Published online Nov 7, 2020. doi: 10.3748/wjg.v26.i41.6455
Peer-review started: July 2, 2020
First decision: July 28, 2020
Revised: August 5, 2020
Accepted: September 18, 2020
Article in press: September 18, 2020
Published online: November 7, 2020
Processing time: 126 Days and 12.4 Hours
Infliximab was the first approved biologic treatment for moderate to severe Crohn’s disease (MS-CD) in China. However, the cost-effectiveness of infliximab maintenance therapy (IMT) for MS-CD relative to conventional maintenance therapy remained unclarified.
To assess the cost-effectiveness of IMT for MS-CD in Chinese patients from the perspective of Chinese public insurance payer.
A cohort of MS-CD patients managed in a Chinese tertiary care hospital was created to compare IMT with conventional maintenance therapy (CMT) for clinical outcomes and direct medical costs over a 1-year observation time using conventional regression analyses. A decision-analytic model with the generated evidence was constructed to assess the cost-effectiveness of IMT relative to CMT using reimbursed medical costs.
Based on the included 389 patients, IMT was associated with significantly higher disease remission chance [odds ratio: 4.060, P = 0.003], lower risk of developing new complications (odds ratio: 0.527, P = 0.010), higher utility value for quality of life (coefficient 0.822, P = 0.008), and lower total hospital costs related to disease management (coefficient -0.378, P = 0.008) than CMT. Base-case cost-effectiveness analysis estimated that IMT could cost Chinese health insurance payers ¥55260 to gain one quality-adjusted life year (QALY). The cost-effectiveness of IMT was mainly driven by the estimate of quality of life, treatment efficacy of maintenance therapy, mortality risk associated with active disease, and unit price of infliximab. The probability that IMT was cost-effective at a willingness-to-pay threshold of three times gross domestic product [2018 Chinese gross domestic product per capita (GDPPC)] was 86.4%.
IMT significantly improved real-world health outcomes and cost the Chinese public health insurance payers less than one GDPPC to gain one QALY in Chinese MS-CD patients.
Core Tip: Infliximab maintenance therapy significantly reduced disease severity, improved quality of life, and reduced outpatient clinic visits and hospitalization related to active disease in Chinese patients with moderate to severe Crohn’s disease. Even though the drug acquisition costs of infliximab could not be fully offset by the saved medical costs, the cost-effectiveness of infliximab maintenance therapy was highly attractive from the perspective of Chinese health care payers.
- Citation: Shi JH, Luo L, Chen XL, Pan YP, Zhang Z, Fang H, Chen Y, Chen WD, Cao Q. Real-world cost-effectiveness associated with infliximab maintenance therapy for moderate to severe Crohn’s disease in China. World J Gastroenterol 2020; 26(41): 6455-6474
- URL: https://www.wjgnet.com/1007-9327/full/v26/i41/6455.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i41.6455
Crohn’s disease (CD) is a chronic disease that can affect any part of the gastrointestinal tract[1]. Even though the causes of CD have not yet been fully clarified, current research has proven that tumor necrosis factor-α (TNF-α) is the proinflammatory cytokine enhancing leukocyte migration, activating leukocytes, inducing acute-phase reactants and metalloproteinases, and inhibiting apoptosis of inflammatory cells in CD patients[2-4]. As the first developed TNF-α blocker, infliximab has been shown to be effective across the spectrum of CD, including refractory luminal CD, steroid-dependent CD, and refractory fistulizing CD. The ACCENT 1 trial demonstrated the clinical benefits of infliximab used as a maintenance therapy. In this trial, there was a significantly higher clinical remission rate, a higher mucosal healing rate, and a lower hospitalization rate associated with 1-year infliximab maintenance therapy (IMT) when compared to placebo[5].
Using a conservative estimate, CD affects at least 200000 patients across China[6]. Similar to CD patients in western countries, Chinese CD patients are relatively young, and their quality of life and social function are significantly impaired by CD. Even though infliximab was approved to treat CD in China shortly after its launch in 2005, access to infliximab in Chinese CD patients was highly limited due to the lack of reimbursement coverage. Thus, anti-inflammatory drugs and immunosuppressants are still the mainstay treatment for CD in China, and the limited clinical benefits and side effects associated with these drugs are still the main concern regarding the utilization of these drugs for moderate to severe Crohn’s disease (MS-CD). The purpose of this study was to clarify the real-world health outcomes, medical costs, and cost-effectiveness associated with IMT for MS-CD in Chinese patients and generate real-world evidence to support reimbursement decision making related to the treatments for MS-CD in China.
This study consisted of a real-world study and cost-effectiveness analysis comparing IMT and conventional maintenance therapy (CMT) for health outcomes and direct medical costs in a clinical cohort of MS-CD patients. Research ethics approval for this study was obtained from Sir Run Run Shaw Hospital, Hangzhou, China.
This study identified MS-CD patients who visited the Inflammatory Bowel Disease clinic of Sir Run Run Shaw Hospital in two time windows: January 1, 2014 to December 31, 2014 and July 1, 2017, to June 30, 2018. This study included all patients with a diagnosis of MS-CD who received maintenance therapy in Sir Run Run Shaw Hospital. To minimize the risk of selection bias, this study only excluded patients with insufficient information for data analysis. Hospital medical records associated with the included patients during the 1-year observation time period were reviewed to extract patient demographics including age, gender, body mass index, socio-economic status (employment, residence, and marital status), lifestyle (smoking and drinking), disease site, history of CD-related surgery, CD-related complications, extraintestinal manifestations, and comorbidities. The prescription records associated with the included patients during the 1-year observation period were the data source for the therapy pattern. The documented telephone follow-up questionnaires of the identified patients from the time window from July 1, 2017 to June 30, 2018 were the data sources to assess disease activity using Harvey-Bradshaw Index and rate quality of life on a 0 to 100 scale (0 indicated the worst health status, and 100 indicated the best health). The measured disease activity and quality of life associated with the followed-up patients were used to develop the prediction formulas from the multiple linear regression analyses that used patient characteristics and treatment pattern as independent variables. The developed prediction formulas for disease activity and utility for quality of life were used to estimate the disease activity and quality of life associated with the identified patients from the time window between January 1, 2014 and December 31, 2014. The billing records associated with the included patients’ outpatient clinic visits and hospitalizations in Sir Run Run Shaw Hospital during the 1-year observation period were used to extract the health resources utilization (outpatient visits, hospital admissions, and hospital stay length) and direct medical costs.
This study stratified the included patients into two groups for the data analysis. The included patients receiving infliximab-contained maintenance therapy were assigned into the IMT group. The other included patients receiving maintenance therapy without containing infliximab were assigned into CMT group. The patient baseline characteristics associated with the two groups were summarized using descriptive statistical methods. Student t test, chi square test, and Wilcoxon rank sum test were used to compare the two groups for their patient characteristics and measured outcomes, which included disease remission, quality of life, health resources utilization, and direct medical costs over 1-year observation time. To adjust the potential confounding effects associated with patient baseline characteristics, this study conducted multivariable conventional regression analyses, including logistic regression analysis, linear regression analysis, Poisson regression analysis, beta-binomial regression analysis, and generalized linear regression analysis, with adjustment of patient baseline characteristics to compare IMT vs CMT for disease remission (defined as Harvey-Bradshaw Index score < 5)[7], utility for quality of life, health resources utilization, and direct medical costs. The statistical significance in these analyses was defined as the two-sided P value less than 0.05.
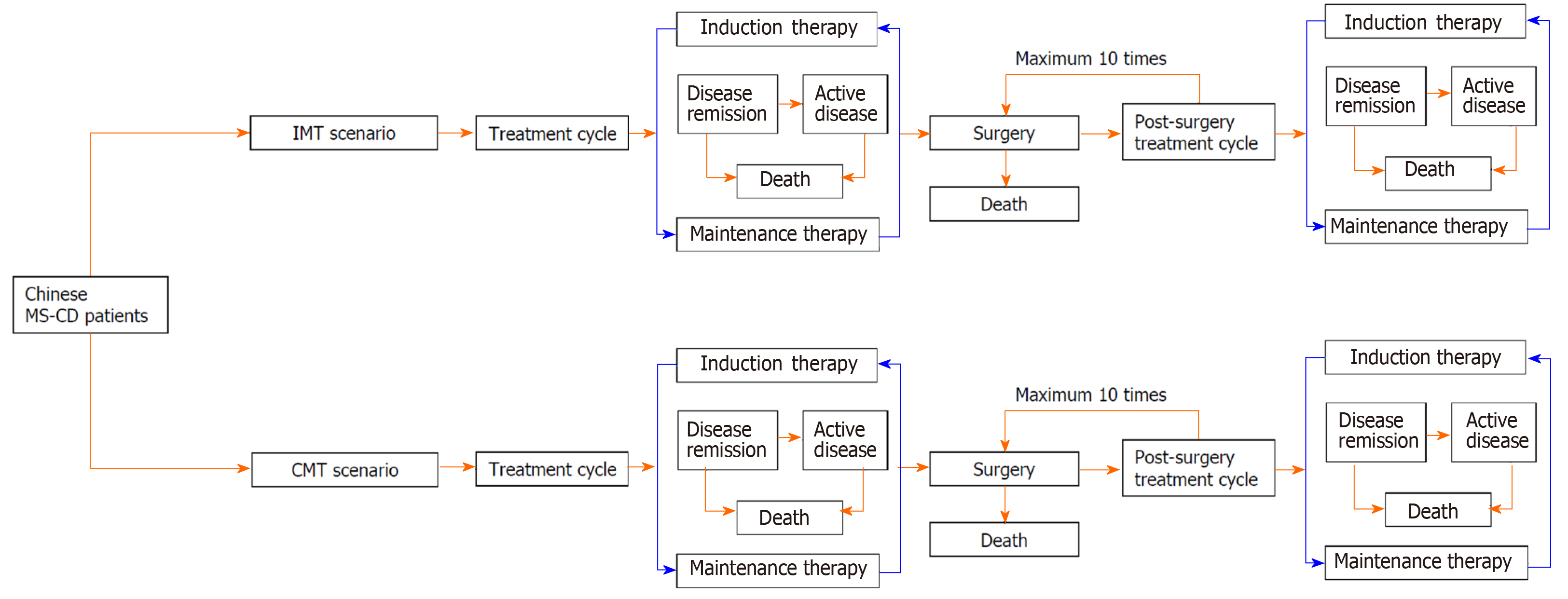
A decision-analytic model was constructed to simulate health outcomes and direct medical costs associated with two model maintenance therapy scenarios: IMT vs CMT. For each model scenario, the decision-analytic model used a Markov model design to simulate treatment cycles between induction therapy and maintenance therapy for treatment response, disease relapse, surgery, and mortality associated with MS-CD patients. The decision analytic model defined the induction therapy as any treatments used with the goal to achieve disease remission in the MS-CD patients who were relapsed from maintenance therapies, which were regularly given to patients to maintain disease remission after induction therapy. The introduction therapies used in real-world study cohort, including steroids (55.2%), infliximab monotherapy (14.3%), a combination of infliximab and immunosuppressant (9.7%) or enteral nutrition (11.7%), and enteral nutrition monotherapy (9.1%), were applied to the decision analytic model to simulate the distribution of introduction therapies in the model cohort. The identified maintenance therapies in the IMT group and CMT group from the real-world study were used to simulate the distributions of IMT and CMT in the model cohort. The administration of infliximab as introduction therapy and maintenance therapy in the real-world study cohort was based on the product monograph of infliximab for MS-CD (5 mg/kg administrated at 0, 2, and 6 wk for introduction therapy, subsequent administration using the same treatment dosage every 8 wk for maintenance therapy). The constructed decision-analytic model allowed patients to receive repeatedly induction therapy and maintenance therapy after disease relapse. The model also assumed that the surgical treatment for the complications only occurred in patients with active CD. The post-surgery patients entered another treatment cycle including induction and maintenance therapy until the occurrence of another surgical treatment in the model.
The decision-analytic model took into account the mortality associated with surgical treatment, disease remission, and active disease to estimate the survival rate associated with each model cycle. The cycle length of the Markov models in the decision-analytic model was 3 mo to align with the patients’ regular follow-up frequency. The simulation time horizon in the decision-analytic model was set to lifetime to estimate overall survival, quality-adjusted life years (QALY), cumulative risk of CD-related surgery, and reimbursed medical costs under the reimbursement policy for CD patients in Zhejiang province (annual co-payment: ¥20000; reimbursement percentage: 80%; annual reimbursement cap: ¥40000). The structure of the decision-analytic model is illustrated in Figure 1.
A literature review was conducted to estimate model variables for the treatment response associated with induction therapy[8-13], risk of disease relapse associated with maintenance therapy[14], surgery risk associated with active CD, perioperative mortality associated with surgery, and the hazard ratio of mortality associated with active CD relative to age and gender-matched general population[15-30]. Meta-analysis was used as the main approach to synthesize the identified evidence from the literature review. The constructed decision analytic model was used to conduct cost-effectiveness analysis, which included base-case analysis, one-way sensitivity analyses, and probabilistic sensitivity analysis (PSA). The point estimates of QALY gains and lifetime reimbursed medical costs from the base-case analysis were used to calculate the incremental cost-effectiveness ratio (ICER) per gained QALY associated with IMT relative to CMT. One-way sensitivity analyses assessed the change of ICER associated with IMT by varying each model variable within its 95% confidence interval (CI) or ± 25% of its baseline value. PSA was conducted using a Monte Carlo simulation method to run 10000 iterations of cost-effectiveness analyses based on the distributions of model variables (beta distributions for probability and utility variables; gamma distributions for cost variables). The cost-effectiveness proportion associated with IMT relative to CMT was calculated under the willingness-to-pay (WTP) of one, two, and three times of the 2018 Chinese gross domestic goods per capita (GDPPC) (¥64644 or $9769)[31], respectively.
The real-world study initially identified 593 MS-CD patients. Of the identified MS-CD patients managed in the study hospital, 393 patients received maintenance therapy for MS-CD. After further exclusion of 4 patients without sufficient information for data analysis, this study eventually included 389 patients to conduct the data analysis. Of the included 389 patients, 259 patients received IMT, including the combination of infliximab and immunosuppressant (38.2%), infliximab monotherapy (44.6%), the combination of infliximab and enteral nutrition (12.4%), and the combination of infliximab, immunosuppressant, and enteral nutrition (4.8%). The other 130 patients received CMT that included immunosuppressant (61.7%), 5-aminosalicylates (25.8%), enteral nutrition (7.0%), the combination of immunosuppressant and enteral nutrition (3.1%), and the combination of immunosuppressant, 5-aminosalicylates, and/or enteral nutrition (2.4%).
The comparisons of the patient baseline characteristics associated with the two study groups for IMT vs CMT identified significantly younger age (34.1 ± 10.9 years vs 37.2 ± 11.2 years, P = 0.004), lower rate of married patients (56.0% vs 67.7%, P = 0.026), higher unemployment rate (6.9% vs 1.5%, P = 0.023), higher proportion of disease site at ileocolon (52.5% vs 33.8%, P < 0.001), higher proportions of historical complications for anus fistula (36.3% vs 14.6%, P < 0.001), perianal abscess (27.4% vs 13.8%, P = 0.003), and intestinal fistula (10.4% vs 3.1%, P = 0.012); and lower proportions of comorbidities including chronic hepatitis B (3.1% vs 8.5%, P = 0.020), gastroenteritis (1.5% vs 4.6%, P = 0.071), and kidney diseases (0.4% vs 3.8%, P = 0.009) in the IMT group. The patient baseline characteristics associated with the two study groups are summarized in Table 1.
| IMT, n = 295 | CMT, n = 130 | P value | |||
| Patient characteristics | Mean/% | SD | Mean/% | SD | |
| Demographics | |||||
| Male proportion | 72.2% | 64.6% | 0.125 | ||
| Age in yr | 34.1 | 10.9 | 37.2 | 11.2 | 0.004b |
| BMI range | |||||
| < 18.5 | 32.8% | 40.0% | 0.162 | ||
| 18.5-23.9 | 56.0% | 53.8% | 0.689 | ||
| ≥ 24 | 10.4% | 6.2% | 0.165 | ||
| Lifestyle | |||||
| Non-smoker | 85.7% | 87.7% | 0.592 | ||
| Non-drinker | 81.9% | 88.5% | 0.093 | ||
| Marital status | |||||
| Unmarried | 43.2% | 32.3% | 0.037a | ||
| Married | 56.0% | 67.7% | 0.026a | ||
| Employment status | |||||
| Student | 13.5% | 7.7% | 0.090 | ||
| Full-time | 59.1% | 54.6% | 0.401 | ||
| Part-time | 2.7% | 2.3% | 0.816 | ||
| Unemployed | 6.9% | 1.5% | 0.023a | ||
| Farmer | 1.5% | 1.5% | 0.996 | ||
| Retired | 3.9% | 4.6% | 0.724 | ||
| Unknown | 5.8% | 18.5% | < 0.001 | ||
| Missing | 6.6% | 9.2% | 0.345 | ||
| Disease site at diagnosis | |||||
| Distal colon | 52.9% | 34.1% | < 0.001 | ||
| Terminal ileum | 19.5% | 48.8% | < 0.001 | ||
| Colon | 9.7% | 10.9% | 0.729 | ||
| Upper gastrointestinal and back colon | 4.3% | 3.1% | 0.572 | ||
| Upper gastrointestinal and ileum end | 4.7% | 0.8% | 0.045a | ||
| Upper gastrointestinal tract | 0.0% | 0.8% | 0.158 | ||
| Previous surgery | 29.0% | 30.0% | 0.831 | ||
| Previous complication | |||||
| Perianal abscess | 27.4% | 13.8% | 0.003b | ||
| Intestinal fistula | 36.3% | 14.6% | < 0.001 | ||
| Intestinal obstruction | 18.1% | 13.1% | 0.203 | ||
| Extra-intestinal manifestation | |||||
| Aphthous stomatitis | 8.9% | 3.8% | 0.070 | ||
| Joint pain | 3.1% | 3.1% | 0.995 | ||
| Comorbidities | |||||
| Gallbladder diseases | 3.9% | 5.4% | 0.488 | ||
| Chronic hepatitis B | 3.1% | 8.5% | 0.020a | ||
| Lung nodes | 3.9% | 2.3% | 0.421 | ||
| Gastroenteritis | 1.5% | 4.6% | 0.071 | ||
The unadjusted comparisons of the measured clinical outcomes, health resources utilization, and hospital costs associated with the two created study groups for IMT vs CMT from the included 389 patients are summarized in Table 2. The multivariate regression analyses with the adjustment of patient demographics, social economic status, disease site at diagnosis, history of CD-related complications, history of CD-related surgery, and extraintestinal manifestation at baseline confirmed that IMT was associated with significantly higher disease remission chance [odds ratio (OR): 4.060, 95%CI: 1.643 to 10.753, P = 0.003], lower risk of developing any new complications (OR: 0.527, 95%CI: 0.323 to 0.858, P = 0.010), and higher utility value for quality of life (coefficient: 0.822, 95%CI: 0.218 to 1.426, P = 0.008) than CMT; IMT was associated with significantly lower outpatient clinic visits (coefficient: -0.564, 95%CI: -0.703 to -0.425) and shorter hospital stay length related to active disease management (coefficient:
| IMT, n = 295 | CMT, n = 130 | P value | |||||
| Outcome measure | Mean/% | SD | Median | Mean/% | SD | Median | |
| Clinical outcomes | |||||||
| Surgery rate | 12.7% | 25.4% | 0.002b | ||||
| Disease remission rate | 94.6% | 86.9% | 0.008b | ||||
| Utility for quality of life | 0.890 | 0.080 | 0.900 | 0.757 | 0.093 | 0.748 | < 0.001 |
| Newly developed complications | |||||||
| Any complications | 27.0% | 42.3% | 0.002b | ||||
| Anus fistula | 17.4% | 14.6% | 0.489 | ||||
| Intestinal fistula | 3.5% | 4.6% | 0.582 | ||||
| Intestinal obstruction | 4.2% | 12.3% | 0.003b | ||||
| Perianal abscess | 5.8% | 6.2% | 0.886 | ||||
| Bowel perforation | 1.9% | 3.8% | 0.260 | ||||
| Health resource utilization | |||||||
| Outpatient clinic visits | 1.9 | 3.3 | 1.0 | 3.7 | 5.2 | 2.0 | < 0.001 |
| Hospital admissions | 5.3 | 2.2 | 6.0 | 1.4 | 1.0 | 1.0 | < 0.001 |
| Hospital admissions for infliximab administration | 4.4 | 2.2 | 5.0 | 0.0 | 0.0 | 0.0 | < 0.001 |
| Hospital admissions for active disease management | 0.9 | 1.0 | 1.0 | 1.4 | 1.0 | 1.0 | < 0.001 |
| Hospital stay days | 15.2 | 11.1 | 14.0 | 14.8 | 12.4 | 9.5 | 0.207 |
| Hospital stay days related to infliximab administration | 5.7 | 3.8 | 6.0 | 0.0 | 0.0 | 0.0 | < 0.001 |
| Hospital stay days for active disease management | 9.5 | 11.3 | 9.0 | 14.8 | 12.4 | 9.5 | < 0.001 |
| Direct medical costs for outpatient clinic visits | |||||||
| Outpatient costs for drugs | ¥710 | ¥4,268 | ¥0 | ¥2,342 | ¥4698 | ¥644 | < 0.001 |
| Outpatient costs for others | ¥232 | ¥589 | ¥0 | ¥130 | ¥446 | ¥0 | 0.008b |
| Total outpatient costs | ¥942 | ¥4371 | ¥54 | ¥2473 | ¥4777 | ¥810 | < 0.001 |
| Direct medical costs for hospitalizations | |||||||
| Hospital costs related to infliximab administration | ¥5,305 | ¥7650 | ¥3577 | ¥0 | ¥0 | ¥0 | < 0.001 |
| Drug acquisition costs of infliximab | ¥39018 | ¥9610 | ¥39200 | ¥0 | ¥0 | ¥0 | < 0.001 |
| Hospital costs for active disease management | ¥11041 | ¥17982 | ¥4090 | ¥24274 | ¥29285 | ¥9321 | < 0.001 |
| Total hospital costs | ¥55365 | ¥22337 | ¥52155 | ¥24274 | ¥29285 | ¥9321 | < 0.001 |
| Total direct medical costs | ¥56307 | ¥23866 | ¥52476 | ¥26747 | ¥30541 | ¥12503 | < 0.001 |
| Outcome type | Disease remission | CD-related surgery | CD-related complications | Utility, quality of life | ||||||||||||||||
| Regression analysis method | Logistic regression analysis | Logistic regression analysis | Logistic regression analysis | Beta-binomial regression analysis | ||||||||||||||||
| Independent variables | Sample size | OR | 95%CI | P value | Sample size | OR | 95%CI | P value | Sample size | OR | 95%CI | P value | Sample size | Coefficient | 95%CI | P value | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | |||||||||||||
| IMT vs CMT | 389 | 4.060 | 1.643 | 10.753 | 0.003a | 389 | 0.658 | 0.349 | 1.249 | 0.196 | 389 | 0.527 | 0.323 | 0.858 | 0.010a | 389 | 0.822 | 0.218 | 1.426 | 0.008a |
| Demographics | ||||||||||||||||||||
| Male gender | 389 | 1.111 | 0.675 | 1.844 | 0.681 | 389 | -0.008 | -0.622 | 0.607 | 0.980 | ||||||||||
| Age in yr | 389 | 0.951 | 0.918 | 0.986 | 0.005 | 389 | 1.027 | 1.002 | 1.052 | 0.035a | 389 | 0.990 | 0.968 | 1.011 | 0.351 | 389 | -0.016 | -0.041 | 0.010 | 0.223 |
| BMI | ||||||||||||||||||||
| < 18.5 | 389 | 1.936 | 0.682 | 6.535 | 0.244 | |||||||||||||||
| 18.5-23.9 | 389 | 0.826 | 0.292 | 2.755 | 0.733 | |||||||||||||||
| Residence area | ||||||||||||||||||||
| Urban city | 389 | 0.521 | 0.246 | 1.035 | 0.073 | |||||||||||||||
| Insurance plan | ||||||||||||||||||||
| Farmar | 389 | 0.384 | 0.098 | 1.634 | 0.178 | |||||||||||||||
| Other plans | 389 | 2.578 | 1.034 | 6.247 | 0.038a | |||||||||||||||
| Disease site at diagnosis | ||||||||||||||||||||
| Terminal ileum | 389 | 1.350 | 0.612 | 3.042 | 0.460 | 389 | 1.246 | 0.732 | 2.104 | 0.414 | 389 | -0.227 | -0.847 | 0.392 | 0.472 | |||||
| Colon | 389 | 0.812 | 0.385 | 1.762 | 0.590 | 389 | 1.581 | 0.756 | 3.247 | 0.216 | ||||||||||
| History of CD-related complications | ||||||||||||||||||||
| Intestinal fistula | 389 | 0.307 | 0.098 | 0.976 | 0.042a | |||||||||||||||
| Intestinal obstruction | 389 | 0.831 | 0.322 | 2.293 | 0.709 | |||||||||||||||
| Extraintestinal abscess | 389 | 5.766 | 1.277 | 25.741 | 0.020a | |||||||||||||||
| Anal fistula | 389 | 0.747 | 0.350 | 1.509 | 0.431 | 389 | 0.142 | -0.546 | 0.829 | 0.687 | ||||||||||
| Joint pain | 389 | 0.318 | 0.059 | 1.866 | 0.187 | |||||||||||||||
| History of CD-related surgery | 389 | 0.158 | 0.056 | 0.407 | 0.000 | 389 | 0.488 | 0.282 | 0.824 | 0.009b | 389 | -0.304 | -0.908 | 0.299 | 0.323 | |||||
| Comorbidities | ||||||||||||||||||||
| Gallbladder disease | 389 | 3.812 | 1.343 | 11.494 | 0.013a | |||||||||||||||
| Kidney disease | 389 | 5.015 | 0.826 | 31.142 | 0.070 | |||||||||||||||
| Outcome type | Outpatient visits | Hospital admissions related to active disease | Hospital stay length related to active disease | ||||||||||||
| Regression analysis method | Poisson regression analysis | Poisson regression analysis | Linear regression analysis | ||||||||||||
| Independent variables | Sample size | Coefficient | 95%CI | P value | Sample size | Coefficient | 95%CI | P value | Sample size | Coefficient | 95%CI | P value | |||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||
| IMT vs CMT | 238 | -0.564 | -0.703 | -0.425 | 0.000 | 291 | -0.074 | -0.276 | 0.128 | 0.470 | 389 | -4.725 | -7.112 | -2.337 | 0.000 |
| BMI | |||||||||||||||
| < 18.5 | 291 | 0.244 | -0.122 | 0.643 | 0.209 | 389 | 5.510 | 1.587 | 9.433 | 0.006b | |||||
| 18.5-23.9 | 291 | -0.020 | -0.382 | 0.375 | 0.917 | 389 | -0.759 | -4.449 | 2.932 | 0.686 | |||||
| Lifestyles | |||||||||||||||
| Smoker | 238 | 0.073 | -0.231 | 0.355 | 0.623 | ||||||||||
| Residence area | |||||||||||||||
| Urban city | 238 | 0.693 | 0.558 | 0.830 | 0.000 | ||||||||||
| Insurance plan | |||||||||||||||
| Urban workers | 238 | -0.070 | -0.230 | 0.092 | 0.392 | ||||||||||
| Urban residents | 238 | -0.442 | -0.696 | -0.196 | 0.001b | ||||||||||
| Disease site at diagnosis | |||||||||||||||
| Terminal ileum | 238 | 0.113 | -0.086 | 0.314 | 0.268 | 389 | -2.100 | -5.085 | 0.884 | 0.167 | |||||
| Colon | |||||||||||||||
| Ileocolon | 238 | 0.128 | -0.057 | 0.317 | 0.179 | 389 | -2.505 | -5.099 | 0.089 | 0.058 | |||||
| End ileum + upper digestive tract | 291 | 0.222 | -0.359 | 0.731 | 0.421 | ||||||||||
| Ileocolon + upper digestive tract | 238 | 0.502 | 0.162 | 0.825 | 0.003b | 291 | 0.253 | -0.218 | 0.670 | 0.263 | |||||
| History of CD-related complications | |||||||||||||||
| Pyloric obstruction | 238 | -0.247 | -1.698 | 0.815 | 0.690 | 291 | 0.819 | 0.080 | 1.450 | 0.018a | |||||
| Intestinal fistula | 238 | 0.028 | -0.272 | 0.312 | 0.850 | ||||||||||
| Intestinal obstruction | 238 | 0.372 | 0.183 | 0.555 | 0.000 | ||||||||||
| Extraintestinal abscess | 389 | 11.363 | 4.696 | 18.030 | 0.001b | ||||||||||
| Anal fistula | 291 | 0.241 | 0.007 | 0.468 | 0.040a | 389 | -0.316 | -2.710 | 2.078 | 0.795 | |||||
| Perianal abscess | 291 | 0.150 | -0.091 | 0.383 | 0.214 | ||||||||||
| Extraintestinal manifestations | |||||||||||||||
| Joint pain | 238 | -0.438 | -0.919 | -0.013 | 0.057 | ||||||||||
| Mouth ulcers | 238 | -0.465 | -0.936 | -0.053 | 0.038a | ||||||||||
| History of CD-related surgery | 238 | -0.117 | -0.291 | 0.054 | 0.184 | ||||||||||
| Comorbidities | |||||||||||||||
| HP infection | 238 | 1.168 | 0.588 | 1.672 | 0.000 | ||||||||||
| Rhinitis | 238 | 1.439 | 0.221 | 2.966 | 0.034a | ||||||||||
| Gallbladder disease | 238 | -0.233 | -0.640 | 0.133 | 0.235 | ||||||||||
| Tuberculosis | 389 | 6.773 | -2.555 | 16.100 | 0.154 | ||||||||||
| Peritonitis | 238 | 1.986 | 1.438 | 2.471 | 0.000 | 291 | 0.872 | -0.321 | 1.759 | 0.091 | 389 | 53.048 | 32.426 | 73.671 | 0.000 |
| Abdominal abscess | 238 | NA | NA | NA | NS | 291 | NA | NA | NA | NS | 389 | NA | NA | NA | NS |
| fracture | 291 | 1.213 | 0.030 | 2.080 | 0.017a | ||||||||||
| Osteoporosis | 238 | -0.159 | -1.626 | 0.945 | 0.802 | ||||||||||
| Muscle atrophy | 389 | 45.956 | 23.426 | 68.486 | 0.000 | ||||||||||
| Arrhythmia | 238 | -1.109 | -2.919 | 0.055 | 0.123 | ||||||||||
| Hepatitis B virus carriers | 238 | -0.358 | -1.062 | 0.235 | 0.275 | ||||||||||
| Outcome type | Outpatient medical costs | Hospital costs related to active disease | Total medical costs | ||||||||||||
| Regression analysis method | Generalized linear regression analysis | Generalized linear regression analysis | Generalized linear regression analysis | ||||||||||||
| Independent variables | Sample size | Coefficient | 95%CI | P value | Sample size | Coefficient | 95%CI | P value | Sample size | Coefficient | 95%CI | P value | |||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||
| IMT vs CMT | 237 | -1.248 | -1.651 | -0.850 | < 0.001 | 293 | -0.117 | -0.387 | 0.150 | 0.384 | 342 | -0.378 | -0.659 | -0.101 | 0.008b |
| BMI | |||||||||||||||
| < 18.5 | 293 | 0.753 | 0.240 | 1.225 | 0.003b | 342 | 0.513 | 0.013 | 0.972 | 0.035a | |||||
| 18.5-23.9 | 293 | 0.214 | -0.276 | 0.657 | 0.367 | 342 | -0.002 | -0.486 | 0.435 | 0.992 | |||||
| Lifestyles | |||||||||||||||
| Smoker | 237 | 0.563 | -0.529 | 1.824 | 0.263 | ||||||||||
| Heavy drinker | 293 | -1.519 | -2.805 | 0.515 | 0.057 | 342 | -1.714 | -2.835 | 0.009 | 0.013a | |||||
| Residence area | |||||||||||||||
| Urban city | 237 | 0.741 | 0.365 | 1.127 | < 0.001 | ||||||||||
| Insurance plan | |||||||||||||||
| Urban residents | 237 | -0.562 | -1.038 | -0.030 | 0.028a | ||||||||||
| Other plans | 293 | 0.395 | -0.071 | 0.915 | 0.111 | 342 | 0.248 | -0.235 | 0.784 | 0.325 | |||||
| Disease site at diagnosis | |||||||||||||||
| Terminal ileum | 342 | 0.183 | -0.204 | 0.564 | 0.343 | ||||||||||
| Colon | 237 | -0.116 | -0.728 | 0.569 | 0.707 | ||||||||||
| Ileocolon | 237 | -0.279 | -0.662 | 0.102 | 0.158 | 293 | -0.252 | -0.513 | 0.010 | 0.060 | 342 | -0.163 | -0.517 | 0.178 | 0.347 |
| Ileocolon + upper digestive tract | 342 | 0.600 | -0.073 | 1.397 | 0.106 | ||||||||||
| CD-related complications | |||||||||||||||
| Intestinal obstruction | 237 | 1.270 | 0.680 | 1.901 | < 0.001 | ||||||||||
| Gastric fistula | 342 | -2.351 | -4.061 | 1.308 | 0.049a | ||||||||||
| Intestinal abscess | 293 | -1.183 | -2.224 | 0.372 | 0.064 | 342 | -1.180 | -2.174 | 0.261 | 0.050 | |||||
| Bowel perforation | 342 | 0.570 | -0.048 | 1.314 | 0.094 | ||||||||||
| Perianal abscess | 237 | -0.058 | -0.495 | 0.409 | 0.795 | ||||||||||
| Intestinal-cutaneous fistula | 293 | -2.539 | -4.153 | 0.711 | 0.022a | ||||||||||
| Extraintestinal manifestations | |||||||||||||||
| Psoriasis | 342 | NA | NA | NA | NS | ||||||||||
| Mouth ulcers | 237 | -0.089 | -0.933 | 1.004 | 0.852 | 293 | -0.781 | -1.253 | -0.241 | 0.002a | 342 | -0.680 | -1.166 | -0.121 | 0.010a |
| History of CD-related surgery | 237 | 0.019 | -0.407 | 0.466 | 0.929 | ||||||||||
| Comorbidities | |||||||||||||||
| Intestinal ulcer | 237 | -4.250 | -6.471 | 0.449 | 0.004b | ||||||||||
| Shingles | 293 | -2.464 | -4.080 | 0.786 | 0.026a | ||||||||||
| Joint pain | 342 | -3.952 | -5.675 | -0.291 | 0.001a | ||||||||||
| Esophageal disease | 342 | -2.696 | -4.467 | 0.976 | 0.026a | ||||||||||
| Diabetes | 342 | -2.147 | -3.910 | 1.523 | 0.076 | ||||||||||
| Asthma | 342 | -3.391 | -5.092 | 0.265 | 0.005 | ||||||||||
Based on the model variables that are summarized in Table 6, the comparisons of the point estimates of the model outputs associated with two model scenarios in the base case analysis without discounting the measured outcomes estimated that the IMT model scenario was associated with the increase of overall survival by 2.871 years (43.815 years vs 40.944 years), QALY by 2.476 years (33.365 QALY vs 30.889 QALY), and reimbursed medical costs by ¥96201 (¥ 469958 vs ¥373757). The cumulative CD-related surgery risk associated with the IMT model scenario was reduced by 39.7%. The discounted point estimates of QALYs and reimbursed medical costs associated with the two model scenarios in the base-case analysis estimated that the ICER associated with the IMT scenario relative to the CMT scenario was ¥55260, 85.5% of the 2018 Chinese GDPPC. The results of the base case analysis before and after discounting are summarized in Table 7.
| Model variable | Base line value | 95%CI | |
| Lower limit | Upper limits | ||
| Treatment efficacies of induction therapy | |||
| Disease remission rate of steroids (reference) | 0.347 | 0.247 | 0.447 |
| Disease remission rate ratio for infliximab relative to reference | 1.476 | 0.620 | 2.090 |
| Disease remission rate ratio for infliximab plus immunosuppressant relative to reference | 2.331 | 1.639 | 3.315 |
| Disease remission rate ratio for infliximab plus enteral nutrition relative to reference | 1.743 | 1.523 | 2.986 |
| Treatment efficacies of maintenance therapy | |||
| Quarterly risk of disease relapse associated with no treatment | 0.207 | 0.146 | 0.284 |
| Relative risk of disease relapse associated with infliximab relative to no treatment | 0.040 | 0.000 | 0.140 |
| Relative risk of disease relapse associated with immunosuppressant relative to no treatment | 0.360 | 0.170 | 0.630 |
| Mortality | |||
| Perioperative mortality rate associated with surgery | 0.014 | 0.007 | 0.030 |
| Hazard ratio of mortality associated with active disease relative to age and gender-matched general population | 3.047 | 2.195 | 4.230 |
| Utility ratio between CD patients and general population | |||
| Disease remission | 0.829 | 0.622 | 0.994 |
| Active disease | 0.743 | 0.565 | 0.926 |
| Direct medical costs | |||
| Annual medical costs related to disease reemission management | ¥9512 | ||
| Annual medical costs related to active disease management | ¥14436 | ||
| Surgery costs per episode | ¥16781 | ||
| Annual drug acquisition costs of infliximab used as induction therapy | ¥49000 | ||
| Drug acquisition costs of infliximab used as MT in the first year | ¥39200 | ||
| Drug acquisition costs of infliximab used as MT beyond the first year | ¥29400 | ||
| Model outputs | Results of base-case analysis without discounting | Results of base-case analysis with discounting | ||||
| IMT | CMT | Difference | IMT | CMT | Difference | |
| Overall survival in yr | 43.815 | 40.944 | 2.871 | 23.858 | 22.947 | 0.911 |
| Disease remission before surgery | 30.433 | 13.157 | 17.276 | 18.102 | 9.392 | 8.710 |
| Active disease before surgery | 1.398 | 2.540 | -1.142 | 0.803 | 1.788 | -0.985 |
| Disease remission after surgery | 11.407 | 20.893 | -9.486 | 4.709 | 9.701 | -4.993 |
| Active disease after surgery | 0.576 | 4.354 | -3.777 | 0.244 | 2.066 | -1.821 |
| Total QALY | 33.365 | 30.889 | 2.476 | 18.392 | 17.491 | 0.901 |
| Disease remission before surgery | 23.450 | 10.220 | 13.230 | 14.066 | 7.288 | 6.778 |
| Active disease before surgery | 0.973 | 1.803 | -0.831 | 0.566 | 1.278 | -0.711 |
| Disease remission after surgery | 8.553 | 15.889 | -7.335 | 3.592 | 7.492 | -3.900 |
| Active disease after surgery | 0.389 | 2.977 | -2.588 | 0.168 | 1.433 | -1.266 |
| Total reimbursed medical costs | ¥469958 | ¥373757 | ¥96201 | ¥242107 | ¥192336 | ¥49771 |
| Drug costs | ¥156606 | ¥48359 | ¥108246 | ¥84553 | ¥25062 | ¥59491 |
| Surgery costs | ¥8261 | ¥28346 | -¥20085 | ¥4019 | ¥14511 | -¥10492 |
| Disease remission management | ¥284675 | ¥226987 | ¥57687 | ¥143601 | ¥116897 | ¥26704 |
| Active disease management | ¥20417 | ¥70065 | -¥49648 | ¥9934 | ¥35866 | -¥25932 |
| Total patient out-of-pocket costs | ¥726433 | ¥163918 | ¥562515 | ¥431392 | ¥109111 | ¥322281 |
| Drug costs | ¥629970 | ¥27629 | ¥602340 | ¥374967 | ¥18346 | ¥356622 |
| Surgery costs | ¥2526 | ¥11736 | -¥9210 | ¥1452 | ¥7840 | -¥6387 |
| Disease remission management | ¥87695 | ¥95541 | -¥7846 | ¥51383 | ¥63547 | -¥12165 |
| Active disease management | ¥6243 | ¥29012 | -¥22769 | ¥3590 | ¥19378 | -¥15789 |
| Total medical costs | ¥1196392 | ¥537676 | ¥658716 | ¥673499 | ¥301447 | ¥372052 |
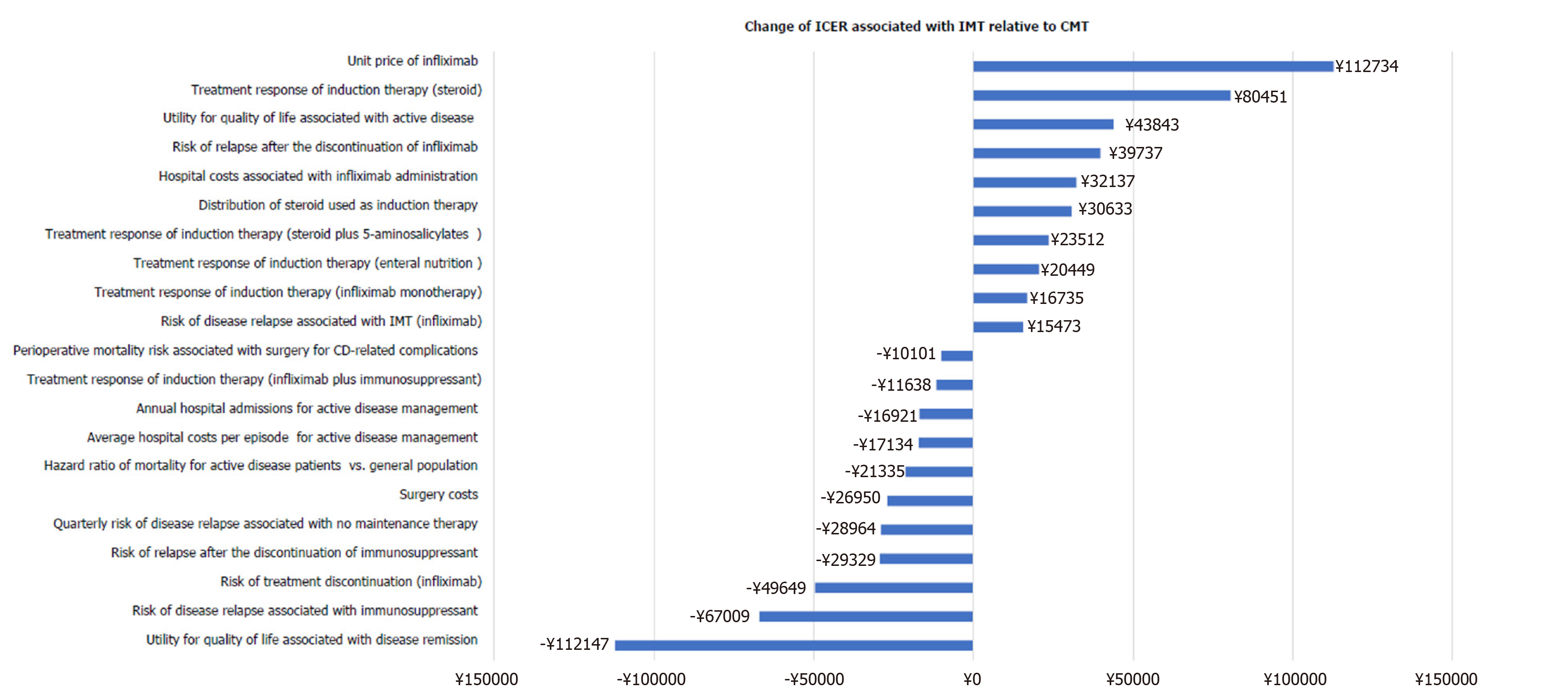
One-way sensitivity analyses indicated that the cost-effectiveness of IMT could be more attractive, indicated by the reduction of ICER over ¥20000, as shown by increasing the following model variables: Quality of life associated with disease remission, relapse risk associated with CMT, treatment discontinuation risk associated with IMT, relapse risk after treatment discontinuation, and hazard ratio of mortality associated with active disease relative to the general population. The cost-effectiveness of IMT was less attractive, indicated by the increase of ICER over ¥20000, when increasing the following model variables: Treatment response of induction therapy with enteral nutrition, steroids plus 5-aminosalicylates, and steroids alone, distribution of induction therapy using steroids, disease relapse risk after the discontinuation of IMT, quality of life associated with active disease, and unit price of infliximab. The impacts of these key model variables on the cost-effectiveness of IMT relative to CMT are illustrated in Figure 2.
The 10000 generated ICER values associated with IMT from the Monte Carlo simulations were ranked to identify the median ICER (¥68512) and its 95% credible interval (¥-238869 to ¥601293). The cost-effectiveness proportions of IMT relative to CMT under the WTP of one, two, and three times of the 2018 Chinese GDPPC were 47.6%, 74.7%, and 86.4%, respectively.
This study observed that IMT was highly effective in a real-world setting by achieving a 94.6% disease remission rate. This is much higher than the reported disease remission rate of infliximab in randomized trials[9,32,33], which reported about 60% disease remission rate associated with 1-year infliximab monotherapy in MS-CD patients. Because the IMT group consisted of approximately 60% of patients receiving a combination of infliximab and immunosuppressants or enteral nutrition in the real-world study, the MT containing infliximab and traditional treatments could be more effective in MS-CD patients. The superior treatment effects associated with the combination of infliximab and immunosuppressants for MS-CD have been proven in randomized clinical trials. However, the reported disease remission rate in these randomized trials was not as high as what was observed in this study. Because the treatment efficacies of infliximab were mainly assessed in randomized clinical trials conducted in western countries, the observed treatment effects of infliximab in this study might suggest that patient ethnicity might play a role in the treatment effects of infliximab. This speculation was supported by another retrospective study that observed nearly the same disease remission rate (97.1%) associated with 1-year treatment with infliximab for MS-CD in Korean patients[34]. Additionally, male gender was found to predict better treatment response of infliximab[35], and the high male proportion in the patient cohort in this study could further increase the disease remission rate. Thus, IMT could gain more clinical benefits and have more attractive cost-effectiveness in Chinese MS-CD patients.
Similar to previous studies reporting reduced health resource utilization associated with infliximab in CD patients, this study confirmed that the high disease remission rate associated with IMT reduced health resources utilization related to active disease management during the 1-year observation period. According to the multivariate regression analysis, IMT significantly reduced outpatient clinic visits and hospital stay days related to active disease management. These impacts on health resources utilization could save direct medical costs and partially offset the high drug acquisition costs of infliximab. However, the drug acquisition costs of infliximab were much higher than conventional medications used for CMT. The overall direct medical costs associated with IMT was about four times of the direct medical costs associated with CMT. Because the cost-effectiveness was assessed by ICER, which is the ratio between the difference in lifetime medical costs and difference in QALY associated with IMT and CMT, the drug acquisition costs of infliximab were likely to be the main driving factor for the cost-effectiveness of IMT for MS-CD in China.
This study constructed a comprehensive decision-analytic model that fully accounted for the induction and maintenance treatment cycles, surgery related to developed complications, and mortality risk related to disease status and surgery to simulate lifetime health outcomes and reimbursed medical costs associated with IMT and CMT. This study leveraged the generated evidence from the real-world study for the estimation of the model variables to maximize the generalizability of the cost-effectiveness analysis. Consistent with real-world studies with long-term follow-ups and the cost-effectiveness analyses assessing IMT for MS-CD in high-income countries[36], the constructed decision-analytic model confirmed that IMT could gain more clinical and health benefits than CMT by increasing overall survival, increasing QALY, and reducing the risk of surgery for CD-related complications. Additionally, IMT was only associated with a modest increase of reimbursed medical costs under current Chinese reimbursement policy. In this case, the cost-effectiveness of IMT relative to CMT for MS-CD in China was highly attractive by having the ICER value less than the 2018 Chinese GDPPC. This result also suggested that the reimbursement coverage in Chinese patients was unlikely to substantially reduce the out-of-pocket costs associated with the disease management. Thus, the affordability of IMT could be still a significant barrier for patient access to infliximab even with reimbursement support. Since the cost-effectiveness of IMT was highly sensitive to the price of infliximab, it might be beneficial to use our constructed decision-analytic model to identify further the appropriate price of infliximab and reimbursement policy to improve patient access to IMT.
This study conducted one-way sensitivity analysis and probability sensitivity analysis to assess the impact of uncertainty associated with the model variables on the cost-effectiveness of IMT relative to CMT in MS-CD patients. The one-way sensitivity analyses clearly demonstrated that quality of life, measured as utility in our study, associated with disease remission and active disease, could substantially change the ICER due to their wide 95%CIs. Thus, the validity of the utility associated with disease remission and active disease in our study was critical for the robustness of our cost-effectiveness analysis. Because the estimated utilities for disease remission and active disease were highly comparable as previously reported results of a meta-analysis[37] based on 17 studies (utility for disease remission: 0.829 vs 0.840; utility for active disease: 0.743 vs 0.753), the utility variables in cost-effectiveness analysis should have sufficient external validity. Our PSA took into account overall uncertainty associated with utility variables and also other model variable to estimate the distribution of the cost-effectiveness of IMT relative to CMT under the 10000 Monte Carlo simulations. Our base-case analysis indicated that IMT was highly cost-effective by having an ICER less than 2018 Chinese GDPPC (85.5%). Our PSA estimated that 47.6% of simulated ICERs less than 2018 Chinese GDPPC. Thus, base-case analysis was likely to overestimate the cost-effectiveness of IMT. As the cost-effectiveness proportion associated with IMT relative to CMT was 86.4% under the recommended cost-effectiveness threshold, both base case analysis and PSA supported the attractive cost-effectiveness of IMT in Chinese MS-CD patients.
Except infliximab, the other launched TNF-alpha inhibitors, such as etanercept and adalimumab, were launched in China as well. However, the approved indications of etanercept and adalimumab did not include MS-CD when this study was conducted. The other biologics indicated for MS-CD, including vedolizumab and ustekinumab, were recently launched in China. Thus, our cost-effectiveness analysis did not include these biologic treatments. Even though the maintenance therapy with these newly approved biologics were reported to have a higher disease remission rate than IMT, the higher acquisition costs associated with these biologics could make their cost-effectiveness relative to IMT unlikely attractive in MS-CD patients. Thus, the newly approved biologics are mainly recommended in the second-line treatment setting after the failure with infliximab treatment.
Even though the cost-effectiveness analysis based on the real-world data minimized the uncertainty and variability associated with the model variables, the real-world observation period was only 1 year, which was not sufficiently long to assess the impact of IMT on long-term clinical outcomes, such as the development of complications, surgeries, and mortality. The predictions of these long-term clinical outcomes in the cost-effectiveness analysis were based on literature evidence. Thus, the generalizability of the cost-effectiveness analysis needs further improvement by future real-world studies assessing these long-term outcomes associated with IMT in Chinese patients with MS-CD. Another main limitation in this study was the small sample size of the study cohort from one tertiary care hospital. The study cohort might not be large enough to represent fully the MS-CD patients across China. As the incidence rate of CD in China was as low as 0.46/1000000[38], it is challengeable to identify a large cohort of MS-CD patients from a single center. However, our study cohort had comparable patient baseline characteristics as the Chinese MS-CD patients in other observational studies[39,40]. Similar to the Chinese MS-CD patients in previously published observational studies, our study cohort was characterized by younger age, more male patients, higher proportion with disease site at colon, and one-third patients with history of surgery for CD-related complications.
In summary, this study confirmed that IMT was superior to CMT regarding disease remission rate, quality of life, and health resources utilization in real-world Chinese patients with MS-CD. The extremely high disease remission rate associated with IMT suggested that Chinese patients might have better treatment response to infliximab. Based on the generated real-world evidence, the cost-effectiveness of IMT relative to CMT was highly attractive as IMT cost the Chinese public health insurance payers less than the 2018 Chinese GDPPC to gain one QALY in Chinese MS-CD patients.
Infliximab was the first approved biologic treatment for moderate to severe Crohn’s disease (MS-CD) in China. Even though infliximab was proven to be clinically more effective and safer than conventional treatments, Chinese MS-CD patients still had limited access to infliximab due to lack of reimbursement for their infliximab treatment.
The conventional treatments could not meet the medical needs of Chinese MS-CD patients. However, the patients could not afford regular infliximab-contained maintenance treatment (IMT) without reimbursement support. Reimbursement decision makers needed evidence to support the reimbursement coverage of infliximab used as maintenance therapy for MS-CD.
This study was designed to leverage the real-world evidence from a clinical cohort of patients with MS-CD in a Chinese tertiary care hospital and existing literature evidence to assess the cost-effectiveness of IMT relative to conventional maintenance therapy (CMT) in Chinese MS-CD patients.
This study conducted a retrospective cohort study to compare IMT vs CMT for disease remission, quality of life, health resource utilizations, and direct medical costs in MS-CD patients who were followed up over one year in a Chinese inflammatory bowel disease treatment center. The generated evidence from the retrospective cohort study were further used to construct a decision analytic model to assess the cost-effectiveness of IMT relative to CMT in Chinese MS-CD patients.
The retrospective data analysis in this study observed significantly better clinical outcomes, including disease remission rate, CD-related complications, and quality of life, and less utilization of health resources associated with IMT. The base case cost-effectiveness analysis estimated that IMT was associated with attractive incremental cost-effectiveness ratio per gained quality-adjusted life year, which was less than one gross domestic products per capita in China. Probabilistic sensitivity analysis confirmed the attractive cost-effectiveness of IMT relative to CMT in Chinese MS-CD patients under the recommended cost-effectiveness threshold.
IMT was confirmed to be superior to CMT in Chinese real-world MS-CD patients. With the overall uncertainty associated with clinical effectiveness, quality of life, and direct medical costs associated with IMT and CMT in Chinese MS-CD patients, the cost-effectiveness of IMT relative to CMT was attractive from the perspective of Chinese health care payers.
This study only followed up a relatively small cohort with MS-CD patients from a single treatment center. The generalizability associated with generated evidence in this study needs confirmation by future studies with large sample size of patients enrolled from more treatment centers. Additionally, this study followed up MS-CD patients for only 1 year. Future studies are needed to follow up patients longer to assess the impact of IMT on long-term clinical outcomes, which should include survival outcomes and CD-related to surgery and complications.
We want to thank Professor Krahn M from THETA Collaborative at the University of Toronto to help with reviewing the statistical methods and proofreading the manuscript of this study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hansson-Hedblom A S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1526] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 2. | Paleolog EM, Delasalle SA, Buurman WA, Feldmann M. Functional activities of receptors for tumor necrosis factor-alpha on human vascular endothelial cells. Blood. 1994;84:2578-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Kuijpers TW, Hakkert BC, Hart MH, Roos D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet-activating factor and IL-8. J Cell Biol. 1992;117:565-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 146] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1844] [Cited by in RCA: 1741] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 5. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P; ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 6. | Zheng JJ, Zhu XS, Huangfu Z, Shi XH, Guo ZR. Prevalence and incidence rates of Crohn's disease in mainland China: a meta-analysis of 55 years of research. J Dig Dis. 2010;11:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol. 2010;8:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 332] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 8. | Lichtenstein GR, Diamond RH, Wagner CL, Fasanmade AA, Olson AD, Marano CW, Johanns J, Lang Y, Sandborn WJ. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30:210-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2373] [Article Influence: 158.2] [Reference Citation Analysis (1)] |
| 10. | D'Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A, Vermeire S, Van De Mierop FJ, Coche JR, van der Woude J, Ochsenkühn T, van Bodegraven AA, Van Hootegem PP, Lambrecht GL, Mana F, Rutgeerts P, Feagan BG, Hommes D; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet. 2008;371:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 940] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 11. | Suzuki Y, Motoya S, Takazoe M, Kosaka T, Date M, Nii M, Hibi T. Efficacy and tolerability of oral budesonide in Japanese patients with active Crohn's disease: a multicentre, double-blind, randomized, parallel-group Phase II study. J Crohns Colitis. 2013;7:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Tromm A, Bunganič I, Tomsová E, Tulassay Z, Lukáš M, Kykal J, Bátovský M, Fixa B, Gabalec L, Safadi R, Kramm HJ, Altorjay I, Löhr H, Koutroubakis I, Bar-Meir S, Stimac D, Schäffeler E, Glasmacher C, Dilger K, Mohrbacher R, Greinwald R; International Budenofalk Study Group. Budesonide 9 mg is at least as effective as mesalamine 4.5 g in patients with mildly to moderately active Crohn's disease. Gastroenterology 2011; 140: 425-434. quiz e13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Yokoyama T, Ohta A, Motoya S, Takazoe M, Yajima T, Date M, Nii M, Nagy P, Suzuki Y, Hibi T. Efficacy and Safety of Oral Budesonide in Patients with Active Crohn's Disease in Japan: A Multicenter, Double-Blind, Randomized, Parallel-Group Phase 3 Study. Inflamm Intest Dis. 2018;2:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus EV Jr. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn's disease after surgery: a systematic review and network meta-analysis. Gastroenterology 2015; 148: 64-76. quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Huang CQ, Wang DX. Analysis of risk factors associated with poor prognosis and prognosis of colonic and non-colon-type Crohn's disease. Weichangbingxue He Ganbingxue Zazhi. 2018;27:45-49. [DOI] [Full Text] |
| 16. | Lei XM, Lu L. Clinical characteristics and prognosis analysis of severe Crohn's disease. Chengde Yixueyuan Xuebao Zazhi. 2017;34:35-37. |
| 17. | Wang M, Ding YB, Xiao WM, Deng B, Zhi JH. The efficacy and safety of azathioprine in a long-term treatment for Crohn’s disease. Weichangbingxue He Ganbingxue Zazhi. 2011;20:647-649. [DOI] [Full Text] |
| 18. | Wang QZ, Wang SJ. Investigation of clinical characteristics and survival conditions of patients with inflammatory bowel disease. Zhongguo Minkang Yixue Zazhi. 2017;29:1-3. [DOI] [Full Text] |
| 19. | Aniwan S, Harmsen WS, Tremaine WJ, Kane SV, Loftus EV Jr. Overall and Cause-Specific Mortality of Inflammatory Bowel Disease in Olmsted County, Minnesota, From 1970 Through 2016. Mayo Clin Proc. 2018;93:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Hovde Ø, Kempski-Monstad I, Småstuen MC, Solberg IC, Henriksen M, Jahnsen J, Stray N, Moum BA. Mortality and causes of death in Crohn's disease: results from 20 years of follow-up in the IBSEN study. Gut. 2014;63:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Caini S, Bagnoli S, Palli D, Saieva C, Ceroti M, Bendinelli B, Assedi M, Masala G. Total and cancer mortality in a cohort of ulcerative colitis and Crohn's disease patients: The Florence inflammatory bowel disease study, 1978-2010. Dig Liver Dis. 2016;48:1162-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Camus M, Seksik P, Bourrier A, Nion-Larmurier I, Sokol H, Baumer P, Beaugerie L, Cosnes J. Long-term outcome of patients with Crohn's disease who respond to azathioprine. Clin Gastroenterol Hepatol. 2013;11:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | D'Haens G, Reinisch W, Colombel JF, Panes J, Ghosh S, Prantera C, Lindgren S, Hommes DW, Huang Z, Boice J, Huyck S, Cornillie F; ENCORE investigators. Five-year Safety Data From ENCORE, a European Observational Safety Registry for Adults With Crohn's Disease Treated With Infliximab [Remicade®] or Conventional Therapy. J Crohns Colitis. 2017;11:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Eshuis EJ, Peters CP, van Bodegraven AA, Bartelsman JF, Bemelman W, Fockens P, D'Haens GR, Stokkers PC, Ponsioen CY. Ten years of infliximab for Crohn's disease: outcome in 469 patients from 2 tertiary referral centers. Inflamm Bowel Dis. 2013;19:1622-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Greener T, Shapiro R, Klang E, Rozendorn N, Eliakim R, Ben-Horin S, Amitai MM, Kopylov U. Clinical Outcomes of Surgery Versus Endoscopic Balloon Dilation for Stricturing Crohn's Disease. Dis Colon Rectum. 2015;58:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Manninen P, Karvonen AL, Huhtala H, Rasmussen M, Salo M, Mustaniemi L, Pirttiniemi I, Collin P. Mortality in ulcerative colitis and Crohn's disease. A population-based study in Finland. J Crohns Colitis. 2012;6:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Rönnblom A, Holmström T, Karlbom U, Tanghöj H, Thörn M, Sjöberg D. Clinical course of Crohn's disease during the first 5 years. Results from a population-based cohort in Sweden (ICURE) diagnosed 2005-2009. Scand J Gastroenterol. 2017;52:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Selinger CP, Andrews J, Dent OF, Norton I, Jones B, McDonald C, Cowlishaw J, Barr G, Selby W, Leong RW; Sydney IBD Cohort Study Group. Cause-specific mortality and 30-year relative survival of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2013;19:1880-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Wang GF, Ren JA, Liu S, Chen J, Gu GS, Wang XB, Fan CG, Li JS. Clinical characteristics of non-perianal fistulating Crohn's disease in China: a single-center experience of 184 cases. Chin Med J (Engl). 2012;125:2405-2410. [PubMed] |
| 30. | Yasukawa S, Matsui T, Yano Y, Sato Y, Takada Y, Kishi M, Ono Y, Takatsu N, Nagahama T, Hisabe T, Hirai F, Yao K, Ueki T, Higashi D, Futami K, Sou S, Sakurai T, Yao T, Tanabe H, Iwashita A, Washio M. Crohn's disease-specific mortality: a 30-year cohort study at a tertiary referral center in Japan. J Gastroenterol. 2019;54:42-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | China Statistical Bulletin of National Economic and Social Development in 2018. [Cited May 20, 2020]. Accessed from: http://www.stats.gov.cn/tjsj/zxfb/201902/t20190228_1651265.html. |
| 32. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE, Rachmilewitz D, Rutgeerts P, Wild G, Wolf DC, Marsters PA, Travers SB, Blank MA, van Deventer SJ. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1581] [Cited by in RCA: 1552] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 33. | Lémann M, Mary JY, Duclos B, Veyrac M, Dupas JL, Delchier JC, Laharie D, Moreau J, Cadiot G, Picon L, Bourreille A, Sobahni I, Colombel JF; Groupe d'Etude Therapeutique des Affections Inflammatoires du Tube Digestif (GETAID). Infliximab plus azathioprine for steroid-dependent Crohn's disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 34. | Choi CH, Song ID, Kim YH, Koo JS, Kim YS, Kim JS, Kim N, Kim ES, Kim JH, Kim JW, Kim TO, Kim HS, Kim HJ, Park YS, Park DI, Park SJ, Song HJ, Shin SJ, Yang SK, Ye BD, Lee KM, Lee BI, Lee SY, Lee CK, Im JP, Jang BI, Jeon TJ, Cho YK, Chang SK, Jeon SR, Jung SA, Jeen YT, Cha JM, Han DS, Kim WH; IBD Study Group of the Korean Association for the Study of the Intestinal Diseases. Efficacy and Safety of Infliximab Therapy and Predictors of Response in Korean Patients with Crohn's Disease: A Nationwide, Multicenter Study. Yonsei Med J. 2016;57:1376-1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Oussalah A, Chevaux JB, Fay R, Sandborn WJ, Bigard MA, Peyrin-Biroulet L. Predictors of infliximab failure after azathioprine withdrawal in Crohn's disease treated with combination therapy. Am J Gastroenterol. 2010;105:1142-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Dretzke J, Edlin R, Round J, Connock M, Hulme C, Czeczot J, Fry-Smith A, McCabe C, Meads C. A systematic review and economic evaluation of the use of tumour necrosis factor-alpha (TNF-α) inhibitors, adalimumab and infliximab, for Crohn's disease. Health Technol Assess. 2011;15:1-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Malinowski KP, Kawalec P. Health utility of patients with Crohn's disease and ulcerative colitis: a systematic review and meta-analysis. Expert Rev Pharmacoecon Outcomes Res. 2016;16:441-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Cui G, Yuan A. A Systematic Review of Epidemiology and Risk Factors Associated With Chinese Inflammatory Bowel Disease. Front Med (Lausanne). 2018;5:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 39. | Gao X, Yang RP, Chen MH, Xiao YL, He Y, Chen BL, Hu PJ. Risk factors for surgery and postoperative recurrence: analysis of a south China cohort with Crohn's disease. Scand J Gastroenterol. 2012;47:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Song XM, Gao X, Li MZ, Chen ZH, Chen SC, Hu PJ, He YL, Zhan WH, Chen MH. Clinical features and risk factors for primary surgery in 205 patients with Crohn's disease: analysis of a South China cohort. Dis Colon Rectum. 2011;54:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |