Published online Dec 7, 2020. doi: 10.3748/wjg.v26.i45.7204
Peer-review started: September 2, 2020
First decision: September 30, 2020
Revised: October 7, 2020
Accepted: November 2, 2020
Article in press: November 2, 2020
Published online: December 7, 2020
Processing time: 93 Days and 4.8 Hours
Esophageal varices (EV) are the most fatal complication of chronic hepatitis B (CHB) related cirrhosis. The prognosis is poor, especially after the first upper gastrointestinal hemorrhage.
To construct nomograms to predict the risk and severity of EV in patients with CHB related cirrhosis.
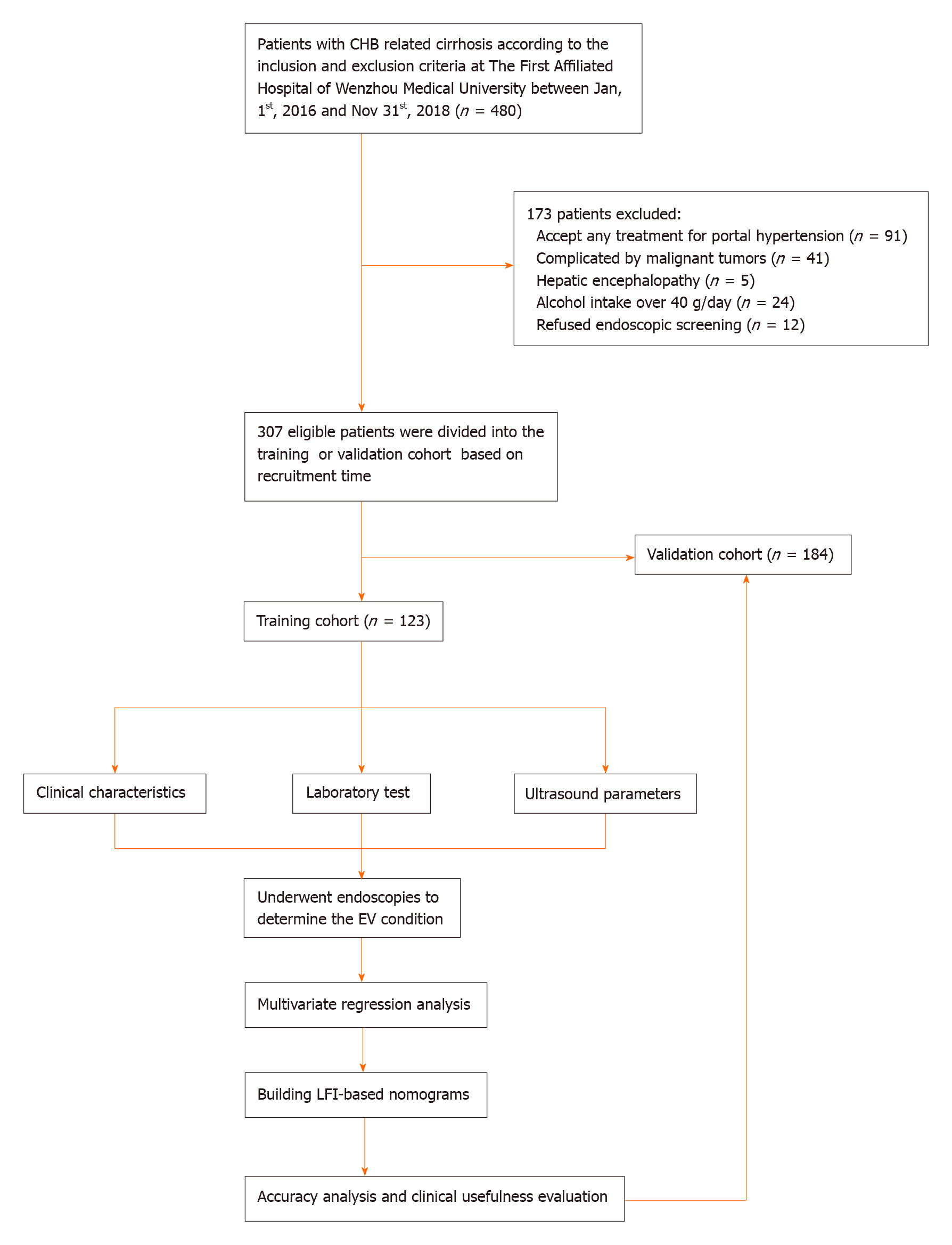
Between 2016 and 2018, the patients with CHB related cirrhosis were recruited and divided into a training or validation cohort at The First Affiliated Hospital of Wenzhou Medical University. Clinical and ultrasonic parameters that were closely related to EV risk and severity were screened out by univariate and multivariate logistic regression analyses, and integrated into two nomograms, respectively. Both nomograms were internally and externally validated by calibration, concordance index (C-index), receiver operating characteristic curve, and decision curve analyses (DCA).
A total of 307 patients with CHB related cirrhosis were recruited. The independent risk factors for EV included Child-Pugh class [odds ratio (OR) = 7.705, 95% confidence interval (CI) = 2.169-27.370, P = 0.002], platelet count (OR = 0.992, 95%CI = 0.984-1.000, P = 0.044), splenic portal index (SPI) (OR = 3.895, 95%CI = 1.630-9.308, P = 0.002), and liver fibrosis index (LFI) (OR = 3.603, 95%CI = 1.336-9.719, P = 0.011); those of EV severity included Child-Pugh class (OR = 5.436, 95%CI = 2.112-13.990, P < 0.001), mean portal vein velocity (OR = 1.479, 95%CI = 1.043-2.098, P = 0.028), portal vein diameter (OR = 1.397, 95%CI = 1.021-1.912, P = 0.037), SPI (OR = 1.463, 95%CI = 1.030-2.079, P = 0.034), and LFI (OR = 3.089, 95%CI = 1.442-6.617, P = 0.004). Two nomograms (predicting EV risk and severity, respectively) were well-calibrated and had a favorable discriminative ability, with C-indexes of 0.916 and 0.846 in the training cohort, respectively, higher than those of other predictive indexes, like LFI (C-indexes = 0.781 and 0.738), SPI (C-indexes = 0.805 and 0.714), ratio of platelet count to spleen diameter (PSR) (C-indexes = 0.822 and 0.726), King’s score (C-indexes = 0.694 and 0.609), and Lok index (C-indexes = 0.788 and 0.700). The areas under the curves (AUCs) of the two nomograms were 0.916 and 0.846 in the training cohort, respectively, higher than those of LFI (AUCs = 0.781 and 0.738), SPI (AUCs = 0.805 and 0.714), PSR (AUCs = 0.822 and 0.726), King’s score (AUCs = 0.694 and 0.609), and Lok index (AUCs = 0.788 and 0.700). Better net benefits were shown in the DCA. The results were validated in the validation cohort.
Nomograms incorporating clinical and ultrasonic variables are efficient in noninvasively predicting the risk and severity of EV.
Core Tip: In this study, we constructed two liver fibrosis index (LFI)-based nomograms for predicting the risk and severity of esophageal varices (EV) in patients with chronic hepatitis B related cirrhosis, through incorporating the clinical and ultrasonic variables screened out by univariate and multivariate logistic regression analyses. The nomograms were well-calibrated and had a good discriminative ability that was validated by receiver operating characteristic curve, concordance index, and decision curve analyses. Both nomograms were more efficient than LFI, splenic portal index, ratio of platelet count to spleen diameter, King’s score, and Lok index in the training and validation cohorts, and can be clinically used for diagnosing EV and making clinical interventions.
- Citation: Xu SH, Wu F, Guo LH, Zhang WB, Xu HX. Liver fibrosis index-based nomograms for identifying esophageal varices in patients with chronic hepatitis B related cirrhosis. World J Gastroenterol 2020; 26(45): 7204-7221
- URL: https://www.wjgnet.com/1007-9327/full/v26/i45/7204.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i45.7204
Chronic hepatitis B (CHB) causes considerable liver-related morbidity and mortality worldwide[1]. CHB is victimizing over 290 million people, and killed 1.34 million in 2015 (96% died from CHB complications)[2]. When CHB progresses to liver cirrhosis, poor prognosis is always suspected for its serious complications, including esophageal varices (EV) that could lead to upper gastrointestinal bleeding[3]. Current primary and secondary treatment strategies cannot prevent the advent of poor prognosis after the first upper gastrointestinal hemorrhage[4].
Hepatic venous pressure gradient and surveillance endoscopy are required to monitor EV in patients with CHB related cirrhosis[5,6]. However, both techniques are invasive and costly[7]. Therefore, there is an urgent need for an effective novel non-invasive tool.
Some clinical indicators are associated with EV, and EV prediction models based on ultrasound imaging have been established[8]. Doppler ultrasonography has been considered an ideal tool for diagnosing portal hypertension. Several studies have evaluated the predictive efficiency of Doppler ultrasound for EV, but the results remain contradictory[9,10]. Compared with portal vein velocity or splenic index alone, splenic portal index (SPI) demonstrated superior diagnostic accuracy [cutoff 3.0, area under the curve (AUC) 0.96], with higher sensitivity, specificity, positive predictive value, and negative predictive value[11]. Supranormal spleen diameter (SD) is regarded as an independent risk marker in diagnosing large esophageal varices for cirrhotic patients[12,13]. Real-time tissue elastography (RTE) is a relatively new non-invasive method for measuring liver tissue elasticity[14-16]. RTE makes the elasticity of the target area visual by capturing secondary echo signals arising from repetitive compression caused by heartbeat[17]. Liver fibrosis index (LFI) is quantitated by RTE and has a good predictive efficiency for liver fibrosis and cirrhosis. As EV always develops as liver cirrhosis progresses, cirrhosis indexes assessed by ultrasonic diagnostic methods were used to evaluate EV in this study.
Nomograms can visualize the complicated predictive models and make the results more readable[18]. In this study, we constructed two nomograms integrating clinical and ultrasonic indicators for determining the risk and severity of EV in patients with CHB related cirrhosis.
A total of 123 consecutive patients were recruited as a training cohort from January 1, 2016 to December 31, 2016, and 184 consecutive patients as a validation cohort from January 1, 2017 to December 31, 2018 at The First Affiliated Hospital of Wenzhou Medical University. The inclusion criteria were: (1) Clear etiological evidence of hepatitis B virus (HBV) infection (i.e., defined as positive HBV surface antigen and HBV DNA ≥ 30 IU/mL); (2) Clinical manifestations and laboratory results of cirrhosis; and (3) Cirrhosis confirmed by liver biopsy or two among ultrasound, computed tomography, and magnetic resonance. The exclusion criteria were: (1) Diagnosed with other liver diseases, such as chronic viral hepatitis C (HCV, defined as positive anti-HCV antibodies and HCV RNA > 1000 IU/mL), autoimmune hepatitis (based on serum autoantibodies or histology), drug-induced hepatic disease, alcoholic liver disease, and cholestatic liver disease; (2) Evidence of hepatic carcinoma, or other malignant or benign tumors; (3) History of endoscopy to determine the condition of EV; and (4) Previous diagnosis of EV and related treatment.
The following variables were collected from each patient within 1 wk before endoscopy: Age, sex, body mass index, systolic blood pressure, diastolic blood pressure, and Child-Pugh class. Also recorded were laboratory results: Total bilirubin, direct bilirubin, total protein, albumin, alanine transaminase (ALT), aspartic transaminase (AST), alkaline phosphatase, gamma-glutamyl transpeptidase, international normalized ratio (INR), platelet count (PLT), mean platelet volume (MPV), and platelet distribution width. All patients underwent endoscopies to determine the EV condition. EV were classified into Degrees 0-3 according to the published criteria[19], with Degrees 2 and 3 defined as severe varices.
After overnight fasting, the patients underwent Doppler ultrasonography operated by two experienced ultrasonographers at The First Affiliated Hospital of Wenzhou Medical University. The patient was placed in a lateral position through a HI-VISION Avius ultrasound system (Hitachi Medical Corporation, Tokyo, Japan) and EUP-C715 phased-array electronic probe (1-5MHz; Hitachi Medical Corporation, Tokyo, Japan). The following indicators were detected: Hepatic artery diameter, hepatic artery peak velocity, portal vein diameter (PVD), mean portal vein velocity (MPVV), splenic artery diameter, splenic artery peak velocity, splenic vein diameters, mean splenic vein velocity, spleen thick (ST), and SD.
RTE was performed using ultrasonography (HI-VISION Avius; Hitachi Medical Corporation, Tokyo, Japan) and an EUP-L52 linear array probe (3-7 MHz; Hitachi Medical Corporation, Tokyo, Japan). The patient was placed in a supine position with maximum right-arm abduction, and required to hold their breath. The right lobe of the liver (the sixth-to-ninth intercostal space) was examined as the examiner gripped the transducer without exerting pressure on the skin. The region of interest (ROI) on the strain image was localized about 1 cm below the margin of the liver, with a size of 2.5 cm × 2.5 cm. Additionally, large blood vessels, lower lobe of the right lung, and ribs should not be included into the ROI, in order that its image quality was not impaired. The LFI was calculated according to a multiple regression equation using nine parameters of each RTE image, including the mean of relative strain value (MEAN), standard deviation of relative strain value (SDV), area ratio of low-strain region (%AREA), complexity of low-strain region (COMP), angular second moment (ASM), entropy (ENT), inverse difference moment (IDM), kurtosis (KURT), and skewness (SKEW)[20].
After the variables with multicollinearity were excluded, those with P < 0.05 in univariate regression analysis were selected for multivariate regression analysis. Then independent clinical and ultrasonic predictive factors were estimated and used to construct the nomograms for assessing the risk and severity of EV, respectively.
Cirrhosis and EV were also predicted with current predictive indexes: LFI[20], SPI[11], ratio of platelet count to spleen diameter (PSR)[21,22], King’s score[23], and Lok index[24]; LFI = −0.009 × MEAN − 0.005 × SDV + 0.023 × %AREA + 0.025 × COMP + 0.775 × SKEW − 0.281 × KURT + 2.083 × ENT + 3.042 × IDM + 39.979 × ASM − 5.542, SPI = ST × SD/MPVV, PSR = PLT/SD, King’s score = Age × AST × INR/PLT, and Lok index = −5.56 − 0.0089 × PLT + 1.26 × AST/ALT + 5.27 × INR.
The receiver operating characteristic (ROC) curve and concordance index (C-index) analyses were used to evaluate the accuracy of nomograms for predicting the risk and severity of EV. The predicted and observed probabilities of the nomograms were illustrated with calibration curves. The potential net benefits of the nomograms were demonstrated by decision curve analysis (DCA). The discrimination performances of LFI, SPI, PSR, King’s score, and Lok index were compared with those of the nomograms.
Statistical analyses were performed using R version 3.6.1. Continuous variables in a normal distribution are expressed as the mean ± SD and were compared using the Student’s t-test, and those in a skewed distribution are presented as median (interquartile range) and were analyzed by the nonparametric Mann-Whitney U test. Categorical variables are expressed as frequencies (%) and were compared using the chi-square test. Univariate and multivariate logistic regression analyses were completed with SPSS statistics version 26. R software was used for building the nomogram using “rms” package. Packages of “pROC” and “rmda” were used in ROC and DCA analyses. The values of AUCs were compared using the DeLong method with MedCalc version 18.11.3. All P values were two-sided.
From 2016 to 2018, we recruited 307 eligible patients. All of them were divided into either a training cohort (n = 123) or a validation cohort (n = 184) based on recruitment time. Figure 1 shows the workflow of our study. Table 1 shows the clinical and ultrasonic characteristics of patients. All clinical, laboratory, and ultrasonic characteristics of both cohorts were comparable (P > 0.05). The mean age of the patients was 54.05 years in the training cohort and 54.54 years in the validation cohort. The majority of patients were men (78.05% vs 79.89%) and had Child-Pugh class A liver function (47.15% vs 46.74%). The percentages of patients suffering from EV (Degrees 0-3) were 26.02%, 13.01%, 17.07%, and 43.90% in the training cohort, and 26.09%, 14.13%, 13.59%, and 46.19% in the validation cohort, respectively.
| Variable | Training cohort (n = 123) | Validation cohort (n = 184) | P value |
| Clinical parameter | |||
| Age (yr) | 54.05 ± 12.02 | 54.54 ± 12.25 | 0.727 |
| Male (%) | 96 (78.05) | 147 (79.89) | 0.697 |
| BMI (kg/m2) | 22.97 ± 3.24 | 22.91 ± 3.41 | 0.877 |
| SBP (mmHg) | 129.29 ± 20.62 | 129.03 ± 20.56 | 0.914 |
| DBP (mmHg) | 71.77 ± 12.16 | 71.41 ± 11.76 | 0.796 |
| Child-Pugh class A (%) | 58 (47.15) | 86 (46.74) | 0.943 |
| Degree of EV (Q1, Q3) | 2 (0, 3) | 2 (0, 3) | 0.873 |
| 0 (%) | 32 (26.02) | 48 (26.09) | |
| 1 (%) | 16 (13.01) | 26 (14.13) | |
| 2 (%) | 21 (17.07) | 25 (13.59) | |
| 3 (%) | 54 (43.90) | 85 (46.19) | |
| Laboratory test | |||
| TBil (µmol/L) | 19.00 (12.00, 31.00) | 18.50 (12.00, 31.00) | 0.943 |
| DBil (µmol/L) | 9.00 (5.00, 16.00) | 9.00 (5.00, 17.00) | 0.766 |
| TP (g/L) | 65.80 ± 10.03 | 66.05 ± 9.95 | 0.831 |
| Albumin (g/L) | 32.85 ± 7.11 | 33.14 ± 7.14 | 0.726 |
| ALT (IU/L) | 27.00 (20.00, 47.00) | 27.50 (20.25, 47.00) | 0.850 |
| AST (IU/L) | 38.00 (28.00, 70.00) | 41.00 (28.00, 70.75) | 0.734 |
| ALP (IU/L) | 94.00 (74.00, 131.00) | 99.00 (73.00, 140.00) | 0.728 |
| GGT (IU/L) | 51.00 (24.00, 126.00) | 50.00 (29.25, 126.75) | 0.865 |
| INR | 1.36 (1.17, 1.54) | 1.36 (1.17, 1.54) | 0.994 |
| PLT (× 109/L) | 82.00 (53.00, 140.00) | 84.00 (56.00, 139.00) | 0.760 |
| MPV (fl) | 11.50 (10.50, 12.50) | 11.30 (10.40, 12.30) | 0.301 |
| PDW (fl) | 14.90 (12.60, 17.30) | 14.65 (12.70, 17.18) | 0.760 |
| Ultrasound parameter | |||
| HAD (mm) | 3.46 ± 0.56 | 3.47 ± 0.57 | 0.914 |
| HAPV (cm/s) | 50.35 ± 12.23 | 50.51 ± 13.33 | 0.915 |
| PVD (mm) | 12.30 ± 1.54 | 12.05 ± 1.61 | 0.186 |
| MPVV (cm/s) | 19.11 ± 5.28 | 19.93 ± 5.59 | 0.196 |
| SAD (mm) | 5.05 ± 1.00 | 5.00 ± 0.98 | 0.678 |
| SAPV (cm/s) | 55.89 ± 15.38 | 55.91 ± 16.03 | 0.990 |
| SVD (mm) | 9.61 ± 1.82 | 9.59 ± 1.70 | 0.899 |
| MSVV (cm/s) | 22.22 ± 4.93 | 22.69 ± 5.20 | 0.428 |
| ST (mm) | 44.47 ± 9.36 | 44.61 ± 9.68 | 0.900 |
| SD (mm) | 110.88 ± 23.73 | 112.27 ± 25.39 | 0.629 |
| SPI | 2.96 ± 1.75 | 2.92 ± 1.96 | 0.871 |
| LFI | 3.74 ± 0.69 | 3.72 ± 0.68 | 0.886 |
Univariate logistic regression analysis in the training cohort indicated that age [odds ratio (OR) = 1.038, 95% confidence interval (CI) = 1.001-1.076, P = 0.043)], Child-Pugh class (OR = 7.990, 95%CI = 2.979-21.426, P < 0.001), albumin (OR = 0.932, 95%CI = 0.877-0.990, P = 0.022), INR (OR = 20.331, 95%CI = 2.870-144.037, P = 0.003), PLT (OR = 0.990, 95%CI = 0.985-0.995, P < 0.001), PVD (OR = 1.505, 95%CI = 1.121-2.021, P = 0.006), MPVV (OR = 0.915, 95%CI = 0.845-0.991, P = 0.029), ST (OR = 1.312, 95%CI = 1.178-1.462, P < 0.001), SD (OR = 1.036, 95%CI = 1.012-1.059, P = 0.003), SPI (OR = 4.183, 95%CI = 2.044-8.559, P < 0.001), and LFI (OR = 7.067, 95%CI = 2.938-16.995, P < 0.001) were risk factors for EV. To reduce multiple collinearity, albumin was excluded in the subsequent multivariate logistic regression analysis, because it was an objective indicator in the Child-Pugh system. Similarly, ST, SD, and MPVV were excluded since they were calculated with SPI formula. On this basis, the multivariate logistic regression analysis identified Child-Pugh class (OR = 7.705, 95%CI = 2.169-27.370, P = 0.002), PLT (OR = 0.992, 95%CI = 0.984-1.000, P = 0.044), SPI (OR = 3.895, 95%CI = 1.630-9.308, P = 0.002), and LFI (OR = 3.603, 95%CI = 1.336-9.719, P = 0.011) as independent indicators for the risk of EV (Table 2).
| Variable | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Clinical parameter | ||||||
| Age (yr) | 1.038 | 1.001-1.076 | 0.043 | |||
| Gender (Female = 0, Male = 1) | 0.994 | 0.375-2.633 | 0.990 | |||
| BMI (kg/m2) | 1.031 | 0.907-1.171 | 0.643 | |||
| SBP (mmHg) | 1.005 | 0.985-1.025 | 0.657 | |||
| DBP (mmHg) | 0.985 | 0.953-1.018 | 0.367 | |||
| Child-Pugh class | 7.990 | 2.979-21.426 | < 0.001 | 7.705 | 2.169-27.370 | 0.002 |
| (Child A = 1, Child B/C = 2) | ||||||
| Laboratory test | ||||||
| TBil (µmol/L) | 1.001 | 0.988-1.013 | 0.930 | |||
| DBil (µmol/L) | 0.998 | 0.981-1.016 | 0.831 | |||
| TP (g/L) | 0.976 | 0.936-1.018 | 0.255 | |||
| Albumin (g/L) | 0.932 | 0.877-0.990 | 0.022 | |||
| ALT (IU/L) | 0.992 | 0.984-1.000 | 0.052 | |||
| AST (IU/L) | 0.997 | 0.990-1.003 | 0.294 | |||
| ALP (IU/L) | 1.009 | 0.999-1.018 | 0.065 | |||
| GGT (IU/L) | 0.998 | 0.995-1.000 | 0.081 | |||
| INR | 20.331 | 2.870-144.037 | 0.003 | |||
| PLT (× 109/L) | 0.990 | 0.985-0.995 | < 0.001 | 0.992 | 0.984-1.000 | 0.044 |
| MPV (fl) | 1.373 | 0.999-1.887 | 0.051 | |||
| PDW (fl) | 1.128 | 0.981-1.297 | 0.092 | |||
| Ultrasound parameter | ||||||
| HAD (mm) | 1.619 | 0.786-3.335 | 0.192 | |||
| HAPV (cm/s) | 1.017 | 0.984-1.051 | 0.318 | |||
| PVD (mm) | 1.505 | 1.121-2.021 | 0.006 | |||
| MPVV (cm/s) | 0.915 | 0.845-0.991 | 0.029 | |||
| SAD (mm) | 0.935 | 0.629-1.390 | 0.740 | |||
| SAPV (cm/s) | 0.995 | 0.970-1.021 | 0.711 | |||
| SVD (mm) | 1.086 | 0.860-1.372 | 0.489 | |||
| MSVV (cm/s) | 1.073 | 0.977-1.180 | 0.142 | |||
| ST (mm) | 1.312 | 1.178-1.462 | < 0.001 | |||
| SD (mm) | 1.036 | 1.012-1.059 | 0.003 | |||
| SPI | 4.183 | 2.044-8.559 | < 0.001 | 3.895 | 1.630-9.308 | 0.002 |
| LFI | 7.067 | 2.938-16.995 | < 0.001 | 3.603 | 1.336-9.719 | 0.011 |
As shown in Table 3, univariate logistic regression analysis indicated that Child-Pugh class (OR = 5.161, 95%CI = 2.344-11.361, P < 0.001), INR (OR = 10.764, 95%CI = 2.342-49.469, P = 0.002), PLT (OR = 0.994, 95%CI = 0.989-0.999, P = 0.016), MPV (OR = 1.399, 95%CI = 1.050-1.865, P = 0.022), PVD (OR = 1.465, 95%CI = 1.126-1.906, P = 0.004), ST (OR = 1.146, 95%CI = 1.077-1.218, P < 0.001), SD (OR = 1.030, 95%CI = 1.010-1.049, P = 0.003), SPI (OR = 1.644, 95%CI = 1.193-2.265, P = 0.002), and LFI (OR = 4.184, 95%CI = 2.080-8.416, P < 0.001) were statistically associated with EV severity in the training cohort. Indicators of ST and SD were also excluded from multivariate logistic regression analysis, since they were calculated with SPI formula. The multivariate logistic regression analysis identified Child-Pugh class (OR = 5.436, 95%CI = 2.112-13.990, P < 0.001), MPV (OR = 1.479, 95%CI = 1.043-2.098, P = 0.028), PVD (OR = 1.397, 95%CI =1.021-1.912, P = 0..037), SPI (OR = 1.463, 95%CI = 1.030-2.079, P = 0.034), and LFI (OR = 3.089, 95%CI = 1.442-6.617, P = 0.004) as independent indicators for EV severity.
| Variable | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Clinical parameter | ||||||
| Age (yr) | 0.997 | 0.967-1.027 | 0.833 | |||
| Gender (Female = 0, Male = 1) | 0.898 | 0.372-2.168 | 0.811 | |||
| BMI (kg/m2) | 1.011 | 0.904-1.132 | 0.844 | |||
| SBP (mmHg) | 1.001 | 0.983-1.019 | 0.928 | |||
| DBP (mmHg) | 0.992 | 0.962-1.022 | 0.584 | |||
| Child-Pugh class | 5.161 | 2.344-11.361 | < 0.001 | 5.436 | 2.112-13.990 | < 0.001 |
| (Child A = 1, Child B/C = 2) | ||||||
| Laboratory test | ||||||
| TBil (µmol/L) | 1.000 | 0.989-1.012 | 0.937 | |||
| DBil (µmol/L) | 0.996 | 0.980-1.012 | 0.643 | |||
| TP (g/L) | 0.978 | 0.942-1.015 | 0.246 | |||
| Albumin (g/L) | 0.987 | 0.937-1.038 | 0.605 | |||
| ALT (IU/L) | 0.994 | 0.986-1.001 | 0.109 | |||
| AST (IU/L) | 0.997 | 0.991-1.003 | 0.282 | |||
| ALP (IU/L) | 1.001 | 0.995-1.007 | 0.637 | |||
| GGT (IU/L) | 0.997 | 0.995-1.000 | 0.064 | |||
| INR | 10.764 | 2.342-49.469 | 0.002 | |||
| PLT (× 109/L) | 0.994 | 0.989-0.999 | 0.016 | |||
| MPV (fl) | 1.399 | 1.050-1.865 | 0.022 | 1.479 | 1.043-2.098 | 0.028 |
| PDW (fl) | 1.036 | 0.920-1.166 | 0.564 | |||
| Ultrasound parameter | ||||||
| HAD (mm) | 1.327 | 0.693-2.541 | 0.393 | |||
| HAPV (cm/s) | 1.025 | 0.995-1.057 | 0.108 | |||
| PVD (mm) | 1.465 | 1.126-1.906 | 0.004 | 1.397 | 1.021-1.912 | 0.037 |
| MPVV (cm/s) | 0.964 | 0.899-1.033 | 0.301 | |||
| SAD (mm) | 0.973 | 0.678-1.396 | 0.880 | |||
| SAPV (cm/s) | 1.000 | 0.977-1.024 | 0.985 | |||
| SVD (mm) | 1.109 | 0.900-1.366 | 0.332 | |||
| MSVV (cm/s) | 1.062 | 0.979-1.151 | 0.148 | |||
| ST (mm) | 1.146 | 1.077-1.218 | < 0.001 | |||
| SD (mm) | 1.030 | 1.010-1.049 | 0.003 | |||
| SPI | 1.644 | 1.193-2.265 | 0.002 | 1.463 | 1.030-2.079 | 0.034 |
| LFI | 4.184 | 2.080-8.416 | < 0.001 | 3.089 | 1.442-6.617 | 0.004 |
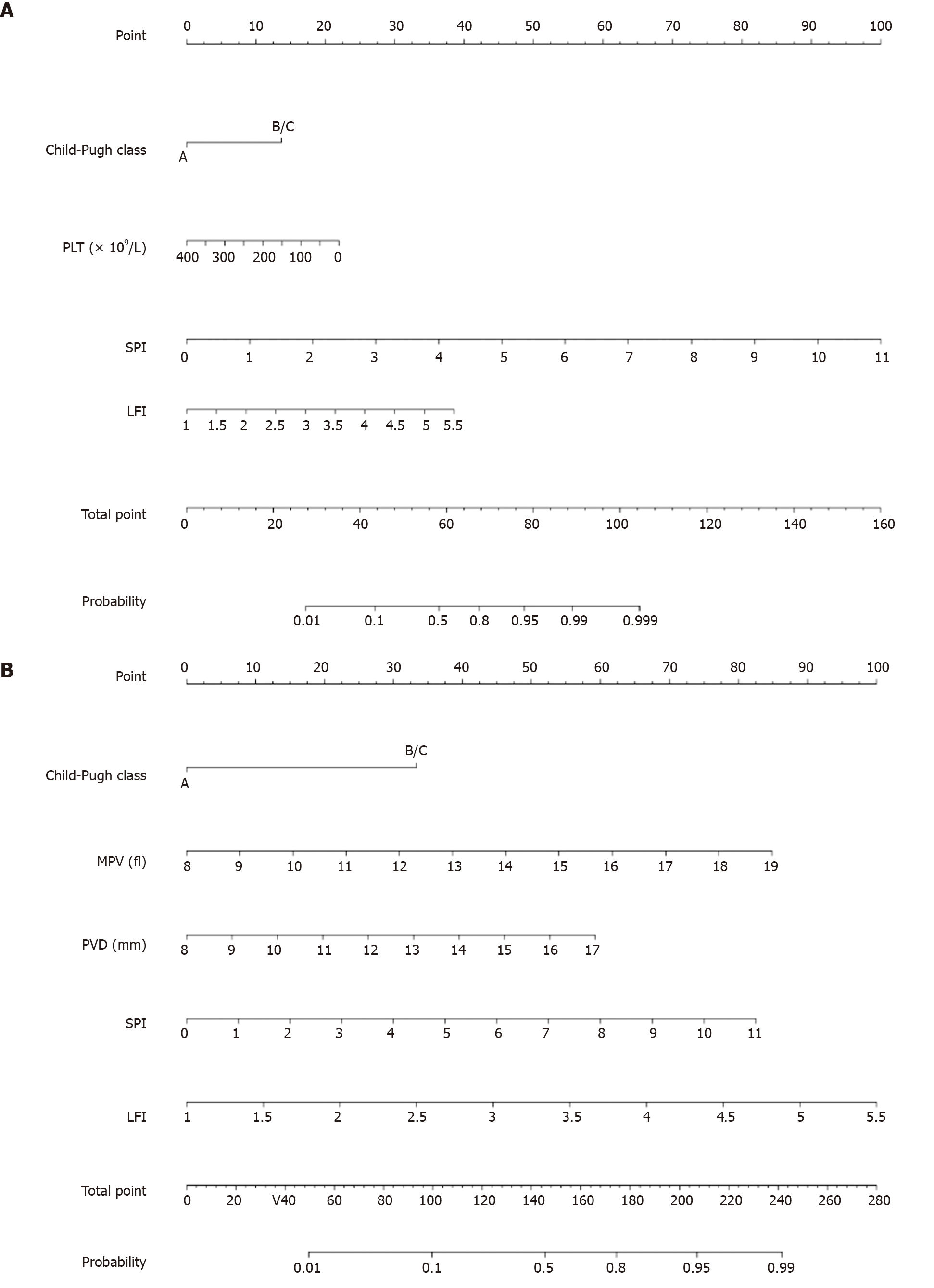
Based on the univariate and multivariate logistic regression analyses, variables that achieved a value of P < 0.05 in multivariate analysis were selected and incorporated into the nomograms for predicting the probability and severity of EV (Figure 2).
The nomograms were used to predict the EV in a patient (PLT, 60 × 109/L; MPV, 10 fl; and Child-Pugh class A). After Doppler ultrasonography and RTE examinations, ultrasound showed PVD of 10 mm, SPI of 3.5, and LFI of 3. In the nomogram predicting the risk of EV, 0 was given to Child-Pugh class A, 18.5 to PLT, 32.5 to SPI, and 18 to LFI. Moreover, in the nomogram predicting the severity of EV, 0 was given to Child-Pugh class A, 15 to MPV, 13 to PVD, 26 to SPI, and 44 to LFI. By summing up all the points, he scored 69 points in the EV risk prediction nomogram and 98 points in the EV severity prediction nomogram. Eventually, his estimated risk of EV occurrence was over 80%, and that of severe EV was slightly lower than 10%.
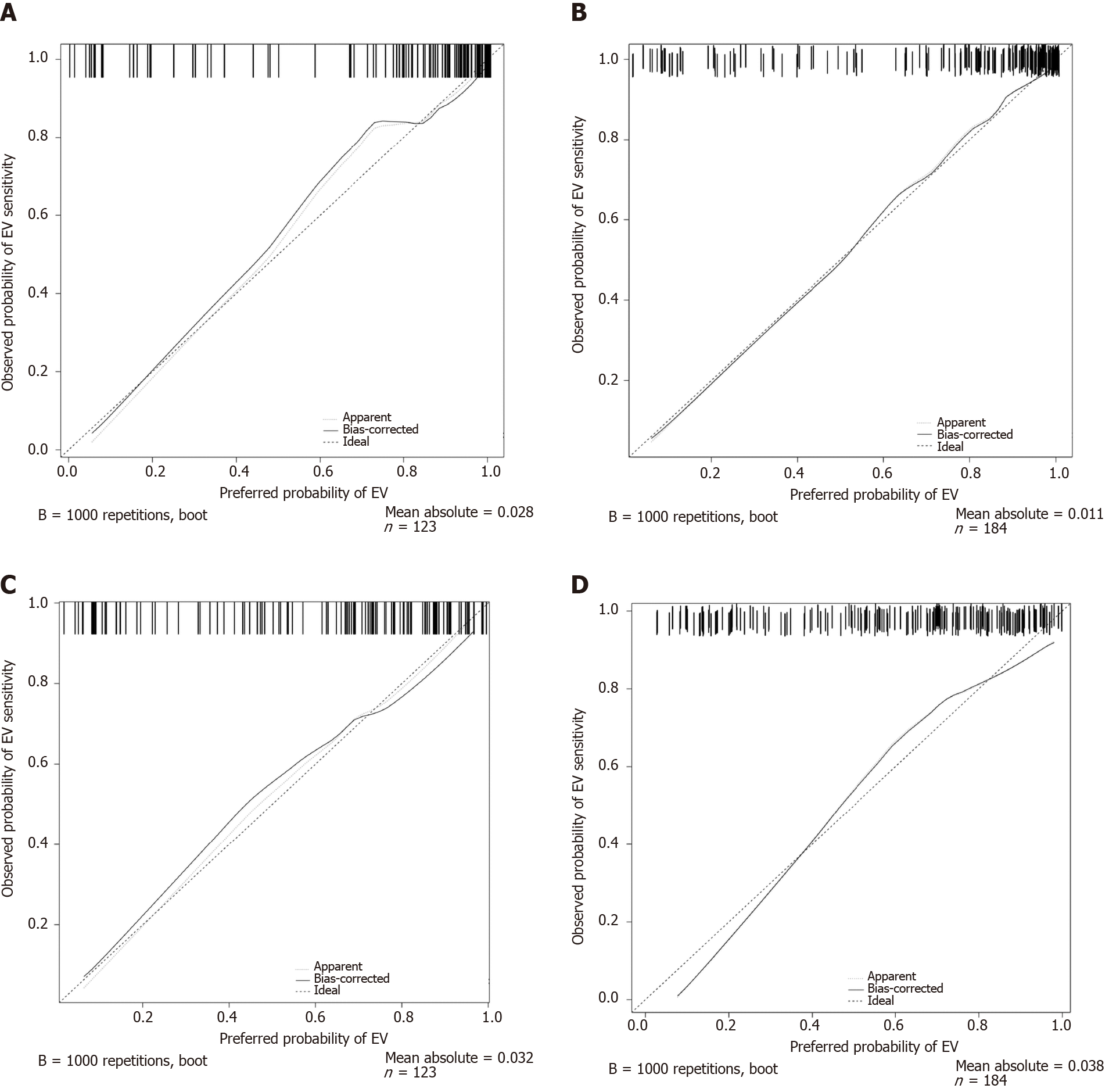
As depicted in Figure 3, the calibration curves of both nomograms were close to the standard curves in the training cohort and validation cohort, which suggested that the nomograms were well-calibrated.
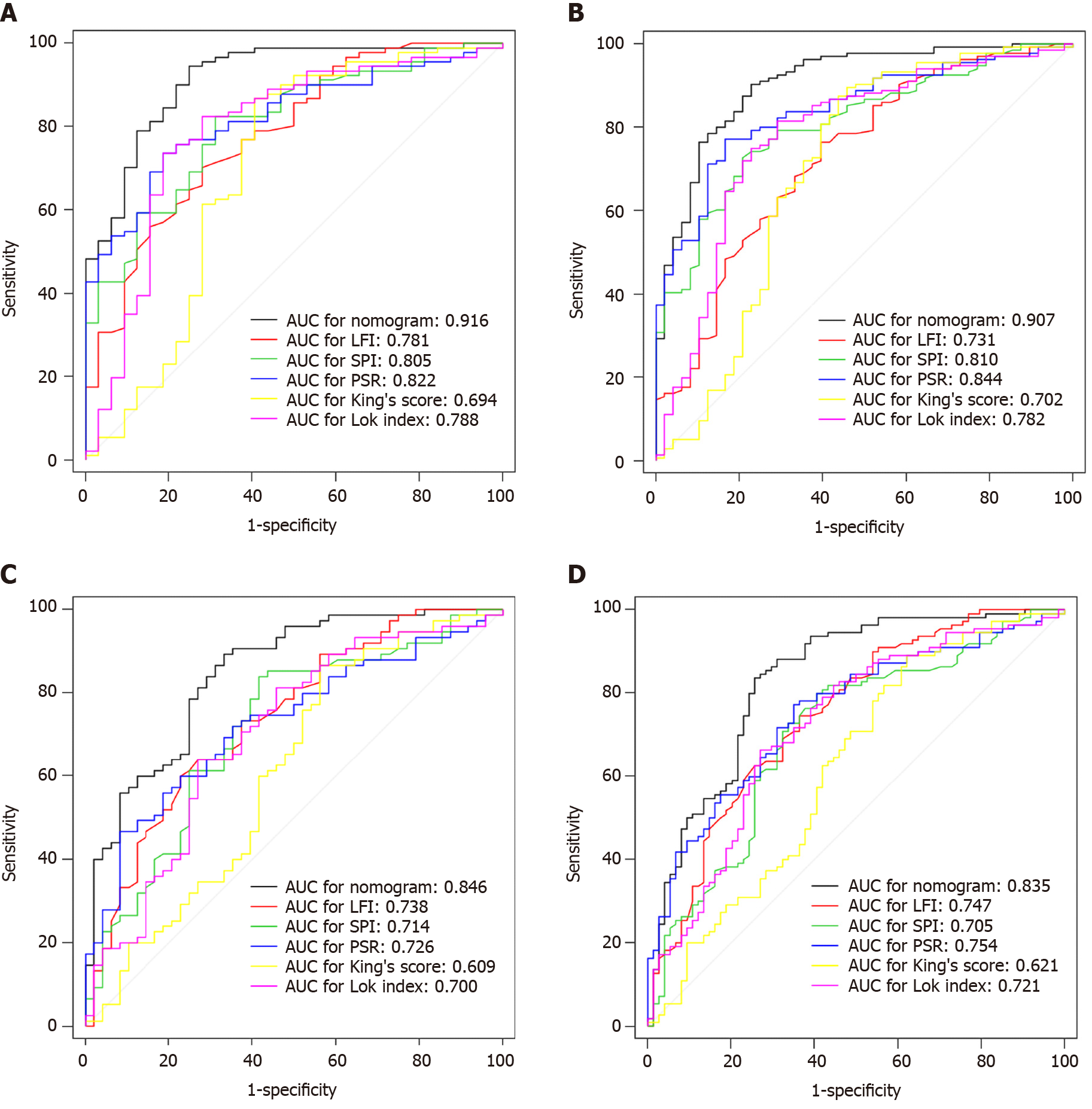
In the training cohort, the C-index values to predict EV risk were 0.916, 0.781, 0.805, 0.822, 0.694, and 0.788 for EV risk prediction nomogram, LFI, SPI, PSR, King’s score, and Lok index, respectively. In the validation cohort, these values were 0.907, 0.731, 0.810, 0.844, 0.702, and 0.782, respectively. In the training cohort, the C-index values to predict EV severity were 0.846, 0.738, 0.714, 0.726, 0.609, and 0.700 for EV severity prediction nomogram, LFI, SPI, PSR, King’s score, and Lok index, respectively. In the validation cohort, these values were 0.835, 0.747, 0.705, 0.754, 0.621, and 0.721, respectively.
AUC of EV risk prediction nomogram was 0.916 in the training cohort and 0.907 in the validation cohort, while AUC of EV severity prediction nomogram was 0.846 in the training cohort and 0.835 in the validation cohort. In the training cohort, the AUCs of LFI, SPI, PSR, King’s score, and Lok index for predicting EV risk were 0.781, 0.805, 0.822, 0.694, and 0.788, respectively. The AUCs of LFI, SPI, PSR, King’s score, and Lok index for predicting EV severity were 0.738, 0.714, 0.726, 0.609, and 0.700, respectively. In the validation cohort, these values were 0.731, 0.810, 0.844, 0.702, and 0.782 in the prediction of EV risk, and 0.747, 0.705, 0.754, 0.621, and 0.721 in the prediction of EV severity, respectively. A pairwise comparison of each index with the nomogram showed statistical significance (P < 0.05), as shown in Figure 4.
DCA, a novel prediction tool, was also used to evaluate the efficiency of both nomograms. The decision curves of both nomograms and other indexes in the training and the validation cohorts are shown in Figure 5. The results indicated that nomograms provided better clinical net benefits within most thresholds.
In this study, nomograms based on clinical and ultrasonic variables showed favorable efficiency in predicting the risk and severity of EV in patients with CHB related cirrhosis.
At present, the diagnosis of EV still relies on endoscopic examination. Since the patients with no varices or mild varices have a low risk for hemorrhage, nonselective beta blocker (NSBB) is not recommended for preventive treatment. Several studies[7,25] have shown that NSBB failed to prevent or delay the progression of EV, and exerted more side effects and complications than the control group. Endoscopic follow-up must be performed every 1-2 years to exclude the occurrence of EV in the low-risk hemorrhagic cirrhotic population[26], while this operation is invasive, expensive, and time-consuming, and some patients even cannot tolerate it. The widespread application of painless endoscopy brings comfortable experience to patients, but anesthesia also increases the risk. The Baveno VI criteria indicated that liver stiffness measurement (LSM) < 20 kPa (an index of transient elastography) and PLT > 150000/mm3 predicted a low risk of varices[26]. Although LSM and PLT cut-offs have been proposed to screen patients with a risk of EV, esophagogastroduodenoscopy still finds no varices in a certain fraction of patients[27,28]. Given the high variance in the results (cut-offs, positive predictive values, and negative predictive values) of LSM, more efficient and non-invasive ways are urgently needed to stratify EV risk. RTE depends on the relative strain within liver tissue caused by external pressure arising from rhythmic heartbeats. Therefore, RTE can reduce intra- and inter-observer variations since this external pressure is stable[29].
After univariate and multivariate logistic regression analyses, LFI, SPI, PVD, PLT, MPV, and Child-Pugh class were found as independent indicators for the risk or severity of EV. This finding was consistent with the result of prior studies that PVD, as a portal hemodynamic indicator, could independently predict EV occurrence and severity[30,31]. PLT decreases in portal hypertension and related hypersplenia, and has been identified as a noninvasive predictive index for the risk of EV in a cross-sectional and longitudinal study[32]. Accordingly, MPV rises as a result of compensatory production of platelets in the peripheral blood[33]. Our outcomes were in line with the finding of prior studies that SPI Doppler index can be used to predict the probability of EV[34,35]. According to our literature review, it was the first time to report the favorable efficiency of SPI in predicting EV severity. Chinese researchers have utilized RTE to examine 71 patients with post-hepatitis B cirrhosis, finding that LFI is positively correlated with EV severity, which is also consistent with our findings. Child-Pugh class is well recognized to evaluate liver function, but in the present study, it is also associated with EV risk and severity. However, what brings forth this association needs to be interpreted.
Various non-invasive and integrated prediction models have been developed. Giannini et al[21] found that PSR, with a cutoff of 909, could keep 27.4% of patients without EV from being screened by endoscopy. However, a meta-analysis containing 1275 patients yielded a pooled positive likelihood ratio of 3.5 and a negative likelihood ratio of 0.1 for predicting EV in cirrhosis of various etiologies, suggesting that PSR could not replace endoscopic screening[36]. According to a study for CHB population, the AUC of PSR for prediction of EV was 0.7095, also indicating that it was better to incorporate PSR with other clinical characteristic[37]. In our study, PSR was efficient for most cases in both the training and the validation cohorts. King’s score has been demonstrated to be a simple and non-invasive model to predict the presence of cirrhosis[38]. However, a study including 39 newly diagnosed cirrhotic patients found that King’s score showed a low specificity of 44% in predicting esophageal variceal bleeding, and no association with EV was verified[39]. Similarly, considering the poor AUC and C-index in this study, King’s score was not a preferred tool for the prediction of EV and its severity in CHB related cirrhosis patients. Additionally, Lok index, as a noninvasive predictive alternative index for liver cirrhosis, was also applied to predict EV in cirrhosis in previous studies. Lok index was proved to be associated with portal hypertension[40]. In a retrospective study including 132 patients, Zhou et al[41] found that in patients who did not meet Baveno VI criteria, Lok index could spare 24.2% of gastroscopy screening without missing high-risk varices which were defined as EV with red wale signs[42]. However, none of PSR, King’s score, or Lok index can take full advantage of imaging examination in the prediction of EV. Our ROC and DCA analyses indicated that our nomograms were superior to PSR, King’s score, and Lok index, which was verified in the validation cohort.
To our knowledge, this is the first study to construct nomograms integrating clinical and ultrasonic parameters to predict the risk and severity of EV. Our nomograms showed a strong discriminative ability and a clinical net benefit compared with other indexes. These predictive nomograms are useful for clinicians to make preventive and therapeutic measures.
Inevitably, the study has several limitations. Intra-observer and inter-observer variations may discount the efficiency of nomograms[43,44]. Next, our study is a single center retrospective study, and needs to be improved into a multi-center prospective study.
The nomograms incorporating clinical and ultrasonic variables are efficient in noninvasively predicting the probability and severity of EV, and can be used in individualized treatment and follow-ups.
Esophageal varices (EV) are an important cause of mortality for patients with chronic hepatitis B (CHB) related cirrhosis.
There is no reliable and non-invasive tool to monitor EV, predict the clinical outcome, and adjust the follow-up strategy.
This study aimed to develop nomogram models including non-invasive and clinically accessible indicators to assess the risk and severity of EV.
Patients with CHB related cirrhosis were retrospectively included and divided into a training or validation cohort. Ultrasound parameters and blood indexes were applied to construct the nomograms, which were subsequently evaluated by receiver operating characteristic, concordance index, and decision curve analyses, and tested in the validation cohort.
The novel nomograms composed of clinical and ultrasonic variables were constructed and proved better than liver fibrosis index, splenic portal index, ratio of platelet count to spleen diameter, King’s score, and Lok index for predicting the risk and severity of EV.
The established novel nomograms are reliable and convenient for clinicians to predict EV in a non-invasive way and make preventive and therapeutic measurements.
The novel models need to be tested by multi-center prospective studies and adjusted for particular groups, such as patients complicated with other liver diseases.
We thank Dr. Fang CY for her assistance in checking and analyzing the data.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boeckxstaens G, Satya R, Schmidt J S-Editor: Chen XF L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 368] [Article Influence: 52.6] [Reference Citation Analysis (2)] |
| 2. | Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1213] [Article Influence: 173.3] [Reference Citation Analysis (2)] |
| 3. | Gracia-Sancho J, Marrone G, Fernández-Iglesias A. Hepatic microcirculation and mechanisms of portal hypertension. Nat Rev Gastroenterol Hepatol. 2019;16:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 4. | Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 527] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 5. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 523] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 6. | Thabut D, Bureau C, Layese R, Bourcier V, Hammouche M, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Goria O, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle-Bladou C, Dao T, Pilette C, Silvain C, Christidis C, Capron D, Bernard-Chabert B, Hillaire S, Di Martino V, Sutton A, Audureau E, Roudot-Thoraval F, Nahon P; ANRS CO12 CirVir group. Validation of Baveno VI Criteria for Screening and Surveillance of Esophageal Varices in Patients With Compensated Cirrhosis and a Sustained Response to Antiviral Therapy. Gastroenterology. 2019;156:997-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M, Christie JM; Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 414] [Article Influence: 41.4] [Reference Citation Analysis (2)] |
| 8. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1438] [Article Influence: 179.8] [Reference Citation Analysis (3)] |
| 9. | Shastri M, Kulkarni S, Patell R, Jasdanwala S. Portal vein Doppler: a tool for non-invasive prediction of esophageal varices in cirrhosis. J Clin Diagn Res. 2014;8:MC12-MC15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Jalli R, Kazem Hosseini-Asl M, Eshraghian A, Rezaei B. Ultrasound based techniques and transient elastography may not be precise methods for the detection of esophageal varices in liver cirrhosis. Med Ultrason. 2014;16:78-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Liu CH, Hsu SJ, Liang CC, Tsai FC, Lin JW, Liu CJ, Yang PM, Lai MY, Chen PJ, Chen JH, Kao JH, Chen DS. Esophageal varices: noninvasive diagnosis with duplex Doppler US in patients with compensated cirrhosis. Radiology. 2008;248:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Madhotra R, Mulcahy HE, Willner I, Reuben A. Prediction of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2002;34:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Sharma SK, Aggarwal R. Prediction of large esophageal varices in patients with cirrhosis of the liver using clinical, laboratory and imaging parameters. J Gastroenterol Hepatol. 2007;22:1909-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Tomeno W, Yoneda M, Imajo K, Suzuki K, Ogawa Y, Shinohara Y, Mawatari H, Fujita K, Shibata W, Kirikoshi H, Maeda S, Nakajima A, Saito S. Evaluation of the Liver Fibrosis Index calculated by using real-time tissue elastography for the non-invasive assessment of liver fibrosis in chronic liver diseases. Hepatol Res. 2013;43:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Tamaki N, Kurosaki M, Matsuda S, Nakata T, Muraoka M, Suzuki Y, Yasui Y, Suzuki S, Hosokawa T, Nishimura T, Ueda K, Tsuchiya K, Nakanishi H, Itakura J, Takahashi Y, Matsunaga K, Taki K, Asahina Y, Izumi N. Prospective comparison of real-time tissue elastography and serum fibrosis markers for the estimation of liver fibrosis in chronic hepatitis C patients. Hepatol Res. 2014;44:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Ochi H, Hirooka M, Koizumi Y, Miyake T, Tokumoto Y, Soga Y, Tada F, Abe M, Hiasa Y, Onji M. Real-time tissue elastography for evaluation of hepatic fibrosis and portal hypertension in nonalcoholic fatty liver diseases. Hepatology. 2012;56:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Hu Q, Zhu SY, Kang LK, Wang XY, Lun HM, Xu CM. Non-invasive assessment of liver fibrosis using real-time tissue elastography in patients with chronic hepatitis B. Clin Radiol. 2014;69:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016;151:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 449] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 19. | Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, Kokudo N, Kokubu S, Sakaida I, Sata M, Tajiri H, Tsukada K, Nonami T, Hashizume M, Hirota S, Murashima N, Moriyasu F, Saigenji K, Makuuchi H, Oho K, Yoshida T, Suzuki H, Hasumi A, Okita K, Futagawa S, Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Yada N, Kudo M, Morikawa H, Fujimoto K, Kato M, Kawada N. Assessment of liver fibrosis with real-time tissue elastography in chronic viral hepatitis. Oncology. 2013;84 Suppl 1:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V, Testa R. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 297] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | González-Ojeda A, Cervantes-Guevara G, Chávez-Sánchez M, Dávalos-Cobián C, Ornelas-Cázares S, Macías-Amezcua MD, Chávez-Tostado M, Ramírez-Campos KM, Ramírez-Arce Adel R, Fuentes-Orozco C. Platelet count/spleen diameter ratio to predict esophageal varices in Mexican patients with hepatic cirrhosis. World J Gastroenterol. 2014;20:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Cross TJ, Rizzi P, Berry PA, Bruce M, Portmann B, Harrison PM. King's Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. 2009;21:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, Everhart JE, Lindsay KL, Bonkovsky HL, Di Bisceglie AM, Lee WM, Morgan TR, Dienstag JL, Morishima C. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42:282-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Qi XS, Bao YX, Bai M, Xu WD, Dai JN, Guo XZ. Nonselective beta-blockers in cirrhotic patients with no or small varices: A meta-analysis. World J Gastroenterol. 2015;21:3100-3108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2290] [Article Influence: 229.0] [Reference Citation Analysis (3)] |
| 27. | Marot A, Trépo E, Doerig C, Schoepfer A, Moreno C, Deltenre P. Liver stiffness and platelet count for identifying patients with compensated liver disease at low risk of variceal bleeding. Liver Int. 2017;37:707-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Llop E, Lopez M, de la Revilla J, Fernandez N, Trapero M, Hernandez M, Fernández-Carrillo C, Pons F, Martinez JL, Calleja JL. Validation of noninvasive methods to predict the presence of gastroesophageal varices in a cohort of patients with compensated advanced chronic liver disease. J Gastroenterol Hepatol. 2017;32:1867-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Tachi Y, Hirai T, Kojima Y, Tachino H, Hosokawa C, Ohya T, Yasue Y, Kurokawa Y, Torii Y, Yamamoto S, Matsuura H, Kobayashi T, Miyoshi H, Inui K, Katano Y. Diagnostic performance of real-time tissue elastography in chronic hepatitis C patients with sustained virological response. Eur J Gastroenterol Hepatol. 2020;32:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Sangma MA, Biswas N, Paul P. Relation of Hepatic Venous Doppler Sonography and Portal Flowmetry in Determination of Severity of Esophageal Varices in Liver Cirrhosis. Mymensingh Med J. 2019;28:727-733. [PubMed] |
| 31. | Hong WD, Dong LM, Jiang ZC, Zhu QH, Jin SQ. Prediction of large esophageal varices in cirrhotic patients using classification and regression tree analysis. Clinics. 66:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Maurer R, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS, Makuch R; Portal Hypertension Collaborative Group. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology. 2008;47:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | A Erdogan M, R Benli A, B Acmali S, Koroglu M, Atayan Y, Danalioglu A, Kayhan B. Predictive Value of Mean Platelet Volume in Variceal Bleeding due to Cirrhotic Portal Hypertension. Euroasian J Hepatogastroenterol. 2017;7:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Bintintan A, Chira RI, Bintintan VV, Nagy GA, Manzat-Saplacan MR, Lupsor-Platon M, Stefanescu H, Duma MM, Valean SD, Mircea PA. Value of hepatic elastography and Doppler indexes for predictions of esophageal varices in liver cirrhosis. Med Ultrason. 2015;17:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Chakrabarti R, Sen D, Khanna V. Is non-invasive diagnosis of esophageal varices in patients with compensated hepatic cirrhosis possible by duplex Doppler ultrasonography? Indian J Gastroenterol. 2016;35:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Chawla S, Katz A, Attar BM, Gupta A, Sandhu DS, Agarwal R. Platelet count/spleen diameter ratio to predict the presence of esophageal varices in patients with cirrhosis: a systematic review. Eur J Gastroenterol Hepatol. 2012;24:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Hong WD, Zhu QH, Huang ZM, Chen XR, Jiang ZC, Xu SH, Jin K. Predictors of esophageal varices in patients with HBV-related cirrhosis: a retrospective study. BMC Gastroenterol. 2009;9:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Pang Q, Bi JB, Xu XS, Liu SS, Zhang JY, Zhou YY, Qu K, Liu C. King's score as a novel prognostic model for patients with hepatitis B-associated hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015;27:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Kraja B, Mone I, Akshija I, Koçollari A, Prifti S, Burazeri G. Predictors of esophageal varices and first variceal bleeding in liver cirrhosis patients. World J Gastroenterol. 2017;23:4806-4814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Wang L, Feng Y, Ma X, Wang G, Wu H, Xie X, Zhang C, Zhu Q. Diagnostic efficacy of noninvasive liver fibrosis indexes in predicting portal hypertension in patients with cirrhosis. PLoS One. 2017;12:e0182969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Zhou H, Long J, Hu H, Tian CY, Lin SD. Liver stiffness and serum markers for excluding high-risk varices in patients who do not meet Baveno VI criteria. World J Gastroenterol. 2019;25:5323-5333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Wang JH, Chuah SK, Lu SN, Hung CH, Chen CH, Kee KM, Chang KC, Tai WC, Hu TH. Transient elastography and simple blood markers in the diagnosis of esophageal varices for compensated patients with hepatitis B virus-related cirrhosis. J Gastroenterol Hepatol. 2012;27:1213-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Maruyama H, Yokosuka O. Ultrasonography for Noninvasive Assessment of Portal Hypertension. Gut Liver. 2017;11:464-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Zhang CX, Xu JM, Li JB, Kong DR, Wang L, Xu XY, Zhao DM. Predict esophageal varices via routine trans-abdominal ultrasound: A design of classification analysis model. J Gastroenterol Hepatol. 2016;31:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |