Published online Nov 7, 2020. doi: 10.3748/wjg.v26.i41.6361
Peer-review started: April 14, 2020
First decision: April 26, 2020
Revised: July 13, 2020
Accepted: September 10, 2020
Article in press: September 10, 2020
Published online: November 7, 2020
Processing time: 205 Days and 17.2 Hours
Acute pancreatitis (AP) is rapid-onset pancreatic inflammation that causes local and systemic inflammatory response syndrome (SIRS) with high morbidity and mortality, but no approved therapies are currently available. P-selectin glycoprotein ligand 1 (PSGL-1) is a transmembrane glycoprotein to initiate inflammatory responses. We hypothesized that PSGL-1 may be involved in the development of AP and would be a new target for the treatment of AP.
To investigate the role and mechanism of PSGL-1 in the development of AP.
The PSGL-1 expression on leukocytes was detected in peripheral blood of AP patients and volunteers. Pancreatic injury, inflammatory cytokines expression, and inflammatory cell infiltration was measured in AP mouse models induced with PSGL-1 knockout (PSGL-1-/-) and wild-type (PSGL-1+/+) mice. Leukocyte-endothelial cell adhesion was measured in a peripheral blood mononuclear cell (PBMC)-endothelial cell coculture system.
The expression of PSGL-1 on monocytes and neutrophils was significantly increased in AP patients. Compared with PSGL-1+/+ mice, PSGL-1-/- AP mice induced by caerulein exhibited lower serum amylase, less Interleukin-1beta (IL-1beta) and Interleukin-6 (IL-6) expression, less neutrophil and macrophage infiltration, and reduced peripheral neutrophil and monocyte accounts. PSGL-1 deficiency alleviated leukocyte-endothelial cell adhesion via IL-6 but not IL-1beta.
PSGL-1 deficiency effectively inhibits the development of AP by preventing leukocyte-endothelial cell adhesion via IL-6 stimulation and may become a potential therapeutic target for treating AP.
Core Tip: The severity of acute pancreatitis (AP) is inversely related to the number of leukocytes that infiltrate pancreatic tissue. P-selectin glycoprotein ligand 1 (PSGL-1) plays an important role in the development of AP by promoting inflammatory cell infiltration initiated by leukocyte and endothelial cell adhesion. PSGL-1 deficiency may protect against the development of AP and may be a new potential target for AP therapy.
- Citation: Zhang X, Zhu M, Jiang XL, Liu X, Liu X, Liu P, Wu XX, Yang ZW, Qin T. P-selectin glycoprotein ligand 1 deficiency prevents development of acute pancreatitis by attenuating leukocyte infiltration. World J Gastroenterol 2020; 26(41): 6361-6377
- URL: https://www.wjgnet.com/1007-9327/full/v26/i41/6361.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i41.6361
Acute pancreatitis (AP) is rapid-onset pancreatic inflammation that causes local and systemic inflammatory response syndrome (SIRS) with high morbidity and mortality[1]. Although most patients with AP experience a mild and self-resolving disease course, 15%–20% of patients develop severe AP that leads to multiple organ dysfunction syndrome and poor prognosis[2]. Uncontrolled systemic inflammation in AP results in a high risk of morbidity and mortality, but no approved therapies are currently available[3]. Immune cells infiltration such as monocytes and neutrophils is the first step of inflammation and results in pancreatic injury[4]. Therefore, blocking immune cell infiltration may be a potential and promising target for AP treatment.
P-selectin glycoprotein ligand 1 (PSGL-1), a transmembrane glycoprotein, is mainly expressed on leukocytes. PSGL-1 regulates leukocyte activation, recruitment, and infiltration via binding to E-selectin and P-selectin, which play an important role in initiating inflammatory responses[5]. Knockdown of PSGL-1 effectively reduces the numbers of circulating neutrophils and monocytes, improves endothelial damage, and decreases blood pressure[6]. Blocking functional PSGL-1 reduces visceral adipose inflammation and ameliorates endothelial dysfunction in atherosclerosis[7]. The contribution of PSGL-1 to the inflammatory response has been demonstrated in various inflammation-related diseases[8]. However, the role of PSGL-1 in the development of AP has not been tested.
This study tested the hypothesis that PSGL-1 plays a significant role in the development of AP by a clinical experiment and animal experiments using caerulein induced AP models in PSGL-1-/- and PSGL-1+/+ mice, which would be a new therapeutic target for the treatment of AP.
The mouse brain endothelial cell line (Bend.3) was purchased from Feng Hui Biological Technology Company Co., Ltd. (Changsha, Hunan Province, China). PSGL-1+/+ and PSGL-1-/- mice were a generous gift from Dr. Xia at the Department of Molecular Biology and Biochemistry, University of Oklahoma Health Science Center. Peripheral blood mononuclear cell (PBMC) extraction kits were purchased from Millipore Sigma Co., Ltd. (St. Louis, MO, United States). Rabbit anti-mouse Interleukin-6 (IL6) and Interleukin-1beta (IL-1beta), as well as the IL-6 inhibitor galiellalactone were purchased from Santa Cruz Biotechnology Co.,Ltd (San Francisco, CA, United States). The IL-1beta inhibitor AS101 was purchased from Selleck Chemicals Co., Ltd. (Huston, TX, United States). PerCP/Cyanine5.5-conjugated anti-human CD162 antibody, APC-conjugated anti-human CD14 antibody, FITC-conjugated anti-human CD33 antibody, PE-conjugated anti-human CD45 antibody, PerCP/Cyanine5.5-conjugated anti-mouse/human CD11b antibody, APC/Cyanine7-conjugated anti-mouse CD45 antibody, PE/Cyanine7-conjugated anti-mouse Ly-6G antibody, and FITC-conjugated anti-mouse Ly-6C antibody were purchased from BioLegend Co., Ltd. (San Diego, CA, United States). A PE-conjugated rat anti-Mouse CD162 was purchased from BD Bioscicences Co., Ltd. (San Jose, CA, United States). IL-1beta enzyme-linked immunosorbent assay (ELISA) kit and IL-6 ELISA kit were purchased from Shanghai Xin Fan Biological Technology Co., Ltd. (Shanghai, China). TUNEL test kits were purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China). Caerulein and lipopolysaccharide (LPS) were purchased from Millipore Sigma Co., Ltd. (St. Louis, MO, United States).
Twenty-one patients with AP who were admitted from January 2018 to January 2019 were recruited. The diagnostic criteria for AP were: (1) Abdominal pain consistent with AP (acute, sudden, persistent, severe upper abdominal pain, often radiating to the back); (2) Serum amylase and/or lipase activity at least three times higher than the normal limit; and (3) Enhanced computed tomography/magnetic resonance imaging (CT/MRI) or abdominal ultrasound showing AP-related imaging changes[9]. Venous blood was collected from patients on an empty stomach in the early morning of the day after admission. Eight healthy volunteers matched by age and sex were recruited as controls. All patients and volunteers signed an informed consent form, which was approved by the hospital's ethics committee (Approval number: QT19012; Approval date: November 15, 2017).
AP was induced in mice by intraperitoneal injection of 0.2 mg/kg caerulein (Millipore Sigma, United States) every hour for 7 h, followed by one injection of 10 mg/kg LPS (Millipore Sigma, United States). Twenty-four hours later, the animals were sacrificed by inhalation of isoflurane (1.5%-2.5%, inhaled). The animals were 6 or 8 wk old at the time of the experiments. Groups (n = 4) of age-matched wild-type and/or PSGL-1-/- mice were used. All experimental protocols were approved by the Animal Care and Use Committee at the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences and Peking Union Medical College (Approval number: YZW19007; Approval date: December 13, 2019).
The levels of the inflammatory cytokines IL-1beta and IL-6 in the serum or supernatant were tested with an IL-1beta ELISA kit (Shanghai Xin Fan Biological Technology, China) and an IL-6 ELISA kit (Shanghai Xin Fan Biological Technology, China), respectively. Briefly, serum and supernatant samples were added to 96-well plate wells that were coated with IL-1beta or IL-6 mAb, incubated for 2 h at 37 °C, washed with PBS four times for 3 min each time, and then incubated with an horseradish peroxidase (HRP) linked streptavidin solution for 30 min at 37 °C in a darkroom. All samples were tested three times, and the absorbance was measured at 450 nm with a microplate reader.
Total protein was extracted using a Total Protein Extraction kit (Beyotime Biotechnology), and protein concentrations were determined using a Bicinchoninic Acid (BCA) Protein Assay kit (Beyotime Biotechnology), and the amount of protein was calculated. Twenty micrograms of protein was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 80 V for 120 min and transferred to a nitrocellulose (NC) membrane, and then the membrane was blocked with 5% skim milk powder at room temperature for 2 h and incubated with a mouse monoclonal IL-6 antibody (Santa Cruz Biotechnology) diluted 1:1000 and an IL-1beta antibody (Santa Cruz Biotechnology) diluted 1:1000, at 4 °C overnight. The next day, the membrane was incubated with an HRP-conjugated anti-rabbit antibody (Santa Cruz Biotechnology, United States) diluted 1:5000 at room temperature for 1 h, and the signals were visualized with an electrochemiluminescence kit (Millipore Sigma).
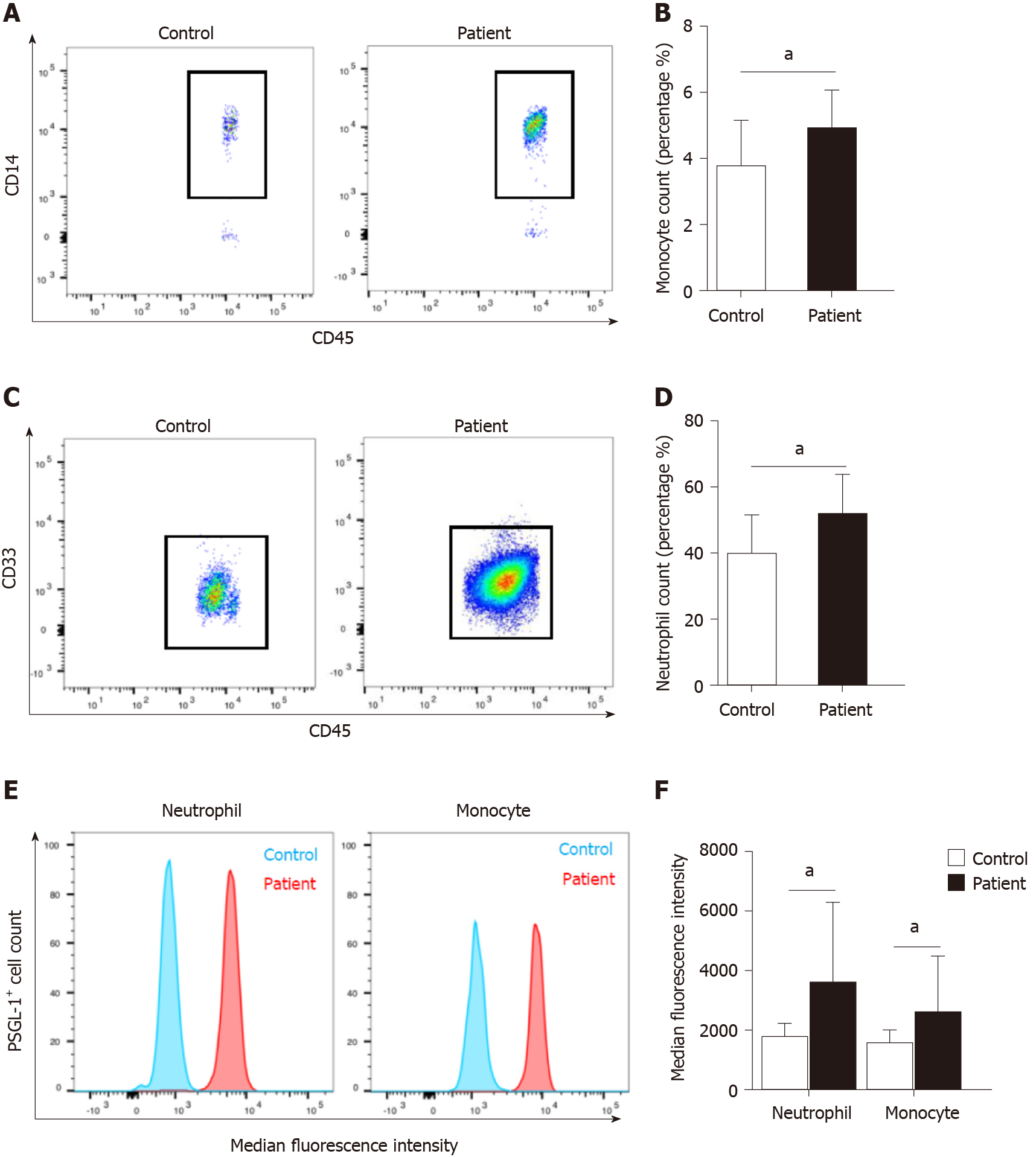
Blood samples were lysed with 1 × BD FACS lysing solution (BD Biosciences, United States) and stained with fluorescently labeled antibodies for 30 min in the dark on ice to identify different leukocyte cell populations. Labeled and fixed samples were analyzed by flow cytometry on a FACSCanto III system (BD Biosciences) immediately or within 24 h. Before each run, BD cytometer setup and tracking beads (BD Biosciences) were used for internal calibration. Appropriate controls were prepared for each sample to allow for compensation and detection of nonspecific binding. Cellular fluorescence was quantified as the mean fluorescence intensity or percentage of double-positive cells at each time point. All results were analyzed by using BD FACS Diva software.
Paraffin-embedded tissues were sectioned, dewaxed, immersed in graded ethanol and 3% hydrogen peroxide solution (twice), washed three times with PBS solution, and subjected to high-temperature and high-pressure antigen retrieval in a weak acid solution. Blocking was performed with 10% goat serum to block nonspecific reactions. The samples were incubated with biotin-labeled solution at 37 °C for 60 min in the dark. Then, the sections were incubated with an HRP-labeled secondary antibody, stained with 3,3′-diaminobenzidine staining solution, counterstained with hematoxylin, dehydrated in ethanol dehydration, mounted with gum, and observed under a microscope. To quantify apoptosis in the tissues, a TUNEL assay was performed according to the instructions of the TUNEL kit (Beyotime Biotechnology).
The pancreatic acinar cell line AR42J was purchased from American Type Culture Collection and cultivated in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C and 5% CO2. A cell model of AP was established by incubating AR42J cells with 100 nmol/L of caerulein for 24 h after the cells reached 80% confluence. The supernatants were collected and frozen at -80 °C. Bend.3 cells were cultured with DMEM containing 10% FBS, 100 μg/mL penicillin, and 10 μg/mL streptomycin in 96-well plates. Peripheral blood monouclear cells (PBMCs) were isolated from the peripheral blood of PSGL-1+/+ and PSGL-1 -/-mice as previously described[10], stained with Green 5-chloromethylformacein diacetate (CMFDA) (Abcam, United Kingdom) for 30 min at 37 °C, and resuspended with normal medium or conditioned medium containing the supernatant from the cell model. Afterwards, the treated PBMCs were incubated with cultured Bend.3 cells grown in monolayers in 96-well plates for 1 h at 37 °C. The IL-1beta inhibitor AS101 (Selleck Chemicals, United States) and IL-6 inhibitor galiellalactone (Santa Cruz Biotechnology, United States) were added simultaneously according to the experimental grouping. After the nonadherent PBMCs were removed by washing with PBS, the fluorescence signals were photographed using an inverted fluorescence microscope.
The data are expressed as the mean ± SD of the mean. Student’s t-test was used to determine significant differences between the control and experimental groups. P < 0.05 was considered statistically significant.
We found that there was a significant increase in the number of circulating monocytes and neutrophils in AP patients compared with volunteers (Figure 1A–D). The expression of PSGL-1 on monocytes and neutrophils in AP patients was significantly elevated than that in volunteers (Figure 1E and F), which suggests that PSGL-1 plays an important role in the development of AP.
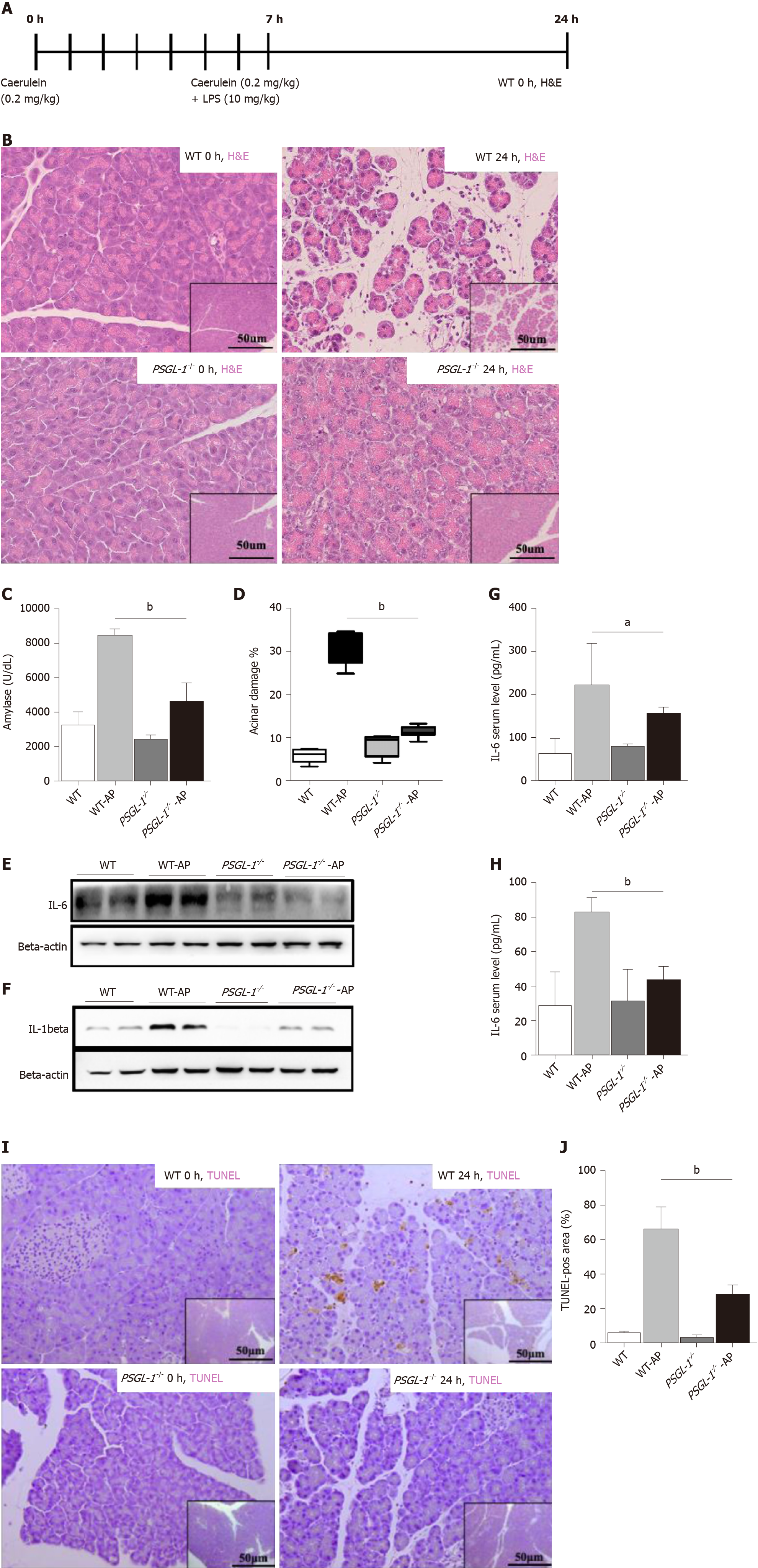
To further examine the role of PSGL-1 in AP, an AP mouse model was induced by caerulein in PSGL-1-/- mice and PSGL-1+/+ mice (Figure 2A). Hematoxylin-eosin staining showed that tissue edema and leukocyte infiltration into the pancreas in PSGL-1+/+ but not in PSGL-1-/- mice (Figure 2B). The level of amylase in PSGL-1+/+ AP mice was significantly higher than that in the control group, while there was no significant increase in amylase in the PSGL-1-/- AP mice (Figure 2C). The pancreases damage rate was approximately three-fold higher in PSGL-1+/+mice than in PSGL-1-/- mice (Figure 2D). The levels of the proinflammatory cytokines IL-1beta and IL-6 were not increased in the pancreatic parenchyma and serum of PSGL-1-/- AP mice compared with those in PSGL-1+/+ AP mice (Figure 2E–H). Caerulein-induced apoptosis of acinar cells quantified by TUNEL assay was blocked, at least partially, by PSGL-1 gene knockout (Figure 2I and J).
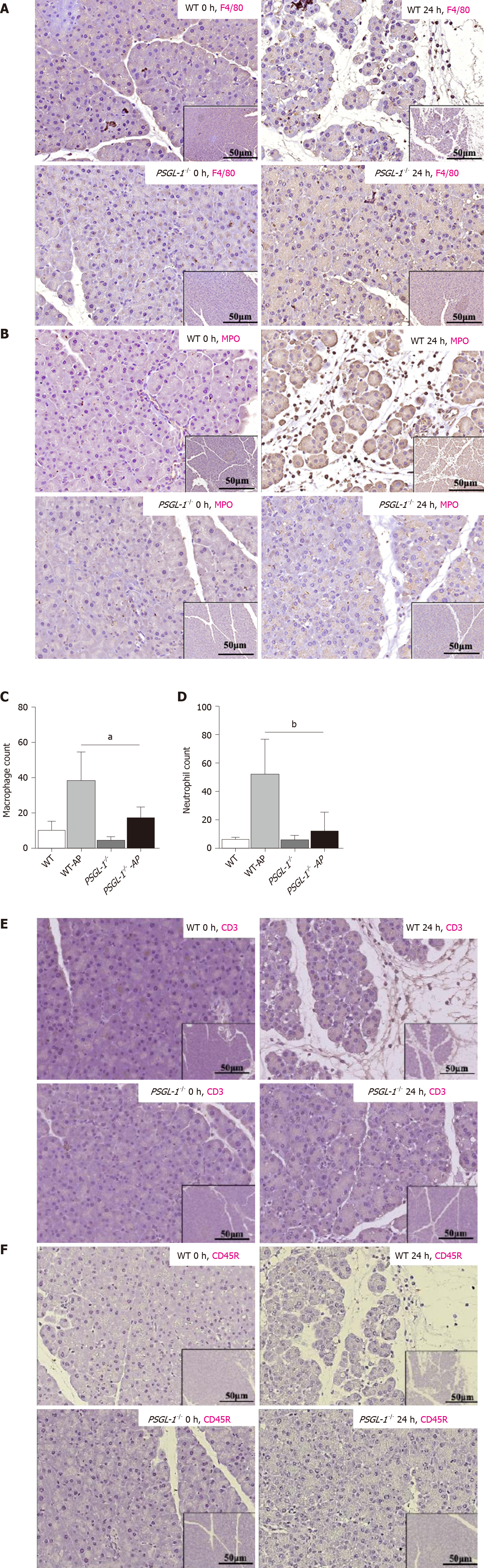
Leukocyte infiltration, especially neutrophils and macrophages, is an important process leading to the occurrence and development of AP. We detected the infiltration of leukocytes in pancreatic tissue from PSGL-1+/+ mice and PSGL-1-/- mice by immunohistochemistry. We found more pancreatic infiltration of myeloperoxidase-positive neutrophils and F4/80-positive monocytes/macrophages in PSGL-1+/+AP mice than in PSGL-1-/- AP mice (Figure 3A-D). However, infiltration of CD3-positive T cells and CD45R-positive B cells was not different (Figure 3E-H) between the two groups of mice. These results indicate that infiltration of neutrophils and macrophages, but not T cells or B cells, is the main pathological change in AP, which is consistent with the results reported in the literature[11]. Thus, PSGL-1 deficiency attenuates infiltration of neutrophils and macrophages in the pancreas.
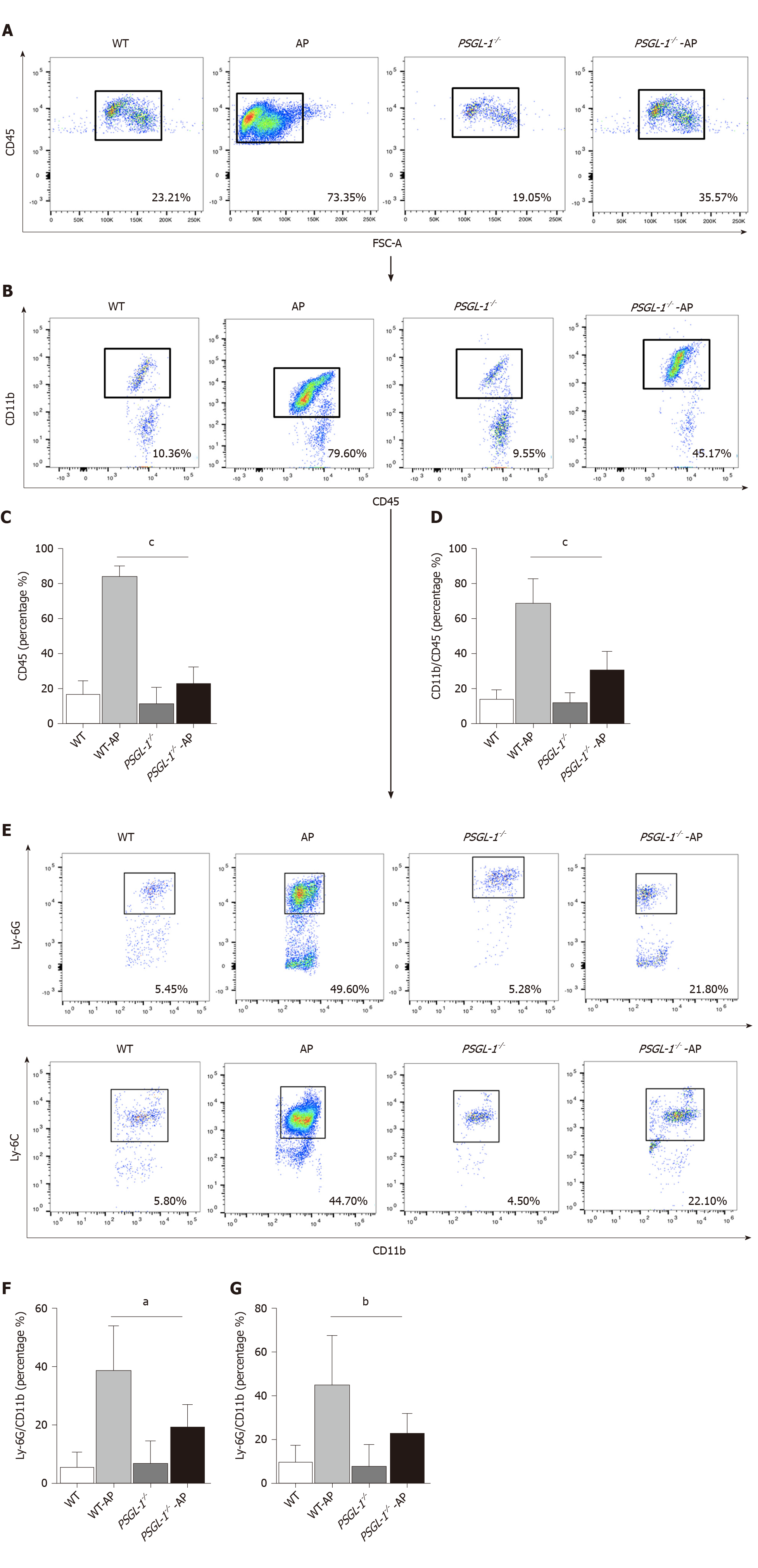
AP occurs early in damaged pancreatic acinar cells, resulting in a local inflammatory response[12]. We investigated the role of PSGL-1 in systemic inflammatory response caused by AP, and found that the number of neutrophils (Ly-6G; Figure 4A and C) and monocytes (Ly-6C; Figure 4B and D) in the peripheral blood was significantly increased in PSGL-1+/+AP mice, with higher expression of PSGL-1 on neutrophils and monocytes cells (Figure 4E and F), compared with PSGL-1-/- AP mice. The results are consistent with our clinical findings, and indicate that PSGL-1 deficiency significantly prevents the systemic inflammatory response caused by AP and blocks the development of AP.
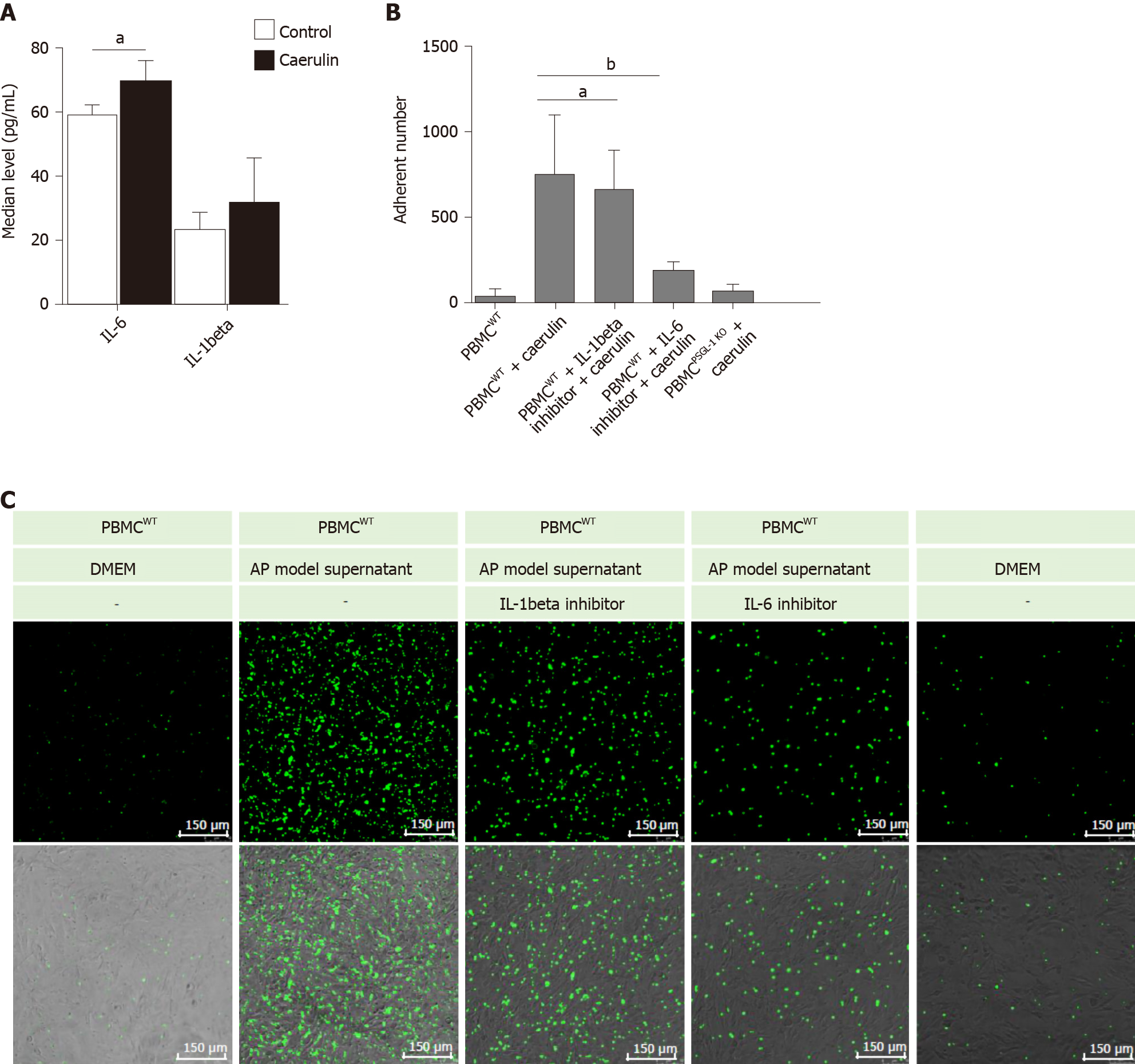
It has been reported that IL-1beta and IL-6 are important proinflammatory factors in AP[13,14]. Therefore, we detected the levels of IL-6 and IL-1beta in the supernatants of AR42J cells treated with caerulein. We found that the change in IL-6 was more obvious than that in IL-1beta (Figure 5A). The result shows that in the early stage of AP, especially within 24 h of onset, IL-6 may play the main promotion role than IL-1beta. We speculated that IL-6 recruits leukocytes and induces leukocyte adhesion and infiltration. We then detected whether PSGL-1 deficiency affects leukocyte-endothelial cell adhesion in an in vitro AP model. As shown in Figure 5B-C the number of PBMCs (green) from wild-type mice that adhered to Bend.3 cells was significantly increased when the cells were cultured with the supernatant from the AP model. Compared with the IL-1beta inhibitor, the IL-6 inhibitor significantly decreased the number of adherent cells when the cells were cultured with the supernatant from the AP model. Furthermore, the number of PBMCs (green) from PSGL-1-/- mice that adhered to Bend.3 cells obviously decreased when the cells were cultured with the supernatant from the AP model. These results indicate that PSGL-1 plays an important role in caerulein-induced AP by regulating leukocyte-endothelial cell adhesion, which is triggered by IL-6 in the early stage of AP.
Severe AP is an acute abdominal disease with a strong systemic inflammatory response. Immune cell infiltration, along with chemokine and cytokine cascade-mediated inflammation, plays a critical role in the development of this disease[15,16]. Although increasing evidence shows that inhibiting the immune cells (neutrophils) or specific adhesion molecules (P-selectin, lymphocyte function antigen-1, and CD11a/CD18) may have a therapeutic effect on excessive inflammatory responses in AP[17-20], no drug that effectively modulates the outcome of AP has been identified[21]. It is urgent to find a novel therapeutic target for the treatment of AP.
PSGL-1, the main ligand of E-selectin and P-selectin, initiates the capture and rolling steps in leukocyte-endothelial cell adhesion and extravasation into tissues, engaging multiple inflammatory signaling pathways[22-24]. Cross-linking between PSGL-1 with P-selectin regulates the rolling and tethering of neutrophils and macrophages /monocytes, plasma B cells, dendritic cells, and T cells during extravasation[25]. Evidence shows that patients with pancreatitis have more P-/E-selectin in their serum than healthy individuals, and that these factors are involved in leukocyte adhesion and inflammation[26]. However, the role of their ligand, PSGL-1, in the development of AP remains poorly understood. Herein, we explored the role of PSGL-1 in AP and found that PSGL-1 was significantly upregulated on circulating monocytes and neutrophils in patients with AP, which indicated the close relationship between PSGL-1 and AP development.
To better understand the function of PSGL-1, we used PSGL-1-/- mice to explore the role of PSGL-1 in the progression of AP. We found that caerulein treatment caused amylase upregulation and acinar damage in PSGL-1+/+ mice, but not in PSGL-1-/- mice. Since acinar cell death is central to AP[27], we also detected whether PSGL-1 deficiency affects acinar cell death by TUNEL assay. We found that PSGL-1 deficiency significantly decreased acinar cell death, indicating the protective effect of PSGL-1 deficiency against acinar cell damage. PSGL-1, as an initiating factor of the leukocyte-endothelial cell adhesion cascade, has been well described to mediate the inflammatory response in immune cells[28]. In this study, we found that PSGL-1 deficiency not only attenuated local inflammation, but also attenuated the systemic inflammation. In addition, fewer PBMCs from PSGL-1-/- mice adhered to endothelial cells than PBMCs from wild-type mice in vitro, which was in consistent with a previous study[10] and further demonstrated the protective effect of PSGL-1 deficiency on AP development by regulating leukocyte-endothelial adhesion, which might become a promising therapeutic target.
In the initial stage of AP, pancreatic acinar cells are damaged mainly due to abnormal activation of enzymes, and circulating neutrophil and monocyte recruitment[29] by cytokines and inflammation factors[30], including IL-6 and IL-1beta, thus leading to a vicious cycle[31]. In our mechanism study, the expression of IL-6 and IL-1beta was significantly increased in the pancreas of AP mouse models and in cell lines. Evidence shows that IL-6 seems to be an effective regulator during systemic inflammation in the pancreas[32]. IL-6-/- mice have a lower death rate than wild-type mice with AP, while injection of IL-6 causes more lethal AP in mice[33]. IL-6 stimulates the phosphorylation of STAT3 and the production of the neutrophil attractant CXCL1 in pancreatic acinar cells[34]. Our study demonstrated that IL-6 inhibitor prevented caerulein-induced adhesion of PBMCs-endothelial cells in vitro, but not IL-1beta inhibitor treatment. Therefore, this study implied that PSGL-1 may initiate leukocyte adhesion and infiltration and promote the development of AP when IL-6 is activated.
In conclusion, PSGL-1 plays an important role in the development of AP by promoting inflammatory cell infiltration initiated by leukocyte and endothelial cell adhesion via IL-6 stimulation. PSGL-1 deficiency may become a new drug target for AP clinical therapy.
Acute pancreatitis (AP)-induced pancreatic injury is positively correlated with the degree of immune cell infiltration. P-selectin glycoprotein ligand 1 (PSGL-1) can regulate leukocyte activation and recruitment in various inflammation-related diseases. Our study showed that the expression of PSGL-1 in leukocytes of AP patients is increased. However, the underlying mechanism has not been fully elucidated.
Uncontrolled systemic inflammation in AP results in a high risk of morbidity and mortality, but no approved therapies are currently available. Elucidating the mechanism of action of PSGL-1 in AP is expected to help identify new targets for the treatment of pancreatitis.
This research aimed to investigate the role and mechanism of PSGL-1 in the inflammatory response during the development of AP.
We used flow cytometry to detect the expression of PSGL-1 in leukocytes from AP patients and a mouse model of caerulein-induced AP. Next, PSGL-1-/- mice administered caerulein were used to detect pancreatic injury, inflammatory cytokine expression, and inflammatory cell infiltration. A peripheral blood mononuclear cell-endothelial cell coculture system was used to clarify the mechanism by which PSGL-1 regulates leukocyte adhesion to endothelial cells.
The results of this study indicated that the numbers of monocytes and neutrophils and the expression of PSGL-1 in the peripheral blood of patients were significantly increased. PSGL-1 deficiency reduced serum amylase levels, the expression of IL-1beta and IL-6 in the serum and pancreas, the number of infiltrated neutrophils and macrophages in the pancreas, and the number of peripheral circulating neutrophils and monocytes in the AP mouse model. PSGL-1 deficiency alleviated caerulein-induced leukocyte-endothelial cell adhesion.
PSGL-1 deficiency protects against the development of AP by inducing leukocyte-endothelial cell adhesion.
Further research will explore the effect of PSGL-1 on leukocyte function and treatments for AP involving drugs targeting PSGL-1.
Thanks are due to Ya-Ping Zhai and Fang-Yuan Qin for assistance with the experiments.
Manuscript source: Unsolicited manuscript
Specialty type: Research and experimental medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Takasawa S S-Editor: Liu M L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Gukovskaya AS, Gukovsky I, Algül H, Habtezion A. Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology. 2017;153:1212-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 2. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 544] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 3. | Ling L, Wang HF, Li J, Li Y, Gu CD. Downregulated microRNA-92a-3p inhibits apoptosis and promotes proliferation of pancreatic acinar cells in acute pancreatitis by enhancing KLF2 expression. J Cell Biochem. 2019 epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, Homuth G, De Freitas Chama LL, Mishra N, Mahajan UM, Bossaller L, Völker U, Bröker BM, Mayerle J, Lerch MM. NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis. Gastroenterology 2020; 158: 253-269. e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 5. | Chen M, Geng JG. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch Immunol Ther Exp (Warsz). 2006;54:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | González-Tajuelo R, de la Fuente-Fernández M, Morales-Cano D, Muñoz-Callejas A, González-Sánchez E, Silván J, Serrador JM, Cadenas S, Barreira B, Espartero-Santos M, Gamallo C, Vicente-Rabaneda EF, Castañeda S, Pérez-Vizcaíno F, Cogolludo Á, Jiménez-Borreguero LJ, Urzainqui A. Spontaneous Pulmonary Hypertension Associated With Systemic Sclerosis in P-Selectin Glycoprotein Ligand 1-Deficient Mice. Arthritis Rheumatol. 2020;72:477-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Ye Z, Zhong L, Zhu S, Wang Y, Zheng J, Wang S, Zhang J, Huang R. The P-selectin and PSGL-1 axis accelerates atherosclerosis via activation of dendritic cells by the TLR4 signaling pathway. Cell Death Dis. 2019;10:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Zarbock A, Müller H, Kuwano Y, Ley K. PSGL-1-dependent myeloid leukocyte activation. J Leukoc Biol. 2009;86:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Li J, Chen J, Tang W. The consensus of integrative diagnosis and treatment of acute pancreatitis-2017. J Evid Based Med. 2019;12:76-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Yang Y, Liu X, Liu Y, Fu H, Gao Y, Liu X, Jiang X. The development of salt-sensitive hypertension regulated by PSGL-1 gene in mice. Cell Biosci. 2018;8:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Geisz A, Sahin-Tóth M. A preclinical model of chronic pancreatitis driven by trypsinogen autoactivation. Nat Commun. 2018;9:5033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 13. | Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24 Suppl 1:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 14. | Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 874] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 15. | Yang ZW, Meng XX, Xu P. Central role of neutrophil in the pathogenesis of severe acute pancreatitis. J Cell Mol Med. 2015;19:2513-2520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Saluja A, Dudeja V, Dawra R, Sah RP. Early Intra-Acinar Events in Pathogenesis of Pancreatitis. Gastroenterology. 2019;156:1979-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 17. | Zhao Q, Wei Y, Pandol SJ, Li L, Habtezion A. STING Signaling Promotes Inflammation in Experimental Acute Pancreatitis. Gastroenterology 2018; 154: 1822-1835. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 18. | Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 19. | Hackert T, Büchler MW, Werner J. Targeting P-selectin in acute pancreatitis. Expert Opin Ther Targets. 2010;14:899-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Bhatia M, Ramnath RD, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1259-G1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Sakikubo M, Furuyama K, Horiguchi M, Hosokawa S, Aoyama Y, Tsuboi K, Goto T, Hirata K, Masui T, Dor Y, Fujiyama T, Hoshino M, Uemoto S, Kawaguchi Y. Ptf1a inactivation in adult pancreatic acinar cells causes apoptosis through activation of the endoplasmic reticulum stress pathway. Sci Rep. 2018;8:15812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Yago T, Liu Z, Ahamed J, McEver RP. Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood. 2018;132:1426-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 23. | Baïsse B, Spertini C, Galisson F, Smirnova T, Spertini O. The function of P-selectin glycoprotein ligand-1 is conserved from ancestral fishes to mammals. J Leukoc Biol. 2019;106:1271-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Tinoco R, Otero DC, Takahashi AA, Bradley LM. PSGL-1: A New Player in the Immune Checkpoint Landscape. Trends Immunol. 2017;38:323-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | Kong L, Wu Q, Zhao L, Ye J, Li N, Yang H. Effect of microRNA-27a-5p on apoptosis and inflammatory response of pancreatic acinar cells in acute pancreatitis by targeting PTEN. J Cell Biochem. 2019;120:15844-15850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Tsaroucha AK, Schizas D, Vailas MG, Rachmani E, Kanavidis P, Asimakopoulos V, Vlachos S, Sotiropoulou M, Pitiakoudis MS, Simopoulos CE. E and P Selectins as Potential Markers in the Assessment of the Severity of Acute Pancreatitis. Pancreas. 2018;47:406-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Hoque R, Sohail M, Malik A, Sarwar S, Luo Y, Shah A, Barrat F, Flavell R, Gorelick F, Husain S, Mehal W. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011;141:358-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 28. | Aleisa FA, Sakashita K, Lee JM, AbuSamra DB, Al Alwan B, Nozue S, Tehseen M, Hamdan SM, Habuchi S, Kusakabe T, Merzaban JS. Functional binding of E-selectin to its ligands is enhanced by structural features beyond its lectin domain. J Biol Chem. 2020;295:3719-3733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Li HY, He HC, Song JF, Du YF, Guan M, Wu CY. Bone marrow-derived mesenchymal stem cells repair severe acute pancreatitis by secreting miR-181a-5p to target PTEN/Akt/TGF-β1 signaling. Cell Signal. 2020;66:109436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Raraty MG, Murphy JA, Mcloughlin E, Smith D, Criddle D, Sutton R. Mechanisms of acinar cell injury in acute pancreatitis. Scand J Surg. 2005;94:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2002;9:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 257] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 32. | Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1126] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 33. | Fischer M, Goldschmitt J, Peschel C, Brakenhoff JP, Kallen KJ, Wollmer A, Grötzinger J, Rose-John S. I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat Biotechnol. 1997;15:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 440] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 34. | Zhang H, Neuhöfer P, Song L, Rabe B, Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regnér S, Thorlacius H, Saur D, Weirich G, Yoshimura A, Halangk W, Mizgerd JP, Schmid RM, Rose-John S, Algül H. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest. 2013;123:1019-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |