report the results of this review[18]. Key research questions were established and approved a priori by the authors.
One reviewer performed a literature search in MEDLINE (PubMed) for studies published from January 1, 2003, to the present. The last search was conducted on April 2, 2019. Search strategies combined MeSH terms and the free text terms “gastrointestinal bleeding” crossed with “iron-deficiency anemia” and “diagnosis” or “treatment” or “management” or “prognosis” or “prevalence” or “safety” or “iron” or “transfusion” or “quality of life” or other terms. Complete search strategies are available in supplemental material SM1. The electronic database search was supplemented by searching for clinical practice guidelines on the websites of worldwide professional associations and reviewing the reference lists of the guidelines for relevant articles. Additional references were included after the peer review process.
The inclusion criteria were as follows: (1) Studies that included adults ≥ 18 years of age; (2) Studies that included patients with gastrointestinal bleeding (all etiologies); (3) Studies that included patients with IDA; (4) Systematic reviews with or without meta-analyses, clinical trials, registry-based studies, cohort studies, population-based studies, and clinical practice guidelines; (5) Studies that were written in English; and (6) Studies that were published after January 1, 2003.
The exclusion criteria were as follows: (1) Studies including children < 18 years of age; (2) Studies including animals; (3) Studies in which abstracts or full-text articles were not available; (4) Studies that included patients under critical care (emergency); (5) Studies that included patients who refuse transfusion treatment; and (6) Review articles, surveys, case reports, case series, case-control studies, comments, letters, conference abstracts or posters, or economic evaluations.
One reviewer screened all titles and abstracts retrieved from the electronic searches to identify potentially eligible articles. Full texts of the potentially eligible articles were retrieved, and the same reviewer classified the articles as eligible, potentially eligible, unclear, or not eligible as well as the reason for exclusion. A second reviewer screened all full-text articles and reviewed the classifications. The second reviewer also screened potentially eligible or unclear full-text articles, determined whether they were eligible or not eligible and recorded the reason for exclusion. Disagreements were resolved by consensus.
One reviewer extracted the relevant data. The extracted data included article or guideline characteristics (author or organization, year of publication, study type, number of patients, gastrointestinal etiology, subject sex, subject age, study period, country or region), incidence of IDA, mortality, rebleeding, rate of screening, rate of diagnosis, rate of treatment, recommended tests and thresholds for hemoglobin and/or serum ferritin for the diagnosis of IDA, target population for treatment, indications for erythrocyte transfusion, indications for intravenous iron treatment, recommendations for ferric carboxymaltose treatment, and treatment targets and timepoints.
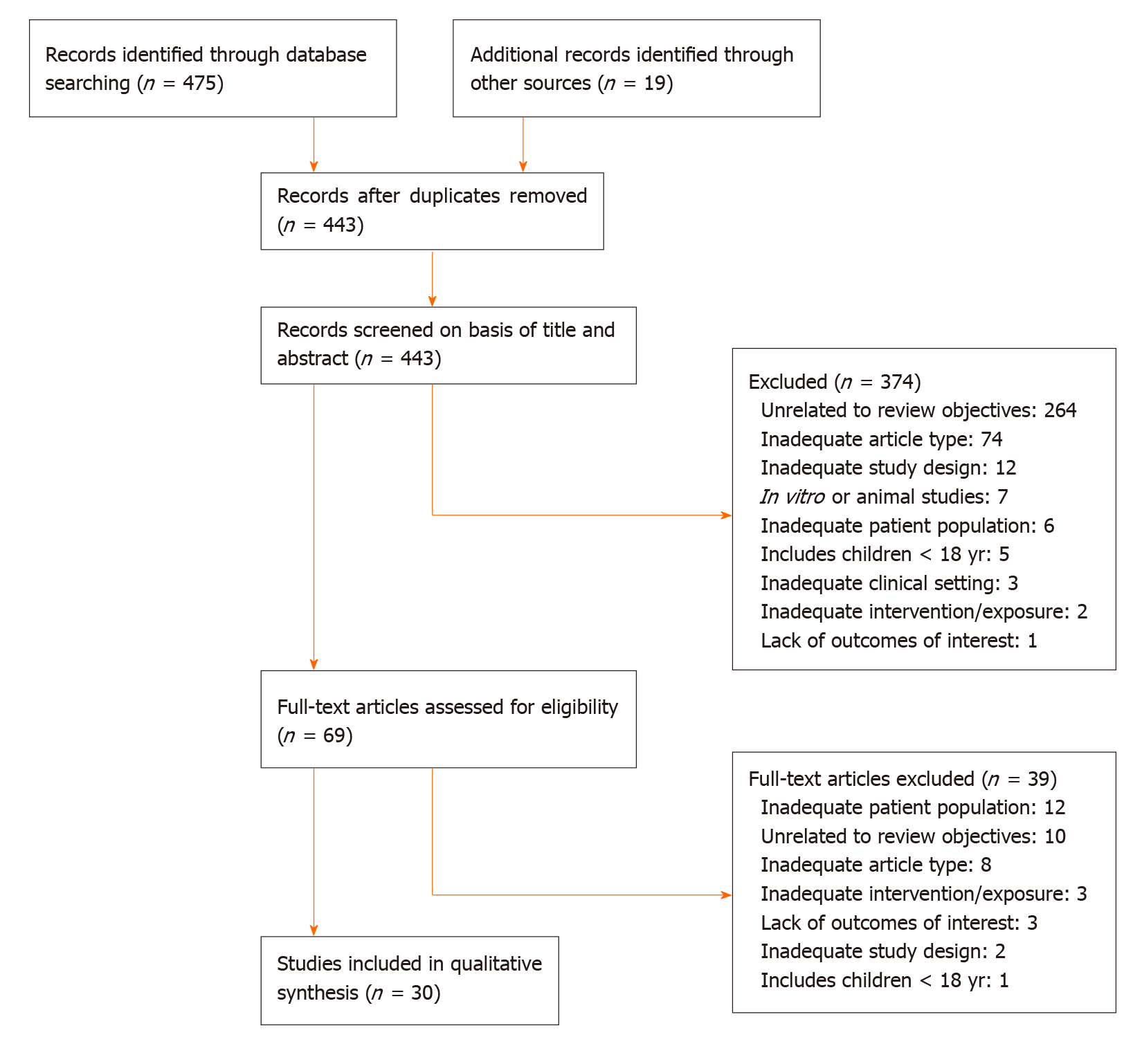
The results of the screening and selection process are shown in the flowchart in Figure 1. Our initial search yielded 494 literature citations, of which 51 were duplicates and were removed. The remaining 443 studies were screened by title and abstract. After excluding irrelevant studies, 69 studies were further assessed by reading the full texts. Of these, 38 were excluded; the remaining 31 studies were included in our analysis and comprised 17 original articles, 1 meta-analysis (Table 1), and 13 clinical practice guidelines (Table 2). Ten additional references regarding the differential diagnosis of IDA and anemia caused by inflammation, the potential role of ascorbic acid in increasing iron absorption, and the characteristics and advantages and disadvantages of oral and intravenous iron preparations were included after the peer review process [Northrop-Clewes[19], World Health Organization (WHO)[20], Shin et al[21], Infusino et al[22], Shubham et al[23], Koulaouzidis et al[24], Muñoz et al[25], Drozd et al[26], Jimenez et al[27], McDonagh et al[28]].
| Ref. | Year | Study type | No of patients | GI etiology | No of males/females | Age | Study period |
| Bager et al[5] | 2013 | Retrospective | 169 | Nonvariceal AUGIB | 86/83 | 70 (22-95)1 | 2009 |
| Bager et al[44] | 2014 | Double-blind, randomized, placebo-control | 97 | Nonvariceal AUGIB | 51/46 | 70 (23-95)2 | 2010-2013 |
| Bager et al[50] | 2014 | Double-blind, randomized, placebo-control | 97 | Nonvariceal AUGIB | 51/46 | 70 (67.4-73.1)3 | - |
| Ballester et al[45] | 2019 | Retrospective, single-center | 84 | Acute GIB | 58/26 | 68.0 (16.9)2 | 2012-2015 |
| Bosch et al[51] | 2017 | Prospective cohort | 2818 | GI diseases known to cause GIB | 1398/1420 | 63.4 (15.7)2 | 2015-2016 |
| Bosch et al[29] | 2017 | Prospective cohort | 4552 | Occult bleeding | 2266/2286 | 63.7 (17.6)2 | 2005-2015 |
| Brooklyn et al[6] | 2003 | N/A | 153 | Occult bleeding | 51/102 | 66 (45-96)2 | 2000 |
| Cheng et al[52] | 2010 | Prospective | 390 | Ulcers | 263/127 | 63 (16)2 | - |
| El-Halabi et al[7] | 2016 | Retrospective, chart review, single-center | 307 | Any GIB | 130/177 | 66.2 (18.6)2 | 2011-2012 |
| Geisser et al[46] | 2010 | Phase I/II, multicenter, open-label, multiple-dose | 46 | Bleeding due to GI disorder | 10/36 | 42.9 (11.0)2 | 2003-2004 |
| Jairath et al[35] | 2010 | Meta-analysis | - | AUGIB | 2731/1710 | Early RBC 67.9 (16.51)2; no early RBC 63.4 (19.19)2 | 2007 |
| Jairath et al[36] | 2015 | Pragmatic, multicentric, open-label, randomized feasibility trial | 936 | AUGIB | 566/370 | Liberal 60.4 (20.0)2; restrictive 58.0 (20.3)2 | 2012-2013 |
| Restellini et al[31] | 2013 | Observational, registry-based | 1677 | Nonvariceal AUGIB | 1035/642 | 66.2 (16.8)2 | 1999-2002 |
| Rockey et al[32] | 2017 | Prospective cohort | 1460 | Acute or chronic GIB | 899/561 | 53 (14)2 | 2006-2011 |
| Salvadori et al[47] | 2016 | Retrospective | 38 | GI chronic blood loss | 22/16 | 78 (54-94)4 | 2014-2015 |
| Schröder et al[48] | 2004 | N/A | 31 | GI blood loss | 12/19 | 43.8 (18.0)2 | - |
| Subramaniam et al[30] | 2016 | Retrospective cohort | 2360 | Nonvariceal AUGIB | 1505/852 | 70 (56-81)4 | 2008-2010 |
| Villanueva et al[37] | 2013 | RCT | 889 | Severe AUGIB | - | - | 2003-2009 |
| Guideline organization/society/authors | Year | GI etiology | Origin | Level of development |
| Iron-Deficiency Anemia Working Group Consensus Report[34] | 2017 | IBD and GIB | Turkey | Scientific committee/expert group |
| The International Consensus Upper Gastrointestinal Bleeding Conference Group[38] | 2010 | Nonvariceal UGIB | International | Scientific committee/expert group |
| Dahlerup et al[17] Guideline approved by the Danish Society of Gastroenterology and Hepatology | 2014 | GIB, various etiologies | Denmark | Independent authors and approved by a professional organization/society |
| Baveno IV Consensus Workshop[43] | 2015 | Variceal bleeding | International | Scientific committee/expert group |
| European Crohn's and Colitis Organization[10] | 2015 | IBD | Europe | Professional organization/society |
| Gasche et al[11] | 2007 | IBD | Europe | Scientific committee/expert group |
| British Society of Gastroenterology[12] | 2011 | - | United Kingdom | Professional organization/society |
| Hong Kong Society of Gastroenterology, the Hong Kong IBD Society, the Hong Kong Society of Digestive Endoscopy, and the Hong Kong Red Cross Blood Transfusion Service[13] | 2018 | Acute and chronic GIB | Hong Kong | Professional organization |
| The 2018 Patient Blood Management International Consensus Conference[39] | 2019 | Acute GIB | Germany | Scientific committee/expert group |
| British Society of Gastroenterology[40] | 2019 | ALGIB | United Kingdom | Professional organization |
| National Institute for Health and Care Excellence[41] | 2015 | - | United Kingdom | Professional organization |
| Strate et al[42] | 2016 | ALGIB | United States and Israel | Independent authors |
| World Health Organization[33] | 2001 | - | International | Professional organization |
Diagnosis: Hemoglobin and iron status should be routinely evaluated in all patients with gastrointestinal bleeding.
Although IDA is common in patients with gastrointestinal bleeding, the rate of IDA screening is generally low[6,7]. Moreover, patients with IDA are less likely to be investigated than patients with iron deficiency according to published guidelines[6].
The recommendation that all patients with gastrointestinal bleeding should be assessed for hemoglobin and iron status is based on data from studies reporting a high prevalence of anemia and IDA and a high incidence of mortality among patients with gastrointestinal bleeding. Two retrospective studies assessed the prevalence of IDA in gastrointestinal bleeding. The first study from Bager et al[5] included 169 patients with nonvariceal acute upper gastrointestinal bleeding (AUGIB) and found that 82% of the patients had anemia at hospital discharge. The second study was a single-center study by El-Halabi et al[7],which included 307 patients with any gastrointestinal bleeding and reported that 47.4% of the patients had IDA.
Moreover, mortality among patients with gastrointestinal bleeding was reported in two observational studies[29,30]. Among patients with nonvariceal upper gastrointestinal bleeding (UGIB), 30 d mortality ranged from 4.9% to 5.4%[30,31], 1-year mortality was 13.9%, and 2-year mortality was 19.6%[30]. For patients with occult gastrointestinal bleeding, 10-year mortality was 13%[29]. In addition, a study by Rockey et al[32] found that 30 d mortality was higher among patients with acute bleeding (7%) than among patients with chronic bleeding (0%).
The WHO defines anemia as a hemoglobin level below 13 g/dL in men and below 12 g/dL in nonpregnant women[33]. Hemoglobin together with serum ferritin are commonly recommended by international and national guidelines as markers for IDA, and most guidelines agree with the cutoff value for hemoglobin as defined by the WHO[10,11,17,34]. According to several guidelines, the recommended serum ferritin cutoff value for diagnosing IDA ranges from 12 to 30 µg/L in the absence of inflammation and from 30 to above 100 µg/L in the presence of inflammation[10-12,17,34]. However, as serum ferritin is an acute-phase reactant, additional markers, such as transferrin saturation, may be required to confirm IDA. Three guidelines recommend measuring transferrin saturation, and the suggested cutoff for diagnosing IDA is below 16%[11,34] or below 20% in the presence of inflammation[10]. To the differential diagnosis of IDA and anemia caused by inflammation, WHO recommends to assess both the concentration of serum transferrin receptor and the serum transferrin receptor/log ferritin ratio[19,20] or log (serum transferrin receptor/ferritin) ratio[19]. Shin and colleagues reported that serum transferrin receptor/log ferritin ratio enabled an accurate diagnosis of IDA, as well as the differential diagnosis between IDA and anemia of chronic disease[21]. Infusino et al[22]. performed a meta-analysis and suggested that both serum transferrin receptor and the serum transferrin receptor/log ferritin ratio are useful to distinguish between patients with IDA and anemia of chronic disease, adding that serum transferrin receptor may be more efficient than the latter[22]. Koulaouzidis and colleagues critically reviewed the use of serum transferrin receptor as a marker for the evaluation of iron stores and suggested a cutoff value of 2.5 mg/L (29.5 nmoL/L) for the identification of IDA[24]. Nevertheless, serum transferrin receptor levels should not be used alone to distinguish between patients with or without iron deficiency in the presence of inflammation, because their levels are affected by the rate of erythropoiesis from any cause[20].
Treatment for IDA should be considered in patients with one or more of the following conditions: Evidence or clinical suspicion of gastrointestinal bleeding, i.e., the presence of melena or hematochezia or hematemesis or if there was IDA (hemoglobin < 13 g/dL in men, < 12 g/dL in women) or a positive fecal occult blood test; previously diagnosed but untreated anemia; hemoglobin levels > 7 g/dL and ≤ 10 g/dL and no indication for erythrocyte transfusion (see indications for erythrocyte transfusion); hemodynamic stability, i.e., the absence of visible active hemorrhaging, with systolic blood pressure > 100 mmHg and a heart rate < 100 beats per minute; comorbidities (for instance, heart or kidney disease); concomitant IDA with erythropoiesis-stimulating agents.
Iron deficiency should be corrected by iron treatment, with the goal of restoring hemoglobin levels, serum ferritin levels, and transferrin saturation to normal levels to avoid or reduce the need for erythrocyte transfusion. The decision to initiate iron treatment should be based on the patient’s history and symptoms and should consider comorbidities, hemodynamic stability, hemoglobin level and additional treatments. Gasche et al[11] suggested that the absolute indications for initiating intravenous iron treatment include hemoglobin levels below 10 g/dL, intolerance or inappropriate response to oral iron, severe intestinal disease activity, concomitant use of an erythropoietic agent, and patient preference. In patients who are not considered for iron treatment, other treatment options should be considered.
The decision to transfuse erythrocytes should be individualized and based on multiple factors related to the patient’s clinical status. In general, erythrocyte transfusion should be considered in patients with: Hemoglobin levels below 7 g/dL; hemoglobin levels above 7 g/dL and below 8 g/dL and comorbidities or under postoperative care; hemoglobin levels between 8 g/dL and below 10 g/dL and symptomatic anemia (i.e., resulting in asthenia and a change in attention capacity), persistent bleeding, or heart disease; in exceptional cases, in patients with hemoglobin levels above 10 g/dL.
Treatment outcomes will depend on etiology, duration, and volume of blood loss. In general, a hemoglobin target of 7 to 9 g/dL should be considered in patients with levels below 7 g/dL and no comorbidities. For patients with comorbid illnesses (cardiovascular disease), hemoglobin target levels of 10 g/dL or above should be considered.
Current clinical practice restricts erythrocyte transfusion to special situations, such as severe anemia or anemia with comorbidities. Treatment with erythrocyte transfusion in patients with UGIB was associated with an increased risk of rebleeding in a meta-analysis of three randomized controlled trials (RCTs)[35] and one observational study[31]. Moreover, erythrocyte transfusion may also be linked to increased mortality, although the evidence is less conclusive than that for rebleeding. The abovementioned meta-analysis found an association between erythrocyte transfusion and increased mortality[35], whereas the observational study did not find an association[31]. Two large RCTs studied the hemoglobin threshold for initiating erythrocyte transfusion in patients with UGIB and its association with treatment complications. The study by Jairath et al[36] did not find any association between a restrictive transfusion strategy (8 g/dL) and rebleeding or mortality. The study by Villanueva et al[37] showed that a restrictive transfusion strategy (7 g/dL) was associated with better survival, a lower rate of further bleeding, and fewer complications than a more liberal transfusion strategy (9 g/dL). Similarly, an observational study by Subramaniam et al[30] found that for patients with hemoglobin levels above 9 g/dL, there was an association between the number of red blood cell units transfused and increased odds of rebleeding.
Most guidelines recommend a restrictive transfusion strategy for patients without comorbid illnesses, although the indications to initiate transfusion vary[10,13,38-42]. Most guidelines recommend transfusion when hemoglobin levels fall below 7 g/dL[10,38,40,41] and the target hemoglobin level is above 7 g/dL[40-43]. The recommended hemoglobin threshold and target levels for the included guidelines are shown in Table 3.
| Professional association | GI etiology | Threshold Hb, g/dL | Threshold Hb cardiovascular disease, g/dL | Target Hb, g/dL | Target Hb cardiovascular disease, g/dL |
| The International Consensus Upper Gastrointestinal Bleeding Conference Group[38] | Nonvariceal UGIB | < 7 | - | - | - |
| Baveno IV Consensus Workshop[43] | Variceal bleeding | 7-8 | - | - | - |
| European Crohn's and Colitis Organization[10] | IDA in IBD | < 7 | - | - | - |
| Hong Kong Society of Gastroenterology, the Hong Kong IBD Society, the Hong Kong Society of Digestive Endoscopy, and the Hong Kong Red Cross Blood Transfusion Service[13] | Acute UGIB | 7-8 | 9-10 | - | - |
| The 2018 Patient Blood Management International Consensus Conference[39] | Acute GIB | 7-8 | - | - | - |
| British Society of Gastroenterology[40] | Acute LGIB | < 7 | 8 | 7-9 | 10 |
| National Institute for Health and Care Excellence[41] | N/A | < 7 | 8 | 7-9 | 8-10 |
| Strate et al[42] | Acute LGIB | - | 9 | > 7 | > 9 |
In addition to the recommendations mentioned above, there are exceptions in which a more liberal transfusion strategy can be adopted, such as in patients with comorbidities such as cardiovascular disease or massive bleeding[13,40-42]. However, the threshold differs between the guidelines, such as hemoglobin concentrations above 8 g/dL in patients with a history of cardiovascular disease[40] or acute coronary syndrome[41], above 9 g/dL in patients with cardiovascular ischemia or massive bleeding[42], or between 9 and 10 g/dL in patients with symptomatic coronary artery disease[13].
The decision to initiate erythrocyte transfusion should be decided on an individual basis after carefully weighing the potential benefits and risks. Patients with heart disease, symptomatic anemia, or persistent bleeding or those receiving postoperative care generally benefit from a less restrictive transfusion strategy. Moreover, the goal of erythrocyte transfusion should be to restore the hemoglobin concentration to a safe level and should generally be followed by iron supplementation to replenish iron stores.
Intravenous iron should be considered for patients undergoing oral iron supplementation but not achieving IDA correction, reporting treatment-emergent adverse events, or reporting nonadherence to oral supplementation.
The goal of iron therapy is to normalize hemoglobin levels, serum ferritin levels, and transferrin saturation to avoid the need for erythrocyte transfusion. Two treatment approaches are available for restoring iron levels in patients with gastrointestinal bleeding: Oral and intravenous iron. Oral iron is the conventional treatment for IDA but has been associated with gastrointestinal side effects, malabsorption, and nonadherence. Intravenous iron, on the other hand, is generally well tolerated and considered safe[44-48]. One of the identified studies directly compared the effects of intravenous iron with oral iron in patients with nonvariceal AUGIB and found that both treatments were equally effective in restoring hemoglobin levels[44]. These findings were supported by clinical trials and retrospective studies showing that intravenous iron (ferric carboxymaltose or iron sucrose) was effective in restoring hemoglobin levels in patients with AUGIB and acute lower gastrointestinal bleeding[45], chronic gastrointestinal bleeding[47], and gastrointestinal disorder[46]. Intravenous iron (ferric carboxymaltose), however, has been shown to be more efficient in restoring ferritin levels and iron stores in patients with nonvariceal AUGIB than oral iron[44]. Although evidence is still scarce, in cases of suspected malabsorption, the co-administration of oral ascorbic acid may be considered to increase oral iron absorption[12,13,23]. Some pharmacological characteristics of worldwide available oral and intravenous iron preparations, as well as their advantages and disadvantages are shown in Table 4.
| Type of preparation | Advantages | Disadvantages |
| Oral | Safe; readily available (does not require a prescription); administered at home; inexpensive; effective when intestinal absorption is not impaired; no need for venous access and infusion monitoring; eliminates the risk of infusion reactions | Slower repletion of iron stores; Intestinal absorption is relatively low, and may be impaired by concomitant food and medications; gastrointestinal adverse events, including constipation, dyspepsia, bloating, nausea, diarrhoea, heartburn, reducing tolerance and adherence to treatment; compliance difficulted by high pill burden (typically three tablets/day) and gastrointestinal intolerance; diminished efficacy when the uptake is impaired (e.g., in celiac disease, autoimmune gastritis, anemia of chronic disease, or post–gastric or duodenal resection) |
| Ferric hydroxide polymaltose complex | ||
| Sodium ferric gluconate | ||
| Ferrous gluconate | ||
| Ferrous sulfate | ||
| Ferrous fumarate | ||
| Intravenous | Fast repletion of iron stores; safe when avoiding preparations with dextran; very effective; gastrointestinal adverse events less frequent; ferric carboxymaltose, iron isomaltoside 1000, and ferumoxytol are considered more stable | Administration by a health care professional, requiring clinic visits; increased costs per dose, but fewer doses required; risk of iron overload and transient increase in oxidative stress; risk of anaphylactic reactions with dextran-containing preparations; risk of hypersensitivity reactions |
| Ferric gluconate | ||
| Iron sucrose | ||
| Low molecular weight iron dextran | ||
| Ferric carboxymaltose | ||
| Iron isomaltoside 1000 | ||
| Ferumoxytol |
The decision to treat patients with intravenous iron should be based on the patient’s clinical history and preference. Moreover, international guidelines recommend intravenous iron as the first-line therapy for patients with inflammatory bowel disease[10,11,13,17,34], intolerance to oral iron[10-13,17], or poor response to oral iron[13,17], or according to patient preference[11].
The recommended maximum cumulative dose of ferric carboxymaltose is 1000 mg of iron (20 mL of ferric carboxymaltose) per week. Ferric carboxymaltose treatment can be administered one or two times, with an interval of at least one week (in cases in which patient iron dose requirements are > 1000 mg).
Patients should be monitored during the infusion and for 30 min after each administration of intravenous ferric carboxymaltose.
Ferric carboxymaltose treatment should not be considered in patients with one or more of the following conditions: (1) active bleeding; (2) first-trimester pregnancy; (3) active bacterial infection; (4) hemochromatosis or hemosiderosis; (5) Evidence of iron overload (ferritin levels > 800 µg/L and transferrin saturation > 50%); (6) hypersensitivity to ferric carboxymaltose preparations or any of its excipients; and (7) known severe hypersensitivity to other intravenous iron formulations.
Concentrations of ferric carboxymaltose up to 1000 mg have been administered in clinical trials without serious adverse events[44-47]. The recommendations regarding the administration of ferric carboxymaltose are based on the Digestive Bleeding and Anemia Workgroup’s clinical experience and the Summary of Product Characteristics[49]. In our opinion, the first step before administering ferric carbo-xymaltose is to evaluate the patients’ iron requirements. Calculations according to patient body weight and hemoglobin level are shown in Table 5. The second step is to calculate the required dosage using the criteria in Table 5 while considering the following conditions: A maximum single dose of ferric carboxymaltose containing 1000 mg of iron (without exceeding 20 mg/kg of body weight) with an infusion duration of 15 min.
| Hemoglobin (g/dL) | Hemoglobin (mmoL/L) | Patient body weight (below 35 kg) | Patient body weight (35 kg to 70 kg) | Patient body weight (70 kg and above) |
| < 10 | < 6.2 | 500 mg | 1500 mg | 2000 mg |
| 10 to 14 | 6.2 to 8.7 | 500 mg | 1000 mg | 1500 mg |
| ≥ 14 | ≥ 8.7 | 500 mg | 500 mg | 500 mg |
The recommendation to monitor patients for 30 min after administration reflects common clinical practice and the findings from a clinical trial showing that ferric carboxymaltose may induce adverse events during drug infusion[47].
After administration of intravenous iron and in the absence of persistent bleeding, the goal of IDA treatment is to increase the hemoglobin level by 1 to 2 g/dL within 2 to 4 wk and maintain a serum ferritin level ≥ 50 ng/mL (in the absence of inflammatory conditions), increase the number of reticulocytes within 3 to 5 d, and maintain transferrin saturation ≥ 30% for 4 to 6 mo after normalization of hemoglobin levels and once the etiological cause of anemia has been corrected.
Clinical trials have shown that increasing hemoglobin levels by 1 to 2 g/dL within 4 wk can be achieved with the administration of intravenous iron (ferric carbo-xymaltose)[44,46]. Similarly, a retrospective study of 38 patients with chronic gastrointestinal bleeding reported a median hemoglobin increase of 2.4 g/dL at 5 wk after ferric carboxymaltose treatment[47]. Moreover, an 2 g/dL increase in hemoglobin levels within 4 wk is also considered an acceptable speed of response according to several guidelines[10,11,34].
Two guidelines recommend serum ferritin target levels. Gasche et al[11] recommend maintaining serum ferritin above 100 µg/L, whereas the European Crohn’s and Colitis Organization guidelines recommend restoring serum ferritin to normal[10].
Reticulocytes are a relatively new biomarker for assessing response to iron treatment. Evidence supporting the monitoring of reticulocytes and their relevant target levels was therefore limited to one small clinical trial and recommendations in one guideline. Geisser et al[46] measured reticulocyte levels at baseline and 14 d after ferric carboxymaltose supplementation and found that the levels increased from 60 × 109/L to 89 × 109/L. Moreover, one guideline recommends measuring reticulocytes at one week after iron treatment to confirm an increase compared to the level before treatment[17]. Generally, reticulocyte levels increase within one week in response to intravenous treatment. We recommend measuring the number of reticulocytes after 3-5 d to assess whether a proper response to initial intravenous iron has been achieved to evaluate further treatment.
Only one guideline recommends measuring transferrin saturation to assess therapeutic response. This guideline recommends target levels of transferrin saturation between 16% and 50% after anemia treatment[11]. Transferrin saturation may temporarily increase in response to intravenous iron treatment and should therefore not be used for monitoring initial treatment response.
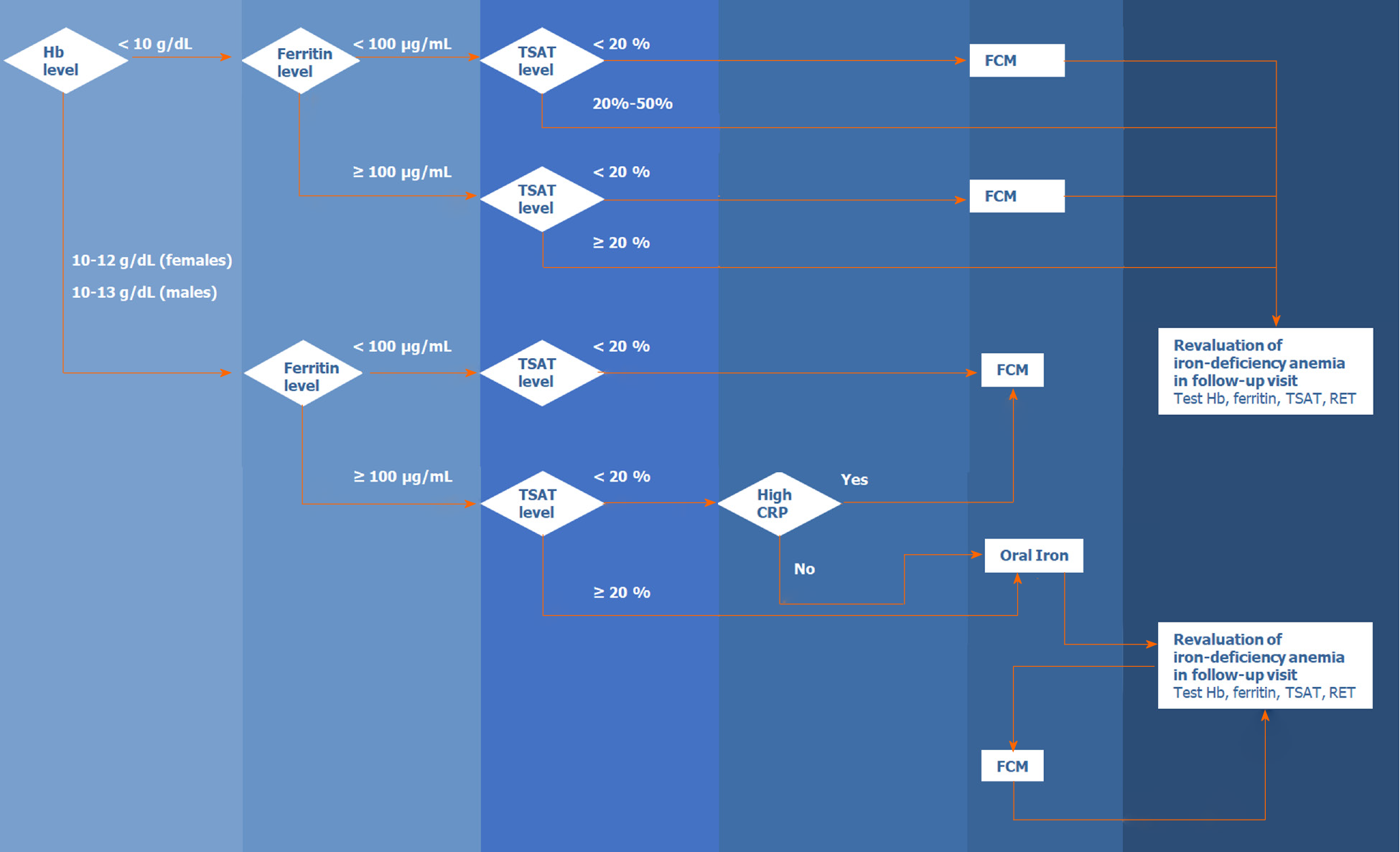
Figure 2 presents the algorithm for the diagnosis and treatment of IDA in patients with acute or chronic gastrointestinal bleeding. The algorithm is based on the literature and the experience of the members of the Digestive Bleeding and Anemia Workgroup. This algorithm should be implemented during the hospital stay and at follow-up visits after patient discharge and is not applicable for emergencies or critical care.
This consensus recommendation had several limitations. First, the quality of the identified studies was generally low, and we found few RCTs on IDA and gastrointestinal bleeding. Therefore, the recommendations are mainly based on observational studies, guidelines, and clinical experience of the members of the Digestive Bleeding and Anemia Workgroup. Second, studies on lower gastrointestinal bleeding are scarce, and our recommendations are mainly based on studies on patients with UGIB. Third, the search strategy included only studies indexed in MEDLINE (PubMed), published from January 2003 until April 2019, and written in English.
Diagnosing and treating patients with IDA is challenging in clinical practice. IDA in patients with gastrointestinal bleeding should be diagnosed and treated promptly to improve the quality of life and reduce morbidity and, eventually, mortality. This consensus recommendation provides a starting point for diagnosing and treating IDA in patients with gastrointestinal bleeding by gastroenterologists and other physicians in daily clinical practice and should serve to optimize the decision-making process for the management of these patients. We believe that this guideline may facilitate improvements in the management of IDA in patients with gastrointestinal bleeding, which ultimately translates to improved health outcomes for these patients.
Anemia is a public health issue affecting approximately 25% of the world’s population, being often caused by iron deficiency. Iron-deficiency anemia (IDA) often originates from blood loss from lesions in the gastrointestinal tract in men and postmenopausal women, and its prevalence among patients with gastrointestinal bleeding has been estimated to be 61%. However, studies have shown that IDA is often underdiagnosed, underrecognized, and undertreated in hospitalized patients with gastrointestinal bleeding, and that therapeutic approaches for iron-deficiency correction have been poorly implemented, and clinical practice guidelines are not being followed. Furthermore, clinical practice recommendations and guidelines on the management of IDA in gastrointestinal bleeding patients are still scarce and there is no standardization on the management of these patients. Therefore, standardized recommendations on the management of IDA in gastrointestinal bleeding patients, based on a systematic review of the current evidence, are needed.
Given the scarcity of clinical practice recommendations and guidelines on the management of IDA in gastrointestinal bleeding patients, and the need of standardization regarding the management of these patients, it is urgent to develop evidence-based standardized diagnostic and therapeutic approaches on the management of patients with IDA due to gastrointestinal bleeding.
With this study, we aimed to review the current evidence and guidelines concerning IDA management in gastrointestinal bleeding patients to develop recommendations for its diagnosis and therapy.
Five gastroenterology experts formed the Digestive Bleeding and Anemia Workgroup and conducted a systematic literature search in PubMed and professional association websites. MEDLINE (via PubMed) searches combined MeSH terms and the keywords “gastrointestinal bleeding” with “iron-deficiency anemia” and “diagnosis” or “treatment” or “management” or “prognosis” or “prevalence” or “safety” or “iron” or “transfusion” or “quality of life”, or other terms to identify relevant articles reporting the management of IDA in patients over the age of 18 years with gastrointestinal bleeding; retrieved studies were published in English between January 2003 and April 2019. Worldwide professional association websites were searched for clinical practice guidelines. Reference lists from guidelines were reviewed to identify additional relevant articles. The recommendations were developed by consensus during two meetings and were supported by the published literature identified during the systematic search.
From 494 Literature citations found during the initial literature search, 17 original articles, one meta-analysis, and 13 clinical practice guidelines were analyzed. Ten additional references were included after the peer review process. Based on the published evidence and clinical experience, the workgroup developed the following ten recommendations for the management of IDA in patients with gastrointestinal bleeding: (1) evaluation of hemoglobin and iron status; (2) laboratory testing; (3) target treatment population identification; (4) indications for erythrocyte transfusion; (5) treatment targets for erythrocyte transfusion; (6) indications for intravenous iron; (7) dosages, (8) monitoring; (9) indications for intravenous ferric carboxymaltose treatment; and (10) treatment targets and monitoring of patients. The workgroup also proposed a summary algorithm for the diagnosis and treatment of IDA in patients with acute or chronic gastrointestinal bleeding, which should be implemented during the hospital stay and follow-up visits after patient discharge.
Ten evidence-based recommendations were developed for screening, treatment indications, appropriate therapies, and treatment goals of IDA in patients with acute or chronic gastrointestinal bleeding. An algorithm for the diagnosis and treatment of these patients was also developed, based on the literature and on the experience of the members of the Digestive Bleeding and Anemia Workgroup. Therefore, this work serves as a starting point for diagnosing and treating IDA in patients with gastrointestinal bleeding by gastroenterologists and other physicians in daily clinical practice and should serve to optimize the decision-making process for the management of these patients. This guideline may facilitate improvements in the management of IDA in patients with gastrointestinal bleeding, which ultimately may improve health outcomes in these patients.
This consensus recommendation provides a starting point for clinicians to better diagnose and treat IDA in patients with gastrointestinal bleeding. Nevertheless, more studies, specially RCTs on IDA and gastrointestinal bleeding are needed to further improve the management of these patients.
The authors would like to thank Scientific Toolbox Consulting (Lisbon, Portugal) for providing medical writing support.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vela D S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12:444-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1381] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 2. | Kepczyk T, Kadakia SC. Prospective evaluation of gastrointestinal tract in patients with iron-deficiency anemia. Dig Dis Sci. 1995;40:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Park JS, Park DI, Park SK, Choi JS, Kim YH, Chang DK, Son HJ, Kim JE, Kim JO, Lee SH, Kim HS, Sin JE, Lee SG, Lee SY, Park SJ, Park CH, Baek IH, Jang BI, Jeen YT, Huh KC. Endoscopic evaluation of significant gastrointestinal lesions in patients with iron deficiency with and without anaemia: a Korean Association for the Study of Intestinal Disease study. Intern Med J. 2009;39:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Planella de Rubinat M, Teixidó Amorós M, Ballester Clau R, Trujillano Cabello J, Ibarz Escuer M, Reñé Espinet JM. [Incidence and predictive factors of iron deficiency anemia after acute non-variceal upper gastrointestinal bleeding without portal hypertension]. Gastroenterol Hepatol. 2015;38:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Bager P, Dahlerup JF. Lack of follow-up of anaemia after discharge from an upper gastrointestinal bleeding centre. Dan Med J. 2013;60:A4583. [PubMed] |
| 6. | Brooklyn TN, Di Mambro AJ, Haslam N. Patients over 45 years with iron deficiency require investigation. Eur J Gastroenterol Hepatol. 2003;15:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 7. | El-Halabi MM, Green MS, Jones C, Salyers WJ Jr. Under-diagnosing and under-treating iron deficiency in hospitalized patients with gastrointestinal bleeding. World J Gastrointest Pharmacol Ther. 2016;7:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011;4:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 9. | Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 482] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 10. | Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, Gomollon F, Iqbal T, Katsanos K, Koutroubakis I, Magro F, Savoye G, Stein J, Vavricka S; European Crohn’s and Colitis Organisation [ECCO]. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 11. | Gasche C, Berstad A, Befrits R, Beglinger C, Dignass A, Erichsen K, Gomollon F, Hjortswang H, Koutroubakis I, Kulnigg S, Oldenburg B, Rampton D, Schroeder O, Stein J, Travis S, Van Assche G. Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:1545-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (3)] |
| 12. | Goddard AF, James MW, McIntyre AS, Scott BB; British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 484] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 13. | Mak LY, Lau CW, Hui YT, Ng C, Shan E, Li MK, Lau JY, Chiu PW, Leong HT, Ho J, Wu JC, Lee CK, Leung WK. Joint recommendations on management of anaemia in patients with gastrointestinal bleeding in Hong Kong. Hong Kong Med J. 2018;24:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36:657-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 868] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 15. | Comin-Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Lüscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study. Eur Heart J. 2013;34:30-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, Chopey IV, Gutzwiller FS, Riopel L, Gasche C; FERGI Study Group. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011; 141: 846-853. e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 17. | Dahlerup JF, Eivindson M, Jacobsen BA, Jensen NM, Jørgensen SP, Laursen SB, Rasmussen M, Nathan T. Diagnosis and treatment of unexplained anemia with iron deficiency without overt bleeding. Dan Med J. 2015;62:C5072. [PubMed] |
| 18. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47037] [Article Influence: 2939.8] [Reference Citation Analysis (0)] |
| 19. | Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response--lessons from malaria and human immunodeficiency virus. Ann Clin Biochem. 2008;45:18-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | World Health Organization (WHO). Serum transferrin receptor levels for the assessment of iron status and iron deficiency in populations. 2014; WHO/NMH/NHD/EPG/14.6: 1-4. Available from: https://apps.who.int/iris/bitstream/handle/10665/133707/WHO_NMH_NHD_EPG_14.6_eng.pdf?sequence=1&isAllowed=y. |
| 21. | Shin DH, Kim HS, Park MJ, Suh IB, Shin KS. Utility of Access Soluble Transferrin Receptor (sTfR) and sTfR/log Ferritin Index in Diagnosing Iron Deficiency Anemia. Ann Clin Lab Sci. 2015;45:396-402. [PubMed] |
| 22. | Infusino I, Braga F, Dolci A, Panteghini M. Soluble transferrin receptor (sTfR) and sTfR/log ferritin index for the diagnosis of iron-deficiency anemia. A meta-analysis. Am J Clin Pathol. 2012;138:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Shubham K, Anukiruthika T, Dutta S, Kashyap AV, Moses JA, Anandharamakrishnan C. Iron deficiency anemia: a comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci Tech. 2020;99:58-75. [RCA] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 24. | Koulaouzidis A, Said E, Cottier R, Saeed AA. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Muñoz M, Gómez-Ramírez S, Besser M, Pavía J, Gomollón F, Liumbruno GM, Bhandari S, Cladellas M, Shander A, Auerbach M. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. 2017;15:422-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 26. | Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron Therapy in Patients with Heart Failure and Iron Deficiency: Review of Iron Preparations for Practitioners. Am J Cardiovasc Drugs. 2017;17:183-201. [PubMed] [DOI] [Full Text] |
| 27. | Jimenez K, Kulnigg-Dabsch S, Gasche C. Management of iron deficiency anemia. Gastroenterol Hepatol (N Y). 2015;11(4):241-250. [PubMed] |
| 28. | McDonagh T, Macdougall IC. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail. 2015;17:248-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Bosch X, Montori E, Guerra-García M, Costa-Rodríguez J, Quintanilla MH, Tolosa-Chapasian PE, Moreno P, Guasch N, López-Soto A. A comprehensive evaluation of the gastrointestinal tract in iron-deficiency anemia with predefined hemoglobin below 9mg/dL: A prospective cohort study. Dig Liver Dis. 2017;49:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Subramaniam K, Spilsbury K, Ayonrinde OT, Latchmiah F, Mukhtar SA, Semmens JB, Leahy MF, Olynyk JK. Red blood cell transfusion is associated with further bleeding and fresh-frozen plasma with mortality in nonvariceal upper gastrointestinal bleeding. Transfusion. 2016;56:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Restellini S, Kherad O, Jairath V, Martel M, Barkun AN. Red blood cell transfusion is associated with increased rebleeding in patients with nonvariceal upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2013;37:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Rockey DC, Hafemeister AC, Reisch JS. Acute on chronic gastrointestinal bleeding: a unique clinical entity. J Investig Med. 2017;65:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | United Nations Children’s Fund, United Nations University, WHO Iron deficiency anemia assessment, prevention and control. A Guide for Programme Managers 2001. Available from: https://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf. |
| 34. | Akpınar H, Çetiner M, Keshav S, Örmeci N, Törüner M. Diagnosis and treatment of iron deficiency anemia in patients with inflammatory bowel disease and gastrointestinal bleeding: iron deficiency anemia working group consensus report. Turk J Gastroenterol. 2017;28:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Jairath V, Hearnshaw S, Brunskill SJ, Doree C, Hopewell S, Hyde C, Travis S, Murphy MF. Red cell transfusion for the management of upper gastrointestinal haemorrhage. Cochrane Database Syst Rev. 2010;(9):CD006613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, Stanley AJ, Everett SM, Bailey AA, Dallal H, Greenaway J, Le Jeune I, Darwent M, Church N, Reckless I, Hodge R, Dyer C, Meredith S, Llewelyn C, Palmer KR, Logan RF, Travis SP, Walsh TS, Murphy MF. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 37. | Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, Guarner-Argente C, Santaló M, Muñiz E, Guarner C. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1068] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 38. | Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P; International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 710] [Article Influence: 47.3] [Reference Citation Analysis (1)] |
| 39. | Mueller MM, Van Remoortel H, Meybohm P, Aranko K, Aubron C, Burger R, Carson JL, Cichutek K, De Buck E, Devine D, Fergusson D, Folléa G, French C, Frey KP, Gammon R, Levy JH, Murphy MF, Ozier Y, Pavenski K, So-Osman C, Tiberghien P, Volmink J, Waters JH, Wood EM, Seifried E; ICC PBM Frankfurt 2018 Group. Patient Blood Management: Recommendations From the 2018 Frankfurt Consensus Conference. JAMA. 2019;321:983-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 409] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 40. | Oakland K, Chadwick G, East JE, Guy R, Humphries A, Jairath V, McPherson S, Metzner M, Morris AJ, Murphy MF, Tham T, Uberoi R, Veitch AM, Wheeler J, Regan C, Hoare J. Diagnosis and management of acute lower gastrointestinal bleeding: guidelines from the British Society of Gastroenterology. Gut. 2019;68:776-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 41. | Padhi S, Kemmis-Betty S, Rajesh S, Hill J, Murphy MF; Guideline Development Group. Blood transfusion: summary of NICE guidance. BMJ. 2015;351:h5832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Strate LL, Gralnek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. 2016;111:755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 43. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2282] [Article Influence: 228.2] [Reference Citation Analysis (3)] |
| 44. | Bager P, Dahlerup JF. Randomised clinical trial: oral vs. intravenous iron after upper gastrointestinal haemorrhage--a placebo-controlled study. Aliment Pharmacol Ther. 2014;39:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Ballester-Clau R, Torres Vicente G, Voltà-Pardo T, López-Barroso L, Cucala-Ramos M, Reñé-Espinet JM, Planella de Rubinat M. Clinical experience with ferric carboxymaltose in the management of anemia in acute gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2019;31:116-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Geisser P, Rumyantsev V. Pharmacodynamics and safety of ferric carboxymaltose: a multiple-dose study in patients with iron-deficiency anaemia secondary to a gastrointestinal disorder. Arzneimittelforschung. 2010;60:373-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Salvadori U, Sandri M, Melli C, Polese F, Simeoni M, Capelli S, Al-Khaffaf A. Ferric carboxymaltose reduces the number of red blood cell units transfused and allows transfusion independence to be obtained in patients with iron deficiency anemia secondary to gastrointestinal chronic blood loss. Transfusion. 2016;56:2720-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Schröder O, Schrott M, Blumenstein I, Jahnel J, Dignass AU, Stein J. A study for the evaluation of safety and tolerability of intravenous high-dose iron sucrose in patients with iron deficiency anemia due to gastrointestinal bleeding. Z Gastroenterol. 2004;42:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Infarmed. Summary of product characteristics (SmPC): Ferinject, Vitor France; 2015 [cited 2019 Jul 9]. Available from: http://app7infarmedpt/infomed/download_ficheirophp?med_id=41653&tipo_doc=fi. |
| 50. | Bager P, Dahlerup JF. Patient-reported outcomes after acute nonvariceal upper gastrointestinal hemorrhage. Scand J Gastroenterol. 2014;49:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Bosch X, Montori E, Guerra-García M, Costa-Rodríguez J, Quintanilla MH, Tolosa-Chapasian PE, Moreno P, Guasch N, López-Soto A. Haemoglobin responses to transfusion in severe iron deficiency anaemia: potential impact of gastrointestinal disorders. Vox Sang. 2017;112:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Cheng CL, Lin CH, Kuo CJ, Sung KF, Lee CS, Liu NJ, Tang JH, Cheng HT, Chu YY, Tsou YK. Predictors of rebleeding and mortality in patients with high-risk bleeding peptic ulcers. Dig Dis Sci. 2010;55:2577-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |