Published online Nov 7, 2020. doi: 10.3748/wjg.v26.i41.6304
Peer-review started: August 3, 2020
First decision: September 12, 2020
Revised: September 30, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: November 7, 2020
Processing time: 94 Days and 9.2 Hours
Recent progress in our understanding of the pathways linked to progression from hepatic insult to cirrhosis has led to numerous novel therapies being investigated as potential cures and inhibitors of hepatic fibrogenesis. Liver cirrhosis is the final result of prolonged fibrosis, which is an intimate balance between fibrogenesis and fibrinolysis. A number of these complex mechanisms are shared across the various etiologies of liver disease. Thankfully, investigation has yielded some promising results in regard to reversal of fibrosis, particularly the indirect benefits associated with antiviral therapy for the treatment of hepatitis B and C and the farnesoid receptor agonist for the treatment of primary biliary cholangitis and metabolic associated fatty liver disease. A majority of current clinical research is focused on targeting metabolic associated fatty liver disease and its progression to metabolic steatohepatitis and ultimately cirrhosis, with some hope of potential standardized therapeutics in the near future. With our ever-evolving understanding of the underlying pathophysiology, these therapeutics focus on either controlling the primary disease (the initial trigger of fibrogenesis), interrupting receptor ligand interactions and other intracellular communications, inhibiting fibrogenesis, or even promoting resolution of fibrosis. It is imperative to thoroughly test these potential therapies with the rigorous standards of clinical therapeutic trials in order to ensure the highest standards of patient safety. In this article we will briefly review the key pathophysiological pathways that lead to liver fibrosis and present current clinical and experimental evidence that has shown reversibility of liver fibrosis and cirrhosis, while commenting on therapeutic safety.
Core Tip: A number of clinical trials have targeted various etiologies of liver fibrosis and cirrhosis. Some have been promising, particularly in metabolic associated fatty liver disease and viral hepatitis. Results from these studies have shown that there are safe treatments available, forming currently practiced therapeutic guidelines, and shining light on the potential reversibility of liver fibrosis and cirrhosis caused by a variety of etiologies.
- Citation: Damiris K, Tafesh ZH, Pyrsopoulos N. Efficacy and safety of anti-hepatic fibrosis drugs. World J Gastroenterol 2020; 26(41): 6304-6321
- URL: https://www.wjgnet.com/1007-9327/full/v26/i41/6304.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i41.6304
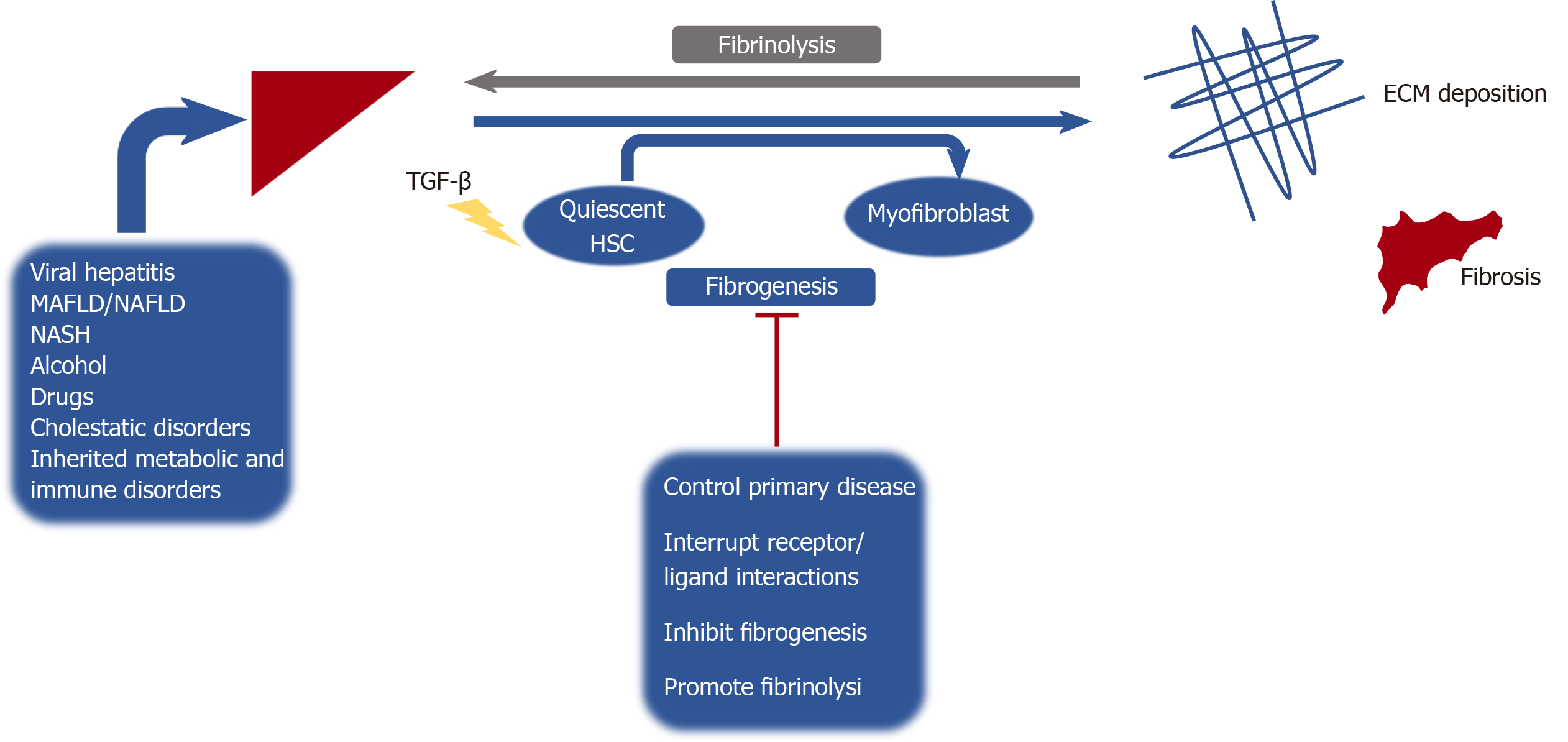
Hepatic fibrosis is the pathologic result of chronic inflammation due to a variety of chronic liver diseases and can progress to liver cirrhosis if the insult is not eliminated[1]. Formation of liver fibrosis represents an imbalance of fibrogenesis and fibrinolysis. If the underlying cause of liver injury is alleviated or cured, fibrosis may be reversible at early stages. However, if the hepatic insult persists, then accumulation of cross-linked extracellular matrix ensues, leading to substitution of liver parenchyma by collagen, making reversibility a challenge[2] (Figure 1). With the rise of metabolic syndrome and obesity globally, metabolic associated fatty liver disease (MAFLD) has been recognized as the most prevalent cause of chronic liver disease in the United States[3]. Globally this disease has a growing impact, with estimates that MAFLD cases will increase from 83.1 million in 2015 to 100.8 million in 2030 in the United States alone[4]. Chronic liver disease and cirrhosis has as a major impact on the health care system and remains an important public health concern, representing the 11th leading cause of death worldwide, and accounting for over 41000 deaths in the United States in 2017[5].
Currently there is no antifibrotic therapy approved by the Food and Drug Administration for clinical use[6]. However, with improved understanding of the cellular and molecular mechanisms underlying hepatic fibrosis, scientists and clinicians can continue to develop therapeutic targets that can inhibit, alter or even revert hepatic fibrosis.
A thorough understanding of the complex mechanisms involved in the pathogenesis of hepatic fibrosis is necessary to develop and investigate therapeutic agents targeting fibrogenesis. The liver is composed of parenchymal cells (i.e., hepatocytes) and non-parenchymal cells. Hepatocytes arranged into lobules compose 60%-80% of the total liver mass, with a portal triad (hepatic artery, hepatic portal vein, bile duct) and the central vein[7]. Kupffer cells are specialized macrophages that reside in the lumen of hepatic sinusoids, and play key roles in the initiation of the immune response[8]. Liver sinusoidal endothelial cells line the sinusoidal wall (endothelium) forming fenestrae allowing for filtration of a variety of solutes and particles between the blood entering the sinusoidal lumen and hepatocytes[9]. Hepatic stellate cells (HSCs) are resident mesenchymal cells found in the subendothelial space of Disse. Following exposure to inflammatory cytokines, chemokines and other noxious stimuli; quiescent HSCs transdifferentiate into proliferative, migratory and contractile myofibroblasts (activated state). These in turn produce components of extracellular matrix; the key driving factor that generates hepatic fibrosis[10].
Fibrosis and ultimately cirrhosis, is elegantly orchestrated by an array of cytokine mediated signaling pathways that regulate the activity of HSCs and fibrogenesis. Transforming growth factor-beta (TGF-β) is one of the most influential cytokines in the induction of fibrogenesis; it participates in HSC activation and myofibroblast formation, leading to extracellular matrix (ECM) deposition[11,12]. This cytokine also induces expression of matrix-producing genes and inhibits its degradation by downregulating matrix metalloproteinases (MMPs) and promoting tissue inhibitors of metalloproteinases (TIMPs), allowing for development of crosslinked collagen, which perpetuates fibrosis[13]. Ultimately the effect of TGF-β on different cell types within the liver results in various biological responses including the promotion of fibrosis, alteration of cell differentiation and even recruitment of other immune cells, which has been shown to inhibit anti-tumor immune response[14]. These mechanisms are mediated through an interplay of intracellular signal transducing SMAD proteins[13].
Additional cytokines that potentiate fibrogenesis include platelet-derived growth factor, tumor necrosis factor-alpha, interferons, and interleukins that can either be pro- or antifibrotic[15]. The mechanism of action of various pharmacological agents that target these cytokine interactions is to interfere with signaling pathways, allowing for the disruption of various transcriptional, translational and post-translational events.
Hepatitis B virus liver disease: Chronic hepatitis B (CHB) (defined as persistence of Australia antigen for more than six months) is a major cause of the worldwide development of cirrhosis and hepatocellular carcinoma[16]. Over the last three decades hepatitis B virus (HBV) infection in the United States has decreased by 82%, although the ability of the virus to progress towards chronic infection and subsequent cirrhosis continues to have profound effects on long term health[17]. Several studies have shown that through viral deoxyribonucleic acid suppression, modern medicine can achieve histological response.
Pegylated Interferon alpha-2b therapy in HBeAg-positive CHB patients has been shown to improve fibrosis (defined as a decrease of at least one point using the Ishak scoring system) and improve necroinflammatory score (defined as a decrease of at least 2 points) in 22% and 53%of tested patients respectively, after 52 wk of therapy. These improvements were more commonly encountered in responders, which was defined as serum seroconversion of the HBeAg antigen to HBeAg not being detected, at the completion of therapy vs non-responders[18]. One of the first oral antivirals, lamivudine, which is a nucleoside analogue has also been shown to improve fibrosis in a variety of studies through its antiviral effects. In a 3-year study, lamivudine resulted in improvement of histologically graded bridging fibrosis and cirrhosis in 63% and 73% of patients with YMDD [tyrosine (Y), methionine (M), aspartate (D), and aspartate (D) amino acid] HBV variants respectively[19]. A similar study showed improvement in both bridging fibrosis and cirrhosis in both YMDD and non-YMDD HBeAg positive patients alike[20]. Long-term treatment with lamivudine (10 years) was associated with complete liver fibrosis/cirrhosis regression or improved Ishak fibrosis score in 21% and 47% of tested patients particularly due to HBeAg loss and seroconversion coupled with extended treatment time allowing for regeneration and histological improvement[21].
Adefovir is a reverse transcriptase inhibitor (nucleotide analogue). Although not a direct anti-fibrotic drug, it’s actions as an antiviral agent can reduce ongoing hepatic inflammation, which has clinical significance in preventing fibrosis, similar to other nucleos(t)ide analogs. In a study performed by Hadziyannis et al[22] Ishak fibrosis score improved in 71% of patients treated with Adefovir dipivoxil for 240 wk. Similar histological improvements were also seen in a subsequent study in which 60% of study participants showed improved fibrosis scores at follow-up biopsy[23].
Entecavir (a nucleoside analog) has also shown similar benefits as an antiviral agent, with one study showing histological improvement (decrease in Knodell necro-inflammatory score and no worsening of Knodell fibrosis score) in 96% of patients, and improved Ishak fibrosis score in 88% of patients, including all 10 patients that had advanced fibrosis or cirrhosis prior to initiation of treatment[24]. In one large, multicenter, phase 2 study (n = 1633) assessing outcomes from three large phase 3 studies; entecavir was found to be superior to lamivudine, leading to improved Ishak fibrosis scores in nucleos(t)ide naïve patients that were either HBeAg-positive (57%), HBeAg-negative (59%) or lamivudine-refractory HBeAg-positive (43%)[25]. Similar findings were found in a Japanese study conducted by Yokosuka et al[26], in which 57% of nucleoside naïve patients treated with 3 years of entecavir showed improvement in fibrosis. A more recent prospective study analyzing 120 treatment-naïve CHB patients, demonstrated both fibrosis regression in 54 patients (45%) and decreased liver stiffness [transient elastography (TE)] in patients with fibrosis regression compared to those without improvement in fibrosis after 78 wk of entecavir treatment (-46.4% vs -28.6%, P < 0.001)[27].
Tenofovir is a commonly used nucleotide analogue that is an effective antiviral treatment for both human immunodeficiency virus-1 and HBV. Marcellin et al[28] evaluated patients with baseline Ishak score 5 or 6 cirrhosis (96 patients), treated with Tenofovir disoproxil fumarate. Histological improvement with resolution of cirrhosis (≥ 1 stage reduction) was noted in 71 patients (74%) after completion of 5 years of treatment. It has been shown that in HBV patients treated with either tenofovir or entecavir; all non-invasive scores [aspartate aminotransferase (AST)/alanine aminotransferase, AST/platelet ratio index, Fibrosis-4, red cell volume distribution width-to-platelet ratio] were significantly lower following treatment at both 12 and 24 mo[29].
Hepatitis C virus liver disease: The advent of new and extremely efficacious compounds at reaching sustained virologic response is promising in the treatment of chronic hepatitis C (CHC) patients with fibrosis and cirrhosis. In one hallmark study including patients with stage two or greater fibrosis at baseline, treatment with combination pegylated interferon alpha (IFN-α) and ribavirin resulted in a 82% decrease in fibrosis scores with some demonstrating normal/near normal liver histology after 5-year follow-up biopsy[30]. In a similar prospective study with patients treated with antiviral regimens (Interferon monotherapy, IFN + ribavirin, or pegylated IFN + ribavirin), 88% demonstrated improved fibrosis scores using TE measurements[31].
Novel direct antiviral agents (DAA) have greatly changed and revolutionized the treatment of CHC. In one multicenter observational study, DAA response was evaluated amongst 392 patients who received DAA-based treatment for CHC. Results demonstrated TE regression (-32.4%) as well as reduced Fibrosis-4 (-29.1%) and AST/platelet ratio index values (-60.9%), which was significantly different between pre and post-treatment groups[32]. Similar findings were reported in a recent study that demonstrated a 40% regression in fibrosis post DAA, using TE measurement. Regression of fibrosis was significantly more prevalent in patients with baseline advanced fibrosis than those with null-mild fibrosis (P < 0.001)[33]. Improvements of hepatic fibrosis have also been observed with 12 wk of sofosbuvir, which demonstrated improved enhanced liver fibrosis and liver stiffness measurement with FibroScan® TE[34].
A published abstract at American Association for the Study of Liver Diseases demonstrated efficacy of a lipid nanoparticle BMS-986263, which delivers a small interfering ribonucleic acid that inhibits heat shock protein 47, in patients who achieved prior sustained virologic response. Improvements in both METAVIR and Ishak fibrosis scores were noted, although some transient infusion related reactions, albeit minor, were also observed (NCT03420768)[35].
Angiotensin II, secreted by stellate cells, has been shown to promote fibrogenesis by binding to the angiotensin II type 1 receptor and activating various intracellular responses mediated by Janus kinase 2[36]. In a small published clinical trial, patients with CHC and fibrosis were treated with losartan, an angiotensin II receptor antagonist, for 18 mo. Post treatment biopsies of the fourteen patients with ≥ F2 fibrosis showed improved fibrosis in 50% of patients, coupled with significant decrease in expression of several profibrogenic genes[37]. Various angiotensin II receptor antagonists will be further discussed later in this review. Recently there have been studies that have demonstrated reduced risk of fibrosis progression[38] as well as decreased cirrhosis development with the utilization of statins in patients with CHC[39]. Possible mechanisms that allow for the reduction of fibrosis progression include their antiviral and immunomodulatory effects, alleviation of portal hypertension by increasing splanchnic nitic oxide formation, and inhibition of hepatic stellate cells through up-regulation of certain transcription factors[39].
Alcoholic liver disease: Time and time again the importance of abstinence from alcohol has been demonstrated to benefit survival in those with alcoholic liver disease. It has been reported that abstinence from alcohol lead to statistically significant improvement in 5-year survival rates of 75% vs 50% in those individuals that continued to consume alcohol. However, histological data was not collected[40]. Rapid decline of liver stiffness using FibroScan® TE was demonstrated in one study, with 1 wk of abstinence resulting in decreased liver stiffness in 45%, and reduction in the estimated stage of fibrosis in 23% of abstinent patients[41]. Continued decrease in transient elastography measurements have been recorded in another study that showed significant decrease in TE in patients day 8 (41.7%) and day 60 post-abstinence (66.7%)[42]. Candesartan, an angiotensin II type 1 receptor blocking agent, has also been shown to significantly decrease fibrosis scores, reduce the area of fibrosis and smooth muscle actin when used in conjunction with ursodeoxycholic acid (UDCA) vs UDCA alone in patients with compensated alcohol-related cirrhosis[43].
Autoimmune hepatitis: The mainstay of treatment in autoimmune hepatitis (AIH) is to reduce inflammation through the use of high dose corticosteroids alone or in combination with immunomodulators such as azathioprine, which may ultimately halt the progression of fibrosis in the liver[44]. In one study, 53% of corticosteroid treated AIH patients demonstrated improved fibrosis scores (Ishak scoring), as well as a decreased frequency of histologically proven cirrhosis from 14 patients (16%) at initiation of treatment to 10 patients (11%) over the 63 mo treatment period[45]. Similar improvements were demonstrated in a group of 19 patients that were treated with cyclosporine A vs prednisolone for 6 mo followed by continued maintenance therapy with azathioprine. Paired liver biopsies both before and after treatment demonstrated a significant improvement in mean fibrosis stage and inflammatory grade[46]. A more recent study confirms the efficacy of immunosuppression in promoting biochemical remission coupled with resolution of fibrosis and even improvement to near-normal liver histology after prolonged treatment of AIH[47]. It has been suggested that corticosteroid therapy allows for histological improvement through both direct and indirect-fibrotic actions; inhibiting the transformation of HSCs into myofibroblasts, reducing liver inflammation, and ultimately hindering molecular signals of fibrosis and facilitating degradation of extracellular matrix[48].
Non-alcoholic fatty liver disease / metabolic associated fatty liver disease: MAFLD also known as non-alcoholic fatty liver disease (NAFLD)[49] has become the most common cause of chronic liver disease in the Western world, however optimal therapy has not been discovered and is currently based on targeting risk factors with diet and lifestyle modification[50].
A multitude of studies have shown the positive effects of weight loss in the improvement of MAFLD and halting the progression towards non-alcoholic steatohepatitis (NASH)/cirrhosis. Weight loss of 5% via caloric restriction has been shown to improve steatosis, weight loss of 5% to 7% resulted in improved inflammation, and a 7%-10% reduction in weight led to possible NAFLD/NASH remission and improved fibrosis[51]. Investigation by Hohenester et al[52], found that 11.8% of patients demonstrated liver fibrosis at baseline via NAFLD fibrosis score. Following weight loss, 0% of patients were fibrotic after 52 wk (P < 0.05). However, an earlier randomized control trial with average weight loss of 9.3% failed to validate a significant change in liver fibrosis, despite improvements in NASH histological activity score in 31 patients over 48 wk[53]. Several studies have shown histological improvement, including decreased fibrosis after bariatric surgery. In one systematic review, pooled analysis of pre and post-surgery histological findings demonstrated a mean decrease in the incidence of fibrosis by 11.9% (P < 0.0001) in obese patients with MAFLD undergoing bariatric surgery[54]. Similar findings were seen in a systematic review and meta-analysis conducted by Mummadi et al[55], in which 65.5% of the pooled proportion of patients demonstrated improvement or resolution of fibrosis after bariatric surgery- induced weight loss.
The results of the PIVENS trial has led to the current AASLD recommendations regarding the use of Vitamin E (800 IU/d) or pioglitazone for treatment of NASH in non-diabetic patients[56]. This trial was a large randomized control trial of vitamin E vs pioglitazone vs placebo, in which 247 adults with biopsy-confirmed, nondiabetic NASH were assigned to either treatment group. Use of both agents was associated with a significant reduction in steatosis, lobular inflammation and liver enzymes compared to placebo, however there was no reduction in hepatic fibrosis[57]. It is important to note that caution needs to be warranted when prescribing these medications as there has been data suggestive of increased all-cause mortality and prostate cancer with Vitamin E[56]. The common side effects of weight gain and bone loss need to also be considered when prescribing thiazolidinediones, such as pioglitazone[56].
Other areas of investigation for fibrosis therapy in NASH include fibroblast growth factor (FGF). FGF19 is a pleotropic protein that has been shown to decrease gluconeogenesis and increase insulin sensitivity, inhibit fatty acid synthesis, increase hepatic synthesis of glycogen and proteins, and regulate bile acid homeostasis[58]. In a recent phase 2 study, Aldafermin (NGM282), a FGF19 analogue, demonstrated histological improvement in patients with NASH. After 12 wk of therapy, paired liver biopsies demonstrated reduced nonalcoholic fatty liver disease activity score (NAS) in both the 1 mg and 3 mg treatment groups (P < 0.001), as well as reduced fibrosis scores in the 3 mg group (P = 0.035)[59]. Results also demonstrated improved NAS by 2 or more points without fibrosis worsening in 50% and 63% of patients in the 1 mg or 3 mg treatment groups, respectively (NCT02443116). During the phase 2 trial, authors noted adverse gastrointestinal effects including nausea, abdominal pain, diarrhea and injection site reactions following administration of NGM282[60]. FGF21 is similar, as it plays many roles in metabolism, including fatty acid oxidation, lipolysis and gluconeogenesis[58]. Pegbelfermin (BMS-986036) is a pegylated human FGF21 analogue, and has shown a significant decrease in absolute hepatic fat fraction in the 10 mg daily group (-6.8% vs -1.3%; P = 0.0004) and in the 20 mg weekly subset (-5.2% vs -1.3%; P = 0.008) compared to placebo in patients with NASH fibrosis stages 1-3 (NCT02413372). Common adverse effects of drug therapy included diarrhea and nausea, which were mild[61].
A recent trial has demonstrated efficacy of PF-05221304, a liver-targeted inhibitor of acetyl- coenzyme A carboxylase, therefore hindering de novo lipogenesis (NCT02871037). Healthy subjects demonstrated dose-responsive reductions in liver fat, reduced liver enzymes and hemoglobin A1c. Minimal adverse effects included a rise in serum triglycerides, headache, diarrhea, and one case of thrombocytopenia leading to withdrawal from the study[62]. Although this study did not directly measure liver fibrosis, future studies may potentially demonstrate that the decreased de novo lipogenesis secondary to this drug leads to fibrosis regression in addition to a reduction of inflammation[63].
Below we will discuss current, key pharmaceuticals in phase 3 clinical trials and other promising therapies for NASH in detail. Additional phase 3 drugs are listed in (Table 1).
| Drug name | Mechanism of action | Estimated completion | Study/identifier |
| Resmetirom (MGL-3196) | Thyroid hormone receptor agonist | March 2024 | [MAESTRO-NASH] NCT03900429 |
| Dapaglifozin | SGLT-2 inhibitor | November 2021 | [DEAN] NCT03723252 |
| Aramchol | SCD1 inhibitor | December 2024 | [ARMOR] NCT04104321 |
| Cenicriviroc | CCR2-CCR5 antagonist | October 2028 | [AURORA] NCT03028740 |
| Elafibranor | PPAR agonist | December 2021 | [RESOLVE-IT] NCT02704403 |
| Obeticholic acid | FXR agonist | October 2022 | [REGENERATE] NCT02548351 |
Farsenoid X receptor is a nuclear hormonal receptor that has been shown to negatively modulate nuclear factor kappa B signaling linked to hepatic inflammation and subsequent role in NASH development[64]. Obeticholic acid (OCA) is an agonist of Farsenoid X receptor and has been shown to increase insulin sensitivity while decreasing hepatic steatosis and fibrosis[65]. In the phase 2 double-blinded placebo-controlled trial (FLINT; NCT01265498), 283 patients were randomized to either receive OCA or placebo for 72 wk. All participants had a baseline NAFLD activity score of 4 or greater, with a score of 1 or greater in each of the three categories (steatosis, ballooning and lobular inflammation). Results were promising with OCA meeting primary outcomes of improved NAS by ≥ 2 points without worsening fibrosis (45% vs 21%, P = 0.002) and improvement in fibrosis (35% vs 19%, P = 0.004) when compared to placebo[66]. Treatment for OCA was associated with a decrease in HDL cholesterol, and higher triglycerides and serum cholesterol. Other adverse events included pruritus, reported in 23% of OCA treated vs 6% in placebo-treated patients (P < 0.0001)[66].
Currently underwork is a phase 3 trial (REGENERATE; NCT02548351), in which non-cirrhotic NASH subjects with stage 2 or 3 fibrosis are randomized to receive OCA 10 mg, OCA 25 mg or placebo. A recently published month-18 interim analysis demonstrates fibrosis improvement (≥ 1 stage) by 18% in the OCA 10 mg group (P = 0.045) and 23% in the OCA 25mg group (P = 0.0002)[67]. The most common adverse event included mild to moderate pruritus, which occurred in 51% of patients receiving 25mg OCA[67]. Investigation on the safety and efficacy of this drug is ongoing in a phase 3 trial (REVERSE; NCT03439254) in patients with compensated cirrhosis due to NASH.
Selonsertib (SEL) is an inhibitor of apoptosis signal-regulating kinase 1 (ASK-1). Under periods of oxidative stress, ASK-1 induces cellular apoptosis, cell death, and production of inflammatory cytokines[68]. In the phase 2 trial, NASH patients with stage 2-3 fibrosis were randomized to receive 6 mg or 18 mg of SEL with or without once weekly injections of 125 mg simtuzumab or simtuzumab alone for 24 wk. Patients treated with selonsertib alone appeared to exhibit improvement of fibrosis and lower rates of fibrosis progression, as indicated by MR elastography and liver biopsy[69]. However, these results were not reproduced in a phase 3 study (STELLAR-3; NCT03053050) of patients with NASH or in patients with bridging fibrosis and compensated cirrhosis (STELLAR-4; NCT03053063), leading to discontinuation of the studies after interim analysis. Importantly, patients in the selonsertib group experienced headache, nausea, abdominal pain, sinusitis, nasopharyngitis and fatigue at greater proportions. There were also instances of severe side effects, and discontinuation of therapy by three patients for worsening schizophrenia, numbness of the face and upper extremities, and an elevation in liver enzymes[69].
Elafibranor is an activator of both the peroxisome proliferator-activated receptor alpha and delta, leading to improved insulin sensitivity and reduced inflammation[70]. In a phase 2 placebo-controlled study (GOLDEN-505; NCT01694849), patients with NASH but without cirrhosis were assigned to receive elafibranor 80 mg or 120 mg daily or placebo. Following post hoc analysis, NASH resolved without worsening fibrosis at a higher rate in the 120 mg group vs placebo (P = 0.045). Patients with NAS ≥ 4, demonstrated resolution of NASH, based on protocol definition, in larger proportions when treated with elafibranor 120 mg (P = 0.018) and modified definitions (P = 0.013). It is important to note that there was no significant difference between treatment and placebo based on the protocol’s primary outcome (resolution of NASH without fibrosis worsening)[70]. Although therapy was well tolerated by recipients, one must continue to monitor for renal impairment as an increase in serum creatinine was noted amongst study participants. Recently an interim analysis was published by GENFIT on the ongoing phase 3 trial (RESOLVE-IT; NCT02704403), analyzing elafibranor 120 mg vs placebo in moderate to severe NASH. Results demonstrated no significant difference between the two groups when analyzing the primary endpoint (NASH resolution without worsening of fibrosis) or the key secondary endpoint (fibrosis improvement of at least one stage).
Cenicriviroc is an antagonist of the chemokine receptor 2 and chemokine receptor 5 receptors, which are binding sites for inflammatory promoting chemokines CCL2 and CCL5 that lead to cellular recruitment promoting hepatic inflammation and injury[71]. A phase 2, randomized, double-blinded study (CENTAUR; NCT02217475), assigned patients with NASH and liver fibrosis (stage 1-3) to receive cenicriviroc 150 mg vs placebo. Subjects who received cenicriviroc displayed reduced fibrosis by 1 stage and no worsening of steatohepatitis compared to placebo (20% vs 10%; P = 0.02). Surprisingly those with higher disease activity (NAS 5) and fibrosis at baseline, showed greater response to therapy[72]. Final data of the 2-year study also demonstrated similar findings with maintained reduced fibrosis in those who responded[73]. Adverse effects included fatigue and diarrhea in a small percentage of patients. Currently there is an ongoing phase 3 trial (AURORA; NCT03028740), recruiting patients with F2-F3 fibrosis to assess histological and clinical outcomes.
Liraglutide and semaglutide are glucagon-like peptide (GLP)-1 analogs (incretin mimetics) used for the treatment of type 2 diabetes. These drugs stimulate the release of endogenous insulin from pancreatic cells and decrease glucagon release[74]. The clinical trial (LEAN; NCT01237119) demonstrated that once daily liraglutide injections led to resolution of NASH with no worsening of fibrosis when compared to placebo (39.1% vs 9%; P = 0.02). In addition, patients in the placebo group had significantly greater progression to fibrosis compared to liraglutide (P = 0.04)[75]. Currently semaglutide is being investigated as a therapeutic agent for NASH resolution without worsening fibrosis with secondary outcomes of liver fibrosis improvement (NCT02970942). Initial data including 230 patients with fibrosis stages F2 to F3, demonstrated NASH resolution in the semaglutide group compared to placebo (59% vs 17%)[76]. GLP-1 receptor agonists have shown beneficial outcomes on cardiovascular, mortality, and renal outcomes in patients with type 2 diabetes, and therefore will likely have promise in the treatment of NASH with diabetes[77].
In a 2017 study the efficacy of atorvastatin in the treatment of NAFLD was evaluated. 57 dyslipidemic patients with histologically confirmed NAFLD received atorvastatin 20 mg/d for 32.8 ± 3.4 wk. Patients treated with atorvastatin demonstrated reduction in alanine aminotransferase, AST, gamma-glutamyl transpeptidase, alkaline phosphatase, total cholesterol and triglycerides in addition to a reduction in steatosis, and overall NAFLD activity score (NAS) (P = 0.00007)[78]. It has been recommended that statin therapy not only helps prevent cardiovascular disease and manage dyslipidemia, but it can also prevent progression of liver fibrosis, making its use highly advantageous[79]. Therefore, in patients with chronic liver disease and compensated cirrhosis, statins should not be avoided as they are a safe and effective treatment modality[80].
Cholestatic liver disease: Primary biliary cholangitis and primary sclerosing cholangitis: Despite decades of use and recent drug development, UDCA has remained the standard of care for primary biliary cholangitis (PBC). UDCA is a hydrophilic bile acid that has shown to improve liver biochemistry and delay the histological progression of disease, improving liver transplantation free survival[81]. In a study conducted by Corpechot et al[82], UDCA therapy was shown to significantly reduce the progression rate from early stage disease to extensive fibrosis or cirrhosis at a rate of 7% per year for UDCA compared to 34% for placebo (P < 0.002). Norursodeoxycholic acid is a C23 homologue of UDCA that has shown in a phase 2, randomized, placebo-control study, significant biochemical improvement including reduced alkaline phosphatase (ALP) and liver enzymes[83]. However, further studies are required to assess histological efficacy. Unlike its front-line role in PBC, UDCA has questionable roles in the treatment of primary sclerosing cholangitis (PSC). The AASLD recommends against the use of UDCA due to the lack of beneficial data and possible worse outcomes[84].
A second line treatment option includes obeticholic acid for UDCA non-responders, which has shown some promising non-histological results. Its approval was based on the POISE study, a one-year placebo-controlled study that demonstrated significant reduction in ALP, total bilirubin and aminotransferases. Investigators also assessed underlying liver pathology with non-invasive measures such as TE and Enhanced Liver Fibrosis scoring, but results did not differ significantly between OCA and placebo groups. It should be noted that patients receiving OCA, experienced dose-dependent increased pruritus compared to placebo[85]. A biopsy substudy of POISE assessed histological outcomes when comparing paired liver biopsies prior to and after 3 years of treatment with OCA in patients with PBC concurrently taking UDCA. Results demonstrated improvement or stabilization of fibrosis (71% of patients), statistically significant reduction in collagen area ratio (-31%, P = 0.013), collagen fiber density (-35%, P = 0.021), collagen reticulation index (-7%, P = 0.008), and fibrosis composite score (-25%, P = 0.002)[86]. Currently OCA is being investigated in a phase 4 study (COBALT; NCT02308111) evaluating clinical outcomes, including non-invasive liver scores, and a biopsy substudy that may further support encouraging clinical outcomes in patients with PBC. In regard to the role of OCA in the treatment of PSC, the AESOP trial demonstrated biochemical improvement, however histological data was not collected[87].
Third line options for the treatment of PBC include bezafibrate, and its efficacy and safety were first demonstrated in the placebo-controlled phase 3 trial (BEZURSO; NCT01654731). Participants were assigned to receive bezafibrate or placebo in addition to UDCA for 24 mo. Results included normalization of ALP and aminotransferases in the bezafibrate group compared to placebo. This included reduction of liver stiffness by 15% and a decrease in Enhanced Liver Fibrosis scores in the treatment group vs placebo. A subset of patients had histologic data before and after the study, and changes in histologic stage, fibrosis stage and activity grade were not significantly different between the two groups. Significant adverse events included an elevation of baseline serum creatinine by 5% compared to a decrease of 3% in the placebo group. Myalgias were also more common in the treatment group, however this was not statistically significant[88].
In summary there are a multitude of drugs that have and continue to be tested in both PBC and PSC. However, many have proven to be ineffective in the treatment of cholestatic liver disease. Studies primarily investigate the biochemical effects of the drugs, and there are limited studies that assess liver fibrosis progression and/or regression.
Iron overload: Hereditary hemochromatosis and beta thalassemia: Accumulation of excess iron is toxic to liver cells as it allows for the generation of excessive reactive oxygen species that lead to damaged cells and secretion of pro-fibrogenic cytokines. The fibrotic response is not only mediated by oxygen species, but from a large array of iron-induced cell signaling pathways, HSC activation by iron related proteins and possibly iron-mediated ECM remodeling[89]. Phlebotomy is a commonly used treatment for hereditary hemochromatosis, as it not only removes excess systemic iron, but it promotes hematopoiesis which utilizes systemic iron stores. In a study conducted by Falize et al[90], 69% of participants with stage F3 and 35% with stage F4 fibrosis demonstrated regression of fibrosis following venesection (decrease of at least 2 METAVIR units). In those who cannot undergo phlebotomy, the iron chelator deferoxamine has been shown to be effective in the removal of excess iron from the liver and ultimately contributing to fibrosis control[91]. Benefits of iron chelation were also demonstrated by Deugnier et al[92] who conducted a study assessing the efficacy of deferasirox in individuals with beta thalassemia. Results demonstrated stability of Ishak fibrosis staging score or improvement (change of ≤ 2) in 82.6% of patients, and improvement of mean Ishak necroinflammatory score by a mean value of -1.3 (P < 0.001) in individuals treated for at least 3 years.
Other antifibrotic therapies: There have been a multitude of studies investigating the consumption of coffee and its protective effects on liver fibrosis and cirrhosis due a variety of etiologies[93]. In a study conducted by Modi et al[94], caffeine consumption above the 75th percentile for the study cohort was associated with reduced liver fibrosis (OR: 0.19; 95%CI: 0.05-0.66; P = 0.009). A recent prospective population- based study assessed liver stiffness in the general population with no established liver disease. The study concluded that the proportion of participants with liver stiffness measurements 8.0 kPa decreased with higher coffee consumption[95].
Inhibitors of the renin-angiotensin system have shown promise in reducing the progression of hepatic fibrosis in experimental models. In a study conducted by Moreno et al[96] targeted delivery of losartan to HSCs in rats with liver fibrosis secondary to bile duct ligation and carbon tetrachloride administration demonstrated a reduction in collagen deposition, myofibroblasts, inflammation and procollagen gene expression; reducing liver fibrosis. Similar results were demonstrated in a rat model of thioacetamide induced liver fibrosis. Rats that received the angiotensin II receptor antagonists losartan or telmisartan displayed decreased concentrations of tumor necrosis factor-alpha, transforming growth factor beta-1, and histologically reduced inflammation and liver fibrosis[97]. A recent systematic review and meta- analysis evaluating the effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on liver fibrosis or cirrhosis patients demonstrated a reduction of serum fibrosis markers including TGF-β, tissue inhibitor of metalloproteinase-1 (TIMP-1), MMP, collagen and displayed significantly lower fibrosis scores and smaller areas of fibrosis[98] A randomized open-label controlled study demonstrated the effects of candesartan in patients with compensated alcoholic liver fibrosis (≥ F2). Candesartan significantly reduced fibrosis score leading to histological improvement, reduced area of fibrosis and smooth muscle actin, and reduced expression of TGF-β, TIMP-1, and MMP (P < 0.05)[43]. Although efficacy has been demonstrated in both animal and human studies, further successful clinical trials are required to safely and confidently implement the use of these drugs in humans.
Hepatocyte growth factor is another agent that has been investigated in animal studies. It exerts its therapeutic affects by suppressing the action of TGF-β, inducing collagenase expression and inhibiting the growth of HSCs, preventing apoptosis[99]. Further human based studies are required to have a better understanding of any potential benefits in liver fibrosis.
Caspase inhibitors are an additional potential group of therapeutic agents. As discussed earlier, hepatocyte apoptosis is an inflammatory process that leads to cellular activation and fibrosis. Animal studies discovered that the use of the pancaspase inhibitor IDN-6556 (Emricasan) reduced hepatocyte apoptosis and liver injury, hepatic inflammation and hepatic fibrogenesis in a mouse model[100]. A recent randomized, placebo-controlled clinical trial (ENCORE-NF; NCT02686762) assessed the effects of emricasan in patients with NASH F1-F3 fibrosis. Emricasan treatment did not improve histology in these patients and even worsened fibrosis[101]. The phase 2 trial (ENCORE-PH; NCT02960204) assessed the effects of emricasan on hepatic venous pressure gradient (HVPG) in patients with cirrhosis and severe portal hypertension, defined as HVPG ≥ 12 mmHg. Results demonstrated no significant difference in the change of HVPG between various emricasan doses vs placebo. Although emricasan did demonstrate reduction in biomarkers such as aminotransferases, caspases and cytokeratins initially; these biomarkers returned to baseline by the end of the 48-wk treatment period[102]. It has also been reported in the media that the primary analysis of the phase 2 trial (ENCORE-LF; NCT03205345) demonstrated failure of emricasan to meet the study’s primary endpoint of event-free survival when compared to placebo.
The hepatic endocannabinoid system is one of the multiple cell-signaling pathways involved in the transformation of quiescent HSCs into myofibroblasts that produce the fibrous matrix. Activation of the CB1 receptor promotes hepatic steatosis, inflammation and subsequent fibrosis; commonly seen in both alcoholic and non-alcoholic fatty liver disease. The CB2 receptor primarily exerts anti-inflammatory and anti-fibrotic effects, displaying protective roles against liver injury[103,104]. In various animal studies inhibition of the CB1 receptor with rimonabant reduced TGF-β levels and fibrosis, as well as MMP activity and pro-fibrogenic and pro-inflammatory factors[105,106]. However, one draw-back to this treatment is abundance of neuropsy-chiatric effects as these receptors are highly expressed in the central nervous system[104]. CB2 agonism has shown to reduce inflammation and fibrosis and even accelerate the regenerative process in various mouse and rat models[105,107]. Although pharmaceuticals targeting both receptors may be promising in animal studies, CB2 agonists may be a more feasible treatment approach.
Other therapies aim to promote degradation of the fibrous matrix. In the 2004 study conducted by Parsons et al[108], blockage of TIMP-1 via anti-TIMP-1 antibody led to a reduction in collagen deposition in a rat model. Despite these findings there are currently no active clinical trials that attempt to target this pathway. Another approach to hinder the ECM is by antagonizing lysyl oxidase-2, one of the enzymes responsible for cross-linkage of fibrillar collagen[109]. A recently completed trial (NCT01672853) assessed the efficacy of Simtuzumab (anti lysyl oxidase-2 Ab) on PSC. After 96 wk of treatment there was no significant change in hepatic collagen content, reduction in Ishak fibrosis stage or progression to cirrhosis[110]. Similar clinical trials investigating simtuzumab in patients with advanced liver fibrosis secondary to NASH have been terminated after failing to show efficacy (NCT01672879 and NCT01672866).
Endothelin stimulates HSC contractility, and antagonists to its receptors initially showed promise for treatment of hepatic fibrosis. In an experimental model of liver fibrosis, scientists demonstrated that with the use of bosentan (an endothelin receptor antagonist) levels of type I collagen and cellular fibronectin mRNAs were reduced[111]. However, evidence of hepatotoxicity and possible teratogenicity developed, and its use was no longer recommended[112].
As discussed earlier, the cytokine TGF-β plays a major role in the development of fibrosis within the liver and other tissues. Therefore, it remains a major focus for various pharmaceutical treatments. There has been concern of systemic inhibition provoking inflammation and epithelial growth and subsequent neoplasia, which has led to further investigations into other treatments[113]. Antagonists to cell surface integrins, which allow for TGF-β activation has become an appealing target. One example is an inhibitory antibody to αVβ6 integrin, which has shown to significantly reduce collagen deposition in a mouse model of bile duct ligation[114]. Inhibition of the α (V) integrin has also been shown to attenuate liver fibrosis in an animal model[115].
Discussion of hepatic anti-fibrotic therapy would not be complete without brief discussion of herbal compounds, some of which have promising results in various studies. Silymarin (Silybum marianum) is a natural herbal flavonoid complex that is extracted from cardoon milk. In a study performed on cultured HSCs from human liver, silybin was found to inhibit pro-fibrogenic actions of HSCs including cell proliferation, cell motility and synthesis of extracellular matrix components[116]. However, a recently completed phase 2 trial titled (SyNCH; NCT00680407) concluded that although safe; there was no statistically significant histological improvement is NAFLD Activity Score in non-cirrhotic patients with NASH[117]. Curcumin (Curcuma longa) is a polyphenol that has shown effectiveness in animal studies. In a study by Shu et al[118], curcumin was shown to prevent liver fibrosis, and inhibit HSCs by triggering apoptosis in a rat model of CCl4 induced hepatic fibrosis. A current single-center, randomized, phase 2 study is underway examining the effects of curcumin on pediatric nonalcoholic fatty liver disease (NCT04109742). Salvianolic acid (Salvia) is extracted from Radix Salviae miltiorrhizae and has been used by traditional Chinese medicine for hundreds of years[119]. A double-blinded, randomized control study compared salvianolic acid to IFN-g for the treatment of hepatitis B. Surprisingly, individuals treated with salvianolic acid demonstrated increased reversal rates of fibrosis and inflammatory alleviation, plus decreased liver fibrotic scores and fibrotic markers. Salvianolic acid showed no side effects compared to IFN-, which had high rates of fever and transient decrease of leukocytes in study participants[120]. A complete list of herbal remedies for liver fibrosis is more thoroughly reviewed by Latief and Ahmad[121].
We have discussed a wide array of ongoing and completed clinical trials targeting various pathways of liver fibrosis. There are currently hundreds of clinical trials listed on ClinicalTrials.gov under the topic of “liver fibrosis,” in various phases of completion. We compiled a list of active phase 2-phase 4 trials, for various etiologies, that are recruiting or not recruiting at the time of this publication (Table 2).
| Drug name | Mechanism of action | Condition(s) | Clinical phase | Study/Identifier |
| Candesartan + Ramipril | Angiotensin II receptor Antagonist, ACE inhibitor | HCV | 3 | NCT03770936 |
| Pirfenidone | Anti-inflammatory + Antifibrotic | Multiple | 2 | [PROMETEO] NCT04099407 |
| BMS-986036, Pegbelfermin | PEG-FGF21Analog | NASH F3 | 2 | [FALCON 1] NCT03486899 |
| NASH + Compensated, Cirrhosis | 2 | [FALCON 2] NCT03486912 | ||
| Aldafermin (NGM282) | FGF19 Analog | NASH | 2 | NCT02443116 |
| CC-90001 | JNK inhibitor | NASH F3/F4 | 2 | NCT04048876 |
| Entecavir | Reverse transcriptase inhibitor | HBV | 4 | NCT02849132 |
| Entecavir + Fuzheng Huaya + TCM granule | Reverse transcriptase inhibitor + Traditional Chinese medicine | HBV | 4 | NCT02241616 |
| Tropifexor + Licoglifozin | FXR agonist + SGLT 1 and 2 inhibitor | NASH F2/F3 | 2 | [ELIVATE] NCT04065841 |
| Tropifexor + Cenicriviroc | FXR agonist + CCR2-CCR5 antagonist | NASH F2/F3 | 2 | [TANDEM] NCT03517540 |
| Nitazoxanide | Anti-protazoal | NASH F2/F3 | 2 | NCT03656068 |
| Mesenchymal stem cells | Umbilical cord mesenchymal stem cells | HBV + Decompensated cirrhosis | 2 | NCT03945487 |
| GXHPC1 | Adipose-derived stem cells | Cirrhosis of multiple etiologies | 2 | NCT04088058 |
| G-CSF | Granulocyte colony stimulating factor | Decompensated cirrhosis of multiple etiologies | 2/3 | NCT03911037 |
| Mesenchymal stem cells | Autologous bone marrow mesenchymal SCs | Cirrhosis of multiple etiologies | 2 | NCT03626090 |
| ADR-001 | Adipose-derived stem cells | HCV or NASH Cirrhosis | 2 | NCT03254758 |
| CD 34 + MSCs | Autologous hematopoietic and mesenchymal SCs | Cirrhosis of multiple etiologies | 4 | NCT04243681 |
| Simvastatin | HMG-CoA Reductase Inhibitor | Cirrhosis of multiple etiologies | 2 | NCT02968810 |
| ORMD-0801 | Oral insulin formulation | NASH + Type 2 Diabetes | 2 | NCT02653300 |
| Atorvastatin | HMG-CoA reductase inhibitor | Cirrhosis of multiple etiologies | 4 | [STATLiver] NCT04072601 |
| Aldafermin (NGM282) | FGF19 analog | NASH F2/F3; NASH + Compensated cirrhosis | 2; 2 | [ALPINE 2/3] NCT03912532; [ALPINE 4] NCT04210245 |
| Obeticholic acid | FXR agonist | PBC + Hepatic impairment | 4 | NCT03633227 |
| Stem cell | Autologous bone marrow mononuclear stem cells | Liver cirrhosis + Biliary atresia | 2 | NCT03468699 |
| VK2809 | Thyroid receptor agonist | NASH | 2 | [VOYAGE] NCT04173065 |
| Spironolactone + Carvedilol | Aldosterone antagonist + Beta receptor blocker | Compensated cirrhosis | 4 | NCT02907749 |
| Cenicriviroc | CCR2-CCR5 antagonist | NASH + Completion of CENTAUR and AURORA Study | 2 | NCT03059446 |
| Selonsertib + Firsocostat + Cilofexor + Fenofibrate + Vascepa® | ASK 1 inhibitor + ACC inhibitor + FXR agonist + Anti-lipid + Anti-lipid | NAFLD, NASH | 2 | NCT02781584 |
| Erlotinib | Tyrosine kinase inhibitor | Fibrosis, Cirrhosis | 1/2 | NCT02273362 |
| Saroglitizar | PPAR agonist | NAFLD, NASH | 2 | NCT03061721 |
Examples of drugs under current investigation include Pirfenidone, a broad spectrum anti-fibrotic drug demonstrating abilities in prevention and removal of collagenous scar tissue in organs such as the lung and kidney[122]. Pegbelfermin (BMS-986036) and Aldafermin (NGM282), analogs of the fibroblast growth factor 21 and 19 respectively, are under investigation as treatment in NASH, as they demonstrate a variety of potentially beneficial biochemical and metabolic effects[123]. Completion of these clinical trials may provide efficacious results and implementation of their use in liver fibrosis, as these drugs are not currently commonly used in the clinical setting.
Formation of liver fibrosis and progression to cirrhosis is a complex mechanism with interplay between formation of the fibrous matrix and its degradation. There is a plethora of etiologies leading to fibrosis and cirrhosis, which vary in prevalence based on worldwide geographic location. Despite the variations in the underlying pathology, many of the common biologic pathways that ultimately lead to HSC activation are shared amongst diseases. Over the years there have been a great number of pharmacologic and non-pharmacologic agents, as well as lifestyle modifications, that have shown some promise in preventing progression and even reversing hepatic fibrosis, ultimately making treatment a reality. As we continue to develop models and learn more about new and existing cellular pathways, newer safe and effective therapeutic targets will likely be developed given the magnitude of their clinical implications. Investigators will not only continue researching individual drug efficacy but should continue to develop combination therapies that target various points of the fibrosis cascade simultaneously. Potential benefit can also be explored by utilizing compounds that are implicated for other systemic forms of fibrosis, as many of the pathways are shared amongst organ systems.
Determination of drug efficacy remains a challenge as there are a variety of different modalities available to assess hepatic fibrosis, veering away from the traditional liver biopsy. As seen in this review there is a multitude of clinical trials that range from therapeutic and observational studies. In our opinion it is imperative that future studies standardize clinical endpoints and fibrosis measurement to allow for better understanding of drug efficacy as single agents or in combination with one another. While most of the trials are currently focused on MAFLD, continued research is needed amongst all etiologies of hepatic fibrosis in order to have improved worldwide benefit.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mao YQ S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Toosi AE. Liver Fibrosis: Causes and Methods of Assessment, A Review. Rom J Intern Med. 2015;53:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1375] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 3. | Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263-8276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 543] [Cited by in RCA: 516] [Article Influence: 64.5] [Reference Citation Analysis (6)] |
| 4. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2908] [Article Influence: 415.4] [Reference Citation Analysis (1)] |
| 5. | Kochanek KD, Murphy SL, Xu J, Arias E. Deaths: Final Data for 2017. Natl Vital Stat Rep. 2019;68:1-77. [PubMed] |
| 6. | Yoon YJ, Friedman SL, Lee YA. Antifibrotic Therapies: Where Are We Now? Semin Liver Dis. 2016;36:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Rodes J, Benhamou J-P, Blei A, Reichen J, Rizzetto M. Textbook of hepatology: from basic science to clinical practice: Blackwell Malden, MA, 2007. |
| 8. | Sato K, Hall C, Glaser S, Francis H, Meng F, Alpini G. Pathogenesis of Kupffer Cells in Cholestatic Liver Injury. Am J Pathol. 2016;186:2238-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 335] [Cited by in RCA: 405] [Article Influence: 36.8] [Reference Citation Analysis (10)] |
| 10. | Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 1054] [Article Influence: 131.8] [Reference Citation Analysis (0)] |
| 11. | Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P; IT-LIVER Consortium. TGF-β signalling and liver disease. FEBS J. 2016;283:2219-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 479] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 12. | Dewidar B, Meyer C, Dooley S, Meindl-Beinker AN. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis-Updated 2019. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 539] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 13. | Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem. 2016;64:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 567] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 14. | Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, Fabregat I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 15. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 780] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 16. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264-1273. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2506] [Article Influence: 192.8] [Reference Citation Analysis (2)] |
| 17. | Thuener J. Hepatitis A and B Infections. Prim Care. 2017;44:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | van Zonneveld M, Zondervan PE, Cakaloglu Y, Simon C, Akarca US, So TM, Flink HJ, de Man RA, Schalm SW, Janssen HL; HBV 99-01 Study Group. Peg-interferon improves liver histology in patients with HBeAg-positive chronic hepatitis B: no additional benefit of combination with lamivudine. Liver Int. 2006;26:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 20. | Rizzetto M, Tassopoulos NC, Goldin RD, Esteban R, Santantonio T, Heathcote EJ, Lagget M, Taak NK, Woessner MA, Gardner SD. Extended lamivudine treatment in patients with HBeAg-negative chronic hepatitis B. J Hepatol. 2005;42:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Xu B, Lin L, Xu G, Zhuang Y, Guo Q, Liu Y, Wang H, Zhou X, Wu S, Bao S, Cai W, Xie Q. Long-term lamivudine treatment achieves regression of advanced liver fibrosis/cirrhosis in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, Brosgart CL, Borroto-Esoda K, Arterburn S, Chuck SL; Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 680] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 23. | Marcellin P, Chang TT, Lim SG, Sievert W, Tong M, Arterburn S, Borroto-Esoda K, Frederick D, Rousseau F. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 24. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, Kreter B. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 772] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 25. | Schiff E, Simsek H, Lee WM, Chao YC, Sette H Jr, Janssen HL, Han SH, Goodman Z, Yang J, Brett-Smith H, Tamez R. Efficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosis. Am J Gastroenterol. 2008;103:2776-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Yokosuka O, Takaguchi K, Fujioka S, Shindo M, Chayama K, Kobashi H, Hayashi N, Sato C, Kiyosawa K, Tanikawa K, Ishikawa H, Masaki N, Seriu T, Omata M. Long-term use of entecavir in nucleoside-naïve Japanese patients with chronic hepatitis B infection. J Hepatol. 2010;52:791-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Wu SD, Liu LL, Cheng JL, Liu Y, Cheng LS, Wang SQ, Ma W, Chen LP, Tseng YJ, Wang JY, Shen XZ, Jiang W. Longitudinal monitoring of liver fibrosis status by transient elastography in chronic hepatitis B patients during long-term entecavir treatment. Clin Exp Med. 2018;18:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1367] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 29. | Koksal AR, Alkim H, Boga S, Ergun M, Bayram M, Ozguven BY, Alkim C. Effect of Entecavir and Tenofovir Treatment on Noninvasive Fibrosis Scores: Which One Is Better? Am J Ther. 2016;23:e429-e438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 31. | Mendoza J, Trapero-Marugán M, González-Moreno L, Jones EA, Gómez-Domínguez E, Moreno-Otero R. Hepatic fibrosis in patients with chronic hepatitis C assessed by transient elastography: implications for determining the efficacy of antiviral therapy. Rev Esp Enferm Dig. 2010;102:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Bachofner JA, Valli PV, Kröger A, Bergamin I, Künzler P, Baserga A, Braun D, Seifert B, Moncsek A, Fehr J, Semela D, Magenta L, Müllhaupt B, Terziroli Beretta-Piccoli B, Mertens JC. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017;37:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 33. | Lledó GM, Carrasco I, Benítez-Gutiérrez LM, Arias A, Royuela A, Requena S, Cuervas-Mons V, de Mendoza C. Regression of liver fibrosis after curing chronic hepatitis C with oral antivirals in patients with and without HIV coinfection. AIDS. 2018;32:2347-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Bernuth S, Yagmur E, Schuppan D, Sprinzl MF, Zimmermann A, Schad A, Kittner JM, Weyer V, Knapstein J, Schattenberg JM, Wörns MA, Galle PR, Zimmermann T. Early changes in dynamic biomarkers of liver fibrosis in hepatitis C virus-infected patients treated with sofosbuvir. Dig Liver Dis. 2016;48:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Oral Abstracts (Abstracts 1–288). Hepatology. 2019;70:1-187. [DOI] [Full Text] |
| 36. | Granzow M, Schierwagen R, Klein S, Kowallick B, Huss S, Linhart M, Mazar IG, Görtzen J, Vogt A, Schildberg FA, Gonzalez-Carmona MA, Wojtalla A, Krämer B, Nattermann J, Siegmund SV, Werner N, Fürst DO, Laleman W, Knolle P, Shah VH, Sauerbruch T, Trebicka J. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology. 2014;60:334-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Colmenero J, Bataller R, Sancho-Bru P, Domínguez M, Moreno M, Forns X, Bruguera M, Arroyo V, Brenner DA, Ginès P. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol. 2009;297:G726-G734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Butt AA, Yan P, Bonilla H, Abou-Samra AB, Shaikh OS, Simon TG, Chung RT, Rogal SS; ERCHIVES (Electronically Retrieved Cohort of HCV Infected Veterans) Study Team. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons: Results from ERCHIVES. Hepatology. 2015;62:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Verrill C, Markham H, Templeton A, Carr NJ, Sheron N. Alcohol-related cirrhosis--early abstinence is a key factor in prognosis, even in the most severe cases. Addiction. 2009;104:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Trabut JB, Thépot V, Nalpas B, Lavielle B, Cosconea S, Corouge M, Vallet-Pichard A, Fontaine H, Mallet V, Sogni P, Pol S. Rapid decline of liver stiffness following alcohol withdrawal in heavy drinkers. Alcohol Clin Exp Res. 2012;36:1407-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Gelsi E, Dainese R, Truchi R, Mariné-Barjoan E, Anty R, Autuori M, Burroni S, Vanbiervliet G, Evesque L, Cherikh F, Tran A. Effect of detoxification on liver stiffness assessed by Fibroscan® in alcoholic patients. Alcohol Clin Exp Res. 2011;35:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Kim MY, Cho MY, Baik SK, Jeong PH, Suk KT, Jang YO, Yea CJ, Kim JW, Kim HS, Kwon SO, Yoo BS, Kim JY, Eom MS, Cha SH, Chang SJ. Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis - a randomized open-label controlled study. Liver Int. 2012;32:977-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis--Update 2015. J Hepatol. 2015;62:S100-S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 45. | Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Mohamadnejad M, Malekzadeh R, Nasseri-Moghaddam S, Hagh-Azali S, Rakhshani N, Tavangar SM, Sedaghat M, Alimohamadi SM. Impact of immunosuppressive treatment on liver fibrosis in autoimmune hepatitis. Dig Dis Sci. 2005;50:547-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Malekzadeh Z, Haghazali S, Sepanlou SG, Vahedi H, Merat S, Sotoudeh M, Nasseri-Moghaddam S, Malekzadeh R. Clinical features and long term outcome of 102 treated autoimmune hepatitis patients. Hepat Mon. 2012;12:92-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Montano-Loza AJ, Thandassery RB, Czaja AJ. Targeting Hepatic Fibrosis in Autoimmune Hepatitis. Dig Dis Sci. 2016;61:3118-3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020; 158: 1999-2014. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2200] [Article Influence: 440.0] [Reference Citation Analysis (1)] |
| 50. | Ganguli S, DeLeeuw P, Satapathy SK. A Review of Current and Upcoming Treatment Modalities In Non-Alcoholic Fatty Liver Disease And Non-Alcoholic Steatohepatitis. Hepat Med. 2019;11:159-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Hannah WN Jr, Harrison SA. Effect of Weight Loss, Diet, Exercise, and Bariatric Surgery on Nonalcoholic Fatty Liver Disease. Clin Liver Dis. 2016;20:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 52. | Hohenester S, Christiansen S, Nagel J, Wimmer R, Artmann R, Denk G, Bischoff M, Bischoff G, Rust C. Lifestyle intervention for morbid obesity: effects on liver steatosis, inflammation, and fibrosis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G329-G338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 53. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 971] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 54. | Bower G, Toma T, Harling L, Jiao LR, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. Bariatric Surgery and Non-Alcoholic Fatty Liver Disease: a Systematic Review of Liver Biochemistry and Histology. Obes Surg. 2015;25:2280-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 55. | Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 351] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 56. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4939] [Article Influence: 705.6] [Reference Citation Analysis (9)] |
| 57. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2467] [Article Influence: 164.5] [Reference Citation Analysis (2)] |
| 58. | Dolegowska K, Marchelek-Mysliwiec M, Nowosiad-Magda M, Slawinski M, Dolegowska B. FGF19 subfamily members: FGF19 and FGF21. J Physiol Biochem. 2019;75:229-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 59. | Harrison SA, Rossi SJ, Paredes AH, Trotter JF, Bashir MR, Guy CD, Banerjee R, Jaros MJ, Owers S, Baxter BA, Ling L, DePaoli AM. NGM282 Improves Liver Fibrosis and Histology in 12 Weeks in Patients With Nonalcoholic Steatohepatitis. Hepatology. 2020;71:1198-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 60. | Harrison SA, Rinella ME, Abdelmalek MF, Trotter JF, Paredes AH, Arnold HL, Kugelmas M, Bashir MR, Jaros MJ, Ling L, Rossi SJ, DePaoli AM, Loomba R. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 348] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 61. | Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, Lawitz EJ, Halegoua-DeMarzio D, Kundu S, Noviello S, Luo Y, Christian R. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 420] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 62. | Bergman A, Carvajal-Gonzalez S, Tarabar S, Saxena AR, Esler WP, Amin NB. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of a Liver-Targeting Acetyl-CoA Carboxylase Inhibitor (PF-05221304): A Three-Part Randomized Phase 1 Study. Clin Pharmacol Drug Dev. 2020;9:514-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 63. | Oseini AM, Sanyal AJ. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. 2017;37 Suppl 1:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 64. | Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632-1643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 496] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 65. | Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013; 145: 574-82. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 734] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 66. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1793] [Article Influence: 179.3] [Reference Citation Analysis (3)] |
| 67. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 922] [Article Influence: 153.7] [Reference Citation Analysis (0)] |
| 68. | Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem. 2004;136:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 266] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 69. | Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP, Subramanian GM, McHutchison JG, Goodman ZD, Afdhal NH, Charlton MR; GS-US-384-1497 Investigators. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology. 2018;67:549-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 440] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 70. | Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A; GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016; 150: 1147-1159. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 819] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 71. | Tacke F. Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis. Expert Opin Investig Drugs. 2018;27:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 72. | Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 533] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 73. | Ratziu V, Sanyal A, Harrison SA, Wong VW, Francque S, Goodman Z, Aithal GP, Kowdley KV, Seyedkazemi S, Fischer L, Loomba R, Abdelmalek MF, Tacke F. Cenicriviroc Treatment for Adults With Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 74. | Kovalic AJ, Satapathy SK, Chalasani N. Targeting incretin hormones and the ASK-1 pathway as therapeutic options in the treatment of non-alcoholic steatohepatitis. Hepatol Int. 2018;12:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team; Abouda G; Aldersley MA; Stocken D; Gough SC; Tomlinson JW; Brown RM; Hübscher SG; Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1464] [Article Influence: 162.7] [Reference Citation Analysis (1)] |
| 76. | Care TAJoM. Novo Nordisk's Semaglutide Shows Promise in Treating NASH. 2020. |
| 77. | Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 1058] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 78. | Cioboată R, Găman A, Traşcă D, Ungureanu A, Docea AO, Tomescu P, Gherghina F, Arsene AL, Badiu C, Tsatsakis AM, Spandidos DA, Drakoulis N, Călina D. Pharmacological management of non-alcoholic fatty liver disease: Atorvastatin vs pentoxifylline. Exp Ther Med. 2017;13:2375-2381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Schierwagen R, Uschner FE, Magdaleno F, Klein S, Trebicka J. Rationale for the use of statins in liver disease. Am J Physiol Gastrointest Liver Physiol. 2017;312:G407-G412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 80. | Kasia C, Scaglione SJ. Patients With Chronic Liver Disease/Cirrhosis Should Not Take Statin Medications. Clin Liver Dis (Hoboken). 2019;13:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Carbone M, Nardi A, Flack S, Carpino G, Varvaropoulou N, Gavrila C, Spicer A, Badrock J, Bernuzzi F, Cardinale V, Ainsworth HF, Heneghan MA, Thorburn D, Bathgate A, Jones R, Neuberger JM, Battezzati PM, Zuin M, Taylor-Robinson S, Donato MF, Kirby J, Mitchell-Thain R, Floreani A, Sampaziotis F, Muratori L, Alvaro D, Marzioni M, Miele L, Marra F, Giannini E, Gaudio E, Ronca V, Bonato G, Cristoferi L, Malinverno F, Gerussi A, Stocken DD, Cordell HJ, Hirschfield GM, Alexander GJ, Sandford RN, Jones DE, Invernizzi P, Mells GF; Italian PBC Study Group and the UK–PBC Consortium. Pretreatment prediction of response to ursodeoxycholic acid in primary biliary cholangitis: development and validation of the UDCA Response Score. Lancet Gastroenterol Hepatol. 2018;3:626-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 82. | Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology. 2000;32:1196-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 83. | Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, Färkkilä M, Schramm C, Spengler U, Chapman R, Bergquist A, Schrumpf E, Nevens F, Trivedi P, Reiter FP, Tornai I, Halilbasic E, Greinwald R, Pröls M, Manns MP, Trauner M; European PSC norUDCA Study Group. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 84. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ; American Association for the Study of Liver Diseases. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 85. | Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D; POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 799] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 86. | Bowlus CL, Pockros PJ, Kremer AE, Parés A, Forman LM, Drenth JPH, Ryder SD, Terracciano L, Jin Y, Liberman A, Pencek R, Iloeje U, MacConell L, Bedossa P. Long-Term Obeticholic Acid Therapy Improves Histological Endpoints in Patients With Primary Biliary Cholangitis. Clin Gastroenterol Hepatol 2020; 18: 1170-1178. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 87. | Goldstein J, Levy C. Novel and emerging therapies for cholestatic liver diseases. Liver Int. 2018;38:1520-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 88. | Corpechot C, Chazouillères O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, Goria O, Potier P, Minello A, Silvain C, Abergel A, Debette-Gratien M, Larrey D, Roux O, Bronowicki JP, Boursier J, de Ledinghen V, Heurgue-Berlot A, Nguyen-Khac E, Zoulim F, Ollivier-Hourmand I, Zarski JP, Nkontchou G, Lemoinne S, Humbert L, Rainteau D, Lefèvre G, de Chaisemartin L, Chollet-Martin S, Gaouar F, Admane FH, Simon T, Poupon R. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N Engl J Med. 2018;378:2171-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 392] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 89. | Mehta KJ, Farnaud SJ, Sharp PA. Iron and liver fibrosis: Mechanistic and clinical aspects. World J Gastroenterol. 2019;25:521-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 132] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (7)] |
| 90. | Falize L, Guillygomarc'h A, Perrin M, Lainé F, Guyader D, Brissot P, Turlin B, Deugnier Y. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology. 2006;44:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 91. | Nielsen P, Fischer R, Buggisch P, Janka-Schaub G. Effective treatment of hereditary haemochromatosis with desferrioxamine in selected cases. Br J Haematol. 2003;123:952-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Deugnier Y, Turlin B, Ropert M, Cappellini MD, Porter JB, Giannone V, Zhang Y, Griffel L, Brissot P. Improvement in liver pathology of patients with β-thalassemia treated with deferasirox for at least 3 years. Gastroenterology 2011; 141: 1202-1211, 1211.e1-1211. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Saab S, Mallam D, Cox GA 2nd, Tong MJ. Impact of coffee on liver diseases: a systematic review. Liver Int. 2014;34:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 94. | Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, Hoofnagle JH. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010;51:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 95. | Alferink LJM, Fittipaldi J, Kiefte-de Jong JC, Taimr P, Hansen BE, Metselaar HJ, Schoufour JD, Ikram MA, Janssen HLA, Franco OH, Darwish Murad S. Coffee and herbal tea consumption is associated with lower liver stiffness in the general population: The Rotterdam study. J Hepatol. 2017;67:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 96. | Moreno M, Gonzalo T, Kok RJ, Sancho-Bru P, van Beuge M, Swart J, Prakash J, Temming K, Fondevila C, Beljaars L, Lacombe M, van der Hoeven P, Arroyo V, Poelstra K, Brenner DA, Ginès P, Bataller R. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology. 2010;51:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Czechowska G, Celinski K, Korolczuk A, Wojcicka G, Dudka J, Bojarska A, Madro A, Brzozowski T. The effect of the angiotensin II receptor, type 1 receptor antagonists, losartan and telmisartan, on thioacetamide-induced liver fibrosis in rats. J Physiol Pharmacol. 2016;67:575-586. [PubMed] |
| 98. | Kim G, Kim J, Lim YL, Kim MY, Baik SK. Renin-angiotensin system inhibitors and fibrosis in chronic liver disease: a systematic review. Hepatol Int. 2016;10:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Ebrahimi H, Naderian M, Sohrabpour AA. New Concepts on Reversibility and Targeting of Liver Fibrosis; A Review Article. Middle East J Dig Dis. 2018;10:133-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004;308:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 101. | Harrison SA, Goodman Z, Jabbar A, Vemulapalli R, Younes ZH, Freilich B, Sheikh MY, Schattenberg JM, Kayali Z, Zivony A, Sheikh A, Garcia-Samaniego J, Satapathy SK, Therapondos G, Mena E, Schuppan D, Robinson J, Chan JL, Hagerty DT, Sanyal AJ. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J Hepatol. 2020;72:816-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 102. | Garcia-Tsao G, Bosch J, Kayali Z, Harrison SA, Abdelmalek MF, Lawitz E, Satapathy SK, Ghabril M, Shiffman ML, Younes ZH, Thuluvath PJ, Berzigotti A, Albillos A, Robinson JM, Hagerty DT, Chan JL, Sanyal AJ; IDN-6556-14 Investigators(‡). Randomized placebo-controlled trial of emricasan for non-alcoholic steatohepatitis-related cirrhosis with severe portal hypertension. J Hepatol. 2020;72:885-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 103. | Dibba P, Li AA, Cholankeril G, Iqbal U, Gadiparthi C, Khan MA, Kim D, Ahmed A. The Role of Cannabinoids in the Setting of Cirrhosis. Medicines (Basel). 2018;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Melgar-Lesmes P, Perramon M, Jiménez W. Roles of the Hepatic Endocannabinoid and Apelin Systems in the Pathogenesis of Liver Fibrosis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 105. | Teixeira-Clerc F, Belot MP, Manin S, Deveaux V, Cadoudal T, Chobert MN, Louvet A, Zimmer A, Tordjmann T, Mallat A, Lotersztajn S. Beneficial paracrine effects of cannabinoid receptor 2 on liver injury and regeneration. Hepatology. 2010;52:1046-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 106. | Giannone FA, Baldassarre M, Domenicali M, Zaccherini G, Trevisani F, Bernardi M, Caraceni P. Reversal of liver fibrosis by the antagonism of endocannabinoid CB1 receptor in a rat model of CCl(4)-induced advanced cirrhosis. Lab Invest. 2012;92:384-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | Guillot A, Hamdaoui N, Bizy A, Zoltani K, Souktani R, Zafrani ES, Mallat A, Lotersztajn S, Lafdil F. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology. 2014;59:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 108. | Parsons CJ, Bradford BU, Pan CQ, Cheung E, Schauer M, Knorr A, Krebs B, Kraft S, Zahn S, Brocks B, Feirt N, Mei B, Cho MS, Ramamoorthi R, Roldan G, Ng P, Lum P, Hirth-Dietrich C, Tomkinson A, Brenner DA. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 109. | Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 670] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 110. | Muir AJ, Levy C, Janssen HLA, Montano-Loza AJ, Shiffman ML, Caldwell S, Luketic V, Ding D, Jia C, McColgan BJ, McHutchison JG, Mani Subramanian G, Myers RP, Manns M, Chapman R, Afdhal NH, Goodman Z, Eksteen B, Bowlus CL; GS-US-321-0102 Investigators. Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results With Insights on the Natural History of the Disease. Hepatology. 2019;69:684-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 111. | Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest. 1996;98:1381-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 152] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 112. | Eriksson C, Gustavsson A, Kronvall T, Tysk C. Hepatotoxicity by bosentan in a patient with portopulmonary hypertension: a case-report and review of the literature. J Gastrointestin Liver Dis. 2011;20:77-80. [PubMed] |
| 113. | Bierie B, Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev. 2006;17:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 325] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 114. | Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology. 2007;46:1404-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 115. | Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 684] [Cited by in RCA: 691] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 116. | Trappoliere M, Caligiuri A, Schmid M, Bertolani C, Failli P, Vizzutti F, Novo E, di Manzano C, Marra F, Loguercio C, Pinzani M. Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J Hepatol. 2009;50:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 117. | Navarro VJ, Belle SH, D'Amato M, Adfhal N, Brunt EM, Fried MW, Reddy KR, Wahed AS, Harrison S; Silymarin in NASH and C Hepatitis (SyNCH) Study Group. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: A randomized, double-blind, placebo controlled trial. PLoS One. 2019;14:e0221683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 118. | Shu JC, He YJ, Lv X, Ye GR, Wang LX. Curcumin prevents liver fibrosis by inducing apoptosis and suppressing activation of hepatic stellate cells. J Nat Med. 2009;63:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 119. | Luk JM, Wang X, Liu P, Wong KF, Chan KL, Tong Y, Hui CK, Lau GK, Fan ST. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: from laboratory discovery to clinical evaluation. Liver Int. 2007;27:879-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 120. | Liu P, Hu YY, Liu C, Zhu DY, Xue HM, Xu ZQ, Xu LM, Liu CH, Gu HT, Zhang ZQ. Clinical observation of salvianolic acid B in treatment of liver fibrosis in chronic hepatitis B. World J Gastroenterol. 2002;8:679-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 121. | Latief U, Ahmad R. Herbal remedies for liver fibrosis: A review on the mode of action of fifty herbs. J Tradit Complement Med. 2018;8:352-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 122. | Lopez-de la Mora DA, Sanchez-Roque C, Montoya-Buelna M, Sanchez-Enriquez S, Lucano-Landeros S, Macias-Barragan J, Armendariz-Borunda J. Role and New Insights of Pirfenidone in Fibrotic Diseases. Int J Med Sci. 2015;12:840-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 123. | Verzijl CRC, Van De Peppel IP, Struik D, Jonker JW. Pegbelfermin (BMS-986036): an investigational PEGylated fibroblast growth factor 21 analogue for the treatment of nonalcoholic steatohepatitis. Expert Opin Investig Drugs. 2020;29:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |