Published online Nov 7, 2020. doi: 10.3748/wjg.v26.i41.6346
Peer-review started: May 19, 2020
First decision: July 29, 2020
Revised: July 30, 2020
Accepted: August 29, 2020
Article in press: August 29, 2020
Published online: November 7, 2020
Processing time: 170 Days and 20.8 Hours
Chronic liver injury (CLI) is now a worldwide disease. However, there is no effective treatment. Pyroptosis plays an essential role in CLI. Dihydromyricetin (DHM) resists oxidation and protects the liver. We hypothesize that the beneficial effect of DHM on CLI is related to its effect on the expression of pyroptosis-related molecules. Therefore, we studied the influence of DHM on CLI and pyroptosis.
To study the role of pyroptosis in the pathogenesis of CLI and the therapeutic mechanism of DHM.
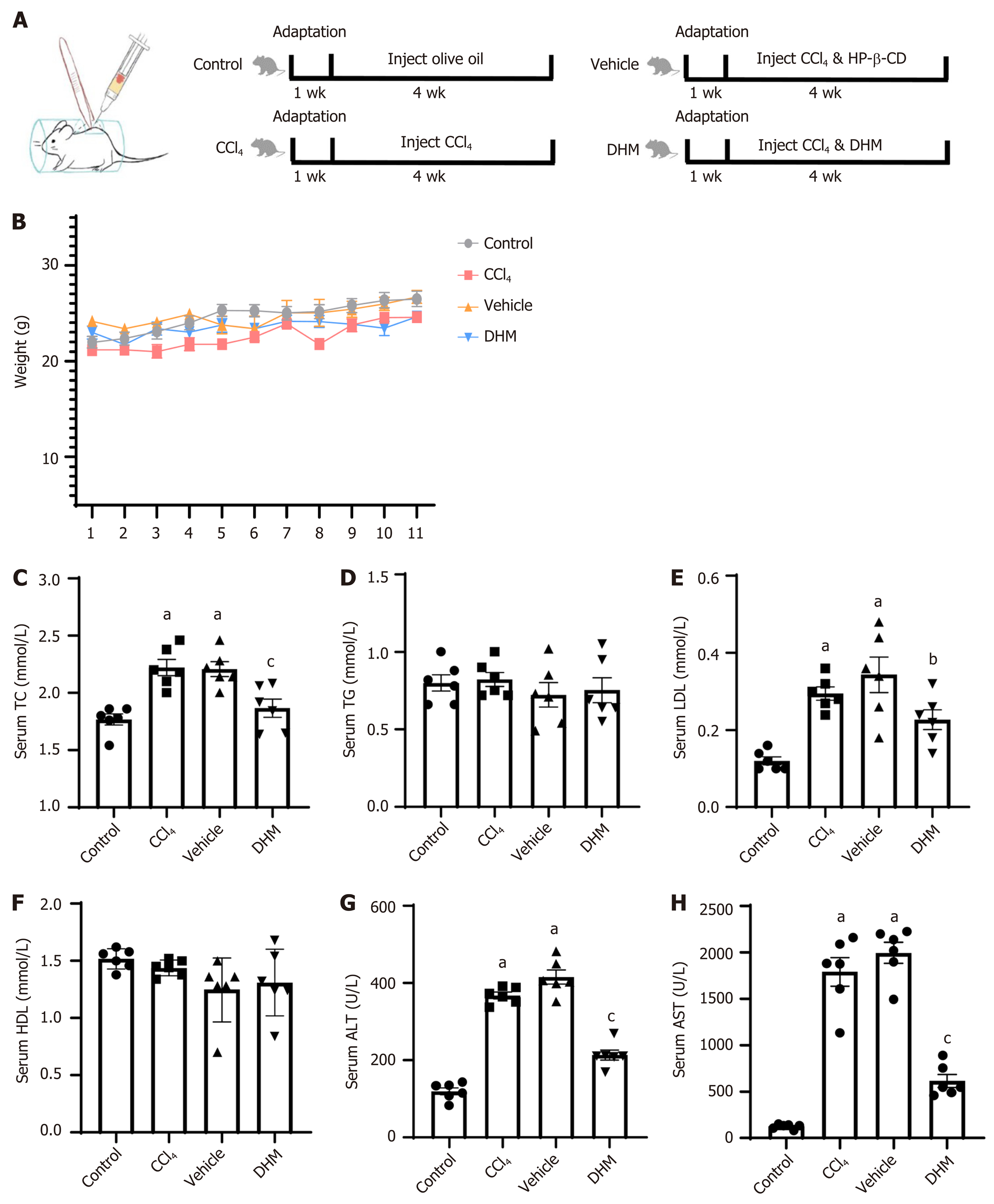
Thirty-two mice were randomly divided into four groups: The control group was injected with olive oil, the carbon tetrachloride (CCl4) group was injected with CCl4, the vehicle group was injected with hydroxypropyl-β-cyclodextrin while injecting CCl4 and the DHM group was injected with DHM while injecting CCl4. After four weeks of treatment, liver tissues from the mice were stained with hematoxylin and eosin, and oil red O. Blood was collected from the angular vein for serological analysis. The severity of CLI was estimated. Some liver tissue was sampled for immunohistochemistry, Western blotting and quantitative reverse transcription PCR to observe the changes in pyroptosis-related molecules.
Serum total cholesterol, low density lipoprotein, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in the CCl4 group were higher than those in the control group, and serum total cholesterol, low density lipoprotein, AST and ALT in the DHM group were lower than those in the vehicle group. Hematoxylin and eosin and oil red O staining showed that there were more lipid droplets in the CCl4 group than in the control group, and there were fewer lipid droplets in the DHM group than in the vehicle group. Western blotting showed that the expression of the pyroptosis-related molecules caspase-1, NOD-, LRR- and pyrin domain-containing 3 (NLRP3) and gasdermin D (GSDMD)-N in the CCl4 group was higher than that in the control group, while expression of these proteins in the DHM group was lower than that in the vehicle group. Quantitative reverse transcription PCR results showed that the expression of the pyroptosis-related genes caspase-1, NLRP3, GSDMD and interleukin-1β (IL-1β) in the CCl4 group was higher than that in the control group, while there was no significant change in NLRP3 and caspase-1 expression in the DHM group compared with that in the vehicle group, and the expression of GSDMD and IL-1β was decreased.
DHM improves CCl4-induced CLI and regulates the pyroptosis pathway in hepatocytes. DHM may be a potential therapeutic agent for CLI.
Core Tip: We established a model of chronic liver injury (CLI) in mice by subcutaneous injection of CCL4 into the back. The effect of dihydromyricetin on improving CLI in terms of morphology and serum level was demonstrated, and the activation of pyroptosis in CLI and the regulation of pyroptosis by dihydromyricetin in terms of protein level and mRNA level were verified, respectively.
- Citation: Cheng QC, Fan J, Deng XW, Liu HC, Ding HR, Fang X, Wang JW, Chen CH, Zhang WG. Dihydromyricetin ameliorates chronic liver injury by reducing pyroptosis. World J Gastroenterol 2020; 26(41): 6346-6360
- URL: https://www.wjgnet.com/1007-9327/full/v26/i41/6346.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i41.6346
Chronic liver injury (CLI) is the beginning of many serious liver diseases. Drugs, viruses, chemical poisons, ethanol and other factors can directly or indirectly cause liver damage[1]. Following liver pathological changes, sustained damage leads to CLI, which causes normal metabolic dysfunction, affects bile excretion and digestive system function, and finally develops into liver failure, which seriously threatens human health[2]. Therefore, the development of new therapeutic drugs is urgently required.
A number of studies have shown that pyroptosis occurs during CLI and that pyroptosis plays an important role in CLI[3-5]. Pyroptosis is a type of cell death that causes a strong inflammatory response, also known as caspase-1-dependent regulatory cell death[6]. It is manifested by cell swelling until the cell membrane bursts, leading to the release of cell contents and resulting in a strong inflammatory response[7]. Excessive pyroptosis can amplify the inflammatory response and cause further damage to the body. Following liver tissue damage, excessive pyroptosis will lead to further damage of liver cells, making it difficult to recover function. Inhibition of excessive pyroptosis can reduce the damage caused by the inflammatory response, but there is little research on the drug mechanism that can interfere with pyroptosis[8]. Therefore, we hope to identify a drug which acts on the pyroptosis process in hepatocytes, inhibits liver tissue damage caused by inflammation, and thus provide protection for CLI.
CCl4 is a hepatotoxic substance that causes liver damage and is often used to induce fatty liver or fibrosis models. The mechanism involved is mainly related to oxidative stress induced by CCl4 in the liver. CCl4 leads to the continuous production and accumulation of harmful lipid and protein peroxidation products and causes severe necrotic reactions, which destroy the structure and function of liver cells[9-11]. Therefore, our research group chose CCl4 to establish an animal model of CLI.
Dihydromyricetin (DHM) is the most abundant natural flavonoid in Ampelopsis grossedentata (A. grossedentata). It has a wide range of pharmacological effects[12]. A number of studies have shown that DHM protects the liver[13-15]. The mechanism may be related to antioxidation and anti-inflammation[12,16,17]. Therefore, our research group aimed to observe the effect of DHM on pyroptosis in hepatocytes in mice with CCl4-induced CLI and determine the relevant mechanism of its protective effect on the liver.
CCl4 (105033) and olive oil (MB13084) were purchased from Beijing Tongguang Fine Chemical Co., Ltd. (Beijing, China) and Dalian Meilun Biotechnology Co., Ltd. Hydroxypropyl-β-cyclodextrin (HP-β-CD) (C7070), DHM (SD8280) and the hematoxylin and eosin (HE) staining kit (SL7070) were purchased from Beijing Solarbio Technology Co., Ltd. (Beijing, China); a total of 0.212 g HP-β-CD was dissolved in 1 mL normal saline to produce the HP-β-CD vehicle solution, and 20 mg DHM was dissolved in 800 μL HP-β-CD solution to produce the DHM solution. Isoflurane was purchased from Shenzhen Reward Life Technology Co., Ltd. (Shenzhen, China), and oil red O (ORO) (o8010-5) was purchased from Sigma (St. Louis, MO, United States). ORO (0.5 g) was dissolved in an appropriate amount of isopropanol, and after full dissolution, the final volume of the solution was increased to 100 mL with isopropanol, which was the preservation ORO solution. When used, the ORO preservation solution was diluted 3:2 with distilled water and was the working solution. HistostainTM-plus kits (SP900) and the DAB substrate kit (ZLI-9018) were purchased from ZSGB-BIO (Beijing, China). RIPA lysis buffer (C1053+ -100) was purchased from Applygen (Beijing, China), and a BCA protein detection kit was purchased from Invitrogen (CA, United States). Anti-caspase-1 p20 and anti-IL-1 antibodies were purchased from Biosource (Beijing, China). Anti-NLRP3, anti-GSDMD, and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were purchased from Abcam (Cambridge, United Kingdom), ABclonal (Beijing, China), and ZSGB-BIO (Beijing, China), respectively. Horseradish peroxidase-conjugated secondary antibodies were purchased from Applygen (Beijing, China). A tissue total RNA extraction kit (RC101-01), reverse transcription kit (R323-01), and ChamQ Universal SYBR qPCR master mix (q711-02) were purchased from Vazyme (Piscataway, United States).
Eight-week-old male C57BL/6J mice with a body weight of 20 ± 2 g were used in the study. All mice were kept in the Department of Laboratory Animal Science of Peking University Health Science Center. The room temperature in the animal center was 23°C, the relative humidity was 50%-55%, and the light cycle was 12 h:12 h. The mice were free to drink water and eat food during the experiment. This experiment was approved by the Local Ethics Committee for Animal Research Studies at the Peking University Health Science Center.
Thirty-two mice were randomly divided into four groups: The control group, CCl4 group, vehicle group, and DHM group. In the control group, the mice received injections of olive oil every three days for up to 4 wk. The CCl4 group was treated with CCl4 injections (40% CCl4 in olive oil was injected subcutaneously into the back at a dose of 30 μL/g body weight) every three days for 4 wk. The vehicle group was treated with CCl4 injections (methods were the same as those for the CCl4 group) every three days, and HP-β-CD solution (the solution was injected intraperitoneally at a dose of 4 μL/g body weight) every day for 4 wk. The DHM group was treated with CCl4 injections (methods were the same as those for the CCl4 group) every three days, and DHM solution (methods were the same as those for the vehicle group) every day for 4 wk. The subcutaneous back injection model is shown in Figure 1A.
We determined the injection dose of DHM based on the study by Shi et al[18]. Weight was monitored every three days throughout the experiment. After 4 wk, the mice were euthanized by inhalation of isoflurane.
After deep anesthesia with isoflurane, blood was collected from the angular vein. The blood was then maintained at 4°C for 30 min, and the supernatant was centrifuged at 4°C and 2500 rpm for 20 min. All serological tests of liver function indices were submitted to the Department of Laboratory Animal Science of Peking University Health Science Center, and the test results are expressed in tables and graphs as the mean ± SEM.
After blood collection, livers were collected and fixed in 10% neutral buffered formalin for histological examination. For the best fixation effect, liver tissue samples of approximately 10 mm × 5 mm × 5 mm were used.
HE and ORO staining were performed using standard procedures. The ORO-positive areas were quantified as described previously. HE-stained sections were observed and photographed under a biological microscope (Olympus, Japan), and the non-alcoholic fatty liver disease activity score was calculated. ORO-stained sections were observed and photographed under a biological microscope. The positive ORO region was divided by the total cell area in each image using ImageJ software, and hepatic steatosis was quantified by image analysis.
For immunostaining of mature caspase-1, liver tissue sections were incubated overnight with anti-caspase-1 p20 antibodies (1:2000), followed by treatment with HistostainTM-plus kits. Antigen-antibody complexes were visualized using a DAB substrate kit.
Following blood collection, livers were collected and stored at -80°C. Livers were lysed with RIPA lysis buffer and centrifuged, and the supernatant was obtained. The protein concentration was measured using a BCA protein detection kit. The proteins were separated by SDS-PAGE and transferred to Immun-Blot PVDF membranes. The membranes were washed, blocked (5% nonfat-dried milk), and incubated with the primary antibody and then with an appropriate horseradish peroxidase-conjugated secondary antibody. The signal was detected with an enhanced/super ECL kit. The images were scanned, and the relative density of the immunoreactive bands was determined using ImageJ software.
According to the manufacturer's instructions, total RNA from mouse liver tissues was extracted with a total RNA extraction kit. cDNA was prepared with a reverse transcription kit, and quantitative reverse transcription-PCR (qRT-PCR) was performed in an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, United States) using ChamQ Universal SYBR qPCR master mix. GAPDH was used as a reference gene to analyze the relative mRNA expression of pyroptosis-related genes. The primer sequences are shown in Table 1.
| Gene | Primers | Sequence (5’to 3’) |
| NLRP3 | Forward | GACCGTGAGGAAAGGACCAG |
| Reverse | GGCCAAAGAGGAATCGGACA | |
| CASP1 | Forward | CGAGGGTTGGAGCTCAAGTT |
| Reverse | AGAAGTCTTGTGCTCTGGGC | |
| GSDMD | Forward | TGCTTGAAGGGTGAAGGCAA |
| Reverse | CACCTGAGGAGGGCTCAAAG | |
| IL-1β | Forward | ATGCCACCTTTTGACAGTGATG |
| Reverse | TGTGCTGCTGCGAGATTTGA | |
| GAPDH | Forward | TCTACATGTTCCAGTATGACTC |
| Reverse | ACTCCACGACATACTCAGCACC |
GraphPad Prism version 8.0 was used to analyze the data, and the results are presented in the table and graphs as the mean ± SEM. Single-factor analysis of variance was used for comparisons between multiple groups, followed by Dunnett's multiple comparison test. The data from two groups were analyzed by the Student's t test (unpaired t test), and P < 0.05 was used to determine significant differences. Each experiment was repeated at least three times to obtain similar results. P values of less than 0.05 were considered significant.
Weight monitoring of mice for one month showed no significant differences in body weight between the four groups (P > 0.05) (Figure 1B). Venous blood serological data were analyzed after 4 wk of modeling (Table 2). Serum total cholesterol was higher in the CCl4 group (P < 0.01) (Figure 1C) compared with that in the control group, and there was no significant difference in triglyceride level (P > 0.05) (Figure 1D). Low density lipoprotein (LDL) was significantly elevated (P < 0.01) (Figure 1E), but high density lipoprotein did not change significantly (P > 0.05) (Figure 1F). Serum total cholesterol decreased significantly (P < 0.01) (Figure 1C) and LDL decreased (P < 0.05) (Figure 1E) compared with those in the vehicle group. Similarly, there was no significant change in triglyceride or high density lipoprotein (P > 0.05) (Figure 1D and F). Elevated serum alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) activity is a hallmark of hepatocyte injury. We found that serum levels of ALT and AST in the CCl4 group were significantly higher than those in the control group (P < 0.01) (Figure 1G and H). Both ALT and AST in the DHM group were significantly lower than those in the vehicle group (P < 0.01) (Figure 1G and H).
| Control | CCl4 | Vehicle | DHM | |
| TC (mmol/L) | 1.77 ± 0.12 | 2.22 ± 0.17a | 2.21 ± 0.16a | 1.87 ± 0.19c |
| TG (mmol/L) | 0.80 ± 0.13 | 0.82 ± 0.11 | 0.72 ± 0.20 | 0.75 ± 0.20 |
| LDL (mmol/L) | 0.12 ± 0.03 | 0.30 ± 0.04a | 0.34 ± 0.11a | 0.23 ± 0.01b |
| HDL (mmol/L) | 1.51 ± 0.09 | 1.44 ± 0.07 | 1.25 ± 0.28 | 1.31 ± 0.29 |
| ALT (U/L) | 119.87 ± 22.26 | 367.40 ± 21.15a | 415.42 ± 45.03a | 213.52 ± 31.82c |
| AST (U/L) | 126.05 ± 25.60 | 1790.30 ± 376.01a | 1995.52 ± 273.80a | 615.65 ± 170.62c |
To further determine whether CCl4 treatment successfully induced significant CLI and whether DHM improved liver injury, analysis of histological staining was performed (Figure 2). HE and ORO staining showed that the control group had normal liver structure and hepatic lobules, and no lipid droplets were found. In contrast, mice in the CCl4 group had large numbers of lipid droplets and inflammatory cell infiltration. However, in the DHM group, the hepatic lobule structure was restored, and the lipid droplets were significantly reduced.
These results strongly suggest that CCl4 treatment of mice induced a stable CLI model, and DHM improves liver damage caused by CCl4 and reduces hepatocyte steatosis.
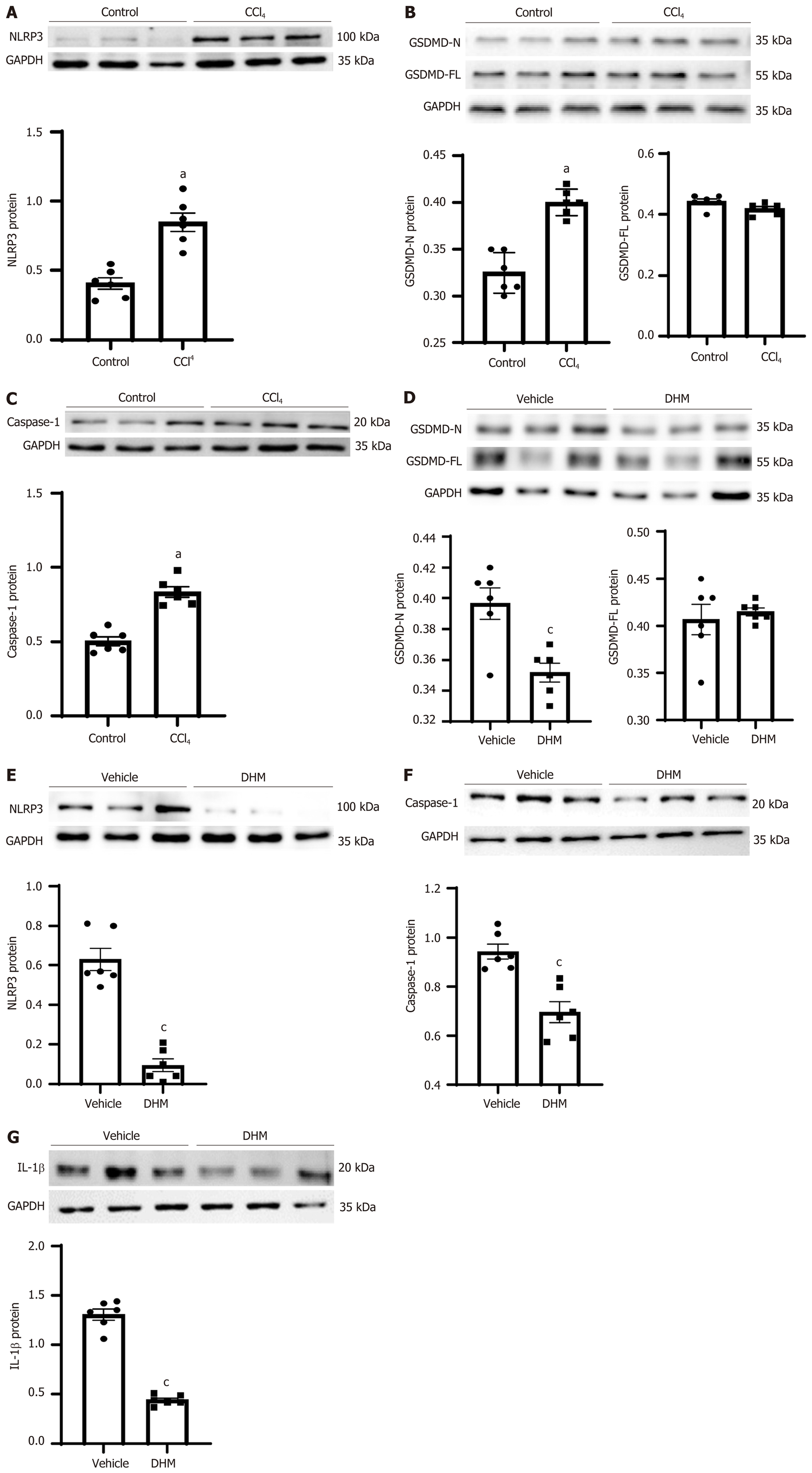
Studies have shown that pyroptosis is involved in the pathogenesis of CLI[19-21]. To investigate whether DHM affects the activation of pyroptosis, the expression levels of pyroptosis-related proteins were examined by Western blotting and immuno-histological analysis. The expression of caspase-1 (p20), the core protein in the pyroptosis pathway, and NLRP3, which forms the inflammatory body, were significantly increased in the CCl4 group compared with that in the control group (P < 0.01) (Figure 3A and C), and the expression level of the effector protein GSDMD was not significantly changed. The full-length GSDMD is indicated as GASMD-FL (P > 0.05) (Figure 3B), but the cleavage product GSDMD-N was significantly increased (P < 0.01) (Figure 3B). After DHM treatment, the protein expression of NLRP3, GSDMD-N and mature caspase-1 was significantly downregulated (P < 0.01) compared with that in the vehicle group (Figure 3D-3D and the inflammatory molecule IL-1β was significantly decreased (P < 0.01) (Figure 3G).
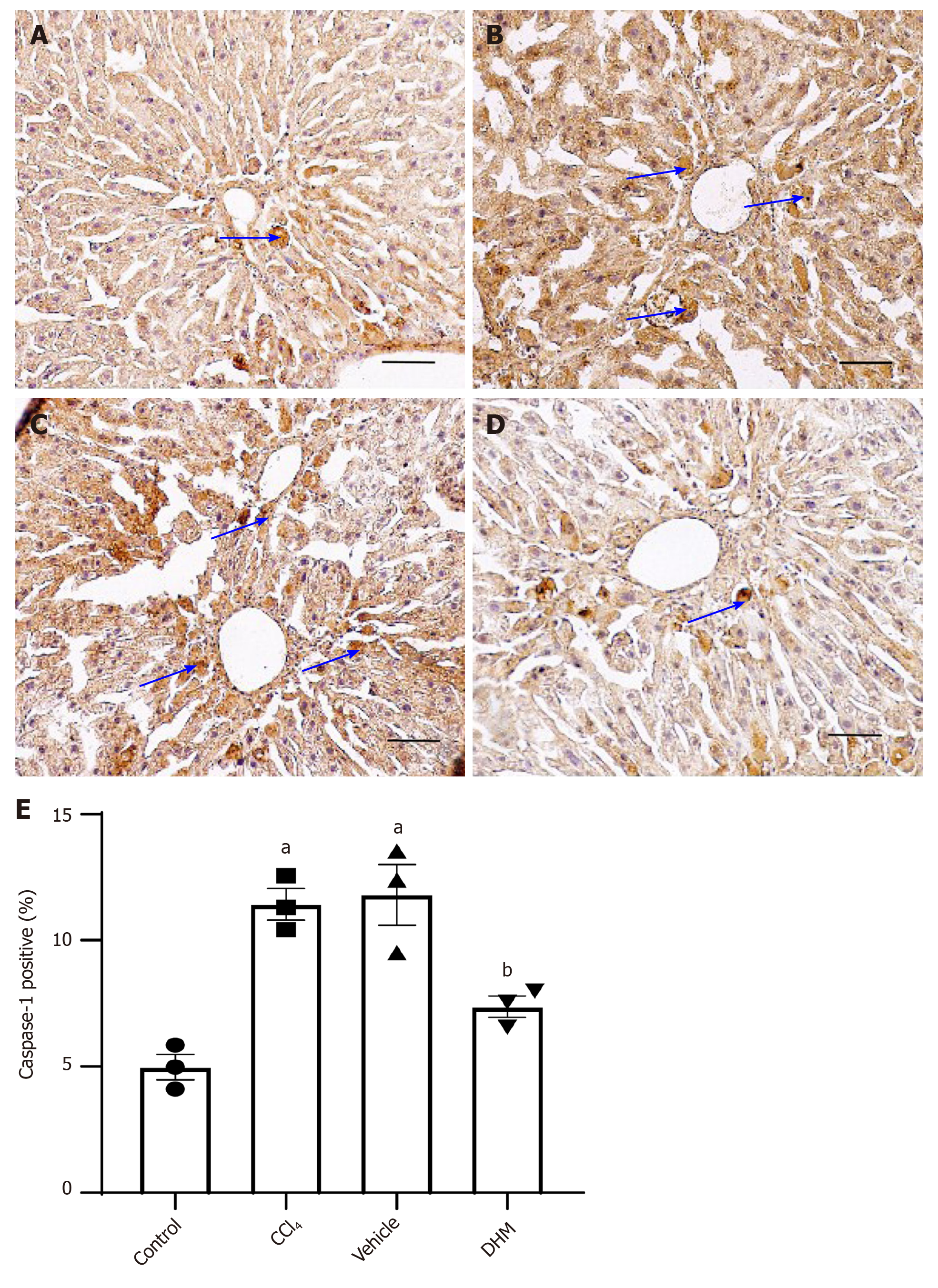
In addition, the expression of mature caspase-1 was examined by immuno-histochemical staining. The expression of mature caspase-1 (p20) was significantly increased in the CCl4 group compared with that in the control group (Figure 4A and B). Mature caspase-1 was downregulated in the DHM group relative to that in the vehicle group (Figure 4C and D).
These results fully demonstrated that CCl4 activates pyroptosis, while DHM significantly reduces pyroptosis.
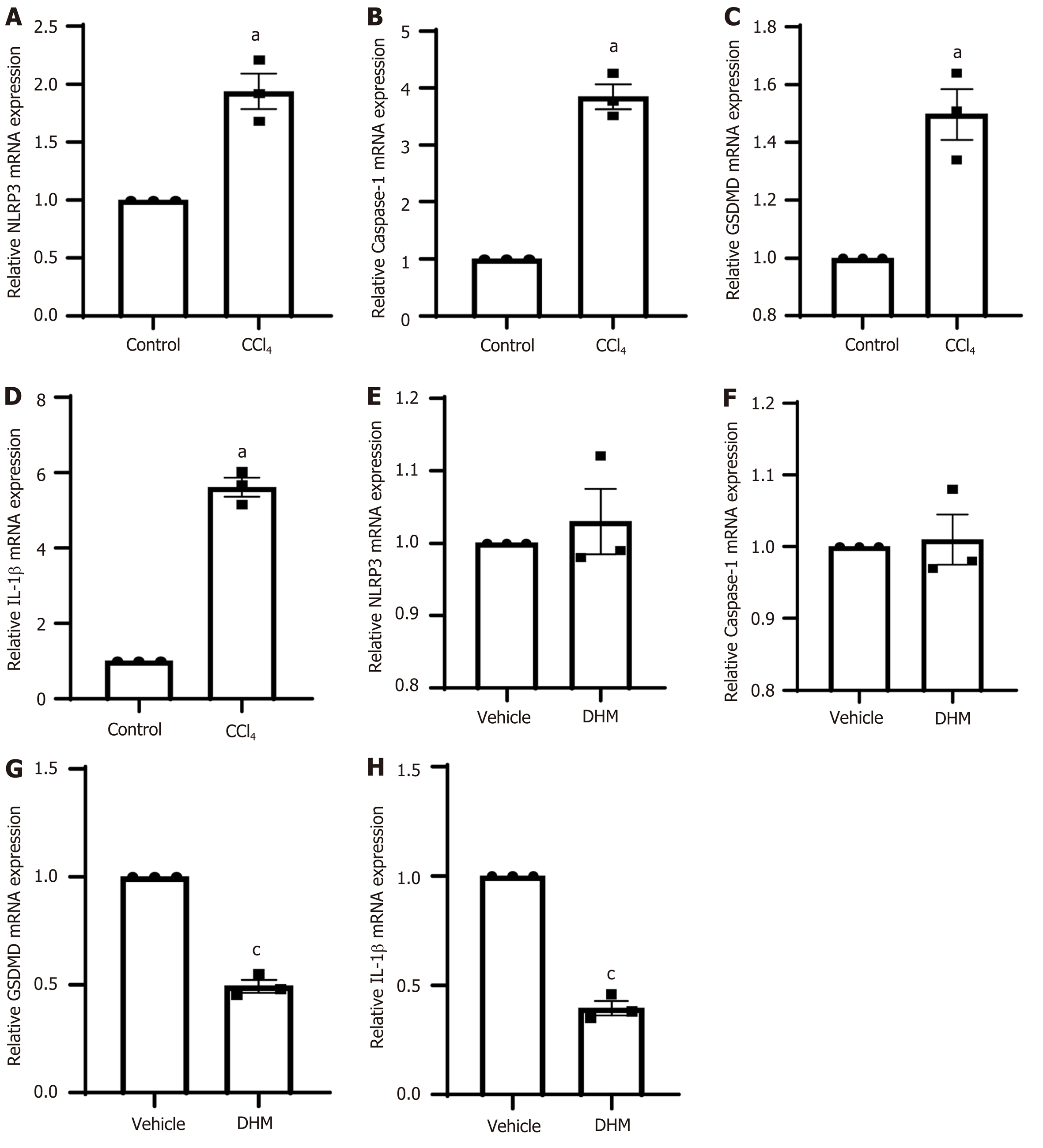
To further clarify the expression level of pyroptosis-related genes, we performed qRT-PCR. The results showed that caspase-1, NLRP3, GSDMD and IL-1β were upregulated at the mRNA level in the CCl4 group (P < 0.01) (Figure 5A-5D which is consistent with the corresponding protein expression levels. However, the DHM group did not show results that were consistent with the Western blotting results compared to those in the vehicle group. The expression of NLRP3 and caspase-1 mRNA in the DHM group was not significantly different compared with that in the vehicle group (P > 0.05) (Figure 5E and F), and GSDMD and IL-IL mRNA decreased (P < 0.01) (Figure 5G and H).
Dihydromyricetin is the main flavonoid in A. grossedentata[12]. A number of studies have shown that flavonoids have many functions, such as scavenging oxygen free radicals and antioxidative, antithrombotic, anti-inflammatory and antitumor activities[16,22,23]. In addition to the general properties of flavonoids, DHM protects against liver I/R injury[13], alcoholic liver disease[24], and non-alcoholic fatty liver disease[15]. Recent studies found that DHM effectively improves the development of CLI[24,25]. DHM attenuates oxidative stress and restores mitochondrial respiration[15]. However, there is a close relationship between oxidative stress and pyroptosis[26]. Whether DHM can intervene in CLI by affecting pyroptosis is not well understood. Therefore, this research was focused on pyroptosis to determine the effect of DHM on CLI and its mechanism.
The mechanism of CCl4-induced CLI includes the production of ROS and inflammatory cytokines, an imbalance in calcium homeostasis, and lipid peroxidation[27], and is thus closely related to pyroptosis. Traditionally, intraperitoneal injection of CCl4 induces CLI, which causes abdominal adhesions and a series of complications that affects the vitality of mice and increases mortality[28]. Our group improved the intraperitoneal injection method by injecting CCl4 subcutaneously into the back (Figure 1A). Body weight monitoring during modeling showed no significant differences in body weight and no deaths, demonstrating the safety of subcutaneous injections into the back. Serological ALT and AST levels increased, LDL increased, and HE/ORO staining results showed that lipid droplets in the CCl4 group increased significantly. These results strongly indicated that CCl4 successfully induced a CLI model.
Cell death can be divided into programmed cell death and necrosis. Programmed cell death can be classified as nonlytic and lytic. Nonlytic cell death, such as apoptosis, does not usually result in a secondary immune response. However, lytic cell death, such as necrotic apoptosis and pyroptosis, can cause a strong inflammatory response. Pyroptosis, also known as caspase-1-dependent programmed cell death, is usually characterized by rapid lysis of pyroptotic cells, which is characterized by pore formation, cell swelling and membrane rupture[29,30]. The process of pyroptosis is mainly divided into the following four steps (Figure 6): Formation of inflammasomes, activation of caspase-1, formation of pores in the cell membrane, and the release of inflammatory factors[31]. The experiments conducted included these four steps to prove the presence of pyroptosis induced by CCl4 in the CLI model and the therapeutic effect of DHM on pyroptosis.
The inflammasome is a cytoplasmic sensor of caspase-1 that activates caspase-1. The inflammasome is composed of three parts: Cytoplasmic receptor molecules (mainly NLRP3), the connective protein ASC, and the effector protein pro-caspase-1[32]. Here, we selected NLRP3 as the target molecule to observe the effect of DHM on CLI pyroptosis. Western blotting results showed that NLRP3 protein expression was upregulated after modeling and decreased to some extent after DHM intervention. The qPCR results showed that mRNA expression was upregulated after modeling; however, DHM did not show results that were consistent with a decrease in protein, and there was no statistically significant difference. We determined that it is possible that DHM only interfered with NLRP3 synthesis or promoted its degradation at the protein level, thus affecting subsequent signaling. Although there was no significant difference in the results, there was a certain upward trend, which also proved that the decrease in NLRP3 protein may negatively regulate expression of the NLRP3 gene.
The effector proteins of the inflammatory body are caspases. Caspases, a group of cysteine proteolytic enzymes, are associated with inflammation and cell death[33]. Caspases mainly play an important role in apoptosis and pyroptosis and do not participate in other programmed cell death processes. According to their functions, caspases are related to apoptosis or inflammation. The NLRP3 inflammasome can be activated by DAMPs and PAMPs[34]. When assembly is complete, caspase-1 is activated, and pyroptosis is activated. Western blot results showed that mature caspase-1 protein (caspase-1 p20) expression was upregulated after modeling and downregulated after DHM intervention, and the immunohistochemical results were consistent with this. The qPCR results showed that caspase-1 mRNA expression was upregulated after modeling but did not decrease after DHM intervention. We determined whether DHM intervention decreased NLRP3 protein, leading to its target protein (pro-caspase 1) not being converted to mature caspase-1. Therefore, at the protein level, caspase-1 expression was downregulated after DHM intervention, but treatment did not affect the mRNA level of pro-caspase-1. Therefore, the mRNA level of pro-caspase-1 did not decrease after DHM intervention, possibly because of the small number of samples measured, which were not sufficient for a statistically significant declining trend.
GSDMD is a protein with 487 amino acid residues that belongs to the gasdermin family[35]. Gasdermin protein binds to lipids on the cell membrane and then produces holes in the cell membrane, leading to the leakage of cell contents and mediating cell death and the inflammatory response[36]. Therefore, GSDMD is the executor of pyroptosis in cells. The complete GSDMD protein consists of two domains that are joined together by joint connections. When the junction is sheared by caspase-1 or caspase-11, it is divided into two parts, the carboxyl terminal (GSDMD-C) and amino terminal (GSDMD-N). GSDMD-C inhibits GSDMD-N, and when the two are separated, GSDMD-N binds to the lipid membrane and polymerizes into an oligomer with a pore in the middle[36]. This channel allows passage of materials < 10 nm in diameter. More pores are formed, which cause cell membrane damage and rupture[37]. In this experiment, no significant change was observed in the complete GSDMD protein after modeling or DHM intervention, but GSDMD-N protein was increased after modeling and downregulated after DHM intervention. We found that GSDMD expression was upregulated at the mRNA level after modeling, and the subsequently translated GSDMD protein was cleaved into GSDMD-C and GSDMD-N by mature caspase-1 protein. Therefore, the full-length GSDMD protein was not significantly upregulated or downregulated, but the effector GSDMD-N protein was significantly upregulated. After DHM intervention, caspase-1 decreased, GSDMD-N decreased, and the mRNA level also decreased.
Mature caspase-1 activates IL-1β. IL-1β, as the main inflammatory molecule of pyroptosis[38], is also one of the main factors that induces inflammation in CLI[38-40]. Therefore, IL-1β was selected as the inflammatory index observed. Western blotting results showed that the expression of IL-1β was downregulated after DHM intervention, and qPCR results showed that the mRNA expression was consistent with the protein changes. Therefore, we believe that DHM improves the inflammation caused by pyroptosis.
In conclusion, this study comprehensively used molecular biology and morphological techniques to fully explore the changes in pyroptosis in CCl4-induced CLI and the influence of DHM on mRNA, protein and tissue morphology and proved that DHM significantly improved CLI by inhibiting pyroptosis. This study provides a new idea for in-depth exploration of the pathogenesis and treatment of CLI and provides an experimental basis for the early prevention and treatment of CLI. As DHM has the effect of anti-oxidative stress, the proportion of DHM's effect on pyroptosis in the treatment of CCl4-induced CLI is not clear, which needs to be further explored. In addition to regulating pyroptosis, whether DHM influences other cell death pathways is also unknown, and should be investigated in further research.
Chronic liver injury (CLI) is the beginning of many serious liver diseases. If not timely managed CLI can seriously threaten human health. It has been reported that pyroptosis occurs in CLI. However, there is no drug that reduces CLI by regulating pyroptosis.
To explore the therapeutic effect and mechanism of dihydromyricetin (DHM) on CLI from a new perspective of the pathogenesis of CLI-pyroptosis.
We mainly focused on the regulatory effect of DHM on pyroptosis in CLI. The effect of DHM on pyroptosis was evaluated at the following three levels: Morphology, protein and mRNA. The related mechanism of DHM treatment for CLI was also studied.
A CLI mouse model was established by subcutaneous injection of carbon tetrachloride (CCl4) into the back. After establishment of the model, the therapeutic effect of DHM on CLI was estimated by serological detection, HE staining and ORO staining. Western blotting, immunohistochemistry and qRT-PCR were used to detect the changes in pyroptosis-related molecules.
DHM improved liver damage caused by CCl4, reduced steatosis in hepatocytes, and inhibited the pyroptosis signal.
DHM can improve CCl4-induced CLI and regulate the pyroptosis pathway in hepatocytes. DHM may be a potential therapeutic drug for CLI.
As a newly discovered programmed cell death, pyroptosis provides a new direction for the study of the pathogenesis of CLI. DHM affects the mechanism of action by regulating pyroptosis to alleviate CLI. This could be a major breakthrough in the development of drugs for CLI.
We thank Dr. Wang SF for skillful technical assistance with statistics.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ho HK, Yildiz K S-Editor: Gao CC L-Editor: Webster JR P-Editor: Zhang YL
| 1. | Tacke F, Weiskirchen R. An update on the recent advances in antifibrotic therapy. Expert Rev Gastroenterol Hepatol. 2018;12:1143-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Roy DN, Goswami R. Drugs of abuse and addiction: A slippery slope toward liver injury. Chem Biol Interact. 2016;255:92-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Davies AP, Gebhart CJ, Meric SA. Campylobacter-associated chronic diarrhea in a dog. J Am Vet Med Assoc. 1984;184:469-471. [PubMed] |
| 4. | Molyvdas A, Georgopoulou U, Lazaridis N, Hytiroglou P, Dimitriadis A, Foka P, Vassiliadis T, Loli G, Phillipidis A, Zebekakis P, Germenis AE, Speletas M, Germanidis G. The role of the NLRP3 inflammasome and the activation of IL-1β in the pathogenesis of chronic viral hepatic inflammation. Cytokine. 2018;110:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 750] [Cited by in RCA: 1052] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 6. | Guo H, Xie M, Zhou C, Zheng M. The relevance of pyroptosis in the pathogenesis of liver diseases. Life Sci. 2019;223:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1330] [Cited by in RCA: 1536] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 8. | Wu J, Lin S, Wan B, Velani B, Zhu Y. Pyroptosis in Liver Disease: New Insights into Disease Mechanisms. Aging Dis. 2019;10:1094-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Kubota N, Kado S, Kano M, Masuoka N, Nagata Y, Kobayashi T, Miyazaki K, Ishikawa F. A high-fat diet and multiple administration of carbon tetrachloride induces liver injury and pathological features associated with non-alcoholic steatohepatitis in mice. Clin Exp Pharmacol Physiol. 2013;40:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Tsuchida T, Lee YA, Fujiwara N, Ybanez M, Allen B, Martins S, Fiel MI, Goossens N, Chou HI, Hoshida Y, Friedman SL. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol. 2018;69:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 393] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 11. | Lee Y, Jee HJ, Noh H, Kang GH, Park J, Cho J, Cho JH, Ahn S, Lee C, Kim OH, Oh BC, Kim H. In vivo (1)H-MRS hepatic lipid profiling in nonalcoholic fatty liver disease: an animal study at 9.4 T. Magn Reson Med. 2013;70:620-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Zhang J, Chen Y, Luo H, Sun L, Xu M, Yu J, Zhou Q, Meng G, Yang S. Recent Update on the Pharmacological Effects and Mechanisms of Dihydromyricetin. Front Pharmacol. 2018;9:1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 13. | Chen Y, Lv L, Pi H, Qin W, Chen J, Guo D, Lin J, Chi X, Jiang Z, Yang H, Jiang Y. Dihydromyricetin protects against liver ischemia/reperfusion induced apoptosis via activation of FOXO3a-mediated autophagy. Oncotarget. 2016;7:76508-76522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Dong S, Ji J, Hu L, Wang H. Dihydromyricetin alleviates acetaminophen-induced liver injury via the regulation of transformation, lipid homeostasis, cell death and regeneration. Life Sci. 2019;227:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Zeng X, Yang J, Hu O, Huang J, Ran L, Chen M, Zhang Y, Zhou X, Zhu J, Zhang Q, Yi L, Mi M. Dihydromyricetin Ameliorates Nonalcoholic Fatty Liver Disease by Improving Mitochondrial Respiratory Capacity and Redox Homeostasis Through Modulation of SIRT3 Signaling. Antioxid Redox Signal. 2019;30:163-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Jia LG, Sheng ZW, Xu WF, Li YX, Liu YG, Xia YJ, Zhang JH. Modulation of anti-oxidation ability by proanthocyanidins during germination of Arabidopsis thaliana seeds. Mol Plant. 2012;5:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Xie C, Chen Z, Zhang C, Xu X, Jin J, Zhan W, Han T, Wang J. Dihydromyricetin ameliorates oleic acid-induced lipid accumulation in L02 and HepG2 cells by inhibiting lipogenesis and oxidative stress. Life Sci. 2016;157:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 18. | Shi L, Zhang T, Liang X, Hu Q, Huang J, Zhou Y, Chen M, Zhang Q, Zhu J, Mi M. Dihydromyricetin improves skeletal muscle insulin resistance by inducing autophagy via the AMPK signaling pathway. Mol Cell Endocrinol. 2015;409:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Beier JI, Banales JM. Pyroptosis: An inflammatory link between NAFLD and NASH with potential therapeutic implications. J Hepatol. 2018;68:643-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 20. | Wang J, Ren H, Yuan X, Ma H, Shi X, Ding Y. Interleukin-10 secreted by mesenchymal stem cells attenuates acute liver failure through inhibiting pyroptosis. Hepatol Res. 2018;48:E194-E202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Kofahi HM, Taylor NG, Hirasawa K, Grant MD, Russell RS. Hepatitis C Virus Infection of Cultured Human Hepatoma Cells Causes Apoptosis and Pyroptosis in Both Infected and Bystander Cells. Sci Rep. 2016;6:37433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Yang N, Jia XB, Zhang ZH, Sun E, Yan HM. [Advance in studies on anti-cancer activity and mechanism of flavonoids]. Zhongguo Zhong Yao Za Zhi. 2015;40:373-381. [PubMed] |
| 23. | de Queiroz AC, Alves Hda S, Cavalcante-Silva LH, Dias Tde L, Santos Mda S, Melo GM, Campesatto EA, Chaves MC, Alexandre-Moreira MS. Antinociceptive and anti-inflammatory effects of flavonoids PMT1 and PMT2 isolated from Piper montealegreanum Yuncker (Piperaceae) in mice. Nat Prod Res. 2014;28:403-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Qiu P, Dong Y, Li B, Kang XJ, Gu C, Zhu T, Luo YY, Pang MX, Du WF, Ge WH. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol-induced hepatic injury. Toxicol Lett. 2017;274:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Silva J, Yu X, Moradian R, Folk C, Spatz MH, Kim P, Bhatti AA, Davies DL, Liang J. Dihydromyricetin Protects the Liver via Changes in Lipid Metabolism and Enhanced Ethanol Metabolism. Alcohol Clin Exp Res. 2020;44:1046-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Robinson N, Ganesan R, Hegedűs C, Kovács K, Kufer TA, Virág L. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019;26:101239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 27. | Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1149] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 28. | Sang L, Wang XM, Xu DY, Sang LX, Han Y, Jiang LY. Morin enhances hepatic Nrf2 expression in a liver fibrosis rat model. World J Gastroenterol. 2017;23:8334-8344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 29. | Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 905] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 30. | Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2573] [Cited by in RCA: 4550] [Article Influence: 455.0] [Reference Citation Analysis (0)] |
| 31. | Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1529] [Cited by in RCA: 2482] [Article Influence: 275.8] [Reference Citation Analysis (0)] |
| 32. | Komada T, Muruve DA. The role of inflammasomes in kidney disease. Nat Rev Nephrol. 2019;15:501-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 33. | Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 548] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 34. | Song N, Li T. Regulation of NLRP3 Inflammasome by Phosphorylation. Front Immunol. 2018;9:2305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 35. | Katoh M, Katoh M. Identification and characterization of human DFNA5L, mouse Dfna5 L, and rat Dfna5 L genes in silico. Int J Oncol. 2004;25:765-770. [PubMed] |
| 36. | Feng S, Fox D, Man SM. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J Mol Biol. 2018;430:3068-3080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 37. | Chen X, He WT, Hu L, Li J, Fang Y, Wang X, Xu X, Wang Z, Huang K, Han J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 624] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 38. | Mirea AM, Tack CJ, Chavakis T, Joosten LAB, Toonen EJM. IL-1 Family Cytokine Pathways Underlying NAFLD: Towards New Treatment Strategies. Trends Mol Med. 2018;24:458-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 39. | DeSantis DA, Ko CW, Liu Y, Liu X, Hise AG, Nunez G, Croniger CM. Alcohol-induced liver injury is modulated by Nlrp3 and Nlrc4 inflammasomes in mice. Mediators Inflamm. 2013;2013:751374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Zhang Y, Cen J, Jia Z, Hsiao CD, Xia Q, Wang X, Chen X, Wang R, Jiang Z, Zhang L, Liu K. Hepatotoxicity Induced by Isoniazid-Lipopolysaccharide through Endoplasmic Reticulum Stress, Autophagy, and Apoptosis Pathways in Zebrafish. Antimicrob Agents Chemother. 2019;63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |