Published online Nov 7, 2020. doi: 10.3748/wjg.v26.i41.6488
Peer-review started: July 10, 2020
First decision: August 8, 2020
Revised: August 21, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: November 7, 2020
Processing time: 119 Days and 4 Hours
Although nonpharmacological interventions (NPI) for irritable bowel syndrome (IBS) have been applied clinically, their relative efficacy and safety are poorly understood.
To compare and rank different NPI in the treatment of IBS.
Five electronic databases were searched from their inception to January 12, 2020. Data of included publications were analyzed using network meta-analysis (NMA). Quality of endpoints were assessed by tools of the Cochrane Handbook and the GRADEpro software. Pooled relative risk or standardized mean difference with their corresponding 95% confidence intervals were used for statistical analysis. Surface under the cumulative ranking curve (SUCRA) probability value was conducted to rank the examined interventions. Sensitivity analysis was performed to verify the robustness of results and test the source of heterogeneity.
Forty randomized controlled trials with 4196 participants were included in this NMA. Compared with routine pharmacotherapies and placebo, acupuncture and cognitive behavioral therapy (CBT) had better efficacy in relieving IBS symptoms. Based on the SUCRA values, acupuncture ranked first in improving overall clinical efficacy and avoiding adverse effects. CBT ranked first in lowering the scores of IBS symptom severity scale, self-rating anxiety scale and self-rating depression scale.
This study confirmed the efficacy and safety of NPI for improving IBS symptoms, which to some extent recommended several interventions for clinical practice.
Core Tip: This is the first study to compare nonpharmacological interventions including biofeedback, cognitive behavioral therapy, probiotics, dietary, acupuncture, and moxibustion using network meta-analysis.
- Citation: Dai YK, Wu YB, Li RL, Chen WJ, Tang CZ, Lu LM, Hu L. Efficacy and safety of non-pharmacological interventions for irritable bowel syndrome in adults. World J Gastroenterol 2020; 26(41): 6488-6509
- URL: https://www.wjgnet.com/1007-9327/full/v26/i41/6488.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i41.6488
Irritable bowel syndrome (IBS) is one of the most common chronic functional gastrointestinal disorders, which is characterized by abdominal pain, irregular defecation or changes in stool property[1,2]. Currently, about 15% of the general population around the world are suffering from this condition[3]. Because of its symptoms IBS affects patients’ work and daily lives and could lead to an increase in healthcare cost[4,5]. According to the latest Rome criteria (Rome IV)[6], IBS is classified into diarrhea predominant, constipation predominant, mixed and unclassified.
However, the pathogenesis of IBS remains unclear. Some factors such as unhealthy lifestyles and diets, psychological factors, visceral allergies, gastrointestinal motility dysfunction and intestinal microbiota alteration have been taken into consideration[7]. Therefore, routine pharmacotherapies (RPs) such as antipsychotics, antispasmodics, promotility agents, laxatives and antidiarrheics are recommended for the management of IBS. Although these interventions can relieve symptoms like abdominal pain, their effects are inadequate and may produce some unwelcome reactions including ischemic colitis and cardiovascular events[8]. Due to the chronicity and recurrence of IBS, many patients are intolerability to pharmacological interventions for a long time and then put their eyes on nonpharmacological interventions (NPI).
As an add-on treatment or alternative option, NPI for IBS include dietary and physical interventions, biofeedback therapy (BFT), cognitive behavioral therapy (CBT), probiotics, acupuncture and moxibustion therapy. Although previous meta-analyses of these therapies showed good efficacy in improving global IBS symptoms[9-14], these studies have concentrated on individual aspects of NPI and are not comprehensive. Therefore, the reliability of the evidence might fluctuate by various assessment outcomes, thereby leading to between-study heterogeneity and mitigating their efficacies in guiding clinical practice.
Network meta-analysis (NMA) is a powerful statistical technique that combines direct and indirect evidence to analyze multiple treatments from different studies and estimate the relative effects of all included treatments in the network simultaneously[15]. Moreover, NMA has the advantage of assisting medical decision-making through providing useful and evidence-based data[16]. Based on these, we used NMA to evaluate the comparative effects and rankings of all known NPIs on IBS.
This study was conducted according to the Cochrane criteria, the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement[17] and relevant meta-analysis guidance[18].
Five electronic databases including OVID EMBASE, MEDLINE, Cochrane Library, PubMed and the Chinese database of CNKI were searched from their inception to January 12, 2020 without language limitation for randomized controlled trials (RCTs). Search strategies were performed with a combination of the following terms: Irritable bowel syndrome, randomized controlled trial, nonpharmacological interventions, biofeedback, cognitive behavioral therapy, probiotics, dietary, acupuncture and moxibustion. Detailed information for each database is displayed in Supporting Information S1. Some unpublished articles were searched in ClinicalTrials.gov and relevant data were obtained through contacting the investigators or authors. In case of duplicates, the most updated one was selected.
Relevant titles and abstracts were blindly evaluated and details of selected studies were independently analyzed by two researchers (Dai YK, Wu YB). Based on the PICOS (participants, interventions, comparisons, outcomes and study design) criteria, the following items were included in this NMA: IBS participants whose ages are 18 years or over should meet one of the Rome criteria versions (Rome II, III or IV)[19-21]; NPI should include at least one of the following treatments: Diet, biofeedback, CBT, probiotics, acupuncture or moxibustion; Outcomes should be at least one of these items such as overall clinical efficacy, IBS-SSS (symptom severity scale), SAS (self-rating anxiety scale) and SDS (self-rating depression scale). Moreover, treatment courses should be 4 wk or over. Studies with a Jadad score above 1 was selected for further analysis.
However, publications would be excluded once the following items appeared: Meeting abstracts; incomplete or imprecise data; ambiguous treatment courses; unavailable full texts; cross-sectional studies or reviews.
Two investigators (Dai YK, Wu YB) independently performed data extraction and methodological quality assessment. The following data should be extracted from each included trial: Study ID (first author and publication year), general characteristics of patients (gender, age and sample size), diagnostic criteria, details of interventions, treatment courses, primary and secondary outcomes and adverse events. Some absent information was obtained by contacting corresponding authors. The risk of bias of each study was assessed using the Cochrane Collaboration Recommendations assessment tool[22]. Six domains with the evaluation of risk bias were as follows: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcomes assessment, incomplete outcome data and selective reporting. Each domain of the included publications was judged as low, unclear or high risk. As for the evaluation of evidence quality, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used with the online guideline development tool (https://gdt.gradepro.org/app/). Quality of evidence in this NMA was assessed as high, moderate, low and very low quality[23].
Compared with results of standard and pairwise analyses, NMA results can afford more precise estimates and rank interventions to inform clinical decisions[24,25]. Therefore, in order to compare the efficacy and safety of each NPI across RCTs, a NMA was conducted using Stata version 13.0 software. For each treatment, we produced a pooled relative risk for dichotomous outcomes or standardized mean difference (SMD) for continuous variable data with their corresponding 95% confidence intervals (CI) to summarize the effect of each comparison tested using a random-effect model as a conservative estimate. Evidence of direct and indirect multiple-intervention comparisons were examined through producing a network plot where node sizes corresponded to the number of study participants while connection sizes referred to the number of studies for each intervention. According to the Bayesian framework and the Markov chain Monte Carlo method, we evaluated and processed research data a priori using WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, United Kingdom). Three Markov chains and noninformative uniform and normal priori distributions were used to fit the model[26,27]. Then, 10 thinning intervals each Markov chain and 50000 iterations were equipped so as to obtain their posterior distributions. Of all the simulation iterations, the first 20000 were applied to annealing for the elimination of impacts of the initial value while the last 30000 were used for sampling. Heterogeneity analysis was quantified using the inconsistency index statistic (I2)[28]. The I2 value above 50% was regarded as heterogeneity throughout the study. Accordingly, we conducted sensitivity analysis to verify the robustness of results and test the source of heterogeneity in each RCT. Surface under the cumulative ranking curve (SUCRA) probability value was used to rank the examined interventions[29].
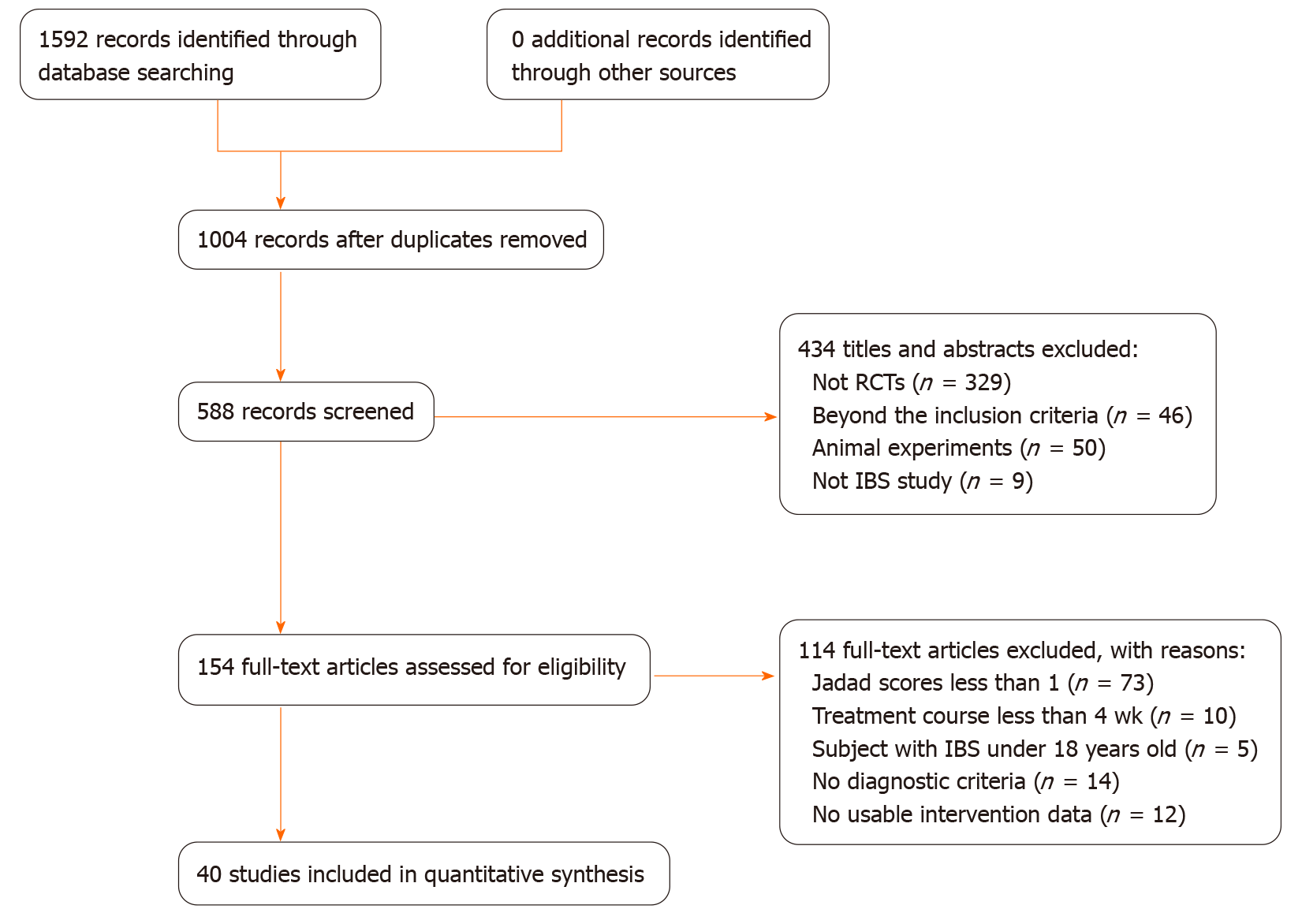
All of the 1592 articles were identified from five data libraries based on the well-established retrieval. Ultimately, 40 RCTs[30-69] including 4196 participants were selected in the NMA according to the inclusion and exclusion criteria. The study selection process is shown in Figure 1. The baseline characteristics of the included studies are summarized in Table 1.
| Ref. | Country | Classification of IBS, criterion | Sample size | Age in yr | Course of disease in yr | Treatment cycle in wk | Intervention | Endpoints | Follow-up | Side effects | ||
| EG, M/F | CG, M/F | EG | CG | |||||||||
| Yang et al[30], 2019 | China | IBS-D (Rome III) | 43/30 | 44/29 | E: 43.93 ± 13.58 C: 45.00 ± 16.67 | E: 3.74 ± 5.02 C: 4.12 ± 4.94 | 4 | AP | Placebo | a, f, h | N/A | N/A |
| He et al[31], 2019 | China | IBS-D (Rome IV) | 13/12 | 14/11 | E: 47.88 ± 15.16 C: 48.56 ± 17.4 | N/A | 4 | AP | Probiotics | a, f, j | N/A | N/A |
| Li[32], 2019 | China | IBS-D (Rome IV) | 15/14 | 15/13 | E: 45.30 ± 11.52 C: 48.33 ± 12.13 mo | E: 10.98 ± 5.12 C: 10.79 ± 5.04 mo | 4 | AP + MB | RPs | a, d, h, i | N/A | N/A |
| Wang et al[33], 2019 | China | IBS (Rome IV) | 25/31 | 23/32 | E: 46.00 ± 2.50 C: 46.80 ± 2.70 | E: 3.20 ± 1.40 C: 3.12 ± 1.38 | 4 | AP + MB | RPs | a, h, j | N/A | N/A |
| Zhang et al[34], 2019 | China | IBS (Rome III) | 23/21 | 25/19 | E: 47.23 ± 2.18 C: 47.66 ± 2.12 | E: 5.22 ± 0.11 C: 5.26 ± 0.16 | 8 | Probiotics | placebo | a, j | N/A | N/A |
| Peng et al[35], 2019 | China | IBS-D (Rome IV) | 14/16 | 16/14 | E: 46.85 ± 14.45 C: 45.43 ± 13.58 | E: 3.65 ± 1.15 C: 3.84 ± 1.32 | 4 | BFT | Probiotics | a, d, f | N/A | N/A |
| Kou et al[36], 2018 | China | IBS-D (Rome III) | 16/29 | 18/27 | E: 38.24 ± 6.58 C: 38.37 ± 6.60 | N/A | 4 | Probiotics + RPs | RPs | a, b, e | N/A | E: 1 C: 2 |
| Sun[37], 2018 | China | IBS-D (Rome III) | 63/42 | 53/42 | E: 43.00 ± 12.45 C: 44.91 ± 13.01 | N/A | 4 | Probiotics | placebo | b, d, f, k, | N/A | E: 6 C: 2 |
| Qin et al[38], 2018 | China | IBS (Rome III) | 45/47 | 45/48 | E: 42.8 ± 8.7 C: 44.2 ± 8.8 | E: 4.5 ± 1.1 C: 4.5 ± 1.2 | 4 | Probiotics + RPs | RPs | a, g, n | N/A | E: 0 C: 0 |
| Zhang et al[39], 2018 | China | IBS (Rome II) | 15/28 | 17/26 | E: 42.16 ± 7.24 C: 43.68 ± 9.09 | N/A | 4 | CBT | RPs | d, o | N/A | N/A |
| Chen et al[40], 2017 | China | IBS-D (Rome III) | 31/13 | 30/14 | E: 46.52 ± 3.75 C: 46.13 ± 3.82 | N/A | 4 | Probiotics + RPs | RPs | a, g, j | N/A | N/A |
| Wang et al[41], 2017 | China | IBS-D (Rome III) | 17/21 | 16/22 | E: 46.5 ± 2.3 C: 46.3 ± 2.2 | E: 3.3 ± 0.8 C: 3.2 ± 0.7 | 4 | Probiotics + RPs | RPs | a, b | N/A | E: 3 C: 1 |
| Hod et al[42], 2017 | United States | IBS-D (Rome III) | 54 | 53 | E: 29.0 C: 30.0 | N/A | 4 | Probiotics | Placebo | a, b, e | N/A | E: 0 C: 0 |
| Joo et al[43], 2017 | Korea | IBS (Rome III) | 9/17 | 5/19 | E: 32.5 C: 33.0 | N/A | 4 | Probiotics | Placebo | a, b, p | N/A | E: 0 C: 0 |
| Liu et al[44], 2017 | China | IBS-C (Rome III) | 17/23 | 17/23 | 43.86 ± 10.29 | 2.93 ± 1.06 | 8 | Probiotics + RPs | RPs | a, b, e, g | N/A | E: 0 C: 0 |
| Huang[45], 2017 | China | IBS-C (Rome III) | 16/23 | 15/25 | E: 44.23 ± 11.92 C: 41.54 ± 12.24 | E: 4.11 ± 1.94 C: 3.54 ± 2.19 | 4 | BFT | RPs | a, e, u | N/A | N/A |
| Cheng et al[46], 2017 | China | IBS-D (Rome III) | 19/22 | 18/21 | E: 36.27 ± 2.78 C: 41.69 ± 12.63 | N/A | 8 | CBT | RPs | d, f, o | N/A | N/A |
| Kang et al[47], 2016 | China | IBS-D (Rome III) | 17/23 | 16/24 | E: 44.5 ± 6.4 C: 42.5 ± 7.2 | N/A | 4 | Probiotic + RPs | RPs | a, i, j | N/A | N/A |
| Robin et al[48], 2016 | France | IBS (Rome III) | 31/161 | 31/156 | E: 45.3 ± 15.7 C: 45.4 ± 14.1 | N/A | 12 | Probiotics | Placebo | a, b, e, m | N/A | E: 10 C: 0 |
| Zhang et al[49], 2016 | China | IBS (Rome III) | 12/18 | 14/16 | E: 40.7 ± 11.4 C: 36.3 ± 14.1 | E: 3.58 ± 2.04 C: 3.88 ± 2.36 | 4 | Probiotics | RPs | a | N/A | E: 0 C: 2 |
| Han et al[50], 2016 | Korea | IBS (Rome III) | 13/10 | 11/12 | E: 45.7 ± 9.55 C: 42.5 ± 10.07 | N/A | 4 | Probiotics | Placebo | a, k, l, p | N/A | N/A |
| Jia et al[51], 2016 | China | IBS (Rome III) | 16/14 | 22/10 | E: 40.08 ± 13.23 C: 41.31 ± 11.82 | N/A | 8 | CBT | RPs | f, o | N/A | N/A |
| Choi et al[52], 2015 | South Korea | IBS (Rome III) | a: 20/34 b: 35/25 C: 35/23 d: 25/31 | 26/31 | E: a: 44.8 ± 13.4 b: 48.9 ± 14.2 C: 46.2 ± 13.8 d: 45.9 ± 12.8 C: 48.5 ± 13.2 | N/A | 6 | Probiotics + RPs | Placebo | a, b, m | N/A | E: 4/8/8/8 C: 6 |
| Jia et al[53], 2015 | China | IBS (Rome III) | N/A | N/A | E: 44.74 ± 11.98 C: 40.85 ± 13.87 | N/A | 8 | CBT | RPs | d, o | N/A | N/A |
| Shi et al[54], 2015 | China | IBS-D (Rome III) | 28/32 | 25/35 | E: 40.2 ± 10.8 C: 38.5 ± 9.1 | E: 8.6 ± 3.8 C: 7.3 ± 2.1 | 4 | AP | RPs | a | N/A | N/A |
| Li[55], 2015 | China | IBS-D (Rome III) | N/A | N/A | E: 46 C: 46 | E: 4.2 C: 4.2 | 4 | AP | RPs + Probiotics | a, e, g | N/A | N/A |
| Ye et al[56], 2015 | China | IBS (Rome III) | N/A | N/A | 43.59 ± 12.17 | 2.42 ± 1.27 | 4 | BFT + Probiotics | Probiotics | o, r, v | N/A | N/A |
| Zheng[57], 2014 | China | IBS-D (Rome III) | 49/40 49/36 40/42 | 52/34 | E: 38.75 ± 18.32 42.66 ± 16.75 42.51 ± 16.78 C: 42.29 ± 18.30 | E: 72.91 ± 76.70 78.83 ± 99.19 77.51 ± 84.56 C: 87.67 ± 90.28 d | 4 | AP | RPs | b, k, l, o, q, s | N/A | E: 3 C: 0 |
| Zhu et al[58], 2014 | China | IBS-D (Rome III) | 9/6 | 7/6 | E: 47.470 ± 0.896 C: 40.920 ± 10.136 | E: 3.0 C: 3.5 | 4 | MB | Placebo | d, t, u | N/A | N/A |
| Kong[59], 2014 | China | IBS-D (Rome III) | 14/16 | 9/21 | E: 40 ± 9 C: 38 ± 11 | E: 5.87 ± 6.52 C: 6.21 ± 6.33 | 4 | AP+MB | RPs | a, d, e | N/A | N/A |
| He et al[60], 2014 | China | IBS-D (Rome III) | N/A | N/A | 37.3 ± 10.4 | 3.7 ± 2.1 | 4 | BFT + RPs | RPs | a, g, i, n, v | N/A | N/A |
| Cheryl et al[61], 2014 | South Africa | IBS (Rome III) | 2/52 | 0/27 | E: 48.15 ± 13.48 C: 47.27 ± 12.15 | E: 9.58 ± 10.32 C: 10.05 ± 9.36 | 6 | Probiotics | Placebo | b, d | N/A | E: 1 C: 0 |
| Lesley et al[62], 2013 | Britain | IBS (Rome III) | 15/73 | 15/76 | E: 44.66 ± 11.98 C: 43.71 ± 12.76 | N/A | 4 | Probiotics | Placebo | a, d, e, f, m | N/A | N/A |
| Ge[63], 2013 | China | IBS (Rome III) | 34/26 | 32/28 | E: 38.9 ± 11.2 C: 39.1 ± 10.3 | E: 6.5 C: 6.4 | 4 | AP | RPs | a, c | E: 6/52 C: 12/43 | N/A |
| Pei et al[64], 2012 | China | IBS-D (Rome III) | 13/17 | 10/20 | E: 39.10 ± 11.80 C: 37.93 ± 11.45 | E: 4.33 ± 3.93 C: 5.23 ± 7.35 | 4 | AP | RPs | a | N/A | N/A |
| Kruis et al[65], 2012 | Germany | IBS (Rome II) | 12/48 | 16/44 | E: 46.3 ± 12.1 C: 45.1 ± 12.7 | E: 12.3 ± 11.5 C: 11.7 ± 12.0 | 12 | Probiotics | Placebo | a, b | N/A | E: 0 C: 1 |
| Sun et al[66], 2011 | China | IBS-D (Rome III) | 13/18 | 20/12 | E: 38.81 ± 11.80 C: 38.59 ± 11.45 | E: 4.23 ± 3.96 C: 5.63 ± 7.35 | 4 | AP | RPs | a, b, d, e | NA | E: 0 C: 0 |
| Zeng et al[67], 2011 | China | IBS-D (Rome III) | 39/30 | 41/28 | E: 38.5 ± 8.4 C: 37.9 ± 9.6 | E: 3.7 ± 1.8 C: 3.5 ± 2.1 | 8 | Probiotics + RPs | RPs | a, b, r | N/A | E: 14 C: 12 |
| Zhao et al[68], 2011 | China | IBS (Rome III) | N/A | N/A | 38.6 ± 11.2 | UN | 4 | BFT | RPs | o, r, v | N/A | N/A |
| Wang et al[69], 2008 | China | IBS-D (Rome II) | N/A | N/A | E: 42.8 ± 12.4 C: 43.7 ± 11.7 | E: 3.41 ± 1.02 C: 3.23 ± 1.31 | 4 | AP | RPs | a | N/A | N/A |
The quality of each included RCT was evaluated using the Cochrane Risk of Bias Assessment Tool[70] including these factors:
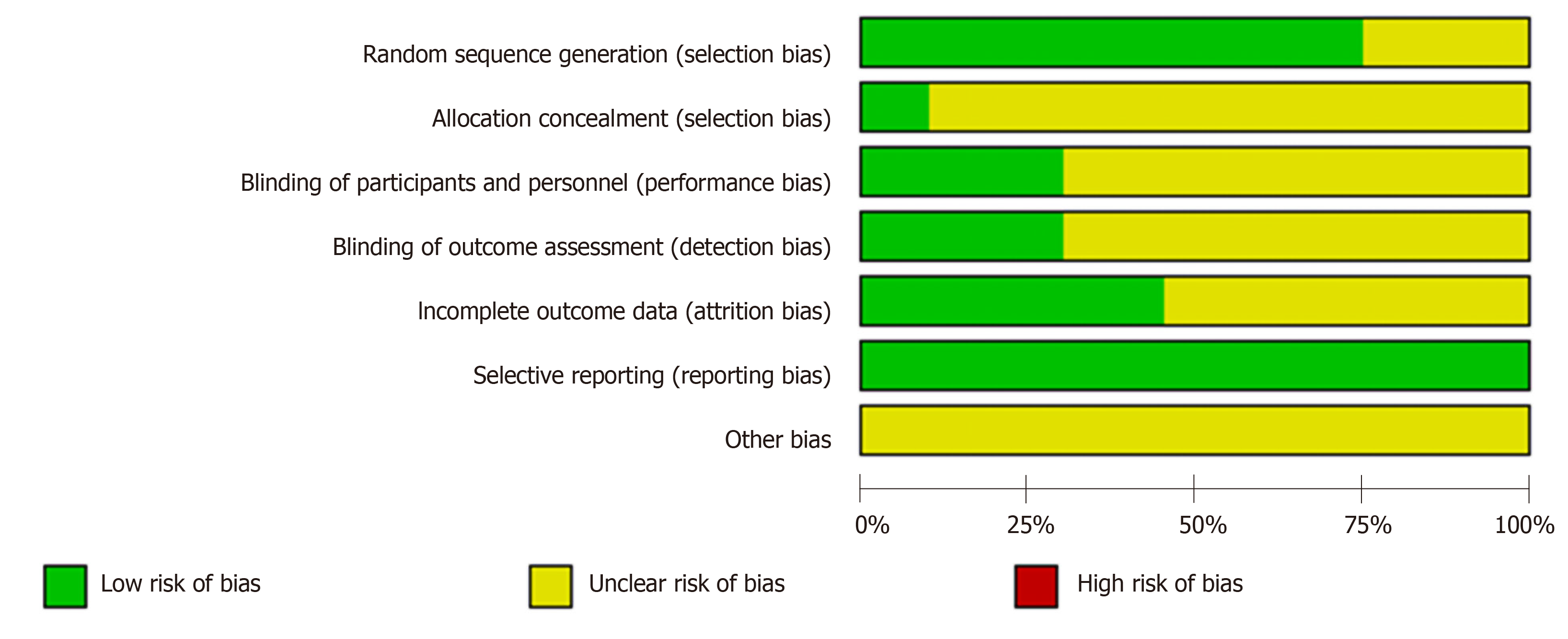
(1) Selection bias: Thirty trials grouped patients according to detailed randomized algorithms while the remaining ten only described “randomization.” Therefore, the thirty trials were assessed as “low risk” while the other ten were viewed as “unclear risk.” As for the allocation concealment, four trials were evaluated as “low risk” within detailed information while the remaining 36 trials were viewed as “unclear risk” because of insufficient information.
(2) Performance bias and detection bias: Twelve trials provided information on blinding and were blinded to the outcome assessors. Therefore, both performance bias and detection bias were assessed as “low risk.” However, the remaining 28 trials failed to provide adequate information on blinding. Therefore, both of the two biases were viewed as “unclear risk.”
(3) Attrition bias: Twenty-three trials were evaluated as “unclear risk” for their incomplete data while the remaining seventeen trials were estimated as “low risk” because they reported withdrawal or dropout.
(4) Reporting bias: Because the complete implementation scheme could be acquired, the bias of all the trials was assessed as “low risk.”
(5) Other bias: Considering the lack of information in this item, all included RCTs were estimated as “unclear risk.” The detailed quality evaluation of the included studies is shown in Figure 2.
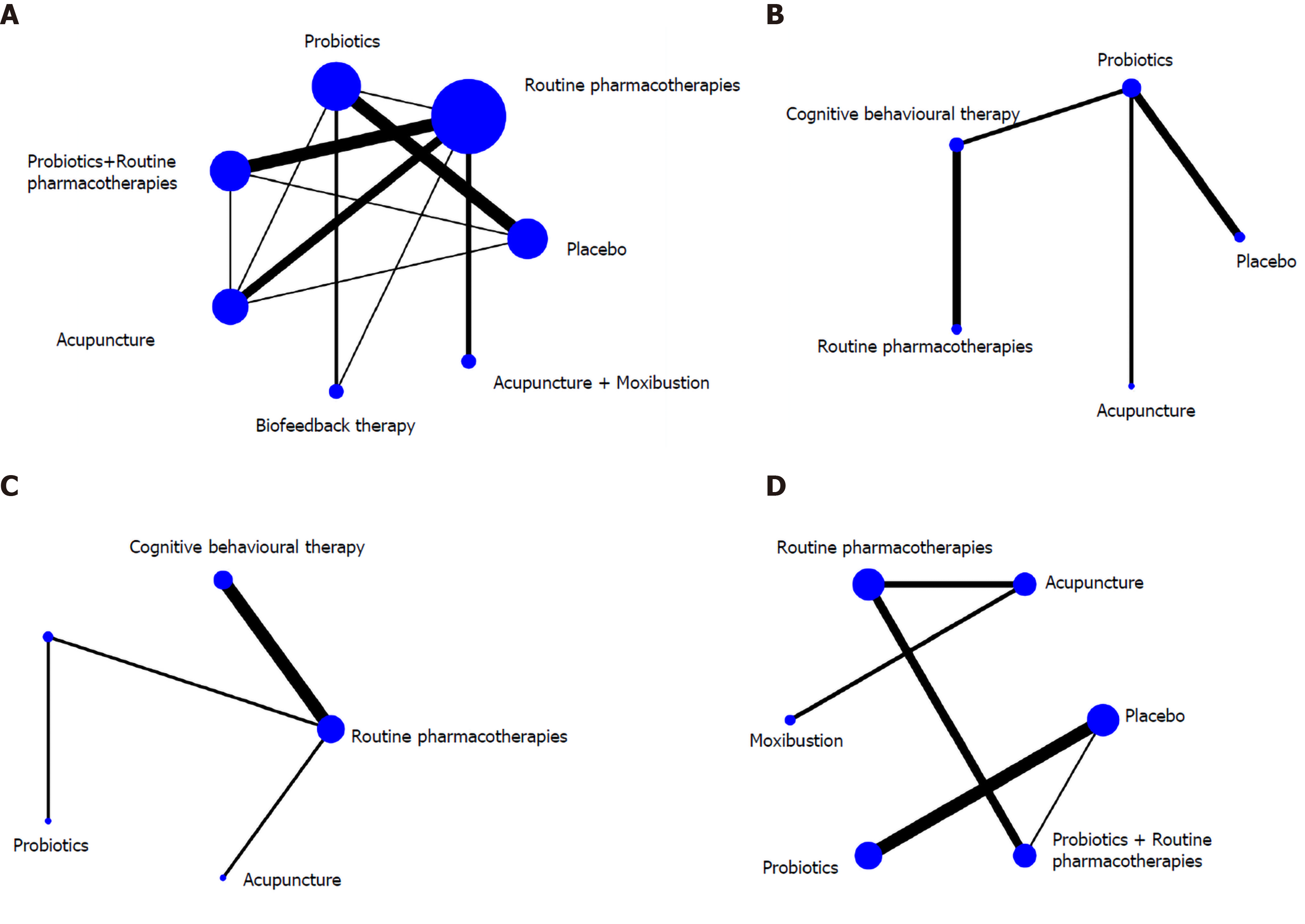
There were ten regimens in this study as follows: RPs, placebo, probiotics, probiotics + RPs, BFT, BFT + probiotics, CBT, acupuncture, moxibustion and acupuncture + moxibustion. The network graphs of these regimens with different outcomes are displayed in Figure 3.
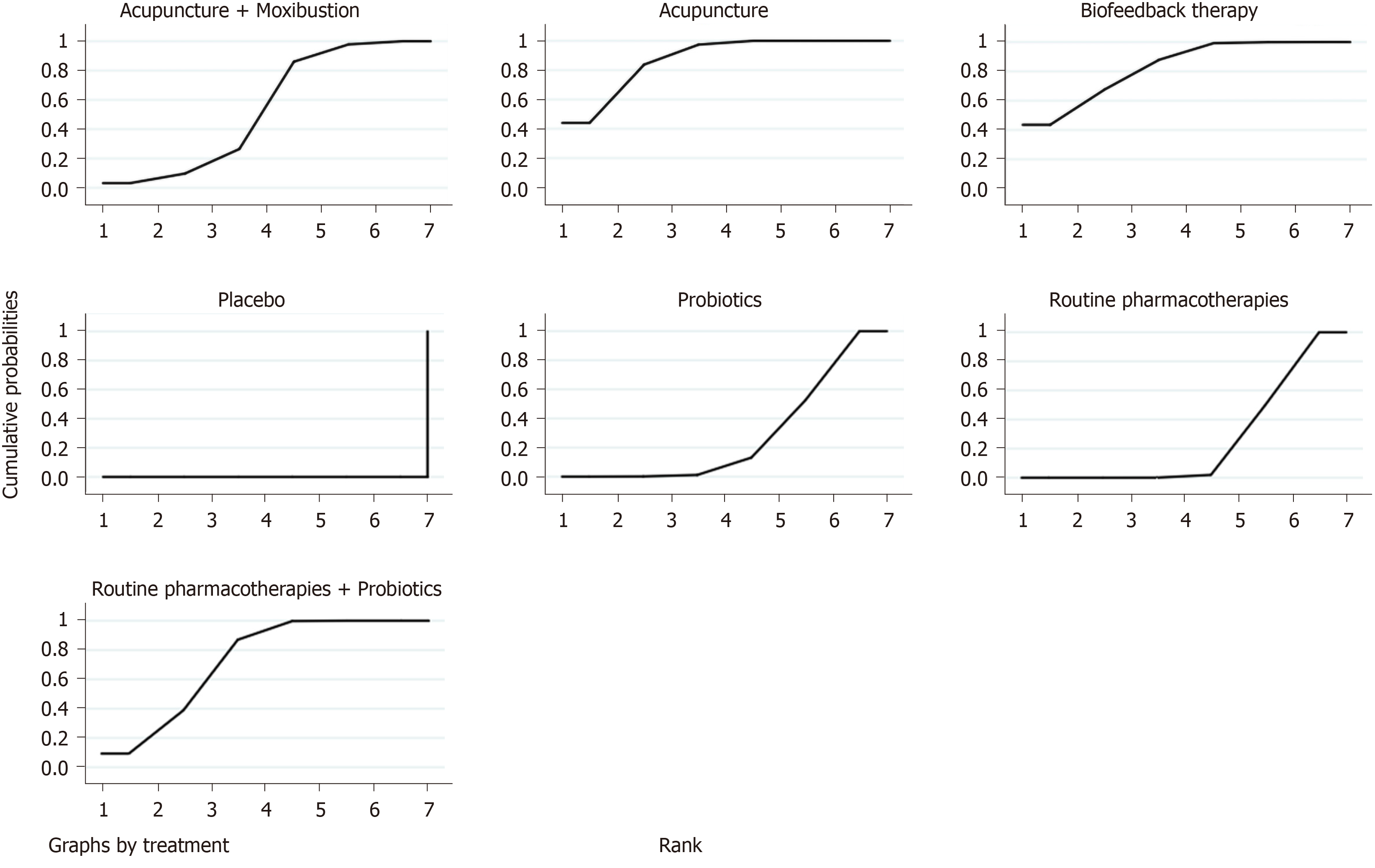
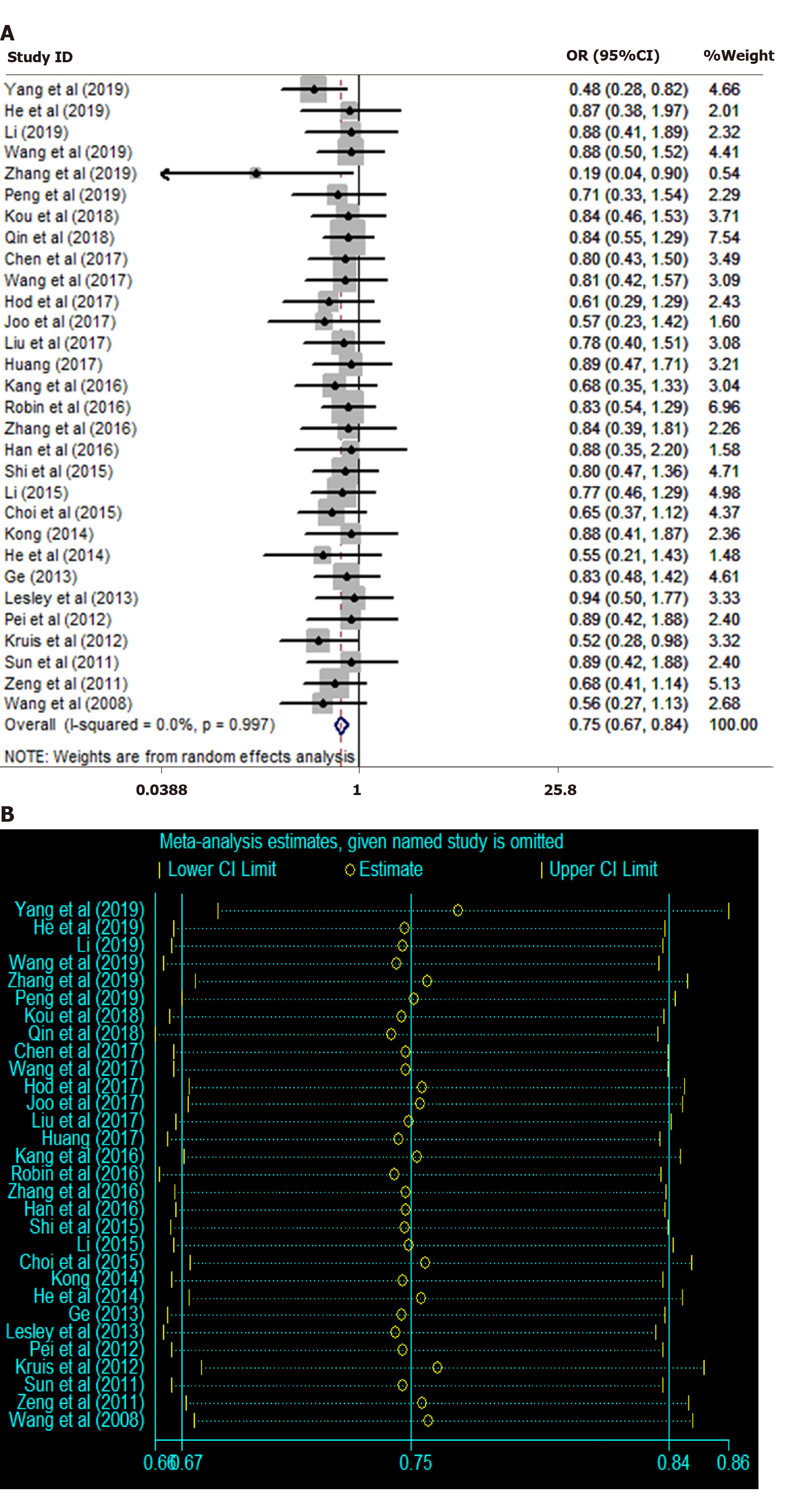
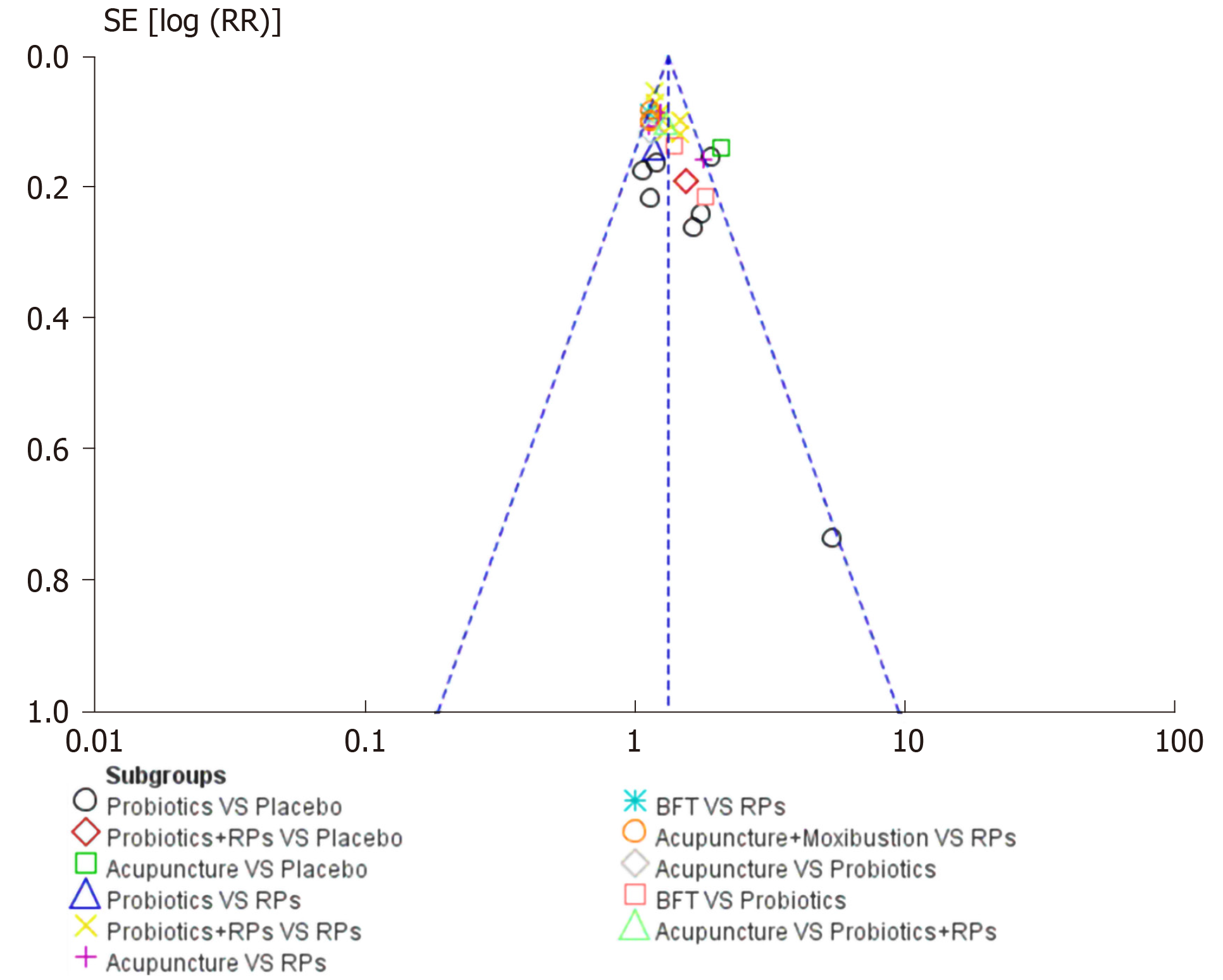
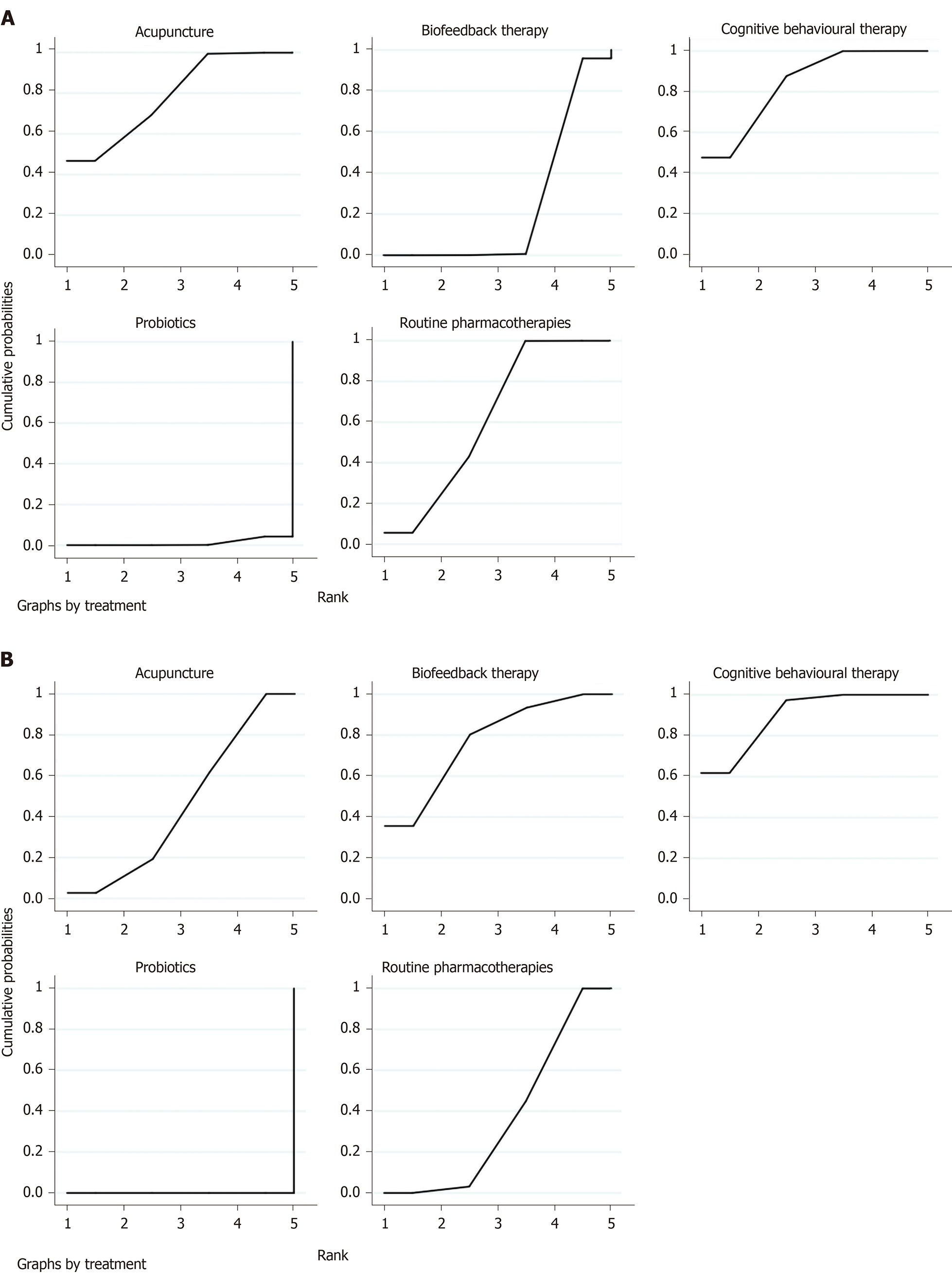
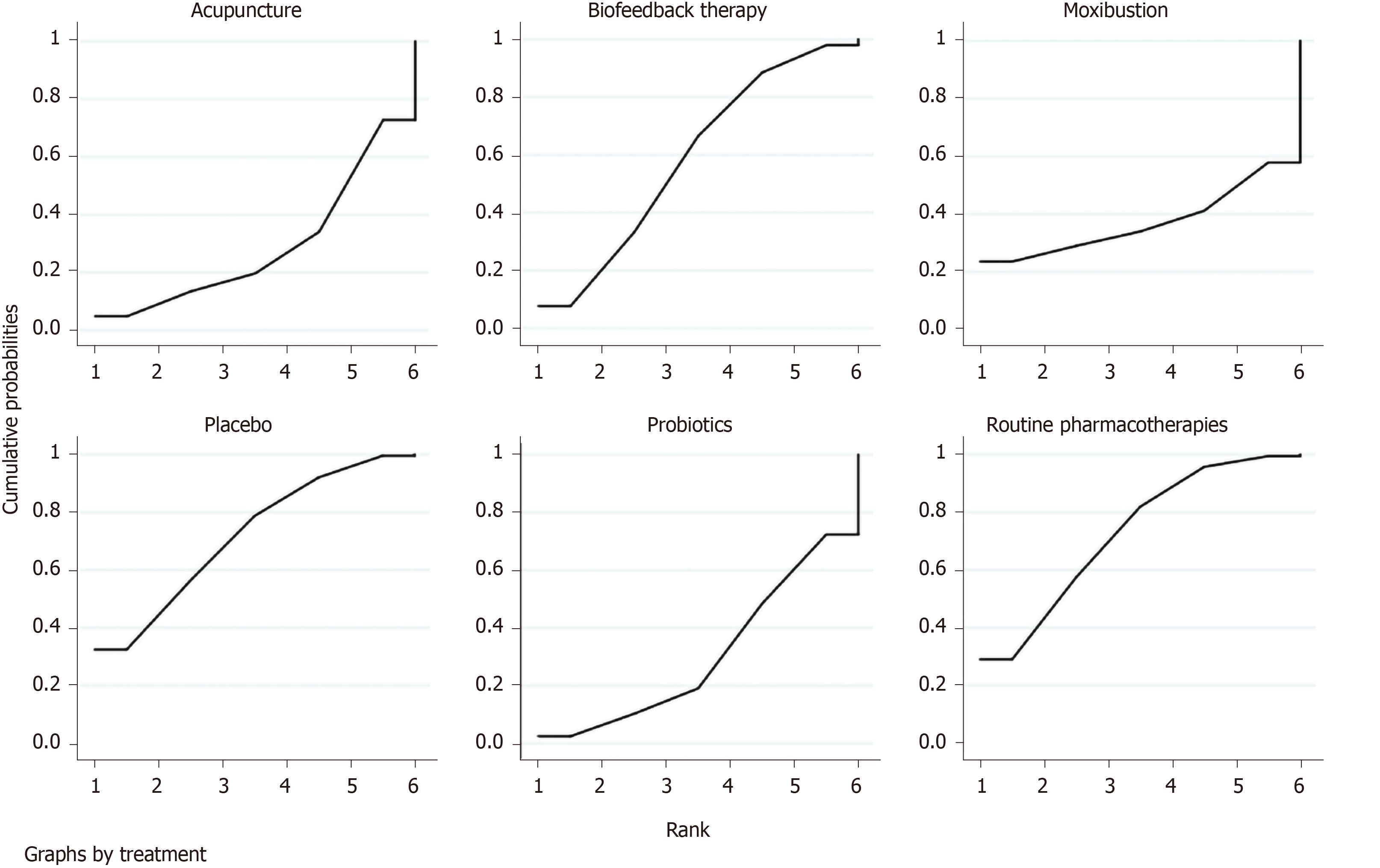
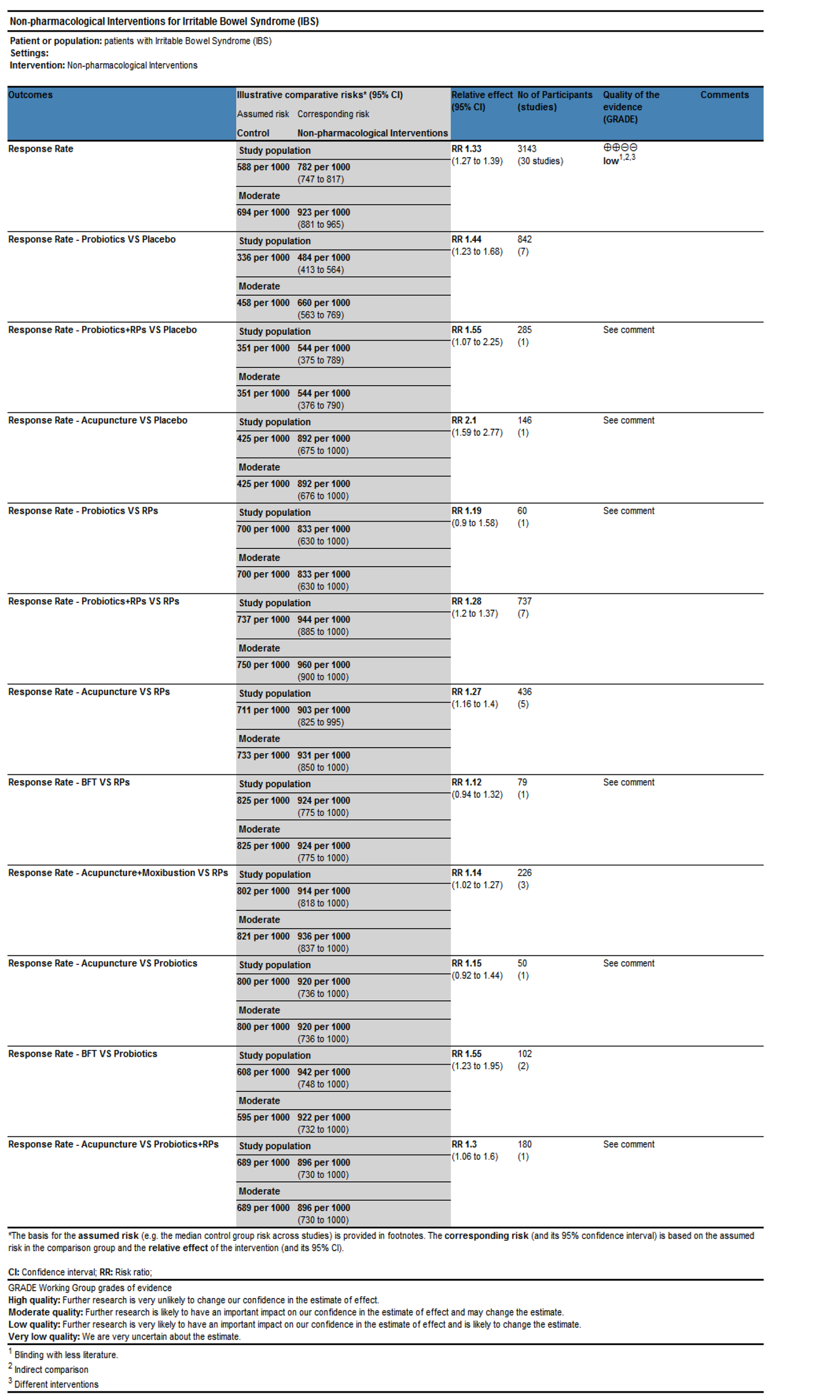
Overall clinical efficacy: There were 30 RCTs reporting overall clinical efficacy. As displayed in Table 2, RPs, probiotics, probiotics + RPs, acupuncture, BFT and acupuncture + moxibustion had better overall clinical efficacy than placebo; Probiotics + RPs, acupuncture and BFT had better overall clinical efficacy than RPs and probiotics. The differences among the above mentioned treatments were statistically significant. As shown in Figure 4, the SUCRA plot indicated that acupuncture ranked first, followed by BFT and probiotics + RPs. Meanwhile, heterogeneity analysis (Figure 5A) showed good homogeneity (I2 = 0.0%, P = 0.997), and sensitivity analysis (Figure 5B) indicated strong stability in the ranking of all treatments for overall clinical efficacy. Furthermore, the symmetry funnel plot of this endpoint was observed in Figure 6.
| RPs | |||||
| 0.99 (0.85, 1.17)a | Probiotics | ||||
| 0.81 (0.75, 0.88)a | 0.82 (0.69, 0.97)a | RPs + probiotics | |||
| 0.77 (0.70, 0.86)a | 0.78 (0.66, 0.91)a | 0.95 (0.84, 1.07) | Acupuncture | ||
| 0.78 (0.64, 0.94)a | 0.78 (0.64, 0.95)a | 0.96 (0.78, 1.17) | 1.01 (0.82, 1.23) | BFT | |
| 0.88 (0.77, 1.01)a | 0.88 (0.72, 1.09) | 1.08 (0.92, 1.27) | 1.14 (0.96, 1.35) | 1.13 (0.89, 1.43) | Acupuncture + moxibustion |
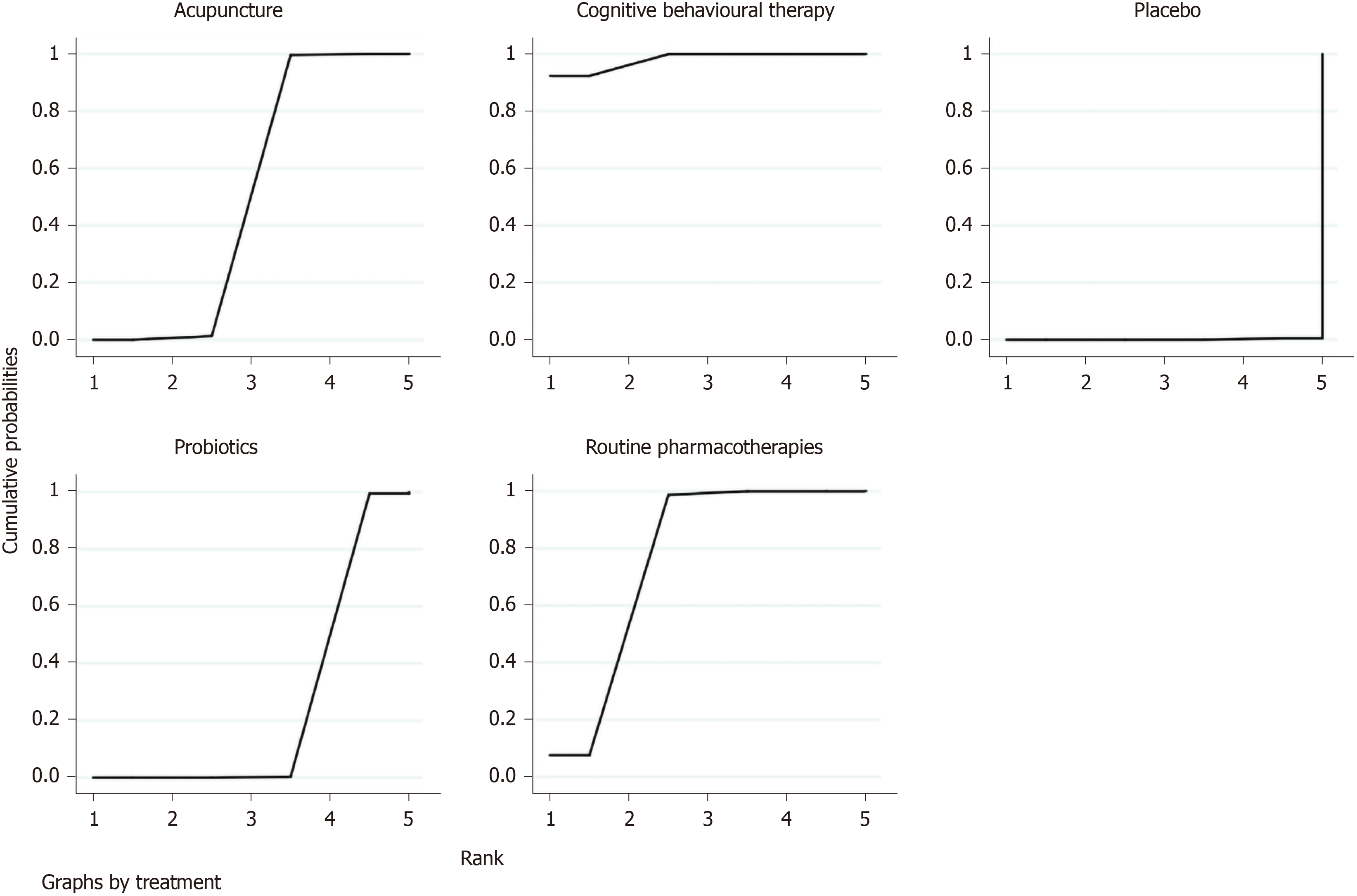
IBS-SSS: The improvement of IBS-SSS was reported in seven RCTs with five interventions (RPs, placebo, probiotics, CBT and acupuncture). Compared with placebo (Table 3), CBT (SMD = 2.39, 95%CI: 1.71, 3.07), RPs (SMD = 2.15, 95%CI: 1.39, 2.90) and probiotics (SMD = 0.30, 95%CI: 0.07, 0.52) had significantly statistical differences. CBT (SMD = 2.09, 95%CI: 1.46, 2.73) and RPs (SMD = 1.85, 95%CI: 1.13, 2.57) were superior to probiotics. CBT (SMD = 0.24, 95%CI: -0.09, 0.57) was better than RPs. According to the SUCRA plot (Figure 7), CBT was the optimal intervention, RPs was the second and acupuncture was the third.
SAS and SDS: In this NMA, seven RCTs with five treatments (RPs, probiotics, BFT, CBT and acupuncture) reported improvement of SAS and SDS. As show in Table 4, CBT (SMD = 3.44, 95%CI: 1.49, 5.39), acupuncture (SMD = 3.39, 95%CI: 1.19, 5.58) and RPs (SMD = 3.13, 95%CI: 1.28, 4.97) had better significant improvement of SAS than probiotics. CBT (SMD = 0.31, 95%CI: -0.31, 0.94) was superior to RPs. As for the improvement of SDS, Table 4 showed that CBT (SMD = 2.97, 95%CI: 1.70, 4.23), BFT (SMD = 2.81, 95%CI: 1.86, 3.77), acupuncture (SMD = 2.36, 95%CI: 1.01, 3.72) and RPs (SMD = 2.27, 95%CI: 1.06, 3.49) were better than probiotics. CBT (SMD = 0.15, 95%CI: -0.68, 0.99) was superior to BFT. Acupuncture (SMD = 0.09, 95%CI: -0.51, 0.69) was better than RPs. Meanwhile, the SUCRA plot suggested that CBT was the most favorable treatment in the improvement of SAS and SDS (Figure 8).
| SMD (95%CI) | ||||
| SAS | ||||
| CBT | ||||
| 0.05 (-1.29, 1.39) | Acupuncture | |||
| 0.31 (-0.31, 0.94)a | 0.26 (-0.92, 1.45) | RPs | ||
| 2.28 (0.83, 3.74) | 2.24 (0.47, 4.01) | 1.97 (0.66, 3.29) | BFT | |
| 3.44 (1.49, 5.39)a | 3.39 (1.19, 5.58)a | 3.13 (1.28, 4.97)a | 1.15 (-0.15, 2.45) | Probiotics |
| SDS | ||||
| CBT | ||||
| 0.15 (-0.68, 0.99)a | BFT | |||
| 0.61 (-0.10, 1.31) | 0.45 (-0.51, 1.42) | Acupuncture | ||
| 0.69 (0.33, 1.06) | 0.54 (-0.21, 1.29) | 0.09 (-0.51, 0.69)a | RPs | |
| 2.97 (1.70, 4.23)a | 2.81 (1.86, 3.77)a | 2.36 (1.01, 3.72)a | 2.27 (1.06, 3.49)a | Probiotics |
A total of sixteen RCTs with six interventions (RPs, placebo, probiotics, probiotics + RPs, acupuncture and moxibustion) reported adverse effects. There were no significant statistical differences among these treatments (Table 5). According to the SUCRA plot (Figure 9), acupuncture was the most favorable intervention, probiotics was the second and moxibustion was the third.
| RPs | |||||
| 0.99 (0.35, 2.81) | Placebo | ||||
| 0.85 (0.45, 1.59) | 0.86 (0.37, 1.97) | BFT | |||
| 0.39 (0.02, 9.12) | 0.39 (0.01, 10.93) | 0.46 (0.02, 11.47) | Moxibustion | ||
| 0.50 (0.13, 1.89) | 0.51 (0.22, 1.15) | 0.59 (0.18, 1.90) | 1.29 (0.04, 39.33) | Probiotics | |
| 0.40 (0.09, 1.88) | 0.41 (0.06, 2.62) | 0.47 (0.09, 2.51) | 1.03 (0.07, 16.13) | 0.80 (0.10, 6.13) | Acupuncture |
For the primary endpoint, the quality of estimates was “low” (Figure 10). Considering the details of GRADE criteria, the result was possibly derived from quality ratings of direct and indirect comparisons within RCTs, thereby leading to imprecision and unclear risk of bias.
NMA is used to analyze trials with multiple interventions and provides rankings for them[71]. Although RPs for IBS can benefit patients, inevitable adverse effects have to be admitted. Accordingly, NPI for IBS have been developed. In this study, to compare the different NPIs, a NMA of multiple NPI comparisons was conducted. Results showed the comprehensive analysis of data for retrievable IBS interventions at present. Based on the SUCRA values, acupuncture was most likely to improve overall clinical efficacy and least likely to result in adverse effects. CBT was most likely to lower the scores of IBS-SSS and SAS and SDS. In summary, when NPIs are used as an alternative therapy in treating IBS, acupuncture and CBT had better efficacy in relieving IBS symptoms.
With the exception of the potential factors mentioned earlier, genetic findings in IBS pathogenesis should also be taken into consideration. Gazouli et al[72] confirmed that single nucleotide polymorphisms in genes of serotonergic signaling pathway are associated with at least a subgroup of IBS. For instance, patients who carry an S allele or S/S genotype have differences in the central processing of visceral pain, which could result in a high susceptibility to negative emotional memory and contribute to enhanced visceral pain perception[73,74]. As is well-known, visceral hypersensitivity has been deemed as an important neurological evidence underlying the pathogenesis of abdominal pain in IBS, and visceral pain is associated with a dysregulation of the brain-gut axis[75,76]. Some clinical investigations have confirmed the efficacy of acupuncture in the regulation of the abnormal brain activities and improving visceral hypersensitivity in IBS sufferers[77,78]. Moreover, numerous animal studies have also suggested that acupuncture could significantly reduce the peripheral blood flow of rats with 5-hydroxytryptamine positive reactant content and improve visceral hypersensitivity[79-81].
As a typical psychosomatic disease, IBS sufferers have more or less cognitive biases and negative coping styles[82,83]. A few studies have shown that CBT could improve these negative emotions and mental tension by means of relaxation training, respiratory training and hypnotherapy, which made them identify uncontrollable stressors[84-86]. Not only that, CBT could also correct their negative coping styles to relieve psychosomatic damage caused by IBS symptoms, thereby improving the overall well-being and quality of life of these patients[87]. Based on this evidence, our findings may supplement the recommendations of existing guidelines and identify specific NPI with better effects.
Consistency is viewed as a one-way comparative relationship between direct and indirect evidence in an NMA[88]. It would be lack of transitivity if there was an inconsistency in a statistical analysis. In this paper, although heterogeneity analysis indicated good homogeneity and sensitivity analysis suggested strong stability in overall clinical efficacy, clinical heterogeneity such as the improvement of IBS-SSS, SAS and SDS, which were evaluated by an excessive personal opinion from professional practitioners or participants should be noticed. Meanwhile, comprehensive evaluation of outcome measurements on different IBS types should also be seriously considered.
There were several limitations in this study. First, although RCTs are insusceptible to many biases, some certain defects in them including design, conduct, analysis and reporting may lead to bias. In this NMA, the methodological quality of all RCTs was moderate and quality estimates based on the GRADE system showed “Low,” which may originate from some overlooked details on randomization and blinding, especially for CBT, BFT, acupuncture and moxibustion that were hard to blind. Second, strict inclusion and exclusion criteria were used in this study, but the number of each NPI in all included trials had relatively large differences (acupuncture /moxibustion: 13 trials, CBT: 4 trials, BFT: 5 trials and probiotics: 18 trials), which was likely to influence the strength of the evidence. Third, although all included RCTs were assessed based on the Cochrane Risk of Bias Assessment Tool, any assessment of bias is subjective. We have to admit that no quantitative index could assess only artificial risk of bias so far. Finally, 32 (80%) of the included RCTs were conducted in China, which may reduce the universality of our results.
In conclusion, evidence from this NMA showed that acupuncture could be beneficial for patients with IBS because of improved overall clinical efficacy and less adverse effects. CBT had preferable effects in lowering the scores of IBS-SSS, SAS and SDS. However, more RCTs should be performed to confirm the impact of NPIs on other IBS symptoms, and additional high-quality clinical research should be conducted to offer more powerful evidence in the future.
Although nonpharmacological interventions (NPI) for irritable bowel syndrome (IBS) have been applied clinically, their relative efficacy and safety are poor understood.
The key significance of this network analysis is to compare and rank different NPIs in the treatment of IBS in clinical practice.
The aim of this study was to determine the rates of overall clinical efficacy and adverse effects, the scores of IBS symptom severity scale (IBS-SSS), self-rating anxiety scale (SAS) and self-rating depression scale (SDS).
Five electronic databases were searched from their inception to January 12, 2020. Data of included publications were analyzed using network meta-analysis (NMA). Quality of endpoints were assessed by tools of the Cochrane Handbook and the GRADEpro software. Pooled relative risk or standardized mean difference with their corresponding 95% confidence intervals were used for statistical analysis. Surface under the cumulative ranking curve (SUCRA) probability value was conducted to rank the examined interventions. Sensitivity analysis was performed to verify the robustness of results and test the source of heterogeneity.
Forty randomized controlled trials with 4196 participants were included in this NMA. Compared with routine pharmacotherapies and placebo, acupuncture and cognitive behavioral therapy (CBT) had better efficacy in relieving IBS symptoms. Based on the SUCRA values, acupuncture ranked first in improving overall clinical efficacy and avoiding adverse effects. CBT ranked first in lowering the scores of IBS-SSS, SAS and SDS.
This study confirmed the efficacy and safety of NPIs for improving IBS symptoms, which to some extent recommended several interventions for clinical practice.
Future large RCTs should be performed to confirm the impact of NPIs on other IBS symptoms, and additional high-quality clinical researches should be conducted to offer more powerful evidence in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Integrative and complementary medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gazouli M, Kang S, Soares RLS S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1898] [Article Influence: 210.9] [Reference Citation Analysis (3)] |
| 2. | Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1416] [Article Influence: 108.9] [Reference Citation Analysis (2)] |
| 3. | Lacy BE. Emerging treatments in neurogastroenterology: eluxadoline - a new therapeutic option for diarrhea-predominant IBS. Neurogastroenterol Motil. 2016;28:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med. 2017;376:2566-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 5. | Sultan S, Malhotra A. Irritable Bowel Syndrome. Ann Intern Med. 2017;166:ITC81-ITC96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1036] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 7. | Oświęcimska J, Szymlak A, Roczniak W, Girczys-Połedniok K, Kwiecień J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 9. | Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:1256-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 10. | Goldenberg JZ, Brignall M, Hamilton M, Beardsley J, Batson RD, Hawrelak J, Lichtenstein B, Johnston BC. Biofeedback for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Radu M, Moldovan R, Pintea S, Băban A, Dumitrascu D. Predictors of outcome in cognitive and behavioural interventions for irritable bowel syndrome. A meta-analysis. J Gastrointestin Liver Dis. 2018;27:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 354] [Article Influence: 50.6] [Reference Citation Analysis (1)] |
| 13. | Zhu L, Ma Y, Ye S, Shu Z. Acupuncture for Diarrhoea-Predominant Irritable Bowel Syndrome: A Network Meta-Analysis. Evid Based Complement Alternat Med. 2018;2018:2890465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Wu IXY, Wong CHL, Ho RST, Cheung WKW, Ford AC, Wu JCY, Mak ADP, Cramer H, Chung VCH. Acupuncture and related therapies for treating irritable bowel syndrome: overview of systematic reviews and network meta-analysis. Therap Adv Gastroenterol. 2019;12:1756284818820438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharm Pract (Granada). 2017;15:943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 256] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 16. | Debray TP, Schuit E, Efthimiou O, Reitsma JB, Ioannidis JP, Salanti G, Moons KG; GetReal Workpackage. An overview of methods for network meta-analysis using individual participant data: when do benefits arise? Stat Methods Med Res. 2018;27:1351-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13355] [Article Influence: 834.7] [Reference Citation Analysis (0)] |
| 18. | Wille-Jørgensen P, Renehan AG. Systematic reviews and meta-analyses in coloproctology: interpretation and potential pitfalls. Colorectal Dis. 2008;10:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Williams RE, Black CL, Kim HY, Andrews EB, Mangel AW, Buda JJ, Cook SF. Stability of irritable bowel syndrome using a Rome II-based classification. Aliment Pharmacol Ther. 2006;23:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Validation of the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gastroenterology. 2013;145:1262-1270.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 1392] [Article Influence: 154.7] [Reference Citation Analysis (1)] |
| 22. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24860] [Article Influence: 1775.7] [Reference Citation Analysis (3)] |
| 23. | Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, Kessels AG, Guyatt GH; GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 1242] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 24. | Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3:80-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 1170] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 25. | Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 584] [Cited by in RCA: 1112] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 26. | Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, Lu G. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics. 2006;24:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 328] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 27. | Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics. 2008;26:753-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 276] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 28. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46546] [Article Influence: 2115.7] [Reference Citation Analysis (3)] |
| 29. | Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2753] [Article Influence: 196.6] [Reference Citation Analysis (0)] |
| 30. | Yang ZT, Wang H, Chen RL, Yin XW, Li X, Zhou T, Tan HC, Zhao XD, Chen J, Wei BH. Therapeutic observation of point-application of spleen-strengthening and kidney-warming cataplasm in the treatment of diarrhea-predominant irritable bowel syndrome. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2019;27:672-675. [DOI] [Full Text] |
| 31. | He WT, Dai GZ, Zhang ZB, Xu C. Therapeutic observation of point-application and Eosinophil-lactobacillus compound tablets in the treatment of diarrhea-predominant irritable bowel syndrome. Jiangsu Zhongyiyao. 2019;51:38-40. [DOI] [Full Text] |
| 32. | Li T. Clinical therapeutic observation of acupuncture on soothing the liver qi stagnation in the treatment of diarrhea-predominant irritable bowel syndrome. Beijing Zhongyiyao Daxue. 2019;. |
| 33. | Wang Q, Chen KJ, Yu AS. Therapeutic effects and mechanism of acupuncture on hepatic spleen deficiency and irritable bowel syndrome. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2019;27:520-524. [DOI] [Full Text] |
| 34. | Zhang T, Chen HM, Cao JY. Clinical efficacy of probiotics for irritable bowel syndrome. Xiandai Yixue Yu Jiankang Yanjiu. 2019;3:80-81. |
| 35. | Peng ZY, Cai LK, Tao LF, Lan SY, Li GX. Clinical research of Zisheng granules combined with biology feedback therapy on diarrhea-predominant irritable bowel syndrome. Yatai Chuantong Yiyao. 2019;15:126-128. [DOI] [Full Text] |
| 36. | Oh JH, Jang YS, Kang D, Chang DK, Min YW. Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Kou SM, Du KL. Clinical effect of probiotics combined with glutamine in the treatment of diarrheal irritable bowel syndrome. Linchuang Yixue Yanjiu Yu Shijian. 2018;3:29-30. [DOI] [Full Text] |
| 38. | Sun YY. The Effect of Clostridium butyricum on Symptoms and Gut Microbiota in Treating Diarrhea-dominant Irritable Bowel Syndrome. Shandong Daxue. 2018;. |
| 39. | Qin Y, Fan JY, Liu J, Li XH, Liu SR, Zhao XR, Wang JH. Clinical Effect and Influence on Inflammatory Factor of Probiotics Combined with Glutamine in the Treatment of Irritable Bowel Syndrome. Xiandai Xiaohua Ji Jieru Zhiliao. 2018;23:339-341. [DOI] [Full Text] |
| 40. | Zhang HY. Clinical Effect and Influence on Inflammatory Factor of Probiotics Combined with Glutamine in the Treatment of Irritable Bowel Syndrome. Xiandai Xiaohua Ji Jieru Zhiliao. 2018;27:2096-2097. [DOI] [Full Text] |
| 41. | Chen WQ. The effect of probiotics and Otilonium Bromide on intestinal microorganisms of patients with under diarrhea irritable bowel syndrome. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2017;25:671-674. [DOI] [Full Text] |
| 42. | Wang JJ. Clinical Therapeutic Effects of Irritable Bowel Syndrome. Neimenggu Yixue Zazhi. 2017;49:952-953. [DOI] [Full Text] |
| 43. | Hod K, Sperber AD, Ron Y, Boaz M, Dickman R, Berliner S, Halpern Z, Maharshak N, Dekel R. A double-blind, placebo-controlled study to assess the effect of a probiotic mixture on symptoms and inflammatory markers in women with diarrhea-predominant IBS. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 44. | Liu M, Wu Y. Effect of probiotics combined with trimebutine maleate on treatment of constipation-irritable bowel syndrome. Guoji Xiaohuabing Zazhi. 2017;37:377-381. [DOI] [Full Text] |
| 45. | Huang ZM. The Anorectal Dynamic Influence and Clinical Study of Regulating-qi and Clearing-heat Decoction with Biofeedback in Qi Stagnation and Intestinal Dryness Pattern of Irritable Bowel Syndrome with Constipation. Guangxi Zhongyiyao Daxue. 2017;. |
| 46. | Cheng YY, Zhang L, Zhang W, Gao FY, Yin JB. The Effects of Cognitive Behavioral Therapy on Quality of Life and Mental Health of Patients with Irritable Bowel Syndrome. Weifang Yixueyuan Xuebao. 2017;39:384-386. [DOI] [Full Text] |
| 47. | Kang NN, Pan D, Tan Y, Fu Y. Influences of probiotics combined with emotional therapy on the efficacy and life quality of IBS-D patients. Shiyong Yaowu Yu Linchuang. 2016;19:879-882. [DOI] [Full Text] |
| 48. | Spiller R, Pélerin F, Cayzeele Decherf A, Maudet C, Housez B, Cazaubiel M, Jüsten P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: improvement in abdominal pain and bloating in those with predominant constipation. United European Gastroenterol J. 2016;4:353-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Zhang X, Jiang HH, Zhao HQ, Du WZ, Ren JX, Wang L, Kang JW. Clinical Study on Bifidobacterium Tetravaccine Capsules for Irritable Bowel Syndrome. Zhongguo Yiyao Daobao. 2016;13:154-157. |
| 50. | Han K, Wang J, Seo JG, Kim H. Efficacy of double-coated probiotics for irritable bowel syndrome: a randomized double-blind controlled trial. J Gastroenterol. 2017;52:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Jia Y, Qin Y, Chen X, Pu P. The long-term influence of cognitive behavioral intervention on negative emotion and quality of life of patients with irritable bowel syndrome. Huli Yanjiu. 2016;30:3227-3230. [DOI] [Full Text] |
| 52. | Choi CH, Kwon JG, Kim SK, Myung SJ, Park KS, Sohn CI, Rhee PL, Lee KJ, Lee OY, Jung HK, Jee SR, Jeen YT, Choi MG, Choi SC, Huh KC, Park H. Efficacy of combination therapy with probiotics and mosapride in patients with IBS without diarrhea: a randomized, double-blind, placebo-controlled, multicenter, phase II trial. Neurogastroenterol Motil. 2015;27:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Jia Y, Wen P, Ji W. Influence of cognitive behavior therapy on mental health and quality of life of patients with diarrhea type of bowel syndrome. Huli Yanjiu. 2015;29:3827-3829. [DOI] [Full Text] |
| 54. | Shi ZM, Li XQ, Liu LN, Liu JP, Guo YJ, Zhou H. Ziwu Liuzhu acupuncture treatment of irritable bowel syndrome. Zhenjiu Linchuang Zazhi. 2015;36:1516-1518. [DOI] [Full Text] |
| 55. | Li XL, Lin Y, Cai JZ, Yang L, Wang SX. Therapeutic Effect of Acupuncture Based on Syndrome Differentiation for Diarrhea-predominant Irritable Bowel Syndrome and Its Influence on Serum 5-Hydroxytryptamine. Guangzhou Zhongyiyao Daxue Xuebao. 2015;32:259-262, 266. [DOI] [Full Text] |
| 56. | Ye DM. Clinical Efficacy of Biology Feedback Therapy on Irritable Bowel Syndrome. Chifeng Xueyuan Xuebao (Ziran Kexue Ban). 2015;31:86-87. [DOI] [Full Text] |
| 57. | Zheng HB. A Multi-center Clinical Randomized Controlled Trial of Acupuncture for Treating Irritable Bowel Syndrome-Diarrhea. Chengdu Zhongyiyao Daxue. 2014;. |
| 58. | Zhu Y, Wu Z, Ma X, Liu H, Bao C, Yang L, Cui Y, Zhou C, Wang X, Wang Y, Zhang Z, Zhang H, Jia H, Wu H. Brain regions involved in moxibustion-induced analgesia in irritable bowel syndrome with diarrhea: a functional magnetic resonance imaging study. BMC Complement Altern Med. 2014;14:500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Kong SP, Wang WQ, Xiao N, Tan QW. Clinical Research of Acupuncture plus Ginger-partitioned Moxibustion for Diarrhea-predominant Irritable Bowel Syndrome. Shanghai Zhenjiu Zazhi. 2014;895-898. [DOI] [Full Text] |
| 60. | He CL. Therapeutic Efficacy of Biology Feedback Therapy for Diarrhea-predominant Irritable Bowel Syndrome. Shantou Daxue Yixueyuan Xuebao. 2014;27:107-108, 122. [DOI] [Full Text] |
| 61. | Stevenson C, Blaauw R, Fredericks E, Visser J, Roux S. Randomized clinical trial: effect of Lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome. Nutrition. 2014;30:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic 'functional food' in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Ge JJ, Zeng KX, Zhou D. Efficacy observation on warm needling for 60 cases of diarrhea irritable bowel syndrome. World J Acupunct Moxibustion. 2013;23:43-45, 51. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Pei LX, Sun JH, Xia C, Xu LZ, Geng H, Chen L, Wu XL. Clinical Evaluation of Acupuncture Treating IBS-D Belonging to Liver Depression and spleen deficiency Syndrome. Nanjing Zhongyiyao Daxue Xuebao. 2012;28:27-29. [DOI] [Full Text] |
| 65. | Kruis W, Chrubasik S, Boehm S, Stange C, Schulze J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int J Colorectal Dis. 2012;27:467-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 66. | Sun JH, Wu XL, Xia C, Xu LZ, Pei LX, Li H, Han GY. Clinical evaluation of Soothing Gan and invigorating Pi acupuncture treatment on diarrhea-predominant irritable bowel syndrome. Chin J Integr Med. 2011;17:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Zeng LR, Li RP, Chen QY, Lin JH. Preventive and therapeutic effect of probiotic preparation on diarrhea-predominant irritable bowel syndrome. Jujie Shoushuxue Zazhi. 2011;20:430-432. |
| 68. | Zhao JH, Mi YG, Dong YM. Clinical Observation of Biology Feedback Therapy in the Treatment of Irritable Bowel Syndrome. Dongnan Daxue Xuebao (Yixue Ban). 2011;30:873-876. [DOI] [Full Text] |
| 69. | Wang YX, Li YK. Clinical Efficacy of Acupuncture and Moxibustion on Irritable Bowel Syndrome. Shanghai Zhenjiu Zazhi. 2007;26:30. [DOI] [Full Text] |
| 70. | Savović J, Weeks L, Sterne JA, Turner L, Altman DG, Moher D, Higgins JP. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. 2014;3:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 71. | Naci H, van Valkenhoef G, Higgins JP, Fleurence R, Ades AE. Evidence-based prescribing: combining network meta-analysis with multicriteria decision analysis to choose among multiple drugs. Circ Cardiovasc Qual Outcomes. 2014;7:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Gazouli M, Wouters MM, Kapur-Pojskić L, Bengtson MB, Friedman E, Nikčević G, Demetriou CA, Mulak A, Santos J, Niesler B. Lessons learned--resolving the enigma of genetic factors in IBS. Nat Rev Gastroenterol Hepatol. 2016;13:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 73. | Fukudo S, Kanazawa M, Mizuno T, Hamaguchi T, Kano M, Watanabe S, Sagami Y, Shoji T, Endo Y, Hongo M, Itoyama Y, Yanai K, Tashiro M, Aoki M. Impact of serotonin transporter gene polymorphism on brain activation by colorectal distention. Neuroimage. 2009;47:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1362] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 75. | Lawal A, Kern M, Sidhu H, Hofmann C, Shaker R. Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology. 2006;130:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19:62-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 267] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 77. | Zhao JM, Lu JH, Yin XJ, Chen XK, Chen YH, Tang WJ, Jin XM, Wu LY, Bao CH, Wu HG, Shi Y. Comparison of electroacupuncture and moxibustion on brain-gut function in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled trial. Chin J Integr Med. 2015;21:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Zhao JM, Lu JH, Yin XJ, Wu LY, Bao CH, Chen XK, Chen YH, Tang WJ, Jin XM, Wu HG, Shi Y. Comparison of Electroacupuncture and Mild-Warm Moxibustion on Brain-Gut Function in Patients with Constipation-Predominant Irritable Bowel Syndrome: A Randomized Controlled Trial. Chin J Integr Med. 2018;24:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Liu HR, Wang XM, Zhou EH, Shi Y, Li N, Yuan LS, Wu HG. Acupuncture at both ST25 and ST37 improves the pain threshold of chronic visceral hypersensitivity rats. Neurochem Res. 2009;34:1914-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Sun J, Wu X, Meng Y, Cheng J, Ning H, Peng Y, Pei L, Zhang W. Electro-acupuncture decreases 5-HT, CGRP and increases NPY in the brain-gut axis in two rat models of Diarrhea-predominant irritable bowel syndrome(D-IBS). BMC Complement Altern Med. 2015;15:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Ma XP, Tan LY, Yang Y, Wu HG, Jiang B, Liu HR, Yang L. Effect of electro-acupuncture on substance P, its receptor and corticotropin-releasing hormone in rats with irritable bowel syndrome. World J Gastroenterol. 2009;15:5211-5217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Nahon S, Lahmek P, Durance C, Olympie A, Lesgourgues B, Colombel JF, Gendre JP. Risk factors of anxiety and depression in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2086-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 83. | Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol. 2014;20:2456-2469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (2)] |
| 84. | Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Holroyd K. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. 2008;6:899-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 85. | Gonsalkorale WM, Toner BB, Whorwell PJ. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J Psychosom Res. 2004;56:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Johnston JM, Shiff SJ, Quigley EM. A review of the clinical efficacy of linaclotide in irritable bowel syndrome with constipation. Curr Med Res Opin. 2013;29:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Zhao SR, Ni XM, Zhang XA, Tian H. Effect of cognitive behavior therapy combined with exercise intervention on the cognitive bias and coping styles of diarrhea-predominant irritable bowel syndrome patients. World J Clin Cases. 2019;7:3446-3462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Madan J, Stevenson MD, Cooper KL, Ades AE, Whyte S, Akehurst R. Consistency between direct and indirect trial evidence: is direct evidence always more reliable? Value Health. 2011;14:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |